Summary
Background
Mitral regurgitation is the most common valve disease worldwide but whether the community-wide prevalence, poor patient outcomes, and low rates of surgical treatment justify costly development of new therapeutic interventions remains uncertain. Therefore, we did an observational cohort study to assess the clinical characteristics, outcomes, and degree of undertreatment of mitral regurgitation in a community setting.
Methods
We used data from Mayo Clinic electronic health records and the Rochester Epidemiology Project to identify all cases of moderate or severe isolated single-valvular mitral regurgitation (with no other severe left-sided valvular disease or previous mitral surgery) diagnosed during a 10-year period in the community setting in Olmsted County (MN, USA). We assessed clinical characteristics, mortality, heart failure incidence, and results of cardiac surgery post-diagnosis.
Findings
Between Jan 1, 2000, and Dec 31, 2010, 1294 community residents (median age at diagnosis 77 years [IQR 66–84]) were diagnosed with moderate or severe mitral regurgitation by Doppler echocardiography (prevalence 0.46% [95% CI 0.42–0.49] overall; 0.59% [0.54–0.64] in adults). Left-ventricular ejection fraction below 50% was frequent (recorded in 538 [42%] patients), and these patients had a slightly lower regurgitant volume than those with an ejection fraction of 50% or higher (mean 39 mL [SD 16] vs 45 mL [21], p<0.0001). Post-diagnosis mortality was mainly cardiovascular in nature (in 420 [51%] of 824 patients for whom the cause of death was available) and higher than expected for residents of the county for age or sex (risk ratio [RR] 2.23 [95% CI 2.06–2.41], p<0.0001). This excess mortality affected all subsets of patients, whether they had a left-ventricular ejection fraction lower than 50% (RR 3.17 [95% CI 2.84–3.53], p<0.0001) or of 50% or higher (1.71 [1.53 –1.91], p<0.0001) and with primary mitral regurgitation (RR 1.73 [95% CI 1.53–1.96], p<0.0001) or secondary mitral regurgitation (2.72 [2.48–3.01], p<0.0001). Even patients with a low comorbidity burden combined with favourable characteristics such as left-ventricular ejection fraction of 50% or higher (RR 1.28 [95% CI 1.10–1.50], p<0.0017) or primary mitral regurgitation (1.29 [1.09–1.52], p=0.0030) incurred excess mortality. Heart failure was frequent (mean 64% [SE 1] at 5 years postdiagnosis), even in patients with left-ventricular ejection fraction of 50% or higher (49% [2] at 5 years postdiagnosis) or in those with primary mitral regurgitation (48% [2]). Mitral surgery was ultimately done in only 198 (15%) of 1294 patients, of which the predominant type of surgery was valve repair (in 149 [75%] patients). Mitral surgery was done in 28 (5%) of 538 patients with left-ventricular ejection fraction below 50% and in 170 (22%) of 756 patients with ejection fraction of 50% or higher, and in 34 (5%) of 723 with secondary mitral regurgitation versus 164 (29%) of 571 with primary regurgitation. All other types of cardiac surgery combined were performed in only 3% more patients (237 [18%] patients) than the number who underwent mitral surgery.
Interpretation
In the community, isolated mitral regurgitation is common and is associated with excess mortality and frequent heart failure postdiagnosis in all patient subsets, even in those with normal left-ventricular ejection fraction and low comorbidity. Despite these poor outcomes, only a minority of affected patients undergo mitral (or any type of cardiac) surgery even in a community with all means of diagnosis and treatment readily available and accessible. This suggests that in a wider population there might be a substantial unmet need for treatment for this disorder.
Funding
Mayo Clinic Foundation.
Introduction
Recent epidemiological studies have shown that the global valvular heart disease burden is high and increasing, with serious implications for affected patients worldwide.1–3 However, the outcomes and treatment standards of each individual patient with heart valve disease cannot be inferred from these studies, which included multi-valvular diseases, previous cardiac surgeries, and associated cardiac disorders, all of which have major confounding effects on outcome and treatment. The standard of care for valve diseases is surgical repair or replacement,4 but many patients do not undergo surgery and remain untreated. Such undertreatment in the community was suspected in the case of aortic stenosis,5 but was ultimately confirmed by increasing numbers of percutaneous aortic valve replacements while the rate of surgical valve replacements remained steady worldwide.6 However, no such data exist for mitral regurgitation, despite its importance as the most common heart valve disease,2 its growing burden,2,3 and favourable reparability.7 Mitral regurgitation is the focus of intense minimally invasive device development,8 but whether serious outcomes or an unmet need for treatment really exist remains unclear.
The 2007 Euro Heart Survey9 suggested that patients referred to cardiology centres with mitral regurgitation in Europe were frequently denied surgical treatment. However, such undertreatment might reflect these patients being referred to cardiology centres too late, with an overwhelming number presenting with heart failure.9 Conversely, it might be a caveat of a brief cross-sectional analysis that did not take into account later treatments given to these patients. In a real-world community setting (in Olmsted County, Rochester, MN, USA), subsets of mitral diseases have been reported,10 but no overall characterisation of isolated, single-valvular mitral regurgitation and its long-term treatment standards has been done. At the national level in the USA, the sharp contrast between the few isolated mitral surgeries performed11 and the large burden of mitral regurgitation inferred from epidemiological studies2 suggest undertreatment in this setting, but comprehensive information is not available.
Olmsted County provides a unique opportunity to study mitral regurgitation because it has a single echocardiographic laboratory centralising diagnoses, large samples of patients with single-valve heart disorders, and health-care providers linked through the Rochester Epidemiology Project.12 Furthermore, facilities and expertise for the diagnosis and treatment of valve diseases are readily available, so that performance versus non-performance of repair or replacement cannot be ascribed to access limitations. Therefore, we aimed to assess the community-wide prevalence, clinical characteristics, survival, and heart-failure post-diagnosis in Olmsted County, to evaluate standards of treatment for native, moderate-to-severe mitral regurgitation, and to ascertain whether or not an unmet need for treatment really exists.
Methods
Study design, eligibility criteria, and source data
In this observational cohort study, we used the Mayo Clinic electronic health records to identify eligible patients who met the following criteria: permanent residents (≥3 months) of Olmsted County (and not solely resident there for medical treatment at the Mayo Clinic) who had undergone clinically indicated Doppler echocardiography between Jan 1, 2000, and Dec 31, 2010, leading to a diagnosis of moderate-to-severe mitral regurgitation that was isolated in nature. We used data from the Rochester Epidemiology Project, which provides record linkage for inpatient and outpatient health-care providers and residency status, to identify permanent residents of Olmsted County.12 We identified patients with moderate or severe mitral regurgitation using Doppler echocardiographic database records. Isolated mitral regurgitation was defined according to the Euro Heart Survey definition:9 no previous valvular, congenital, myocardial or pericardial surgery; no mitral stenosis (mean gradient <5 mm Hg); and no aortic valve disease that was more than moderate in severity. All eligible patients had to have consented for their data to be used for research.
