Abstract
Background
Delivering therapeutic materials, like stem cells or gene vectors, to the myocardium is difficult in the setting of ischemic heart failure because of decreased coronary flow and impaired microvascular perfusion (MP). The aim of this study was to determine if mechanical left ventricular (LV) unloading with the Impella increases coronary flow and MP in a subacute myocardial infarction.
Methods and Results
Anterior transmural myocardial infarction (infarct size, 26.0±3.4%) was induced in Yorkshire pigs. At 2 weeks after myocardial infarction, 6 animals underwent mechanical LV unloading by Impella, whereas 4 animals underwent pharmacological LV unloading using sodium nitroprusside for 2 hours. LV unloading with Impella significantly reduced end‐diastolic volume (−16±11mL, P=0.02) and end‐diastolic pressure (EDP; −32±23 mm Hg, P=0.03), resulting in a significant decrease in LV end‐diastolic wall stress (EDWS) (infarct: 71.6±14.7 to 43.3±10.8 kdynes/cm2 [P=0.02]; remote: 66.6±20.9 to 40.6±13.3 kdynes/cm2 [P=0.02]). Coronary flow increased immediately and remained elevated after 2 hours in Impella‐treated pigs. Compared with the baseline, MP measured by fluorescent microspheres significantly increased within the infarct zone (109±81%, P=0.003), but not in the remote zone. Although sodium nitroprusside effectively reduced LV‐EDWS, 2 (50%) of sodium nitroprusside–treated pigs developed profound systemic hypotension. A significant correlation was observed between the infarct MP and EDWS (r 2=0.43, P=0.03), suggesting an important role of EDWS in regulating MP during LV unloading in the infarcted myocardium.
Conclusions
LV unloading using an Impella decreased EDWS and increased infarct MP without hemodynamic decompensation. Mechanical LV unloading is a novel and efficient approach to increase infarct MP in patients with subacute myocardial infarction.
Keywords: coronary flow, left ventricular unloading, percutaneous left ventricular assist device, perfusion, wall stress
Subject Categories: Catheter-Based Coronary and Valvular Interventions, Heart Failure, Coronary Circulation, Animal Models of Human Disease
Clinical Perspective
What Is New?
We showed that mechanical left ventricular unloading in subacute myocardial infarction using Impella increases infarct microvascular perfusion by reducing the left ventricular end‐diastolic wall stress.
What Are the Clinical Implications?
Impella‐mediated left ventricular unloading in subacute myocardial infarction may be a viable option to mechanically improve postinfarct tissue perfusion and, therefore, enable more efficient delivery of therapeutic materials, like stem cells or gene therapy vectors.
Introduction
Patients who survive myocardial infarction (MI) often develop signs of heart failure (HF) in the subacute MI setting. These signs appear within 2 to 4 weeks after MI and are associated with worse clinical outcomes.1, 2 Current treatment paradigms make use of medical therapies to hemodynamically stabilize these patients, but they are limited in fundamentally improving native heart function. In fact, 25% of patients develop clinical HF within 1 year of the index MI event, and 75% will do so within 5 years.3 Preventing the development of HF in patients after MI poses a substantial challenge, and new therapeutic approaches are needed.
Catheter‐based left ventricular (LV) unloading has become a realistic option for treating patients with MI complicated by cardiogenic shock and progressive HF.4 Impella is among the most frequently used devices in interventional clinics, and emerging data have suggested that LV unloading with an Impella during acute MI may reduce reperfusion injury and final infarct size.5, 6, 7, 8 However, mechanical LV unloading may also have a therapeutic impact beyond the acute MI setting.
In addition to increasing cardiac output and providing hemodynamic stability, Impella actively aspirates blood directly from the LV into the aorta. This decreases LV end‐diastolic wall stress (EDWS) by reducing both the size and the pressure of the LV dramatically.9 Increased wall stress is a pathological feature of HF and is a well‐established stimulus for maladaptive ventricular remodeling after MI, including hypertrophic responses and remodeling of the microvasculature.10 Increased EDWS also directly diminishes coronary flow because of increased mechanical compressive forces placed on the coronaries and microvasculature.11 As a consequence, cardiac delivery of novel therapeutics that rely on efficient coronary access and perfusion, such as stem cells12 or regenerative gene therapies,13 to patients with HF faces difficulties. Temporally unloading the LV and decreasing wall stress may reverse elevated wall stress and improve cardiac perfusion, opening the possibility of a more safe and efficient application of these therapies in the nonacute MI patient population. Mechanical LV unloading is a unique and novel approach that simultaneously decreases ventricular wall stress and maintains sufficient arterial perfusion pressure. This is in contrast to current pharmacological approaches of unloading, like sodium nitroprusside (SNP), that effectively reduce cardiac afterload and wall stress, but only do so at the expense of significant reduction in blood pressure, risking patients to profound hypotension.
We hypothesized that mechanical LV unloading with the Impella will safely decrease LV wall stress and increase coronary flow and microvascular perfusion (MP) within the infarct, peri‐infarct, and remote regions in a porcine model of ischemic HF. We compared the effects of this mechanical ventricular unloading with those of afterload reduction by SNP, a current standard‐of‐care approach to pharmacologically unload the LV. This is the first study to investigate the effects of temporal mechanical LV support on myocardial MP in the nonacute MI setting.
Methods
The data, analytic methods, and study materials will not be made publically available to other researchers for purposes of reproducing the results or replicating the procedure. However, researchers may contact the corresponding author for any questions related to the article.
Animal Procedures
The experimental protocols involving animals complied with the Guide for the Care and Use of Laboratory Animals’ regulations and US regulatory agencies. They were approved by the Icahn School of Medicine at Mount Sinai Institutional Animal Care and Use Committee. A total of 11 Yorkshire pigs (5 male and 6 female) that survived MI were included in the study. All pigs underwent percutaneous MI induction and, 2 weeks later, LV unloading experiments were conducted. Animals were intubated and ventilated with 100% oxygen, while anesthesia was maintained with propofol (8–10 mg/kg per hour) throughout the procedure. Low‐dose inhaled isoflurane (0.5%) was added for the LV unloading experiments to reduce hemodynamic instability associated with fluctuations in anesthesia depth during the long procedures.
