Abstract
Objectives
To examine whether previously reported results indicating that PSA screening can reduce PC mortality regardless of sociodemographic inequality and if they could be corroborated in an 18-yr follow-up.
Material and methods
In 1994, 20,000 men aged 50–64 yr were randomized from the population register to PSA screening or control (1:1). Men in the screening group (n=9950) were invited for biennial PSA testing up to the median age of 69 yr. Prostate biopsy was recommended for men with PSA ≥2.5 ng/ml. Last follow up was Dec 31, 2012. Study ID: ISRCTN54449243
Results
In the screening group, 77% (7647/9950) attended at least once. After 18 yr, 1396 men in the screening group and 962 in the control group had been diagnosed with PC (hazard ratio [HR] 1.51, 95% confidence interval [CI] 1.39–1.64). Cumulative PC mortality was 0.98% (95% CI 0.78–1.22%) in the screening group versus 1.50% (95% CI 1.26–1.79%) among controls, an absolute reduction of 0.52% (95% CI 0.17–0.87%). The rate ratio (RR) for PC death was 0.65 (95% CI 0.49–0.87). To prevent one death from PC, the number needed to invite was 231 and the number needed to diagnose was 10. Systematic PSA screening demonstrated even greater benefit in PC mortality for men who started screening at age 55–59 yr (RR 0.47, 95% CI 0.29–0.78) and men with low education (RR 0.49, 95% CI 0.31–0.78).
Conclusions
These data corroborate previous findings that systematic PSA screening reduces PC mortality and suggests that systematic screening may reduce sociodemographic inequality in PC mortality.
Keywords: mass screening, socioeconomic factors, prostate cancer, prostate-specific antigen
Introduction
In 2010, the Göteborg screening trial reported a 44% relative risk reduction (rate ratio [RR] 0.56) in prostate cancer (PC) mortality after 14-yr follow-up, due to systematic, organized prostate-specific antigen (PSA) screening [1]. Since then, the data reported from 11- and 13-yr follow-up of the European Randomised Study of Screening for Prostate Cancer (ERSPC) each showed 21% relative risk reduction [2,3]. Nevertheless, the controversy whether to screen for PC with PSA continues. Advocates refer to the reported reduction in advanced disease and PC mortality; critics argue that the associated harmful effects, such as psychological consequences of screening, biopsy complications, overdiagnosis, and overtreatment, might outweigh the benefits [4,5].
In Sweden, organized PC screening has never been recommended. The Swedish National Board of Health and Welfare recommends informed decision-making where individual men decide whether to be screened after receiving information on advantages and disadvantages of PSA screening [6-8]. This form of non-organized, opportunistic PSA screening increased during the late 1990s and became common in Sweden during the 2000s, similar to other Western countries [9,10].
Previous studies has demonstrated inequalities in screening use correlating with sociodemographic factors [11-13]; but there are contradictory findings whether the effects of sociodemographic factors are reduced with an organized screening program, compared to opportunistic screening [14,15].
Here we update the PC mortality data after 18-yr follow-up of the Göteborg screening trial, and report outcomes of systematic biennial PSA screening in sociodemographic subgroups.
Material and methods
The Göteborg randomized, population-based PC screening trial (ISRCTN54449243) started in 1995, after approval from the local Ethics committee at the University of Göteborg. Since 1996, the Göteborg screening trial has constituted the Swedish arm of the ERSPC. Details of the study protocol for the Göteborg screening trial have been published previously [1].
In summary, from the population register of males aged 50–64 yr living in Göteborg on Dec 31, 1994, 20,000 men were randomly selected for this study, then randomized 1:1 to screening and control groups. Men in the screening group received written information about PSA screening together with an invitation to participate every 2 yr until the upper age limit for invitation (median 69 yr, range 67–71). Men with PSA at or above threshold (3.4 ng/ml between 1995 and 1998, 2.9 ng/ml between 1999 and 2004, and 2.5 ng/ml after 2004) were invited for clinical follow-up with digital rectal examination, transrectal ultrasound, and laterally directed sextant biopsies (10-core biopsy after 2009). Men with PSA levels below threshold and those with a benign biopsy were re-invited after 2 yr. Men in the control group were not invited to participate in screening. The tenth and final screening round was finalized in spring 2014. Minor changes in the screening algorithm have been performed during the study period [16]. We continue to follow the study population with quarterly updates of the study database and linkage to the Swedish population register and the Swedish Cancer Register to receive information on mortality, emigration, and PC diagnoses. The present report includes information obtained until Dec 31, 2012.
Outcomes
The primary endpoint of the Göteborg screening trial was absolute and relative risk reduction in PC mortality between study arms. All PC deaths in the screening and control group were included, regardless of method of PC diagnosis (screen-detected, interval cancer, autopsy-detected, etc.). Cause of death for men diagnosed with PC was determined by an independent committee using a standardized algorithm and review of medical charts. Secondary outcomes in the present study were attendance, PC incidence, mortality rate, and RR in sociodemographic subgroups.
A separate ethical approval was obtained in 2012 from the local Ethics committee at the University of Göteborg (ID 204-12) to link the screening database with official registers. Linkage to the registers used the Swedish personal identification number, a 10-digit unique identifier for all individuals residing in Sweden. Cohabitation status, level of education, and information on country of birth were retrieved from the longitudinal integration database for health insurance and labor market studies at Statistics Sweden and were based on registered data in 1994. For cohabitation status, yes included cohabitant and/or married men but not divorced or widowed. This categorization was chosen because living with another person without being married is common in Sweden. Level of education was selected as the indicator of socioeconomic status (SES), as this variable was available for almost all individuals and is believed to be one of the socioeconomic indicators most likely to capture aspects of lifestyle and behaviour [17]. The highest level of formal education registered was stratified as low (≤9 years, mandatory school) or medium/high (≥10 years, high school, college or university). Comorbidity information, based on inpatient care from 1986 to 1994, was retrieved from the National Patient Register at the National Board of Health and Welfare. Comorbidity was calculated using a modified version of the Charlson comorbidity index. The only difference from the original Charlson comorbidity index [18] was that age did not add any points to the total score; comorbidity index was stratified as 0 (no comorbidities) or ≥1.
