Abstract
Background
Worsening renal function (WRF) is associated with adverse outcomes in patients with heart failure. We investigated the predictors and prognostic value of WRF during admission, in patients with preserved ejection fraction (HFpEF) versus those with reduced ejection fraction (HFrEF).
Methods and Results
A total of 5625 patients were enrolled in the KorAHF (Korean Acute Heart Failure) registry. WRF was defined as an absolute increase in creatinine of ≥0.3 mg/dL. Transient WRF was defined as recovery of creatinine at discharge, whereas persistent WRF was indicated by a nonrecovered creatinine level. HFpEF and HFrEF were defined as a left ventricle ejection fraction ≥50% and ≤40%, respectively. Among the total population, WRF occurred in 3101 patients (55.1%). By heart failure subgroup, WRF occurred more frequently in HFrEF (57.0% versus 51.3%; P<0.001 in HFrEF and HFpEF). Prevalence of WRF increased as creatinine clearance decreased in both heart failure subgroups. Among various predictors of WRF, chronic renal failure was the strongest predictor. WRF was an independent predictor of adverse in‐hospital outcomes (HFrEF: odds ratio; 2.75; 95% confidence interval, 1.50–5.02; P=0.001; HFpEF: odds ratio, 9.48; 95% confidence interval, 1.19–75.89; P=0.034) and 1‐year mortality (HFrEF: hazard ratio, 1.41; 95% confidence interval, 1.12–1.78; P=0.004 versus HFpEF: hazard ratio, 1.72; 95% confidence interval, 1.23–2.42; P=0.002). Transient WRF was a risk factor for 1‐year mortality, whereas persistent WRF had no additive risk compared to transient WRF.
Conclusions
In patients with acute heart failure patients, WRF is an independent predictor of adverse in‐hospital and follow‐up outcomes in both HFrEF and HFpEF, though with a different effect size.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT01389843.
Keywords: heart failure, heart failure with preserved ejection fraction, heart failure with reduced ejection fraction, renal function
Subject Categories: Heart Failure
Clinical Perspective
What Is New?
From a prospective, multicenter cohort, the KorAHF (Korean Acute Heart Failure) registry, worsening renal function (WRF) occurred in 55.1%, and patients with reduced ejection fraction had a higher rate of WRF than did the patients with preserved ejection fraction (HFpEF).
WRF was an independent predictor of adverse in‐hospital outcomes (defined as all‐cause mortality or aggravation of heart failure during hospitalization) in both patients with reduced ejection fraction and HFpEF, whereas the effect size of WRF was 3‐fold larger in HFpEF.
WRF was associated with 3‐month and 1‐year mortality in both patients with reduced ejection fraction and HFpEF. Regarding the degree of WRF (transient WRF and persistent WRF), transient WRF was an independent risk factor for long‐term mortality, whereas persistent WRF had no additive risk compared with transient WRF.
What Are the Clinical Implications?
Our results reflect the rate of WRF in real‐world heart failure patients; more than one half of acute heart failure patients experienced WRF, which was associated with adverse in‐hospital outcomes and adverse 1‐year mortality.
We compared the effect size of WRF for adverse outcomes in acute heart failure patients with patients with reduced ejection fraction versus HFpEF, showing that WRF had a larger effect size on adverse outcomes in HFpEF patients.
Patients with transient WRF had a higher risk for 1‐year mortality compared with those without WRF, whereas persistent WRF was not a risk factor compared with transient WRF.
Introduction
Worsening renal function (WRF) represents a deterioration of renal function over time, and it is associated with worse outcomes in patients with acute and chronic heart failure (HF).1, 2 Previous reports have shown that WRF is associated with death and HF hospitalization.3, 4 Bidirectional interactions exist between heart disease and kidney disease, referred to as cardiorenal syndrome,5 and neurohumoral maladaptation and decreased renal perfusion are 2 of the discussed mechanisms.6
Among patients with HF, up to 50% of the patients have normal or near normal left ventricular ejection fraction (LVEF), known as HF with preserved ejection fraction (HFpEF).7 They have a prognosis similar to those with HF with reduced ejection fraction (HFrEF).8 Whether HFpEF and HFrEF represent distinct forms of HF, or exist as part of an “HF spectrum,” has yet to be established.9 However, the different patterns of chamber remodeling and disparate responses to medical therapies suggest that they are 2 discrete disease processes. Furthermore, patients with HFrEF have higher B‐type natriuretic peptide (BNP) levels than patients with HFpEF, which suggests that they have a higher degree of neurohumoral activity.10, 11 This prompts consideration of whether the clinical significance of WRF may be dependent on HF type.
Until now, the predictors of WRF and prognostic value of WRF on outcomes have not been well established in either type of HF. Therefore, in this study, we sought to evaluate the influence of WRF in acute HF patients according to HF type.
Methods
Data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure, because the data are protected by the Korean Ministry of Health and Welfare.
