Abstract
Background:
Special consideration should be given when creating and selecting cytopathology specimens for digitization to maximize quality. Advances in scanning and viewing technology can also improve whole-slide imaging (WSI) output quality.
Methods:
Accumulated laboratory experience with digitization of glass cytopathology slides was collected.
Results:
This paper describes characteristics of a cytopathology glass slide that can reduce quality on resulting WSI. Important points in the glass cytopathology slide selection process, preparation, scanning, and WSI-editing process that will maximize the quality of the resulting acquired digital image are covered. The paper outlines scanning solutions which have potential to predict issues with a glass cytopathology slide before image acquisition, allowing for adjustment of the scanning approach. WSI viewing solutions that better simulate the traditional microscope experience are also discussed.
Conclusion:
In addition to taking advantage of technical advances, practical steps can taken to maximize quality of cytopathology WSI.
Keywords: Cytopathology, digital pathology, quality assurance, virtual microscopy, virtual pathology, virtual slides, vslides, whole-slide imaging
INTRODUCTION
Digital surgical pathology is now accepted as equivalent to traditional microscopy for diagnostic accuracy,[1,2,3,4,5,6] with z-axis capability[7] or specific scanning parameters[5] required for accuracy in some small biopsy specimens. Overall, studies show that diagnostic accuracy using digital cytopathology is not inferior and is sometimes superior,[8] to using a glass slide and microscope,[8,9,10,11,12] but results are dependent on the number of planes acquired in the z-axis on whole-slide imaging (WSI), the distance between planes and whether the z-stacks are attached as focal annotations.[8,9,10,12] Although acceptance of digital cytopathology is increasing (in parallel with z-axis capability),[9] perception overall is that it is still inadequate for diagnosis and proficiency testing.[12,13,14] The flaws in using digital cytopathology are regularly listed.[14] Reasons have been succinctly outlined by Hanna and Pantanowitz[9,15] and include cytopathology specimens containing three-dimensional cell groups and dispersed cells at different focal planes. There are also a variety of cytological specimen types and preparation methods, the diversity of which can produce a challenge when digitizing the slides. Navigating a digital slide can be slow, and this is exacerbated when material on the slide is scant.[8,9,13,14] Studies analyzing which imaging system produces more truthful digital replicas of their glass counterparts are emerging and are a step toward improving quality.[9,16,17] Useful information on the optimal z-axis scanning parameters is being collected.[8,10] However, we need to move closer toward a z-axis image acquisition and viewing solution that better simulates the smooth exploration of a glass slide when a traditional microscope is used.
We also need to modify our approach and concentrate on how to increase the quality of the original glass specimen to maximize the quality of its digital counterpart. Although Hanna et al. and Van Es. have touched on this,[9,15,18,19] there is scant literature covering these last two points.[9,15,18,19,20] The answer to perfecting digital cytopathology may well be in the modification of the specimen to improve the suitability for scanning.[15,18,19] In other words, improve the quality of the original specimen and preparation as well as the resulting WSI.
Historically with cytopathology samples, it can be difficult to predict how well the slides will scan. Due to the three-dimensional nature of the cytological material which is often red blood cell contaminated, the preliminary scans are often unsuitable. Special consideration should be given to the original specimen when selecting cytopathology glass slides for digitization. In addition, this selection process should feedback into modifying our technique of specimen acquisition to ultimately improve the quality of the resulting digitized slide.
Research tells us that accuracy, efficiency, and acceptance of digital pathology improve with time and experience.[2,12,21,22] Thus, the aim of this article is to report our collective experience in improving the quality of the digital cytopathology slide from specimen collection, glass slide selection, and preparation through to our experience with scanning parameters, troubleshooting, editing, and finally, viewing of the final WSI.
APPROACH: HOW DO WE IMPROVE THE QUALITY OF CYTOPATHOLOGY WHOLE-SLIDE IMAGING?
