Summary
Purpose
The oral PI3K inhibitor BKM120 has been reported as safe and well tolerated in early phase clinical trials of advanced cancer patients. We performed a phase I trial of BKM120 plus mFOLFOX6 (5-FU/LV + oxaliplatin), a common chemotherapeutic backbone in GI malignancies, to establish the maximum tolerated dose (MTD) and characterize the safety and tolerability of the combination.
Methods
Patients with advanced solid tumors received oral BKM120 daily combined with standard doses of mFOLFOX6 every 2 weeks of a 28 day cycle. The study utilized a standard 3 + 3 dose escalation schema.
Results
A total of 17 patients received treatment with BKM120, 13 of which were evaluate for dose limited toxicity (DLT). The most common tumor types were colorectal cancer, cholangiocarcinoma, pancreatic cancer and hepatocellular carcinoma. DLT included grade 3 hyperglycemia, grade 3 AST/ALT elevation, grade 4 neutropenia and grade 4 thrombocytopenia. A total of 76 % of patients experienced treatment related grade 3/4 adverse events (AEs), the most common of which were neutropenia, fatigue, leukopenia, hyperglycemia and thrombocytopenia. One patient demonstrated an unconfirmed partial response and three patients had stable disease.
Discussion
The MTD of BKM120 in combination with standard doses of mFOLFOX6 was 40 mg daily, which is well below the 100 mg daily dose proven effective and tolerable both as a single agent and in combination with other chemotherapeutics. In addition, the regimen of BKM120 with mFOLFOX6 in patients with refractory solid tumors resulted in increased toxicity than would be expected from either the PI3K inhibitor or the chemotherapy backbone alone.
Keywords: BKM120, PI3K pathway, 5-fluorouracil, Oxaliplatin, Gastrointestinal malignancies
Introduction
The phosphatidyl inositol 3 kinase (PI3K)/AKT signaling pathway plays an integral role in the tumorigenesis of many human gastrointestinal (GI) cancers [1]. The PIK3CA gene encodes the catalytic subunit of PI3K (p110α) and is responsible for the conversion of phosphatidylinositol 4,5-biphosphate (PIP2) to phosphatidylinositol-3,4,5-trisphosphate (PIP3). Amplifications, gain-of-function mutations, and activation by upstream signaling of PIK3CA lead to the dysregulated activation of AKT allowing for the constitutive upregulation of multiple AKT-directed oncogenic pathways including the mammalian target of rapamycin (mTOR) pathway [2]. Increased signaling through mTOR leads to the transcription of genes such as vascular endothelial growth factor (VEGF) and hypoxia inducible factor (HIF) that are involved in angiogenesis and cell survival. In vitro disruption of PI3K signaling results in increased apoptosis and impaired cancer cell survival in a variety of GI tumor types; hence, the targeted inhibition of the PI3K/AKT pathway has emerged as an attractive treatment strategy [3–6].
The combination of 5-fluorouracil (5-FU) with leucovorin plus oxaliplatin (mFOLFOX6) is commonly used to treat patients with a variety of GI cancers. Overcoming intrinsic and acquired resistance to chemotherapy remains a challenging aspect of caring for these patients. Emerging evidence suggests that activation of the PI3K/AKT pathway contributes to the development of resistance to many anti-cancer therapies. For example, in patients with colon cancer treated with chemotherapy, response rates were lower in those whose tumors expressed ANGPTL2, a known downstream effector of the PI3K pathway [7]. In an esophageal tumor xenograft model, acquired 5-FU resistance was reversed after treating the animals with wortmannin, a chemical PI3K inhibitor [8]. The same compound markedly augmented apoptosis of pancreatic cancer cells compared to cells treated with gemcitabine alone [9]. Sparse clinical trial data exists, however, that evaluates PI3K inhibition combined with commonly used chemotherapy agents.
