Abstract
Dysregulation of the immune system contributes to the breakdown of immune regulation, leading to autoimmune diseases, such as type 1 diabetes (T1D). Current therapies for T1D include daily insulin, due to pancreatic β-cell destruction to maintain blood glucose levels, suppressive immunotherapy to decrease the symptoms associated with autoimmunity, and islet transplantation. Genetic risks for T1D have been linked to IL-2 and IL-2R signaling pathways that lead to the breakdown of self-tolerance mechanisms, primarily through altered regulatory T cell (Treg) function and homeostasis. In attempt to correct such deficits, therapeutic administration of IL-2 at low-doses has gained attention due to the capacity to boost Tregs without the unwanted stimulation of effector T cells. Preclinical and clinical studies utilizing low-dose IL-2 have shown promising results to expand Tregs due to their high selective sensitivity to respond to IL-2. These results suggest that low-dose IL-2 therapy represents a new class of immunotherapy for T1D by promoting immune regulation rather than broadly suppressing unwanted and beneficial immune responses.
Keywords: type 1 diabetes, IL-2, Tregs, low-dose IL-2 therapy, IL-2 receptor, and tolerance
Introduction
Type 1 diabetes (T1D) is an autoimmune disorder primarily mediated by the adaptive immune responses against several islet cell autoantigens, which eventually leads to the destruction of pancreatic β cells and in turn severe insulin deficiency [1–3]. Individuals with this disorder must take insulin daily to maintain normal blood glucose levels. Decades of research have led to improved management of T1D but there is still no cure. In this review we will discuss how the IL-2 receptor (IL-2R) may represent a therapeutic target for controlling islet autoimmunity and restoring self-tolerance in patients with T1D, which in turn would preserve a functional mass of pancreatic β cells. IL-2 not only causes proliferation of regulatory T cells (Tregs) but may compensate for a genetic defect associated with T1D, as single nucleotide polymorphisms (SNPs) in IL2RA represent a genetic risk [4]. A completed clinical trial has demonstrated the safety of administering IL-2 to T1D patients and defined a low dose range at which IL-2 increases Tregs without reactivating autoreactive T cells and in general effector T cells [5]. Patients with recent onset T1D are now being enrolled in a low-dose IL-2 phase 2 clinical trial to test the efficacy of this therapy (NTC02411253). Before describing ongoing work concerning low-dose IL-2 in the clinic, we will first briefly discuss our current understanding of the function of IL-2 in the immune system and how altered activity of the IL-2 pathway contributes to T1D.
The role of interleukin-2 in Tregs
IL-2R signaling plays a non-redundant role for the development of CD4+ Foxp3+ Tregs and importantly contributes to Treg homeostasis [6–8]. IL-2−/−, IL-2Rα−/−, and IL-2Rβ−/− mice are characterized by severe systemic autoimmunity and lymphoproliferation that rapidly leads to the death of these mice [9–11]. Our laboratory showed nearly 14 years ago that the transfer of purified Tregs was sufficient to fully protect IL-2-sufficient IL-2Rβ−/− mice from disease [12]. This work and that of others directly demonstrated that impaired Tregs were the fundamental reason for lethal autoimmunity in these mice [13, 14]. In the complete absence of IL-2R signaling, mice harbor a low proportion of non-functional immature CD4+ CD25− Foxp3lo cells [15]. During thymic development, IL-2 is a necessary second signal after TCR signaling that upregulates CD25 and Foxp3 in developing Tregs by a STAT5-mediated mechanism, leading to functional Tregs [16–19]. Under the appropriate conditions, conventional peripheral T cells can also develop into suppressive Tregs. These induced or peripheral Tregs, similar to thymic-derived Tregs, also require IL-2 for development in addition to retinoic acid and TGFβ to upregulate Foxp3 [20–22]. This pathway may also counteract RORγt expression and production of IL-17 to reinforce Treg suppressive function [22].
In the periphery, IL-2 regulates a number of activities in Tregs. During the neonatal period, blockade of IL-2 severely affects the initial peripheral amplification of Tregs [23], whereas interfering with IL-2R signaling in adult mice lowers the numbers of Tregs, but many Tregs are still detected [24, 25]. IL-2 mediates Treg homeostasis primarily by STAT5 activation of cell cycle progression and promoting expression of cell survival molecules such as Bcl-2 and Mcl-1 [26, 27]. Recent work suggests that IL-2 is most critical for the homeostasis of central Tregs, those Tregs that are CCR7+ and CD62Lhi and primarily reside in secondary lymphoid tissues [28]. The homeostasis of activated or effector Tregs, e.g. CCR7−, CD62Llo, ICOShi Tregs, are much more dependent on TCR signaling [29, 30]. However, the development of activated, terminally differentiated Tregs, characterized by expression of Klrg1 and high levels of several key Tregs suppressive molecules, also depends on IL-2 [31].
Another important property of IL-2 in the periphery is to reinforce the expression of Foxp3 [32]. This function is mediated by direct IL-2-dependent STAT5 binding to regulatory regions within the promoter and conserved noncoding sequence 2 (CNS2) of Foxp3 [33]. Foxp3 directly represses key genes related to T effector (Teff) cells, e.g. IL-2 and IL-17, and upregulates the expression of several Treg suppressive molecules, e.g. CTLA-4, IL-10, and IL-35 [34, 35]. Moreover, Foxp3 and IL-2 are in a positive regulatory loop through direct effects of Foxp3 and STAT5 to upregulate CD25 expression. Besides effects on Foxp3, IL-2 may directly regulate some aspects of the Treg suppressive program. For example, a mechanism by which Tregs suppress autoimmunity involves perforin/granzyme B cytotoxicity, and both are partially regulated by IL-2-dependent STAT5 activation [36–38]. Besides activating mechanisms to promote Treg suppressive function, the IL-2R pathway passively promotes suppression of autoreactive T cells due to the high level of CD25 expression and the high affinity IL-2R on Treg cells. This property promotes Tregs to preferentially consume IL-2 and sequester IL-2 from autoreactive T cells [39, 40].
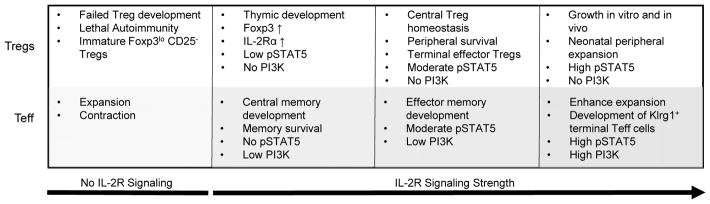
A very important aspect of Treg immunobiology uncovered by our laboratory is that Treg development and homeostasis are effectively supported with low levels of IL-2R signaling whereas Teff responses require more extensive signaling [41, 26] (Fig. 1). This conclusion stems from studies of genetically engineered mice in which T cells expressed mutated IL-2Rβ cytoplasmic tails resulting in lowered IL-2-dependent pSTAT5 and PI3K activation. This study also established that a gradient of IL-2R signaling impacts Treg cells. Some fundamental Treg properties, such as regulation of Foxp3, CD25, and CTLA-4 expression, are supported by low IL-2R signaling whereas other functions such as development of Klrg1+ effector Tregs and expression of some Treg functional molecules, e.g. IL-10 and granzyme B, require more extensive IL-2R signal transduction. Importantly, these findings provide strong conceptual support for using low levels of IL-2 to selectively stimulate Tregs.