Study Design and Population
The KorAHF (Korean Acute Heart Failure) registry was a prospective, multicenter cohort study that was designed to describe patient characteristics, current treatments, and short‐ and long‐term patient outcomes among Korean patients with acute HF. A total of 5625 patients were enrolled from the KorAHF registry from March 2011 to February 2014 at 10 tertiary centers. Detailed information on the study design and results has been previously reported elsewhere.4, 12 Briefly, patients with signs or symptoms of HF and either lung congestion, objective findings of left ventricular systolic dysfunction, or structural heart disease were eligible for the study. Detailed variables were collected at admission, and events including all‐cause mortality, mortality from HF aggravation, and rehospitalization for HF aggravation were recorded after discharge. Follow‐up data were collected from patients by the attending physician at 30 days and 3, 6, 12, 24, 36, 48, and 60 months after discharge. The study is currently continuing follow‐up.
The study protocol was approved by the ethics committee at each participating center and was conducted according to the principles of the Declaration of Helsinki. All patients provided written informed consent for participation in the registry.
Study End Points and Definitions
Clinical outcomes included adverse in‐hospital outcomes (defined as all‐cause mortality or aggravation of HF during hospitalization) and all‐cause mortality during follow‐up. All‐cause mortality was analyzed by short‐term (3‐month) and long‐term (1‐year) events. Despite the absence of expert consensus in terms of the definition of WRF, WRF was defined as an absolute increase in creatinine of 0.3 mg/dL or more, as per previous studies.13 In the present study, WRF was limited to those occurring during admission. Transient WRF was defined as a recovery of creatinine level at discharge, by more than 0.3 mg/dL compared with the peak level during admission. Persistent WRF was defined as a nonrecovered creatinine level. Stages of glomerular filtration rate (GFR) were defined according to the Kidney Disease Outcomes Quality Initiative (KDOQI) Clinical Practice Guidelines for chronic renal failure (CRF). According to GFR at admission, GFR ≥60 mL/min per 1.73 m2, GFR 30 to 59 mL/min per 1.73 m2, and GFR <30 mL/min per 1.73 m2 were defined as “mildly,” “moderately,” and as “severely decreased GFR,” respectively. HFpEF and HFrEF were defined as LVEF ≥50% and LVEF≤40%, respectively.7 Patients with a 40% <LVEF <50% were excluded from the study.
Statistical Analyses
All variables and outcome analyses were based on WRF. Data are presented as numbers and frequencies for categorical variables and as means±SD for continuous variables. For comparison among groups, the χ2 test (or Fisher's exact test when any expected count was <5 for a 2×2 table) for categorical variables and the unpaired Student t test or 1‐way analysis of variance for continuous variables were applied.
To estimate the predictors of WRF, and to predict the independent effect of WRF on adverse in‐hospital outcomes, we used the multivariable logistic regression model using a step‐wise algorithm. Variables found to be statistically significant in the univariate analysis were included in the multivariable model, excluding variables that were closely related to other clinical variables. As a result, variables such as sex, old age, body mass index, hypertension, diabetes mellitus, CRF, previous myocardial infarction, New York Heart Association grade, LVEF (HFrEF or HFpEF), laboratory results at admission (ie, white blood cell count, hemoglobin, sodium, BNP (or N‐terminal pro‐brain‐type natriuretic peptide, C‐reactive protein levels), and intravenous medications during admission (ie, diuretics, inotropes, and vasodilators) were included in the model. Assumptions of the logistic regression model (eg, dichotomous dependent variable, independence in each observation, linear relationship between continuous independent variables and the logit transformation of the dependent variable, and multicollinearity) were tested. The Cox and Snell R Square, Nagelkerke R square, and Hosmer‐Lemeshow goodness‐of‐fit test were used to evaluate model calibration. A 2‐sided probability value <0.05 was considered to estimate statistically significant differences. Statistical tests were performed using SPSS (v22.0; IMB Corporation, Armonk, NY) and Stata (version 10; 2007; StataCorp LP, College Station, TX).
Results
A total of 5625 patients were enrolled in the KorAHF registry. Mean age was 68.5±14.5 years, with a mean LVEF of 37.7±15.6%, and the most common etiology of HF was ischemic heart disease (2113 patients [37.6%]; Table 1). By definition, 3105 patients (55.2%) had HFrEF, 1295 (23.0%) had HFpEF, and the remaining patients had HF with mid‐range ejection fraction (40% <LVEF <50%). Characteristics of patients with HFpEF and HFrEF at admission (Table 2) showed that the 2 groups had distinct characteristics.
Table 1.
Etiology of the Total Population and HFrEF, HFpEF Subgroups
| Etiology | Total Population | HFrEF | HFpEF | P Value |
|---|---|---|---|---|
| Ischemic heart disease | 2113 (37.6) | 1381 (43.0) | 295 (21.7) | <0.001 |
| Valvular heart disease | 804 (14.3) | 232 (7.5) | 405 (31.3) | <0.001 |
| Congenital heart disease | 63 (1.1) | 15 (0.5) | 28 (2.2) | <0.001 |
| Cardiomyopathy | 1159 (20.6) | 933 (30.0) | 82 (6.3) | <0.001 |
| Hypertensive | 222 (3.9) | 91 (2.9) | 82 (6.3) | <0.001 |
| Myositis | 78 (1.4) | 49 (1.6) | 13 (1.0) | 0.141 |
| Infiltrative disease | 70 (1.2) | 23 (0.7) | 26 (2.0) | <0.001 |
| Tachycardia related | 600 (10.7) | 250 (8.1) | 182 (14.1) | <0.001 |
| Thyroid related | 30 (0.5) | 12 (0.4) | 11 (0.8) | 0.052 |
| Toxic related | 59 (1.0) | 44 (1.4) | 3 (0.2) | <0.001 |
| Unknown | 178 (4.0) | 109 (3.5) | 69 (5.3) | 0.005 |
| Others | 152 (3.5) | 30 (1.0) | 122 (9.4) | <0.001 |
Values are expressed in n (%). HFpEF indicates heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Table 2.