The specimen
Slide selection
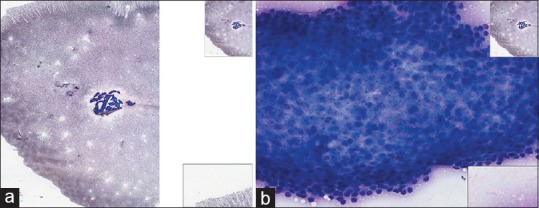
What makes good glass slides, usually also makes good WSI [Table 1]. To maximize the quality of the final image, glass slides need evidence of good cellular preservation, and ideally, the slide should have been recently stained. The slide needs to be free of dense tissue fragments [Figure 1a and b]. Marked red cell contamination on the glass slide [Figure 2a and b] may cause the scanner to base the focal plane on the red cells in the background, rather than on the diagnostic cells, making effective scanning of the cells of interest more difficult. In addition, red cell contamination can produce dense-clotted aggregates of diagnostic material making the diagnostic cells more difficult to see on the WSI. Overstained glass slides also do not scan well, with the nuclear detail often obscured.
Table 1.
Ideal characteristics of a glass slide to acquire good quality whole-slide imaging
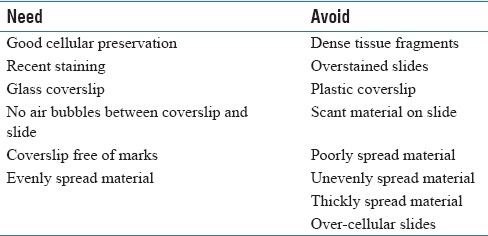
Figure 1.
(a) Fine-needle aspiration arm (Glomus tumor), Diff-Quik, low power. Dense-tissue fragments are difficult to interpret on the whole-slide imaging. (b) Fine-needle aspiration arm (Glomus tumor), Diff-Quik, high power. Dense tissue fragments are difficult to interpret on the whole slide imaging
Figure 2.
(a) Fine-needle aspiration thyroid (cyst), Papanicolaou, low power. This Papanicolaou-stained specimen is heavily red blood cell contaminated. (b) Fine-needle aspiration thyroid (cyst), Papanicolaou, high power. This Papanicolaou stained specimen is heavily red blood cell contaminated obscuring focus on diagnostic cells at high power
The scant material on a slide presents issues as well. Glass slides containing scant material can result in cells that are often widely dispersed over multiple focal planes in the z-axis. This makes the resulting WSI difficult to maneuver and navigate increasing the time to screen a slide and increasing the risk of missing diagnostic material. In addition, the more focal planes in the z-axis over which the cells are dispersed in the specimen, the more likely diagnostic material may not be included in the acquired focal planes, even when z-stacking is available. In contrast, a hypercellular slide may not scan well, and diagnostic cells may be missed in the dark aggregated material.
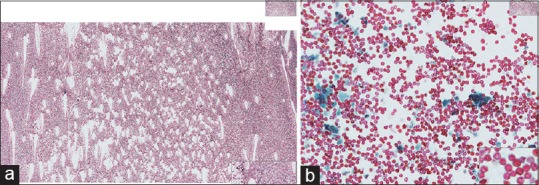
It is important to note that even cellular smears will scan very well if the material is spread evenly [Figure 3a and b]. Specimens need to consist of a thin layer of specimen free of dark tissue cells groups [Figure 4]. A specimen that is poorly spread or thickly spread can make scanning and viewing of cytopathology WSI suboptimal [Figure 5a and b and 6a and b].
Figure 3.
