Abstract
Background
The Hypertension, Abnormal renal/liver function, Stroke, Bleeding, Labile International Normalized Ratio (INR), Elderly, Drugs or alcohol use (HAS‐BLED) score has strong predictive validity for major bleeding complications, but limited validation has been conducted in venous thromboembolism (VTE). This study evaluates the HAS‐BLED score in a large cohort of VTE patients.
Methods and Results
A retrospective cohort of adults ≥18 years with primary diagnosis of VTE between January 1, 2010 and November 31, 2013 were identified in an insurance claims database. Patients were tracked until death, any bleed event, or end of study period. HAS‐BLED score and components were evaluated via proportional hazard models. Cumulative incidence functions were reported at 30, 60, 90, and 180 days. N=132 280 patients with a VTE were identified, with 73.8% having HAS‐BLED scores of 0 to 2, 3.6% score ≥4, and 4789 bleeding events (3.6% all patients). A 1‐point HAS‐BLED score increase was associated with 20% to 30% bleeding rate increase overall, but in a cancer cohort only the increase from 3‐ to 4‐points was significant for all bleeds (csHR=1.41, 95% CI: 1.17–1.69; sdHR=1.40, 95% CI: 1.17–1.69) and major bleeds (csHR=1.66, 95% CI: 1.26–2.20; sdHR=1.66, 95% CI: 1.25–2.19). Adding cancer to the model as an independent covariate provided the strongest association among all covariates, with csHR=2.25 (95% CI: 1.98–2.56) and sdHR=2.11 (95% CI: 1.85–2.41) in the model for major bleeds.
Conclusions
The HAS‐BLED score has good predictive validity for bleeding risks in patients with VTE. The addition of cancer as an independent bleeding risk factor merits consideration, possibly as part of the “B” criterion (“bleeding tendency or predisposition”).
Keywords: HAS‐BLED score, risk stratification, venous thromboembolism
Subject Categories: Thrombosis, Risk Factors
Clinical Perspective
What Is New?
The HAS‐BLED score has been widely used for risk stratification in patients with atrial fibrillation receiving anticoagulation.
This study shows that HAS‐BLED has high predictive validity for bleeding events in venous thromboembolism (VTE) patients receiving anticoagulation.
Further, we show that cancer is a strong independent risk factor for bleeding in this population and should be included in the risk score.
What Are the Clinical Implications?
HAS‐BLED can be used in patients with VTE to predict the risk of a bleeding event while being anticoagulated.
Bleed risk stratification scores should not be used to withhold treatment.
Rather, risk scores can be used to identify patients for monitoring or modified therapeutic approaches as well as identification of some modifiable risk factors (eg, blood pressure, medication use).
Introduction
Venous thromboembolism (VTE), including both deep vein thrombosis and pulmonary embolism affects around 1 to 2 adults per 1000 every year.1 The most recent American College of Chest Physicians (ACCP) guidelines recommend treatment with non‐vitamin K antagonist oral anticoagulants over vitamin K antagonists (eg, warfarin) in patients without cancer for at least 3 months.2 In patients with cancer, low‐molecular weight heparins (LMWH) are recommended over other treatments. The goal of anticoagulation is to treat the current VTE and to prevent recurrent VTE. However, anticoagulation also imposes an increased risk for bleeding events and this risk must be assessed to determine appropriateness of a given treatment plan for each patient.
In an analogous treatment paradigm, individuals with atrial fibrillation (AF) often receive long‐term anticoagulation for prevention of cerebrovascular events. In the AF population, risk scores are now commonly used as clinical decision support tools to initiate anticoagulation based on stroke risk (eg, CHADSs and CHA2DS2‐VASc).3 More recently, bleeding risk scores have been developed to go hand‐in‐hand with stroke risk scores to determine the potential net clinical benefit of anticoagulation and to guide patient follow‐up throughout therapy.3
The Hypertension, Abnormal renal/liver function, Stroke, Bleeding, Labile International Normalized Ratio (INR), Elderly, Drugs or alcohol use (HAS‐BLED) score is one such score and has been shown to have strong predictive validity for major bleeding complications.4 HAS‐BLED has been validated across several AF cohorts4, 5 and has recently been applied to patients with VTE to determine its ability to identify those at the highest risk.6 These prior studies of VTE cohorts have been limited in size and number of events, and have thus been unable to establish a clear ability of the HAS‐BLED score to assess bleeding risk in a VTE population.
This study's primary objective was to evaluate the HAS‐BLED score in a large cohort of VTE patients receiving outpatient anticoagulation treatment. Second, the HAS‐BLED score was evaluated in a subgroup of VTE patients with cancer. The latter objective fulfills a crucial gap in the literature, given that cancer is one of the strongest risk factors for VTE and presents a more complicated patient population in regards to additional risk factors for adverse bleeding events.
Methods
This retrospective cohort study used the Truven Health MarketScan Commercial Claims and Medicare Supplemental Databases from the years 2010–2014. The MarketScan data include ≈40 million individuals from >160 large employers and health plans across the United States. The data represent an individual's healthcare utilization including medical claims with diagnosis and procedure codes for medical encounters and all prescription medication fills, as well as in‐hospital mortality. These data are de‐identified in compliance with the Health Insurance Portability and Accountability Act regulations (HIPAA) and the University of Kentucky Institutional Review Board approved the use of the database for this study. The data are licensed and are not available for public dissemination. Codes for analytic methods are available from the authors on request for purposes of reproducing the study results.
