Abstract
Background
Previous studies have reported that atrial fibrillation (AF) is associated with cognitive decline and dementia. These studies, however, had limited follow‐up, were based mostly on white and highly selected populations, and did not account for attrition. We evaluated the association of incident AF with 20‐year change in cognitive performance (accounting for attrition) and incident dementia in the ARIC (Atherosclerosis Risk in Communities) Study.
Methods and Results
We analyzed data from 12 515 participants (mean age, 56.9 [SD, 5.7] years in 1990–1992; 56% women and 24% black) from 1990 to 1992 through 2011 to 2013. Incident AF was ascertained from study ECGs and hospital discharge codes. Cognitive tests were performed in 1990 to 1992, 1996 to 1998, and 2011 to 2013. Incident dementia was clinician adjudicated. We used generalized estimating equations and Cox proportional hazards models to assess the association of time‐dependent AF with change in Z scores of cognitive tests and incident dementia, respectively. During 20 years, 2106 participants developed AF and 1157 participants developed dementia. After accounting for cardiovascular risk factors, including ischemic stroke, the average decline over 20 years in global cognitive Z score was 0.115 (95% confidence interval, 0.014–0.215) greater in participants with AF than in those without AF. Further adjustment for attrition by multiple imputation by chained equations strengthened the association. In addition, incident AF was associated with an increased risk of dementia (hazard ratio, 1.23; 95% confidence interval, 1.04–1.45), after adjusting for cardiovascular risk factors, including ischemic stroke.
Conclusions
AF is associated with greater cognitive decline and increased risk of dementia, independent of ischemic stroke. Because cognitive decline is a precursor to dementia, our findings prompt further investigation to identify specific treatments for AF that will delay the trajectory of cognitive decline and, thus, prevent dementia in patients with AF.
Keywords: atrial fibrillation, cognition, cohort study, dementia, epidemiology
Subject Categories: Atrial Fibrillation, Epidemiology
Clinical Perspective
What Is New?
In contrast to previous studies that had limited follow‐up, were based mostly on white and highly selected populations, and did not account for attrition, this study evaluated the association of incident atrial fibrillation with both 20‐year change in cognitive performance (accounting for attrition) and incident dementia in a community‐based cohort, including whites and blacks.
What Are the Clinical Implications?
Given that cognitive decline is a precursor to dementia, our findings motivate further research to identify strategies that will delay the trajectory of cognitive decline and, thus, prevent dementia in patients with atrial fibrillation.
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia, and its prevalence is increasing over time.1, 2 AF is associated with an increased risk of stroke,3 heart failure,4 and death.5, 6 Data are emerging to indicate that AF is associated with another growing public health problem (namely, cognitive decline and dementia); the evidence, however, is conflicting.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Methodologic differences and limitations that may explain the inconsistent evidence include small sample sizes, cross‐sectional designs, short follow‐up, lack of rigorous adjustment for potential confounding variables, and highly selected populations (eg, primarily white populations, hospitalized patients, or elderly populations, where associations tend to be weaker). More important, existing studies have not accounted for attrition bias: participants who are ill or have cognitive impairment are less likely to return for study measurements, hence introducing bias in epidemiological associations. Furthermore, existing studies have only examined AF in relation to either cognitive decline or dementia, but not both. Because cognitive decline is a precursor to dementia, demonstrating AF as a risk factor for cognitive decline and dementia in the same cohort will emphasize AF as a potential target for early intervention to prevent dementia.
We had previously shown in the ARIC (Atherosclerosis Risk in Communities) Study that incident AF was associated with greater cognitive decline after a median follow‐up of 10.6 years between 1993 to 1995 and 2004 to 2006.7 In 2011 to 2013, ARIC Study participants underwent a comprehensive cognitive assessment in the ARIC‐NCS (ARIC Neurocognitive Study), allowing us to extend our investigation on this subject over a 20‐year period. Incident dementia was also adjudicated as part of the ARIC‐NCS. Thus, we are uniquely positioned to evaluate the association of incident AF with cognitive change and dementia onset among black and white adults in a community‐based cohort over 20 years. Given the burgeoning public health problem of dementia in elderly people, identifying midlife risk factors for cognitive decline and dementia in older age is an important priority; a study with follow‐up over 20 years can address this priority. In this study, we used methods to account for attrition from the cohort during follow‐up, which is important in quantifying the long‐term relationship of AF to cognitive decline and dementia.
Methods
Study Population
The study data will not be made available to other researchers for purposes of reproducing the results or replicating the procedure because of human subjects’ restrictions. However, interested investigators can request overall access to the ARIC Study data by contacting the ARIC Study Coordinating Center at the University of North Carolina–Chapel Hill.19
The ARIC Study is a predominantly biracial community‐based, prospective, cohort study from 4 communities in Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis suburbs, Minnesota; and Washington County, Maryland; the study consisted of 15 792 men and women, aged 45 to 64 years at baseline or visit 1 (1987–1989).20 The ARIC Study field centers in all 4 communities selected participants by probability sampling; the Mississippi field center recruited only black people, the Forsyth County site recruited black and white people, and the racial distribution in the other locations resulted in a small percentage of nonwhite participants. After the visit 1 examination, there were 4 additional examinations: visit 2 (1990–1992), visit 3 (1993–1995), visit 4 (1996–1998), and visit 5 (2011–2013). Cognitive function was evaluated at visits 2, 4, and 5. Detailed information about the ARIC Study can be found elsewhere.20 The study was approved by each participating institution's institutional review board, and all participants provided written informed consent.
Baseline for the present analysis was visit 2, the first visit at which cognitive data were collected. Of the 14 348 participants who attended visit 2, we excluded those not identified as black or white and the small number of black people in Minneapolis and Washington County (n=103), those with prevalent AF (n=110), those with prevalent dementia or with race‐sex specific lowest fifth percentile cognitive test scores (n=679), those missing or with uninterpretable ECGs (n=202), those missing cognitive scores (n=203), and those missing covariates of interest (n=536). After exclusions, 12 515 participants remained for analysis. A flow diagram of the study population is provided in the Figure.
Figure 1.
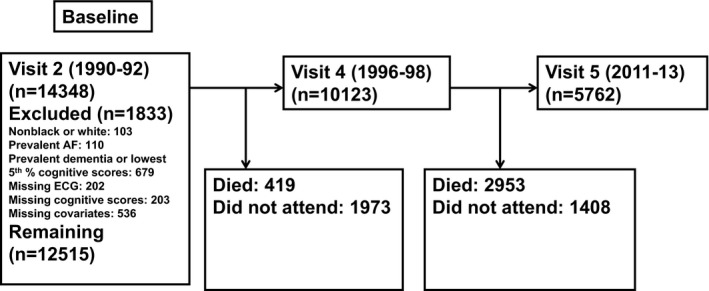
Flow diagram of participants in the study, ARIC‐NCS (Atherosclerosis Risk in Communities Neurocognitive Study). AF indicates atrial fibrillation.
Ascertainment of AF
AF diagnoses were obtained from ECGs at study visits and hospital discharge records through December 31, 2013.21 All ECGs from study visits, automatically coded as AF, were visually rechecked by a cardiologist to confirm the diagnosis.22 Hospitalizations in the ARIC Study are identified by participant or proxy report in annual follow‐up telephone calls and by surveillance of local hospital discharge lists. A trained abstractor obtained and recorded all International Classification of Diseases, Ninth Revision (ICD‐9) hospital discharge diagnoses from each hospitalization. AF was defined as the presence of ICD‐9 code 427.31 or 427.32 in the discharge codes. By physician review of a sample of 125 discharge summaries with ICD codes indicating possible AF, we confirmed the presence of AF in ≈90%.21
Assessment of Cognitive Function
We used 3 neuropsychological tests to assess cognitive function: the Delayed Word Recall Test (DWRT), the Digit Symbol Substitution Test (DSST) of the Wechsler Adult Intelligence Scale–Revised, and the Word Fluency Test (WFT). Protocols for the tests were standardized, and trained examiners administered the tests in a fixed order during 1 session in a quiet room.
