Abstract
Deuterium- and tritium-labeled pharmaceutical compounds are pivotal diagnostic tools in drug discovery research, providing vital information about the biological fate of drugs and drug metabolites. Herein we demonstrate that a photoredox-mediated hydrogen atom transfer protocol can efficiently and selectively install deuterium (D) and tritium (T) at α-amino sp3 carbon-hydrogen bonds in a single step, using isotopically labeled water (D2O or T2O) as the source of hydrogen isotope. In this context, we also report a convenient synthesis of T2O from T2, providing access to high-specific-activity T2O. This protocol has been successfully applied to the high incorporation of deuterium and tritium in 18 drug molecules, which meet the requirements for use in ligand-binding assays and absorption, distribution, metabolism, and excretion studies.
An essential component of the drug discovery and development process is the thorough elucidation of a drug molecule’s action and toxicity (1–9). Despite rapid advancements in analytical techniques over the past 20 years, the introduction of isotopic labels, which integrates a distinguishing signal into a molecule without drastically altering its function, remains the most effective method to detect and quantify drugs and drug metabolites both in vivo and in vitro (1–6). Recently, the ability to introduce hydrogen isotopes at nonlabile C–H moieties has led to the emergence of deuterium (2H, D) and tritium (3H, T) as cheaper and more readily accessible alternatives to 13C and 14C for the synthesis of isotopically labeled drug analogs, especially when high isotopic incorporation is desired (1–6). In particular, highly deuterated analogs of drug molecules are used in absorption, distribution, metabolism, and excretion (ADME) studies, where they are ideal internal standards for mass spectrometry–based quantification in animal and human samples, because they negate matrix effects that can interfere with accurate quantification (10, 11). High-specific-activity tritium analogs are critical for accurate quantification in nanomolar ligand-binding affinity studies, as well as the imaging of in vivo compound distribution by autoradiography (7–9).
A large majority of deuterium- and tritium-labeled pharmaceutical compounds are synthesized in multistep procedures involving the reduction of halogenated or unsaturated drug precursors (1–6). However, advances in transition metal–catalyzed C–H activation have allowed for direct hydrogen isotope exchange (HIE) at C–H bonds for deuterium or tritium, enabling the synthesis of labeled compounds in a single step without the need for resynthesis (12–14). This straightforward approach has recently found widespread application in the pharmaceutical industry, where the increasing impact of early-stage ligand-binding assays and ADME studies on drug discovery has led to a rising demand for the efficient synthesis of isotopically labeled pharmaceutical compounds (2).
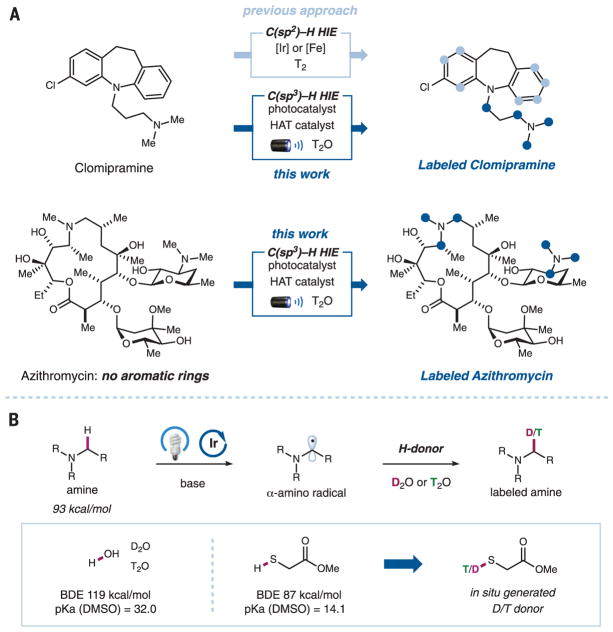
Transition metal–catalyzed HIE reactions at aromatic C(sp2)–H moieties are well established (Fig. 1A). For example, cationic iridium(I) complexes have long been used to selectively label sites ortho to directing groups on aromatic rings (12, 13). More recently, Chirik and co-workers reported the use of an iron catalyst for the deuteration and tritiation of pharmaceutical drugs at aromatic C–H moieties without the need for a directing group, enabling orthogonal site selectivity relative to the iridium catalyst (14). In contrast, the direct HIE at aliphatic C(sp3)–H moieties remains a challenge in the field (15–20). With more than 50% of the top-selling commercial drugs containing at least one alkyl amine moiety (21), the development of a HIE reaction targeting α-amino C(sp3)–H bonds could potentially provide a general method for the isotopic labeling of the aliphatic positions of a drug molecule. The site of the isotopic label can be of vital importance, depending on the nature of the study and the metabolic pathways of the substrate of interest (1–6). In addition, the inherently larger number of exchangeable hydrogens at α-amino C–H moieties relative to aromatic C–H moieties enables high incorporation of the desired isotopic label, which is often necessary for the labeled compound to be of practical use in pharmaceutical studies. Therefore, the development of a mild and efficient HIE reaction targeting α-amino C(sp3)–H bonds is particularly attractive for the pharmaceutical industry.