Cohort Selection
Adults aged ≥18 years diagnosed with a VTE event between January 1, 2010 and November 31, 2013 were identified. The date of the first qualifying diagnosis of VTE was defined as the index date, requiring that the diagnosis was in the primary position on an inpatient hospital record. VTE was identified by International Classification of Disease, 9th revision (ICD‐9) codes based on previously validated coding algorithms.7 Patients were further required to have at least 12 months of pre‐index and a 1‐month minimum of post‐index continuous enrollment with medical and outpatient pharmacy information included in the database. Patients were also required to be treatment‐naïve (ie, no anticoagulant therapy during the baseline period), to present an anticoagulant treatment naïve cohort.
Cohort Characteristics
The cohort was described by age (18–64, 65–74, ≥75 years) and sex. All categories for the HAS‐BLED score were identified by ICD‐9 codes including: hypertension, liver disease, history of stroke, history of bleeding,8 alcohol abuse, and drug abuse.9 NSAIDs or antiplatelet medications were identified from pharmacy records. The “labile INR” criterion of HAS‐BLED was not assessed given that the cohort was required to be anticoagulant treatment‐naïve and no such records would exist.
Patients were assigned a total HAS‐BLED score of 1‐point each for hypertension, renal disease, liver disease, history of stroke, history of bleeding, aged >65 years, medication use predisposing to bleeding (NSAIDs and antiplatelets), and alcohol and/or drug abuse. Patients with malignant neoplasms and/or metastatic disease during the baseline period were also identified using ICD‐9 codes 140.x to 209.x. Anticoagulant treatment was identified as the first observed LMWH, non‐vitamin K antagonist oral anticoagulant, or warfarin, allowing for bridge therapy from LMWH to warfarin.
Patient Follow‐Up and Outcomes
All bleeding events were tracked during the 180 days following the initial VTE diagnosis. Patients were followed up until they experienced a bleeding event, were censored due to dropping out of the database, they died, or until the end of the study period or follow‐up time. Bleeding events were classified as “all” if they met the coding algorithm used or “major” if they occurred during an inpatient stay, were associated with a critical site, resulted in need for transfusion, or lead to death while in the hospital.10
Statistical Analysis
A time‐to‐event, survival analytic approach was taken. Given that there is a high risk of death in the overall cohort, and an especially elevated risk in the cancer sub‐group, it was deemed necessary to assess outcomes in a competing risks framework, taking death into account as a competing event to the primary bleeding outcome.11
The cumulative incidence function (CIF) of all bleeding events and major bleeding events were modeled using Fine and Gray's method for competing risks which reported the incidence proportion at multiple time periods (30, 60, 90, 180 days).12 CIF plots were stratified by HAS‐BLED scores and show the incidence of bleeding complications, taking into account both censoring and competing events during follow‐up. Gray's statistical tests for the equality of CIF curves between HAS‐BLED stratifications were conducted.
Two proportional hazard regression models specifications were used. A cause‐specific model was used which treats the competing risk as a censoring event. This models the instantaneous rate of bleeding events among subjects who are event free at a given time point (no bleeding, no death). The cause‐specific model is comparable to a typical Cox proportional hazard model. A sub‐distribution model was also used which accounts directly for death as a competing risk by retaining those that die within a given time point in the risk set. Such a model provides an estimate of the instantaneous rate of bleeding events in those who have not experienced a bleed or have died within the time interval. Including both models allowed for broader interpretation of results as each has unique implications and applications.11 Specifically, using both approaches allows for an assessment of how the risk of death interplays with the risk of bleeding and the association of each with the included covariates.
From each model, cause‐specific (csHR) and sub‐distribution (sdHR) hazard ratios and 95% confidence intervals (CI) are reported for the HAS‐BLED score, individual components of the HAS‐BLED score, and for cancer. To better compare HAS‐BLED scores and potential low/high risk cut‐offs, hazard ratios are presented incrementally (ie, comparing the level with the previous level, rather than having a common reference group). Anticoagulant treatment was included as a covariate to control for any selection bias in treatment selection. C‐indices, a metric of model concordance or, the ability of the model to identify those who will have outcomes, are reported.
All data analysis was performed using SAS Enterprise Guide (SAS Institute, Cary, NC) version 7.1 with an a priori alpha of 0.05 for statistical significance.
Results
Over the study period, N=132 280 patients with VTE were identified including 60 930 (46.1%) with deep vein thrombosis and 71 350 (53.9%) with pulmonary embolism (Table 1). Nearly two‐thirds were aged 18 to 64 years (63.7%), and 45.4% were male. The most common HAS‐BLED component was hypertension (44.5%), followed by NSAID or antiplatelet medication use (27.5%), cancer (18.8%), and a history of bleeding (11.7%). The large majority of patients had HAS‐BLED scores of 0 to 2, 10.2% had a score of 3, and 3.6% had a score ≥4. The majority of patients received warfarin (64.6%), 15.5% received non‐vitamin K antagonist oral anticoagulants, and 7.5% received LMWH. Treatment did not differ based on the HAS‐BLED score in the overall cohort but cancer patients were more likely to receive LMWH than non‐cancer patients, which is consistent with US treatment guidelines.2, 13
Table 1.