Methods for MI induction were previously described in detail,14, 15 with an additional thrombus injection to induce transmural MI. Briefly, after basal cardiac performance evaluation, a bolus of amiodarone (1 mg/kg) was given over 10 minutes, followed by a continuous infusion at the rate of 1 mg/min for the duration of the procedure. Heparin was administered to maintain an activated coagulation time of 250 to 300 seconds. Through the vascular access established in the femoral artery, a 7F hockey‐stick catheter (Cordis, Miami, FL) was advanced to the left coronary artery and a 0.014‐inch guide wire (Abbott, Abbott Park, IL) was advanced into the left anterior descending artery (LAD). An 8‐mm‐long, 4.0‐mm VOYAGER over‐the‐wire balloon (Abbott) was advanced to the proximal LAD. The balloon was then inflated to 4 atm for 90 minutes, followed by a thrombus injection through the balloon lumen (Figure 1). For the thrombus injection, ≈1‐cm3 clot was added to 3 mL of iodine contrast and mixed thoroughly to crush the clot into small pieces to enable injection through the balloon wire lumen. Clot‐contrast solution (1 mL) was injected through the balloon lumen just before balloon deflation. All pigs immediately developed TIMI (Thrombolysis in Myocardial Infarction) 0 flow, and animals were allowed to recover. Intramuscular injections of nitroglycerin and furosemide were administered to prevent development of acute congestive HF.
Figure 1.
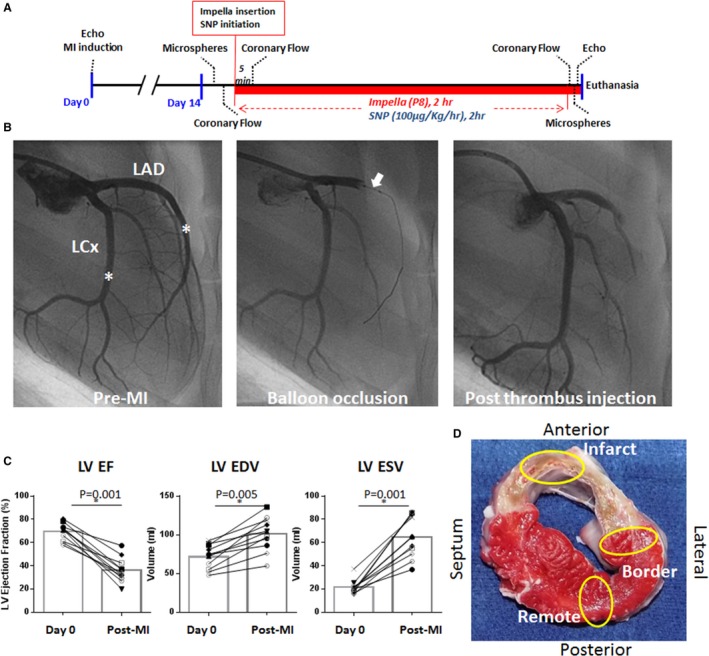
Animal model and methods used in the study. A, Scheme of the study protocol at 2 weeks after myocardial infarction (MI). After the baseline measurements, Impella or sodium nitroprusside (SNP; 100 μg/kg per hour) was initiated to unload the left ventricle (LV) and was continued for 2 hours. Coronary flow measurements with a flow wire were repeated at 5 minutes and at 2 hours after the initiation of the Impella or SNP. Microvascular perfusion was assessed by fluorescent microspheres before and 2 hours after Impella or SNP initiation. B, Coronary angiograms during the induction of MI (Right anterior oblique view, 90°). Blood (3 mL) was drawn from the femoral sheath before heparin injection to allow thrombus formation. After 90 minutes of coronary balloon occlusion at the proximal left anterior descending artery (LAD; white arrow), the thrombus was mixed with contrast agent (1 mL) and injected through the balloon lumen. Injection of a thrombus (1 mL) results in a total occlusion of the LAD immediately. The asterisk indicates the location of the coronary flow wire tip for flow measurements at the 2‐week time point. C, Echocardiographic parameters before and 2 weeks after MI, exhibiting decreased systolic function and LV enlargement, suggesting that these animals have an ischemic heart failure. Closed symbols are those in the Impella group, and open symbols are in the SNP group. Pig died before Impella insertion is shown as *. D, A representative cross‐sectional image of the explanted heart after the LV unloading study. The yellow ovals indicate the area of tissue collection for microsphere analyses at the infarct, border, and remote myocardium. EDV indicates end‐diastolic volume; EF, ejection fraction; ESV, end‐systolic volume; and LCx, left circumflex artery. *P<0.05.