Demographic and Laboratory Characteristics Between HFpEF and HFrEF Subgroups
| HFrEF | HFpEF | P Value | |
|---|---|---|---|
| Sex, male | 1872 (60.3%) | 496 (38.3%) | <0.001 |
| Age, y | 66.3±14.7 | 72.0±13.5 | <0.001 |
| BMI, kg/m2 | 23.1±3.8 | 23.8±4.0 | <0.001 |
| LVEF, % | 27.0±7.7 | 59.5±6.6 | <0.001 |
| Risk factors | |||
| Hypertension, n (%) | 1832 (59.0) | 865 (66.8) | <0.001 |
| DM, n (%) | 1297 (41.8) | 435 (33.6) | <0.001 |
| Smoking, %a | 21.3/22.5/56.2 | 11.9/16.5/7106 | <0.001 |
| Previous MI, n (%) | 590 (19.0) | 112 (8.6) | <0.001 |
| Previous PCI, n (%) | 55 (18.2) | 151 (11.7) | <0.001 |
| Previous CABG, n (%) | 181 (5.8) | 34 (2.6) | <0.001 |
| COPD, n (%) | 328 (10.6) | 174 (13.4) | 0.006 |
| CRF, n (%) | 449 (14.5) | 150 (11.6) | 0.011 |
| Previous CVA, n (%) | 442 (14.2) | 206 (15.9) | 0.154 |
| Alcohol, %b | 7.5/35.0/57.5 | 5.3/25.6/69.2 | <0.001 |
| Valve disease, n (%) | 279 (9.0) | 348 (26.9) | <0.001 |
| Arrythmia, n (%) | 898 (28.9) | 531 (41.0) | <0.001 |
| NYHA at admission, % | 13.7/37.0/49.3 | 18.5/37.5/43.9 | <0.001 |
| Initial SBP, mm Hg | 128±29 | 136±30 | <0.001 |
| Initial DBP, mm Hg | 79±19 | 77±18 | <0.001 |
| Initial HR | 95±25 | 86±26 | <0.001 |
| Laboratory analysis | |||
| WBC, 109/L | 8770±4220 | 8160±3500 | <0.001 |
| Hb, g/dL | 12.7±2.3 | 12.0±2.2 | <0.001 |
| Platelet, 109/L | 216±92 | 202±89 | <0.001 |
| Total cholesterol, mg/dL | 153±43 | 149±43 | 0.020 |
| Triglyceride, mg/dL | 99±58 | 101±61 | 0.556 |
| HDL, mg/dL | 41±14 | 42±13 | 0.165 |
| LDL, mg/dL | 96±37 | 90±37 | 0.001 |
| Na, mEq/L | 137±5 | 138±5 | 0.697 |
| Uric acid, mg/dL | 7.3±3.1 | 6.7±2.6 | <0.001 |
| BUN, mg/dL | 26.7±16.8 | 24.5±14.9 | <0.001 |
| Creatinine, mg/dL | 1.51±1.47 | 1.28±1.07 | <0.001 |
| Glucose, mg/dL | 158±78 | 145±68 | <0.001 |
| CRP, mg/L | 2.25±4.11 | 2.39±4.28 | 0.328 |
| BNP, pg/mL | 1600±1410 | 810±850 | <0.001 |
| NTproBNP, pg/mL | 10 690±11 700 | 5280±6870 | <0.001 |
BMI indicates body mass index; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CABG, coronary artery bypass graft surgery; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; CRF, chronic renal failure; CVA, cerebrovascular accident; DBP, diastolic blood pressure; DM, diabetes mellitus; Hb, hemoglobin; HDL, high‐density lipoprotein; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, heart rate; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; MI, myocardial infarction; Na, natrium; NTproBNP, N‐terminal pro‐brain‐type natriuretic peptide; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; WBC, white blood cell.
Smoking: current smoker/ex‐smoker/never smoker.
Alcohol: heavy drinker/social drinker/never drinker.
WRF and Risk Factors
At admission, creatinine level was 1.49±1.47 mg/dL, which peaked to 1.83±1.78 mg/dL during hospitalization and decreased to 1.40±1.36 mg/dL before discharge. WRF during admission occurred in 3101 patients (55.1%), of which 980 (17.4%) had a creatinine increase greater than 1.0 mg/dL. Patients with HFrEF had a higher rate of WRF than did those with HFpEF (56.9% versus 50.3%; P<0.001). Among patients with WRF, 1919 (61.9%) showed transient WRF; rates of persistent WRF were higher in patients with HFrEF (38.7% versus 34.3%; P=0.045; Figure 1).