Demographic and Clinical Characteristics of Patients With an Index Venous Thromboembolism
| DVT Only | PE With or Without DVT | Overall | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Total | 60 930 | 46.1 | 71 350 | 53.9 | 132 280 | 100 |
| Age, y | ||||||
| 18 to 64 | 36 731 | 60.3 | 47 520 | 66.6 | 84 251 | 63.7 |
| 65 to 74 | 7793 | 12.8 | 8978 | 12.6 | 16 771 | 12.7 |
| ≥75 | 16 406 | 26.9 | 14 852 | 20.8 | 31 258 | 23.6 |
| Sex (male) | 27 734 | 52.0 | 32 257 | 51.5 | 59 991 | 45.4 |
| Hypertension | 28 682 | 53.8 | 30 127 | 48.1 | 58 809 | 44.5 |
| Renal disease | 6365 | 11.9 | 3770 | 6.0 | 10 135 | 7.7 |
| Liver disease | 3826 | 7.2 | 3733 | 6.0 | 7559 | 5.7 |
| History of stroke | 6943 | 13.0 | 5470 | 8.7 | 12 413 | 9.4 |
| History of bleeding | 8076 | 15.2 | 7405 | 11.8 | 15 481 | 11.7 |
| Medication use (NSAIDs, antiplatelets) | 16 443 | 30.8 | 19 908 | 31.8 | 36 351 | 27.5 |
| Alcohol or drug use | 827 | 1.6 | 843 | 1.4 | 1670 | 1.3 |
| Cancer | 12 586 | 23.6 | 12 329 | 19.7 | 24 915 | 18.8 |
| Metastatic cancer | 4197 | 7.9 | 4317 | 6.9 | 8514 | 6.4 |
| HAS‐BLED Score | ||||||
| 0 | 12 564 | 23.6 | 18 023 | 28.8 | 30 587 | 23.1 |
| 1 | 16 424 | 30.8 | 20 858 | 33.3 | 37 282 | 28.2 |
| 2 | 14 536 | 27.3 | 15 282 | 24.4 | 29 818 | 22.5 |
| 3 | 7048 | 13.2 | 6389 | 10.2 | 13 437 | 10.2 |
| 4 | 2320 | 4.4 | 1786 | 2.9 | 4106 | 3.1 |
| 5 | 397 | 0.7 | 251 | 0.4 | 648 | 0.5 |
| 6 | 28 | 0.1 | 19 | 0.0 | 47 | 0.0 |
| 7 | 2 | 0.0 | ··· | ··· | 2 | 0.0 |
| Anticoagulation | ||||||
| LMWH | 5955 | 11.2 | 3980 | 6.4 | 9935 | 7.5 |
| NOAC | 8465 | 15.9 | 12 100 | 19.3 | 20 565 | 15.5 |
| Warfarin | 38 899 | 73.0 | 46 528 | 74.3 | 85 427 | 64.6 |
DVT indicates deep vein thrombosis; HAS‐BLED, Hypertension, Abnormal renal/liver function, Stroke, Bleeding, Labile International Normalized Ratio (INR), Elderly, Drugs or alcohol use; LMWH, low molecular weight heparin; NOAC, non‐vitamin K antagonist oral anticoagulant; PE, pulmonary embolism.
There were a total of 4789 (3.6% of all patients) bleeding events of which, 1847 (38.6%) were classified as major bleeds. Median time to bleed was 50 days (mean 62 days) for all bleeds as well as major bleeds. An additional 2739 patients (2.1% of all patients) died in the hospital during follow‐up with a median time to death of 66 days after index. The remainder of patients were censored with mean follow‐up of 160±32 days.
Table 2 shows the 30, 60, 90, and 180‐day CIF estimates of all bleeds and major bleeds stratified by the HAS‐BLED scores with the corresponding CIF plots in Figure. The incidence of bleeding events increased with increasing HAS‐BLED scores with statistically significant differences between HAS‐BLED scores (P<0.001). Directly comparing the HAS‐BLED scores of 3 and 4, which have been investigated previously as cut points for distinguishing high risk, at 180‐days a score of 4 had cumulative incidence for major bleeding of 3.1% (2.7–3.6%) and all bleeding of 7.3% (6.6–8.0%). A HAS‐BLED score of 3 was associated with a cumulative incidence of 5.3% (4.9–5.6%), which was significantly lower than the HAS‐BLED ≥4 group (P<0.001). The cancer cohort had a higher risk of bleeding than the overall and non‐cancer cohort and there was a consistent correlation between increases in HAS‐BLED scores and the risk of bleeding events.
Table 2.