The Institutional Review Board at the Mayo Clinic approved this study and, since it is a low-risk study, waived the need for written consent. According to Minnesota state law, patients who denied authorisation for their data to be used for research were excluded from the study.
Procedures
Throughout the study period, Mayo Echocardiographic Laboratory was the only provider of echocardiography in Olmsted County, and mitral regurgitation grading criteria remained consistent, with the same criteria used for all patients examined, ensuring that all diagnoses were standardised and centralised. Diagnosis date was the date the patient first experienced moderate or severe mitral regurgitation. Clinical characteristics were retrieved from electronic health records using validated natural language processing algorithms, and echocardiographic characteristics were downloaded without alteration (ie, as initially stored) from the Doppler echocardiographic database.13 Symptoms of dyspnoea, chest pain, palpitations, syncope, and oedema were identified. Comorbidities were summarised using the Charlson index14 and details about surgical procedures were retrieved using ICD9, billing codes, and procedural databases. Surgical procedures (mitral, aortic, or tricuspid valve repair or replacement; coronary bypass grafting; MAZE procedure [surgical ablation of atrial fibrillation]); left-ventricular-assist device; and heart transplantation were all recorded. Detailed Doppler echocardiographic characteristics were obtained from a quantitative and qualitative computerised repository. Atrial fibrillation was diagnosed by electrocardiograph. Plasma creatinine and haemoglobin concentrations closest to the index date (ie, the date of the diagnosis by Doppler echocardiography) were retrieved. Deaths were ascertained from a nationwide death location database (Accurint) which uses patented big-data technology platform linking multiple civil sources and from Olmsted County by traditional Rochester Epidemiology Project methods. Occurrence of heart failure was based on clinical diagnosis by patients’ treating physicians, and excluded diagnoses during the first month postdiagnosis to avoid overlap with events leading to the diagnosis of mitral regurgitation.
Doppler echocardiography is essential to obtain a specific diagnosis of mitral regurgitation.15,16 Examinations were done by registered cardiac sonographers according to recommendations15 and under the supervision of the attending cardiologist present during examinations. Diagnosis of moderate-to-severe mitral regurgitation was based on comprehensive grading by specific, supportive, and quantitative signs and measurements recommended by the American Society of Echocardiography.16 Measured left atrium size, mitral regurgitant volume and orifice, left ventricular ejection fraction, diameters and muscle mass, stroke volume, cardiac index and pulmonary pressure were obtained by direct electronic transfer without alteration. Measurements were also normalised for body surface area.
Statistical analysis
We used the Shapiro–Wilk test to assess normality of data. Continuous variables are presented as mean (standard deviation [SD]) or median (IQR). Categorical variables are presented as counts and percentages. For group comparisons, we used Student’s t test for continuous variables and the χ2 test for categorical variables. For the main stratification, standardised in all patients, we used left-ventricular ejection fraction (<50% vs ≥50%). For alternative stratification (primary vs secondary mitral regurgitation), we used valvular lesions (full echocardiography description in 56% of patients) completed by left-ventricular ejection fraction below 50% as a marker of secondary mitral regurgitation to classify mitral regurgitation as primary or secondary according to clinical guidelines.4,17 We used the USA 2010 white population census to calculate prevalence adjusted by age and sex distribution. We used the Kaplan-Meier method to estimate heart failure and survival post-diagnosis, which was compared to expected rates in Olmsted County using the log-rank test and expressed as a risk ratio. We used Cox-proportional hazard models to assess event determinants with calculated hazard ratios. A two-tailed p value less than 0.05 was regarded as statistically significant. JMP 10.0, SAS 9.4, and R-3.1.1 were used for all statistical analyses.
Role of the funding source
Mayo Clinic Foundation provided funding for data collection and analysis but did not have a role in data interpretation or reporting. VD, MD, PT, and ME-S had full access to all the data. ME-S was ultimately responsible for all data interpretation and manuscript formulation, and had final responsibility for the decision to submit for publication.
Results
Between Jan 1, 2000, and Dec 31, 2010, 29 390 Olmsted County residents underwent Doppler echocardiography and 1294 residents fulfilled eligibility criteria with isolated (single-valvular) moderate-to-severe mitral regurgitation. During the study period, more than 30 staff cardiologists were assigned to the Echocardiographic Laboratory, and nine staff surgeons performed around 3000 cardiac surgeries at that centre per year.
The prevalence of isolated moderate-to-severe mitral regurgitation in the community was 0.46% (95% CI 0.42–0.49) overall and 0.59% (0.54–0.64) in adults. Prevalence was similar in men (0.46% [95% CI 0.41–0.51] and women (0.45% [0.40–0.50]) and increased with age (figure 1).
Figure 1.
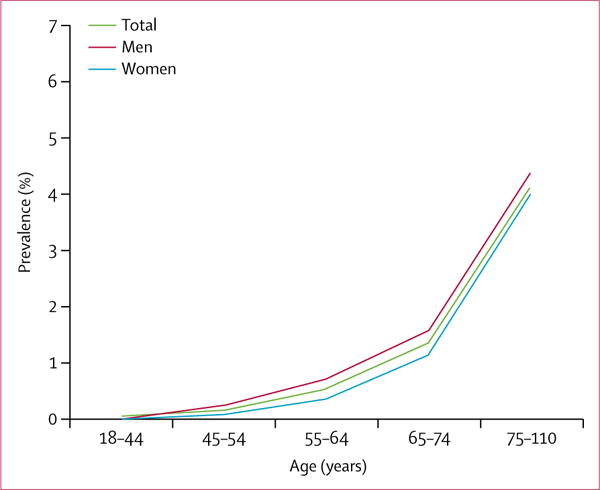
Prevalence of isolated mitral regurgitation in the community by age
At diagnosis, the median age of the patients was 77 years (IQR 66–84), 682 (53%) of 1294 were women, and the patients had frequent but expected associated comorbidities (eg, hypertension in 828 [64%] patients and diabetes in 247 [19%]). Patients were often symptomatic, mostly for dyspnoea (785 [63%] of 1294), with 359 patients in New York Heart Association class II, 355 in class III, and 71 in class IV. Cardiovascular treatment, involving any angiotensin inhibitors, beta-blockers, diuretics, calcium channel blockers, nitrates, and digoxin, was administered in 1220 (94%) patients.
Mean regurgitant volume (43 mL [SD 19]), and orifice size (0.25 cm2 [0.14]), which were measurable in 822 (64%) patients, confirmed moderate to severe grading with left ventricular enlargement (mean end-diastolic size 53 mm [SD 9] and end-systolic size 48 mm [8]). Mean left-ventricular ejection fraction was borderline for the 50% cutoff (48% [SD 17]) but widely distributed (538 [42%] of 1294 patients had an ejection fraction <50%). A comparison of baseline echocardiographic characteristics between patients with versus without quantitative assessment of mitral regurgitation showed no differences (appendix), indicating no bias related to mitral regurgitation quantification.