At 2 weeks after MI induction, animals were brought back to the catheterization laboratory. A total of 6 animals (2 male and 4 female pigs) underwent mechanical LV unloading using Impella CP (Impella group), whereas 4 animals (3 male and 1 female pig) underwent pharmacological LV unloading with intravenous SNP infusion (100 μg/kg per hour). This SNP dose is a relatively low dose compared with the regimen used commonly for the clinical management of hypertension or acute HF. Comprehensive transthoracic echocardiographic assessments using 2‐dimensional, 3‐dimensional, and Doppler echocardiography were performed.16 LV volume and functional data were obtained by acquiring 3‐dimensional images with iE‐33 (Phillips Medical Systems, Andover, MA) before and after LV unloading. Analysis of 3‐dimensional images was conducted with Q‐Lab software (Philips Medical Systems). The volume data were analyzed by 2 independent investigators (S.W. and K.I.), and the interobserver variability of LV volumes was similar to our previous report (interclass correlation coefficient, 0.91 [95% confidence interval, 0.81–0.96]).16 Vascular access was established at both the left and right carotid arteries, as well as at the femoral artery and the femoral vein. A Swan‐Ganz catheter was inserted through the femoral venous access site to measure cardiac output by the thermodilution method. A Millar pressure catheter (Millar Instruments Inc, Houston, TX) was advanced to the LV through the right carotid sheath. After all of the baseline hemodynamic measurements were collected, MP was evaluated by injecting fluorescent microspheres. Subsequently, coronary flow was assessed using a coronary flow wire (FloWire; Philips Volcano, Delmar, CA) at the middle coronary arteries, as shown in Figure 1. For the Impella group, the Impella was inserted through the left carotid sheath and initiated with the maximal pump flow (P8). Coronary flow measurements were repeated at 5 minutes and at 2 hours after the initiation of Impella support, whereas MP and echocardiography were repeated at 2 hours after Impella initiation. The SNP group underwent the same protocol, except for receiving continuous IV SNP infusion (100 μg/kg per hour) instead of Impella support. A schematic timeline of the experimental protocol is shown in Figure 1.
LV wall stress was calculated using the formulas:
where EDP (mm Hg) is LV EDP, EDV is LV end‐diastolic volume assessed by 3‐dimensional echocardiography, LVIDd (mm) is LV diastolic internal diameter, and WT (mm) is wall thickness. WT was determined from the 2‐dimensional echocardiography at the infarct and remote myocardium. MI was induced in a total of 13 pigs; however, 2 pigs died immediately after MI induction (within 48 hours) and 1 female pig in the Impella group died during the experiment because of a carotid arterial dissection and subsequent blood loss from technical difficulty in delivering the Impella device. Pre‐Impella data from this pig were included in the microperfusion analysis, but they are not included in Figures 2, 3 through 4, because changes could not be evaluated.
Figure 2.
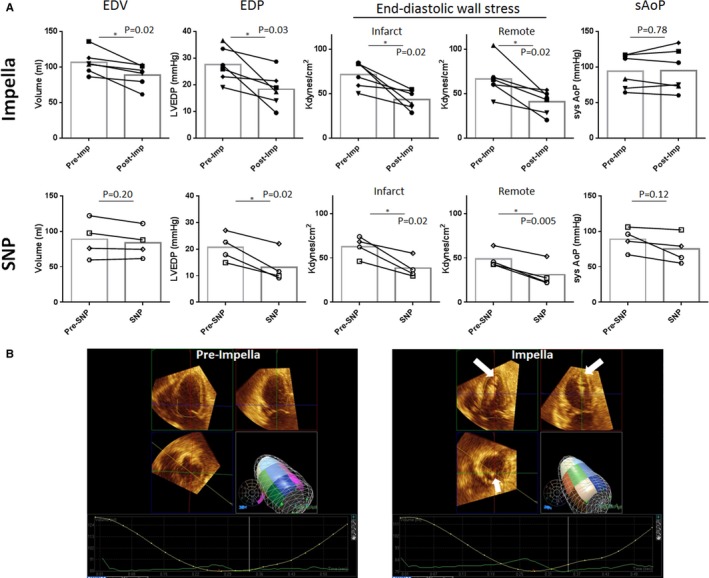
Effects of left ventricular (LV) unloading on end‐diastolic parameters. A, LV unloading with an Impella CP (imp) reduced LV end‐diastolic volume (EDV) and end‐diastolic pressure (EDP), resulting in significantly reduced end‐diastolic wall stress in both the infarct and remote myocardium. Sodium nitroprusside (SNP) also reduced LVEDP, resulting in reduced end‐diastolic wall stress. Systolic aortic pressure (sysAoP) decreased in the SNP group but was maintained in the Impella group without statistical difference (P=0.07 for intergroup comparison). B, Representative 3‐dimensional echocardiographic images before and 2 hours after mechanical LV unloading with Impella. White arrows indicate the Impella catheter. *P<0.05.
Figure 3.
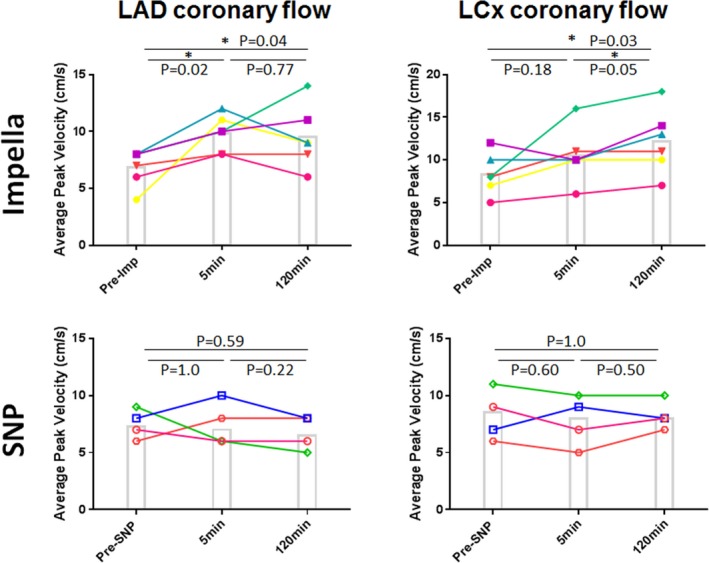
Changes in epicardial coronary flow. Epicardial coronary flow measured by coronary flow wire at the mid–left anterior descending artery (LAD) increased at 5 minutes after Impella (imp)‐mediated left ventricular unloading. Increased flow sustained until the end of the protocol in both LAD and left circumflex artery (LCx; P=0.04 for LAD and P=0.03 for LCx with repeated‐measures ANOVA). In contrast, sodium nitroprusside (SNP) failed to increase epicardial coronary flow in both the LAD and LCx (P=0.59 for LAD and P=1.00 for LCx with repeated‐measures ANOVA). Each line shows individual pigs at respective time points. Post hoc test results are shown in the figure. *P<0.05.