Fig. 1. Photoredox-catalyzed deuteration and tritiation of pharmaceutical compounds.
(A) The merger of photoredox and hydrogen atom transfer (HAT) catalysis enables α-amino C(sp3)–H selective hydrogen isotope exchange (HIE) of alkyl amine–based drugs. Light and dark blue circles represent the positions of isotopically labeled C(sp2)–H and C(sp3)–H bonds, respectively. (B) Hypothesis for the proposed photoredox-catalyzed deuteration and tritiation. Me, methyl; Et, ethyl; R, alkyl or aryl group; DMSO, dimethyl sulfoxide; BDE, bond dissociation energy.
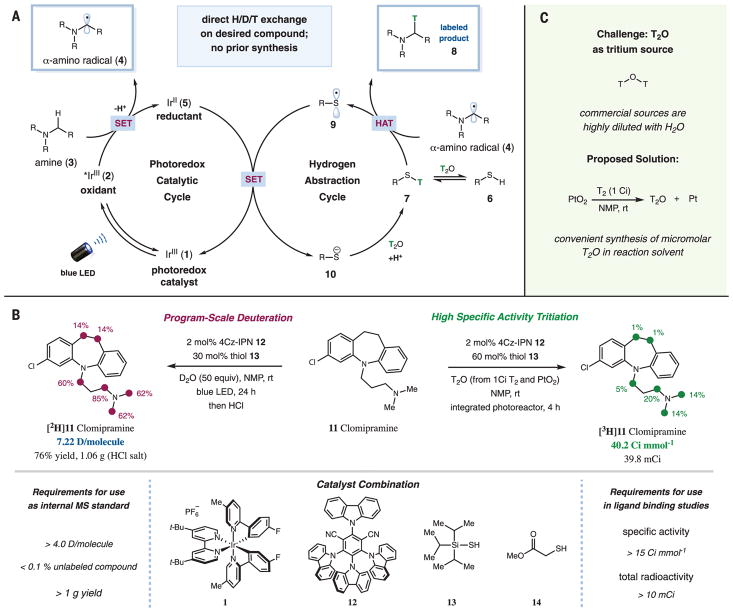
Visible light–mediated photoredox catalysis has emerged in recent years as an enabling platform to access new organic transformations through single-electron transfer events (22–24). In particular, our laboratory and others have demonstrated that tertiary amines can be activated by single-electron oxidation to give an amine radical cation, which can undergo facile α-deprotonation to yield carbon-centered α-amino radicals. The synthetic utility of α-amino radicals has been showcased by its ability to couple with various electrophilic partners (25–27). On this basis, we questioned whether we could exploit α-amino radicals to access α-deuterated or α-tritiated products (Fig. 1B). Specifically, we hypothesized that, instead of trapping the α-amino radical with a carbon electrophile, the use of an appropriate hydrogen atom transfer (HAT) catalyst in equilibrium with D2O or T2O could facilitate the abstraction of deuterium or tritium to yield labeled products. We recognized that the choice of the HAT catalyst would be heavily influenced by thermodynamic factors, particularly the bond dissociation energy (BDE) relative to that of the α-amino C–H bond, as well as its pKa (where Ka is the acid dissociation constant) relative to water (Fig. 1B) (28–30). With these considerations in mind, we postulated that thiols, which have been demonstrated to be good hydrogen atom donors (31), would be suitable HAT catalysts for this transformation. Herein we describe direct HIE at α-amino C(sp3)–H bonds mediated by the synergistic merger of photoredox and HAT catalysis. This methodology was readily applied to the program-scale deuteration (32) and high-specific-activity tritiation of 18 representative drugs and drug candidates.