Cumulative Incidence Proportion of Bleeding Events at 30, 60, 90, and 180 Days of Follow‐Up by HAS‐BLED Score
| Time to Bleeding Event | HAS‐BLED Score | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ≥4 | |
| (A) Major bleeds, overall | |||||
| 30 d | 0.5% (0.4–0.5%) | 0.5% (0.5–0.6%) | 0.6% (0.5–0.7%) | 0.6% (0.5–0.8%) | 1.1% (0.9–1.4%) |
| 60 d | 0.6% (0.5–0.7%) | 0.7% (0.6–0.8%) | 0.8% (0.8–0.9%) | 0.8% (0.7–1.0%) | 1.7% (1.4–2.0%) |
| 90 d | 0.7% (0.6–0.8%) | 0.9% (0.8–1.0%) | 1.1% (1.0–1.2%) | 1.1% (1.0–1.3%) | 2.3% (2.0–2.7%) |
| 180 d | 0.9% (0.8–1.1%) | 1.3% (1.1–1.4%) | 1.7% (1.5–1.8%) | 2.0% (1.8–2.3%) | 3.4% (3.0–3.9%) |
| (B) Major bleeds, no cancer | |||||
| 30 d | 0.4% (0.3–0.5%) | 0.5% (0.4–0.5%) | 0.5% (0.4–0.6%) | 0.6% (0.5–0.8%) | 1.0% (0.7–1.3%) |
| 60 d | 0.5% (0.4–0.6%) | 0.6% (0.5–0.7%) | 0.7% (0.6–0.8%) | 0.8% (0.6–0.9%) | 1.5% (1.2–1.9%) |
| 90 d | 0.6% (0.5–0.7%) | 0.8% (0.7–0.9%) | 0.9% (0.8–1.0%) | 1.0% (0.9–1.2%) | 2.1% (1.7–2.6%) |
| 180 d | 0.7% (0.6–0.8%) | 1.0% (0.9–1.2%) | 1.3% (1.2–1.5%) | 1.8% (1.6–2.1%) | 3.1% (2.6–3.7%) |
| (C) Major bleeds, cancer only | |||||
| 30 d | 0.9% (0.7–1.3%) | 0.7% (0.6–0.9%) | 0.9% (0.7–1.1%) | 0.7% (0.5–0.9%) | 1.3% (0.9–1.9%) |
| 60 d | 1.3% (1.0–1.7%) | 1.1% (0.8–1.3%) | 1.3% (1.1–1.6%) | 1.0% (0.8–1.3%) | 1.9% (1.4–2.6%) |
| 90 d | 1.7% (1.3–2.1%) | 1.3% (1.1–1.6%) | 1.7% (1.5–2.0%) | 1.3% (1.1–1.7%) | 2.7% (2.1–3.4%) |
| 180 d | 2.4% (1.9–2.9%) | 2.1% (1.8–2.5%) | 2.6% (2.2–2.9%) | 2.5% (2.1–3.0%) | 4.1% (3.3–5.0%) |
| (D) All bleeds, overall | |||||
| 30 d | 1.2% (1.1–1.4%) | 1.4% (1.3–1.5%) | 1.5% (1.4–1.6%) | 1.5% (1.4–1.7%) | 2.5% (2.2–2.9%) |
| 60 d | 1.6% (1.5–1.8%) | 1.9% (1.8–2.1%) | 2.1% (1.9–2.2%) | 2.2% (2.0–2.4%) | 3.7% (3.2–4.2%) |
| 90 d | 1.9% (1.8–2.1%) | 2.4% (2.2–2.5%) | 2.7% (2.6–2.9%) | 3.1% (2.8–3.3%) | 5.0% (4.5–5.6%) |
| 180 d | 2.6% (2.4–2.8%) | 3.4% (3.2–3.6%) | 4.2% (4.0–4.4%) | 5.3% (4.9–5.6%) | 8.0% (7.3–8.7%) |
| (E) All bleeds, no cancer | |||||
| 30 d | 1.0% (0.9–1.2%) | 1.3% (1.1–1.4%) | 1.3% (1.2–1.5%) | 1.6% (1.4–1.8%) | 2.3% (1.9–2.8%) |
| 60 d | 1.4% (1.2–1.5%) | 1.7% (1.6–1.9%) | 1.8% (1.7–2.0%) | 2.1% (1.9–2.4%) | 3.5% (3.0–4.1%) |
| 90 d | 1.6% (1.4–1.8%) | 2.1% (1.9–2.2%) | 2.3% (2.1–2.5%) | 2.9% (2.6–3.2%) | 4.8% (4.2–5.5%) |
| 180 d | 2.1% (2.0–2.3%) | 2.9% (2.7–3.1%) | 3.6% (3.4–3.9%) | 4.8% (4.4–5.2%) | 7.6% (6.8–8.5%) |
| (F) All bleeds, cancer only | |||||
| 30 d | 2.5% (2.0–3.0%) | 1.9% (1.6–2.2%) | 1.9% (1.6–2.1%) | 1.4% (1.2–1.8%) | 2.9% (2.2–3.6%) |
| 60 d | 3.3% (2.7–3.8%) | 2.8% (2.4–3.1%) | 2.8% (2.5–3.2%) | 2.4% (2.1–2.9%) | 4.1% (3.3–4.9%) |
| 90 d | 4.2% (3.6–4.9%) | 3.5% (3.1–3.9%) | 3.8% (3.4–4.2%) | 3.5% (3.0–4.0%) | 5.4% (4.5–6.4%) |
| 180 d | 5.9% (5.2–6.7%) | 5.4% (4.9–6.0%) | 5.8% (5.4–6.3%) | 6.3% (5.6–7.0%) | 8.6% (7.4–9.9%) |
HAS‐BLED indicates Hypertension, Abnormal renal/liver function, Stroke, Bleeding, Labile International Normalized Ratio (INR), Elderly, Drugs or alcohol use.
Figure 1.