Stratification of patients by left-ventricular ejection fraction (<50% vs ≥50%) is presented in table 1. Patients with left-ventricular ejection fraction below 50% were older, more likely to be male, with more frequent symptoms and comorbidities, and had lower regurgitant volume, despite larger left atrial and ventricular remodelling (table 1). Patients with left-ventricular ejection fraction of 50% or higher were more likely to have primary mitral regurgitation (table 1).
Table 1.
Clinical and echocardiographic characteristics of isolated mitral regurgitation in the community
| All patients (n=1294) | Ejection fraction <50% (n=538) | Ejection fraction ≥50% (n=756) | p value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
|
| ||||
| Age at diagnosis, years | 77 (66–84) | 77–5 (67–84) | 77 (65–84) | 0.0449 |
| Men | 612 (47%) | 311 (58%) | 301 (40%) | <0.0001 |
| Primary mitral regurgitation | 571 (44%) | 20 (4%) | 551 (73%) | <0.0001 |
| History of heart failure | 447 (35%) | 279 (52%) | 168 (22%) | <0.0001 |
| History of myocardial infarction | 150 (12%) | 101 (19%) | 49 (6%) | <0.0001 |
| Dyspnoea | 785 (63%) | 390 (75%) | 395 (55%) | <0.0001 |
| Chest pain | 367 (30%) | 159 (30%) | 208 (29%) | 0.5536 |
| Leg oedema | 542 (44%) | 260 (50%) | 282 (39%) | 0.0002 |
| Palpitations | 192 (15%) | 65 (12%) | 127 (18%) | 0.0124 |
| Syncope | 101 (8%) | 40 (8%) | 61 (8%) | 0.6046 |
| Atrial fibrillation or flutter | 335 (26%) | 151 (29%) | 184 (24%) | 0.0087 |
|
| ||||
| Associated disorders and variables | ||||
|
| ||||
| Hypertension | 828 (64%) | 357 (66%) | 471 (62%) | 0.1342 |
| Hyperlipidaemia | 569 (44%) | 271 (50%) | 298 (39%) | <0.0001 |
| Cerebral vascular disease | 182 (14%) | 85 (16%) | 100 (13%) | 0.1927 |
| Peripheral vascular disease | 107 (8%) | 61 (11%) | 46 (6%) | 0.0010 |
| COPD | 165 (13%) | 93 (17%) | 72 (10%) | <0.0001 |
| Diabetes | 247 (19%) | 135 (25%) | 112 (15%) | <0.0001 |
| Charlson index | 2 (1–4) | 3 (2–5) | 2 (0–3) | <0.0001 |
| Creatinine concentration (mg/dL) | 1.3 (0.7) | 1.4 (0.8) | 1.2 (0.6) | <0.0001* |
| Haemoglobin concentration (g/dL) | 12.5 (1.8) | 12.5 (1.8) | 12.5 (1.9) | 0.7884 |
|
| ||||
| Echocardiographic characteristics | ||||
|
| ||||
| Left atrium diameter (mm) | 48 (8) | 50 (8) | 47 (9) | 0.0006 |
| Left atrium volume (mL) | 93 (36) | 100 (35) | 88 (36) | <0.0001* |
| Regurgitant volume (mL/beat) | 43 (19) | 39 (16) | 45 (21) | <0.0001* |
| Effective regurgitant orifice volume (cm2) | 0.25 (0.14) | 0.25 (0.12) | 0.26 (0.15) | 0.3367 |
| Left-ventricular ejection fraction % | 48 (17) | 30 (10) | 61 (7) | <0.0001* |
| Left ventricle end diastolic diameter (mm) | 53 (9) | 59 (9) | 49 (7) | <0.0001* |
| Left ventricle end systolic diameter (mm) | 48 (8) | 50 (8) | 47 (9) | 0.0005 |
| Left ventricle mass index (g/m2) | 120 (38) | 135 (39) | 108 (32) | <0.0001* |
| Pulmonary artery pressure (mm Hg) | 49 (16) | 52 (15) | 46 (16) | <0.0001* |
Data are median (IQR), n (%), or mean (SD). COPD=chronic obstructive pulmonary disease.
Indicates for continuous variables >10% differences between groups; extended table with 95% CIs is in the appendix.
Stratification by sex (612 [47%] men vs 682 [53%] women) showed that men had a larger body surface area (mean 1.95 m2 [SD 0.33] vs 1.68 m2 [0.26], p<0.0001), were younger (median age 75 years [IQR 63–82] vs 80 years [69–86], p<0.0001), but had more comorbidities (median Charlson index score 3 [IQR 1–4] in men vs 2 [1–4] in women, p=0.0075). Men had a larger regurgitant volume (mean 46 mL [SD 22], vs 40 mL [15] in women, p<0.0001) and orifice size (mean 0.29 cm2 [SD 0.16] vs 0.22 cm2 [0.11], p<0.0001), more remodelling (mean left atrial volume 105 mL [SD 38] vs 83 mL [30] and mean left ventricular end-diastolic diameter 57 mm [9] vs 50 mm [8]; both p<0.0001). However, the consequences of mitral regurgitation were similar in men and women, with no difference between the sexes in pulmonary pressure (mean 48 mm Hg [SD 17] in men vs 50 mm Hg [15] in women, p=0.12), or the prevalence of dyspnoea (recorded in 362 [62%] of 612 men vs 423 [64%] of 682 women, p=0.64), or atrial fibrillation (167 [27%] vs 168 [25%], p=0.36). Most differences between the sexes were related to body size, with similar values normalised to body surface area for mean regurgitant volume (24 mL/m2 [SD 11] in men vs 24 mL/m2 [12] in women, p=0.62) and left-ventricular end-diastolic diameter (30 mm/m2 [9] vs 31 mm/m2 [9], p=0.71), underscoring the similarity of mitral regurgitation characteristics between the sexes when body size is accounted for.
When stratified by severe mitral regurgitation (394 [30%] of 1294 patients) versus moderate mitral regurgitation (900 [70%]), the 394 patients with severe disease were noted to have a larger left atrium volume (mean 103 mL [SD 39] vs 89 mL [34], p<0.0001), higher regurgitant volume (56 mL [23] vs 35 mL [10], p<0.0001), larger left-ventricular end-diastolic diameter (56 mm [10] vs 52 mm [9], p<0.0001), and higher pulmonary artery systolic pressure (52 mm Hg [17] vs 47 mm Hg [15], p<0.0001) than the 900 patients with moderate mitral regurgitation. No differences in age (median age 77 years [IQR 67–84] in patients with moderate regurgitation vs 78 years [63–85] in those with severe regurgitation, p=0.13), comorbidity (median Charlson index 2 [IQR 1–4] for both, p=0.15), atrial fibrillation (86 [22%] of 394 patients with severe disease vs 249 [28%] of 900 with moderate disease, p=0.12), dyspnoea (245 [65%] vs 450 [62%], p=0.28) and left-ventricular ejection fraction (mean 48% [SD 19] vs 49% [17], p=0.53) were noted between patients with moderate versus severe mitral regurgitation.