Figure 1.
Varied IL-2R signaling strength leads to distinct outcome in Treg and Teff cells
The role of IL-2 in Teff cells
IL-2 is also an important cytokine for mounting an optimal immune response. Recent work, however, shows that substantial T cell expansion and contraction occurs even in the absence of IL-2 [42]. Nevertheless, IL-2 promotes more robust T cell growth in vivo and sensitizes cells for apoptosis, which also facilitates contraction of an immune response [43]. IL-2 provides critical signals for the development of several key Teff cell functions and promotes Th1 cells by positive regulation of IFNγ, IL-12Rβ2 and T-bet [44, 45]. Th2 differentiation is promoted by IL-2 through positive regulation of IL-4 and IL-4Rα via STAT5-mediated mechanisms [46, 47]. Moreover, IL-2 is critical for development of cytotoxic T lymphocytes (CTLs) though regulation of granzyme B and perforin [38] by promoting the development of terminally differentiated CTLs through strong IL-2 signaling [48–50]. In striking contrast, the development of Th17 and Tfh subsets are favored in the absence of IL-2 [51, 52]. IL-2R signaling constrains Th17 development by activating STAT5 that directly competes with STAT3 for binding to regulatory regions in IL-17 [53]. Tfh development is also constrained by IL-2R signaling through STAT5-dependent activation of Blimp-1, which represses Bcl-6 that is necessary for Tfh development [51]. Thus, IL-2 therapy may promote tolerance not only through its effects of enhancing Tregs but also by lowering Th17 and Tfh activity.
IL-2 provides important signals for optimal T memory responses and acts during the primary response to promote a strong memory recall response [54]. The development of central memory CD8+ T cells are readily supported by low IL-2Rβ signaling whereas the development effector and effector/memory CD8+ T cells require more intensive IL-2R signaling [41]. IL-2 also impacts the survival of CD4+ T memory cells through the upregulation of IL-7Rα [55, 56]. Overall, just like for Tregs, IL-2 critically impacts the Teff compartment. However most of these activities in Teff cells depend on persistent and intense IL-2R signaling (Fig. 1). Thus, at a proper low dose, IL-2 is expected to primarily affect the Treg compartment.
IL2/IL2RA as a genetic risk for T1D
The non-obese diabetic (NOD) mouse model has many properties that are similar to human disease, including key components of the genetic susceptibility. Among the insulin-dependent diabetes (Idd) risk loci identified in the NOD mouse, the Idd3 region, which encodes IL2 and IL21, is a major contributor to diabetes susceptibility [57–59]. Lower IL-2 levels represent an important aspect by which Idd3 contributes to diabetes. In fact, further lowering IL-2 activity in female NOD mice by neutralization with anti-IL-2 accelerates the onset of diabetes [25]. Congenic NOD mice that contain the Idd3 interval from diabetes-free C57BL/6 mice, i.e. NODB6Idd3 mice, are largely protected from diabetes and exhibit reduced insulitis [60]. These protective effects are due to approximately two-fold increased IL-2 production when compared to NOD mice [61] that leads to improved Treg function and reduced pro-inflammatory cytokine production [62, 63, 61].
The first and most prominent genetic risk factor for human T1D is localized in the HLA complex with odds ratio values close to 7. The HLA complex is a multigene risk locus in which the most important genes are polymorphic variants of HLA class I and class II antigen-presenting molecules [64]. Underscoring the complexity of T1D, more than 50 loci have now been identified as genetic risks for T1D development [65–67], most individually providing small contributions to risk (odds ratio below 1.5). Among these, the four non-HLA genetic polymorphisms with stronger association (odds ratios in the 1.5–2.5 range) have been mapped to CTLA4 [68], INS [69], PTPN22 and IL2RA [65, 4]. Statistically, each of these loci individually represents a small risk toward developing T1D. Furthermore, individuals express distinct patterns of these risk loci. However, by targeting important pathways that regulate the immune system, the cumulative biological effects of disease-related genetic polymorphisms in conjunction with environmental factors may trigger T1D. So far, the precise biological contributions of these genetic risks have been difficult to precisely define.
Genome-wide association studies (GWAS) and tag-SNPs have identified key SNPs associated with T1D and sometimes have defined variation in transcription or protein function. Using tag-SNP technology, 54 SNPs were identified near the regulatory regions or exons of IL2RA [70], initially implicating IL2RA as a genetic risk for T1D. GWAS established two specific SNPs (ss52580101 and ss52580109) in the region containing intron 1 of IL2RA and the 5′ upstream regions of IL2RA and RBM17 that specifically correlate with T1D disease susceptibility [4]. However, these two SNPs are not associated with eight regions known to control IL2RA transcription [4]. Susceptible SNP ss52580101, however, has been linked to reduced serum concentrations of soluble IL-2Rα in patients with T1D, consistent with individuals with fewer IL-2Rα+ T cells, i.e. Tregs or Teff cells. Indeed, individuals with the IL2RA T1D susceptible SNPs have reduced CD25 expression on Tregs and T memory cells, and reduced IL-2-induced tyrosine phosphorylated STAT5 (pSTAT5) activity [71]. Overall, lower IL-2R signaling in Tregs leads to decreased Foxp3 expression and impaired Treg function [71, 72]. Thus, low-dose IL-2 therapy has the potential to selectively correct potential genetic defects associated with altered IL-2R signaling in Tregs.
IL-2 in the therapy of autoimmunity
We still lack a robust therapy for islet autoimmunity, to prevent progression to diabetes symptoms or to preserve residual β-cell mass after diagnosis. Current treatments focus on targeting symptoms or consequences of the disease, from daily injections of insulin, use of immunosuppressive drugs, strategies to deplete autoreactive T cells [73–76], islet transplantation to repair disease tissues [77, 78], and the use of biologics to suppress the inflammatory response [79–81]. There is much interest in providing [82, 83] or boosting Tregs in patients with T1D as this may induce immunoregulation by restoring tolerance and leave beneficial aspects of the immune system intact that protect one from infectious disease [65, 84]. Based on the studies discussed above, IL-2 represents a prime candidate to mediate these effects as it can directly increase Tregs. Accordingly, IL-2 may reestablish impaired immune regulation and may correct impaired Treg activities associated with the IL-2/IL-2R genetic risk in this disease.