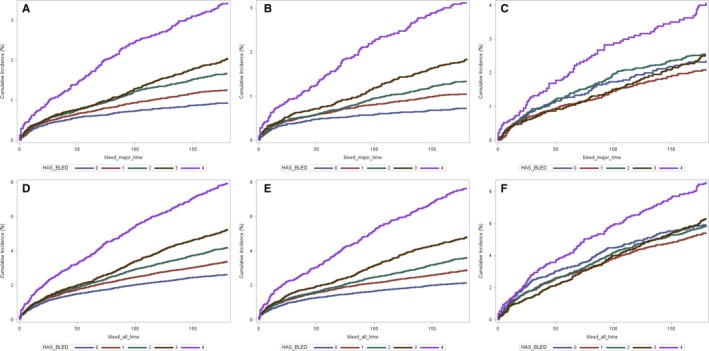
Cumulative incidence function plots of major (A through C) and all (D through F) bleeding events stratified by HAS‐BLED scores for overall (A and D), non‐cancer (B and E), and cancer groups (C and F). HAS‐BLED indicates Hypertension, Abnormal renal/liver function, Stroke, Bleeding, Labile International Normalized Ratio (INR), Elderly, Drugs or alcohol use. Graph lablel of HAS‐BLED score of “4” indicates HAS‐BLED ≥ 4.
The results of the regression analyses are provided in Table 3 for the HAS‐BLED score and Table 4 for individual components and cancer variables. Overall, an increase of 1 point in the HAS‐BLED score increased the rate of bleeding by 20% to 30%. In the cancer only cohort, increases from 0 points to 3 points were not statistically significant. For cancer, only increasing from 3 points to 4 points total was significant. In Table 4, most of the HAS‐BLED's individual components were significant predictors of major and all bleeding with hazard ratios ranging from 1.35 to 1.90. Age and high bleed risk medication use were not found to be significantly predictive in these results.
Table 3.
Survival Regression Model Showing the Incremental Cause‐Specific and Sub‐Distribution Hazard Ratios Comparing HAS‐BLED Scores Risk of Bleeding Events
| HAS‐BLED Score Comparison | Cox PH | Competing Risks | ||
|---|---|---|---|---|
| csHR | 95% CI | sdHR | 95% CI | |
| (A) Major bleeds, overall | ||||
| HAS‐BLED 1 vs 0 | 1.34 | 1.15 to 1.56 | 1.34 | 1.15 to 1.55 |
| HAS‐BLED 2 vs 1 | 1.31 | 1.16 to 1.49 | 1.31 | 1.16 to 1.48 |
| HAS‐BLED 3 vs 2 | 1.22 | 1.06 to 1.39 | 1.21 | 1.06 to 1.39 |
| HAS‐BLED ≥4 vs 3 | 1.71 | 1.44 to 2.04 | 1.71 | 1.44 to 2.03 |
| (B) Major bleeds, no cancer | ||||
| HAS‐BLED 1 vs 0 | 1.44 | 1.20 to 1.73 | 1.44 | 1.20 to 1.72 |
| HAS‐BLED 2 vs 1 | 1.26 | 1.08 to 1.47 | 1.26 | 1.08 to 1.47 |
| HAS‐BLED 3 vs 2 | 1.37 | 1.16 to 1.63 | 1.37 | 1.15 to 1.63 |
| HAS‐BLED ≥4 vs 3 | 1.72 | 1.38 to 2.14 | 1.71 | 1.38 to 2.14 |
| (C) Major bleeds, cancer only | ||||
| HAS‐BLED 1 vs 0 | 0.88 | 0.67 to 1.14 | 0.89 | 0.68 to 1.15 |
| HAS‐BLED 2 vs 1 | 1.22 | 0.99 to 1.49 | 1.23 | 1.00 to 1.50 |
| HAS‐BLED 3 vs 2 | 0.95 | 0.77 to 1.19 | 0.95 | 0.77 to 1.19 |
| HAS‐BLED ≥4 vs 3 | 1.66 | 1.26 to 2.20 | 1.66 | 1.25 to 2.19 |
| (D) All bleeds, overall | ||||
| HAS‐BLED 1 vs 0 | 1.29 | 1.18 to 1.41 | 1.28 | 1.17 to 1.41 |
| HAS‐BLED 2 vs 1 | 1.24 | 1.15 to 1.33 | 1.24 | 1.14 to 1.33 |
| HAS‐BLED 3 vs 2 | 1.24 | 1.14 to 1.35 | 1.24 | 1.14 to 1.35 |
| HAS‐BLED ≥4 vs 3 | 1.55 | 1.38 to 1.73 | 1.54 | 1.38 to 1.73 |
| (E) All bleeds, no cancer | ||||
| HAS‐BLED 1 vs 0 | 1.35 | 1.21 to 1.50 | 1.34 | 1.21 to 1.50 |
| HAS‐BLED 2 vs 1 | 1.24 | 1.13 to 1.36 | 1.24 | 1.13 to 1.36 |
| HAS‐BLED 3 vs 2 | 1.33 | 1.20 to 1.48 | 1.33 | 1.20 to 1.48 |
| HAS‐BLED ≥4 vs 3 | 1.62 | 1.41 to 1.86 | 1.61 | 1.40 to 1.86 |
| (F) All bleeds, cancer only | ||||
| HAS‐BLED 1 vs 0 | 0.89 | 0.76 to 1.05 | 0.90 | 0.76 to 1.06 |
| HAS‐BLED 2 vs 1 | 1.07 | 0.94 to 1.22 | 1.08 | 0.95 to 1.23 |
| HAS‐BLED 3 vs 2 | 1.05 | 0.92 to 1.21 | 1.05 | 0.92 to 1.21 |
| HAS‐BLED ≥4 vs 3 | 1.41 | 1.17 to 1.69 | 1.40 | 1.17 to 1.69 |
C‐indices (95% CI) for Cox PH and competing risks models, respectively: A=0.710 (0.698–0.723), 0.700 (0.690–0.710); B=0.730 (0.712–0.748), 0.720 (0.710–0.730); C=0.685 (0.665–0.705), 0.670 (0.658–0.682); D=0.725 (0.715–0.735), 0.715 (0.705–0.725); E=0.735 (0.728–0.742), 0.730 (0.720–0.740); F=0.670 (0.650–0.690), 0.660 (0.635–0.685). CI indicates confidence interval; csHR, cause‐specific hazard ratio; HAS‐BLED, Hypertension, Abnormal renal/liver function, Stroke, Bleeding, Labile International Normalized Ratio (INR), Elderly, Drugs or alcohol use; PH, proportional hazards; sdHR, sub‐distribution hazard ratio.