When stratified by primary regurgitation (571 [44%] of 1294 patients overall) versus secondary regurgitation (723 [56%]), primary regurgitation was associated with smaller left ventricle diameter (mean 50 mm [SD 7] vs 56 mm [10], p<0.0001) and left atrium (mean 86 mL [SD 39] vs 98 mL [33], p<0.0001) but a larger regurgitant volume (mean 49 mL [SD 24] vs 38 mL [14], p<0.0001)
Mitral valve surgery was ultimately (throughout the median duration of follow-up of 4.8 years [IQR 1.7–8.2]) performed in 198 (15%) of the 1294 residents of Olmsted County diagnosed with mitral regurgitation. In these 198 patients who underwent surgery, mitral repair was preferred (149 [75%]) over mitral replacement (49 [25%]). Other cardiac surgeries were done rarely (table 2). Mitral surgery was done more frequently in younger patients (adjusted hazard ratio [HR] 0.99 [95% CI 0.98–0.996] per year of age, p=0.0024), in those with a lower comorbidity index (0.86 [0.78–0.93] per point, p=0.0007), in men (vs women; 2.41 [1.78–3.33], p<0.0001), left-ventricular ejection fraction of 50% or higher (4.81 [3.22–7.41], p<0.0001), and severe (vs moderate) regurgitation (5.37 [3.97–7.41], p<0.0001), but not with symptoms of dyspnoea (1.32 [0.96–1.82], p=0.09) or chest pain (1.04 [0.73–1.46], p=0.83). Less mitral surgery in women was confirmed adjusting for regurgitant volume (adjusted HR 1.82 [95% CI 1.22–2.73], p=0.0035). Table 2 shows the distribution of cardiac surgery performed at any timepoint according to left-ventricular ejection fraction, with mitral surgery performed in 28 (5%) of 538 patients with ejection fraction below 50% and in 170 (22%) of 756 with ejection fraction of 50% or higher. Stratified by primary versus secondary regurgitation, mitral surgery was performed in 164 (29%) of 571 patients with primary regurgitation and in 34 (5%) of 723 with secondary regurgitation. Mitral surgery was also performed almost twice as often in men than in women (123 [20%] of 612 men vs 75 [11%] of 682 women) and more frequently in severe versus moderate mitral regurgitation (in 123 [31%] of 394 patients with severe regurgitation vs 75 [8%] of 900 with moderate regurgitation). In the 394 patients with severe mitral regurgitation, 107 (48%) of 225 with left-ventricular ejection fraction of 50% or higher versus 16 (9%) of 169 with ejection fraction below 50% versus and 106 (50%) of 210 with primary and 17 (9%) of 184 with secondary mitral regurgitation received mitral surgery at any point (both p<0.0001). Only 79 (39%) of 204 men and 44 (23%) of 190 women with severe mitral regurgitation had mitral surgery at any time (p=0.0011). Guideline-based class I surgical indications were present in 562 patients with a left-ventricular ejection fraction of 50% or higher but only 118 (21%) of these patients actually underwent mitral surgery. The same surgical indications were present in 425 patients with primary regurgitation, of whom only 113 (27%) underwent mitral surgery. Including all interventions (listed in table 2), cardiac surgery was performed in a minority of patients with moderate or severe mitral regurgitation and left-ventricular ejection fraction of 50% or higher (188 [24%] of 756) or less than 50% (49 [8%] of 538, p<0.0001).
Table 2.
Type of intervention in isolated mitral regurgitation
| All patients (n=1294) |
Ejection fraction <50% (n=538) |
Ejection fraction ≥50% (n=756) |
|
|---|---|---|---|
| Mitral surgery | |||
|
| |||
| Total | 198 (15%) | 28 (5%) | 170 (22%) |
| Repair | 149 (12%) | 18 (3%) | 131 (17%) |
| Replacement | 49 (4%) | 10 (2%) | 39 (5%) |
| Tissue | 23 (2%) | 3 (<1%) | 20 (3%) |
| Mechanical | 26 (2%) | 7 (1%) | 19 (3%) |
|
| |||
| Other cardiac procedures* | |||
|
| |||
| Any cardiac surgery | 237 (18%) | 49 (9%) | 188 (25%) |
| Coronary artery bypass grafting | 88 (7%) | 27 (5%) | 61 (8%) |
| Aortic valve surgery | 27 (2%) | 6 (1%) | 21 (3%) |
| Tricuspid valve surgery | 32 (2%) | 9 (2%) | 23 (3%) |
| Left ventricular assist device | 6 (<1%) | 6 (1%) | 0 |
| MAZE | 19 (1%) | 2 (<1%) | 17 (2%) |
| Heart transplant | 6 (<1%) | 6 (1%) | 0 |
Data are n (%). MAZE=surgical ablation of atrial fibrillation.
The numbers for the different types of cardiac surgery do not add up to the totals in the ‘any cardiac surgery’ row because some patients might have had various different interventions.
Surgical treatment with valve repair, performed in 146 (75%) of 198 surgical cases, is standardised with almost uniform annuloplasty (almost universally with a 63 mm band),18 with resection or plication depending on excess tissue and with subvalvular support depending on redundancy. Valve replacement in 49 (25%) of 198 patients always preserved posterior leaflet chordae with mechanical prosthesis in 26 (53%) patients and biological valve in 23 (47%).