(a) Fine-needle aspiration (metastatic melanoma), Papanicolaou, high power. This slide ticks all the boxes. It is well-spread, well-fixed, and well-stained, resulting in a high-quality whole-slide imaging even though the slide is cellular. (b) Fine-needle aspiration (metastatic melanoma), Diff-Quik, high power. This slide ticks all the boxes. It is well-spread, well-fixed, and well-stained, resulting in a high-quality whole slide imaging even though the slide is cellular
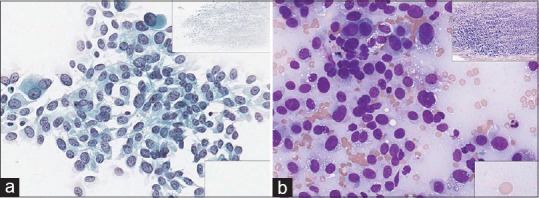
Figure 4.
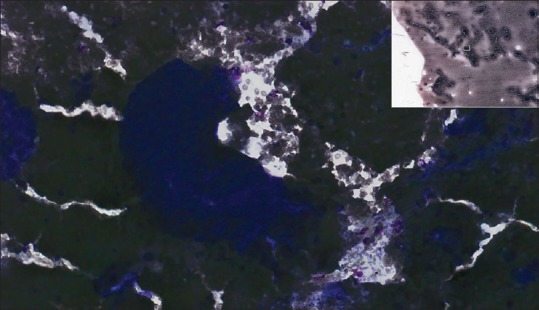
Fine-needle aspiration lung (adenocarcinoma), Diff-Quik, high power. This slide is hypercellular and over-stained with dark aggregated material and did not scan well
Figure 5.
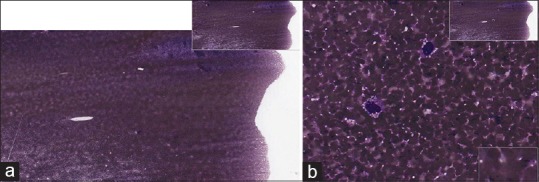
(a) Fine-needle aspiration breast implant seroma (anaplastic large cell lymphoma), Diff-Quik, low power. The material on this slide is unevenly spread, thick and red blood cell contaminated. (b) Fine-needle aspiration breast implant seroma (anaplastic large cell lymphoma), Diff-Quik, high power. The material on this slide is unevenly spread, thick, and red blood cell contaminated
Figure 6.
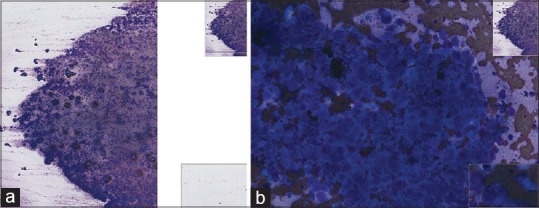
(a) Fine-needle aspiration submandibular mass (small cell carcinoma), Diff-Quik, low power. The material on this slide is thickly and unevenly spread resulting in poor quality whole slide imaging. (b) Fine-needle aspiration submandibular mass (small cell carcinoma), Diff-Quik, high power. The material on this slide is thickly and unevenly spread resulting in poor quality whole slide imaging
There must be no lifting or air bubbles between the slide and cover. Glass coverslips tend to produce better quality WSI compared to plastic, which scratches more easily during laboratory processing as well as during the cleaning and preparation process. This can result in suboptimal grainy image acquisition or WSI that are out of focus with the scanner having concentrated on the marked coverslip rather than the cellular material [Figure 7a and b].
Figure 7.
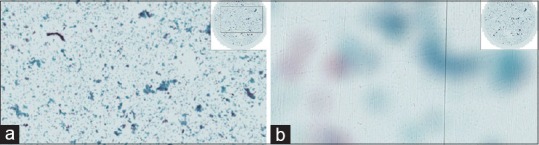
(a) Plastic coverslips sometimes scratch during cleaning resulting in whole slide imaging quality issues; large scratches can be seen even at low power resulting in a grainy appearance to the image. Papanicolaou, low power. (b) This is the same slide with plastic scratched coverslip-note how the scanner has focused on the scratches in the plane of the coverslip rather than on the sample itself. Papanicolaou, high power
Glass slide and coverslip (whether glass or plastic) need to be free of marks. This includes nonintentional scratches and cracks as well as screening marks acquired during the diagnostic process. Having a record of the diagnostic screening marks is useful, for example, by taking a photocopy, scan, or photo before the marks are wiped from the coverslip. The photographic record may need to be part of the medical record for recent cases or may be an aid when creating instructive educational annotations for the WSI teaching cases.