Figure 4.
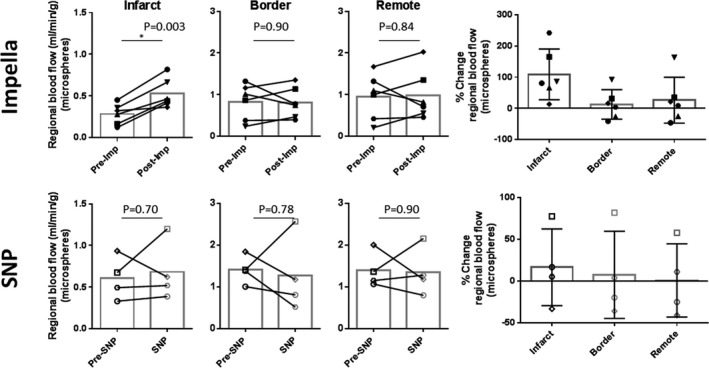
Changes in myocardial perfusion at different regions of the heart 2 hours after left ventricular unloading. Regional myocardial perfusion measured by fluorescent microspheres revealed significantly increased myocardial perfusion at the infarct region in Impella (imp)‐treated animals. Although there was some variability between the animals, no clear changes were found in the myocardial perfusion at the infarct border and the remote myocardium of Impella pigs and all the regions of sodium nitroprusside (SNP)–treated pigs. *P<0.05.
Microsphere Injection
Regional MP was quantified using fluorescently colored microspheres.17 Briefly, 1×107 polystyrene fluorescent microspheres (15 μm; Interactive Medical Technologies, Irvine, CA) were injected into the left atrium through transseptal access via the femoral vein. Reference blood was withdrawn from a femoral artery sheath using a specialized pump for 2 minutes at a rate of 2.9 mL/min. Microspheres with different wave lengths were used for measurements before and 2 hours after LV unloading. The number of fluorescent microspheres trapped in the infarct, infarct border, and remote region (areas of analyses depicted in Figure 1) were sent out for quantification by flow cytometric analysis (Interactive Medical Technologies). Regional blood flow (RBF) was calculated using the following formula:
where R is blood reference withdrawal rate (2.9 mL/min); lt and Ibr are fluorescent counts in the tissue and the blood reference sample, respectively; and Wt is the weight of the tissue sample (g).
Statistical Analysis
Data were expressed as mean±SD. Statistical analysis was performed with SPSS V.22 for Windows (IBM Corp, Armonk, NY) and R. Graphs were created by using GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla, CA). Paired t test was used to compare the differences between the 2 time points of identical animals. A 1‐factor repeated‐measures ANOVA was used to compare the 3 time points of the coronary flow data, followed by post hoc test using Fisher's least significant difference. Effect of Impella and SNP was compared using repeated‐measures ANOVA, and the statistic results are shown as group×time in the Table. Correlation analysis was performed by repeated‐measures correlation using R.18 P<0.05 was considered statistically significant.
Table 1.
LV Parameters Before and 2 Hours After LV Unloading
| Parameters | Impella Group | SNP Group | Group×Time | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 120 Min | P Value | Baseline | 120 Min | P Value | P Value | |
| 3D echocardiography | |||||||
| Ejection fraction, % | 38.8±13.2 | 31.7±10.4 | 0.36 | 33.4±7.3 | 32.0±5.2 | 0.57 | 0.30 |
| End‐diastolic volume, mL | 106±17 | 89±16 | 0.02 | 89±27 | 84±21 | 0.20 | 0.10 |
| End‐systolic volume, mL | 66±18 | 62±18 | 0.67 | 59±19 | 57±15 | 0.48 | 0.99 |
| Stroke volume, mL | 41±13 | 27±7 | 0.09 | 31±12 | 27±8 | 0.25 | 0.09 |
| LV pressure catheter | |||||||
| Maximum pressure, mm Hg | 101±22 | 91±22 | 0.33 | 90±16 | 72±22 | 0.10 | 0.46 |
| End‐diastolic pressure, mm Hg | 27.6±6.5 | 18.3±6.6 | 0.03 | 20.6±5.3 | 13.2±6.0 | 0.02 | 0.66 |
| dP/dt maximum, mm Hg/s | 1326±326 | 1097±384 | 0.10 | 1355±299 | 1087±345 | 0.27 | 0.86 |
| dP/dt minimum, mm Hg/s | −1508±388 | −1077±370 | 0.01 | −1264±445 | −896±417 | 0.14 | 0.75 |
| Stroke work, mm Hg×mL | 2856±1743 | 1935±1322 | 0.34 | 2280±1568 | 2175±1727 | 0.62 | 0.48 |
| Other hemodynamics | |||||||
| Mean aortic pressure, mm Hg | 76±22 | 83±29 | 0.20 | 89±17 | 75±22 | 0.46 | 0.15 |
| Diastolic aortic pressure, mm Hg | 61±18 | 72±27 | 0.10 | 51±18 | 48±21 | 0.65 | 0.14 |
| Total cardiac output, L/min | 3.03±0.54 | 3.49±1.13 | 0.24 | 3.10±1.19 | 3.18±1.11 | 0.87 | 0.51 |
| Impella flow, L/min | 0 | 2.8±0.2 | 0 | 0 | |||
| Heart rate, bpm | 81±17 | 89±6 | 0.32 | 89±21 | 85±13 | 0.75 | 0.84 |
Data are given as mean±SD. P values are from paired t test. The group×time P values are derived from repeated‐measures ANOVA using group (Impella vs SNP) and time point (baseline vs 120 minutes) as variables. 3D indicates 3 dimensional; bpm, beats per minute; dP/dt, rate of left ventricle LV pressure change; LV, left ventricular; and SNP, sodium nitroprusside.