Death after diagnosis of mitral regurgitation occurred in 844 patients during follow-up; the cause of death was available in 824 patients, in whom death was due to cardiovascular causes in 420 (51%). The proportion of patients alive at 5 years was 53% (vs expected 78% in the rest of the community) and at 10 years was 30% (vs expected 63%, p<0.0001; figure 2). Adjusted for age, sex, and comorbidity, the following factors were associated with an increased risk of mortality post diagnosis: left-ventricular ejection fraction 50% or higher (adjusted HR 0.72 [0.63–0.84], p<0.0001); atrial fibrillation (1.17 [0.99–1.37], p=0.06); severe regurgitation (1.21 [1.04–1.41], p=0.0152); and non-performance of mitral surgery (1.97 [1.51–2.60], p<0.0001). Excess mortality versus that expected for people of the same age and sex in the same community was high overall (risk ratio [RR] 2.23 [95% CI 2.06–2.41], p<0.0001), especially in patients with left-ventricular ejection fraction below 50% (RR 3.17 [95% CI 2.84–3.53], p<0.0001) but was still notable in those with a left-ventricular ejection fraction of 50% or higher (1.71 [1.53–1.91], p<0.0001; figure 3). Excess mortality persisted in subsets of patients with an ejection fraction threshold of 60% or higher (RR 1.51 [95% CI 1.31–1.82], p<0.0001) or with either severe regurgitation (2.33 [2.02–2.68]; p<0.001) or moderate regurgitation (2.22 [2.04–2.41]); p<0.001). Excess mortality persisted in both primary regurgitation (RR 1.73 [95% CI 1.53–1.96], p<0.0001) and secondary regurgitation (2.72 [2.48–3.01], p<0.0001) and even in subsets of patients with low comorbidity (Charlson score <3) plus a left-ventricular ejection fraction of 50% or higher (RR 1.28 [95% CI 1.10–1.50], p=0.0017) or plus primary regurgitation (1.29 [1.09–1.52], p=0.0030). Estimated 5-year survival was 63% (SE 2) in patients with primary regurgitation and 46% (2) in those with secondary regurgitation, and 10-year survival was 41% (SE 2) for primary regurgitation and 23% (2) for secondary regurgitation.
Figure 2. Survival after diagnosis of isolated moderate or severe mitral regurgitation.
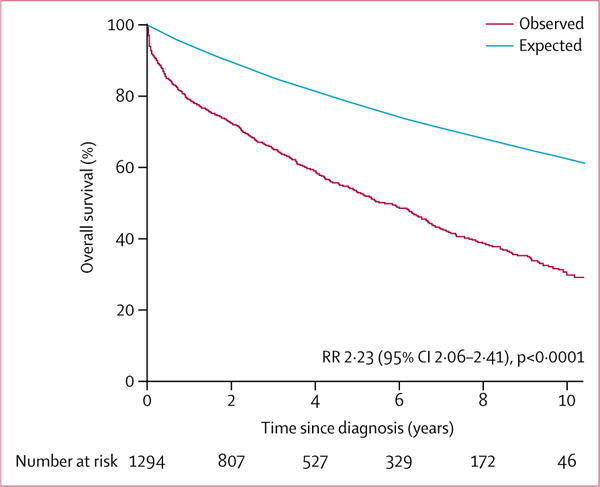
The red Kaplan-Meier curve represents observed survival in Olmsted County residents diagnosed with isolated moderate or severe mitral regurgitation and the blue line represents the expected survival of the general Olmsted County population of same age and sex. RR=risk ratio indicating the excess mortality of patients with mitral regurgitation over the general population of the county.
Figure 3. Survival after diagnosis of isolated moderate or severe mitral regurgitation, stratified by left-ventricular ejection fraction at diagnosis.
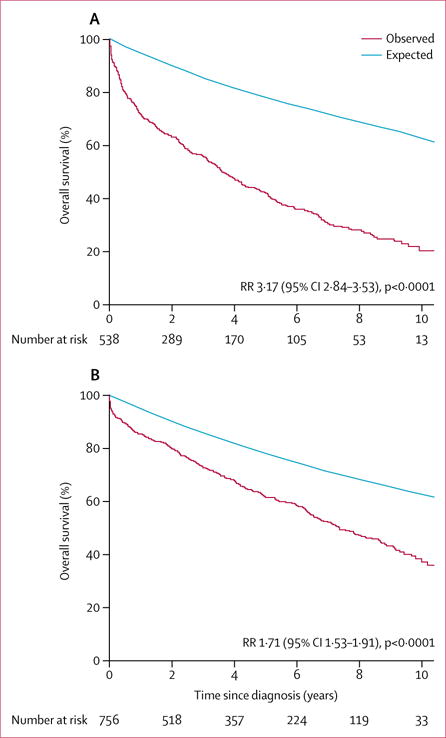
(A) Survival in residents with left-ventricular ejection fraction <50%. (B) Survival in residents with left-ventricular ejection fraction ≥50%. In each panel, the red Kaplan-Meier curve represents observed survival in Olmsted County residents diagnosed with isolated moderate or severe mitral regurgitation and the blue line represents the expected survival of the general Olmsted County population of same age and sex. RR=risk ratio indicating the excess mortality of patients with mitral regurgitation over the general population of the county.
Heart failure occurring more than 1 month after mitral regurgitation diagnosis was reported in 836 patients during follow-up. Overall, the proportion of patients with heart failure at 5 years was 64% (SE 1) and at 10 years was 76% (1), and was higher in patients with a left-ventricular ejection fraction below 50% (86% [SE 2]) but remained notable in those with an ejection fraction of 50% or higher (49% [2], p<0.0001). Similarly, the proportion of patients with heart failure at 5 years was higher in those with secondary mitral regurgitation (78% [SE 2]) but was also substantial in those with primary regurgitation (48% [2], p<0.0001; appendix p 3). In a multivariable analysis adjusting for age and sex, the risk of heart failure was independently linked to left-ventricular ejection fraction below 50% (adjusted HR 2.39 [95% CI 2.06–2.79], p<0.0001), severe regurgitation (1.33 [1.13–1.56], p=0.0005), dyspnoea at diagnosis (1.79 [1.54–2.09], p<0.0001), atrial fibrillation (1.25 [1.08–1.44], p=0.0020), and lack of mitral surgery (1.28 [1.01–1.63], p=0.0404). The combined endpoint of death or heart failure after diagnosis was very frequent, ultimately affecting 1084 (84%) of the 1294 patients with mitral regurgitation, with a mean 5-year rate of 73% (SE 1) and 10-year rate of 85% (1).
Discussion
The results of our study show that mitral regurgitation, even isolated, is associated with sizeable excess mortality, notable subsequent heart failure, and is rarely treated by the only approach recommended—ie, mitral valve surgery. No subgroup of patients is spared, with these results recorded in all possible subsets and classifications of mitral regurgitation. Although rationales for non-referral are undefined, such undertreatment is not a simple delay but rather affects patients throughout their lives and is especially remarkable in this Olmsted County community, where state-of-the-art valvular diagnosis, treatment, and expertise, good access to treatment (with high numbers of medically insured residents) are all immediately available.19,20 These results are also remarkable because, although in this cohort of patients in their mid-70s, frequent comorbidities are present as expected, there is a clear predominance of cardiovascular mortality, sizeable excess mortality compared with other residents of the county, frequent heart failure preceding death, and rare surgical treatment, even in subsets of patients with primary mitral regurgitation, normal ejection fraction, and low comorbidity. Therefore, comorbid disorders are unlikely targets in assigning responsibility for the unmet need for treatment of mitral regurgitation, warranting comprehensive efforts to improve outcomes of this disorder.
