Abstract
Background
Previous studies have suggested that metformin may be useful for preventing and treating endometrial cancer (EC), while the results have been inconsistent. This systematic review and meta-analysis aimed to investigate the association between metformin use and risk and prognosis of patients with EC.
Methods
PubMed, Embase, and the Cochrane Library databases were searched for observational studies evaluating the effect of metformin on EC prevention or treatment. The odds ratio (OR) was used for analyzing risks, and the hazard ratio (HR) was used for analyzing survival outcomes. A random-effects model was used for data analysis.
Results
Seven studies reported data on EC risk. The pooled results suggested that metformin was not significantly associated with a lower risk of EC [OR = 1.05, 95% confidence interval (CI) 0.82–1.35, P = 0.70]. For patients with diabetes, metformin showed no advantage in reducing the EC risk compared with other interventions (OR = 0.99, 95% CI 0.78–1.26, P = 0.95). Further, seven studies were included for survival analysis. The pooled data showed that metformin could significantly improve the overall survival of patients with EC (HR = 0.61, 95% CI 0.48–0.77, P < 0.05) and reduce the risk of EC recurrence (OR = 0.50, 95% CI 0.28–0.92, P < 0.05) Finally, we noted metformin was associated with significantly improving the overall survival of EC patients among diabetes (HR = 0.47; 95%CI 0.33–0.67, P < 0.05).
Conclusions
This meta-analysis did not prove that metformin was beneficial for preventing EC. However, metformin could prolong the overall survival of patients with EC and reduce their risk of cancer relapse.
Electronic supplementary material
The online version of this article (10.1186/s12885-018-4334-5) contains supplementary material, which is available to authorized users.
Keywords: Endometrial cancer, Metformin, Risk, Prognosis, Meta-analysis
Background
Endometrial cancer (EC), a tumor originating from the endometrium, is a major cause of morbidity and mortality in women. Hyperplastic endometrium may be a result of exposure to unopposed estrogen, leading to the progression of cancer. It is the most common malignancy of the female genital tract in the United States, with approximately 54,870 new cases and 10,170 related deaths in 2015 [1]. The incidence of EC is lower in developing countries compared with developed countries. However, EC was associated with higher cancer mortality and poor prognosis in developing countries [1–3]. Further, despite advances in the treatment of EC, the prognosis for stages III–IV EC remains poor [4].
Various adjuvant medications have been suggested for preventing and treating EC, including aromatase inhibitors [5], aspirin [6], statins [7], hormone therapy [8], and metformin [9, 10]. Metformin has several advantages in addition to its anticancer activity. First, it is a first-line pharmacologic treatment for patients with type 2 diabetes mellitus [11]. Second, in addition to metformin use for diabetes, it is also safely prescribed for various nondiabetic conditions, including polycystic ovarian syndrome [12], primary prevention of type 2 diabetes mellitus and cardiovascular diseases [13, 14], and obesity control [15]. Finally, metformin is readily available worldwide at low cost.
Type 2 diabetes mellitus is a well-established risk factor for EC [16, 17]. Insulin resistance has been suggested to be one of the critical biological processes that contribute to EC [18, 19]. Approximately 30% of patients with EC have type 2 diabetes mellitus, and up to 36% have undiagnosed insulin resistance [20]. Metformin use could reduce the risk of type 2 diabetes mellitus and delay its progression. It reduces insulin resistance by increasing insulin receptor tyrosine kinase activity, enhancing glycogen synthesis, and promoting the recruitment and increasing the activity of glucose transporter type 4 [21]. Moreover, it affects endometrial maturation, proliferation, and implantation process [22–24]. Finally, the risk of EC is increased in women who have higher endogenous estrogen levels [25], and metformin has been reported to hinder estrogen-mediated endometrial proliferation [26].
Several studies have reported that metformin is a promising intervention for preventing and treating EC. However, these studies had conflicting results, and no relevant meta-analyses have been conducted. Therefore, this systematic review and meta-analysis was performed to evaluate the effectiveness of metformin for the risk and survival outcomes in patients with EC.
Methods
Search strategy
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines (Additional file 1) [27]. PubMed, Embase, and the Cochrane Library databases were searched for eligible studies between 1980 and July 2016. The following key words and medical terms were used for the literature search: (metformin OR glucophage OR dimethylbiguanide OR dimethylguanylguanidine) AND (endometrial cancer OR endometrial carcinoma OR endometrial hyperplasia OR endometrial proliferation OR endometrial thickness). The language was limited to English. Manual searching was also conducted on the reference lists of included studies and reviews for potentially relevant studies.
Inclusion criteria
Articles were included in the study if they met the following criteria: (1) used metformin for preventing or treating EC; (2) evaluated the incidence of EC or survival outcomes; the survival endpoints were overall survival (OS), recurrence-free survival (RFS)/disease-free survival, or the recurrence rate; (3) directly reported the effect estimates of odds ratio (OR), hazard ratio (HR), or relative risk (RR); (4) indirectly reported data allowing for the calculation of these effect estimates; and (5) the study with observational design. Abstracts, unpublished data, and studies not published in English were excluded.