IL-2 has been an approved for clinical use for over 20 years with the first immunotherapeutic approaches to boost an immune responses in patients with cancer and HIV/AIDS [85–88]. In early clinical trials of renal cancer and metastatic melanoma, IL-2 was administered at high doses in attempts to maintain efficacious circulatory levels of IL-2 to boost immune responses, which was believed essential due to the relatively short half-life of IL-2 [85–87]. At such high doses, IL-2 stimulates an immune response in some patients but leads to extreme toxicities. Moreover the accompany increase in Tregs inhibit the capacity to boost immunity in patients with cancer and HIV/AIDS. [89, 88].
Several preclinical studies raised the possibility that much lower doses of IL-2 might selectively boost Tregs while avoiding effect on Teff or T autoreactive T cells. One such study, described above, showed that low levels of IL-2R signaling was effective for the development and homeostasis of Tregs, but this low signaling did not support Teff cells [26]. More directly, administering low levels of recombinant IL-2 or in the form of agonist IL-2/anti-IL-2 complexes [90] to NOD mice increased Tregs and prevented the development of diabetes [91]. This type of treatment was also effective in NOD mice with recent onset diabetes [92]. Pancreas-targeted adeno-associated virus expressing IL-2 also effectively controlled diabetes in NOD mice [93]. Low levels of IL-2 has also been shown to increase Tregs, prolong allogenic islet allografts, and suppress disease in mouse models of experimental autoimmune encephalomyelitis (EAE), systemic lupus erythematosus (SLE), and muscular dystrophy [94–97].
Given the promise of low-dose IL-2 therapy in these preclinical studies, the focus has now shifted to the utilization of IL-2 in phase I/II clinical trials for autoimmune diseases. The first reports of the use of low levels of IL-2 in patients with overactive immune responses were in chronic graft versus host disease (GvHD) and hepatitis c virus (HCV) induced vasculitis [98, 99]. Patients suffering with chronic GvHD received IL-2 s.c. at 0.3×106, 1×106, 3×106 IU/m2 of body surface area daily for 8 weeks. For HCV, IL-2 was administered s.c. at 1.5×106 IU/day for 5 days followed by 3 additional applications of IL-2 at 3×106 IU/day for 5 days at 3 week intervals. These levels of IL-2 were substantially lower than those given to patients with cancer and HIV/AIDS, where the goal was to boost immunity. Evaluation of these patients revealed that low-dose IL-2 was safe and the highest tolerated dose was 1×106 IU/m2 in the GvHD study. Importantly both studies found no indication that self-reactive T cells were activated. These levels of IL-2 led to sustained Treg expansion with clinical improvement of disease manifestations in many patients. The one off-target effect was increases in CD56bright NK cells that was more prominent at the higher doses of IL-2.
There have been several additional reports using low dose IL-2 in a limited number of patients with alopecia areata, SLE, and acute GvHD [100, 101, 98]. Dosing of IL-2 in these studies ranged from 2×105 to 3 x 106 IU s.c. per injection. The frequency of administering IL-2 varied in these studies, but generally several injections were administered closely spaced, e.g daily or every other day, followed by a rest period and repeat administration of IL-2. Again Tregs levels increased in all patients and clinical improvement was noted in approximately 80% of the patients. The main adverse reaction was inflammation at the injection sites; importantly, grade 3 or 4 toxicities were not seen. When low-dose IL-2 was administered to patients undergoing allogeneic hematopoietic stem cells transplants, no patients developed grade 2–4 acute GvHD, whereas 12% of the patients in the control group developed severe acute GvHD. Low-dose IL-2 in SLE led to amelioration of clinical symptoms and decrease in disease-associated autoantibodies. The results with alopecia areata were particularly impressive in that before IL-2 administration skin biopsies revealed numerous CD4+ and CD8+ T cells, with few Tregs. After IL-2 treatment, inflammatory infiltrates were markedly reduced and Tregs were now readily detected as Foxp3-positive cells. Remarkably, significant hair regrowth was also noted. Thus, all these studies point to the potential benefit of low-dose IL-2 to inhibit pathogenic effects mediated by self-reactive T cells through improved immune regulation via enhanced Treg function.
Low-Dose IL-2 Therapy in T1D
Substantial efforts are now under way to test low-dose IL-2 in patients with T1D. The rationale for these efforts, as discussed above, is that Tregs are impaired in T1D and this therapy targets a known genetic risk in T1D. Thus, expanding Tregs may restore tolerance mechanisms and preserve this residual islet activity to benefit the health of these patients. A phase I/II dose-limiting study aimed at establishing an optimal IL-2 therapeutic dose has already been completed. Twenty-four participants were randomly assigned to placebo or IL-2 groups where they received 0.33×106, 1×106, or 3×106 IU/day for 5 consecutive days. The participants were monitored for 60 days [5]. No participants exhibited severe adverse effects, but an injection-site reaction was often seen. A dose-dependent increase in Tregs was seen in all participants with minimal NK cell expansion, especially when using 1×106 IU of IL-2 or lower. Importantly, the IL-2-treated participants did not exhibit any detrimental changes in glucose metabolism, supporting the safety of using these levels of IL-2 in participants with T1D.
More detailed examination of samples from this trial [102] showed that all participants with T1D treated with low-dose IL-2 trial upregulated CD25 and Foxp3 on Tregs but not on CD4+ T effector memory (TEM) cells. This is particularly relevant for CD25 as this target is substantially upregulated by IL-2 in Tregs and TEM, although TEM depend on a higher amount of IL-2. Importantly, low-dose IL-2 selectively induces pSTAT5 signaling in Treg cells ex-vivo. Proportions of Tregs increased and remained elevated in patients given IL-2 at 1 or 3×106 IU at 60 days post-treatment, but these levels were lower than detected immediately after the end of IL-2 administration (this was a 5 day course). Further characterization showed that expanded Tregs were largely of a CD45RO+ memory phenotype with some features that suggested heightened activation. Plasma proteomics of cytokines were consistent with a shift towards a more regulatory environment. Transcriptome analysis of PBMCs showed up-regulation of genes associated with cell cycle and transcription and down-regulation of B cell signatures. At only the highest dose of IL-2, a NK gene signature was detected. Although IL-2 was safe at all treatments, it appears that dosing at 1×106 IU of IL-2 is the higher end where Tregs are most selectively targeted with negligible effects on NK cells. However, this trial was not powered to assess effects on insulin secretion, a question that will be addressed by a clinical trial in Europe that is enrolling children and adults with recent onset T1D (NCT02411253).