Knowledge about the epidemiology of mitral regurgitation is scarce.1,2,21 Although it is the most common heart valve disease in the global population and community,2 inclusion of the disorder in previous studies of multi-valvular diseases was problematic because it increased the natural and surgical risks incurred, yielding uncertainties about which valve disease predominated or caused complications. Therefore, it is essential to analyse isolated (not multi-valvular) moderate or severe mitral regurgitation, which is the main candidate for surgical treatment. Heart valve diseases are diagnosed in 1.8% of adults worldwide,2 and isolated (single-valvular) mitral regurgitation affects around 0.6% of adults; at present, about 1.3 million US adults have the disorder, with the burden projected to increase to an estimated 2.3 million affected US adults by 2030. Preponderance and high burden of mitral regurgitation has been confirmed in the OxValve study,3 which did systematic echocardiographic scanning of elderly patients. By contrast with estimates based on qualitative assessment,2 with frequent quantitative assessment we found a similar prevalence of the disorder in both sexes, emphasising the large burden of mitral regurgitation in women, which is generally not reflected in surgical series.22 Although isolated mitral regurgitation means a single-valvular condition, it does not exist in vacuum, and in this population with a median age at diagnosis of 77 years, comorbidities are frequent but similar to those that occur with other heart valve diseases.5 Clinically, chronic obstructive pulmonary disease or atrial fibrillation make interpretation of dyspnoea (which is present in around two-thirds of patients) challenging.23 However, our data show that even in those with low comorbidity, mitral regurgitation has substantial outcome implications.
The rationale for studying isolated (single-valvular) mitral regurgitation is directly related to outcomes and treatment standards. Mixed valve diseases incur mediocre survival, as has been shown in predominant aortic stenosis,24 and double-valve surgery, which increases operative risk, is a deterrent to surgical treatment, emphasising the importance of an outcome and treatment analysis solely focused on isolated mitral regurgitation.
Indeed, this community study shows that isolated mitral regurgitation is associated with excess mortality and frequent heart failure postdiagnosis overall and in all subsets of patients—those with high or low left-ventricular ejection fraction, with moderate or severe regurgitation, with or without comorbidity, and with secondary or primary regurgitation. Predominantly cardiovascular causes of death—often preceded by heart failure and excess mortality (compared with residents in the same community of the same age and sex)—provide robust evidence that mitral regurgitation itself is serious and the very high rates of these cardiac complications warrant serious consideration for treatment in all subsets of patients. While referral centre series mostly19,25 (but not all26) suggest such an effect on survival, these can raise concerns about mortality overestimation and bias because patients distantly referred to academic institutions might have particularly severe disease and subsequently fare poorly.27 Such is not the case in our geographically defined study, whereby all patients are local and enrolled from first diagnosis. Hence, this study provides unique and hitherto unavailable evidence that mitral regurgitation, in and by itself and in all possible subsets of patients, is associated with excess mortality and high rates of heart failure. This serious outcome contrasts with very infrequent use of the only recommended treatment,4 mitral surgery. Despite availability and known safety of cardiac surgery in the county, where neither access to treatment nor expertise is an issue, overall only 15% of patients diagnosed with isolated mitral regurgitation ever underwent mitral surgery. Previously published data about undertreatment were cross-sectional and might have misrepresented simply delayed treatment but also, because of their hospital-based nature, profoundly underestimated the degree of undertreatment in the community.9 Conversely, our real-world community demonstration of very low use of the only recommended treatment for mitral regurgitation,4 despite affected patients having symptoms and classic indications for surgery, justifies use of the terms undertreatment or unmet need for treatment.9,22,28 Although cases labelled as severe regurgitation, primary regurgitation, and those with a left-ventricular ejection fraction of 50% or higher tend to be operated on more frequently than those classified as moderate regurgitation, secondary regurgitation, or with an ejection fraction below 50%, no single subset of patients exists in whom a majority undergoes mitral surgery in their lifetime.
Excess mortality, frequent heart failure, and undertreatment are compelling reasons to develop strategies to improve outcomes for mitral regurgitation. First, enforcement of guideline-based indications is essential.4 Older age, which affects undertreatment,9,29 implies operative risk and comorbidity concerns,30 increasing fears of treatment failure. However, to leave patients untreated despite a condition with excess mortality and an almost universal risk of death or heart failure within a few years, as demonstrated by our study, is woefully inadequate. We believe that defining objective outcome markers to alleviate the challenging interpretation of the presence or absence of symptoms and developing risk scores for medical or surgical treatment could help increase compliance to guidelines. Second, the rate of mitral surgery in women was half that of men22 despite the favourable comorbidity burden in women and after all adjustments for covariables. Male predominance in mitral surgery is widespread,31,32 pertaining to the smaller body and heart size of women (meaning that surgical indications are more often missed in women than in men), and this issue should be formally addressed in clinical guidelines.33 Third, moderate mitral regurgitation, not included in indications for surgery in guidelines, but for which prognostic data are accruing,19,25 warrants well-designed clinical trials to assess treatment suitability. Fourth, a major issue is minimally invasive treatment availability. As an example, undertreatment of aortic stenosis, suspected in our community,5 was clearly demonstrated when effective percutaneous treatment became available.34 This issue of undertreatment is particularly relevant to secondary mitral regurgitation,9 whereby only 5% of patients with this type of the disorder ever underwent mitral surgery. This extremely low figure might relate to uncertainties regarding effectiveness of surgery,35,36 but it shows, particularly for future clinical trials, that the treatment standard for secondary or reduced ejection fraction regurgitation cannot be considered surgical.37 Although percutaneous treatment of mitral regurgitation8 causes financial burdens, new strategies must be tested to improve the substantial undertreatment of mitral regurgitation that persists even in the best of circumstances. Various devices aiming at mitral repair or replacement are being developed and tested.8 Although rational dispersion of technology will require careful planning,38 consideration for accelerated approval testing should be given.
In the present study, we analysed community management of mitral regurgitation without interfering with the care provided, to detect undertreatment. However, future clinical trials of community interventions should be considered. As recommended in guidelines, comprehensive grading incorporating all elements of judgment of mitral regurgitation severity was used in all cases.16
Undertreatment would be doubtful if massive out-migration of patients were noted. However, long follow-up and ascertainment of low out-migration in the Olmsted County population make this issue irrelevant.12 Olmsted County has all the required facilities and expertise of heart valve centres4,39 for mitral regurgitation diagnosis and treatment, with a high insurance rate, easy access to care (93% of population seen over 3 years),12 a valve clinic and specialists, invasive and interventional facilities, cardiac surgeons who have done a large number of valvular surgeries with low mortality rates from these surgeries, and state-of-the-art imaging laboratories, emphasising the depth of mitral regurgitation un dertreatment despite these optimal circumstances. The population of Olmsted County is predominantly white or of European ancestry, but generalisability of our findings to other settings is likely in view of very similar prevalence and survival impact of heart valve diseases in this county compared with across the rest of the USA,2 as well as general life expectancy being similar.40 It is unlikely that similarly centralised and available diagnosis and treatment facilities will easily allow study of another entire community that would better meet needs for mitral regurgitation treatment than is available in Olmsted County.9,28 Hence, we believe that our findings of high prevalence, excess mortality, frequent heart failure, and severe lifelong undertreatment of isolated mitral regurgitation in this community are in all likelihood generalisable to other settings and are quite concerning.