Data collection and quality assessment
Two reviewers independently examined the included studies for eligibility and extracted the data. Any disagreement was resolved by consensus. The following information from each included study was extracted into standardized tables: author, publication year, region, study design, sample size, age, patient characteristics, EC incidence, percentage of metformin use in DM patients, proportion of patients with diabetes, reported effect estimates, degree of adjustment, and study period. When multiple studies for the same cohort were found, the most comprehensive or most recent data were used. The same reviewers independently evaluated the risk of bias using the Newcastle–Ottawa Scale [28]. This scale assigned 0–9 points based on three items: selection, comparability, and outcome assessment. Studies with 0–3, 4–6, and 7–9 points were classified as low-, medium-, and high-quality studies, respectively.
Statistical analysis
ORs and their associated 95% confidence intervals (CIs) were used as the effect measures for the outcome of EC risk. HRs and associated 95% CIs were used as the effect measures for the survival outcomes. Adjusted ORs or HRs were preferred for the analyses. However, if the adjusted effect estimates were not directly presented, they were calculated from the crude data available. HR was considered to be equivalent to RR in cohort studies. Given the low incidence of EC, HRs could be assumed to be accurate estimates of ORs. When a study presented only Kaplan–Meier curves, HRs and 95% CIs were calculated based on published methods [29]. A fixed-effects model was used for the data combination of different subgroups in a single study. The result from a random-effects meta-analysis is more conservative than that from a fixed-effects model. Thus, the between-study results were pooled using the random-effects model proposed by DerSimonian and Laird [30]. The heterogeneity between studies was evaluated with Q and I2 statistics [31]. I2 values between 0% and 25% were designated as a low level, values more than 25% as a moderate level, and values more than 75% as a high level of heterogeneity. The heterogeneity was explored by subgroup, meta-regression, and sensitivity analyses. The publication bias was examined visually by the symmetry of funnel plots and statistically by Egger’s or Begg’s tests [32, 33]. All statistical analyses were performed using Stata software (version 12.0, Stata Corporation, TX, USA). All statistical tests were two sided. A P value less than 0.05 was considered statistically significant.
Results
Study selection
The PRISMA flow diagram of the identification and selection of studies is shown in Fig. 1. From a total of 246 publications (141 from PubMed, 97 from Embase, and 8 from the Cochrane Library), duplicates were removed and irrelevant studies or those without sufficient data discarded. Finally, 13 studies were pooled in the meta-analysis, including 7 studies on the risk of EC [34–40] and 7 studies on the survival outcomes of EC [9, 10, 41–45].
Fig. 1.

Flow diagram of the study selection process
Study characteristics and quality evaluation
The characteristics of the seven studies on EC risk are shown in Table 1, including two case–control studies [34, 39], four retrospective studies [36–38, 40], and one prospective study [35]. These studies were all published between 2013 and 2017. Two studies were conducted in the United States [35–37], three in Europe [34, 39, 40], and one in China [38]. The sample size ranged from 7861 to 4,478,921. Six studies reported the adjusted ORs [34–36, 38–40], and one study reported only the crude data [37]. The incidence of EC ranged from 0.1% to 14.3% of included studies. The features of the studies on EC prognosis are shown in Table 2. All of the studies were retrospectively designed, including five studies in the United States [9, 10, 41, 43, 44] and two studies in Europe [42, 45]. The sample size ranged from 107 to 1303. Except for a study that included only cases of advanced EC [44], most of the studies primarily included patients with early-stage EC (70%–82%). The proportion of patients with diabetes ranged from 17% to 100%. Hall et al. only presented crude data on recurrence rates [10], but the other studies reported adjusted data. The quality appraisal of the included studies is summarized in Additional file 2. All studies were of high quality, with a score of 8–9.
Table 1.
Characteristics of included studies on the risk of endometrial cancer
| Author (year) | Region | Design | No. of patients | Mean/Median age (year) | EC incidence | Percentage of metformin use in DM patients (%) | Measurement of outcome | Adjusted variables | Subgroups | Study period |
|---|---|---|---|---|---|---|---|---|---|---|
| Becker et al. (2013) [34] | UK | Case–control | 17,878 | 63 | 14.3% | 51.5 | Adjusted OR | BMI, smoking, DM | Metformin intensity, DM state | 1995–2012 |
| Luo et al. (2014) [35] | USA | Prospective | 88,107 | 63 | 1.4% | 12.5 | Adjusted OR | Age, ethnicity, education, smoking, PA, alcohol, hormone use, parity, contraception, BMI | BMI | 1993–1998 |
| Ko et al. (2015) [36] | USA | Retrospective | 541,128 | 52 | 0.1% | 84.4 | Adjusted HR | Age, Charlson index, fibroid, infertility, PCOS, DM, hypertension, endometrial hyperplasia, connective tissue disease, oral contraceptive, HRT, ultrasound | Age, DM state, PCOS, EH | 2000–2011 |
| Soffer et al. (2015) [37] | USA | Retrospective | 66,778 | 55 | 0.5% | 40.3 | Unadjusted OR | None | None | 1998–2004 |
| Tseng et al. (2015) [38] | China | Retrospective | 4,478,921 | 56 | 0.6% | 40.3 | Adjusted HR | Age, hypertension, COPD, stroke, heart disease, obesity, metabolic profiles, various drugs | Duration, dose | 1998–2002 |
| Franchi et al. (2016) [39] | Italy | Case–control | 7861 | > 40 | 4.8% | 36.1 | Adjusted OR | Charlson index, medical conditions, prescription of selected drugs, antidiabetic drugs | Duration | 2002–2007 |
| Arima et al. (2017) [40] | Finland | Retrospective | 92,366 | > 40 | 0.6% | 63.8 | Adjusted HR | Age, duration of DM and use at any time of other forms of medication. | None | 1996–2011 |
BMI Body mass index, COPD chronic obstructive pulmonary disease, DM diabetes mellitus, EC endometrial cancer, EH endometrial hyperplasia, HR hazard ratio, HRT hormone replacement therapy, OR odds ratio, PA physical activity, PCOS polycystic ovary syndrome, UK United Kingdom, USA the United States of America
Table 2.