Statistical analysis
Analyses of PC mortality were performed as intention-to-screen analysis comparing the two study arms. Incidence and mortality rates were calculated by dividing the number of events and number of observed person-years at 14, 16 and 18 yr after randomization. Corresponding relative RR were calculated using Mantel-Haenzsel stratification by 5-yr age groups. The cumulative hazard for PC incidence and mortality were calculated and plotted with the Nelson-Aalen method [19]. To calculate the absolute differences in PC incidence and mortality between the study arms, 1-Kaplan Meier estimator was used. RR for subgroup analyses were calculated with Poisson regression. RR was also calculated for subgroups containing attendees only. The Cox proportional hazards model was used to estimate hazard ratio (HR). The validity of the proportional hazards assumption was established by means of the Schoenfeld residuals and the Grambsch and Therneau significance test. Piecewise constant HR models were used for PC incidence analysis. Number needed to invite (NNI) was calculated as 1 divided by the absolute risk reduction in PC mortality. Number needed to diagnose (NND) was calculated as 1 divided by (the absolute risk reduction in PC mortality multiplied by the excess incidence). In alternate analyses, to achieve more comparable groups, the control group was adjusted by subtracting the PC incidence and mortality rates among non-attendees in the screening group from the control group. Stata statistical software version 13.1 was used for all statistical analyses (StataCorp. 2015, College Station, TX).
Results
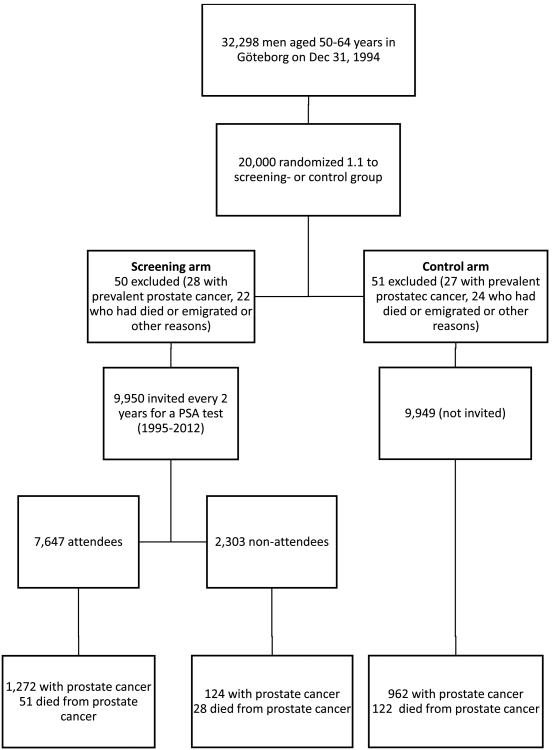
Of the 20,000 men randomized, 101 were excluded: 55 men with prevalent PC and 46 men who had died or emigrated before randomization (Figure 1). Of the 9950 men in the screening group, 7647 (77%) attended at least one screening. During 18-yr follow-up (Jan 1, 1995 to Dec 31, 2012), a total of 34,442 PSA tests were performed, with 5365 (16%) showing results above threshold. These elevated PSA results led to 4654 prostate biopsies. Among the 7647 attendees, 2651 men (35%) screened positive at least once, of whom 2482 (94%) underwent at least one prostate biopsy. The median number of screening rounds invited and attended was 6 (range 3-9) and 3 (range 0-9) respectively.
Figure 1. Trial profile of the Göteborg screening trial. PSA = prostate-specific antigen.
At 18 yr, a total of 1396 (14%) men had been diagnosed with PC in the screening group, compared with 962 (9.7%) men in the control group (Table 1). For the screening and control groups, respectively, median time from randomization to PC diagnosis was 8.6 yr (interquartile range [IQR] 4.7–12.8) versus 10.3 yr (IQR 7.0–14.0), and median age at diagnosis was 66 yr versus 68 yr. PC incidence rate at 18 yr was 9.7 (95% confidence interval [CI] 9.2–10.2) per 1000 person-yr in the screening group and 6.5 (95% CI 6.1–6.9) per 1000 person-yr in the control group (Table 2). When calculated as 1-Kaplan Meier, PC incidence was 16.2% (95% CI 15.4–17.0) in the screening group and 11.5% (95% CI 10.8–12.2) in the control group. The cumulative hazard of PC and the PC incidence RR for sociodemographic subgroups are shown in Figures 2a and 2b, respectively. The HR for PC incidence in the screening versus control group was 5.2 during the first year after randomization but decreased to 1.9 at 5 yr and 1.1 at 15 yr. PC incidence rate at different lengths of follow-up for the two arms and for age subgroups is shown in Table 2. Median time from PC diagnosis to last follow-up or death was longer in the screening group than in the control group (8.2 vs 6.0 yr). Table 1 shows the marked difference in tumor risk group distribution between the study arms. In accordance with the risk group distribution, treatment patterns differed between the study arms.
Table 1. Prostate cancer (PC) cases, risk group distribution, management, and deaths after 18 yr for screening and control groups.
| Control (n=9949) | Screening (n=9950) | |||
|---|---|---|---|---|
|
| ||||
| All (n=9950) | Attendees (n=7647) | Non-attendees (n=2303) | ||
|
| ||||
| n (%) | n (%) | n (%) | n (%) | |
| PC cases | 962 (9.7) | 1396 (14) | 1272 (17) | 124 (5.4) |
|
| ||||
| PC stage, | ||||
| Low-riska | 254 (2.6) | 699 (7.0) | 682 (8.9) | 17 (0.74) |
| Intermediate-riskb | 359 (3.6) | 470 (4.7) | 438 (5.7) | 32 (1.4) |
| High-riskc | 170 (1.7) | 135 (1.4) | 103 (1.3) | 32 (1.4) |
| Advancedd | 118 (1.2) | 67 (0.67) | 34 (0.44) | 33 (1.4) |
| Missinge | 61 (0.61)e | 25 (0.25) | 15 (0.20) | 10 (0.43) |
|
| ||||
| Primary treatment | ||||
| Surveillancef | 321/962 (33) | 608/1396 (44) | 583/1272 (46) | 25/124 (20) |
| Radical prostatectomyg | 304/962 (32) | 529/1396 (38) | 499/1272 (39) | 30/124 (24) |
| Radiation | 95/962 (9.9) | 109/1396 (7.8) | 95/1272 (7.5) | 14/124 (11) |
| Endocrine treatment | 223/962 (23) | 137/1396 (9.8) | 84/1272 (6.6) | 53/124 (43) |
| Not treatede | 19/962 (2.0)e | 10/1396 (0.72) | 9/1272 (0.71) | 1/124 (0.81) |
| Missing | - | 3/1396 (0.21) | 2/1272 (0.16) | 1/124 (0.81) |
|
| ||||
| Metastatic prostate cancer | 179 (1.8) | 116 (1.2) | 69 (0.9) | 47 (2.0) |
|
| ||||
| Deaths from PC | 122 (1.23) | 79 (0.79) | 51 (0.67) | 28 (1.2) |
| Deaths from other causes | 2735 (27) | 2765 (28) | 1712 (22) | 1053 (46) |
T1, not N1 or M1, and Gleason score ≤6 and prostate-specific antigen (PSA) <10 ng/ml.