We also believe that low dose IL-2 therapy could be beneficial in patients with established T1D, beyond the immediate post-diagnosis period, as long as there is still residual insulin secretion. In support of this belief, we note that levels of stimulated C-peptide at diagnosis are only partially reduced in many patients [103–105]. While C-peptide production declines after diagnosis, it persists in many patients for several years. In a two-year follow-up of 191 newly diagnosed patients conducted by the Type 1 Diabetes TrialNet, 93% of the patients had detectable C-peptide 2 years after diagnosis, with 88% and 66% of patients maintaining a peak stimulated C-peptide ≥0.2 nmol/L at 1 year and 2 years after onset, respectively; this level is used as entry criteria by most clinical trials in recently diagnosed patients. Emerging pathology data indicate that β-cell loss at diagnosis may be much less severe than previously believed [106–108]. Pathology studies also show that islet autoimmunity may persist for many years after diagnosis [109–112]. Thus, if low-dose IL-2 could simply prevent further loss of insulin secretion and maintain a ≥0.2 nmol/L C-peptide response, this would be clinically significant because at this level of C-peptide there is an association with lower incidence of complications [113].
Low dose IL-2: Immunoregulation without a compromised immune response
One concern with administration of IL-2 to stimulate a Treg response is its potential stimulate a Teff immune response. Excess IL-2 accelerates T1D in NOD mice [91]. In a T1D study using IL-2 in combination with rapamycin, 9 participants were treated orally with 2–4mg/day rapamycin for 3 months and 4.5×106 IU IL-2 three times per week for 1 month [114]. The goal in this trial was to inhibit Teff cells with rapamycin and to sensitize Teff cells to apoptosis through the action of IL-2, [115, 116]. However, a decline in insulin C-peptide levels was noted in participants with T1D that received IL-2 and rapamycin. It should be noted that this is not considered a low-dose regimen, and represents 4.5-fold-higher initial and 9-fold higher cumulative dose of IL-2 during the same time frame than is currently being used in ongoing efficacy trial of new onset participants with T1D (NTC02411253). Tregs also increased after IL-2/rapamycin, but glucose metabolism worsened in these participants, raising concerns that these higher levels of IL-2 may have led to a net increase in the activity of autoreactive T cells. Whether this actually explains this adverse reaction remains unclear, as rapamycin is known to cause β cell toxicity, leading to reduced β cell size, mass, proliferation, impaired insulin secretion, increased apoptosis, autophagy, and peripheral insulin resistance [117–119]. Robust preclinical data about the combined use of IL-2 with rapamycin also show that rapamycin impairs β cell function in NOD mice [119]. The overall experience with low-dose IL-2, however, points to its safety, including the inability to promote Teff responses at the levels currently used.
The above concerns, nevertheless, make plain that a more detailed understanding is required concerning the selectivity of human Tregs to low-dose IL-2. We quantified the levels by which IL-2 selectively activated human Tregs at initial proximal signaling and down-stream gene activation [120]. IL-2 optimally stimulated tyrosine phosphorylated STAT5 (pSTAT5) at approximately 10-fold lower levels of IL-2 than CD45RO+ CD4+ memory T cells. With respect to gene activation, quantitative analysis of 12 of 388 IL-2-dependent genes in human Tregs indicated that 10/12 were highly upregulated at a 100-fold lower level of IL-2 when compared to CD4+ T memory cells. These included Foxp3 and CD25 which work together to reinforce the Treg suppressive program in response to therapeutically administered or endogenous IL-2. Thus, a substantial window exists in which Tregs are available to selectively respond to IL-2 during low-dose IL-2 therapy. This was shown in both healthy subjects and patients with T1D. This increase in IL-2 sensitivity was due to in part to higher levels of IL-2Rα and γc subunits on Tregs. Moreover, PP2A activity may be higher in Tregs due to increased levels of the PP2A inhibitor, SET. This may lead to lower serine/threonine phosphorylation of IL-2R and/or associated signaling molecules, which would promote IL-2R signal transduction [121].
Another concern with boosting Tregs with low-dose IL-2 is the increased Tregs might limit beneficial immune responses. Several studies suggest that this may not be a serious problem. First, administration of low-dose IL-2 to patients undergoing an allogenic hematopoietic stem cell transplanted showed increases in Tregs and lower instances of acute GvHD [122]. Importantly, these patients did not experience diminished anti-tumor and anti-viral responses. Second, administering 1010 viral genomes of a recombinant adeno-associated viral (AAV) vector containing the IL-2 cDNA to NOD mice leads to continuous IL-2 production at levels that increased Tregs and protected mice from diabetes [123]. Although Tregs increased, protective immunity was elicited against influenza infection, growth of transplanted or chemically-induced tumors were not accelerated, and allogenic pregnancy were normal with regard to number of offspring and the male/female ratio. Overall, low-dose IL-2 promotes immune tolerance through selective action on Tregs while not obviously impairing immunity.
Concluding Remarks
Boosting Tregs in patients with autoimmunity has been a major clinical objective since CD4+ Foxp3+ Tregs were accepted as an important population of T cells that critically maintain peripheral tolerance to self. Based on our much better understanding of the immunobiology of IL-2, new clinical approaches have been devised to take advantage of the powerful activity of IL-2 on Tregs. The current experience with low-dose IL-2 therapy is very promising (Table 1). So far clinical trials have been completed where 99 participants with autoimmunity or overactive immune responses have been treated with low-dose IL-2. The experience is that this therapy is safe, Tregs increase, and depending upon the trial design, therapeutic benefit has often been noted (Table 1). Nevertheless the jury is still out on whether the promise of low-dose IL-2 therapy as an entirely new approach to treat autoimmunity, including T1D, becomes a reality. Clearly the next key step is larger scale efficacy trials. The research teams of David Klatzmann, John Todd and Linda Wicker, and ours are actively involved in such trials for T1D, focusing on IL-2 as a monotherapy. Thus, it will be a relatively short time before we learn whether this approach benefits patients with T1D. A major concern that will be more definitely answered in these and other trials is related to the potential of IL-2 to reactivate autoreactive T cells and worsen disease. We have defined a therapeutic window where low levels of IL-2 preferentially stimulate Tregs. This finding and the current experience in patients with low-dose IL-2 suggest that such selectivity is achievable, especially a doses of IL-2 at <1×106 IU/injection for an adult patient.
Table 1.