BKM120 is an oral pan-class 1 PI3K inhibitor that works via inactivation of the p110α subunit. The first phase I trial of BKM120 enrolled 35 patients with advanced solid tumors and established the maximum tolerated dose (MTD) as 100 mg daily [10]. Dose limiting toxicity (DLT) included hyperglycemia, skin rash, epigastric pain, and mood disorders. Two subsequent studies have confirmed that BKM120 is well tolerated when combined with both the aromatase inhibitor letrozole in estrogen receptor-positive metastatic breast cancer and carboplatin plus paclitaxel in advanced solid tumors [11, 12]. Based on the above rationale, we conducted a phase I trial of BKM120 in combination with mFOLFOX6 in order to determine the MTD of the combination in patients with refractory advanced solid tumors.
Methods
Patient population
Patients eligible for enrollment on this study had histologically confirmed advanced solid tumors refractory to standard therapy or for which no accepted standard therapy exists. Other inclusion criteria included: measurable or evaluable disease, Eastern Cooperative Oncology Group (ECOG) performance status ≤1, and complete recovery from all reversible toxicities related to their previous treatments. Patients were required to have an absolute neutrophil count (ANC) ≥1500 cells/mm3, platelet count ≥100,000/m3, and hemoglobin ≥9 g/dL. All patients had normal kidney function. For patients with liver metastases, total bilirubin ≤1.5 × the upper limit of normal (ULN) was required as well as an AST and ALT ≤3 × ULN. Pertinent exclusion criteria included prior treatment with a PI3K inhibitor, patients with known coagulopathies on coumarin-derived anticoagulants, chronic corticosteroid treatment, patients with documented mood disorders, and GI dysfunction resulting in impaired absorption. Initially, the protocol excluded patients solely based on a history of poorly controlled diabetes mellitus defined by fasting plasma glucose ≤120; the protocol was subsequently amended to only include patients with a fasting plasma glucose ≤120 and a hemoglobin A1c (HgA1c) ≤8.0 %.
This study received approval by the Institutional Review Board (IRB) of the University of North Carolina at Chapel Hill. It was registered with the United States National Institutes of Health (trial number: NCT01571024). Written informed consent was obtained from all patients prior to the initiation of any study related procedures or treatments.
Study design
This trial was a single arm, open-label phase I study with a primary objective to determine the MTD of BKM120 when administered concomitantly with mFOLFOX6 in patients with advanced solid tumors. Secondary objectives included characterizing the safety and tolerability of the combination via an adverse event (AE) profile as well as exploring any anti-tumor activity using RECIST 1.1 criteria.
BKM120 was initiated at an oral dose of 40 mg once a day continuously in combination with standard dose mFOLFOX6 (oxaliplatin 85 mg/m2, leucovorin 400 mg/m2, 5-FU bolus 400 mg/m2 and 5-FU infusion 2400 mg/m2 over 46 h) administered IV every 2 weeks of a 28 day cycle. It was recommended that BKM120 be taken each morning, after a light breakfast with a glass of water. Patients were instructed to fast for 2 h after each BKM120 dose. To receive days 1 and 15 of treatment, patients were required to have an ANC ≥1,500/mm3 and a platelet count ≥75,000/mm3. Patients continued on protocol-based treatment until documented disease progression, unacceptable toxicity, patient preference or patient death.
The study utilized a standard 3 + 3 dose escalation scheme outlined in Table 1. If none of the first three patients enrolled in cohort 1 experienced DLT within the first 4 week cycle, then the next group of patients were permitted to enroll to cohort 2. If one of the three patients in any cohort experienced DLT, then three additional patients were enrolled into that same cohort. If only one of these six patients experienced DLT, subsequent patients were enrolled into the next cohort. If two or more of these six patients experienced DLT, or two patients experienced DLT in a cohort of three patients, the MTD was considered to have been exceeded. Therefore, the estimated MTD was the highest dose at which ≤1 out of six patients experienced a DLT. No intra-patient dose escalation was allowed.