Table 4.
Survival Regression Model Results Showing Individual HAS‐BLED Components and Cancer Variables
| Variable | Major Bleeds | All Bleeds | ||||||
|---|---|---|---|---|---|---|---|---|
| Cox PH (c‐Index=0.722) | Competing Risks (c‐Index=0.690) | Cox PH (c‐Index=0.710) | Competing Risks (c‐Index=0.685) | |||||
| csHR | 95% CI | sdHR | 95% CI | csHR | 95% CI | sdHR | 95% CI | |
| Age 65 to 74 vs ≤64, y | 0.93 | 0.81 to 1.07 | 0.94 | 0.82 to 1.08 | 0.88 | 0.81 to 0.97 | 0.89 | 0.82 to 0.98 |
| Age 75+ vs ≤64, y | 0.87 | 0.78 to 0.98 | 0.88 | 0.79 to 0.99 | 0.85 | 0.79 to 0.91 | 0.85 | 0.80 to 0.92 |
| Hypertension | 1.35 | 1.22 to 1.50 | 1.35 | 1.21 to 1.49 | 1.29 | 1.21 to 1.37 | 1.28 | 1.21 to 1.37 |
| Renal disease | 1.55 | 1.36 to 1.76 | 1.54 | 1.35 to 1.75 | 1.59 | 1.46 to 1.72 | 1.58 | 1.45 to 1.71 |
| Liver disease | 1.40 | 1.20 to 1.62 | 1.38 | 1.19 to 1.60 | 1.43 | 1.31 to 1.57 | 1.41 | 1.29 to 1.55 |
| Prior stroke | 1.87 | 1.66 to 2.10 | 1.86 | 1.65 to 2.09 | 1.47 | 1.36 to 1.59 | 1.46 | 1.36 to 1.58 |
| Prior bleeding | 1.69 | 1.51 to 1.88 | 1.69 | 1.51 to 1.88 | 1.91 | 1.78 to 2.04 | 1.91 | 1.78 to 2.04 |
| High risk medication use | 0.92 | 0.83 to 1.02 | 0.92 | 0.84 to 1.02 | 0.97 | 0.92 to 1.04 | 0.98 | 0.92 to 1.04 |
| Alcohol/drug abuse | 1.55 | 1.16 to 2.08 | 1.55 | 1.16 to 2.07 | 1.59 | 1.33 to 1.90 | 1.58 | 1.32 to 1.89 |
| Cancer | 2.25 | 1.98 to 2.56 | 2.11 | 1.85 to 2.41 | 2.07 | 1.91 to 2.25 | 1.94 | 1.78 to 2.11 |
CI indicates confidence interval; csHR, cause‐specific hazard ratio; HAS‐BLED, Hypertension, Abnormal renal/liver function, Stroke, Bleeding, Labile International Normalized Ratio (INR), Elderly, Drugs or alcohol use; PH, proportional hazards; sdHR, sub‐distribution hazard ratio.
Adding cancer to the model as an independent covariate provided the strongest association of all included covariates, with hazard ratios of 2.25 (95% CI: 1.98–2.56) in the cause‐specific model for major bleeds and 2.11 (95% CI: 1.85–2.41) in the sub‐distribution model for major bleeds. Similar results were observed for all bleeds, with hazard ratios of 2.07 (95% CI: 1.91–2.25) in the cause‐specific model and 1.94 (95% CI: 1.78–2.11) in the sub‐distribution model. C‐indices ranged between 0.66 to 0.73 and were highest for the non‐cancer cohort versus the cancer cohort.
Discussion
To our knowledge this is the largest and most comprehensive analysis using the HAS‐BLED score to assess bleeding in patients with VTE. Our principal findings are that the HAS‐BLED score has good predictive validity for bleeding risks in patients with VTE, where a HAS‐BLED score of ≥4 indicated a clear delineation for those at high risk for major bleeding among the total cohort. This observation remained consistent when comparing risk for major bleeding among cancer and non‐cancer patients. Second, the addition of cancer as an independent risk factor to bleeding risk merits consideration, possibly as part of the B criterion (“bleeding tendency or predisposition”) of the HAS‐BLED score.
The use of the HAS‐BLED score, or any risk stratification tool, in patients with VTE should be appropriately applied and not misused.14 A designation of “high risk” in this population should be used to “flag up” high risk patients for additional review and follow‐up. More importantly, the management of reversible bleeding risk factors (and the HAS‐BLED score contains most of the more common modifiable bleeding risk factors) should be performed in all patients, and the designation of high bleed risk is not intended for the withholding of anticoagulation therapy in many patients with VTE.