Figure 1.
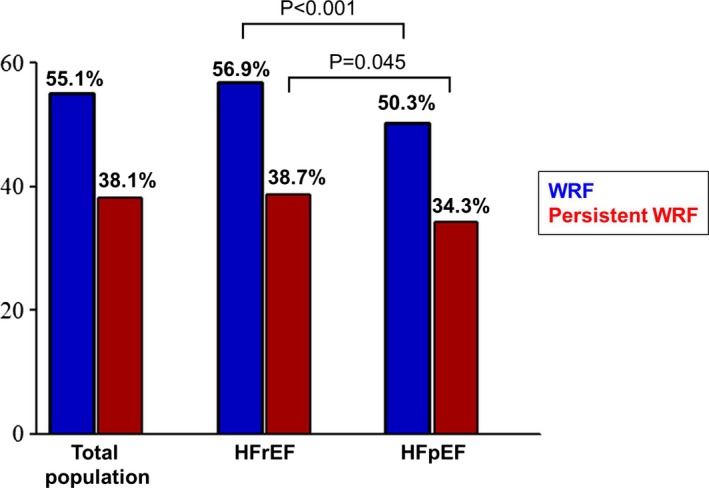
Prevalence of WRF and persistent WRF. WRF occurred in 55.1% of the total population, among which 38.1% showed persistent WRF. In subgroups of HFrEF and HFpEF, WRF and persistent WRF were more common in the HFrEF group. Abbreviations: HFrEF indicates heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; WRF, worsening renal function.
Characteristics of the “no WRF,” “transient WRF,” and “persistent WRF” groups at admission are shown in Table 3. Patients with persistent WRF were more “sick” with a greater number of risk factors, compared with those in the “no WRF” and “transient WRF” groups. Prevalence of WRF increased as GFR decreased, and among the types of WRF, persistent WRF was shown to increase gradually along with grade of GFR decrease (Figure 2A). This trend was also present in both HFrEF and HFpEF subgroups (Figure 2B and 2C).
Table 3.
Demographic and Laboratory Characteristics According to the Severity of WRF
| No WRF (n=2520) | Transient WRF (n=1919) | Persistent WRF (n=1182) | P Value | |
|---|---|---|---|---|
| Sex, male | 1270 (50.4%) | 1046 (54.5%) | 675 (57.1%) | <0.001 |
| Age, y | 67.4±14.8 | 69.0±14.6 | 70.0±13.4 | <0.001 |
| BMI, kg/m2 | 23.6±3.9 | 23.1±3.9 | 23.1±3.7 | <0.001 |
| LVEF, % | 38.8±15.7 | 37.0±16.0 | 36.3±14.8 | <0.001 |
| Risk factors | ||||
| Hypertension, n (%) | 1461 (58.0) | 1188 (61.9) | 845 (71.5) | <0.001 |
| DM, n (%) | 800 (31.7) | 833 (43.4) | 614 (51.9) | <0.001 |
| Smoking, %a | 17.8/19.4/62.8 | 17.8/21.8/60.3 | 17.0/22.9/60.1 | 0.107 |
| Previous MI, n (%) | 355 (14.1) | 327 (17.0) | 235 (19.9) | <0.001 |
| Previous PCI, n (%) | 371 (14.7) | 321 (16.7) | 251 (21.3) | <0.001 |
| Previous CABG, n (%) | 102 (4.1) | 116 (6.0) | 72 (6.1) | 0.003 |
| COPD, n (%) | 273 (10.8) | 220 (11.5) | 140 (11.8) | 0.627 |
| CRF, n (%) | 115 (4.6) | 312 (16.3) | 378 (32.0) | <0.001 |
| Previous CVA, n (%) | 340 (13.5) | 318 (16.6) | 194 (16.4) | 0.007 |
| Alcohol, %b | 6.8/33.3/59.9 | 6.8/29.7/63.5 | 5.8/31.9/62.4 | 0.075 |
| Valve disease, n (%) | 352 (14.0) | 296 (15.4) | 160 (13.5) | 0.252 |
| Arrhythmia, n (%) | 827 (32.8) | 674 (35.1) | 369 (31.2) | 0.064 |
| NYHA II/III/IV at admission, % | 19.6/41.8/38.6 | 13.0/34.2/52.8 | 9.2/30.8/60.0 | <0.001 |
| Initial SBP, mm Hg | 132±28 | 128±32 | 134±33 | <0.001 |
| Initial DBP, mm Hg | 80±18 | 76±19 | 79±20 | <0.001 |
| Initial HR | 92±26 | 93±27 | 93±25 | 0.054 |
| Laboratory analysis | ||||
| WBC, 109/L | 7850±3150 | 9400±4440 | 9250±4840 | <0.001 |
| Hb, g/dL | 12.9±2.1 | 12.2±2.4 | 11.6±2.3 | <0.001 |
| Platelet, 109/L | 213±79 | 211±98 | 205±95 | 0.001 |
| Total cholesterol, mg/dL | 156±42 | 147±44 | 149±44 | <0.001 |
| Triglyceride, mg/dL | 100±60 | 100±61 | 97±54 | 0.247 |