A proposed mechanism for the photoredox- and HAT-catalyzed HIE of α-amino C(sp3)–H bonds is shown in Fig. 2A (33). Initial photoexcitation of the iridium(III) photocatalyst Ir(F-Meppy)2 (dtbbpy)PF6 [F-Meppy, 2-(4-fluorophenyl)-5-(methyl)pyridine; dtbbpy, 4,4′-di-tert-butyl-2,2′-bipyridine] (1) would generate the long-lived (lifetime τ = 1.1 μs) triplet-excited-state IrIII complex 2 (34). This species is a strong single-electron oxidant [reduction potential E1/2red(*IrIII/IrII) = +0.94 V versus saturated calomel electrode (SCE) in acetonitrile] (34) and can oxidize amine 3, which undergoes facile deprotonation at the α-position to give α-amino radical 4. At the same time, a thiol HAT catalyst (6) would undergo exchange with T2O to give the tritiated thiol 7, which would serve as the source of tritium. In addition to the reported BDE for typical α-amino C–H and thiol S–H bonds, we reasoned that HAT can proceed between the polarity-matched (35) nucleophilic α-amino radical 4 and tritiated thiol 7, which would furnish α-tritiated amine product 8 and the electrophilic thiol radical 9 [α-amino C–H BDE = 93.0 kcal mol−1 (36) versus S–H BDE = 87.0 kcal mol−1 (37)]. Both catalytic cycles can then converge to undergo a second single-electron transfer between 5 and 9 to regenerate photocatalyst 1 and tritiated thiol 7 through protonation of thiol anion 10 [E1/2red(IrIII/IrII) = −1.50 V versus SCE in acetonitrile (31); E1/2red(thiol) = −0.85 V versus SCE for cysteine] (38).
Fig. 2. Reaction development.
(A) Proposed catalytic cycle for the photoredox-catalyzed HAT protocol. SET, single-electron transfer; LED, light-emitting diode. (B) Procedures and requirements for program-scale deuteration and high-specific-activity tritiation. The colored circles (maroon or green) and numbers denote the positions of the C–H bonds that are labeled and the percent incorporation of the hydrogen isotope, respectively. NMP, N-methyl-2-pyrrolidone; t-Bu, tert-butyl group; rt, room temperature; h, hours. (C) High-specific-activity T2O is accessible from T2 and PtO2 at a micromolar scale and can be used for photoredox-catalyzed tritiation in a one-pot procedure.
We began our investigations into the proposed isotopic-labeling protocol by first examining the deuteration of clomipramine (11) hydrochloride (Anafranil), a commercially available antidepressant. For the application of deuterated compounds as internal standards in pharmaceutical studies, each compound should ideally have an isotopic incorporation of more than 4.0 deuteriums per molecule, with less than 0.1% of the unlabeled compound remaining, so as to avoid peak overlaps on the mass spectrum and allow for accurate quantification (11). In addition, to facilitate long-term studies, the program-scale synthesis of a uniformly deuterated batch of compound is also highly desirable (Fig. 2B, left). A variety of photoredox and thiol catalysts and their respective loadings were evaluated, using N-methyl-2-pyrrolidone (NMP) as the solvent (figs. S1 and S2). In our preliminary studies conducted at a 0.1-mmol scale, we observed that the use of 2 mol % of organic photo-catalyst 4Cz-IPN (12) [1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene; excited-state τ = 5.1 μs; E1/2red(*4Cz-IPN/4Cz-IPN–) = +1.35 V; E1/2red(4Cz-IPN–/4Cz-IPN) = +1.21 V] (39) and 30 mol % triisopropylsilanethiol (13), along with 1.2 equivalents of lithium carbonate, afforded the corresponding deuterated product in 79% yield, with 9.2 deuteriums incorporated per molecule and less than 0.1% of unlabeled compound remaining. Scaling the reaction up resulted in a comparable efficiency of deuterium incorporation, enabling the program-scale synthesis of [2H]11 with 7.2 deuteriums per molecule, no detectable unlabeled compound remaining, and an isolated yield of 76% as its HCl salt (1.06 g) (Fig. 2B, left). The use of the sterically hindered tri-isopropylsilanethiol was critical to prevent a deleterious thiol-substrate coupling pathway (fig. S2). Control experiments also revealed that light, photoredox catalyst, and thiol are all essential for deuteration to proceed (table S1).