Summary of completed clinical trials using low-dose IL-2 therapy
| Autoimmune Disease | Participants |
|---|---|
| Chronic graft vs. host disease (GvHD) | 23 |
| Hepatitis C virus-induced vasculitis | 10 |
| GvHD after allogeneic hematopoietic stem cell transplant | 16 |
| Type 1 diabetes | 24 |
| Systemic Lupus Erythematosus | 21 |
| Alopecia areata | 5 |
| Major Clinical Outcomes | |
| Optimal dosing: 0.3–1 × 106 IU of IL-2 s.c. | |
| Frequency: Daily or 5 day induction and biweekly maintenance | |
| Therapy well tolerated, minor injection site reaction | |
| Treg expansion in PBMCs of most patients | |
| No reactivation of self-reactive T cells | |
| Main off target effect: Increase in CD56hi NK cells | |
| Improvement of clinical outcomes in many patients |
Current results indicate that IL-2 must be frequently administered at low doses to maintain increases in Tregs. Another key point, therefore, is the extent that low-dose IL-2 therapy on its own can induce robust immune tolerance after a limited course of therapy or whether chronic administration may be required. There is also increasing consensus that combination therapies may be more effective in controlling autoimmunity and supporting β-cell function in T1D [124]. Low-dose IL-2 may be combined with other agents, as well as with the tolerogenic administration of autoantigens to drive the specificity of Tregs. It is known that Tregs specific for a given autoantigen are much more effective in controlling the relevant autoimmune responses than polyclonal Treg cells, and in much lower numbers [125]. Thus, what may ultimately be critical in developing robust long lasting tolerance is induction of autoantigen specific Tregs where low-dose IL-2 may help to increase their numbers. A clinical trial in patients with T1D, who by necessity immunize themselves with a key autoantigen, insulin [126], on a daily basis, present a unique opportunity to test this concept and may be relevant to other autoimmune diseases. Lastly, IL-2 exhibits poor pharmacokinetic with very short-half life in the blood, approximately 30 min. Thus, new analogs of IL-2 may improve low-dose IL-2 therapy by extending its half-life and potency to boost Tregs, perhaps as novel fusion proteins, agonist IL-2/anti-IL2 complexes or superkines, the latter to enhance selectively toward Treg
Acknowledgments
Our work was supported by the National Institutes of Health (R01 DK093866; R01 AI055815), the American Diabetes Association (1-15-BS-125), Wallace H. Coulter Center for Translational Research, and Diabetes Research Institute Foundation, Hollywood, FL, the Peacock Foundation, Inc., Miami, FL.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Connor J. Dwyer, Natasha C. Ward, Alberto Pugliese, and Thomas R. Malek declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–85. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 2.Bach JF, Chatenoud L. Tolerance to islet autoantigens in type 1 diabetes. Annu Rev Immunol. 2001;19:131–61. doi: 10.1146/annurev.immunol.19.1.131. [DOI] [PubMed] [Google Scholar]
- 3.Pugliese A. Advances in the etiology and mechanisms of type 1 diabetes. Discov Med. 2014;18:141–50. [PubMed] [Google Scholar]
- 4.Lowe CE, Cooper JD, Brusko T, Walker NM, Smyth DJ, Bailey R, et al. Large-scale genetic fine mapping and genotype-phenotype associations implicate polymorphism in the IL2RA region in type 1 diabetes. Nat Genet. 2007;39:1074–82. doi: 10.1038/ng2102. [DOI] [PubMed] [Google Scholar]
- 5••.Hartemann A, Bensimon G, Payan CA, Jacqueminet S, Bourron O, Nicolas N, et al. Low-dose interleukin 2 in patients with type 1 diabetes: a phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2013;1:295–305. doi: 10.1016/S2213-8587(13)70113-X. This study is the first clinical trial in which low-dose IL-2 therapy was used in participants with T1D. [DOI] [PubMed] [Google Scholar]
- 6.Fehervari Z, Sakaguchi S. CD4+ Tregs and immune control. J Clin Invest. 2004;114:1209–17. doi: 10.1172/JCI23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gavin M, Rudensky A. Control of immune homeostasis by naturally arising regulatory CD4+ T cells. Curr Opin Immunol. 2003;15:690–6. doi: 10.1016/j.coi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665–74. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 9.Sadlack B, Lohler J, Schorle H, Klebb G, Haber H, Sickel E, et al. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur J Immunol. 1995;25:3053–9. doi: 10.1002/eji.1830251111. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki H, Kundig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor β. Science. 1995;268:1472–6. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 11.Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor α chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–30. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 12.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rβ-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–78. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 13.Almeida AR, Legrand N, Papiernik M, Freitas AA. Homeostasis of peripheral CD4+ T cells: IL-2R alpha and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J Immunol. 2002;169:4850–60. doi: 10.4049/jimmunol.169.9.4850. [DOI] [PubMed] [Google Scholar]
- 14.Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4+ regulatory T cell function. J Exp Med. 2002;196:851–7. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bayer AL, Lee JY, de la Barrera A, Surh CD, Malek TR. A function for IL-7R for CD4+CD25+Foxp3+ T regulatory cells. J Immunol. 2008;181:225–34. doi: 10.4049/jimmunol.181.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burchill MA, Yang J, Vang KB, Moon JJ, Chu HH, Lio CW, et al. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 2008;28:112–21. doi: 10.1016/j.immuni.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor β-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–90. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 18.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–11. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng G, Yu A, Dee MJ, Malek TR. IL-2R signaling is essential for functional maturation of regulatory T cells during thymic development. J Immunol. 2013;190:1567–75. doi: 10.4049/jimmunol.1201218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, et al. TGF-β-induced Foxp3 inhibits Th17 cell differentiation by antagonizing RORγ function. Nature. 2008;453:236–40. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayer AL, Yu A, Adeegbe D, Malek TR. Essential role for interleukin-2 for CD4+CD25+ T regulatory cell development during the neonatal period. J Exp Med. 2005;201:769–77. doi: 10.1084/jem.20041179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng G, Yu A, Malek TR. T-cell tolerance and the multi-functional role of IL-2R signaling in T-regulatory cells. Immunol Rev. 2011;241:63–76. doi: 10.1111/j.1600-065X.2011.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3+ CD25+ CD4+ regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–35. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu A, Zhu L, Altman NH, Malek TR. A low interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells. Immunity. 