Table 1.
Dose escalation table
| Dose level | BKM120 (mg daily) |
100 % mFOLFOX6 (D1, D15) |
Total patients | Number of evaluable patients for DLT |
Number of patients with DLT |
|---|---|---|---|---|---|
| 1 | 40 | Oxaliplatin: 85 mg/m2 | 4 | 3 | 0 |
| Leucovorin: 400 mg/m2 | |||||
| 5-FU bolus: 400 mg/m2 | |||||
| 5-FU infusion: 2400 mg/m2 | |||||
| 2 | 60 | Oxaliplatin: 85 mg/m2 | 10 | 8 | 4 |
| Leucovorin: 400 mg/m2 | Hyperglycemia (grade 3) | ||||
| 5-FU bolus: 400 mg/m2 | AST/ALT elevation (grade 3) | ||||
| 5-FU infusion: 2400 mg/m2 | neutropenia (grade 4) | ||||
| febrile neutropenia (grade 4) | |||||
| thrombocytopenia (grade 4) | |||||
| 3 | 80 | Oxaliplatin: 85 mg/m2 | 3 | 2 | 1 |
| Leucovorin: 400 mg/m2 | neutropenia (grade 4) | ||||
| 5-FU bolus: 400 mg/m2 | |||||
| 5-FU infusion: 2400 mg/m2 |
Assessments
Safety assessments
Toxicity was assessed using the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. DLT was evaluated during the first cycle of treatment (28 days) and was defined as an AE or abnormal laboratory value unrelated to disease, disease progression, concurrent illness or concomitant medications. Any AE that resulted in a dose interruption of BKM120 for more than 7 days or a dose reduction of BKM120 within the first treatment cycle was also considered a DLT. Any patient who received at least 1 day of BKM120 treatment was evaluable for toxicity. Patients who received at least one cycle of therapy (or whose treatment was discontinued as a result of DLT) were evaluable for DLT.
Regarding hyperglycemia, the protocol originally defined DLT as a single occurrence of grade 3 hyperglycemia OR grade 2 hyperglycemia that did not resolve to grade 0 within 14 consecutive days. After enrollment to cohort 3 was initiated, the protocol was amended to re-define DLT as a single occurrence of grade 4 hyperglycemia only, as grade 3 hyperglycemia can be successfully managed by close monitoring and anti-diabetic medications.
Clinical assessments
Baseline assessments included a complete history and physical exam (including ECOG performance status), laboratory measurements, 12-lead EKG, echocardiogram, and radiographic imaging of disease. Mood questionnaires were completed using the Patient Health Questionnaire (PHQ-9) and Generalized Anxiety Disorder 7-item (GAD-7) scales. Patients were seen weekly for the first cycle to complete history and physical exams, as well as toxicity assessments (including mood questionnaires), and then on D1 and D15 of cycles 2 and beyond. Efficacy was determined via tumor measurements every two cycles (8 weeks) according to RECIST 1.1 criteria.
Results
Baseline patient characteristics
A total of 17 patients received treatment with BKM120 and mFOLFOX6 on this trial from August 2012 through April 2015. Their baseline characteristics are summarized in Table 2. Four patients were replaced before completing cycle one and therefore were not evaluable for DLT. Reasons for their replacement included 1) an oxaliplatin infusion reaction, 2) rapid decline in performance status, 3) patient preference due to grade 2 nausea and 4) patient preference due to chemotherapy delays secondary to grade 2 neutropenia and grade 2 thrombocytopenia. The median age was 56 years (range 25–74) and 53 % of patients were male. The most common tumor types were colorectal cancer (n = 5), cholangiocarcinoma (n = 4), pancreatic adenocarcinoma (n = 2) and hepatocellular carcinoma (n = 2). Patients received a median of two lines of therapy prior to enrollment.
Table 2.