Next, we sought to extend this HIE protocol to the high-specific-activity tritiation of amine-containing drugs with T2O. In contrast to the deuterium HIE reactions, the analogous tritium HIE reactions are predominantly run on a micro-molar scale because (i) the tritium isotope is easily detectable and only a small amount of radioactive product is required, and (ii) safety and cost concerns favor limiting the amount of tritium used to 1.0 Ci (17.2 μmol) per reaction. An additional complication is that commercially available T2O is typically highly diluted with natural-abundance H2O to prevent the decomposition of T2O through autoradiolysis (Fig. 2C) (3). To overcome this issue, we set out to establish a convenient process to synthesize high-specific-activity T2O using only 1 Ci of T2 gas, which could be performed in a common laboratory setting and used immediately in our tritium HIE reaction. Industrially, neat T2O is produced in bulk from the reaction between T2 and PtO2 before being distilled and diluted (40). To facilitate the transfer of micromolar-scale T2O into our photo-redox reaction mixture, we proposed to adapt this procedure for the generation of T2O in a potential reaction solvent. Among the solvents evaluated, we observed that the use of NMP as the reaction solvent enabled the formation of high-specific-activity tritiated water in 61% yield (0.51 Ci, 8.8 μmol) from 1.0 Ci T2 gas and PtO2 (fig. S3).
With this convenient method to access T2O in hand, we proceeded to optimize the proposed photoredox-HAT–catalyzed tritium-labeling protocol. In general, high-specific-activity tritium–labeled compounds are required to have a specific activity of at least 15 Ci mmol−1 (equivalent to an incorporation of 0.5 tritiums per molecule) and are isolated in an appreciable radiochemical yield of at least 10 mCi (Fig. 2B, right). Under the dilute micromolar conditions (using ~4.4 equivalents of T2O), we observed that the use of 11 in its free base form—along with increased loadings of the photo-redox (4 mol %) and thiol (60 mol %) catalysts—and the use of the integrated photoreactor developed at Merck & Co., Inc. (41), as the visible light source greatly enhanced the incorporation of tritium, yielding [3H]11 with a high specific activity of 40.2 Ci mmol−1 and an isolated yield of 39.8 mCi (Fig. 2B, right).