In conclusion, our study shows that isolated mitral regurgitation is prevalent in the community, equally in men and women, and increases with age. It is first diagnosed in patients when they are in their 70s, often with associated symptoms and decreased left-ventricular ejection fraction. Isolated mitral regurgitation is associated with poor outcome with excess mortality and notable rates of heart failure after diagnosis, overall and in all subsets of patients irrespective of mitral regurgitation type. This serious outcome contrasts with very low numbers of patients being treated by mitral surgery irrespective of mitral regurgitation type, even those with a low comorbidity burden. This undertreatment is not a delayed treatment but rather a lifelong absence of surgical treatment. Excess mortality and heart failure and substantial surgical undertreatment underscore the limits of current mitral regurgitation management standards and suggests that new strategies to improve treatment and outcomes of mitral regurgitation should be tested.
Research in context.
Evidence before this study
We searched the Cochrane Library and PubMed for publications about the prevalence and outcomes of isolated moderate to severe mitral regurgitation between Jan 1, 1990, and June 1, 2016, using the search terms “epidemiology”, “prevalence”, “outcomes”, “mitral insufficiency”, “regurgitation”, and “isolated”. Mitral regurgitation is generally considered the most common heart valve disorder worldwide, but only a few studies have reported on its epidemiology. In particular, outcome after diagnosis, which has been analysed previously only in selected populations, remains undefined in the community setting. Although mitral surgery is established as the only guideline-recommended treatment for moderate to severe mitral regurgitation, access to care and treatment might be insufficient in some settings. However, access to care and treatment for isolated mitral regurgitation has not been evaluated in an entire community with state-of-the-art facilities, good access to treatment, and high availability of specialised providers, or over the long-term. In summary, prevalence, survival, heart failure rates, and surgical outcomes of rigorously defined isolated mitral regurgitation (diagnosed by Doppler echocardiography) over the long-term, in an entire community with no or minimal impediment to access to care, is unknown; therefore, the unmet need for treatment for this heart valve disorder remains uncertain.
Added value of this study
We analysed a cohort of patients with isolated mitral regurgitation in Olmsted County, MN, USA, that included all community-wide consecutive cases diagnosed, to define the prevalence of the disorder and—most importantly—to assess its management and outcomes across the community and in the long term. Because this community has modern facilities, diagnostic methods, expertise, and cardiac surgery easily accessible, it provides an ideal setting to ascertain whether mitral regurgitation therapeutic needs are met. In this context, we can report a high community prevalence of isolated mitral regurgitation. We also found that from all diagnosed cases, isolated moderate or severe mitral regurgitation is associated with excess mortality compared to that expected in the same county, both overall and in all subsets of patients, even those who seem to have the most benign types of the disorder. Similarly, heart failure is very frequent in all subsets of patients with mitral regurgitation, even in those without any other predisposing factor or comorbidity. Most importantly, long-term outcome analysis showed that despite all available facilities and expertise, only 15% of patients ultimately underwent surgical correction of the mitral regurgitation. It is particularly notable that women were operated on less than half as often as men. Hence, for the first time, we show that in a community with very well-equipped medical facilities and good access to treatment, moderate-to-severe isolated mitral regurgitation is common, and is associated with a high incidence of heart failure, and severe excess mortality, and is substantially undertreated.
Implications of all the available evidence
All evidence, especially our lifelong population-based data, point towards a substantial unmet need for treatment of mitral regurgitation, which contrasts with the high excess mortality and high frequency of heart failure, with few patients receiving the only treatment available (surgery) and represents a call for action. Beyond the necessary education of care providers regarding medical knowledge and clinical guidelines for mitral regurgitation, referral of patients with the disorder to cardiology teams for decision-making, integrating all clinical information and therapeutic approaches available, is a critical step for these patients to obtain access to care. Simultaneously, new approaches to treatment of mitral regurgitation in all subsets of patients warrant development and testing in appropriate clinical trials.
Acknowledgments
ME-S reports grants from Edwards LLC, outside the current work.
Footnotes
See Online for appendix
Contributors
VD and MD contributed to data collection analysis and interpretation, to manuscript drafting and revisions, and to the final version. JRM-I contributed to data collection and analysis. M-AC, HM, JM, and VN contributed to data interpretation, review, revision, and final approval of the manuscript. PT contributed to data analysis, interpretation of results, and final approval of the manuscript. ME-S contributed to study conception and design; data collection, analysis, and interpretation; and to drafting, revision and final approval of the report.
Declaration of interests
All other authors declare no competing interests.