Results
A total of 6 pigs in the Impella group and 4 pigs in the SNP group completed the protocol. At 2 weeks after MI induction, pigs presented with an enlarged LV (EDV: 72±15 to 101±22 mL [P=0.002]; end‐systolic volume: 21±6 to 65±18 mL [P<0.001]) and impaired LV systolic function (LV ejection fraction: 69.4±7.4% to 36.0±10.7% [P<0.001], pre‐MI to 2 weeks after MI, respectively) (Figure 1). At 2 weeks after MI, end‐diastolic WT of the anterior wall (infarct zone) decreased significantly, whereas the remote myocardial WT remained unchanged (anterior: 7.2±0.9 to 6.3±1.0 mm [P=0.03]; remote: 7.2±0.7 to 7.1±0.7 mm [P=0.89], before MI to 2 weeks after MI, respectively). Infarct size measured by digital planimetry15 after heart explantation was 26.0±3.4% of the total LV. These data are consistent with previously published reports using a similar approach to induce ischemic HF.13, 15 At 2 weeks after MI, coronary angiograms revealed TIMI 2 flow in 8 animals and TIMI 3 flow in 2 animals (1 each in Impella and SNP groups) in the LAD. No collaterals from other branches were found in any animal.
LV Unloading Reduces LV EDWS
After the baseline echocardiographic and hemodynamic measurements, pigs with ischemic HF underwent LV unloading using an Impella CP6 or LV afterload reduction by intravenous SNP. Average Impella flow shown on the Impella console was 2.8±0.2 L/min under P8 support. As a result, total CO was higher, as measured by a Swan‐Ganz catheter, at 2 hours after LV unloading (Table). Consistent with direct ventricular unloading, both EDV (−16±11mL, P=0.02) and EDP (−32±23 mm Hg, P=0.03) were significantly decreased in the Impella group (Figure 2 and Table), resulting in a significant reduction in EDWS within both the infarct and the remote myocardium (Figure 2). In the SNP‐treated pigs, there was no significant difference in EDV. However, EDP was decreased significantly. As a result, EDWS was also decreased significantly with the intravenous SNP administration. However, this was accompanied by profound systemic hypotension (systolic blood pressure, <60 mm Hg) in 2 of 4 pigs. Systemic pressure gradually recovered during the 2‐hour period, but only to 60 to 65 mm Hg in these 2 pigs (Figure 2). These data indicate that both mechanical and pharmacological LV unloading decrease LV wall stress globally in ischemic HF associated with recent MI, whereas Impella supports systemic hemodynamics better than SNP in pigs with severe cardiac dysfunction.
Coronary Flow Increased With Impella Support
Increased wall stress is known to impede coronary flow,11 thereby exacerbating myocardial ischemia after MI. Therefore, we measured epicardial coronary flow using a FloWire before unloading and this flow was compared with the flow at 5 minutes and 2 hours after LV unloading. Most of the pigs in the Impella group presented with increased coronary flow velocity at the 5‐minute time point in both LAD (6.84±1.61 to 9.83±1.60 cm/s; P=0.02) and left circumflex artery (8.33±2.42 to 10.5±3.21 cm/s; P=0.18), before Impella and 5 minutes after Impella, respectively, except for 1 pig that showed a mild decrease in the left circumflex artery flow (Figure 3). Epicardial coronary flow velocity remained elevated at the 2‐hour time point in both the LAD (9.50±2.73 cm/s, P=0.04) and the left circumflex artery (12.17±3.76 cm/s, P=0.03) compared with the baseline. The mean and diastolic aortic pressures at 2 hours after Impella initiation were numerically higher, but not different from the baseline (Table). In contrast, pigs treated with SNP did not show significant differences in the epicardial coronary flows between the time points (Figure 3).
Ventricular Unloading Increases Perfusion of the Infarct Zone
To determine if increased coronary flow is associated with better perfusion of the tissue, we injected fluorescent microspheres into the left atrium before and 2 hours after LV unloading to directly measure the MP.17 An average 2‐fold increase in the perfusion of the infarcted area (109±81%, P=0.003) was found after LV unloading with the Impella compared with perfusion before mechanical support. No significant differences in MP before and after support were observed in either the infarct border (13±47%, P=0.90) or remote myocardium (26±74%, P=0.84) after Impella (Figure 4). MP exhibited variable response to pharmacological LV unloading by SNP in all the myocardial segments analyzed, with no statistical differences. Interestingly, MP within the infarct zone before and after LV unloading correlated with EDWS in all animals studied and over all time points (R 2=0.43, P=0.03). However, no clear correlation between EDWS and MP of either the border zone or the remote myocardium was observed (Figure 5). Diastolic aortic pressure did not correlate with infarct MP (R 2=0.02, P=0.68).
Figure 5.
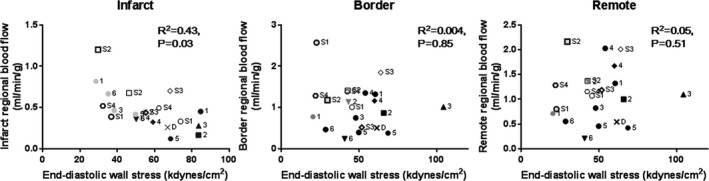
Relationship between end‐diastolic wall stress and myocardial perfusion. Repeated‐measures correlation analysis revealed a significant correlation between regional myocardial perfusion and end‐diastolic wall stress at the infarcted myocardium. No clear correlations were found in the infarct border and the remote myocardium. Closed symbols indicate Impella‐treated animals (black, pre‐Impella; gray, post‐Impella), and open symbols indicate sodium nitroprusside (SNP)–treated animals (thin marks, pre‐SNP; bold marks, post‐SNP). One pig died during the experiment and is shown as x (pre‐Impella data). Numbers on the side represent individual pigs.