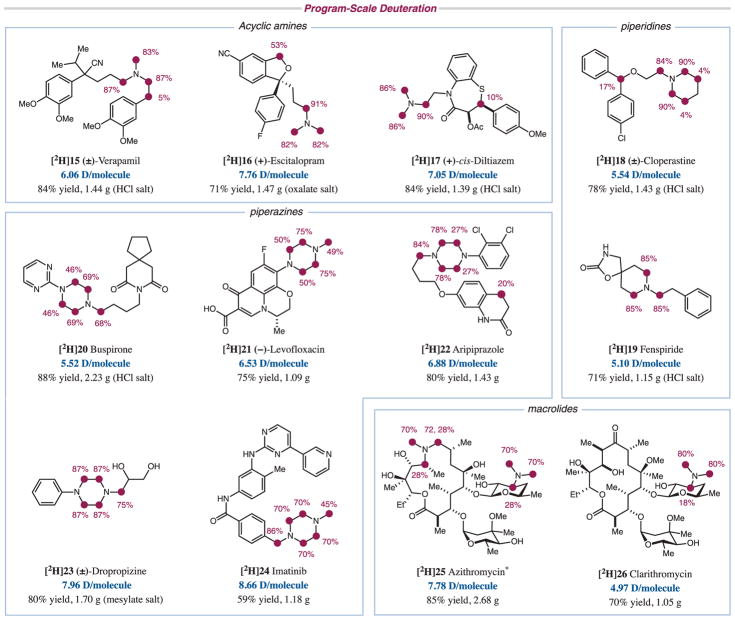
Under these optimized conditions, we explored the generality of the photoredox-mediated deuteration and tritiation protocols with a library of commercially available drugs containing a variety of alkyl amine scaffolds. For the deuteration of these substrates (Fig. 3), the best conditions were found to be substrate-dependent, given a choice of two photocatalysts [Ir(F-Meppy)2(dtbbpy)PF6 (1) and 4Cz-IPN (12)], two thiols (13 and 14), and two light sources [a 34-W blue light-emitting diode (LED) or the integrated photoreactor; see the supplementary materials]. Acyclic trialkyl amines ([2H]15 to [2H]17) worked well, as did piperidine ([2H]18 and [2H]19) and piperazine rings ([2H]20 to [2H]24). The reaction also showed good tolerance for a variety of functional groups. Aryl fluoride ([2H]16) and aryl chloride ([2H]18 and [2H]22) substituents were well tolerated under the reaction conditions. Carboxylic acid ([2H]21), ester ([2H]17), amide ([2H]20, [2H]22, and [2H]24), and nitrile ([2H]15 and [2H]16) functionalities were also amenable to the photoredox conditions, as were free hydroxyl groups ([2H]23, [2H]25, and [2H]26). In addition, chiral centers away from the reactive sites were unperturbed during the reaction ([2H]16, [2H]17, and [2H]21). Our deuteration protocol was also applicable to macrolide drugs such as azithromycin ([2H]25) and clarithromycin ([2H]26). These high-molecular-weight macro-cycles with no C(sp2)–H bonds are particularly challenging substrates for HIE through conventional transition metal catalysis. The preparation of deuterated azithromycin would have previously involved a multistep sequence and the use of costly deuterated reagents (42). In contrast, our photoredox protocol enables access to [2H]25 in a single step using D2O, a readily available source of deuterium. Stereochemistry was retained even when H/D exchange occurred at chiral centers on these macrocycles, likely owing to substrate control in the HAT process. In addition to α-amino positions, benzylic and β-amino positions were also deuterated in certain instances ([2H]15, [2H]16, [2H]18, and [2H]22; figs. S4 and S5). In summary, all substrates gave excellent deuterium incorporations on a program scale, with incorporations of more than 5.0 deuteriums per molecule and, importantly, less than 0.1% of the unlabeled compound, meeting the minimum requirement for their use as internal standards.
Fig. 3. Scope of program-scale deuteration.
Reaction conditions: photoredox catalyst 1 or 12 (2 mol %), triisopropylsilanethiol 13 (30 mol %), D2O (50 equivalents), Li2CO3 (1.2 equivalents, added when substrate is used as its acid salt), NMP, rt, 34-W blue LED or integrated photoreactor. The products were isolated as the appropriate acid salts shown in parentheses. Ac, acetyl group. *The two protons at the α-amino methylene position are diastereotopic and are labeled to different extents.