The glass specimen also needs to be one slide thick. Common laboratory practice with a broken slide is to reinforce it by gluing to a second glass slide. This type of specimen is unsuitable for scanning.
The preparation
Glass slides need to be cleaned and checked to be free of marks both to the macroscopic and microscopic eye. This includes removing screening marks, fingerprints, and dust, before scanning. An ideal cleaning solution is isopropyl alcohol (alcohol wipes) with glass slides then being dried with soft-tissue wipes.
Not all of the glass slide may be suitable for scanning. There may be areas of “dead space” on the slide or there may also be suboptimal areas that predictably may not scan well. Demarcating, defining, or “marking-up” suitable areas on the glass slide with a marking pen to flag these optimal areas to the scientist responsible for scanning the slide, may improve the quality of the WSI.
Scanning
The cleaned and “marked up” glass slides should be photographed again at this step of the preparation process. The marked up area should be noted and the glass slide cleaned again with an alcohol wipe to remove marks completely. An image is acquired of the areas that have been “marked up” by referring to the previous photographic record of the glass slide.
Papanicolaou (Pap)-stained wet-fixed specimens usually contain cells in multiple planes and do benefit from z-stacking to improve WSI quality. Air-dried Romanowsky-stained smears, in contrast, often scan well using only the x-and y-axis. The air-drying process tends to flatten-out cell collections and enlarges them, minimizing the need for acquiring the image in the z-axis [Figure 8]. Hematoxylin and eosin and immunoperoxidase-stained slides from cell blocks often present in a monolayer and acquiring the image in the x-and y-axes only are usually sufficient [Figure 9].
Figure 8.
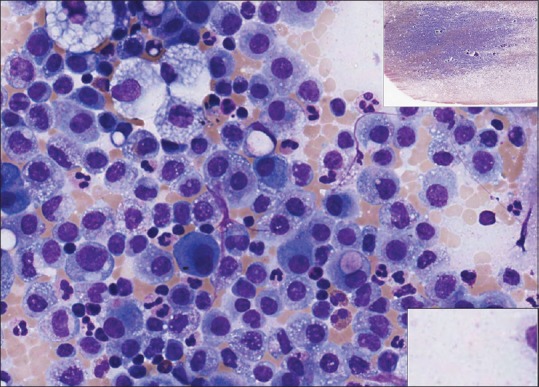
Ascitic fluid (metastatic adenocarcinoma), Diff-Quik, high power. Air drying tends to flatten out cell collections and enlarge them, minimizing the need for z-axis scanning
Figure 9.
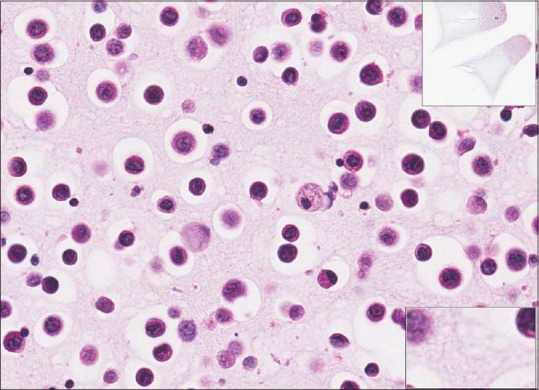
Ascitic fluid (metastatic gastric adenocarcinoma) H&E, high power. Slides prepared from cell blocks are presented mostly in a monolayer and scanning in x-and y-axes only may produce good quality whole slide imaging
The scanner
Aperio ScanScope™ XT Digital Slide Scanning System (Leica Biosystems North Ryde, Sydney, Australia) is used in the RCPAQAP (Royal College of Pathologists of Australasia Quality Assurance Programs) laboratory. It is employed with a ×20 lens and a doubler to ×40 for air-dried specimens and slides resulting from cell blocks. The Aperio scanner can z-stack annotations. These annotated z-stacks need to be inserted manually and are placed focally across the slide, thereby creating a diagnostic cytopathology image rather than a screening image because the areas of interest are highlighted in advance for the viewer. This can be an issue for WSI being used in an external quality assurance or examination setting.