T1–2, not N1 or M1, with Gleason score ≤7, PSA <20 ng/ml or both; and not meeting the criteria for low risk.
T1–4, not N1 or M1, with Gleason score ≥8, PSA <100 ng/ml, or both; and not meeting the criteria for low or intermediate risk.
N1 or M1, or PSA ≥100 ng/ml.
Includes eight cases detected at autopsy.
Includes active surveillance and watchful waiting.
Includes nine cryosurgeries and 12 cystoprostatectomies.
Table 2. Prostate cancer (PC) incidence rate, PC mortality rate, and rate ratio at different lengths of follow-up for the screening and control groups and for subgroups stratified by age.
| Rate per 1000 person-years (95% CI) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| 14yr follow-up | 16yr follow-up | 18yr follow-up | ||||||||||
| Number of PC/person years |
PC incidence |
Number of PC deaths/person years |
PC mortality |
Number of PC/person years |
PC incidence |
Number of PC death/person years |
PC mortality |
Number of PC/person years |
PC incidence |
Number of PC deaths/person years |
PC mortality |
|
| Screening | 1140/132199 | 8.6 (8.1–9.1) | 44/125973 | 0.35 (0.26–0.47) | 1288/131683 | 9.8 (9.3–10.3) | 60/141118 | 0.43 (0.33–0.55) | 1396/143776 | 9.7 (9.2–10.2) | 79/155374 | 0.51 (0.41–0.64) |
|
| ||||||||||||
| Control | 722/136840 | 5.3 (4.9–5.7) | 78/125914 | 0.62 (0.50–0.77) | 860/136317 | 6.3 (5.9–6.7) | 98/141035 | 0.69 (0.57–0.85) | 962/149129 | 6.5 (6.1–6.9) | 122/155245 | 0.79 (0.66–0.94) |
|
| ||||||||||||
| Rate ratio | ||||||||||||
| All men | 1.63 (1.49–1.80) | 0.56 (0.38– 0.83) | 1.55 (1.42–1.69) | 0.61 (0.44–0.85) | 1.51 (1.39–1.64) | 0.65 (0.48–0.87) | ||||||
| 50–54 yr | 1.85 (1.56–2.20) | 0.62 (0.16–2.15) | 1.80 (1.55–2.10) | 0.54 (0.18–1.44) | 1.77 (1.54–2.04) | 0.50 (0.20–1.16) | ||||||
| 55–59 yr | 1.67 (1.43–1.97) | 0.35 (0.16–0.68) | 1.45 (1.25–1.69) | 0.35 (0.18–0.65) | 1.37 (1.19–1.59) | 0.47 (0.28–0.79) | ||||||
| 60–64 yr | 1.43 (1.22–1.69) | 0.77 (0.45–1.31) | 1.43 (1.22–1.67) | 0.90 (0.57–1.43) | 1.39 (1.19–1.62) | 0.85 (0.56–1.28) | ||||||
CI = confidence interval. Rate ratios were calculated with Mantel-Haenzsel stratification by 5-yr age groups.
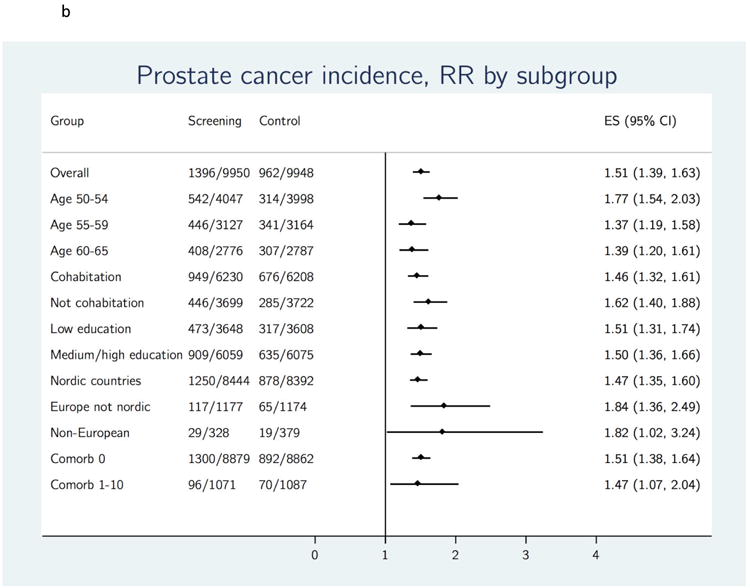
Figure 2.
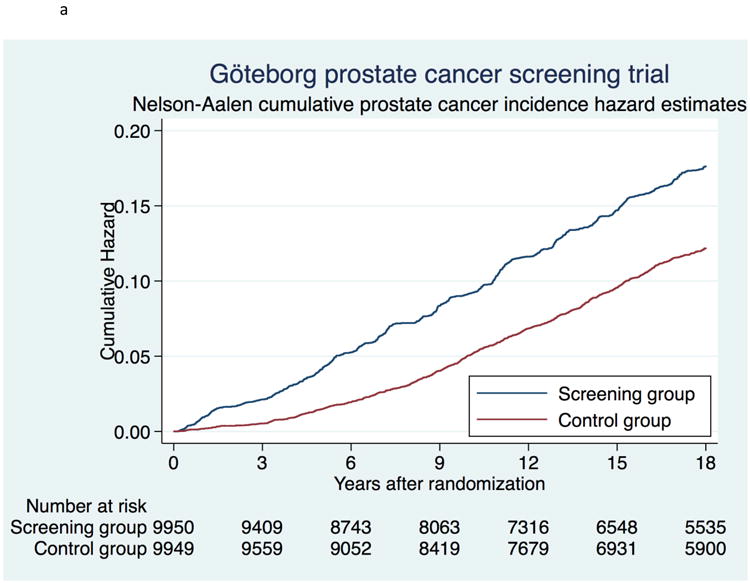
a. Cumulative risk of prostate cancer using Nelson-Aalen cumulative hazard estimates.
b. Effect of organized PSA screening on prostate cancer incidence, stratified by sociodemographic variables. ES = Estimated risk ratio; RR = risk ratio.