Characteristics of included studies on the survival outcomes of endometrial cancer
| Author (year) | Region | Design | Percentage of stages I–II, % | No. of patients | Age (year) | Diabetic patients, % | Adjusted variables | Outcomes | Subgroups | Follow-up duration |
|---|---|---|---|---|---|---|---|---|---|---|
| Ko et al. (2014) [41] | USA | Retrospective | 82 | 363 | 63 | 100 | Age, stage, grade, histology, adjuvant treatment | TTR, RFS, OS | None | 2005–2010 |
| Nevadunsky et al. (2014) [9] | USA | Retrospective | 75 | 985 | 64 | 75 | Age, clinical stage, grade, chemotherapy, radiation, hyperlipidemia | OS | Diabetes | 1999–2009 |
| Lemanska et al. (2015) [42] | Poland | Retrospective | 75 | 107 | 64 | 64 | Age, diabetes, hypertension, BMI, glucose level, grade, stage, hysterectomy, radiation, endometrial cancer type | OS | Histological type | 2002–2010 |
| Ezewuiro et al. (2016) [44] | USA | Retrospective | 0 | 349 | 63 | 17 | study site, BMI, race, age, stage | OS, recurrence | Diabetes | 1992–2011 |
| Hall et al. (2016) [10] | USA | Retrospective | 79 | 351 | 58 | 18 | None | Recurrence | None | 2005–2011 |
| Al Hilli et al. (2016) [43] | USA | Retrospective | 81 | 1303 | 65 | 21 | Age, BMI, smoking, ASA score, cardiopulmonary state, various tumor features, surgery, adjuvant therapy | OS, PFS | Histological type, diabetes | 1999–2008 |
| Seebacher et al. (2016) [45] | Austria | Retrospective | 70 | 465 | 65 | 19 | Age, tumor stage, grade, histological subtype | CSS, OS, RFS, recurrence | None | 1995–2011 |
ASA American Society of Anesthesiologists, BMI body mass index, CSS cancer-specific survival, EC endometrial cancer, OS overall survival, PFS progression -free survival, RFS recurrence-free survival, TTR time to recurrence, USA the United States of America
Metformin and the risk of EC
Seven studies were eligible for the meta-analysis. Becker et al. analyzed the risk based on different metformin prescriptions (1–24 and ≥ 25) [34]. The data were first pooled in a fixed-effects model. Six studies presented the adjusted ORs. The pooled data showed that metformin use was not associated with the risk of EC (OR = 1.05, 95% CI 0.82–1.35, P = 0.70) (Fig. 2). High heterogeneity was shown among the studies (I2 = 90.9%, P < 0.05). In the sensitivity analysis, the exclusion of any single study did not markedly alter the overall effect size, and in stratified analyses, the overall effect was not significantly changed for the subgroups by geographic region (USA, Europe, or Asia) and study design (case–control, prospective, or retrospective). However, no heterogeneity was detected in two European case–control studies (I2 = 0). In the meta-regression analysis, the sample size could not explain the source of heterogeneity (P > 0.05). The funnel plot appeared to be symmetrical (Fig. 3a). Notably, a significant publication bias was revealed by the Egger’s test (P < 0.05), but not by the Begg’s test (P = 1.00). The conclusions were not changed after adjustment for publication bias by using the trim and fill method [46].
Fig. 2.

Forest plot of the association between metformin use and risk of endometrial cancer
Fig. 3.

Funnel plot of the included studies: a Studies presenting the association between metformin use and risk of endometrial cancer (EC). b Studies presenting an adjusted hazard ratio for the association between metformin use and overall survival of EC
The association between metformin and EC risk was further investigated in patients with diabetes. Franchi et al., Tseng et al. and Arima et al. included only patients with type 2 diabetes mellitus [37–40]. Three other studies reported data for subpopulations with type 2 diabetes mellitus [34–36]. The pooled data showed that patients with diabetes using metformin did not have a substantially lower risk of EC compared with those receiving other interventions (OR = 0.99, 95% CI 0.78–1.26, P = 0.95) (Fig. 4). However, high heterogeneity was detected (I2 = 89.9%, P < 0.05).
Fig. 4.