The DWRT evaluates verbal learning and short‐term memory.23 Participants learn 10 nouns, use them in sentences, and, after 5 minutes, are asked to recall them. The score is the number of nouns recalled (maximum of 10). The DSST evaluates executive function and processing speed.24 Participants use a key to write symbols corresponding to numbers in 90 seconds. The score, ranging from 0 to 93, is the number of correctly written symbols. The WFT evaluates executive function and expressive language.25 Participants generate as many words as possible within 60 seconds, starting with F, A, and S, with 1 trial per letter. The total score is the sum of all correct words generated. Mean test scores in a normative healthy sample of ARIC Study participants have been published elsewhere.26
To facilitate comparison across cognitive tests, Z scores standardized to visit 2 were calculated for each test by subtracting the participant's test score at each visit from the mean score at visit 2 and dividing by the SD of the visit 2 scores. A composite global cognitive Z score was calculated by averaging the Z scores of the 3 tests and was then standardized to visit 2 by using the mean and SD of the global Z scores at visit 2. Thus, a Z score of −1 would describe cognitive performance that is 1 SD below the mean score at visit 2. Composite global scores derived in this manner have been used in analyses of cognitive change in the ARIC Study27, 28 and elsewhere.29
Ascertainment of Dementia
At ARIC Study visit 5 (ARIC‐NCS, 2011–2013), in‐person assessment of dementia was conducted using an algorithm that was based on the formulations of dementia as laid out in the National Institute on Aging–Alzheimer's Association work groups30 and the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition.31 The algorithm used the following scores: Mini‐Mental State Examination, the sum of the 6 individual domain ratings in the Clinical Dementia Rating interview (“Clinical Dementia Rating sum of boxes”), Z scores from the visit 5 neuropsychological test battery, change in scores from the serial 3‐test ARIC Study cognitive assessments, and the Functional Activities Questionnaire. The algorithmic approach generated 37 possible combinations of cognitive‐functional profiles that were reviewed by a committee of clinicians who then rendered diagnoses of dementia or no dementia (cognitively normal or mild cognitive impairment).32
For ARIC Study participants who were alive at the time of ARIC‐NCS but who declined to be seen in person, 3 strategies were used to establish diagnoses of dementia: the telephonic instrument of cognitive status–modified score, an informant interview, and review of ICD‐9 hospital discharge diagnostic codes. The ICD‐9 codes used to define dementia referred to Alzheimer disease (code 331.0), vascular dementia (code 290.4), or any other code that could have been used for dementia of other cause, including senile, presenile, frontotemporal dementias, and dementias secondary to a general medical condition (eg, Parkinson's disease) (codes 290.0, 290.1, 290.2, 290.3, 290.9, 294.1, 294.2, 294.8, 294.9, 331.1, 331.2, 331.8, and 331.9).32
Covariates
All covariates used in the regression models were assessed at visit 2, except race, sex, education, and occupation, which were assessed at visit 1. We evaluated the following covariates as confounders: age, sex, race‐field center, education (less than high school; high school, high school equivalent, or vocational school; or college, graduate, or professional school), occupation (9 categories), cigarette smoking status (current, former, or never), body mass index (kg/m2), use of blood pressure–lowering medication, systolic blood pressure (mm Hg), diastolic blood pressure (mm Hg), diabetes mellitus, history of coronary heart disease, history of ischemic stroke, history of heart failure, and apolipoprotein E ε4 genotype (0, 1, or 2 alleles). Incident ischemic stroke was evaluated as a potential mediator. For the analysis of cognitive change, we also included interaction terms between each of these variables and time to allow for different rates of decline by these covariates.
Body mass index was calculated as the ratio of weight in kilograms/height in meters squared. Hypertension was defined as use of medication to treat high blood pressure, systolic blood pressure ≥140 mm Hg, or diastolic blood pressure ≥90 mm Hg. Diabetes mellitus was defined as a self‐reported physician's diagnosis of diabetes mellitus, use of hypoglycemic medications, nonfasting serum glucose levels ≥200 mg/dL, or fasting serum glucose level ≥126 mg/dL. Heart failure at baseline was defined as the reported use of medications to treat heart failure in the previous 2 weeks or the presence of heart failure according to Gothenburg criteria.33 Prevalent coronary heart disease was defined as physician‐diagnosed coronary heart disease or the presence of a previous myocardial infarction by ECG, plus adjudicated cases between visit 1 and visit 2. Questionnaires during study visits assessed self‐reported race, smoking status, occupation, and educational level.
To identify incident stroke, cohort participants were followed up over time through annual telephone interviews, field center examinations, surveillance of the ARIC Study community hospitals for all cohort members’ hospitalizations, and the review of death certificates, physician questionnaires, coroner/medical examiner reports, and informant interviews. Hospital reports were reviewed for evidence of acute stroke if the discharge diagnosis included a cerebrovascular disease code (ICD‐9 codes 430–438), if a cerebrovascular procedure was mentioned in the summary, or if the computed tomographic or magnetic resonance imaging report showed evidence of cerebrovascular disease. Medical records for potential stroke events were forwarded to a single nurse abstractor at a central ARIC Study office who abstracted each record for number, type, and severity of neurological deficits and supporting angiographic, computed tomographic, magnetic resonance imaging, spinal tap, or autopsy evidence. The ARIC Study adapted National Survey of Stroke criteria for its stroke definition.34 A computerized algorithm and physician reviewer independently confirmed the diagnosis of stroke, with disagreements adjudicated by a second physician reviewer.
Statistical Analysis
We report means with SDs or medians and interquartile ranges for continuous variables and counts with percentages for categorical variables.
We used linear models to estimate the association between AF (modeled as a time‐dependent variable) and cognitive decline and fit them with generalized estimating equations to account for the within‐person correlations of test scores arising from the repeated measures across time. We used unstructured correlation matrices and robust variance estimates. Time since baseline was modeled by using a linear spline with a knot at 6 years (the mean duration between visits 2 and 4). The spline term allowed for a nonlinear association between time and cognitive decline, more appropriately fit the study design than a quadratic term, and was supported by diagnostic Lowess smoothers. The primary coefficients of interest were the interactions between AF and the time spline terms, which address the hypothesis of greater decline among participants with AF, versus those without AF, after adjustment for other covariates. In model 1, we adjusted for age, sex, and race‐field center. In model 2, we additionally adjusted for education, occupation, cigarette smoking status, body mass index, use of blood pressure–lowering medication, systolic blood pressure, diastolic blood pressure, prevalent coronary heart disease, prevalent heart failure, and apolipoprotein E ε4 genotype. Finally, to determine whether the association between AF and cognitive decline was independent of stroke, we additionally adjusted for prevalent and incident ischemic stroke in model 3.
To account for attrition attributable to death or dropout, we used multiple imputation by chained equations.35 Missing values were imputed with multiple imputation by chained equations, creating 20 imputed data sets using Stata, version 14.0 (StataCorp). Imputation models included all variables listed in Table 1 and additional information previously described in the ARIC Study.36 Briefly, information about cognitive function for participants who did not attend visits after baseline was available through the telephonic instrument of cognitive status–modified questionnaire, suspect dementia status, the Clinical Dementia Rating scale, and telephone interviews with the participant or his or her proxy.
Table 1.