Of the 9950 men in the screening group, 2844 (28.6%) died within 18 yr, of whom 79 (0.79%) died from PC. The corresponding figures in the control group were 2857 (28.7%) and 122 (1.23%) (Table 1). Prostate cancer mortality rate at 18 years was 0.51 (95% CI 0.41–0.64) per 1000 person-years in the screening group and 0.79 (95% CI 0.66–0.94) in the control group, corresponding to a statistically significant, absolute reduction in PC mortality of 0.28 per 1000 person-years (Table 2). When calculated as 1-Kaplan Meier, PC mortality was 0.98% (95% CI 0.78%–1.22%) in the screening group and 1.50% (95% CI 1.26%–1.79%) in the control group, corresponding to an absolute cumulative risk reduction of 0.52 (95% CI 0.17–0.87). The cumulative hazard of PC mortality is shown in Figure 3a. The RR and the HR for PC mortality were almost identical: 0.65 (95% CI 0.49–0.87, p=0.003) and 0.65 (95% CI 0.49–0.86) respectively. Table 2 shows PC mortality rate at different lengths of follow-up and for subgroups stratified by age. At 18 years, NNI was 231 and NND 10.
Figure 3.
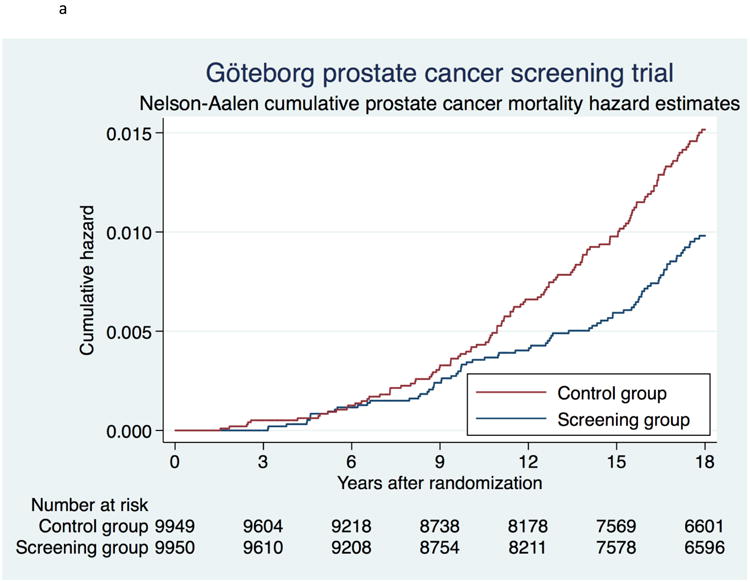
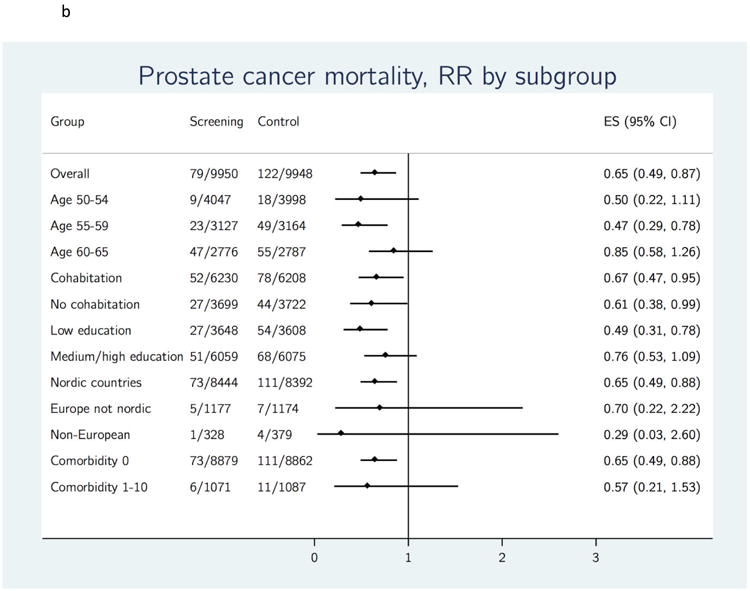
a. Cumulative risk of death from prostate cancer using Nelson-Aalen cumulative hazard estimates.
b. Effect of organized PSA screening on prostate cancer mortality, stratified by sociodemographic variables. ES = Estimated risk ratio; RR = risk ratio.
Screening attendance in the different sociodemographic subgroups ranged from 45% to 83% (Table 3a). Prostate cancer incidence rate in screening subgroups followed the level of attendance, apart from older men who had lower attendance but higher incidence rate (Table 3a). The RR for incidence ranged from 1.37 to 1.84 (Figure 2b).
Table 3a. Attendance, prostate cancer (PC) incidence, PC mortality, and overall mortality in sociodemographic subgroups at 18-yr follow-up.