Patient characteristics
| Characteristics | |
|---|---|
| Total number of patients | 17 |
| Median age (years) | 56 (range 25–74) |
| Gender | |
| Male | 9 |
| Female | 8 |
| Race | |
| White | 12 |
| Black | 3 |
| Hispanic | 1 |
| American Indian | 1 |
| Median number of previous therapies | 2 (range 1–7) |
| Tumor type | |
| Colorectal | 5 |
| Wildtype | 3 |
| Mutant | 2 |
| Cholangiocarcinoma | 4 |
| Pancreatic adenocarcinoma | 2 |
| Hepatocellular carcinoma | 2 |
| Esophageal squamous cell carcinoma | 1 |
| Gastroesophageal junction adenocarcinoma | 1 |
| Breast | 1 |
| Ocular melanoma | 1 |
Dose escalation and determination of MTD
A total of 13 patients were evaluable for DLT associated with BKM120 plus mFOLFOX6. DLT occurring during the first cycle of therapy are summarized in Table 1. In cohort 1, a total of three patients completed cycle one without DLT. In cohort 2, one patient experienced DLT (grade 3 hyperglycemia) during cycle 1. Therefore, three additional patients (for a total of 6) were enrolled to cohort 2. A second patient experienced grade 3 hyperglycemia on day 1 of cycle 2. This patient remained on study for five additional cycles with the initiation of metformin for glucose control. Three patients were enrolled into cohort 3, one of whom experienced DLT of grade 4 neutropenia. The other two patients discontinued therapy electively due to grade 1 and grade 2 toxicities (fatigue and nausea).
As none of the three patients in cohort 3 were able to complete two cycles of treatment, we elected to not expand cohort 3, but rather to enroll additional patients to cohort 2. However, the first two patients enrolled in this expanded cohort experienced DLT. Both patients had cholangiocarcinoma and had received first line therapy with gemcitabine and cisplatin. The first patient was admitted on day 14 of cycle 1 with fever. She developed grade 3 ALT and AST elevation. Although these levels resolved to baseline within 7 days of stopping BKM120, the protocol mandated a dose reduction of BKM120 upon re-initiation, which was considered a DLT. The second patient was admitted on day 21 of cycle 1 with esophagitis secondary to gastric outlet obstruction. He developed grade 4 neutropenia with fever and grade 4 thrombocytopenia that resulted in permanent discontinuation of therapy. Therefore, out of eight evaluable patients who received BKM120 in cohort 2 at 60 mg per day, four patients experienced DLT. As such, the MTD of BKM120 with standard doses of mFOLFOX6 was determined to be 40 mg daily (cohort 1). As this dose was well below the 100 mg daily dose that has been achievable in combination with other chemotherapeutic agents, we elected not to proceed with a planned expansion cohort of patients with metastatic pancreatic cancer.
Adverse events
Of the 17 patients evaluable for toxicity, all patients reported at least one treatment related AE with 76 % of patients (13/17) experiencing ≥ grade 3 toxicity (Table 3). Overall, the most common AE reported was nausea (82 % of patients) with the majority of cases being grade 1 or 2 that was well managed with anti-emetics. Other commonly reported non-laboratory based AEs included fatigue (53 %), anorexia (35 %), mucositis (29 %), vomiting (29 %), peripheral neuropathy (24 %) and epistaxis (18 %).
Table 3.