Baseline Characteristics by AF, ARIC Study, 1990 to 2013
| Characteristics | All (N=12 515) | No Incident AF (n=10 409) | Incident AF (n=2106) |
|---|---|---|---|
| Age, mean (SD), y | 56.9 (5.7) | 56.4 (5.6) | 59.3 (5.4) |
| Male sex | 5534 (44) | 4424 (43) | 1110 (53) |
| Race/field center | |||
| White/Minneapolis, MN | 3437 (27) | 2878 (28) | 559 (27) |
| White/Washington County, MD | 3198 (26) | 2537 (24) | 661 (31) |
| White/Forsyth County, NC | 2876 (23) | 2348 (23) | 528 (25) |
| Black/Forsyth County, NC | 352 (3) | 307 (3) | 45 (2) |
| Black/Jackson, MS | 2652 (21) | 2339 (22) | 313 (15) |
| Visit 5 attendance | |||
| Died before visit | 4136 (33) | 3071 (30) | 1065 (51) |
| Alive but did not attend | 2617 (21) | 2245 (22) | 372 (18) |
| Attended | 5762 (46) | 5093 (49) | 669 (32) |
| Education | |||
| Less than high school degree | 2396 (19) | 1913 (18) | 483 (23) |
| High school, GED, or vocational school | 5297 (42) | 4393 (42) | 904 (43) |
| College, graduate, or professional school | 4822 (39) | 4103 (39) | 719 (34) |
| Occupation | |||
| Managerial and professional specialty | 3040 (24) | 2637 (25) | 403 (19) |
| Technical, sales, and administrative support | 2650 (21) | 2274 (22) | 376 (18) |
| Service | 1231 (10) | 1050 (10) | 181 (9) |
| Farming, forestry, and fishing | 86 (1) | 73 (1) | 13 (1) |
| Precision production, craft, and repair | 958 (8) | 764 (7) | 194 (9) |
| Operators, fabricators, and laborers | 1126 (9) | 943 (9) | 183 (9) |
| Homemakers | 1182 (9) | 973 (9) | 209 (10) |
| Retired | 1853 (15) | 1373 (13) | 480 (23) |
| Missing job code for nonhomemakers or homemakers with job code | 389 (3) | 322 (3) | 67 (3) |
| APOE ε4 alleles | |||
| 0 | 8640 (69) | 7156 (69) | 1484 (70) |
| 1 | 3543 (28) | 2960 (28) | 583 (28) |
| 2 | 332 (3) | 293 (3) | 39 (2) |
| Cigarette smoking status | |||
| Current | 2752 (22) | 2250 (22) | 502 (24) |
| Former | 4753 (38) | 3880 (37) | 873 (41) |
| Never | 5010 (40) | 4279 (41) | 731 (35) |
| Body mass index, mean (SD), kg/m² | 28.0 (5.4) | 27.7 (5.3) | 29.1 (5.9) |
| Systolic blood pressure, mean (SD), mm Hg | 121.4 (18.7) | 120.4 (18.2) | 126.4 (20.3) |
| Diastolic blood pressure, mean (SD), mm Hg | 72.2 (10.2) | 72.1 (10.1) | 72.4 (10.9) |
| Use of hypertensive medication | 4011 (32) | 3064 (29) | 947 (45) |
| Use of oral anticoagulants | 961 (8) | 328 (3) | 633 (30) |
| Diabetes mellitus | 1818 (15) | 1404 (13) | 414 (20) |
| Prevalent coronary heart disease | 673 (5) | 468 (5) | 205 (10) |
| Prevalent heart failure | 546 (4) | 388 (4) | 158 (8) |
| Prevalent stroke | 190 (2) | 142 (1) | 48 (2) |
| Cognitive score, mean (SD) | |||
| Global Z score | 0.08 (0.94) | 0.10 (0.95) | −0.03 (0.90) |
| DWRT, no. of words | 6.7 (1.5) | 6.7 (1.5) | 6.5 (1.5) |
| DWRT, Z score | 0.04 (0.98) | 0.06 (0.98) | −0.06 (0.96) |
| DSST, no. of symbols | 45.3 (13.8) | 45.6 (14.0) | 43.9 (12.8) |
| DSST, Z score | 0.05 (0.97) | 0.07 (0.98) | −0.05 (0.90) |
| WFT, no. of words | 34.4 (11.6) | 34.6 (11.7) | 33.7 (11.2) |
| WFT, Z score | 0.10 (0.93) | 0.11 (0.93) | 0.04 (0.89) |
Data are presented as number (percentage) unless otherwise stated. AF indicates atrial fibrillation; APOE, apolipoprotein E; ARIC, Atherosclerosis Risk in Communities; DSST, Digit Symbol Substitution Test; DWRT, Delayed Word Recall Test; GED, general equivalency diploma; and WFT, Word Fluency Test.
To estimate the association between incident AF and incident dementia, we calculated hazard ratios and 95% confidence intervals using Cox proportional hazards model with AF as a time‐dependent variable to account for person‐years both before and after AF diagnoses. Person‐years at risk for dementia were calculated from the date of baseline until the date of dementia, death, unavailability for follow‐up, or end of follow‐up, whichever occurred first. Model covariates were the same as the ones for evaluation of cognitive change. The proportional hazards assumption was assessed with scaled Schoenfeld residuals for both graphical and numerical tests, time interaction terms, and inspection of log‐negative log survival curves. Modeling assumptions were not violated in any model.
Age‐ (stratified at a median of 57 years), sex‐ and race‐stratified analyses were conducted for both cognitive change and dementia. Statistical analysis was performed using SAS, version 9.2 (SAS Institute Inc, Cary, NC). All P values reported were 2 sided, and the statistical significance threshold was chosen as 5%.
Results
Study Population
The analysis cohort consisted of 12 515 participants (mean age, 56.9 [SD, 5.7] years at visit 2; 56% women and 24% black). Of the 12 515 participants who attended visit 2, 17% did not attend visit 4 or 5. Among the remaining 83% of participants who had at least 1 follow‐up visit (10 123 attended visit 4 and 5762 attended visit 5), the median (interquartile range) follow‐up was 20.2 (17.0–21.3) years (Figure); maximum follow‐up was 24 years. During follow‐up, 2106 participants developed AF and 1157 participants developed dementia. Participants who developed AF were older, were more likely to be men and white, and had an overall higher burden of cardiovascular risk factors than those who did not develop AF. Participants who developed AF were less likely to attend visit 5 than those who did not develop AF (32% versus 49%), which was largely attributable to the cumulative incidence of mortality (51% versus 30%) rather than study withdrawal (18% versus 22%) (Table 1).
AF and Cognitive Decline
Table 2 shows the estimated 20‐year decline by AF status from our linear models for global cognitive, DWRT, DSST, and WFT Z scores. AF was associated with significantly greater decline in the scores of all tests except the DWRT. After accounting for cardiovascular risk factors and condition (Table 2, model 2), the average decline over 20 years in global cognitive Z score was 0.123 (95% confidence interval, 0.027–0.230) greater in participants with AF than in those without AF. After further adjustment for prevalent and incident stroke, the association was slightly attenuated but remained significant: the average decline over 20 years in global cognitive Z score was 0.742 in participants without AF and 0.857 in those with AF (difference, −0.115 [95% confidence interval, −0.215 to −0.014], a 16% greater decline among the latter).
Table 2.
Additional Adjusted 20‐Year Cognitive Change Associated With AF, ARIC Study, 1990 to 2013
| Variable | No AF | Additional Cognitive Change in Z Score (95% CI) for AF | |||||
|---|---|---|---|---|---|---|---|
| Model 1 | P Value | Model 2 | P Value | Model 3 | P Value | ||
| Unweighted | |||||||
| Global Z score | 0 (Reference) | −0.198 (−0.299 to −0.098) | <0.001 | −0.123 (−0.230 to −0.027) | 0.01 | −0.115 (−0.215 to −0.014) | 0.03 |
| DWRT Z score | 0 (Reference) | −0.141 (−0.289 to 0.007) | 0.06 | −0.114 (−0.266 to 0.038) | 0.14 | −0.106 (−0.258 to 0.046) | 0.17 |
| DSST Z score | 0 (Reference) | −0.212 (−0.289 to −0.135) | <0.001 | −0.131 (−0.208 to −0.054) | 0.001 | −0.118 (−0.194 to −0.041) | 0.002 |
| WFT Z score | 0 (Reference) | −0.162 (−0.259 to −0.066) | 0.001 | −0.104 (−0.202 to −0.006) | 0.04 | −0.093 (−0.191 to 0.004) | 0.06 |
| MICE | |||||||
| Global Z score | 0 (Reference) | −0.225 (−0.346 to −0.103) | <0.001 | −0.142 (−0.260 to −0.023) | 0.02 | −0.127 (−0.245 to −0.010) | 0.03 |
| DWRT Z score | 0 (Reference) | −0.186 (−0.345 to −0.027) | 0.02 | −0.157 (−0.314 to −0.001) | 0.05 | −0.144 (−0.300 to 0.012) | 0.07 |
| DSST Z score | 0 (Reference) | −0.214 (−0.293 to −0.132) | <0.001 | −0.114 (−0.195 to −0.032) | 0.006 | −0.101 (−0.181 to −0.020) | 0.01 |
| WFT Z score | 0 (Reference) | −0.143 (−0.244 to −0.041) | 0.006 | −0.096 (−0.196 to 0.004) | 0.06 | −0.086 (−0.185 to 0.014) | 0.09 |
Model 1 is adjusted for age (centered at 60 years), sex, and race‐field center, time as a linear spline with a knot at 6 years, age by time spline terms, sex by time spline terms, and race‐field center by time spline terms. Model 2 is additionally adjusted for education, occupation, apolipoprotein E, the time‐dependent variables of smoking, body mass index, systolic blood pressure, diastolic blood pressure, use of hypertensive medication, diabetes mellitus, prevalent coronary heart disease, and prevalent heart failure, plus all these variables by spline terms. Model 3 is model 2 and additionally adjusted for prevalent and incident stroke, plus stroke by spline terms. AF indicates atrial fibrillation; ARIC, Atherosclerosis Risk in Communities; CI, confidence interval; DSST, Digit Symbol Substitution Test; DWRT, Delayed Word Recall Test; MICE, multiple imputation by chained equations; and WFT, Word Fluency Test.