| HDL, mg/dL | 44±14 | 39±14 | 40±14 | <0.001 |
| LDL, mg/dL | 96±36 | 91±38 | 93±39 | 0.066 |
| Na, mEq/L | 138±4 | 137±5 | 137±5 | <0.001 |
| Uric acid, mg/dL | 6.3±2.5 | 7.7±3.1 | 7.4±2.9 | <0.001 |
| BUN, mg/dL | 19.2±8.4 | 31.1±18.0 | 33.3±20.4 | <0.001 |
| Creatinine, mg/dL | 0.99±0.53 | 1.63±1.32 | 2.31±2.38 | <0.001 |
| Glucose, mg/dL | 142±62 | 164±84 | 170±88 | <0.001 |
| CRP, mg/L | 1.50±3.07 | 2.89±4.77 | 3.24±5.02 | <0.001 |
| BNP, pg/mL | 980±890 | 1510±1410 | 1850±1620 | <0.001 |
| NTproBNP, pg/mL | 5560±6670 | 11 000±11 100 | 13 710±14 050 | <0.001 |
| During Adm. | ||||
| Mech. Ventil., n (%) | 63 (2.5) | 453 (23.6) | 346 (29.3) | <0.001 |
| Transfusion, n (%) | 140 (5.6) | 599 (31.2) | 464 (39.3) | <0.001 |
| Intravascular diuretics, n (%) | 1671 (66.3) | 1579 (82.3) | 963 (81.5) | <0.001 |
| Intravascular inotropics, n (%) | 361 (14.3) | 849 (44.2) | 539 (45.6) | <0.001 |
| Intravascular vasodilators, n (%) | 871 (34.6) | 848 (44.2) | 582 (49.2) | <0.001 |
BMI indicates body mass index; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; CRF, chronic renal failure; CRP, C‐reactive protein; DBP, diastolic blood pressure; DM, diabetes mellitus; HDL, high‐density lipoprotein cholesterol; HR, heart rate; LDL, low‐density lipoprotein cholesterol; LVEF, left ventricle ejection fraction; MI, myocardial infarction; NTproBNP, N‐terminal pro‐brain‐type natriuretic peptide; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; WBC, white blood cell.
Smoking: current smoker/ex‐smoker/never smoker.
Alcohol: heavy drinker/social drinker/never drinker.
Figure 2.
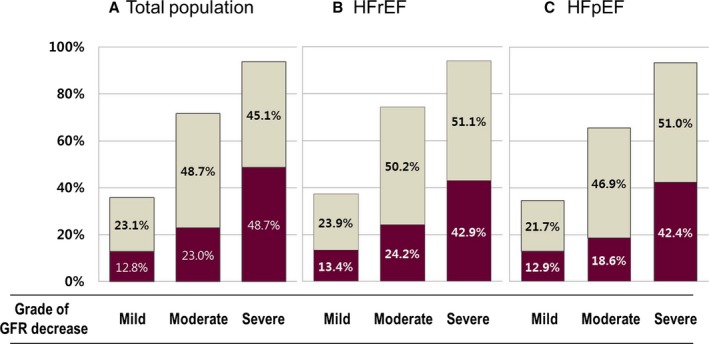
Prevalence of WRF by CRF stage. Prevalence of WRF increased along with the decrease of initial GFR at admission. Dark wine color represents persistent WRF, and gray color represents transient WRF. Both transient and persistent WRF increased as GFR grade decreased, in the (A) total study population, (B) HFrEF group, and (C) HFpEF group. GFR indiactes glomerular filtration rate; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; WRF, worsening renal function.
Among various risk factors identified for WRF, CRF and usage of inotropes were the strongest predictors of WRF (Table 4). When the population was divided into patients with HFrEF and those with HFpEF, the 2 populations showed different risk‐factor profiles; CRF, hyponatremia, anemia, high BNP or N‐terminal pro‐brain‐type natriuretic peptide, high C‐reactive protein, high uric acid levels, and usage of diuretics and inotropes were common risk factors, whereas low body mass index, hypertension, diabetes mellitus, and vasodilator usage during admission were predictors of WRF in patients with HFrEF only.
Table 4.