Most frequent adverse events
| Adverse event | Total patients (%) | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|---|
| Nausea | 14 (82) | 6 | 7 | 1 | |
| Hyperglycemia | 12 (71) | 8 | 2 | 2 | |
| Neutropenia | 11 (65) | 3 | 6 | 2 | |
| Thrombocytopenia | 11 (65) | 4 | 5 | 1 | 1 |
| Fatigue | 9 (53) | 4 | 2 | 3 | |
| Leukopenia | 9 (53) | 4 | 2 | 3 | |
| Elevated alkaline phosphatase | 6 (35) | 5 | 1 | ||
| Anemia | 6 (35) | 6 | |||
| Anorexia | 6 (35) | 3 | 3 | ||
| AST elevation | 6 (35) | 5 | 1 | ||
| Increased lipase | 6 (35) | 2 | 4 | ||
| Lymphopenia | 6 (35) | 2 | 4 | ||
| Mucositis | 5 (29) | 2 | 2 | 1 | |
| Vomiting | 5 (29) | 1 | 3 | 1 | |
| Hypertriglyeridemia | 4 (24) | 1 | 3 | ||
| Peripheral neuropathy | 4 (24) | 3 | 1 | ||
| ALT elevation | 3 (18) | 2 | 1 | ||
| Epistaxis | 3 (18) | 3 |
Laboratory abnormalities comprised the majority of the treatment related AEs. A total of 71 % of patients experienced hyperglycemia (eight grade 1, two grade 2 and two grade 3). Out of the 12 patients that reported hyperglycemia, three had a pre-existing diagnosis of diabetes. All four patients in the lowest dose cohort (BKM120 at 40 mg) reported either grade 1 or 2 hyperglycemia (none of whom had diabetes at baseline). Other commonly reported lab based AEs included neutropenia (65 %), thrombocytopenia (65 %), leukopenia (53 %), elevated alkaline phosphatase (35 %), anemia (35 %), AST elevation (35 %), increased lipase (35 %) and lymphopenia (35 %).
The most common grade 3/4 AEs were neutropenia, fatigue, leukopenia, hyperglycemia and thrombocytopenia. Two patients developed grade 4 neutropenia, one with fever and the other with concomitant grade 4 thrombocytopenia. Other grade 3 AEs (nausea, vomiting, mucositis, AST/ALT elevation, and GGT elevation) occurred in one patient each. Three patients died while on study or within 30 days of receiving treatment on study: one during cycle 3 after presenting with pneumonia and septic shock (not deemed to be drug related) and two from disease progression.
Clinical efficacy
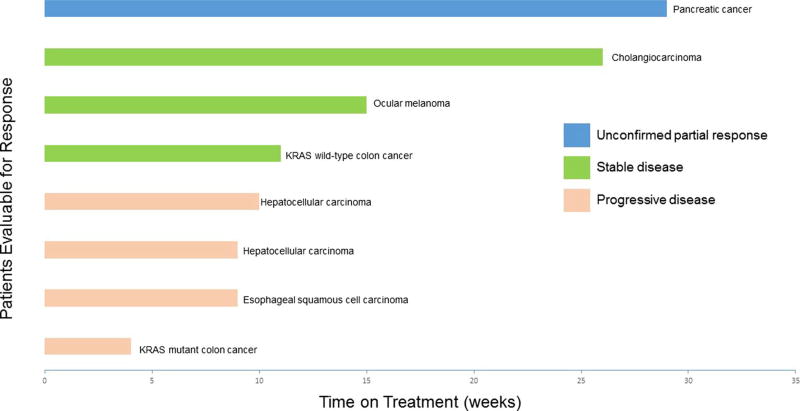
The 17 patients enrolled on trial received a median of 6 weeks of treatment (range 1–29). Of those, eight patients were evaluable for response, having remained on treatment for at least two cycles (8 weeks) of treatment or in whom treatment was discontinued due to disease progression. The median time on treatment for these eight patients was 10 weeks (range 4–29) (Fig. 1). One patient with stage IV pancreatic cancer experienced an unconfirmed partial response after cycle 4 with a 47 % decrease in measurable disease from baseline. She then developed progression after cycle 6 and her treatment was discontinued at 29 weeks. Three patients (38 %) demonstrated stable disease and remained on treatment for 9, 15 and 26 weeks, respectively. These patients had intrahepatic cholangiocarcinoma, ocular melanoma and KRAS wild-type colon cancer (with previous FOLFOX treatment). Four patients (50 %) had progressive disease at the time of their first evaluation.