| Number of men |
Attendance (proportion) |
Number of PCs |
PC incidence rate per 1000 person- years |
Rate ratio PC incidence (95% CI) |
Number of PC deaths |
PC mortality rate per 1000 person- years |
Rate ratio PC mortality (95% CI) |
Number of deaths |
Overall mortality rate per 1000 person- years |
Rate ratio overall mortality (95% CI) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | |||||||||||
| Screening group | 9950 | 7647 (77%) | 1396 | 9.7 | 1.51 (1.39–1.63) | 79 | 0.51 | 0.65 (0.49–0.87) | 2844 | 18.3 | 0.99 (0.94–1.05) |
| Control group | 9949 | – | 962 | 6.5 | 122 | 0.79 | 2857 | 18.4 | |||
|
| |||||||||||
| Subgroup analyses | Scr/Ctrl | Scr | Scr/Ctrl | Scr/Ctrl | Scr/Ctrl | Scr/Ctrl | Scr/Ctrl | Scr/Ctrl | Scr/Ctrl | Scr/Ctrl | |
|
| |||||||||||
| Age | |||||||||||
| 50–54 | 4047/3998 | 3159 (78%) | 542/314 | 8.7/4.9 | 1.77 (1.54–2.03) | 9/18 | 0.14/0.27 | 0.50 (0.22–1.11) | 751/703 | 11.3/10.7 | 1.06 (0.96–1.18) |
| 55–59 | 3127/3164 | 2423 (77%) | 446/341 | 9.9/7.2 | 1.37 (1.19–1.58) | 23/49 | 0.47/1.00 | 0.47 (0.29–0.78) | 910/930 | 18.7/18.9 | 0.99 (0.90–1.08) |
| 60–64 | 2776/2787 | 2065 (74%) | 408/307 | 11.2/8.1 | 1.39 (1.20–1.61) | 47/55 | 1.17/1.37 | 0.85 (0.58–1.26) | 1183/1224 | 29.4/30.5 | 0.96 (0.89–1.04) |
|
| |||||||||||
| Cohabitation | |||||||||||
| Yes | 6230/6208 | 5151 (83%) | 949/676 | 10.2/7.0 | 1.46 (1.32–1.61) | 52/78 | 0.52/0.78 | 0.67 (0.47–0.95) | 1468/1409 | 14.6/14.0 | 1.04 (0.97–1.12) |
| No | 3699/3722 | 2484 (67%) | 446/285 | 8.8/5.4 | 1.62 (1.40–1.88) | 27/44 | 0.50/0.81 | 0.61 (0.38–0.99) | 1373/1445 | 25.2/26.5 | 0.95 (0.88–1.02) |
| Missing | 21/19 | 12 (57%) | 1/1 | 3.2/3.6 | – | – | – | – | 3/3 | 9.4/10.4 | – |
|
| |||||||||||
| Education | |||||||||||
| Low | 3648/3608 | 2675 (73%) | 473/317 | 9.2/6.1 | 1.51 (1.31–1.74) | 27/54 | 0.49/1.00 | 0.49 (0.31–0.78) | 1257/1329 | 22.8/24.7 | 0.92 (0.86–1.00) |
| Medium/high | 6059/6075 | 4862 (80%) | 909/635 | 10.2/6.8 | 1.50 (1.36–1.66) | 51/68 | 0.53/0.69 | 0.76 (0.53–1.09) | 1498/1436 | 15.4/14.7 | 1.05 (0.98–1.13) |
| Missing | 243/266 | 110 (45%) | 14/10 | 4.4/2.9 | – | 1/– | 0.31/– | – | 89/92 | 27.4/26.7 | – |
|
| |||||||||||
| Country of origin | |||||||||||
| Nordic countries | 8444/8392 | 6551 (78%) | 1250/878 | 10.3/7.0 | 1.47 (1.35–1.60) | 73/111 | 0.55/0.84 | 0.65 (0.49–0.88) | 2454/2449 | 18.6/18.6 | 1.00 (0.94–1.05) |
| Europe (not Nordic countries) | 1177/1174 | 865 (74%) | 117/65 | 6.8/3.7 | 1.84 (1.36–2.49) | 5/7 | 0.27/0.39 | 0.70 (0.22–2.22) | 318/318 | 17.4/17.7 | 0.99 (0.84–1.15) |
| Non-European countries | 328/379 | 231 (70%) | 29/19 | 6.3/3.4 | 1.82 (1.02–3.24) | 1/4 | 0.21/0.71 | 0.29 (0.03–2.60) | 72/90 | 14.8/15.9 | 0.93 (0.67–1.28) |
| Missing | 1/4 | – | – | – | – | – | – | – | – | – | – |
|
| |||||||||||
| Comorbidity index | |||||||||||
| 0 | 8879/8862 | 6915 (78%) | 1300/892 | 9.9/6.5 | 1.51 (1.38–1.64) | 73/111 | 0.51/0.78 | 0.65 (0.49–0.88) | 2225/2242 | 15.6/15.8 | 0.99 (0.93–1.05) |
| ≥ 1 | 1071/1087 | 732 (68%) | 96/70 | 7.8/5.3 | 1.47 (1.07–2.04) | 6/11 | 0.46/0.80 | 0.57 (0.21–1.53) | 619/615 | 47.0/44.9 | 1.05 (0.94–1.17) |
CI = confidence interval; Ctrl = control group; PC = prostate cancer; Scr = screening group. Rate ratios were calculated with Poisson regression.
Prostate cancer mortality rate also varied with baseline factors (Table 3a). A high PC mortality was observed in the control group among men who were older at study entry and men with a low level of education (Table 3a). The RR for PC mortality did not reach significance in all subgroups, but ranged from 0.47 to 0.67 across the subgroups that had a significant reduction (Figure 3b). Overall mortality also varied with baseline factors but was not statistically different between the screening and control groups in any of the subgroups (Table 3a). Differences in screening outcomes across subgroups were more pronounced in analyses restricted to attendees versus an adjusted control group (Table 3b). Large and statistically significant relative reductions in PC mortality were observed for men aged 50–54 (RR 0.31; 95% CI 0.10–0.94), men aged 55–59 (RR 0.37; 95% CI 0.20–0.67), and men with a low level of education (RR 0.33; 95% CI 0.18–0.61) (Table 3b).
Table 3b. Prostate cancer (PC) incidence and PC-specific mortality among attendees in sociodemographic subgroups.