Discussion
The main finding of the present study is that LV unloading using an Impella transaortic microaxial pump reduces LV EDWS and increases perfusion to the infarcted region in a porcine model of ischemic HF. Pharmacological LV unloading with SNP was also effective in reducing LV EDWS, at the cost of profound systemic hypotension in some of the pigs. We observed a significant correlation between infarct perfusion and EDWS, but this relationship was not observed within the border zone or the remote myocardium. Our data suggest that MP of the infarct region is at least partly regulated by the EDWS, but not by coronary perfusion pressure (ie, diastolic aortic pressure). In contrast, perfusion of other regions is likely controlled by multiple mechanisms, such as autoregulation of vascular tone within the coronary arteries and veins.19, 20 These data have implications on the development and delivery of stem cell therapies or the delivery of gene therapy vectors targeting infarct zone repair. Gaining differential access to the infarct zone by means of mechanical unloading would allow a more effective means of delivering these therapies to their targeted tissue.
This is the first study to report that coronary flow can be improved by mechanical LV unloading in a subacute MI, where scar formation and remodeling have taken place for 2 weeks. Although the effect of Impella on coronary flow was only tested for 2 hours in this study, we believe this demonstrates that the effect of mechanical LV unloading on coronary flow is not only a short‐term phenomenon that has been already documented,21, 22 but one that can be maintained more long‐term in an unloaded heart. Longer‐duration experiments are needed to prove this. Data on the impact of Impella‐mediated LV unloading on coronary flow are limited. In animal models of acute HF induced by MI or mitral regurgitation, LV unloading was associated with increased epicardial coronary flow.5, 22, 23 However, Remmelink et al21 reported that, in patients undergoing high‐risk percutaneous coronary interventions, there was no change in the resting coronary flow under Impella support in nonstenotic arteries, whereas hyperemic flow measured after adenosine administration was associated with significantly higher values under Impella. Our results are consistent with the former. This discrepancy is likely attributable to differences in myocardial function in the acute HF versus stable coronary disease setting. In acute HF induced by MI, ventricular wall stress is immediately increased because of the inability of the myocardium to effectively compensate for the loss of viable muscle tissue, a process that takes longer periods of time. As ejection fraction immediately decreases, the wall stress is increased, resulting in a compression of the myocardial vasculature. Our data demonstrate that unloading the ventricle using the Impella is an effective approach to relieving wall stress and restoring coronary flow.
Our study demonstrates that there is a significant degree of MP in the infarcted tissue in a subacute MI, albeit to a lower perfusion than remote nonischemic myocardium. Furthermore, we demonstrated that mechanical LV unloading by Impella improves infarct perfusion for the first time in a quantitative manner. We did not observe large differences in the mean and diastolic aortic pressures before and 2 hours after Impella. This suggests that the increased MP is not solely from the increased perfusion pressure derived from Impella support per se, but rather associated with modulation of cardiac condition, such as decrease in EDWS. Consistently, there was no correlation between diastolic aortic pressure and MP. Lack of significant improvement in infarct MP after SNP despite decreased EDWS, however, suggests that there are also other factors involved in infarct perfusion regulation. Identifying the difference between mechanical and pharmacological LV unloading requires further research focusing on this aspect. More important, preferentially increasing tissue perfusion within the infarct zone by mechanical LV unloading may be useful for more effectively delivering therapies, such as stem cells or drugs, through the intracoronary route. It is notable, however, that even with improved MP with mechanical LV unloading, it is still around half of that in the nonischemic areas, leaving opportunities for further improvements.
Our results also indicate that increase in epicardial coronary flow to the noninfarcted regions of the heart does not necessarily lead to improved tissue perfusion. Although we observed a significant increase in coronary flow supplying the remote myocardium, we could not find a significant change in myocardial perfusion within the microvasculature of this same region. Lack of significant change in MP despite increased epicardial coronary flow seems paradoxical. In flow wire measurements, we assumed that the diameter of the vessels remains the same before and after unloading. Because the flow is a function of pressure and resistance, reduction in vessel diameter can result in increased flow velocity. It is likely that there exists autoregulatory mechanisms in the noninfarcted myocardium to maintain sufficient, but not excessive, MP to the tissue. Increased blood oxygenation, reduced cardiac oxygen consumption, decreased microvascular resistance, and reduced neurohormonal activation have been shown to trigger autoregulation at the microvascular level in the normal myocardium.19 Increased epicardial coronary flow only during the hyperemic condition in the aforementioned study by Remmelink et al21 also supports our hypothesis. In contrast, both coronary flow and MP improved in the infarct tissue after Impella, suggesting that these mechanisms may be disrupted in the infarcted myocardium, leaving EDWS as a key mechanism controlling perfusion within the infarct region. More important, these results indicate the importance of evaluating not only the epicardial coronary flow, but also the tissue perfusion.
Clinical Implications and Future Directions
Whether improving infarct perfusion is beneficial to infarct healing and preventing myocardial remodeling remains to be determined in future studies. However, higher perfusion of the infarct has been shown to be associated with better myocardial recovery at the long‐term stage.24 Moreover, meta‐analysis of percutaneous coronary intervention studies targeting totally occluded infarct‐related artery reported that restoring the coronary flow at an average of 10 days after an MI is associated with improved cardiac function and reduced LV remodeling in the long‐term stages.25 These data suggest that increasing the infarct perfusion with mechanical LV unloading may be favorable for treating recent MI. In addition, Impella support can be combined with other newer therapeutic approaches, such as stem cell therapy and gene therapy at the subacute MI phase. More important, these therapies are expected to benefit from increased coronary flow when delivered through the coronary route.26, 27, 28, 29 Moreover, supporting the hemodynamic decompensation for the short‐term, while providing therapies with less immediate effects, is an attractive new approach that takes advantage of this new catheter‐based LV assist device for treating patients with decompensated HF. Future studies should test this combination therapy with temporal LV unloading and stem/gene therapies.