Fig. 1.
Time on treatment for patients evaluable for response
Discussion
This study represents the first published trial of the oral PI3K inhibitor BKM120 with the chemotherapeutic combination mFOLFOX6. The MTD of BKM120 in this trial was 40 mg daily (cohort 1) with only 20 % of patients receiving treatment beyond cycle 3. This is in stark contrast to the phase I dose-escalation study of single agent BKM120 in which MTD was established to be 100 mg daily with 20 % of enrolled patients remaining on treatment for over 8 months [10]. Effects of BKM120 on downstream target inhibition in that trial suggested that doses of at least 80 mg were required for more than a 40 % decrease in pS6 levels. Therefore, although none of the three evaluable patients in cohort 1 experienced DLT, further expansion was not recommended due to concerns for lack of target effect at the MTD of 40 mg.
A review of the literature of BKM120 in combination with cytotoxic chemotherapy reveals only a handful of trials that can provide insight into the increased toxicity seen in our trial. Two studies have evaluated BKM120 with paclitaxel or paclitaxel/carboplatin [11, 13]. In both studies, the MTD of BKM120 was the same as the single-agent MTD (100 mg daily) and the combination was reported to be well tolerated. A review of the patient demographics in these trials confirms a heavily pretreated patient population; hence, it is difficult to ascribe the toxicity seen in our trial to the phenotype of the refractory patient cohort.
Almost half of patients on our trial (47 %) experienced grade 3/4 neutropenia. This is in line with the frequency of hematologic toxicity reported in trials of mFOLFOX6 and is hence more likely related to the effects of chemotherapy, not the combined effect of PI3K inhibition [14, 15]. A higher percentage of patients in our study reported hyperglycemia (71 %) compared to 40 % in the single agent first-in-man trial. While this is a known class effect of PI3K inhibitors, impaired fasting glucose is not considered a common side effect of mFOLFOX6. However, in a cohort study of over 400 patients with colorectal cancer who received 5-FU based chemotherapy (two thirds of which received FOLFOX), approximately 25 % developed diabetes or elevated fasting glucose levels while on treatment [16]. Pre-clinical studies suggest that in rats treated with 5-FU, fasting blood glucose levels are significantly higher when compared to untreated controls with lower insulin levels detected in animals exposed to 5-FU [17]. While no gross pathologic damage was observed within the pancreas of these mice, diminished secretory granules from islet cells were observed microscopically at day 7, potentially as a direct cytotoxic effect from the chemotherapy. Interestingly, few if any mood disorders were documented in our trial, an AE that has been more frequently reported in the breast cancer literature in which BKM120 is combined with endocrine therapy [12]. This may reflect that the majority of patients on our study were treated with BKM120 doses less than the standard 100 mg used in these other trials.
In conclusion, the combination of BKM120 with mFOLFOX6 in patients with refractory solid tumors resulted in increased toxicity compared to that which would be expected from either the PI3K inhibitor or the chemotherapy alone. The MTD of BKM120 in combination with standard doses of mFOLFOX6 was 40 mg daily, which is well below the 100 mg daily dose proven effective and tolerable both as a single agent and in combination with other chemotherapeutics. Therefore, further study of BKM120 is not recommended in combination with mFOLFOX6 in GI malignancies.
Acknowledgments
The authors would like to acknowledge grant funding support for this project. Dr. McRee receives support from the UNC Calabresi K12 Career Development Grant 2K12CA120780-06. Dr. Sanoff receives support from the National Cancer Institute K07CA160722.
Dr. Hanna Sanoff has received research funding from Novartis.
Footnotes
Conflict of interest The remaining authors declare that they have no conflict of interest.