Forest plot of the association between metformin use and risk of endometrial cancer among patients with diabetes
Metformin and OS of EC
Six retrospective studies were included in this analysis. Ezewuiro et al. and Al Hilli et al. separately reported data for patients with diabetes not using metformin and those without diabetes [43, 44]. The data from the subgroups within a single study were first pooled using a fixed-effects model. The pooled data showed that metformin use in patients with EC was significantly associated with longer OS compared with patients with EC not using metformin (HR = 0.61, 95% CI 0.48–0.77, P < 0.05) (Fig. 5). Low heterogeneity was identified, which was not significant (I2 = 8.1%, P = 0.36). No single study markedly changed the overall effect in the sensitivity analysis. In the subgroup analysis, the results were not significant for two European studies (HR = 0.74, 95% CI 0.37–1.49, P = 0.41), but were significant for the four studies conducted in the USA (HR = 0.59, 95% CI 0.45–0.76, P < 0.05). When stratified by the percentage of patients with type 2 diabetes mellitus (< 50% vs ≥50%), the overall effect had no substantial change. The meta-regression analysis revealed no significant role of sample size (P = 0.81), proportion of stages I–II patients (P = 0.88), or percentage of patients with type 2 diabetes mellitus (P = 0.84) to account for the heterogeneity. The funnel plot seemed to be symmetrical (Fig. 3b). No significant publication bias was shown by the Egger’s test (P = 0.78) or the Begg’s test (P = 0.45).
Fig. 5.
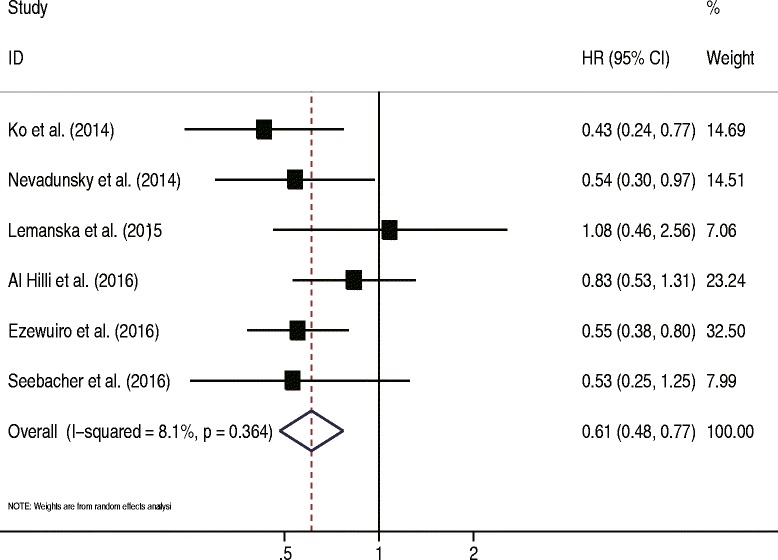
Forest plot of the association between metformin use and overall survival of endometrial cancer
The efficacy of metformin was further investigated among patients with EC having diabetes. Three studies reported relevant data. Metformin use was significantly associated with improved OS compared with other antidiabetic regimens (OR = 0.47, 95% CI 0.33–0.67, P < 0.05) (Fig. 6). No heterogeneity was detected (I2 = 0%, P = 0.69).
Fig. 6.

Forest plot of the association between metformin use and overall survival of endometrial cancer among patients with diabetes
Metformin and recurrence of EC
Three studies reported data on the recurrence of EC [10, 44, 45]. The pooled results suggested that metformin use in patients with EC did not significantly reduce the risk of recurrence (OR = 0.50, 95% CI 0.28–0.92, P < 0.05) (Fig. 7). No heterogeneity was identified (I2 = 0%, P = 0.98).
Fig. 7.
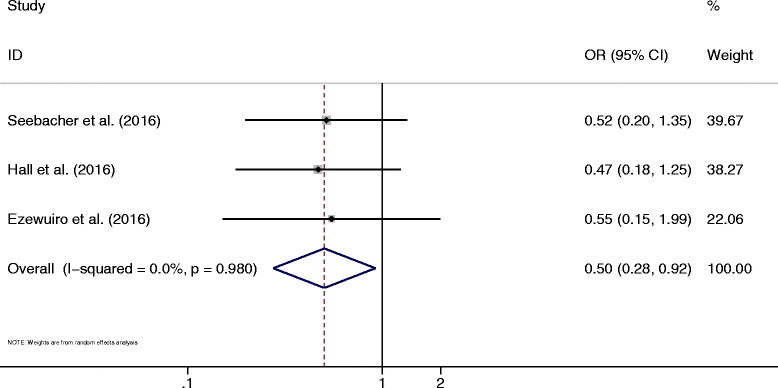
Forest plot of the association between metformin use and recurrence of endometrial cancer
Discussion
This meta-analysis on the prevention of EC with metformin included 7 studies and a total of 5,293,039 participants. The pooled data suggested that the use of metformin could not substantially prevent the development of EC. When analyzing the subgroup of patients with diabetes, who were at a higher risk of EC, a significant protective effect of metformin against EC still could not be detected compared with patients with diabetes treated with other antidiabetic therapies. Differences in the duration of use and dose of metformin might have limited the statistical power of this study. The protective effects of metformin might be time and dose dependent [47]. However, most included studies failed to conduct dose-escalation analyses. Further, this meta-analysis also comprised 7 studies with a total of 3923 patients with EC who were treated with metformin. It was found that metformin could substantially improve the OS and reduce the risk of recurrence. The benefit for OS remained significant for the subgroup of patients with diabetes.