Wet-fixed Pap-stained specimens are ideally acquired in the z-axis. To better simulate a traditional microscope, smooth three-dimensional focus over the entire WSI is important in cytopathology. Zeiss Axio imager Z2 microscope (Carl Zeiss, Germany) and Metafer Vslide scanning system (MetaSystems, Altlussheim, Germany) have given the laboratory the ability to automatically z-stack entire Pap-stained wet-fixed slides allowing focus into cell collections on different planes in the z-axis on appropriately selected slides. In practice, we have found the ideal number of focal planes to be seven providing a practical balance between quality versus file size and storage. This latest scanning technology (Metasystems/Zeiss) also analyses cell distribution topography on the glass slide before scanning. This allows those specimens with a narrow range of cell distribution planes to be selected for the scanning process [Figure 10a and b] and those with a wide range to be rejected before scanning. Those specimens with a wide range of cell distribution planes may cause quality issues on the WSI; capturing a desirable number of cells in the specified number of z-axis planes may be more difficult [Figure 11a–c]. This technology gives the option of rejecting such slides before scanning in z-axis.
Figure 10.
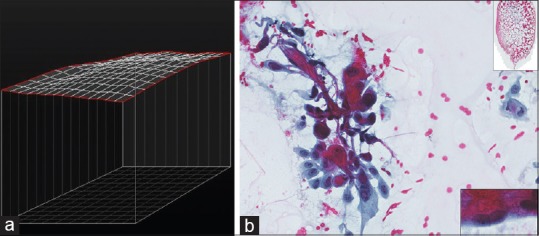
(a) Fine-needle aspiration bone (metastatic squamous cell carcinoma). Narrower distribution planes of cells is reflected in a flatter topography map and result in a better scan. (b) Fine-needle aspiration bone (metastatic squamous cell carcinoma), Papanicolaou, high power with low-power inset. Narrower distribution planes of cells result in a better scan
Figure 11.
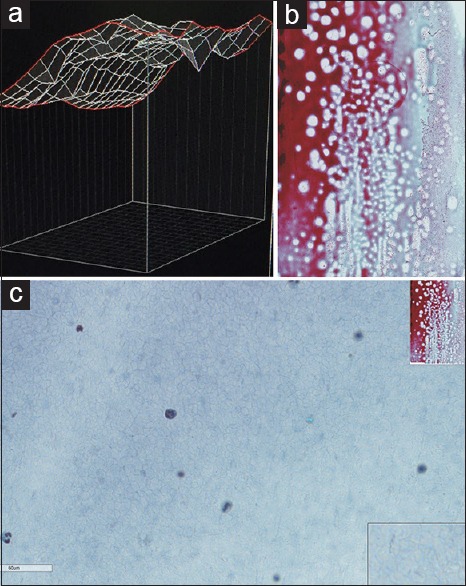
(a) Ascitic fluid (carcinosarcoma) cell distribution analysis showing the distribution of cells across a wide range of planes in the topography map. (b) Ascitic fluid (carcinosarcoma), Papanicolaou, low power. The whole slide imaging related to the topographical image in Figure 11a. (c) Ascitic fluid (carcinosarcoma), Papanicolaou, high power. High power of the same whole-slide imaging reveals scant cells captured in the acquired z-axis focal planes
The editing and troubleshooting
Checking the final WSI to identify problems and troubleshoot issues with the scanner or slide itself are important steps in the quality process for cytopathology WSI. WSI are assessed for quality issues such as poorly focused areas or unusual marks either from the slide itself or created due to an error in the image acquisition [Figure 12]. WSI can then be cropped if necessary to remove nonfocused or non-diagnostic areas. They then need to be quality approved by trained staff.