References
- 1.Brown KK, Toker A. The phosphoinositide 3-kinase pathway and therapy resistance in cancer. F1000Prime Rep. 2015;7:13. doi: 10.12703/P7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 3.Cai X, et al. Synergistic inhibition of colon carcinoma cell growth by Hedgehog-Gli1 inhibitor arsenic trioxide and phosphoinositide 3-kinase inhibitor LY294002. Onco Targets Ther. 2015;8:877–883. doi: 10.2147/OTT.S71034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng HB, et al. Longikaurin E induces apoptosis of pancreatic cancer cells via modulation of the p38 and PI3K/AKT pathways by ROS. Naunyn Schmiedeberg's Arch Pharmacol. 2015;388(6):623–634. doi: 10.1007/s00210-015-1107-4. [DOI] [PubMed] [Google Scholar]
- 5.Simioni C, et al. The novel dual PI3K/mTOR inhibitor NVP-BGT226 displays cytotoxic activity in both normoxic and hypoxic hepatocarcinoma cells. Oncotarget. 2015;6:17147–17160. doi: 10.18632/oncotarget.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng YB, et al. Paeoniflorin inhibits human gastric carcinoma cell proliferation through up-regulation of microRNA-124 and suppression of PI3K/Akt and STAT3 signaling. World J Gastroenterol. 2015;21(23):7197–7207. doi: 10.3748/wjg.v21.i23.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horiguchi H, et al. Angiopoietin-like protein 2 renders colorectal cancer cells resistant to chemotherapy by activating spleen tyrosine kinase-phosphoinositide 3-kinase-dependent anti-apoptotic signaling. Cancer Sci. 2014;105(12):1550–1559. doi: 10.1111/cas.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li B, et al. Suppression of esophageal tumor growth and chemoresistance by directly targeting the PI3K/AKT pathway. Oncotarget. 2014;5(22):11576–11587. doi: 10.18632/oncotarget.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng SSW, et al. Inhibition of phosphatidylinositide 3-kinase enhances gemcitabine-induced apoptosis in human pancreatic cancer cells. Cancer Res. 2000;60(19):5451–5455. [PubMed] [Google Scholar]
- 10.Bendell JC, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30(3):282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 11.Hyman DM, et al. Parallel phase Ib studies of two schedules of buparlisib (BKM120) plus carboplatin and paclitaxel (q21 days or q28 days) for patients with advanced solid tumors. Cancer Chemother Pharmacol. 2015;75(4):747–755. doi: 10.1007/s00280-015-2693-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayer IA, et al. Stand up to cancer phase Ib study of panphosphoinositide-3-kinase inhibitor buparlisib with letrozole in estrogen receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2014;32(12):1202–1209. doi: 10.1200/JCO.2013.54.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zambrano CC, Schuler MH, Machiels J-PH, Hess D, Paz-Ares L, Awada A, von Moos R, Steeghs N, Ahnert JR, De Mesmaeker P, Richly H, Herremans C, Joerger M, Jaime JC, Alsina M, Baffert F, Demanse D, Duval V, Morozov A, Dirix L. Phase lb study of buparlisib (BKM120) plus either paclitaxel (PTX) in advanced solid tumors (aST) or PTX plus trastuzumab (TZ) in HER2+ breast cancer (BC) J Clin Oncol. 2014;32(5s) abstract 627. [Google Scholar]
- 14.Goldberg RM, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22(1):23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 15.Tournigand C, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22(2):229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 16.Feng JP, et al. Secondary diabetes associated with 5-fluorouracil-based chemotherapy regimens in non-diabetic patients with colorectal cancer: results from a single-centre cohort study. Color Dis. 2013;15(1):27–33. doi: 10.1111/j.1463-1318.2012.03097.x. [DOI] [PubMed] [Google Scholar]
- 17.Feng JP, et al. Impact of 5-fluorouracil on glucose metabolism and pancreatic pathology in rats. Zhonghua Wei Chang Wai Ke Za Zhi. 2010;13(12):935–938. [PubMed] [Google Scholar]