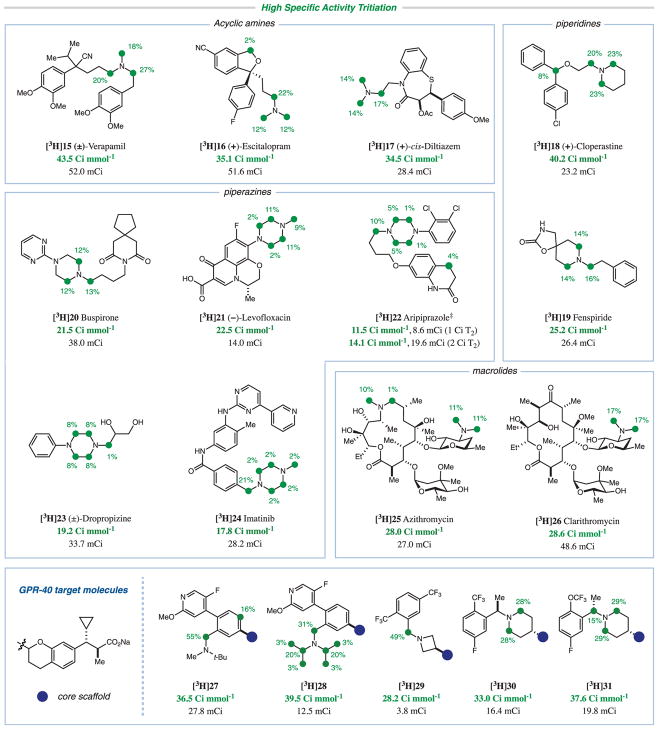
With the same library of 13 pharmaceutical drugs, we demonstrated similar functional group tolerance for the photoredox-mediated tritium HIE protocol (Fig. 4). As with the deuteration reaction, the best conditions were substrate-dependent, and combinations of the two photocatalysts 1 and 12 and the two thiols triisopropylsilanethiol (13) and methyl thioglycolate (14) were used. Despite the smaller excess of T2O in the reaction, the presence of acidic hydrogens from carbamate ([3H]19), amide ([3H]22 and [3H]24), carboxylic acid ([3H]21), and alcohol ([3H]23, [3H]25, and [3H]26) functionalities did not have a major impact on tritium incorporation. Again, we observed high tritium incorporations with the high-molecular-weight macrocycles azithromycin ([3H]25) and clarithromycin ([3H]26); these substrates have no aromatic rings and cannot be tritiated using existing HIE protocols. For [3H]22, when the amount of T2 gas was increased to 2 Ci, specific activity was boosted from 11.5 to 14.6 Ci mmol−1, with a large increase in yield (8.6 to 19.6 mCi). This demonstrates the tunability of our protocol with respect to the amount of T2 used, enabling single-step tritiation even for particularly recalcitrant substrates. We achieved high specific activities for all substrates, meeting the typical standards for the preparation of high-specific-activity pharmaceutical drugs in appreciable yield for use in ligand-binding studies.
Fig. 4. Scope of high-specific-activity tritiation.
Reaction conditions: substrate (2 μmol), photoredox catalyst 1 or 12 (4 mol %), thiol catalyst 13 or 14 (60 mol %), T2O (preformed from 1 Ci T2 and PtO2), NMP, rt, 34-W blue LED or integrated photoreactor. ‡Incorporation depicted is for the 2 Ci reaction with [3H]22.
In addition to the library of commercially available drug molecules, we further evaluated the utility of this photoredox-catalyzed tritiation with a series of potential GPR-40 agonists with positive allosteric modulation (agoPAMs) (Fig. 4) developed in the research laboratories of Merck & Co., Inc. (43). The characterization of G protein–coupled receptor allostery is critical to advancing this agoPAM chemical class. Augmentation of binding in the presence of orthosteric ligands has been previously demonstrated using radio-labeled analogs in radioligand-binding assays and subsequently visualized in the GPR-40 ternary crystal structure (44). As such, we envision that the radiolabeling methodology described herein might be used to enable metabolite identification both in vitro and in vivo, as well as the elucidation of the extent of covalent protein binding in human hepatocytes to lower the risk of any bioactivation toward reactive electrophiles (45).