2009;30:204–17. doi: 10.1016/j.immuni.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pierson W, Cauwe B, Policheni A, Schlenner SM, Franckaert D, Berges J, et al. Antiapoptotic Mcl-1 is critical for the survival and niche-filling capacity of Foxp3+ regulatory T cells. Nat Immunol. 2013;14:959–65. doi: 10.1038/ni.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Smigiel KS, Richards E, Srivastava S, Thomas KR, Dudda JC, Klonowski KD, et al. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J Exp Med. 2014;211:121–36. doi: 10.1084/jem.20131142. This study demonstrates how IL-2 is differentially used by peripheral Treg subpopulations for thier homeostasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levine AG, Arvey A, Jin W, Rudensky AY. Continuous requirement for the TCR in regulatory T cell function. Nat Immunol. 2014;15:1070–8. doi: 10.1038/ni.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vahl JC, Drees C, Heger K, Heink S, Fischer JC, Nedjic J, et al. Continuous T cell receptor signals maintain a functional regulatory T cell pool. Immunity. 2014;41:722–36. doi: 10.1016/j.immuni.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 31•.Cheng G, Yuan X, Tsai MS, Podack ER, Yu A, Malek TR. IL-2 receptor signaling is essential for the development of Klrg1+ terminally differentiated T regulatory cells. J Immunol. 2012;189:1780–91. doi: 10.4049/jimmunol.1103768. This study defines the contribution of IL-2 for peripheral Tregs and shows that IL-2 is required for the development of terminally-differentated effector Tregs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–51. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 33.Feng Y, Arvey A, Chinen T, van der Veeken J, Gasteiger G, Rudensky AY. Control of the inheritance of regulatory T cell identity by a cis element in the Foxp3 locus. Cell. 2014;158:749–63. doi: 10.1016/j.cell.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marson A, Kretschmer K, Frampton GM, Jacobsen ES, Polansky JK, MacIsaac KD, et al. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature. 2007;445:931–5. doi: 10.1038/nature05478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–40. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- 36.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Janas ML, Groves P, Kienzle N, Kelso A. IL-2 regulates perforin and granzyme gene expression in CD8+ T cells independently of its effects on survival and proliferation. J Immunol. 2005;175:8003–10. doi: 10.4049/jimmunol.175.12.8003. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Scordi I, Smyth MJ, Lichtenheld MG. Interleukin 2 receptor signaling regulates the perforin gene through signal transducer and activator of transcription (Stat)5 activation of two enhancers. J Exp Med. 1999;190:1297–308. doi: 10.1084/jem.190.9.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–62. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 40.Sitrin J, Ring A, Garcia KC, Benoist C, Mathis D. Regulatory T cells control NK cells in an insulitic lesion by depriving them of IL-2. J Exp Med. 2013;210:1153–65. doi: 10.1084/jem.20122248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castro I, Yu A, Dee MJ, Malek TR. The basis of distinctive IL-2- and IL-15-dependent signaling: weak CD122-dependent signaling favors CD8+ T central-memory cell survival but not T effector-memory cell development. J Immunol. 2011;187:5170–82. doi: 10.4049/jimmunol.1003961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–79. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 43.Lenardo MJ. Interleukin-2 programs mouse αβ T lymphocytes for apoptosis. Nature. 1991;353:858–61. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 44.Liao W, Lin JX, Wang L, Li P, Leonard WJ. Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nat Immunol. 2011;12:551–9. doi: 10.1038/ni.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi M, Lin TH, Appell KC, Berg LJ. Janus-kinase-3-dependent signals induce chromatin remodeling at the Ifng locus during T helper 1 cell differentiation. Immunity. 2008;28:763–73. doi: 10.1016/j.immuni.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cote-Sierra J, Foucras G, Guo L, Chiodetti L, Young HA, Hu-Li J, et al. Interleukin 2 plays a central role in Th2 differentiation. Proc Natl Acad Sci U S A. 2004;101:3880–5. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liao W, Schones DE, Oh J, Cui Y, Cui K, Roh TY, et al. Priming for T helper type 2 differentiation by interleukin 2-mediated induction of interleukin 4 receptor α-chain expression. Nat Immunol. 2008;9:1288–96. doi: 10.1038/ni.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 50.Mitchell DM, Ravkov EV, Williams MA. Distinct roles for IL-2 and IL-15 in the differentiation and survival of CD8+ effector and memory T cells. J Immunol. 2010;184:6719–30. doi: 10.4049/jimmunol.0904089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. J Exp Med. 2012;209:243–50. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–81. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 53.Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12:247–54. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–3. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dooms H, Kahn E, Knoechel B, Abbas AK. IL-2 induces a competitive survival advantage in T lymphocytes. J Immunol. 2004;172:5973–9. doi: 10.4049/jimmunol.172.10.5973. [DOI] [PubMed] [Google Scholar]
- 56.Dooms H, Wolslegel K, Lin P, Abbas AK. Interleukin-2 enhances CD4+ T cell memory by promoting the generation of IL-7R α-expressing cells. J Exp Med. 2007;204:547–57. doi: 10.1084/jem.20062381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Denny P, Lord CJ, Hill NJ, Goy JV, Levy ER, Podolin PL, et al. Mapping of the IDDM locus Idd3 to a 0.35-cM interval containing the interleukin-2 gene. Diabetes. 1997;46:695–700. [Google Scholar]
- 58.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–77. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 59.Lyons PA, Armitage N, Argentina F, Denny P, Hill NJ, Lord CJ, et al. Congenic mapping of the type 1 diabetes locus, Idd3, to a 780-kb region of mouse chromosome 3: identification of a candidate segment of ancestral DNA by haplotype mapping. Genome Res. 2000;10:446–53. doi: 10.1101/gr.10.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wicker LS, Todd JA, Prins JB, Podolin PL, Renjilian RJ, Peterson LB. Resistance alleles at two non-major histocompatibility complex-linked insulin-dependent diabetes loci on chromosome 3, Idd3 and Idd10, protect nonobese diabetic mice from diabetes. J Exp Med. 1994;180:1705–13. doi: 10.1084/jem.180.5.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamanouchi J, Rainbow D, Serra P, Howlett S, Hunter K, Garner VE, et al. Interleukin-2 gene variation impairs regulatory T cell function and causes autoimmunity. Nat Genet. 2007;39:329–37. doi: 10.1038/ng1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kornete M, Sgouroudis E, Piccirillo CA. ICOS-dependent homeostasis and function of Foxp3+ regulatory T cells in islets of nonobese diabetic mice. J Immunol. 2012;188:1064–74. doi: 10.4049/jimmunol.1101303. [DOI] [PubMed] [Google Scholar]