Limitation
Our LV unloading studies were conducted at the 2‐week post‐MI time point. Because infarct healing is a dynamic process that takes place during the entire peri‐MI period, the impact of an Impella on coronary flow and myocardial perfusion at more immediate or later time points needs to be examined in future studies. The subacute MI phase is currently treated with only pharmacological interventions. Our results suggest that LV unloading with Impella can increase infarct perfusion and may be useful to promote faster healing and provide myocardial salvage as an option to intervene mechanically. We also used a transmural infarction model by injecting thrombus to induce more severe HF phenotype as those are the likely target patient population in clinic. Whether our findings apply to nontransmural infarction model, such as ischemia/reperfusion15 or chronic ischemia,17 needs to be addressed in the future studies. The coronary flow wire was removed after the measurements at 5 minutes and reintroduced at 120 minutes after Impella initiation. Although careful attention was paid to place the wire at the same locations, the precise location and the angle of the wire relative to the coronary artery may have been changed and resulted in the fluctuation of the coronary flow data for individual animals. However, averaging the data of all the animals should reduce the impact of these factors, and our data suggest that increased coronary flow was sustained up to 120 minutes. Finally, we included only 4 animals in the SNP group; thus, the study may be underpowered to detect differences in some of the parameters in this group. Nevertheless, severe hypotension that is unlikely to be accepted for clinical practice in half of the pigs with relatively low dose of SNP renders this approach challenging to apply in patients with severe cardiac dysfunction.
Conclusion
LV unloading using an Impella CP reduces LV EDP and EDV, which leads to the reduction of EDWS. MP within the infarct region was significantly increased by mechanical LV unloading, which was associated with the reduction of EDWS. These data suggest that LV support by the Impella CP may offer new and unique opportunities to mechanically intervene infarcted myocardium during the subacute MI stage.
Sources of Funding
This study was partly supported by a research grant from ABIOMED Inc (Danvers, MA) (Ishikawa); National Institutes of Health (NIH) grants R01 HL139963 (Ishikawa) and HL117505, HL119046, HL129814, 128072, HL131404, R01HL135093, and P50 HL112324 (Hajjar); American Heart Association–Scientist Development Grant 17SDG33410873 (Ishikawa); and 2 Transatlantic Fondation Leducq grants. Bikou was supported by the Deutsche Herzstiftung. We would like to acknowledge the Gene Therapy Resource Program of the National Heart, Lung, and Blood Institute, NIH.
Disclosures
Ishikawa received a research grant from ABIOMED Inc (Danvers, MA). The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2018;7:e006462 DOI: 10.1161/JAHA.117.006462.)29514806
References
- 1. Ito H, Yu H, Tomooka T, Masuyama T, Aburaya M, Sakai N, Watada H, Hori M, Higashino Y, Fujii K, Minamino T. Incidence and time course of left ventricular dilation in the early convalescent stage of reperfused anterior wall acute myocardial infarction. Am J Cardiol. 1994;73:539–543. [DOI] [PubMed] [Google Scholar]
- 2. Iwahashi N, Kimura K, Kosuge M, Tsukahara K, Hibi K, Ebina T, Saito M, Umemura S. E/e’ two weeks after onset is a powerful predictor of cardiac death and heart failure in patients with a first‐time ST elevation acute myocardial infarction. J Am Soc Echocardiogr. 2012;25:1290–1298. [DOI] [PubMed] [Google Scholar]
- 3. Ezekowitz JA, Kaul P, Bakal JA, Armstrong PW, Welsh RC, McAlister FA. Declining in‐hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J Am Coll Cardiol. 2009;53:13–20. [DOI] [PubMed] [Google Scholar]
- 4. Burzotta F, Trani C, Doshi SN, Townend J, van Geuns RJ, Hunziker P, Schieffer B, Karatolios K, Moller JE, Ribichini FL, Schafer A, Henriques JP. Impella ventricular support in clinical practice: collaborative viewpoint from a European expert user group. Int J Cardiol. 2015;201:684–691. [DOI] [PubMed] [Google Scholar]
- 5. Yoshitake I, Hata M, Sezai A, Unosawa S, Wakui S, Kimura H, Nakata K, Hata H, Shiono M. The effect of combined treatment with Impella((R)) and landiolol in a swine model of acute myocardial infarction. J Artif Organs. 2012;15:231–239. [DOI] [PubMed] [Google Scholar]
- 6. Kapur NK, Qiao X, Paruchuri V, Morine KJ, Syed W, Dow S, Shah N, Pandian N, Karas RH. Mechanical pre‐conditioning with acute circulatory support before reperfusion limits infarct size in acute myocardial infarction. JACC Heart Fail. 2015;3:873–882. [DOI] [PubMed] [Google Scholar]
- 7. Sun X, Li J, Zhao W, Lu S, Guo C, Lai H, Wang C. Early assistance with left ventricular assist device limits left ventricular remodeling after acute myocardial infarction in a swine model. Artif Organs. 2016;40:243–251. [DOI] [PubMed] [Google Scholar]
- 8. Meyns B, Stolinski J, Leunens V, Verbeken E, Flameng W. Left ventricular support by catheter‐mounted axial flow pump reduces infarct size. J Am Coll Cardiol. 2003;41:1087–1095. [DOI] [PubMed] [Google Scholar]