Initially, we observed photoredox-mediated decarboxylation of the aliphatic carboxylic acid moiety in the drug candidates. However, this was overcome by using a less oxidizing photo-catalyst, Ir(ppy)2(dtbbpy)PF6 [E1/2red(*IrIII/IrII) = +0.66 V versus SCE in acetonitrile] (34) or a Lewis acid additive (supplementary materials). We found that sterically hindered amines ([3H] 27 and [3H]28) and azetidine rings ([3H]29) were also amenable substrates for this protocol. Tritiation was also observed at a C(sp2)–H moiety in [3H]27, possibly owing to conjugation of the benzylic α-amino radical. For [3H]31, epimerization likely occurred at the chiral center adjacent to nitrogen as a result of the introduction of tritium (46). All five compounds were satisfactorily labeled and met the requirements for use in preclinical candidate selection studies. We anticipate that facile access to highly deuterated and tritiated compounds based on the method described herein will enable accelerated and broader interrogation of the biological activity of molecules in the pursuit of the development of new molecular therapies.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institutes of Health (NIH) under award number R01 GM103558-04 (D.W.C.M., Y.Y.L., and K.N.). Y.Y.L. thanks the Agency for Science, Technology and Research (A*STAR) for a graduate fellowship. K.N. thanks the Japan Society for the Promotion of Science for an overseas postdoctoral fellowship. Additional funding was provided by kind gifts from Merck, Abbvie, BMS, and Janssen. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Additional data supporting the conclusions are available in the supplementary materials.
Footnotes
www.sciencemag.org/content/358/6367/1182/suppl/DC1
Materials and Methods
NMR Spectra
References (47–54)
REFERENCES AND NOTES
- 1.Isin EM, Elmore CS, Nilsson GN, Thompson RA, Weidolf L. Chem Res Toxicol. 2012;25:532–542. doi: 10.1021/tx2005212. [DOI] [PubMed] [Google Scholar]
- 2.Lockley WJS, McEwen A, Cooke R. J Labelled Comp Radiopharm. 2012;55:235–257. [Google Scholar]
- 3.Voges R, Heys JR, Moenius T. Preparation of Compounds Labeled with Tritium and Carbon-14. John Wiley & Sons; 2009. [Google Scholar]
- 4.Allen PH, Hickey MJ, Kingston LP, Wilkinson DJ. J Labelled Comp Radiopharm. 2010;53:731–738. doi: 10.1002/jlcr.3385. [DOI] [PubMed] [Google Scholar]
- 5.Elmore CS, Bragg RA. Bioorg Med Chem Lett. 2015;25:167–171. doi: 10.1016/j.bmcl.2014.11.051. [DOI] [PubMed] [Google Scholar]
- 6.Elmore CS. Annu Rep Med Chem. 2009;44:515–534. [Google Scholar]
- 7.Hulme EC, Trevethick MA. Br J Pharmacol. 2010;161:1219–1237. doi: 10.1111/j.1476-5381.2009.00604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meleza C, et al. Anal Biochem. 2016;511:17–23. doi: 10.1016/j.ab.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 9.Stumpf WE. J Pharmacol Toxicol Methods. 2005;51:25–40. doi: 10.1016/j.vascn.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Atzrodt J, Derdau V, Fey T, Zimmermann J. Angew Chem Int Ed. 2007;46:7744–7765. doi: 10.1002/anie.200700039. [DOI] [PubMed] [Google Scholar]
- 11.Derdau V, Atzrodt J, Zimmermann J, Kroll C, Brückner F. Chemistry. 2009;15:10397–10404. doi: 10.1002/chem.200901107. [DOI] [PubMed] [Google Scholar]
- 12.Hesk D, Das PR, Evans B. J Labelled Comp Radiopharm. 1995;36:497–502. [Google Scholar]
- 13.Heys JR. J Labelled Comp Radiopharm. 2007;50:770–778. [Google Scholar]
- 14.Yu RP, Hesk D, Rivera N, Pelczer I, Chirik PJ. Nature. 2016;529:195–199. doi: 10.1038/nature16464. [DOI] [PubMed] [Google Scholar]
- 15.For transition metal–catalyzed deuteration of C(sp3)–H bonds, see (16–19). For transition metal–catalyzed tritiation of C(sp3)–H bonds, see (20).