Figure 12.
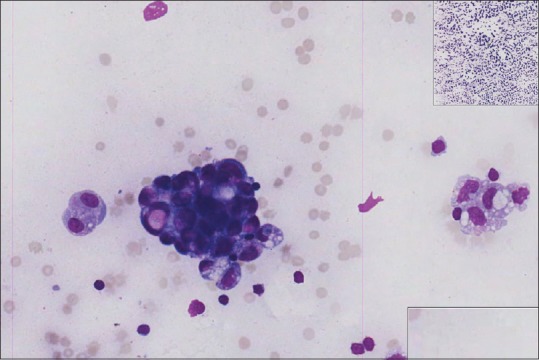
Ascitic fluid (metastatic ovarian carcinoma), Diff-Quik, high power. Editing: The cause of pink vertical lines needs to be identified (is the origin from the glass slide or a technical problem with the scanner?) and resolved
The viewing solution
The cytopathology WSI are stored in the Sectra digital pathology solution (Sectra AB, Linköping, Sweden) and viewed with Sectra Uniview. Sectra's digital pathology solution and zero-footprint viewer improves on RCPAQAP's previous imaging solution by delivering a smooth view of whole-slide z-stacked images using smart streaming technology and negating the need for large software installations. The zero-footprint viewer allows for the use of mobile devices and viewing over lower bandwidth connections, enabling access to digital pathology images in remote areas, and providing a more consistent user experience in difficult image disciplines, such as cytopathology.
CONCLUSION
Hanna and Pantanowitz[15] are correct in their statement that cytopathology includes a variety of specimen types, including Pap tests, fine-needle aspirations, and exfoliative samples. This is part of the cytopathology digitization problem and each of these unique samples needs to be approached differently during image acquisition. Different preparations such as direct smears, liquid-based cytology, and cell blocks also require a unique approach. Hurdles need to be overcome in digital cytopathology to maximize quality.
Rapid technological advances have helped to improve the quality of cytopathology WSI. However, there are further potential steps that can be taken to maximize quality. Improving practitioner technique in acquiring our cytological specimens, based on the points raised in this article, as well as modifying the type of cytological preparations that we use for scanning, will go a long way toward improving quality of digital cytopathology for diagnosis, proficiency testing, and education. Improving practitioner technique during collection, transportation, and laboratory preparation is important. Minimizing trauma at the collection of an fine-needle aspiration and thus reducing red blood cell contamination, as well transporting the specimen to the laboratory for processing in a timely manner to minimize cell deterioration are both important. Rapid on-site evaluation for immediate triage and slide preparation may also reduce specimen clotting, allow removal of tissue fragments for embedding and immediate and appropriate fixation to maintain cellular preservation. Improving practitioner technique in the laboratory preparation of specimens is important to produce the well-fixed, evenly spread, and well-stained specimens that maximize the resulting WSI quality.
To maximize the quality of cytopathology WSI, cytopathology specimens also need to be specifically chosen, favoring specimens with a more monolayered topography if possible. Utilization of liquid-based cytology and cell blocks may help to maximize the quality of the digital result. Additional slide characteristics also need to be considered such as the age of stained slide and coverslip characteristics.