A previous meta-analysis based on 19 studies and illustrated effects of metformin on reversal of atypical endometrial hyperplasia, cellular proliferation biomarkers expression and overall survival. Further, they point out metformin could reverse atypical endometrial hyperplasia to normal endometrial histology, reduction of cell proliferation biomarkers, and improvement of OS. However, mostly investigated outcomes focused on precancerous indicators, while the risk of certain cancer was not evaluated [48]. The study conducted by Perez-Lopez et al. suggested metformin therapy was associated with a reduced risk of overall mortality in T2DM women with EC. Whereas this study focused on patients with T2DM and the preventive effect of metformin on EC risk was not illustrated [49]. Tang et al. conducted a meta-analysis based on 11 studies and indicated metformin therapy are significantly improvement EC risk and prognosis of EC. However, this study with incomplete electronic searches and the result of recurrence of EC were not calculated [50]. This novel meta-analysis of metformin use for preventing and treating EC analyzed both risk and survival scenarios. The most comprehensive up-to-date relevant studies were included. The sample sizes of most studies were sufficiently large, and the studies were of high quality. The inclusion of participants from all parts of the world meant that the present study results should be generalizable to the general population. Most studies sufficiently adjusted for various clinicopathological confounding factors. Moreover, the role of metformin was specifically assessed among patients with diabetes.
Abundant preclinical in vitro and in vivo studies have reported the anticancer effect of metformin on various malignancies. Nevertheless, the exact molecular mechanisms remain unknown. Metformin may inhibit cancer stem cell–like subpopulations in cases of intraepithelial neoplasia [51]. Metformin may also prevent the conversion of epithelial cells into mesenchymal cells [52]. Several studies have reported that metformin can reverse endometrial hyperplasia [53, 54]. Thus, metformin may have multiple functions mediated through direct and indirect mechanisms [51]. The indirect effect is insulin dependent. Metformin helps control the circulating glucose level and improve insulin sensitivity. The direct effect is insulin independent. Metformin exerts its effects on tumor cells primarily through the adenosine 5′-monophosphate–activated protein kinase and phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin signaling pathways [55]. Notably, these molecular targets are similar to the targets of current drugs, such as sorafenib and everolimus. A phase I clinical trial of 21 cases, including 4 patients with advanced EC, showed that the combination of temsirolimus and metformin was a promising treatment [56]. Metformin is nontoxic and may be extremely useful for enhancing the treatment efficacy of the targeted drugs [51]. Since metformin has been used for more than 50 years, its safety profile has been well established. Although it can cause potentially dangerous toxicity from lactic acidosis, the risk is mainly confined to patients aged more than 80 years, patients with alcohol abuse, or those who have comorbidities of renal, hepatic, or cardiac insufficiency [57].
This meta-analysis had several limitations. It only identified a small number of studies exploring the role of metformin for prevention and treatment. Further, stratified results according to individuals characteristics were not reported. Therefore, a subgroup or meta-regression analysis could not identify the sources of heterogeneity. Many of the included studies were retrospectively designed, which might have led to recall and selection bias, and those study are associated with low level of evidence. Furthermore, whether patients had taken different antidiabetic drugs before metformin administration could not be determined. Their glycemic control might also be inadequate. Moreover, this meta-analysis was based on observational data, which was associated with higher indication bias. Such as, more “healthier patients” always receive the best treatment. No randomized controlled trial has been conducted on patients with EC. Interestingly, a previous large randomized controlled trial that enrolled patients with gastrointestinal malignancies showed that metformin was helpful for the chemoprevention of colorectal cancer [58], but did not significantly improve the OS of patients with pancreatic cancer [59]. Most of the studies included in this meta-analysis did not report the effect of metformin dose or duration. Although most of the studies performed sufficient adjustment of variables, little information was available to evaluate the potential influence of other drugs such as aspirin or statins. Moreover, stratified analyses were not conducted based on study design and other patient characteristics, since a smaller number of cohorts were included. Therefore, this comprehensive meta-analysis just provided relative results on metformin use for EC prevention and treatment.
Conclusions
In conclusion, a preventive effect of metformin on the development of EC was not observed in this meta-analysis. However, metformin was beneficial in improving the OS and reducing the relapse risk for patients with EC. More prospective long-term studies should be conducted to verify the findings of the present study.
Additional files
PRISMA Checklist. (DOC 65 kb)
Newcastle–Ottawa scale for quality assessment of the included studies. (DOC 36 kb)
Acknowledgments
Funding
National Natural Science Foundation of China (No. 31670844) and Program for Science and Technology Innovation Teams in Universities of Henan Province (No.17IRTSTHN021) provides financial supports in the study and the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- CI
Confidence interval
- EC
Endometrial cancer
- HR
Hazard ratio
- OR
Odds ratio
- OS
Overall survival
- RFS
Recurrence-free survival
- RR
Relative risk
Authors’ contributions
DC conceived and coordinated the study, designed, performed and analyzed the data, wrote the paper. JW, KW, MZ, CW, LL and RG carried out the data collection, data analysis, and revised the paper. All authors reviewed the results and approved the final version of the manuscript.
Ethics approval and consent to participate
All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12885-018-4334-5) contains supplementary material, which is available to authorized users.
Contributor Information
Danxia Chu, Email: chudanxia@126.com.