| PC incidence rate per 1000 person- years |
Rate ratio PC incidence (95% CI) |
PC-specific mortality rate per 1000 person-years |
Rate ratio PC- specific mortality (95% CI) |
Overall mortality rate per 1000 person-years |
Rate ratio overall mortality (95% CI) |
|
|---|---|---|---|---|---|---|
| Overall | ||||||
| Attendees | 11.1 | 1.59 (1.46–1.73) | 0.41 | 0.54 (0.39–0.76) | 14.0 | 0.99 (0.93–1.06) |
| Adjusted control group | 7.0 | 0.75 | 14.2 | |||
|
| ||||||
| Subgroup analyses | Att/Adj ctrl | Att/Adj ctrl | Att/Adj ctrl | Att/Adj ctrl | Att/Adj ctrl | Att/Adj ctrl |
|
| ||||||
| Age | ||||||
| 50–54 | 10.1/5.4 | 1.87 (1.62–2.17) | 0.07/0.24 | 0.31 (0.10–0.94) | 8.1/7.3 | 1.15 (0.97–1.28) |
| 55–59 | 11.3/7.9 | 1.43 (1.23–1.66) | 0.38/1.02 | 0.37 (0.20–0.67) | 14.4/14.7 | 0.98 (0.87–1.09) |
| 60–64 | 12.7/8.6 | 1.47 (1.25–1.73) | 1.00/1.26 | 0.80 (0.50–1.27) | 23.6/24.9 | 0.94 (0.86–1.04) |
|
| ||||||
| Cohabitation | ||||||
| Yes | 11.2/7.4 | 1.52 (1.37–1.68) | 0.43/0.73 | 0.59 (0.39–0.88) | 12.1/11.4 | 1.06 (0.97–1.16) |
| No | 10.9/6.1 | 1.78 (1.52–2.10) | 0.35/0.79 | 0.45 (0.24–0.85) | 18.3/20.1 | 0.91 (0.82–1.01) |
| Missing | 5.2/6.2 | 0.83 (0.05–13.3) | – | – | 9.9/11.8 | 0.84 (0.12–5.97) |
|
| ||||||
| Education | ||||||
| Low | 10.7/6.7 | 1.61 (1.38–1.88) | 0.33/0.98 | 0.33 (0.18–0.61) | 16.8/19.0 | 0.88 (0.80–0.98) |
| Medium/high | 11.4/7.2 | 1.58 (1.42–1.75) | 0.46/0.66 | 0.69 (0.46–1.05) | 12.4/11.5 | 1.08 (0.99–1.18) |
| Missing | 7.2/4.2 | 1.72 (0.70–4.20) | – | – | 21.3/20.7 | 1.03 (0.66–1.61) |
|
| ||||||
| Country of origin | ||||||
| Nordic countries | 11.6/7.5 | 1.55 (1.41–1.70) | 0.43/0.78 | 0.54 (0.38–0.78) | 14.1/14.2 | 1.00 (0.93–1.07) |
| Europe (not Nordic countries) | 8.2/4.1 | 1.98 (1.44–2.74) | 0.28/0.43 | 0.65 (0.18–2.32) | 13.6/13.9 | 0.98 (0.80–1.20) |
| Non–European countries | 7.4/3.6 | 2.03 (1.09–3.79) | 0.27/0.89 | 0.30 (0.03–2.71) | 12.3/14.2 | 0.87 (0.60–1.27) |
|
| ||||||
| Comorbidity index | ||||||
| 0 | 11.2/7.1 | 1.59 (1.45–1.74) | 0.41/0.74 | 0.55 (0.39–0.79) | 12.3/12.5 | 0.98 (0.91–1.06) |
| ≥ 1 | 9.3/6.0 | 1.55 (1.12–2.14) | 0.39/0.83 | 0.47 (0.14–1.52) | 33.5/31.5 | 1.06 (0.92–1.23) |
PC, prostate cancer. Analyses restricted to attendees (Att) versus an adjusted control group (Adj ctrl). The control group was adjusted by subtracting the incidence and mortality rate of prostate cancer among non-attendees in the screening group from the control group. Rate ratios were calculated with Poisson regression.
Discussion
This analysis consolidates our previous finding that an organized PSA screening program effectively reduces PC mortality [1]. In addition, introducing an organized screening program seems to be able to reduce sociodemographic inequalities in PC mortality. Compared to the results from the 14-year follow-up [1], absolute risk reduction has increased (0.52 versus 0.40), which is also evident from the improvement in NNI and NND (231 versus 293 and 10 versus 12). However, relative risk reduction has decreased (RR 0.65 versus 0.56).
The Göteborg screening trial is truly population-based, with high attendance (77%), intense screening design (biennial and PSA threshold of 2.5 ng/ml), and a long duration of screening (up to 20 years). This 18-yr report constitutes the longest follow-up to date for a PSA screening trial. Data on sociodemographic variables and comorbidity were retrieved from official registers in Sweden and not from self-reported data, which should minimize the risk of bias. However, we lack data on the level of contamination by opportunistic PSA testing in the control group. The rate of PSA testing in Sweden before this trial began in 1995 was estimated at only 3% (estimated by a questionnaire sent out to a sample of the study population) but opportunistic PSA screening in Sweden has increased substantially since [9]. Another limitation is that the study population is very homogenous ethnically, with only 15% of subjects born outside the Nordic countries.
Our finding that the absolute risk reduction increased while relative risk reduction decreased is consistent with the idea that the beneficial effect of screening would start to decline in men who were the oldest at the start of the study and stopped screening 10–12 yr ago; a previous report from this trial suggested that the protective effect of screening lasts approximately 9 years after screening ends [20]. A complementary explanation is increased opportunistic screening during the last decades leading to more contamination in the control group. This opportunistic screening likely requires many years of follow-up before any potential reduction in PC mortality can be observed. Another report from the Göteborg trial showed that it was after 14 years that a small, but non-significant, reduction in PC mortality was first observed in the control group as a result of the last decades of opportunistic screening [21].
The Göteborg screening trial, the Swedish arm of ERSPC, is the ERSPC center reporting the largest reduction in PC mortality [1,22,23]. The study arm in Finland, despite being the largest component of ERSPC, found a non-significant 15% relative reduction in PC mortality at 12 yr [22], while the Rotterdam center reported a 32% relative reduction for men aged 55–69 years at 13-yr follow-up [23]. Possible explanations for the different screening effects include differences in randomization, screening intensity, and duration as well as differences in screening compliance, and contamination. For example, the Rotterdam center performed randomization after written informed consent [23] which was not necessary in Finland or Sweden where the control group was not contacted [1,22]. The contrasting findings of the Göteborg screening trial and the ERSPC in comparison to the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial have been intensely debated [24]. However, the ERSPC and Göteborg trials introduced organized screening to a previously unscreened population while the PLCO trial introduced organized PSA screening to a population already undergoing opportunistic PSA screening [25]. Other differences between the trials include PSA threshold, screening interval and the execution of prostate biopsies which were carried out in the screening centers in the ERSPC but were left to the regional health care providers in the PLCO [24].