- 63.Sgouroudis E, Albanese A, Piccirillo CA. Impact of protective IL-2 allelic variants on CD4+ Foxp3+ regulatory T cell function in situ and resistance to autoimmune diabetes in NOD mice. J Immunol. 2008;181:6283–92. doi: 10.4049/jimmunol.181.9.6283. [DOI] [PubMed] [Google Scholar]
- 64.Redondo MJ, Fain PR, Eisenbarth GS. Genetics of type 1A diabetes. Recent Prog Horm Res. 2001;56:69–89. doi: 10.1210/rp.56.1.69. [DOI] [PubMed] [Google Scholar]
- 65.Concannon P, Rich SS, Nepom GT. Genetics of type 1A diabetes. N Engl J Med. 2009;360:1646–54. doi: 10.1056/NEJMra0808284. [DOI] [PubMed] [Google Scholar]
- 66.Cooper JD, Smyth DJ, Smiles AM, Plagnol V, Walker NM, Allen JE, et al. Meta-analysis of genome-wide association study data identifies additional type 1 diabetes risk loci. Nat Genet. 2008;40:1399–401. doi: 10.1038/ng.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pociot F, Akolkar B, Concannon P, Erlich HA, Julier C, Morahan G, et al. Genetics of type 1 diabetes: what’s next? Diabetes. 2010;59:1561–71. doi: 10.2337/db10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nistico L, Buzzetti R, Pritchard LE, Van der Auwera B, Giovannini C, Bosi E, et al. The CTLA-4 gene region of chromosome 2q33 is linked to, and associated with, type 1 diabetes. Belgian Diabetes Registry. Hum Mol Genet. 1996;5:1075–80. doi: 10.1093/hmg/5.7.1075. [DOI] [PubMed] [Google Scholar]
- 69.Bell GI, Horita S, Karam JH. A polymorphic locus near the human insulin gene is associated with insulin-dependent diabetes mellitus. Diabetes. 1984;33:176–83. doi: 10.2337/diab.33.2.176. [DOI] [PubMed] [Google Scholar]
- 70.Vella A, Cooper JD, Lowe CE, Walker N, Nutland S, Widmer B, et al. Localization of a type 1 diabetes locus in the IL2RA/CD25 region by use of tag single-nucleotide polymorphisms. Am J Hum Genet. 2005;76:773–9. doi: 10.1086/429843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garg G, Tyler JR, Yang JH, Cutler AJ, Downes K, Pekalski M, et al. Type 1 diabetes-associated IL2RA variation lowers IL-2 signaling and contributes to diminished CD4+CD25+ regulatory T cell function. J Immunol. 2012;188:4644–53. doi: 10.4049/jimmunol.1100272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Long SA, Cerosaletti K, Bollyky PL, Tatum M, Shilling H, Zhang S, et al. Defects in IL-2R signaling contribute to diminished maintenance of FOXP3 expression in CD4+CD25+ regulatory T-cells of type 1 diabetic subjects. Diabetes. 2010;59:407–15. doi: 10.2337/db09-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, et al. A single course of anti-CD3 monoclonal antibody hOKT3γ1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54:1763–9. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–8. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 75.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 76.Keymeulen B, Walter M, Mathieu C, Kaufman L, Gorus F, Hilbrands R, et al. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual β cell mass. Diabetologia. 2010;53:614–23. doi: 10.1007/s00125-009-1644-9. [DOI] [PubMed] [Google Scholar]
- 77.Harlan DM, Kenyon NS, Korsgren O, Roep BO. Current advances and travails in islet transplantation. Diabetes. 2009;58:2175–84. doi: 10.2337/db09-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–9. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 79.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, et al. Rituximab, B-lymphocyte depletion, and preservation of β-cell function. N Engl J Med. 2009;361:2143–52. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mastrandrea L, Yu J, Behrens T, Buchlis J, Albini C, Fourtner S, et al. Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes Care. 2009;32:1244–9. doi: 10.2337/dc09-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Orban T, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:412–9. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015;7:315ra189. doi: 10.1126/scitranslmed.aad4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Techmanska I, Juscinska J, et al. Administration of CD4+CD25highCD127− regulatory T cells preserves β-cell function in type 1 diabetes in children. Diabetes Care. 2012;35:1817–20. doi: 10.2337/dc12-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wicker LS, Clark J, Fraser HI, Garner VE, Gonzalez-Munoz A, Healy B, et al. Type 1 diabetes genes and pathways shared by humans and NOD mice. J Autoimmun. 2005;25(Suppl):29–33. doi: 10.1016/j.jaut.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 85.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–16. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 86.Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313:1485–92. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- 87.Rosenberg SA, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA. 1994;271:907–13. [PubMed] [Google Scholar]
- 88.Group I-ES, Committee SS, Abrams D, Levy Y, Losso MH, Babiker A, et al. Interleukin-2 therapy in patients with HIV infection. N Engl J Med. 2009;361:1548–59. doi: 10.1056/NEJMoa0903175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25hi Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–14. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–7. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 91.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–97. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grinberg-Bleyer Y, Baeyens A, You S, Elhage R, Fourcade G, Gregoire S, et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med. 2010;207:1871–8. doi: 10.1084/jem.20100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goudy KS, Johnson MC, Garland A, Li C, Samulski RJ, Wang B, et al. Inducible adeno-associated virus-mediated IL-2 gene therapy prevents autoimmune diabetes. J Immunol. 2011;186:3779–86. doi: 10.4049/jimmunol.1001422. [DOI] [PubMed] [Google Scholar]
- 94.Rouse M, Nagarkatti M, Nagarkatti PS. The role of IL-2 in the activation and expansion of regulatory T-cells and the development of experimental autoimmune encephalomyelitis. Immunobiology. 2013;218:674–82. doi: 10.1016/j.imbio.2012.08.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, et al. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206:751–60. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mizui M, Koga T, Lieberman LA, Beltran J, Yoshida N, Johnson MC, et al. IL-2 protects lupus-prone mice from multiple end-organ damage by limiting CD4-CD8- IL-17-producing T cells. J Immunol. 2014;193:2168–77. doi: 10.4049/jimmunol.1400977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Villalta SA, Rosenthal W, Martinez L, Kaur A, Sparwasser T, Tidball JG, et al. Regulatory T cells suppress muscle inflammation and injury in muscular dystrophy. Sci Transl Med. 2014;6:258ra142. doi: 10.1126/scitranslmed.3009925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98••.Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP, 3rd, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365:2055–66. doi: 10.1056/NEJMoa1108188. This study shows the efficacy of using low-dose IL-2 therapy to selectively boost Tregs in an setting were there are many allo-antigen self-reactive T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99•.Saadoun D, Rosenzwajg M, Joly F, Six A, Carrat F, Thibault V, et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med. 2011;365:2067–77. doi: 10.1056/NEJMoa1105143. This study and reference 98 were the first clincial trials showing that low-dose IL-2 increases Tregs and benefits patients undergoing a pathological self-reactive T cell response. [DOI] [PubMed] [Google Scholar]