Jie Wu, Email: wujiezzu@163.com.
Kaili Wang, Email: wkl19930609@163.com.
Mengling Zhao, Email: 1310468432@qq.com.
Chunfang Wang, Email: wangchunfangys@qq.com.
Liuxia Li, Email: llxia698@163.com.
Ruixia Guo, Phone: +86-13525569376, Email: grxcdxzzu@163.com.
References
- 1.Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387:1094–1108. doi: 10.1016/S0140-6736(15)00130-0. [DOI] [PubMed] [Google Scholar]
- 2.Koh WJ, Greer BE, Abu-Rustum NR, Apte SM, Campos SM, Chan J, et al. Uterine neoplasms, version 2.2016. J Natl Compr Cancer Netw. 2016;12:248–280. doi: 10.6004/jnccn.2014.0025. [DOI] [PubMed] [Google Scholar]
- 3.Tangjitgamol S, Anderson BO, See HT, Lertbutsayanukul C, Sirisabya N, Manchana T, et al. Management of endometrial cancer in Asia: consensus statement from the Asian oncology summit 2009. Lancet Oncol. 2009;10:1119–1127. doi: 10.1016/S1470-2045(09)70290-6. [DOI] [PubMed] [Google Scholar]
- 4.Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, et al. Carcinoma of the corpus uteri. FIGO 26th annual report on the results of treatment in gynecological Cancer. Int J Gynaecol Obstet. 2006;95 Suppl 1:S105–S143. doi: 10.1016/S0020-7292(06)60031-3. [DOI] [PubMed] [Google Scholar]
- 5.Chlebowski RT, Schottinger JE, Shi J, Chung J, Haque R. Aromatase inhibitors, tamoxifen, and endometrial cancer in breast cancer survivors. Cancer. 2015;121:2147–2155. doi: 10.1002/cncr.29332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang D, Bai B, Xi Y, Zhao Y. Can aspirin reduce the risk of endometrial Cancer?: a systematic review and meta-analysis of observational studies. Int J Gynecol Cancer. 2016;26:1111–1120. doi: 10.1097/IGC.0000000000000731. [DOI] [PubMed] [Google Scholar]
- 7.Feng CH, Miller CM, Tenney ME, Lee NK, Yamada SD, Hasan Y. Statin use significantly improves overall survival in high-grade endometrial Cancer. Int J Gynecol Cancer. 2016;26:1642–1649. doi: 10.1097/IGC.0000000000000819. [DOI] [PubMed] [Google Scholar]
- 8.Chlebowski RT, Anderson GL, Sarto GE, Haque R, Runowicz CD, Aragaki AK, Thomson CA, Howard BV, Wactawski-Wende J, Chen C, Rohan TE, Simon MS, Reed SD, Manson JE. Continuous combined estrogen plus progestin and endometrial Cancer: the Women's Health Initiative randomized trial. J Natl Cancer Inst. 2015;108(3). 10.1093/jnci/djv350. [DOI] [PMC free article] [PubMed]
- 9.Nevadunsky NS, Van Arsdale A, Strickler HD, Moadel A, Kaur G, Frimer M, et al. Metformin use and endometrial cancer survival. Gynecol Oncol. 2014;132:236–240. doi: 10.1016/j.ygyno.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall C, Stone RL, Gehlot A, Zorn KK, Burnett AF. Use of metformin in obese women with type I endometrial Cancer is associated with a reduced incidence of Cancer recurrence. Int J Gynecol Cancer. 2016;26:313–317. doi: 10.1097/IGC.0000000000000603. [DOI] [PubMed] [Google Scholar]
- 11.Handelsman Y, Bloomgarden ZT, Grunberger G, Umpierrez G, Zimmerman RS, Bailey TS, et al. American association of clinical endocrinologists and american college of endocrinology - clinical practice guidelines for developing a diabetes mellitus comprehensive care plan - 2015. Endocr Pract. 2015;21 Suppl 1:1–87. doi: 10.4158/EP15672.GLSUPPL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCartney CR, Marshall JC. CLINICAL PRACTICE. Polycystic ovary syndrome. N Engl J Med. 2016;375:54–64. doi: 10.1056/NEJMcp1514916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diabetes Prevention Program Research G Within-trial cost-effectiveness of lifestyle intervention or metformin for the primary prevention of type 2 diabetes. Diabetes Care. 2003;26:2518–2523. doi: 10.2337/diacare.26.9.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fontbonne A, Diouf I, Baccara-Dinet M, Eschwege E, Charles MA. Effects of 1-year treatment with metformin on metabolic and cardiovascular risk factors in non-diabetic upper-body obese subjects with mild glucose anomalies: a post-hoc analysis of the BIGPRO1 trial. Diabetes Metab. 2009;35:385–391. doi: 10.1016/j.diabet.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Seifarth C, Schehler B, Schneider HJ. Effectiveness of metformin on weight loss in non-diabetic individuals with obesity. Exp Clin Endocrinol Diabetes. 2013;121:27–31. doi: 10.1055/s-0032-1327734. [DOI] [PubMed] [Google Scholar]
- 16.Rosato V, Zucchetto A, Bosetti C, Dal Maso L, Montella M, Pelucchi C, et al. Metabolic syndrome and endometrial cancer risk. Ann Oncol. 2011;22:884–889. doi: 10.1093/annonc/mdq464. [DOI] [PubMed] [Google Scholar]
- 17.Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia. 2007;50:1365–1374. doi: 10.1007/s00125-007-0681-5. [DOI] [PubMed] [Google Scholar]