Our results were robust to further analysis that accounted for attrition. By using the multiple imputation by chained equations approach to account for attrition, we observed that AF was associated with significantly greater decline in the scores of all tests; the association with greater decline in WFT Z score was of borderline significance (Table 2, model 2). After further adjustment for prevalent and incident stroke, AF remained significantly associated with greater decline in global and DSST Z scores.
To provide context for these results and because age‐related decline in cognitive function is well established, we used our linear model to estimate how much older a participant without AF would need to be at baseline to have, on average, a Z score that was 0.11 SDs lower; we estimated that a participant had to be 2.75 years older. In other words, a Z score that is 0.11 SDs lower is equivalent to the difference in cognitive performance of a 63‐year‐old participant versus a 60‐year‐old participant if they were otherwise similar.
In age‐stratified analysis (stratified at the median of 57 years), AF was associated with greater decline in global Z, DSST Z, and WFT Z scores in participants aged >57 years but not in those aged ≤57 years (Table 3, model 3). In sex‐stratified analysis, AF‐related decline in cognitive scores was of consistent magnitude in women and men, with the exception of WFT Z score: the decline was greater in women than in men (−0.191 versus −0.023, P=0.06 for interaction) (Table 4, model 3). We did not observe any race‐based interactions (Table 5).
Table 3.
Age‐Stratified Additional Adjusted 20‐Year Cognitive Change Associated With AF, ARIC Study, 1990 to 2013
| Variable | No AF | Additional Cognitive Change in Z Score (95% CI) for AF | |||||
|---|---|---|---|---|---|---|---|
| Model 1 | P Value | Model 2 | P Value | Model 3 | P Value | ||
| Baseline age ≤57 y | |||||||
| Global Z score | 0 (Reference) | −0.102 (−0.267 to 0.064) | 0.23 | −0.001 (−0.164 to 0.162) | 0.99 | 0.008 (−0.154 to 0.170) | 0.92 |
| DWRT Z score | 0 (Reference) | −0.100 (−0.342 to 0.141) | 0.42 | −0.057 (−0.301 to 0.187) | 0.65 | −0.048 (−0.292 to 0.195) | 0.70 |
| DSST Z score | 0 (Reference) | −0.184 (−0.306 to −0.063) | 0.003 | −0.052 (−0.176 to 0.073) | 0.42 | −0.041 (−0.166 to 0.083) | 0.51 |
| WFT Z score | 0 (Reference) | −0.094 (−0.246 to 0.057) | 0.22 | −0.024 (−0.176 to 0.128) | 0.76 | −0.017 (−0.168 to 0.134) | 0.83 |
| Baseline age >57 y | |||||||
| Global Z score | 0 (Reference) | −0.289 (−0.446 to −0.132) | <0.001 | −0.231 (−0.389 to −0.073) | 0.004 | −0.216 (−0.374 to −0.059) | 0.007 |
| DWRT Z score | 0 (Reference) | −0.243 (−0.462 to −0.025) | 0.03 | −0.221 (−0.437 to −0.006) | 0.04 | −0.206 (−0.420 to 0.008) | 0.06 |
| DSST Z score | 0 (Reference) | −0.209 (−0.318 to −0.101) | <0.001 | −0.142 (−0.253 to −0.031) | 0.03 | −0.130 (−0.240 to −0.020) | 0.02 |
| WFT Z score | 0 (Reference) | −0.184 (−0.319 to −0.050) | 0.007 | −0.170 (−0.305 to −0.035) | 0.01 | −0.160 (−0.293 to −0.026) | 0.02 |
Model 1 is adjusted for age (centered at 60 years), sex, and race‐field center, time as a linear spline with a knot at 6 years, age by time spline terms, sex by time spline terms, and race‐field center by time spline terms. Model 2 is additionally adjusted for education, occupation, apolipoprotein E, the time‐dependent variables of smoking, body mass index, systolic blood pressure, diastolic blood pressure, use of hypertensive medication, diabetes mellitus, prevalent coronary heart disease, and prevalent heart failure, plus all these variables by spline terms. Model 3 is model 2 and additionally adjusted for prevalent and incident stroke, plus stroke by spline terms. Age is stratified at the median age at visit 2 of 57 years. P value for age interaction (on the basis of model 3): global Z score, P=0.06; DWRT Z score, P=0.21; DSST Z score, P=0.14; WFT Z score, P=0.07. Analyses performed using multiple imputation by chained equations. AF indicates atrial fibrillation; ARIC, Atherosclerosis Risk in Communities; CI, confidence interval; DSST, Digit Symbol Substitution Test; DWRT, Delayed Word Recall Test; and WFT, Word Fluency Test.
Table 4.
Sex‐Stratified Additional Adjusted 20‐Year Cognitive Change Associated With AF, ARIC Study, 1990 to 2013
| Variable | No AF | Additional Cognitive Change in Z Score (95% CI) for AF | |||||
|---|---|---|---|---|---|---|---|
| Model 1 | P Value | Model 2 | P Value | Model 3 | P Value | ||
| Women | |||||||
| Global Z score | 0 (Reference) | −0.286 (−0.478 to −0.094) | 0.003 | −0.206 (−0.399 to −0.013) | 0.04 | −0.187 (−0.379 to 0.004) | 0.06 |
| DWRT Z score | 0 (Reference) | −0.212 (−0.466 to 0.041) | 0.10 | −0.205 (−0.461 to 0.052) | 0.12 | −0.185 (−0.442 to 0.071) | 0.16 |
| DSST Z score | 0 (Reference) | −0.219 (−0.347 to −0.091) | 0.001 | −0.119 (−0.248 to 0.011) | 0.07 | −0.103 (−0.232 to 0.025) | 0.12 |
| WFT Z score | 0 (Reference) | −0.244 (−0.404 to −0.084) | 0.003 | −0.200 (−0.364 to −0.037) | 0.02 | −0.191 (−0.353 to −0.029) | 0.02 |
| Men | |||||||
| Global Z score | 0 (Reference) | −0.183 (−0.322 to −0.043) | 0.01 | −0.081 (−0.218 to 0.054) | 0.24 | −0.071 (−0.206 to 0.064) | 0.30 |
| DWRT Z score | 0 (Reference) | −0.169 (−0.366 to 0.028) | 0.09 | −0.093 (−0.288 to 0.102) | 0.35 | −0.084 (−0.279 to 0.110) | 0.40 |
| DSST Z score | 0 (Reference) | −0.195 (−0.292 to −0.098) | <0.001 | −0.107 (−0.203 to −0.011) | 0.03 | −0.095 (−0.190 to −0.001) | 0.05 |
| WFT Z score | 0 (Reference) | −0.079 (−0.208 to 0.050) | 0.23 | −0.032 (−0.160 to 0.096) | 0.63 | −0.023 (−0.150 to 0.103) | 0.72 |
Model 1 is adjusted for age (centered at 60 years), sex, and race‐field center, time as a linear spline with a knot at 6 years, age by time spline terms, sex by time spline terms, and race‐field center by time spline terms. Model 2 is additionally adjusted for education, occupation, apolipoprotein E, the time‐dependent variables of smoking, body mass index, systolic blood pressure, diastolic blood pressure, use of hypertensive medication, diabetes mellitus, prevalent coronary heart disease, and prevalent heart failure, plus all these variables by spline terms. Model 3 is model 2 and additionally adjusted for prevalent and incident stroke, plus stroke by spline terms. P value for sex interaction (on the basis of model 3): global Z score, P=0.60; DWRT Z score, P=0.78; DSST Z score, P=0.76; WFT Z score, P=0.06. Analyses performed using multiple imputation by chained equations. AF indicates atrial fibrillation; ARIC, Atherosclerosis Risk in Communities; CI, confidence interval; DSST, Digit Symbol Substitution Test; DWRT, Delayed Word Recall Test; and WFT, Word Fluency Test.