- 9. Remmelink M, Sjauw KD, Henriques JP, de Winter RJ, Vis MM, Koch KT, Paulus WJ, de Mol BA, Tijssen JG, Piek JJ, Baan J Jr. Effects of mechanical left ventricular unloading by Impella on left ventricular dynamics in high‐risk and primary percutaneous coronary intervention patients. Catheter Cardiovasc Interv. 2010;75:187–194. [DOI] [PubMed] [Google Scholar]
- 10. Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. [DOI] [PubMed] [Google Scholar]
- 11. Mann DL. Mechanisms and models in heart failure: a combinatorial approach. Circulation. 1999;100:999–1008. [DOI] [PubMed] [Google Scholar]
- 12. Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. Ishikawa K, Fish K, Aguero J, Yaniz‐Galende E, Jeong D, Kho C, Tilemann L, Fish L, Liang L, Eltoukhy AA, Anderson DG, Zsebo K, Costa KD, Hajjar RJ. Stem cell factor gene transfer improves cardiac function after myocardial infarction in swine. Circ Heart Fail. 2015;8:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ishikawa K, Ladage D, Tilemann L, Fish K, Kawase Y, Hajjar RJ. Gene transfer for ischemic heart failure in a preclinical model. J Vis Exp. 2011:2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ishikawa K, Aguero J, Tilemann L, Ladage D, Hammoudi N, Kawase Y, Santos‐Gallego CG, Fish K, Levine RA, Hajjar RJ. Characterizing preclinical models of ischemic heart failure: differences between LAD and LCX infarctions. Am J Physiol Heart Circ Physiol. 2014;307:H1478–H1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shimada YJ, Ishikawa K, Kawase Y, Ladage D, Tilemann L, Shiota T, Hajjar RJ. Comparison of left ventricular stroke volume assessment by two‐ and three‐dimensional echocardiography in a swine model of acute myocardial infarction validated by thermodilution method. Echocardiography. 2012;29:1091–1095. [DOI] [PubMed] [Google Scholar]
- 17. Ishikawa K, Ladage D, Takewa Y, Yaniz E, Chen J, Tilemann L, Sakata S, Badimon JJ, Hajjar RJ, Kawase Y. Development of a preclinical model of ischemic cardiomyopathy in swine. Am J Physiol Heart Circ Physiol. 2011;301:H530–H537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bakdash JZ, Marusich LR. Repeated measures correlation. Front Psychol. 2017;8:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duncker DJ, Koller A, Merkus D, Canty JM Jr. Regulation of coronary blood flow in health and ischemic heart disease. Prog Cardiovasc Dis. 2015;57:409–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van de Hoef TP, Nolte F, Rolandi MC, Piek JJ, van den Wijngaard JP, Spaan JA, Siebes M. Coronary pressure‐flow relations as basis for the understanding of coronary physiology. J Mol Cell Cardiol. 2012;52:786–793. [DOI] [PubMed] [Google Scholar]
- 21. Remmelink M, Sjauw KD, Henriques JP, de Winter RJ, Koch KT, van der Schaaf RJ, Vis MM, Tijssen JG, Piek JJ, Baan J Jr. Effects of left ventricular unloading by Impella recover LP2.5 on coronary hemodynamics. Catheter Cardiovasc Interv. 2007;70:532–537. [DOI] [PubMed] [Google Scholar]
- 22. Sauren LD, Accord RE, Hamzeh K, de Jong M, van der Nagel T, van der Veen FH, Maessen JG. Combined Impella and intra‐aortic balloon pump support to improve both ventricular unloading and coronary blood flow for myocardial recovery: an experimental study. Artif Organs. 2007;31:839–842. [DOI] [PubMed] [Google Scholar]
- 23. Reesink KD, Dekker AL, Van Ommen V, Soemers C, Geskes GG, van der Veen FH, Maessen JG. Miniature intracardiac assist device provides more effective cardiac unloading and circulatory support during severe left heart failure than intraaortic balloon pumping. Chest. 2004;126:896–902. [DOI] [PubMed] [Google Scholar]
- 24. Steigen TK, Buller CE, Mancini GB, Jorapur V, Cantor WJ, Rankin JM, Thomas B, Webb JG, Kronsberg SS, Atchison DJ, Lamas GA, Hochman JS, Dzavik V. Myocardial perfusion grade after late infarct artery recanalization is associated with global and regional left ventricular function at one year: analysis from the Total Occlusion Study of Canada‐2. Circ Cardiovasc Interv. 2010;3:549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Appleton DL, Abbate A, Biondi‐Zoccai GG. Late percutaneous coronary intervention for the totally occluded infarct‐related artery: a meta‐analysis of the effects on cardiac function and remodeling. Catheter Cardiovasc Interv. 2008;71:772–781. [DOI] [PubMed] [Google Scholar]
- 26. Takigawa M, Yamada T, Yoshida Y, Ishikawa K, Aoyama Y, Yamamoto T, Inoue N, Tatematsu Y, Nanasato M, Kato K, Tsuboi N, Hirayama H. The incidence and clinical significance of non‐isolation of the pulmonary vein carina after encircling ipsilateral pulmonary veins isolation for paroxysmal atrial fibrillation: a pitfall of the double‐Lasso technique. Europace. 2013;15:33–40. [DOI] [PubMed] [Google Scholar]
- 27. Llano R, Epstein S, Zhou R, Zhang H, Hamamdzic D, Keane MG, Freyman T, Wilensky RL. Intracoronary delivery of mesenchymal stem cells at high flow rates after myocardial infarction improves distal coronary blood flow and decreases mortality in pigs. Catheter Cardiovasc Interv. 2009;73:251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Emani SM, Shah AS, Bowman MK, Emani S, Wilson K, Glower DD, Koch WJ. Catheter‐based intracoronary myocardial adenoviral gene delivery: importance of intraluminal seal and infusion flow rate. Mol Ther. 2003;8:306–313. [DOI] [PubMed] [Google Scholar]
- 29. Logeart D, Hatem SN, Heimburger M, Le Roux A, Michel JB, Mercadier JJ. How to optimize in vivo gene transfer to cardiac myocytes: mechanical or pharmacological procedures? Hum Gene Ther. 2001;12:1601–1610. [DOI] [PubMed] [Google Scholar]