- 18.Soliman PT, Wu D, Tortolero-Luna G, Schmeler KM, Slomovitz BM, Bray MS, et al. Association between adiponectin, insulin resistance, and endometrial cancer. Cancer. 2006;106:2376–2381. doi: 10.1002/cncr.21866. [DOI] [PubMed] [Google Scholar]
- 19.Mu N, Zhu Y, Wang Y, Zhang H, Xue F. Insulin resistance: a significant risk factor of endometrial cancer. Gynecol Oncol. 2012;125:751–757. doi: 10.1016/j.ygyno.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 20.Burzawa JK, Schmeler KM, Soliman PT, Meyer LA, Bevers MW, Pustilnik TL, et al. Prospective evaluation of insulin resistance among endometrial cancer patients. Am J Obstet Gynecol. 2011;204:355. doi: 10.1016/j.ajog.2010.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giannarelli R, Aragona M, Coppelli A, Del Prato S. Reducing insulin resistance with metformin: the evidence today. Diabetes Metab. 2003;29:6S28–6S35. doi: 10.1016/S1262-3636(03)72785-2. [DOI] [PubMed] [Google Scholar]
- 22.Yanokura M, Irie H, Masuda K, Kobayashi Y, Tominaga E, Aoki D, et al. Modulation of the IGF system and proliferation in human endometrial stromal cells by metformin: a dose-dependent effect. ScientificWorldJournal. 2015;292:465–472. doi: 10.1007/s00404-015-3650-0. [DOI] [PubMed] [Google Scholar]
- 23.Sivalingam VN, Kitson S, McVey R, Roberts C, Pemberton P, Gilmour K, et al. Measuring the biological effect of presurgical metformin treatment in endometrial cancer. Br J Cancer. 2016;114:281–289. doi: 10.1038/bjc.2015.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuler KM, Rambally BS, Difurio MJ, Sampey BP, Gehrig PA, Makowski L, et al. Antiproliferative and metabolic effects of metformin in a preoperative window clinical trial for endometrial cancer. Cancer Medicine. 2015;4:161–173. doi: 10.1002/cam4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao H, Jiang Y, Liu Y, Yun C, Li L. Endogenous estrogen metabolites as biomarkers for endometrial cancer via a novel method of liquid chromatography-mass spectrometry with hollow fiber liquid-phase microextraction. Horm Metab Res. 2015;47:158–164. doi: 10.1055/s-0034-1371865. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Q, Celestino J, Schmandt R, McCampbell AS, Urbauer DL, Meyer LA, et al. Chemopreventive effects of metformin on obesity-associated endometrial proliferation. Am J Obstet Gynecol. 2013;209:24.e1–24.e12. doi: 10.1016/j.ajog.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://wwwohrica/programs/clinical_epidemiology/oxfordasp.
- 29.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 30.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 31.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 32.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 34.Becker C, Jick SS, Meier CR, Bodmer M. Metformin and the risk of endometrial cancer: a case-control analysis. Gynecol Oncol. 2013;129:565–569. doi: 10.1016/j.ygyno.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Luo J, Beresford S, Chen C, Chlebowski R, Garcia L, Kuller L, et al. Association between diabetes, diabetes treatment and risk of developing endometrial cancer. Br J Cancer. 2014;111:1432–1439. doi: 10.1038/bjc.2014.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ko EM, Sturmer T, Hong JL, Castillo WC, Bae-Jump V, Funk MJ. Metformin and the risk of endometrial cancer: a population-based cohort study. Gynecol Oncol. 2015;136:341–347. doi: 10.1016/j.ygyno.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Soffer D, Shi J, Chung J, Schottinger JE, Wallner LP, Chlebowski RT, et al. Metformin and breast and gynecological cancer risk among women with diabetes. BMJ Open Diabetes Res Care. 2015;3:e000049. doi: 10.1136/bmjdrc-2014-000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tseng CH. Metformin and endometrial cancer risk in Chinese women with type 2 diabetes mellitus in Taiwan. Gynecol Oncol. 2015;138:147–153. doi: 10.1016/j.ygyno.2015.03.059. [DOI] [PubMed] [Google Scholar]
- 39.Franchi M, Asciutto R, Nicotra F, Merlino L, La Vecchia C, Corrao G, et al. Metformin, other antidiabetic drugs, and endometrial cancer risk: a nested case-control study within Italian healthcare utilization databases. Eur J Cancer Prev. 2016;26:225–231. doi: 10.1097/CEJ.0000000000000235. [DOI] [PubMed] [Google Scholar]
- 40.Arima R, Marttila M, Hautakoski A, Arffman M, Sund R, Ilanne-Parikka P, et al. Antidiabetic medication, statins and the risk of endometrioid endometrial cancer in patients with type 2 diabetes. Gynecol Oncol. 2017;146:636–641. doi: 10.1016/j.ygyno.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 41.Ko EM, Walter P, Jackson A, Clark L, Franasiak J, Bolac C, et al. Metformin is associated with improved survival in endometrial cancer. Gynecol Oncol. 2014;132:438–442. doi: 10.1016/j.ygyno.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 42.Lemanska A, Zaborowski M, Spaczynski M, Nowak-Markwitz E. Do endometrial cancer patients benefit from metformin intake? Ginekol Pol. 2015;86:419–423. doi: 10.17772/gp/2397. [DOI] [PubMed] [Google Scholar]