The HAS‐BLED score has been most validated in patients with AF, where it was predictive of bleeding in patients on no antithrombotic therapy, aspirin and anticoagulants (whether warfarin or non‐warfarin anticoagulants).4, 15 Data in VTE patients are more limited. For example, Kooiman, et al found an increased risk of major bleeding at a HAS‐BLED score of ≥3 in a cohort of 537 acute VTE patients.6 The present study greatly extends the work by Kooiman et al by replicating the study design using a substantially larger cohort, where estimates could be stabilized and individual elements of the risk score could be examined.
The implications of these findings are that the current designation of “high risk” for major bleeds may perhaps be better attributed to those VTE patients with a HAS‐BLED score ≥4 as Kooiman, et al postulated.6 As mentioned above, we should not focus on designating a cut point for what is high risk, but to provide further evidence for continued review or for follow up and action to reduce the elements of the HAS‐BLED score that are modifiable (eg, alcohol excess, concomitant NSAIDs or antiplatelets, uncontrolled hypertension, etc).14 In the current analysis, utilizing a high‐risk cut‐off of HAS‐BLED ≥4 would have a sensitivity of <5% for identifying true positive cases given the distribution of the HAS‐BLED scores in the cohort studied (≈5% of patients had a HAS‐BLED ≥4). Thus, to prevent the most bleed events in a patient population, the range of HAS‐BLED scores should be considered, and modifiable risk factors considered, for clinical intervention.
Cancer was included as a means to stratify the cohort given the possible difference in baseline hazard for the cancer population and also included as a covariate in the regression models. Cancer is among the strongest risk factors for VTE,16, 17 and was present in roughly 20% of the cohort studied. When included as an individual covariate along with components of the HAS‐BLED score, cancer was the strongest predictor of major and overall bleeding. In the cancer cohort, the HAS‐BLED score without consideration of cancer appeared to be less predictive of bleeding events compared with the non‐cancer cohort (Table 3). Along with the finding that age and medication use were not strong predictors, a bleeding risk score specifically tailored to a cancer population with VTE as risk factors could inherently be different compared with an AF population or a general VTE patient group. Alternatively, cancer could be added as an additional risk factor in a modified HAS‐BLED score specific for patients with VTE, where it could be part of the B criterion (“bleeding tendency or predisposition”) of the HAS‐BLED score. Further work is needed to evaluate cancer as a risk factor for bleeding, its suitability in a modified HAS‐BLED score, and the development of other risk scores specifically for the VTE patient population. Of note, cancer has been included as an independent risk factor in a recently developed, VTE‐specific bleed risk stratification score, VTE‐BLEED.18
Validating an existing risk stratification tool like HAS‐BLED across multiple indications may help improve clinical adoption and encourage its uptake in practice.19 Some urge caution in applying bleeding risk scores in the clinical environment, due to the development of these scores based on initial clinical decisions to prescribe anticoagulants which necessitated that the patients were considered lower bleed risk.20 Additionally, bleed risk scores have only been modestly predictive of bleeding in anticoagulated patients with VTE in a prior validation study.21 Also, there is the perception that bleeding risk scores are inappropriately used to deny patients anticoagulation because of their perceived “high risk” although bleeding risk is highly dynamic and modifiable, and the reversible risk factors for bleeding should be addressed in all patients rather than just those at high risk.14 HAS‐BLED has been used widely in clinical and research settings,20 and performs better than other bleeding risk scores (including ATRIA).19 HAS‐BLED should be compared with other bleed scores specifically developed in the VTE population18 to determine the comparative predictive validity of these similar clinical tools.
Limitations
There are several limitations within this study. First, administrative claims data may not reliably capture all diagnoses and procedures within the patient population.22 Second, previous examination of cumulative incidence in HAS‐BLED scoring of major bleed risk have not incorporated the death as a competing risk in the cohort, which suggests that our analysis results may not be directly comparable to previous studies. Our measure of death included only in‐hospital death as death data are not readily available in the MarketScan data. This will have caused an underestimation of the number of major bleeding events detected in this study and some misclassification of follow‐up status as censored rather than the competing risk of death. Misclassification of death is unlikely to have impacted the magnitude of effect between the HAS‐BLED score categories given how few (≈2%) patients died during follow‐up. Misclassifying death would have underestimated the effect size between higher HAS‐BLED scores versus lower scores as the lower scores likely have a smaller proportion of in‐hospital deaths and, thus, an overestimated cumulative incidence of bleeding.11, 23 Further, there were no clear differences between Cox and competing risks regression models which suggested little effect of missing death data. Lastly, the database primarily contains patients with employer‐sponsored health insurance plans, which means that lower income patients, who tend to have worse health outcomes, are likely under‐represented.
Conclusions
The HAS‐BLED score has good predictive validity for bleeding risks in patients with VTE. The addition of cancer as an independent risk factor to bleeding risk merits consideration, possibly as part of the B criterion (“bleeding tendency or predisposition”) of the HAS‐BLED score.
Sources of Funding
This study was partially funded by a grant from the Hematology/Oncology Pharmacy Association (V.R.A. and J.D.B.). Publication of this article was funded in part by the University of Florida Open Access Publishing Fund.