Table 5.
Race‐Stratified Additional Adjusted 20‐Year Cognitive Change Associated With AF, ARIC Study, 1990 to 2013
| Variable | No AF | Additional Cognitive Change in Z Score (95% CI) for AF | |||||
|---|---|---|---|---|---|---|---|
| Model 1 | P Value | Model 2 | P Value | Model 3 | P Value | ||
| White | |||||||
| Global Z score | 0 (Reference) | −0.198 (−0.322 to −0.074) | 0.002 | −0.109 (−0.232 to 0.014) | 0.08 | −0.097 (−0.219 to 0.025) | 0.12 |
| DWRT Z score | 0 (Reference) | −0.158 (−0.327 to 0.010) | 0.07 | −0.119 (−0.286 to 0.048) | 0.16 | −0.107 (−0.273 to 0.059) | 0.21 |
| DSST Z score | 0 (Reference) | −0.194 (−0.276 to −0.112) | <0.001 | −0.097 (−0.183 to −0.011) | 0.03 | −0.085 (−0.171 to 0.001) | 0.05 |
| WFT Z score | 0 (Reference) | −0.134 (−0.243 to −0.029) | 0.01 | −0.090 (−0.195 to 0.016) | 0.10 | −0.081 (−0.185 to 0.024) | 0.13 |
| Black | |||||||
| Global Z score | 0 (Reference) | −0.347 (−0.710 to 0.015) | 0.06 | −0.328 (−0.670 to 0.014) | 0.06 | −0.282 (−0.616 to 0.051) | 0.10 |
| DWRT Z score | 0 (Reference) | −0.426 (−0.864 to 0.01) | 0.06 | −0.405 (−0.859 to 0.050) | 0.08 | −0.371 (−0.821 to 0.079) | 0.11 |
| DSST Z score | 0 (Reference) | −0.302 (−0.641 to 0.037) | 0.08 | −0.200 (−0.504 to 0.104) | 0.20 | −0.164 (−0.457 to 0.129) | 0.27 |
| WFT Z score | 0 (Reference) | −0.138 (−0.553 to 0.276) | 0.51 | −0.103 (−0.492 to 0.287) | 0.61 | −0.071 (−0.465 to 0.323) | 0.72 |
Model 1 is adjusted for age (centered at 60 years), sex, and race‐field center, time as a linear spline with a knot at 6 years, age by time spline terms, sex by time spline terms, and race‐field center by time spline terms. Model 2 is additionally adjusted for education, occupation, apolipoprotein E, the time‐dependent variables of smoking, body mass index, systolic blood pressure, diastolic blood pressure, use of hypertensive medication, diabetes mellitus, prevalent coronary heart disease, and prevalent heart failure, plus all these variables by spline terms. Model 3 is model 2 and additionally adjusted for prevalent and incident stroke, plus stroke by spline terms. P values for race interaction (on the basis of model 3): global Z score, P=0.63; DWRT Z score, P=0.44; DSST Z score, P=0.53; WFT Z score, P=0.57. Analyses performed using multiple imputation by chained equations. AF indicates atrial fibrillation; ARIC, Atherosclerosis Risk in Communities; CI, confidence interval; DSST, Digit Symbol Substitution Test; DWRT, Delayed Word Recall Test; and WFT, Word Fluency Test.
AF and Dementia
Compared with participants without AF, those with incident AF had a higher incidence rate of dementia (Table 6). In the multivariable model (Table 6, model 2), AF was significantly associated with a 1.31‐fold higher risk of dementia. Although additional adjustment for prevalent and incident ischemic stroke attenuated this risk estimate (Table 6, model 3), the association remained statistically significant: incident AF was associated with 23% higher risk of dementia.
Table 6.
Association of AF With Incident Dementia, ARIC Study, 1990 to 2013
| Variable | No AF (n=10 504) | AF (n=2011) | P Value |
|---|---|---|---|
| No. of dementia events | 977 | 180 | … |
| Person‐years | 218 165 | 8485 | … |
| Incidence rate (95% CI)a | 4.48 (4.20–4.77) | 21.21 (18.28–24.49) | … |
| HR (95% CI) | |||
| Model 1 | 1 (Reference) | 1.37 (1.16–1.61) | <0.001 |
| Model 2 | 1 (Reference) | 1.31 (1.11–1.55) | 0.001 |
| Model 3 | 1 (Reference) | 1.23 (1.04–1.45) | 0.02 |
Model 1 is adjusted for age, sex, and race‐field center. Model 2 is additionally adjusted for education, occupation, apolipoprotein E, smoking, body mass index, systolic blood pressure, diastolic blood pressure, use of hypertensive medication, diabetes mellitus, prevalent coronary heart disease, and prevalent heart failure. Model 3 is model 2 and additionally adjusted for prevalent and incident stroke. AF indicates atrial fibrillation; ARIC, Atherosclerosis Risk in Communities; CI, confidence interval; and HR, hazard ratio.
Incidence rate is per 1000 person‐years.
In age‐stratified analysis, we evaluated both incidence rate differences and hazard ratios. The incidence rate difference (the difference in incidence rates of dementia in those with and without AF) was greater in participants aged >57 years than in those aged ≤57 years (P=0.01 for age interaction) (Table 7). On the basis of hazard ratios, AF was associated with higher risk of dementia in both >57 and ≤57 years groups (Table 7, model 3). In sex‐stratified analysis, we found that the risk of dementia associated with AF in women to be comparable with men (Table 8, model 3, P=0.96 for interaction by sex). Similarly, race‐stratified analysis showed that AF‐related dementia was of consistent magnitude in blacks and whites (Table 9, model 3, P=0.67 for interaction by race).
Table 7.
Association of AF With Incident Dementia by Age, ARIC Study, 1990 to 2013
| Variable | No AF | AF | Rate Difference | P Value |
|---|---|---|---|---|
| Baseline age ≤57 y | ||||
| No. of participants | 6080 | 737 | … | … |
| No. of dementia events | 272 | 34 | … | … |
| Person‐years | 126 825 | 3102 | … | … |
| Incidence rate (95% CI)a | 2.22 (1.96–2.52) | 8.64 (6.12–12.18) | 6.42 (3.45–9.37) | <0.001 |
| HR (95% CI) | ||||
| Model 1 | 1 (Reference) | 1.81 (1.26–2.61) | … | 0.001 |
| Model 2 | 1 (Reference) | 1.65 (1.14–2.40) | … | 0.008 |
| Model 3 | 1 (Reference) | 1.52 (1.04–2.20) | … | 0.03 |
| Baseline age >57 y | ||||
| No. of participants | 4424 | 1274 | … | … |
| No. of dementia events | 705 | 146 | … | … |
| Person‐years | 91 341 | 5384 | … | … |
| Incidence rate (95% CI)a | 9.19 (8.52–9.92) | 22.95 (19.45–27.09) | 13.76 (9.92–17.60) | <0.001 |
| HR (95% CI) | ||||
| Model 1 | 1 (Reference) | 1.32 (1.10–1.59) | … | 0.003 |
| Model 2 | 1 (Reference) | 1.28 (1.06–1.54) | … | 0.009 |
| Model 3 | 1 (Reference) | 1.21 (1.01–1.46) | … | 0.04 |
Model 1 is adjusted for age, sex, and race‐field center. Model 2 is additionally adjusted for education, occupation, apolipoprotein E, smoking, body mass index, systolic blood pressure, diastolic blood pressure, use of hypertensive medication, diabetes mellitus, prevalent coronary heart disease, and prevalent heart failure. Model 3 is model 2 and additionally adjusted for prevalent and incident stroke. Age is stratified at the median age at visit 2 of 57 years. P=0.01 for age interaction in incidence rate difference. AF indicates atrial fibrillation; ARIC, Atherosclerosis Risk in Communities; CI, confidence interval; and HR, hazard ratio.