Men with a low SES, as indicated by a low level of education, especially appeared to benefit from organized screening. Though the Swedish healthcare system is free and tax-funded, in the control group less-educated men had much higher PC mortality than more highly-educated men (1.00 per1000 person-years versus 0.69 per 1000 person-years). However, in our screening group, PC mortality was similar in men with low versus medium/high education, suggesting that organized screening could possibly diminish differences in PC mortality across SES groups. These results are in line with those by Kilpeläinen et al. who investigated the association between SES and screening outcomes in the Finnish arm of the ERSPC [11]. Although they did not observe a significant reduction in PC mortality in any SES subgroup, the largest relative protective effect was seen in men with low income and short education. In addition, they found that screening diluted the risk difference for advanced PC between income groups suggesting that introducing organized screening could possibly lead to increased equality. The authors concluded that special attention should be directed toward recruiting men with low SES to participate in population-based PSA-screening. Possible explanations for a greater effect in men with low SES include that they are less likely to attend opportunistic PSA screening, receive less rigorous follow-up after an elevated PSA, and less aggressive treatment in an opportunistic setting; these theories are supported by several previous studies [26,27]. For example, a study by Berglund et al. showed that for Swedish men with high-risk prostate cancer, white-collar workers had a higher likelihood of having a bone scan, a higher likelihood of intention to treat, and a lower overall mortality and prostate cancer-specific mortality, compared to blue-collar workers [26]. Rapiti and colleagues found similar results for Swiss men: those with low SES had higher risk of dying from their PC and the increased mortality was largely attributed to diagnostic delay, poor diagnostic work-up and less invasive treatment [27].
Several previous studies reported sociodemographic variables such as age, SES, and ethnicity being associated with the level of PSA testing [11,28-30]. In the present analysis, we found similar associations between sociodemographic variables and organized screening participation rate. There were also indications in the control group of an association between sociodemographics and contamination by opportunistic screening: cohabiting men or those with a medium/high level of education had higher incidence, probably indicating higher diagnostic activity due to opportunistic screening.
Men who were 55–59 years of age at the start of screening experienced a large reduction in PC mortality (RR 0.47, 95% CI, 0.29 to 0.78). This large mortality reduction was observed despite the fact that the RR for PC incidence was relatively low (RR 1.37, 95% CI, 1.19 to 1.58). For the youngest group of men (50–54 years at the start of screening), an organized screening program led to a relatively large increase in PC incidence (RR 1.77, 95% CI, 1.54 to 2.03) and a non-significant reduction in PC mortality (RR 0.50, 95% CI, 0.22 to 1.11). The reason for not observing a statistically significant effect on PC mortality for men aged 50–54 is probably a “power” problem with too few deaths in this group as they were 68–72 years old at the end of follow-up. In a separate report, when we compared men aged 50–54 randomized to screening in Göteborg, to unscreened men in a cohort from Malmö, we noted a substantial decrease in PC mortality at 17 years (RR 0.29, 95% CI, 0.11 to 0.67) [31]. When the current analysis was restricted to attendees among men 50–54, a large and statistically significant reduction in PC mortality was observed (RR 0.31, 95% CI, 0.10 to 0.94), an effect also seen among men aged 55–59 (RR 0.37, 95% CI, 0.20 to 0.67). This is consistent with the hypothesis that younger age at screening entry allows for reaping the benefits of detection at a curable stage. Interestingly, the oldest age group only had a small, non-significant, PC mortality reduction (all men: 0.85, 95% CI, 0.58 to 1.26, attendees: 0.80, 95% CI, 0.50 to 1.27). This could be due to a high rate of incurable disease at diagnosis or a shorter duration of screening. The duration of screening appears to be important, and the two centers in ERSPC with largest mortality reduction, Göteborg and Rotterdam, have durations of screening of up to 20 years, in contrast to Finland, which only has 3 rounds of screening, spanning 8 years [1,22,23]. Healthy men without comorbidities had a significant reduction in PC mortality (0.65, 95% CI, 0.49 to 0.88) but no such effect could be observed in men with comorbidities (0.57 95% CI, 0.21 to 1.53). One possible explanation could be that men with comorbidities are also likely to be older and older men did not have any significant effect on PC mortality due to the reasons discussed above.
This analysis confirms our previous findings that population-based, systematic PSA screening increases PC incidence and reduces PC mortality. At 18-yr follow-up, there was increased absolute reduction in risk of PC death for the screening group compared to the control group. Although it was difficult to demonstrate significant differences between sociodemographic subgroups the results suggest that the effect of screening varies: less-educated men in particular appeared to benefit from organized PC screening. Our findings suggest that implementing an organized screening program for PC will increase PC incidence while decreasing PC mortality, and may perhaps also diminish socioeconomic inequalities.
Acknowledgments
We thank the cause of death committee (Bo Johan Norlén, Silas Petterson, Eberhard Varenhorst, and Per Folmerz); data base manager Helén Ahlgren, and study nurse Maria Nyberg. We also thank Amy Plofker at MSKCC for polishing the manuscript.
Funding details: This work was supported by the Swedish Cancer Society [contract numbers 14 0694, 11 0178 and 14 0722], Swedish Research Council (VR-MH 2016-02974), Sahlgrenska University Hospital, SC is funded by a postdoctoral research grant from AFA insurance, HL is supported in part by Oxford Biomedical Research Centre Program in UK, and the work of SC and HL is supported in part by a Cancer Center Support Grant from the National Institutes of Health/National Cancer Institute (NIH/NCI) made to Memorial Sloan Kettering Cancer Center [P30 CA008748], the MSKCC SPORE in Prostate Cancer (P50CA092629), and the Sidney Kimmel Center for Prostate and Urologic Cancers, David H. Koch through the Prostate Cancer Foundation.
Footnotes
Disclosure of interests: HL holds patents for free PSA, human kallikrein-related peptidase 2 and intact PSA assays. HL is named on a patent for a statistical method to detect PC that is commercialized by OPKO Health. HL owns stock in OPKO and receives royalties from any sales of the test. HL has served on an advisory panel for Roche Diagnostics during 2014, and an immediate family member of HL is an employee at Ferring Pharmaceuticals. There are no other relationships or activities that have influenced the submitted work. The funders of the study had no role in the design of the study, the collection, analysis and interpretation of data, the writing of the report or the decision to submit the article for publication. The researchers of this study are independent from the funders.
References
- 1.Hugosson J, Carlsson S, Aus G, et al. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010;11:725–32. doi: 10.1016/S1470-2045(10)70146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schröder FH, Hugosson J, Roobol MJ, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366:981–90. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027–35. doi: 10.1016/S0140-6736(14)60525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Preventive Services Task Force. Draft Recommendation Statement: Prostate Cancer: Screening. [accessed April 24rd, 2017];2017 Apr; https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementDraft/prostate-cancer-screening1.