Multivariate Analysis for Predictors of WRF
| Factor | Whole | HFrEF | HFpEF | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Sex, female | 1.26 | 1.05 to 1.52 | 0.015 | ||||||
| BMI <25 kg/m2 | 1.20 | 1.00 to 1.45 | 0.049 | 1.26 | 1.01 to 1.58 | 0.041 | |||
| Hypertension | 1.22 | 1.02 to 1.45 | 0.030 | 1.27 | 1.03 to 1.56 | 0.028 | |||
| Diabetes mellitus | 1.30 | 1.09 to 1.55 | 0.003 | 1.26 | 1.03 to 1.55 | 0.027 | |||
| CRF | 4.62 | 3.36 to 6.34 | <0.001 | 5.24 | 3.55 to 7.75 | <0.001 | 2.97 | 1.69 to 5.23 | <0.001 |
| Na <135 mmol/L | 1.78 | 1.44 to 2.19 | <0.001 | 1.64 | 1.28 to 2.11 | <0.001 | 2.20 | 1.49 to 3.24 | <0.001 |
| Hb <12 g/dL | 1.63 | 1.36 to 1.96 | <0.001 | 1.73 | 1.38 to 2.17 | <0.001 | 1.52 | 1.10 to 2.09 | 0.011 |
| High BNP or NTproBNP | 1.68 | 1.41 to 2.00 | <0.001 | 1.52 | 1.24 to 1.87 | <0.001 | 2.17 | 1.52 to 3.09 | <0.001 |
| Uric acid >7 mg/dL | 2.28 | 1.92 to 2.70 | <0.001 | 2.32 | 1.90 to 2.84 | <0.001 | 2.54 | 1.83 to 3.52 | <0.001 |
| CRP >0.5 mg/dL | 1.63 | 1.36 to 1.95 | <0.001 | 1.73 | 1.39 to 2.16 | <0.001 | 1.43 | 1.02 to 1.99 | 0.037 |
| Diuretic usage | 1.55 | 1.26 to 1.90 | <0.001 | 1.32 | 1.03 to 1.69 | 0.027 | 2.43 | 1.67 to 3.53 | <0.001 |
| Inotropic usage | 4.88 | 4.03 to 5.92 | <0.001 | 4.32 | 3.48 to 5.36 | <0.001 | 8.65 | 5.45 to 13.74 | <0.001 |
| Vasodilator usage | 1.30 | 1.10 to 1.54 | 0.001 | 1.45 | 1.19 to 1.78 | <0.001 | |||
| HFrEF (vs HFpEF) | 0.84 | 0.70 to 1.02 | 0.077 | ||||||
BMI indicates body mass index; BNP, brain natriuretic peptide; CI, confidence interval; CRF, chronic renal failure; CRP, C‐reactive protein; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; NTproBNP, N‐terminal pro‐brain‐type natriuretic peptide; WBC, white blood cell; WRF, worsening renal function.
Adverse In‐Hospital Outcomes
Among the entire study population, the mean hospitalization duration was 14.0±17.6 days; overall, it was longer in patients with HFrEF than in those with HFpEF (14.8±17.7 versus 12.9±16.9 days; P=0.001). It was also longer in patients with WRF than in those without WRF (7.9±5.2 versus 18.9±22.0 days; P<0.001).
Adverse in‐hospital outcomes occurred in 292 patients (5.2%). Patients with WRF during admission had a higher number of in‐hospital outcomes (24/2520 [1.0%] versus 268/3101 [8.6%], P<0.001 in WRF (−) and WRF (+), respectively) in both the total population and the subgroups of HFrEF and HFpEF (Table 5). In the multivariate analysis, WRF was independently associated with adverse in‐hospital outcomes in the total patient population (odds ratio, 3.18; 95% confidence interval [CI], 1.78–5.67; P<0.001), as well as in the subgroups of HFrEF (odds ratio, 2.73; 95% CI, 1.48–5.01; P=0.001) and HFpEF (odds ratio, 8.53; 95% CI, 1.06–68.48; P=0.044; Table 6).
Table 5.
Adverse in‐Hospital Clinical Outcomes
| No WRF | WRF | P Value | |
|---|---|---|---|
| Total population | 24/2520 (1.0%) | 268/3101 (8.6%) | <0.001 |
| HFrEF | 16/1339 (1.2%) | 149/1765 (8.4%) | <0.001 |
| HFpEF | 3/643 (0.5%) | 36/651 (5.5%) | <0.001 |
HFpEF indicates heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; WRF, worsening renal function.
Table 6.
Multivariate Analysis for Predictors of Adverse in‐Hospital Outcomes
| Total | HFrEF | HFpEF | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value | |
| WRF | 3.18 | 1.78 to 5.67 | <0.001 | 2.73 | 1.48 to 5.01 | 0.001 | 8.53 | 1.06 to 68.48 | 0.044 |
| Age >70 y | 2.08 | 1.40 to 3.10 | <0.001 | 2.29 | 1.48 to 3.54 | <0.001 | NA | ||
| Arrhythmia | 1.72 | 1.13 to 2.62 | 0.011 | 2.05 | 1.25 to 3.37 | 0.004 | NA | ||
| CRP >0.5 mg/dL | 1.66 | 1.16 to 2.37 | 0.005 | 1.60 | 1.07 to 2.37 | 0.021 | NA | ||
| High BNP or NTproBNP | 1.61 | 1.07 to 2.43 | 0.022 | 1.61 | 1.01 to 2.57 | 0.044 | NA | ||
| Inotropics usage | 6.74 | 4.39 to 10.33 | <0.001 | 6.36 | 3.95 to 10.26 | <0.001 | 8.45 | 3.16 to 22.63 | <0.001 |
| HFrEF (vs HFpEF) | 1.14 | 0.71 to 1.83 | 0.590 | NA | NA | ||||
BNP indicates brain natriuretic peptide; CI, confidence interval; CRF, chronic renal failure; CRP, C‐reactive protein; Hb, hemoglobin; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; Na, sodium; NA, not applicable; NTproBNP, N‐terminal pro‐brain‐type natriuretic peptide; OR, odds ratio; UA, uric acid; WRF, worsening renal function.