- 100.Castela E, Le Duff F, Butori C, Ticchioni M, Hofman P, Bahadoran P, et al. Effects of low-dose recombinant interleukin 2 to promote T-regulatory cells in alopecia areata. JAMA Dermatol. 2014;150:748–51. doi: 10.1001/jamadermatol.2014.504. [DOI] [PubMed] [Google Scholar]
- 101.von Spee-Mayer C, Siegert E, Abdirama D, Rose A, Klaus A, Alexander T, et al. Low-dose interleukin-2 selectively corrects regulatory T cell defects in patients with systemic lupus erythematosus. Ann Rheum Dis. 2015 doi: 10.1136/annrheumdis-2015-207776. [DOI] [PubMed] [Google Scholar]
- 102.Rosenzwajg M, Churlaud G, Mallone R, Six A, Derian N, Chaara W, et al. Low-dose interleukin-2 fosters a dose-dependent regulatory T cell tuned milieu in T1D patients. J Autoimmun. 2015;58:48–58. doi: 10.1016/j.jaut.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sherry NA, Tsai EB, Herold KC. Natural history of β-cell function in type 1 diabetes. Diabetes. 2005;54(Suppl 2):S32–9. doi: 10.2337/diabetes.54.suppl_2.s32. [DOI] [PubMed] [Google Scholar]
- 104.Greenbaum CJ, Beam CA, Boulware D, Gitelman SE, Gottlieb PA, Herold KC, et al. Fall in C-peptide during first 2 years from diagnosis: evidence of at least two distinct phases from composite Type 1 Diabetes TrialNet data. Diabetes. 2012;61:2066–73. doi: 10.2337/db11-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sherr JL, Ghazi T, Wurtz A, Rink L, Herold KC. Characterization of residual β cell function in long-standing type 1 diabetes. Diabetes Metab Res Rev. 2014;30:154–62. doi: 10.1002/dmrr.2478. [DOI] [PubMed] [Google Scholar]
- 106.Klinke DJ., 2nd Extent of β cell destruction is important but insufficient to predict the onset of type 1 diabetes mellitus. PLoS One. 2008;3:e1374. doi: 10.1371/journal.pone.0001374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Krogvold L, Edwin B, Buanes T, Ludvigsson J, Korsgren O, Hyoty H, et al. Pancreatic biopsy by minimal tail resection in live adult patients at the onset of type 1 diabetes: experiences from the DiViD study. Diabetologia. 2014;57:841–3. doi: 10.1007/s00125-013-3155-y. [DOI] [PubMed] [Google Scholar]
- 108.Campbell-Thompson M, Fu A, Kaddis JS, Wasserfall C, Schatz DA, Pugliese A, et al. Insulitis and β-Cell Mass in the Natural History of Type 1 Diabetes. Diabetes. 2015 doi: 10.2337/db15-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Coppieters KT, Dotta F, Amirian N, Campbell PD, Kay TW, Atkinson MA, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med. 2012;209:51–60. doi: 10.1084/jem.20111187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sorensen JS, Vaziri-Sani F, Maziarz M, Kristensen K, Ellerman A, Breslow N, et al. Islet autoantibodies and residual β cell function in type 1 diabetes children followed for 3–6 years. Diabetes Res Clin Pract. 2012;96:204–10. doi: 10.1016/j.diabres.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 111.Hilbrands R, Huurman VA, Gillard P, Velthuis JH, De Waele M, Mathieu C, et al. Differences in baseline lymphocyte counts and autoreactivity are associated with differences in outcome of islet cell transplantation in type 1 diabetic patients. Diabetes. 2009;58:2267–76. doi: 10.2337/db09-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jaeger C, Brendel MD, Eckhard M, Bretzel RG. Islet autoantibodies as potential markers for disease recurrence in clinical islet transplantation. Exp Clin Endocrinol Diabetes. 2000;108:328–33. doi: 10.1055/s-2000-8125. [DOI] [PubMed] [Google Scholar]
- 113.Steffes MW, Sibley S, Jackson M, Thomas W. β-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26:832–6. doi: 10.2337/diacare.26.3.832. [DOI] [PubMed] [Google Scholar]
- 114.Long SA, Rieck M, Sanda S, Bollyky JB, Samuels PL, Goland R, et al. Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs β-cell function. Diabetes. 2012;61:2340–8. doi: 10.2337/db12-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–37. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rabinovitch A, Suarez-Pinzon WL, Shapiro AM, Rajotte RV, Power R. Combination therapy with sirolimus and interleukin-2 prevents spontaneous and recurrent autoimmune diabetes in NOD mice. Diabetes. 2002;51:638–45. doi: 10.2337/diabetes.51.3.638. [DOI] [PubMed] [Google Scholar]
- 117.Tanemura M, Saga A, Kawamoto K, Machida T, Deguchi T, Nishida T, et al. Rapamycin induces autophagy in islets: relevance in islet transplantation. Transplant Proc. 2009;41:334–8. doi: 10.1016/j.transproceed.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 118.Barlow AD, Nicholson ML, Herbert TP. Evidence for rapamycin toxicity in pancreatic β-cells and a review of the underlying molecular mechanisms. Diabetes. 2013;62:2674–82. doi: 10.2337/db13-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Baeyens A, Perol L, Fourcade G, Cagnard N, Carpentier W, Woytschak J, et al. Limitations of IL-2 and rapamycin in immunotherapy of type 1 diabetes. Diabetes. 2013;62:3120–31. doi: 10.2337/db13-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120••.Yu A, Snowhite I, Vendrame F, Rosenzwajg M, Klatzmann D, Pugliese A, et al. Selective IL-2 responsiveness of regulatory T cells through multiple intrinsic mechanisms supports the use of low-dose IL-2 therapy in type 1 diabetes. Diabetes. 2015;64:2172–83. doi: 10.2337/db14-1322. This study quantifies a therapeutic window in which Tregs selectively respond to IL2 and provides a mechanistic basis for this selective response. [DOI] [PubMed] [Google Scholar]
- 121.Ross JA, Cheng H, Nagy ZS, Frost JA, Kirken RA. Protein phosphatase 2A regulates interleukin-2 receptor complex formation and JAK3/STAT5 activation. J Biol Chem. 2010;285:3582–91. doi: 10.1074/jbc.M109.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kennedy-Nasser AA, Ku S, Castillo-Caro P, Hazrat Y, Wu MF, Liu H, et al. Ultra low-dose IL-2 for GVHD prophylaxis after allogeneic hematopoietic stem cell transplantation mediates expansion of regulatory T cells without diminishing antiviral and antileukemic activity. Clin Cancer Res. 2014;20:2215–25. doi: 10.1158/1078-0432.CCR-13-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Churlaud G, Jimenez V, Ruberte J, Amadoudji Zin M, Fourcade G, Gottrand G, et al. Sustained stimulation and expansion of Tregs by IL-2 control autoimmunity without impairing immune responses to infection, vaccination and cancer. Clin Immunol. 2014;151:114–26. doi: 10.1016/j.clim.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 124.Skyler JS. Prevention and reversal of type 1 diabetes--past challenges and future opportunities. Diabetes Care. 2015;38:997–1007. doi: 10.2337/dc15-0349. [DOI] [PubMed] [Google Scholar]
- 125.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–65. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pugliese A. Insulin: a critical autoantigen and potential therapeutic agent in Type 1 diabetes. Expert Rev Clin Immunol. 2006;2:419–31. doi: 10.1586/1744666X.2.3.419. [DOI] [PubMed] [Google Scholar]