- 5.Ilic D, Neuberger MM, Djulbegovic M, Dahm P. Screening for prostate cancer. Cochrane Database Syst Rev. 2013;1:CD004720. doi: 10.1002/14651858.CD004720.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter HB, Albertsen C, Barry MJ, et al. American Urological Association. Early Detection of Prostate Cancer: AUA Guidelines. [accessed June 28th, 2017]; https://www.auanet.org/guidelines/early-detection-of-prostate-cancer-(2013-reviewed-and-validity-confirmed-2015.
- 7.Mottet N, Bellmunt J, Briers E, et al. European Association of Urology. Guidelines on Prostate Cancer. [accessed June 28th, 2017]; http://uroweb.org/wp-content/uploads/09-Prostate-Cancer_LR.pdf.
- 8.The Swedish National Board of Health and Welfare. [accessed June 28th, 2017];Nationella Screeningprogram. http://www.socialstyrelsen.se/riktlinjer/nationellascreeningprogram/prostatacancer-screeningmedpsa.
- 9.Nordström T, Aly M, Clements MS, Weibull CE, Adolfsson J, Grönberg H. Prostate-specific antigen (PSA) testing is prevalent and increasing in Stockholm County, Sweden, Despite no recommendations for PSA screening: results from a population-based study, 2003-2011. Eur Urol. 2013;63:419–25. doi: 10.1016/j.eururo.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Jemal A, Fedewa SA, Ma J, et al. Prostate Cancer Incidence and PSA Testing Patterns in Relation to USPSTF Screening Recommendations. JAMA. 2015;314:2054–61. doi: 10.1001/jama.2015.14905. [DOI] [PubMed] [Google Scholar]
- 11.Kilpeläinen TP, Talala K, Raitanen J, et al. Prostate Cancer and Socioeconomic Status in the Finnish Randomized Study of Screening for Prostate Cancer. Am J Epidemiol. 2016 doi: 10.1093/aje/kww084. DOI: https://doi-org.ezproxy.ub.gu.se/10.1093/aje/kww084 [Epub ahead of print] [DOI] [PubMed]
- 12.World Health Organization, International Agency for Research on Cancer (IARC) IARC Handbooks of Cancer Prevention, Volume 10, Cervix Cancer Screening. Lyon: IARC Press; 2005. [Google Scholar]
- 13.World Health Organization, International Agency for Research on Cancer (IARC) IARC Handbooks of Cancer Prevention, Volume 7, Breast Cancer Screening. Lyon: IARC Press; 2002. [Google Scholar]
- 14.Palencia L, Espelt A, Rodriguez-Sanz M, et al. Socio-economic inequalities in breast and cervical cancer screening practices in Europe: influence of the type of screening program. Int J Epidemiol. 2010;39:757–65. doi: 10.1093/ije/dyq003. [DOI] [PubMed] [Google Scholar]
- 15.Spadea T, Bellini S, Kunst A, Stirbu I, Costa G. The impact of interventions to improve attendance in female cancer screening among lower socioeconomic groups: a review. Prev Med. 2010;50:159–64. doi: 10.1016/j.ypmed.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 16.ERSPC website. [accessed September 22nd, 2017]; http://www.esrpc.org/sweden.
- 17.Shavers VL. Measurement of socioeconomic status in health disparities research. J Natl Med Assoc. 2007;99:1013–23. [PMC free article] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Aalen O. Nonparametric inference for a family of counting processes. Ann Stat. 1978;6:701–26. [Google Scholar]
- 20.Grenabo Bergdahl A, Holmberg E, Moss S, Hugosson J. Incidence of prostate cancer after termination of screening in a population-based randomised screening trial. Eur Urol. 2013;64:703–9. doi: 10.1016/j.eururo.2013.05.024. [DOI] [PubMed] [Google Scholar]
- 21.Arnsrud Godtman R, Holmberg E, Lilja H, Stranne J, Hugosson J. Opportunistic Testing Versus Organized Prostate-specific Antigen Screening: Outcome After 18 Years in the Goteborg Randomized Population-based Prostate Cancer Screening Trial. Eur Urol. 2015;68:354–60. doi: 10.1016/j.eururo.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Kilpeläinen TP, Tammela TL, Malila N, et al. Prostate cancer mortality in the Finnish randomized screening trial. J Natl Cancer Inst. 2013;105:719–25. doi: 10.1093/jnci/djt038. [DOI] [PubMed] [Google Scholar]
- 23.Roobol MJ, Kranse R, Bangma CH, et al. Screening for prostate cancer: results of the Rotterdam section of the European randomized study of screening for prostate cancer. Eur Urol. 2013;64:530–9. doi: 10.1016/j.eururo.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 24.Schröder FH, Roobol MJ. ERSPC and PLCO prostate cancer screening studies: what are the differences? Eur Urol. 2010;58:46–52. doi: 10.1016/j.eururo.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 25.Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125–32. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berglund A, Garmo H, Robinson D, et al. Differences according to socioeconomic status in the management and mortality in men with high risk prostate cancer. Eur J Cancer. 2012;48:75–84. doi: 10.1016/j.ejca.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Rapiti E, Fioretta G, Schaffar R, et al. Impact of socioeconomic status on prostate cancer diagnosis, treatment, and prognosis. Cancer. 2009;115:5556–65. doi: 10.1002/cncr.24607. [DOI] [PubMed] [Google Scholar]
- 28.Morgan RM, Steele RJ, Nabi G, McCowan C. Socioeconomic variation and prostate specific antigen testing in the community: a United Kingdom based population study. J Urol. 2013;190:1207–12. doi: 10.1016/j.juro.2013.04.044. [DOI] [PubMed] [Google Scholar]
- 29.Drazer MW, Huo D, Schonberg MA, Razmaria A, Eggener SE. Population-based patterns and predictors of prostate-specific antigen screening among older men in the United States. J Clin Oncol. 2011;29:1736–43. doi: 10.1200/JCO.2010.31.9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guessous I, Cullati S, Fedewa SA, et al. Prostate cancer screening in Switzerland: 20-year trends and socioeconomic disparities. Prev Med. 2016;82:83–91. doi: 10.1016/j.ypmed.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Carlsson S, Assel M, Ulmert D, et al. Screening for Prostate Cancer Starting at Age 50-54. A Population-based Cohort Study. Eur Urol. 2017;71:46–52. doi: 10.1016/j.eururo.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]