References
- 1.Singh JP, Evans JC, Levy D, et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study) Am J Cardiol. 1999;83:897–902. doi: 10.1016/s0002-9149(98)01064-9. [DOI] [PubMed] [Google Scholar]
- 2.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–11. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 3.d’Arcy JL, Coffey S, Loudon MA, et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE Population Cohort Study. Eur Heart J. 2016;37:3515–22. doi: 10.1093/eurheartj/ehw229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2438–88. doi: 10.1016/j.jacc.2014.02.537. [DOI] [PubMed] [Google Scholar]
- 5.Malouf J, Le Tourneau T, Pellikka P, et al. Aortic valve stenosis in community medical practice: Determinants of outcome and implications for aortic valve replacement. J Thorac Cardiovasc Surg. 2012;144:1421–27. doi: 10.1016/j.jtcvs.2011.09.075. [DOI] [PubMed] [Google Scholar]
- 6.Makkar RR, Fontana GP, Jilaihawi H, et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366:1696–704. doi: 10.1056/NEJMoa1202277. [DOI] [PubMed] [Google Scholar]
- 7.Suri RM, Vanoverschelde JL, Grigioni F, et al. Association between early surgical intervention vs watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. JAMA. 2013;310:609–16. doi: 10.1001/jama.2013.8643. [DOI] [PubMed] [Google Scholar]
- 8.Maisano F, Alfieri O, Banai S, et al. The future of transcatheter mitral valve interventions: competitive or complementary role of repair vs. replacement? Eur Heart J. 2015;36:1651–59. doi: 10.1093/eurheartj/ehv123. [DOI] [PubMed] [Google Scholar]
- 9.Mirabel M, Iung B, Baron G, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J. 2007;28:1358–65. doi: 10.1093/eurheartj/ehm001. [DOI] [PubMed] [Google Scholar]
- 10.Avierinos JF, Gersh BJ, Melton LJ, 3rd, et al. Natural history of asymptomatic mitral valve prolapse in the community. Circulation. 2002;106:1355–61. doi: 10.1161/01.cir.0000028933.34260.09. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee S, Rankin JS, Gammie JS, et al. Isolated mitral valve surgery risk in 77,836 patients from the society of thoracic surgeons database. Ann Thorac Surg. 2013;96:1587–95. doi: 10.1016/j.athoracsur.2013.06.051. [DOI] [PubMed] [Google Scholar]
- 12.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202–13. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H, Bielinski SJ, Sohn S, et al. An information extraction framework for cohort identification using electronic health records. AMIA Jt Summits Transl Sci Proc. 2013;2013:149–53. [PMC free article] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. JASE. 2002;15:167–84. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 16.Quinones MA, Douglas PS, Foster E, et al. ACC/AHA clinical competence statement on echocardiography: a report of the American College of Cardiology/American Heart Association/American College of Physicians-American Society of Internal Medicine Task Force on Clinical Competence. J Am Coll Cardiol. 2003;41:687–708. doi: 10.1016/s0735-1097(02)02885-1. [DOI] [PubMed] [Google Scholar]
- 17.Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet. 2009;373:1382–94. doi: 10.1016/S0140-6736(09)60692-9. [DOI] [PubMed] [Google Scholar]
- 18.Suri RM, Clavel MA, Schaff HV, et al. Effect of recurrent mitral regurgitation following degenerative mitral valve repair: long-term analysis of competing outcomes. J Am Coll Cardiol. 2016;67:488–98. doi: 10.1016/j.jacc.2015.10.098. [DOI] [PubMed] [Google Scholar]
- 19.Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med. 2005;352:875–83. doi: 10.1056/NEJMoa041451. [DOI] [PubMed] [Google Scholar]
- 20.Suri RM, Schaff HV, Enriquez-Sarano M. Mitral valve repair in asymptomatic patients with severe mitral regurgitation: pushing past the tipping point. Semin Thorac Cardiovasc Surg. 2014;26:95–101. doi: 10.1053/j.semtcvs.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Jones EC, Devereux RB, Roman MJ, et al. Prevalence and correlates of mitral regurgitation in a population-based sample (the Strong Heart Study) Am J Cardiol. 2001;87:298–304. doi: 10.1016/s0002-9149(00)01362-x. [DOI] [PubMed] [Google Scholar]
- 22.Avierinos JF, Inamo J, Grigioni F, Gersh B, Shub C, Enriquez-Sarano M. Sex differences in morphology and outcomes of mitral valve prolapse. Ann Intern Med. 2008;149:787–95. doi: 10.7326/0003-4819-149-11-200812020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24:1231–43. doi: 10.1016/s0195-668x(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 24.Bedogni F, Latib A, De Marco F, et al. Interplay between mitral regurgitation and transcatheter aortic valve replacement with the CoreValve Revalving System: a multicenter registry. Circulation. 2013;128:2145–53. doi: 10.1161/CIRCULATIONAHA.113.001822. [DOI] [PubMed] [Google Scholar]
- 25.Grigioni F, Enriquez-Sarano M, Zehr K, Bailey K, Tajik A. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759–64. doi: 10.1161/01.cir.103.13.1759. [DOI] [PubMed] [Google Scholar]
- 26.Rosenhek R, Rader F, Klaar U, et al. Outcome of watchful waiting in asymptomatic severe mitral regurgitation. Circulation. 2006;113:2238–44. doi: 10.1161/CIRCULATIONAHA.105.599175. [DOI] [PubMed] [Google Scholar]
- 27.Al-Hasan MN, Eckel-Passow JE, Baddour LM. Influence of referral bias on the clinical characteristics of patients with Gram-negative bloodstream infection. Epidemiol Infect. 2011;139:1750–56. doi: 10.1017/S095026881100001X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bach DS, Awais M, Gurm HS, Kohnstamm S. Failure of guideline adherence for intervention in patients with severe mitral regurgitation. J Am Coll Cardiol. 2009;54:860–65. doi: 10.1016/j.jacc.2009.03.079. [DOI] [PubMed] [Google Scholar]
- 29.Iung B, Baron G, Tornos P, Gohlke-Barwolf C, Butchart EG, Vahanian A. Valvular heart disease in the community: a European experience. Curr Prob Cardiol. 2007;32:609–61. doi: 10.1016/j.cpcardiol.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Avierinos JF, Tribouilloy C, Grigioni F, et al. Impact of ageing on presentation and outcome of mitral regurgitation due to flail leaflet: a multicentre international study. Eur Heart J. 2013;34:2600–09. doi: 10.1093/eurheartj/eht250. [DOI] [PubMed] [Google Scholar]
- 31.Mohty D, Orszulak TA, Schaff HV, Avierinos JF, Tajik JA, Enriquez-Sarano M. Very long-term survival and durability of mitral valve repair for mitral valve prolapse. Circulation. 2001;104(12 suppl 1):I1–7. doi: 10.1161/hc37t1.094903. [DOI] [PubMed] [Google Scholar]
- 32.Suri RM, Schaff HV, Dearani JA, et al. Determinants of early decline in ejection fraction after surgical correction of mitral regurgitation. J Thorac Cardiovasc Surg. 2008;136:442–47. doi: 10.1016/j.jtcvs.2007.10.067. [DOI] [PubMed] [Google Scholar]
- 33.Mantovani F, Clavel MA, Michelena HI, Suri RM, Schaff HV, Enriquez-Sarano M. Comprehensive imaging in women with organic mitral regurgitation: implications for clinical outcome. JACC Cardiovasc Imaging. 2016;9:388–96. doi: 10.1016/j.jcmg.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 34.Makkar RR, Fontana GP, Jilaihawi H, et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366:1696–704. doi: 10.1056/NEJMoa1202277. [DOI] [PubMed] [Google Scholar]
- 35.Acker MA, Parides MK, Perrault LP, et al. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med. 2014;370:23–32. doi: 10.1056/NEJMoa1312808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michler RE, Smith PK, Parides MK, et al. Two-year outcomes of surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med. 2016;374:1932–41. doi: 10.1056/NEJMoa1602003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stone GW, Adams DH, Abraham WT, et al. Clinical trial design principles and endpoint definitions for transcatheter mitral valve repair and replacement: part 2: endpoint definitions: a consensus document from the Mitral Valve Academic Research Consortium. J Am Coll Cardiol. 2015;66:308–21. doi: 10.1016/j.jacc.2015.05.049. [DOI] [PubMed] [Google Scholar]
- 38.Mack MJ, Holmes DR., Jr Rational dispersion for the introduction of transcatheter valve therapy. JAMA. 2011;306:2149–50. doi: 10.1001/jama.2011.1675. [DOI] [PubMed] [Google Scholar]
- 39.Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–91. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 40.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:151–60. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