Disclosures
Dr Lip reports receiving consulting feesfrom Bayer/Janssen, BMS/Pfizer, Biotronik, Medtronic, Boehringer Ingelheim, Novartis, Verseon and Daiichi‐Sankyo and speaker fees from Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi‐Sankyo. No fees were directly received personally. Other authors report no conflicts of interest.
(J Am Heart Assoc. 2018;7:e007901 DOI: 10.1161/JAHA.117.007901.)29514808
References
- 1. Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. 2016;41:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, Stevens SM, Vintch JRE, Wells P, Woller SC, Moores L. Antithrombotic therapy for VTE disease. Chest. 2016;149:315–352. [DOI] [PubMed] [Google Scholar]
- 3. You JJ, Singer DE, Howard PA, Lane DA, Eckman MH, Fang MC, Hylek EM, Schulman S, Go AS, Hughes M, Spencer FA, Manning WJ, Halperin JL, Lip GYH. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:e531S–e575S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 5. Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS‐BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol. 2011;57:173–180. [DOI] [PubMed] [Google Scholar]
- 6. Kooiman J, van Hagen N, Iglesias Del Sol A, Planken EV, Lip GY, van der Meer FJ, Cannegieter SC, Klok FA, Huisman MV. The HAS‐BLED score identifies patients with acute venous thromboembolism at high risk of major bleeding complications during the first six months of anticoagulant treatment. PLoS One. 2015;10:e0122520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. White RH, Garcia M, Sadeghi B, Tancredi DJ, Zrelak P, Cuny J, Sama P, Gammon H, Schmaltz S, Romano PS. Evaluation of the predictive value of ICD‐9‐CM coded administrative data for venous thromboembolism in the United States. Thromb Res. 2010;126:61–67. [DOI] [PubMed] [Google Scholar]
- 8. Graham DJ, Reichman ME, Wernecke M, Hsueh YH, Izem R, Southworth MR, Wei Y, Liao J, Goulding MR, Mott K, Chillarige Y, MaCurdy TE, Worrall C, Kelman JA. Stroke, bleeding, and mortality risks in elderly Medicare beneficiaries treated with dabigatran or rivaroxaban for nonvalvular atrial fibrillation. JAMA Intern Med. 2016;176:1662–1671. [DOI] [PubMed] [Google Scholar]
- 9. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 10. Hernandez I, Zhang Y. Comparing stroke and bleeding with rivaroxaban and dabigatran in atrial fibrillation: analysis of the US Medicare Part D data. Am J Cardiovasc Drugs. 2017;17:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 13. Lyman GH, Bohlke K, Khorana AA, Kuderer NM, Lee AY, Arcelus JI, Balaban EP, Clarke JM, Flowers CR, Francis CW, Gates LE, Kakkar AK, Key NS, Levine MN, Liebman HA, Tempero MA, Wong SL, Somerfield MR, Falanga A; American Society of Clinical Oncology . Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update 2014. J Clin Oncol. 2015;33:654–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lip GYH, Lane DA. Bleeding risk assessment in atrial fibrillation: observations on the use and misuse of bleeding risk scores. J Thromb Haemost. 2016;14:1711–1714. [DOI] [PubMed] [Google Scholar]
- 15. Roldan V, Marin F, Fernandez H, Manzano‐Fernandez S, Gallego P, Valdes M, Vicente V, Lip GY. Predictive value of the HAS‐BLED and ATRIA bleeding scores for the risk of serious bleeding in a “real‐world” population with atrial fibrillation receiving anticoagulant therapy. Chest. 2013;143:179–184. [DOI] [PubMed] [Google Scholar]
- 16. Hisada Y, Geddings JE, Ay C, Mackman N. Venous thrombosis and cancer: from mouse models to clinical trials. J Thromb Haemost. 2015;13:1372–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer‐associated venous thrombosis. Blood. 2013;122:1712–1723. [DOI] [PubMed] [Google Scholar]
- 18. Klok FA, Hösel V, Clemens A, Yollo WD, Tilke C, Schulman S, Lankeit M, Konstantinides SV. Prediction of bleeding events in patients with venous thromboembolism on stable anticoagulation treatment. Eur Respir J. 2016;48:1369–1376. [DOI] [PubMed] [Google Scholar]
- 19. Zhu W, He W, Guo L, Wang X, Hong K. The HAS‐BLED score for predicting major bleeding risk in anticoagulated patients with atrial fibrillation: a systematic review and meta‐analysis. Clin Cardiol. 2015;38:555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parks AL, Fang MC. Scoring systems for estimating the risk of anticoagulant‐associated bleeding. Semin Thromb Hemost. 2017;43:514–524. [DOI] [PubMed] [Google Scholar]
- 21. Riva N, Bellesini M, Di Minno MN, Mumoli N, Pomero F, Franchini M, Fantoni C, Lupoli R, Brondi B, Borretta V, Bonfanti C, Ageno W, Dentali F. Poor predictive value of contemporary bleeding risk scores during long‐term treatment of venous thromboembolism. A multicentre retrospective cohort study. Thromb Haemost. 2014;112:511–521. [DOI] [PubMed] [Google Scholar]
- 22. Johnson EK, Nelson CP. Utility and pitfalls in the use of administrative databases for outcomes assessment. J Urol. 2013;190:17–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brown JD, Adams VR. Incidence and risk factors of thromboembolism with multiple myeloma in the presence of death as a competing risk: an empirical comparison of statistical methodologies. Healthcare (Basel). 2016;4:E16. [DOI] [PMC free article] [PubMed] [Google Scholar]