- 16.Takahashi M, Oshima K, Matsubara S. Chem Lett. 2005;34:192–193. [Google Scholar]
- 17.Neubert L, et al. J Am Chem Soc. 2012;134:12239–12244. doi: 10.1021/ja3041338. [DOI] [PubMed] [Google Scholar]
- 18.Pieters G, et al. Angew Chem Int Ed. 2014;53:230–234. doi: 10.1002/anie.201307930. [DOI] [PubMed] [Google Scholar]
- 19.Hale LVA, Szymczak NK. J Am Chem Soc. 2016;138:13489–13492. doi: 10.1021/jacs.6b07879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lockley WJS, Hesk D. J Labelled Comp Radiopharm. 2010;53:704–715. [Google Scholar]
- 21.McGrath NA, Brichacek M, Njardarson JT. J Chem Educ. 2010;87:1348–1349. [Google Scholar]
- 22.Prier CK, Rankic DA, MacMillan DWC. Chem Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw MH, Twilton J, MacMillan DWC. J Org Chem. 2016;81:6898–6926. doi: 10.1021/acs.joc.6b01449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kärkäs MD, Porco JA, Jr, Stephenson CRJ. Chem Rev. 2016;116:9683–9747. doi: 10.1021/acs.chemrev.5b00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNally A, Prier CK, MacMillan DWC. Science. 2011;334:1114–1117. doi: 10.1126/science.1213920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noble A, MacMillan DWC. J Am Chem Soc. 2014;136:11602–11605. doi: 10.1021/ja506094d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prier CK, MacMillan DWC. Chem Sci. 2014;5:4173–4178. doi: 10.1039/C4SC02155J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanksby SJ, Ellison GB. Acc Chem Res. 2003;36:255–263. doi: 10.1021/ar020230d. [DOI] [PubMed] [Google Scholar]
- 29.Olmstead WN, Margolin Z, Bordwell FG. J Org Chem. 1980;45:3295–3299. [Google Scholar]
- 30.Yu HZ, Yang YM, Zhang L, Dang ZM, Hu GH. J Phys Chem A. 2014;118:606–622. doi: 10.1021/jp410274n. [DOI] [PubMed] [Google Scholar]
- 31.Escoubet S, et al. J Org Chem. 2006;71:7288–7292. doi: 10.1021/jo061033l. [DOI] [PubMed] [Google Scholar]
- 32.A scale that will allow enough material to support a drug discovery program (in this case, more than 1 g of material).
- 33.Although we favor the mechanism outlined in Fig. 2A, we cannot rule out the possibility that a mechanism similar to that outlined in fig. S4 is a competing pathway. In this instance, thiol radical generated in situ can abstract from α-amino C–H bonds in the substrate to form the key α-amino radical intermediate.
- 34.Lowry MS, et al. Chem Mater. 2005;17:5712–5719. [Google Scholar]
- 35.Le C, Liang Y, Evans RW, Li X, MacMillan DWC. Nature. 2017;547:79–83. doi: 10.1038/nature22813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wayner DDM, Clark KB, Rauk A, Yu D, Armstrong DA. J Am Chem Soc. 1997;119:8925–8932. [Google Scholar]
- 37.Hadad CM, Rablen PR, Wiberg KB. J Org Chem. 1998;63:8668–8681. [Google Scholar]
- 38.Shaidarova LG, Ziganshina SA, Budnikov GK. J Anal Chem. 2003;58:577–582. [Google Scholar]
- 39.Luo J, Zhang J. ACS Catal. 2016;6:873–877. [Google Scholar]
- 40.Morimoto H, Williams PG. Fus Sci Technol. 1992;21:256–261. [Google Scholar]
- 41.Le CC, et al. ACS Cent Sci. 2017;3:647–653. doi: 10.1021/acscentsci.7b00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Czarnik AW. 20,090, 062220. US Patent. 2009
- 43.Plummer CW, et al. ACS Med Chem Lett. 2017;8:221–226. doi: 10.1021/acsmedchemlett.6b00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu J, et al. Nat Struct Mol Biol. 2017;24:570–577. doi: 10.1038/nsmb.3417. [DOI] [PubMed] [Google Scholar]
- 45.Baillie TA. Chem Res Toxicol. 2006;19:889–893. doi: 10.1021/tx060062o. [DOI] [PubMed] [Google Scholar]
- 46.For the use of tracers or reagents in early in vitro screening, epimerization is less of an issue because the experimental error can be high (>2× in discovery studies for binding or ex vivo occupancy studies). It should be noted that racemic tracers have been used in preclinical and clinical settings (e.g., positron emission tomography).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.