- 43.Al Hilli MM, Bakkum-Gamez JN, Mariani A, Cliby WA, Mc Gree ME, Weaver AL, et al. The effect of diabetes and metformin on clinical outcomes is negligible in risk-adjusted endometrial cancer cohorts. Gynecol Oncol. 2016;140:270–276. doi: 10.1016/j.ygyno.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 44.Ezewuiro O, Grushko TA, Kocherginsky M, Habis M, Hurteau JA, Mills KA, et al. Association of Metformin use with outcomes in advanced endometrial Cancer treated with chemotherapy. PLoS One. 2016;11:e0147145. doi: 10.1371/journal.pone.0147145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seebacher V, Bergmeister B, Grimm C, Koelbl H, Reinthaller A, Polterauer S. The prognostic role of metformin in patients with endometrial cancer: a retrospective study. Eur J Obstet Gynecol Reprod Biol. 2016;203:291–296. doi: 10.1016/j.ejogrb.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 46.Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication Bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98. [Google Scholar]
- 47.Chen HP, Shieh JJ, Chang CC, Chen TT, Lin JT, Wu MS, et al. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2013;62:606–615. doi: 10.1136/gutjnl-2011-301708. [DOI] [PubMed] [Google Scholar]
- 48.Meireles CG, Pereira SA, Valadares LP, Rêgo DF, Simeoni LA, Guerra ENS, et al. Effects of metformin on endometrial cancer: systematic review and meta-analysis. Gynecol Oncol. 2017;147:167–180. doi: 10.1016/j.ygyno.2017.07.120. [DOI] [PubMed] [Google Scholar]
- 49.Perez-Lopez FR, Pasupuleti V, Gianuzzi X, Palma-Ardiles G, Hernandez-Fernandez W, Hernandez AV. Systematic review and meta-analysis of the effect of metformin treatment on overall mortality rates in women with endometrial cancer and type 2 diabetes mellitus. Maturitas. 2017;101:6–11. doi: 10.1016/j.maturitas.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 50.Tang YL, Zhu LY, Li Y, Yu J, Wang J, Zeng XX, et al. Metformin use is associated with reduced incidence and improved survival of endometrial Cancer: a meta-analysis. Biomed Res Int. 2017;2017:5905384. doi: 10.1155/2017/5905384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Del Barco S, Vazquez-Martin A, Cufi S, Oliveras-Ferraros C, Bosch-Barrera J, Joven J, et al. Metformin: multi-faceted protection against cancer. Oncotarget. 2011;2:896–917. doi: 10.18632/oncotarget.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cufi S, Vazquez-Martin A, Oliveras-Ferraros C, Martin-Castillo B, Joven J, Menendez JA. Metformin against TGFbeta-induced epithelial-to-mesenchymal transition (EMT): from cancer stem cells to aging-associated fibrosis. Cell Cycle. 2010;9:4461–4468. doi: 10.4161/cc.9.22.14048. [DOI] [PubMed] [Google Scholar]
- 53.Shen ZQ, Zhu HT, Lin JF. Reverse of progestin-resistant atypical endometrial hyperplasia by metformin and oral contraceptives. Obstet Gynecol. 2008;112:465–467. doi: 10.1097/AOG.0b013e3181719b92. [DOI] [PubMed] [Google Scholar]
- 54.Shan W, Wang C, Zhang Z, Gu C, Ning C, Luo X, et al. Conservative therapy with metformin plus megestrol acetate for endometrial atypical hyperplasia. J Gynecol Oncol. 2014;25:214–220. doi: 10.3802/jgo.2014.25.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin-Castillo B, Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. Metformin and cancer: doses, mechanisms and the dandelion and hormetic phenomena. Cell Cycle. 2010;9:1057–1064. doi: 10.4161/cc.9.6.10994. [DOI] [PubMed] [Google Scholar]
- 56.Khawaja MR, Nick AM, Madhusudanannair V, Fu S, Hong D, LM MQ, et al. Phase I dose escalation study of temsirolimus in combination with metformin in patients with advanced/refractory cancers. Cancer Chemother Pharmacol. 2016;77:973–977. doi: 10.1007/s00280-016-3009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCormack J, Johns K, Tildesley H. Metformin's contraindications should be contraindicated. CMAJ. 2005;173:502–504. doi: 10.1503/cmaj.045292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Higurashi T, Hosono K, Takahashi H, Komiya Y, Umezawa S, Sakai E, et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: a multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016;17:475–483. doi: 10.1016/S1470-2045(15)00565-3. [DOI] [PubMed] [Google Scholar]
- 59.Kordes S, Pollak MN, Zwinderman AH, Mathot RA, Weterman MJ, Beeker A, et al. Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2015;16:839–847. doi: 10.1016/S1470-2045(15)00027-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist. (DOC 65 kb)
Newcastle–Ottawa scale for quality assessment of the included studies. (DOC 36 kb)
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