Editing WSI postscanning with crop and extract of adequately scanned areas may also improve the quality of the result. This procedure may be an option not only for educational and proficiency testing in cytopathology but also for diagnosis. Similarly, traditional glass slide and microscope evaluation of specimens may not always utilize the entire specimen collection for diagnosis as the diagnostic material may remain unprocessed due to volume and cellularity. Others have found that digitizing less than the entire glass specimen did not compromise accuracy.[12]
Hanna and Pantanowitz refer to litigation cells.[15] They correctly point out that a troublesome specimen is one where there are scant cells, particularly where it is difficult to maneuver the computer mouse and keyboard arrows to screen and locate these diagnostic cells that may be hidden among background material. This is where automated topographical analysis may help. The glass slide is rejected for scanning, and the original specimen is revisited (and if possible a repeat more optimal slide preparation utilized).
With the use of flat-tissue sections in surgical pathology, there are fewer topographic variations. However, with cytology samples importance of z-axis scanning is underscored by the distribution of cells in a range of focal planes in the z-axis. With advances in cell layer topographical analysis in scanning solutions, cytological samples appropriate for scanning can be selected or rejected in advance, speeding up the workflow, as well as providing valuable feedback to the laboratory as to which specimens need to be modified or even which pathologists or cytologists creating the specimens need to modify their technique to make glass slide specimens more suitable for scanning in the future.
Scanning the specimen in multiple z-axis planes and compiling these images into a composite for viewing can address some of the cytopathology quality issues. Z-stacking does produce larger digital files which can be an issue resulting with slow loading of image files and pixilation of images. However, the combined Zeiss/MetaSystems and Sectra viewing solution discussed provides a seamless experience even with heavily z-stacked slides.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Available FREE in open access from: http://www.jpathinformatics.org/text.asp?2018/9/1/13/229631.
REFERENCES
- 1.Houghton JP, Ervine AJ, Kenny SL, Kelly PJ, Napier SS, McCluggage WG, et al. Concordance between digital pathology and light microscopy in general surgical pathology: A pilot study of 100 cases. J Clin Pathol. 2014;67:1052–5. doi: 10.1136/jclinpath-2014-202491. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen PS, Lindebjerg J, Rasmussen J, Starklint H, Waldstrøm M, Nielsen B, et al. Virtual microscopy: An evaluation of its validity and diagnostic performance in routine histologic diagnosis of skin tumors. Hum Pathol. 2010;41:1770–6. doi: 10.1016/j.humpath.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Fallon MA, Wilbur DC, Prasad M. Ovarian frozen section diagnosis: Use of whole-slide imaging shows excellent correlation between virtual slide and original interpretations in a large series of cases. Arch Pathol Lab Med. 2010;134:1020–3. doi: 10.5858/2009-0320-OA.1. [DOI] [PubMed] [Google Scholar]
- 4.Griffin J, Treanor D. Digital pathology in clinical use: Where are we now and what is holding us back? Histopathology. 2017;70:134–45. doi: 10.1111/his.12993. [DOI] [PubMed] [Google Scholar]
- 5.Snead DR, Tsang YW, Meskiri A, Kimani PK, Crossman R, Rajpoot NM, et al. Validation of digital pathology imaging for primary histopathological diagnosis. Histopathology. 2016;68:1063–72. doi: 10.1111/his.12879. [DOI] [PubMed] [Google Scholar]