Per 1000 person‐years.
Table 8.
Association of AF With Incident Dementia by Sex, ARIC Study, 1990 to 2013
| Variable | No AF | AF | P Value |
|---|---|---|---|
| Women | |||
| No. of participants | 6032 | 949 | … |
| No. of dementia events | 576 | 91 | … |
| HR (95% CI) | |||
| Model 1 | 1 (Reference) | 1.38 (1.10–1.73) | 0.005 |
| Model 2 | 1 (Reference) | 1.36 (1.08–1.71) | 0.009 |
| Model 3 | 1 (Reference) | 1.26 (1.00–1.59) | 0.05 |
| Men | |||
| No. of participants | 4472 | 1062 | … |
| No. of dementia events | 401 | 89 | … |
| HR (95% CI) | |||
| Model 1 | 1 (Reference) | 1.37 (1.08–1.74) | 0.001 |
| Model 2 | 1 (Reference) | 1.24 (0.98–1.59) | 0.08 |
| Model 3 | 1 (Reference) | 1.20 (0.94–1.53) | 0.14 |
Model 1 is adjusted for age, sex, and race‐field center. Model 2 is additionally adjusted for education, occupation, apolipoprotein E, smoking, body mass index, systolic blood pressure, diastolic blood pressure, use of hypertensive medication, diabetes mellitus, prevalent coronary heart disease, and prevalent heart failure. Model 3 is model 2 and additionally adjusted for prevalent and incident stroke. P=0.96 for sex interaction (on the basis of model 3). AF indicates atrial fibrillation; ARIC, Atherosclerosis Risk in Communities; CI, confidence interval; and HR, hazard ratio.
Table 9.
Association of AF With Incident Dementia by Race, ARIC Study, 1990 to 2013
| Variable | No AF | AF | P Value |
|---|---|---|---|
| White race | |||
| No. of participants | 7836 | 1675 | … |
| No. of dementia events | 660 | 140 | … |
| HR (95% CI) | |||
| Model 1 | 1 (Reference) | 1.36 (1.13–1.64) | 0.001 |
| Model 2 | 1 (Reference) | 1.24 (1.03–1.50) | 0.02 |
| Model 3 | 1 (Reference) | 1.16 (0.96–1.40) | 0.13 |
| Black race | |||
| No. of participants | 2668 | 336 | … |
| No. of dementia events | 317 | 40 | … |
| HR (95% CI) | |||
| Model 1 | 1 (Reference) | 1.41 (1.00–1.98) | 0.05 |
| Model 2 | 1 (Reference) | 1.44 (1.02–2.04) | 0.04 |
| Model 3 | 1 (Reference) | 1.33 (0.94–1.89) | 0.10 |
Model 1 is adjusted for age, sex, and race‐field center. Model 2 is additionally adjusted for education, occupation, apolipoprotein E, smoking, body mass index, systolic blood pressure, diastolic blood pressure, use of hypertensive medication, diabetes mellitus, prevalent coronary heart disease, and prevalent heart failure. Model 3 is model 2 and additionally adjusted for prevalent and incident stroke. P=0.67 for race interaction (on the basis of model 3). AF indicates atrial fibrillation; ARIC, Atherosclerosis Risk in Communities; CI, confidence interval; and HR, hazard ratio.
Discussion
In a large biracial population‐based cohort study that comprised middle‐aged individuals, we observed that participants who developed AF had greater cognitive decline over 20 years, compared with participants who did not develop AF. The AF‐related decline in global score was 16% greater and was augmented after accounting for attrition. In addition, incident AF was associated with 23% higher risk of dementia. Although adjustment for prevalent and incident ischemic stroke attenuated the associations slightly, they remained significant. This indicates that although clinical stroke can explain the associations of AF with cognitive decline and dementia, it does so only partially. Other factors may play a role, such as subclinical cerebral infarcts.7 The age‐cognitive decline and dementia relationship was stronger in participants aged >57 years than ≤57 years; the former group was, on average, 10 years older. Our findings were generally consistent in women and men, and in blacks and whites.
Previous studies on the relationship of AF to cognitive decline or dementia have reported inconsistent findings; some have reported an increased risk,7, 8, 9, 17, 37 whereas others have not.11, 12, 38 Methodologic limitations and differences may explain the inconsistent findings. For example, smaller sample sizes or shorter follow‐up periods might have resulted in inadequate statistical power for some studies to find an association. A recent report from the Rotterdam Study addressed these limitations by evaluating the association of prevalent and incident AF with incident dementia in a sample of 6514 white participants over 20 years of follow‐up.18 In the entire sample, after accounting for stroke, AF was not significantly associated with an increased risk of dementia. Only in a subgroup analysis that stratified age at the median (67 years) was incident AF associated, with 1.81 (95% confidence interval, 1.11–2.94) higher risk of dementia in those aged <67 years.
Our study advances current knowledge on several fronts by addressing 3 relatively unexplored areas: (1) evaluation of both cognitive change and dementia in the same sample, (2) consideration of attrition, and (3) evaluation in a biracial population. First, in contrast to the Rotterdam Study,18 we found that incident AF was significantly associated with an increased risk of dementia, after adjustment for ischemic stroke. In addition, incident AF was also associated with greater decline in global cognitive and DSST Z scores, after adjustment for ischemic stroke. Because cognitive decline is a precursor to dementia, our findings underscore AF as a potential modifiable target, providing an important opportunity to prevent cognitive decline and delay progression to dementia. Moreover, although AF was associated with greater cognitive decline and risk of dementia in both younger (≤57 years) and older (>57 years) individuals, the associations were stronger in the older group, emphasizing the importance of diagnosing and treating AF to prevent dementia in older age. Second, an inherent challenge to accurately quantifying long‐term risk factor associations in observational studies is that ill participants are less likely to return for study visits. Thus, attrition is an important concern in any long‐term observational study. To the best of our knowledge, our study is the first to use methods to account for attrition, which provides more accurate estimates of the effect of AF on cognitive function than when attrition is ignored, as in prior reports. Third, previous studies have not explored race‐based differences. We did not find any difference between blacks and whites in AF‐related cognitive decline and dementia.
The pattern of cognitive decline is worth noting. Cognitive decline associated with AF was reflected in a greater average change in DSST and WFT, but not DWRT. In general, DWRT is a test of recent memory, DSST is a test of executive function and processing speed, and WFT is a test of executive function and language. The ARIC Study has recently reported that over a 20‐year follow‐up, diabetes mellitus and hypertension were also associated with greater decline in DSST and WFT, but not DWRT.27, 28 The APOE ε4 genotype, most strongly associated with the risk of Alzheimer disease, was associated with declining performance in DWRT.39 These patterns are consistent with a model of pathoanatomic relationships, in which lacunar infarcts40 and vascular risk factors41, 42 are associated with executive function deficits, whereas Alzheimer disease–related risk is associated with decline in memory functioning.43, 44 These findings suggest that shared vascular risk factors (eg, diabetes mellitus and hypertension) may be important mechanisms underlying the AF–cognitive decline association, although we adjusted for these risk factors. Other mechanisms, however, should be considered. We had previously shown among stroke‐free participants in the ARIC Study that incident AF was associated with greater cognitive decline only in participants who had silent cerebral infarcts on brain magnetic resonance imaging but not in those without silent cerebral infarcts,7 suggesting that asymptomatic or subclinical stroke may explain the link between AF and cognitive decline or dementia. Decreased cerebral perfusion attributable to lower cardiac output from AF,45 chronic subcortical ischemia that manifests as white matter hyperintensities on brain magnetic resonance imaging,46 microbleeds, cortical atrophy,47, 48 reduced brain volume,47, 48 and inflammation49 are other possible mechanisms.