Short‐ and Long‐Term Follow‐up Mortality by WRF
During the 1‐year follow‐up duration, 970 patients (17.2%) died. Regarding short‐term (3‐month) and long‐term (1‐year) mortality, mortality increased with severity of WRF in the total population, and in the HFrEF and HFpEF subgroups (Figure 3; Table 7). Using a Cox proportional hazard model, WRF was independently associated with short‐term mortality (hazard ratio [HR], 1.66; 95% CI, 1.22–2.25; P=0.001) and long‐term mortality (HR, 1.39; 95% CI, 1.14–1.69; P=0.001). When stratified according to HF type, WRF remained a significant risk factor in both the HFrEF and HFpEF subgroups (Table 8). Regarding the association with 3‐ and 12‐month mortality, WRF has a larger effect size in HFpEF compared with HFrEF (3‐month mortality: HR 1.59 for HFrEF and HR 1.86 for HFpEF; 12‐month mortality: HR 1.35 for HFrEF and HR 1.54 for HFpEF; Table 8).
Figure 3.
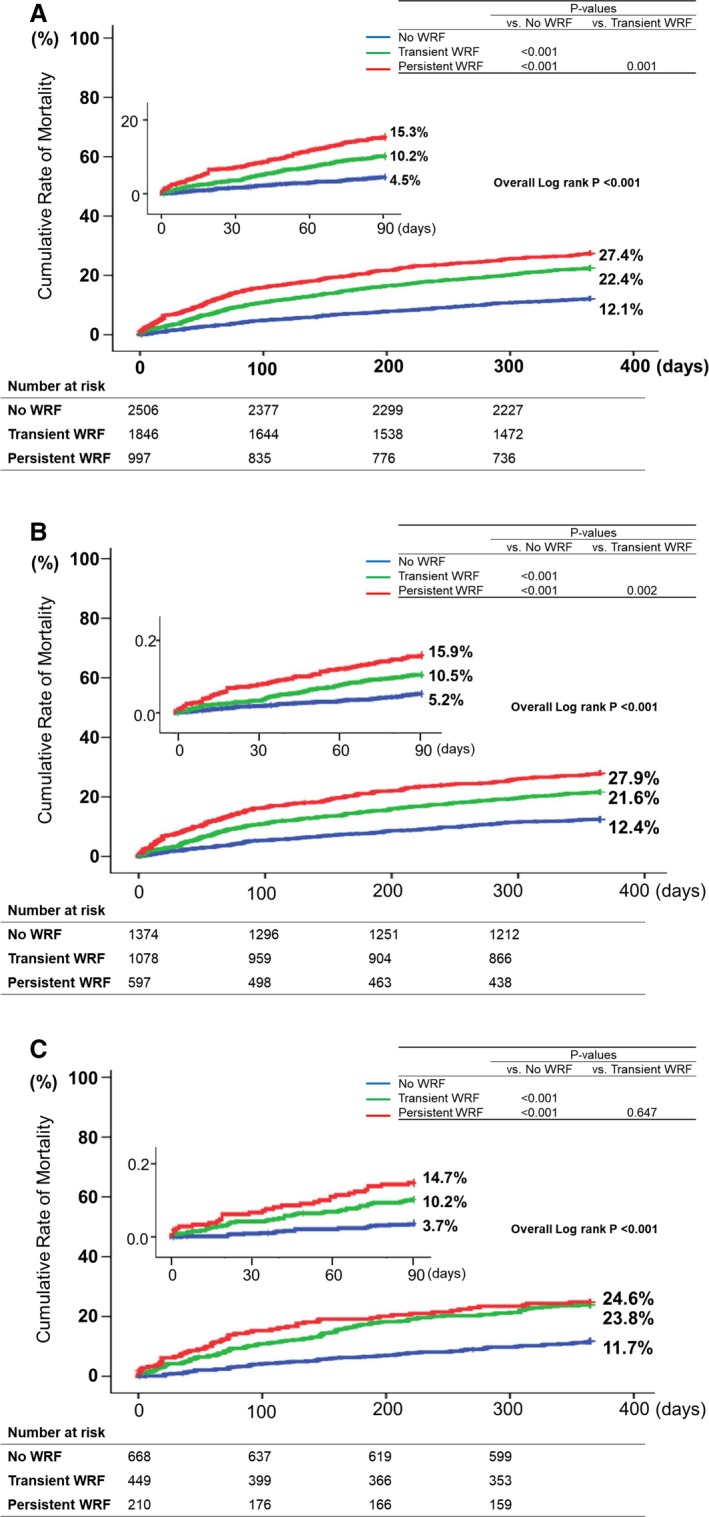
Survival curve of the total population by the severity of WRF. Short‐term (3‐month) and long‐term (1‐year) mortality increased with severity of WRF in (A) the total study population, (B) the HFrEF group, and (C) the HFpEF group. HFpEF indicates heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; WRF, worsening renal function.
Table 7.