- 6.Thorstenson S, Molin J, Lundström C. Implementation of large-scale routine diagnostics using whole slide imaging in Sweden: Digital pathology experiences 2006-2013. J Pathol Inform. 2014;5:14. doi: 10.4103/2153-3539.129452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalinski T, Zwönitzer R, Sel S, Evert M, Guenther T, Hofmann H, et al. Virtual 3D microscopy using multiplane whole slide images in diagnostic pathology. Am J Clin Pathol. 2008;130:259–64. doi: 10.1309/QAM22Y85QCV5JM47. [DOI] [PubMed] [Google Scholar]
- 8.Evered A, Dudding N. Accuracy and perceptions of virtual microscopy compared with glass slide microscopy in cervical cytology. Cytopathology. 2011;22:82–7. doi: 10.1111/j.1365-2303.2010.00758.x. [DOI] [PubMed] [Google Scholar]
- 9.Hanna MG, Monaco SE, Cuda J, Xing J, Ahmed I, Pantanowitz L, et al. Comparison of glass slides and various digital-slide modalities for cytopathology screening and interpretation. Cancer Cytopathol. 2017;125:701–9. doi: 10.1002/cncy.21880. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly AD, Mukherjee MS, Lyden ER, Bridge JA, Lele SM, Wright N, et al. Optimal z-axis scanning parameters for gynecologic cytology specimens. J Pathol Inform. 2013;4:38. doi: 10.4103/2153-3539.124015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gagnon M, Inhorn S, Hancock J, Keller B, Carpenter D, Merlin T, et al. Comparison of cytology proficiency testing: Glass slides vs. virtual slides. Acta Cytol. 2004;48:788–94. doi: 10.1159/000326447. [DOI] [PubMed] [Google Scholar]
- 12.Dee FR, Donnelly A, Radio S, Leaven T, Zaleski MS, Kreiter C, et al. Utility of 2-D and 3-D virtual microscopy in cervical cytology education and testing. Acta Cytol. 2007;51:523–9. doi: 10.1159/000325788. [DOI] [PubMed] [Google Scholar]
- 13.Mori I, Nunobiki O, Ozaki T, Taniguchi E, Kakudo K. Issues for application of virtual microscopy to cytoscreening, perspectives based on questionnaire to Japanese cytotechnologists. Diagn Pathol. 2008;3(Suppl 1):S15. doi: 10.1186/1746-1596-3-S1-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Es SL, Kumar RK, Pryor WM, Salisbury EL, Velan GM. Cytopathology whole slide images and adaptive tutorials for postgraduate pathology trainees: A randomized crossover trial. Hum Pathol. 2015;46:1297–305. doi: 10.1016/j.humpath.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Hanna MG, Pantanowitz L. Why is digital pathology in cytopathology lagging behind surgical pathology? Cancer Cytopathol. 2017;125:519–20. doi: 10.1002/cncy.21855. [DOI] [PubMed] [Google Scholar]
- 16.Walkowski S, Szymas J. Quality evaluation of virtual slides using methods based on comparing common image areas. Diagn Pathol. 2011;6(Suppl 1):S14. doi: 10.1186/1746-1596-6-S1-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shrestha P, Kneepkens R, Vrijnsen J, Vossen D, Abels E, Hulsken B, et al. Aquantitative approach to evaluate image quality of whole slide imaging scanners. J Pathol Inform. 2016;7:56. doi: 10.4103/2153-3539.197205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Es SL. Why is digital pathology in cytopathology lagging behind surgical pathology? Cancer Cytopathol. 2017;125:731. doi: 10.1002/cncy.21889. [DOI] [PubMed] [Google Scholar]
- 19.Hanna MG, Pantanowitz L. Reply to why is digital pathology in cytopathology lagging behind surgical pathology? Cancer Cytopathol. 2017;125:732. doi: 10.1002/cncy.21890. [DOI] [PubMed] [Google Scholar]
- 20.Tawfik O, Davis M, Dillon S, Tawfik L, Diaz FJ, Amin K, et al. Whole-slide imaging of pap cellblock preparations is a potentially valid screening method. Acta Cytol. 2015;59:187–200. doi: 10.1159/000430082. [DOI] [PubMed] [Google Scholar]
- 21.Jara-Lazaro AR, Thamboo TP, Teh M, Tan PH. Digital pathology: Exploring its applications in diagnostic surgical pathology practice. Pathology. 2010;42:512–8. doi: 10.3109/00313025.2010.508787. [DOI] [PubMed] [Google Scholar]
- 22.Van Es SL, Kumar RK, Pryor WM, Salisbury EL, Velan GM. Cytopathology whole slide images and adaptive tutorials for senior medical students: A randomized crossover trial. Diagn Pathol. 2016;11:1. doi: 10.1186/s13000-016-0452-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