Other strengths of the study should be noted. First, in contrast to evaluating dementia or cognitive performance at a single time point, we evaluated cognitive change at several time points over 20 years of follow‐up, thus reducing the influence of confounding factors, such as cultural factors. Second, the extensive and rigorous measurement of covariates allows us to perform comprehensive statistical adjustment, thus reducing confounding. Finally, we assessed cognitive function in >1 domain, which might shed some light on pathoanatomic relationships. Some limitations of our study should be noted. First, incident AF was identified mostly from hospitalization discharges, and we could have missed asymptomatic AF or AF managed exclusively in an outpatient setting. However, we and others have previously shown that the validity of AF ascertainment using hospitalizations is acceptable21, 50 (incidence rates of AF in the ARIC Study are consistent with other population‐based studies).21, 50 Second, because we are unable to classify AF type (paroxysmal, persistent, or permanent AF) or assess the burden of AF (percentage time a person is in AF) accurately in the ARIC Study, we did not assess the relationship of AF type or burden to cognitive decline and dementia. However, in a small cross‐sectional subset of the ARIC Study, we reported that persistent, but not paroxysmal, AF was associated with lower cognitive function.51 Third, some dementia diagnoses were ascertained from ICD‐9 hospital discharge diagnostic codes; thus, we could have underascertained incident dementia and dates of onset are uncertain. Furthermore, causative categories of dementia were available only for participants who attended visit 5; hence, we could not evaluate longitudinal association of AF with specific types of dementia. Fourth, we did not have information on treatment for AF (eg, catheter ablation); thus, we were not able to account for the possible effect of treatment on AF‐related cognitive decline or dementia. Fifth, our analyses were not adjusted for the use of anticoagulants. This may be important because silent cerebral infarcts may play a role in AF‐related cognitive decline.7 Finally, given the ARIC Study's design, our comparison showing no difference in the associations between blacks and whites is potentially confounded by geographical and sociocultural differences among the ARIC Study field centers.
In conclusion, our report, based on a large biracial population‐based cohort study of middle‐aged individuals with 20 years of follow‐up, provides evidence that incident AF is associated with both greater cognitive decline and increased risk of dementia, independent of clinical ischemic stroke. Because cognitive decline is a precursor to dementia, our findings beg the questions of whether treatment for AF and what treatments (eg, anticoagulation or rhythm control) will delay the trajectory of cognitive decline, thus preventing dementia in patients with AF. Given the burgeoning twin epidemics of AF and dementia, more research to address these questions and discover novel dementia prevention strategies is increasingly urgent.
Sources of Funding
The ARIC (Atherosclerosis Risk in Communities) Study is performed as a collaborative study supported by the National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN2682011000010C, HHSN2682011000011C, and HHSN2682011000012C). Neurocognitive data are collected by the support of the National Heart, Lung, and Blood Institute U01 HL096812, HL096814, HL096899, HL096902, and HL096917, with previous brain magnetic resonance imaging examinations funded by R01‐HL70825. This work was additionally supported by National Heart, Lung and Blood Institute R01HL126637 (Chen) and American Heart Association grant 16EIA26410001 (Alonso).
Disclosures
None.
Acknowledgments
We thank the staff and participants of the ARIC (Atherosclerosis Risk in Communities) Study for their important contributions.
(J Am Heart Assoc. 2018;7:e007301 DOI: 10.1161/JAHA.117.007301.)29514809
References
- 1. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 2. Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TSM. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. [DOI] [PubMed] [Google Scholar]
- 3. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. PMID:1866765. [DOI] [PubMed] [Google Scholar]
- 4. Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. [DOI] [PubMed] [Google Scholar]
- 5. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 6. Chen LY, Sotoodehnia N, Bůžková P, Lopez FL, Yee LM, Heckbert SR, Prineas R, Soliman EZ, Adabag AS, Konety S, Folsom AR, Siscovick D, Alonso A. Atrial fibrillation and the risk of sudden cardiac death: the Atherosclerosis Risk in Communities (ARIC) Study and Cardiovascular Health Study (CHS). JAMA Intern Med. 2013;173:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen LY, Lopez FL, Gottesman RF, Huxley RR, Agarwal SK, Loehr L, Mosley T, Alonso A. Atrial fibrillation and cognitive decline‐the role of subclinical cerebral infarcts: the Atherosclerosis Risk in Communities study. Stroke. 2014;45:2568–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bunch TJ, Weiss JP, Crandall BG, May HT, Bair TL, Osborn JS, Anderson JL, Muhlestein JB, Horne BD, Lappe DL, Day JD. Atrial fibrillation is independently associated with senile, vascular, and Alzheimer's dementia. Heart Rhythm. 2010;7:433–437. [DOI] [PubMed] [Google Scholar]
- 9. Dublin S, Anderson ML, Haneuse SJ, Heckbert SR, Crane PK, Breitner JC, McCormick W, Bowen JD, Teri L, McCurry SM, Larson EB. Atrial fibrillation and risk of dementia: a prospective cohort study. J Am Geriatr Soc. 2011;59:1369–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elias MF, Sullivan LM, Elias PK, Vasan RS, D'Agostino RB Sr, Seshadri S, Au R, Wolf PA, Benjamin EJ. Atrial fibrillation is associated with lower cognitive performance in the Framingham offspring men. J Stroke Cerebrovasc Dis. 2006;15:214–222. [DOI] [PubMed] [Google Scholar]
- 11. Marengoni A, Qiu CX, Winblad B, Fratiglioni L. Atrial fibrillation, stroke and dementia in the very old: a population‐based study. Neurobiol Aging. 2011;32:1336–1337. [DOI] [PubMed] [Google Scholar]
- 12. Peters R, Poulter R, Beckett N, Forette F, Fagard R, Potter J, Swift C, Anderson C, Fletcher A, Bulpitt CJ. Cardiovascular and biochemical risk factors for incident dementia in the Hypertension in the Very Elderly Trial. J Hypertens. 2009;27:2055–2062. [DOI] [PubMed] [Google Scholar]
- 13. Ott A, Breteler MM, de Bruyne MC, van Harskamp F, Grobbee DE, Hofman A. Atrial fibrillation and dementia in a population‐based study: the Rotterdam Study. Stroke. 1997;28:316–321. [DOI] [PubMed] [Google Scholar]
- 14. Cacciatore F, Abete P, Ferrara N, Calabrese C, Napoli C, Maggi S, Varricchio M, Rengo F; Osservatorio Geriatrico Campano Study Group. Congestive heart failure and cognitive impairment in an older population. J Am Geriatr Soc. 1998;46:1343–1348. [DOI] [PubMed] [Google Scholar]
- 15. Tilvis RS, Kahonen‐Vare MH, Jolkkonen J, Valvanne J, Pitkala KH, Strandberg TE. Predictors of cognitive decline and mortality of aged people over a 10‐year period. J Gerontol A Biol Sci Med Sci. 2004;59:268–274. [DOI] [PubMed] [Google Scholar]
- 16. Forti P, Maioli F, Pisacane N, Rietti E, Montesi F, Ravaglia G. Atrial fibrillation and risk of dementia in non‐demented elderly subjects with and without mild cognitive impairment (MCI). Arch Gerontol Geriatr. 2007;44:155–165. [DOI] [PubMed] [Google Scholar]
- 17. Marzona I, O'Donnell M, Teo K, Gao P, Anderson C, Bosch J, Yusuf S. Increased risk of cognitive and functional decline in patients with atrial fibrillation: results of the ONTARGET and TRANSCEND studies. CMAJ. 2012;184:E329–E336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Bruijn RF, Heeringa J, Wolters FJ, Franco OH, Stricker BH, Hofman A, Koudstaal PJ, Ikram MA. Association between atrial fibrillation and dementia in the general population. JAMA Neurol. 2015;72:1288–1294. [DOI] [PubMed] [Google Scholar]
- 19. Collaborative Studies Coordinating Center . Atherosclerosis Risk in Communities Study. University of North Carolina at Chapel Hill. https://www2.cscc.unc.edu/aric/desc. Accessed January 8, 2018.
- 20. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 21. Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African‐Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC Jr. Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2009;40:1204–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Knopman DS, Ryberg S. A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Arch Neurol. 1989;46:141–145. [DOI] [PubMed] [Google Scholar]
- 24. Wechsler D. Wechsler Adult Intelligence Scale–Revised. New York, NY: Psychological Corp; 1981. [Google Scholar]
- 25. Lezak MD. Neuropsychological Assessment. 3rd ed New York, NY: Oxford University Press; 1995. [Google Scholar]
- 26. Schneider ALC, Sharrett AR, Gottesman RF, Coresh J, Coker L, Wruck L, Selnes OA, Deal J, Knopman D, Mosley TH. Normative data for 8 neuropsychological tests in older blacks and whites from the Atherosclerosis Risk in Communities (ARIC) study. Alzheimer Dis Assoc Disord. 2015;29:32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rawlings AM, Sharrett AR, Schneider AL, Coresh J, Albert M, Couper D, Griswold M, Gottesman RF, Wagenknecht LE, Windham BG, Selvin E. Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med. 2014;161:785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gottesman RF, Schneider ALC, Albert M, Alonso A, Bandeen‐Roche K, Coker L, Coresh J, Knopman D, Power MC, Rawlings A, Sharrett AR, Wruck LM, Mosley TH. Midlife hypertension and 20‐year cognitive change: the Atherosclerosis Risk in Communities (ARIC) Neurocognitive Study. JAMA Neurol. 2014;71:1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Elias PK, Elias MF, DAgostino RB, Cupples LA, Wilson PW, Silbershatz H, Wolf PA. NIDDM and blood pressure as risk factors for poor cognitive performance: the Framingham Study. Diabetes Care. 1997;20:1388–1395. [DOI] [PubMed] [Google Scholar]
- 30. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging–Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging–Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Knopman DS, Gottesman RF, Sharrett AR, Wruck LM, Windham BG, Coker L, Schneider ALC, Hengrui S, Alonso A, Coresh J, Albert MS, Mosley TH Jr. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study. Alzheimers Dement (Amst). 2016;2:1–11. DOI: 10.1016/j.dadm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eriksson H, Caidahl K, Larsson B, Ohlson LO, Welin L, Wilhelmsen L, Svardsudd K. Cardiac and pulmonary causes of dyspnea—validation of a scoring test for clinical‐epidemiologic use—the Study of Men Born in 1913. Eur Heart J. 1987;8:1007–1014. [DOI] [PubMed] [Google Scholar]
- 34. Weinfeld FD, Adelman SM, Baum HM, Robins M, Walker AE, Weiss W. The national survey of stroke. Stroke. 1981;12:I1–I91.7222163 [Google Scholar]
- 35. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. [DOI] [PubMed] [Google Scholar]
- 36. Rawlings AM, Sang Y, Sharrett AR, Coresh J, Griswold M, Kucharska‐Newton AM, Palta P, Wruck LM, Gross AL, Deal JA, Power MC, Bandeen‐Roche KJ. Multiple imputation of cognitive performance as a repeatedly measured outcome. Eur J Epidemiol. 2017;32:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thacker EL, McKnight B, Psaty BM, Longstreth WT Jr, Sitlani CM, Dublin S, Arnold AM, Fitzpatrick AL, Gottesman RF, Heckbert SR. Atrial fibrillation and cognitive decline: a longitudinal cohort study. Neurology. 2013;81:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rastas S, Verkkoniemi A, Polvikoski T, Juva K, Niinisto L, Mattila K, Lansimies E, Pirttila T, Sulkava R. Atrial fibrillation, stroke, and cognition: a longitudinal population‐based study of people aged 85 and older. Stroke. 2007;38:1454–1460. [DOI] [PubMed] [Google Scholar]
- 39. Knopman DS, Mosley TH, Catellier DJ, Coker LH. Fourteen‐year longitudinal study of vascular risk factors, APOE genotype, and cognition: the ARIC MRI Study. Alzheimers Dement. 2009;5:207–214. [DOI] [PubMed] [Google Scholar]
- 40. Reed BR, Eberling JL, Mungas D, Weiner M, Kramer JH, Jagust WJ. Effects of white matter lesions and lacunes on cortical function. Arch Neurol. 2004;61:1545–1550. [DOI] [PubMed] [Google Scholar]
- 41. Roberts RO, Geda YE, Knopman DS, Christianson TJ, Pankratz VS, Boeve BF, Vella A, Rocca WA, Petersen RC. Association of duration and severity of diabetes mellitus with mild cognitive impairment. Arch Neurol. 2008;65:1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Haan MN, Shemanski L, Jagust WJ, Manolio TA, Kuller L. The role of APOE epsilon 4 in modulating effects of other risk factors for cognitive decline in elderly persons. JAMA. 1999;282:40–46. [DOI] [PubMed] [Google Scholar]
- 43. Wilson RS, Schneider JA, Barnes LL, Beckett LA, Aggarwal NT, Cochran EJ, Berry‐Kravis E, Bach J, Fox JH, Evans DA, Bennett DA. The apolipoprotein E epsilon 4 allele and decline in different cognitive systems during a 6‐year period. Arch Neurol. 2002;59:1154–1160. [DOI] [PubMed] [Google Scholar]
- 44. Mayeux R, Small SA, Tang MX, Tycko B, Stern Y. Memory performance in healthy elderly without Alzheimer's disease: effects of time and apolipoprotein‐E. Neurobiol Aging. 2001;22:683–689. [DOI] [PubMed] [Google Scholar]
- 45. Lavy S, Stern S, Melamed E, Cooper G, Keren A, Levy P. Effect of chronic atrial‐fibrillation on regional cerebral blood‐flow. Stroke. 1980;11:35–38. PMID:7355427. [DOI] [PubMed] [Google Scholar]
- 46. de Leeuw FE, de Groot JC, Oudkerk M, Kors JA, Hofman A, van Gijn J, Breteler MM. Atrial fibrillation and the risk of cerebral white matter lesions. Neurology. 2000;54:1795–1801. [DOI] [PubMed] [Google Scholar]
- 47. Piers RJ, Nishtala A, Preis SR, DeCarli C, Wolf PA, Benjamin EJ, Au R. Association between atrial fibrillation and volumetric magnetic resonance imaging brain measures: Framingham Offspring Study. Heart Rhythm. 2016;13:2020–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stefansdottir H, Arnar DO, Aspelund T, Sigurdsson S, Jonsdottir MK, Hjaltason H, Launer LJ, Gudnason V. Atrial fibrillation is associated with reduced brain volume and cognitive function independent of cerebral infarcts. Stroke. 2013;44:1020–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Anderson JL, Maycock CAA, Lappe DL, Crandall BG, Horne BD, Bair TL, Morris SR, Li QY, Muhlestein JB; Intermountain Heart Collaborative Study Group . Frequency of elevation of C‐reactive protein in atrial fibrillation. Am J Cardiol. 2004;94:1255–1259. [DOI] [PubMed] [Google Scholar]
- 50. Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. [DOI] [PubMed] [Google Scholar]
- 51. Chen LY, Agarwal SK, Norby FL, Gottesman RF, Loehr LR, Soliman EZ, Mosley TH, Folsom AR, Coresh J, Alonso A. Persistent but not paroxysmal atrial fibrillation is independently associated with lower cognitive function: ARIC Study. J Am Coll Cardiol. 2016;67:1379–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]