1‐Year Follow‐up Clinical Outcomes
| No WRF | Transient WRF | Persistent WRF | P Value | |
|---|---|---|---|---|
| 3‐month mortality | ||||
| Total population | 109 (4.3) | 185 (10.0) | 145 (14.5) | <0.001 |
| HFrEF | 69 (5.2) | 106 (10.2) | 89 (15.3) | <0.001 |
| HFpEF | 24 (3.7) | 38 (9.0) | 28 (14.6) | <0.001 |
| 1‐year mortality | ||||
| Total population | 298 (11.9) | 407 (22.0) | 265 (26.6) | <0.001 |
| HFrEF | 161 (12.1) | 224 (21.5) | 157 (26.9) | <0.001 |
| HFpEF | 74 (11.5) | 93 (21.9) | 48 (25.0) | <0.001 |
Values are expressed in n (%). HFpEF indicates heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; WRF, worsening renal function.
Table 8.
Multivariate Analysis for Predictors of All‐Cause Mortality During Follow‐up
| Total | HFrEF | HFpEF | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| 3‐month mortality | |||||||||
| WRF | 1.66 | 1.22 to 2.25 | 0.001 | 1.59 | 1.10 to 2.29 | 0.013 | 1.86 | 1.06 to 3.27 | 0.031 |
| Age >70 y | 2.46 | 1.81 to 3.35 | <0.001 | 2.50 | 1.77 to 3.55 | <0.001 | 2.36 | 1.22 to 4.60 | 0.011 |
| Female sex | 1.37 | 1.04 to 1.80 | 0.028 | NA | NA | ||||
| BMI <25 kg/m2 | 1.69 | 1.21 to 2.36 | 0.002 | 1.73 | 1.14 to 2.63 | 0.010 | NA | ||
| Hb <12 g/dL | 1.30 | 0.99 to 1.70 | 0.062 | 1.41 | 1.02 to 1.95 | 0.038 | NA | ||
| Na <135 mmol/L | 1.67 | 1.28 to 2.18 | <0.001 | 1.87 | 1.37 to 2.54 | <0.001 | NA | ||
| UA >7 mg/dL | 1.34 | 1.04 to 1.74 | 0.024 | NA | 1.86 | 1.14 to 3.03 | 0.013 | ||
| CRP >0.5 mg/dL | 1.46 | 1.13 to 1.89 | 0.004 | 1.43 | 1.06 to 1.94 | 0.020 | 1.63 | 1.00 to 2.64 | 0.049 |
| High BNP or NTproBNP | 1.76 | 1.32 to 2.36 | <0.001 | 1.95 | 1.35 to 2.84 | <0.001 | NA | ||
| 12‐month mortality | |||||||||
| WRF | 1.39 | 1.14 to 1.69 | 0.001 | 1.35 | 1.06 to 1.71 | 0.014 | 1.54 | 1.08 to 2.18 | 0.016 |
| Age >70 y | 2.20 | 1.80 to 2.69 | <0.001 | 2.30 | 1.83 to 2.90 | <0.001 | 1.95 | 1.28 to 2.98 | 0.002 |
| Female sex | 1.29 | 1.07 to 1.55 | 0.007 | NA | NA | ||||
| BMI <25 kg/m2 | 1.47 | 1.20 to 1.82 | <0.001 | 1.31 | 1.02 to 1.68 | 0.038 | 1.94 | 1.33 to 2.84 | 0.001 |
| Diabetes mellitus | 1.18 | 1.00 to 1.41 | 0.053 | 1.28 | 1.04 to 1.58 | 0.022 | NA | ||
| CRF | 1.36 | 1.10 to 1.69 | 0.005 | 1.43 | 1.11 to 1.84 | 0.006 | NA | ||
| Hb <12 g/dL | 1.43 | 1.19 to 1.73 | <0.001 | 1.46 | 1.17 to 1.83 | 0.001 | NA | ||
| Na <135 mmol/L | 1.64 | 1.37 to 1.96 | <0.001 | 1.73 | 1.39 to 2.14 | <0.001 | 1.42 | 1.01 to 2.00 | 0.047 |
| UA >7 mg/dL | 1.20 | 1.01 to 1.43 | 0.036 | NA | 1.57 | 1.15 to 2.16 | 0.005 | ||
| CRP >0.5 mg/dL | 1.32 | 1.11 to 1.56 | 0.002 | 1.34 | 1.09 to 1.65 | 0.005 | NA | ||
| High BNP or NTproBNP | 1.49 | 1.23 to 1.79 | <0.001 | 1.68 | 1.33 to 2.14 | <0.001 | NA | ||
BNP indicates brain natriuretic peptide; CI, confidence interval; CRF, chronic renal failure; CRP, C‐reactive protein; Hb, hemoglobin; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; Na, sodium; NA, not applicable; NTproBNP, N‐terminal pro‐brain‐type natriuretic peptide; OR, odds ratio; UA, uric acid; WRF, worsening renal function.
We also analyzed whether transient WRF and persistent WRF had different effects on long‐term mortality. Transient WRF, when compared with the “no WRF” group, was a significantly associated with long‐term mortality after adjusting for other risk factors (Table 9). However, persistent WRF was not associated with mortality, when compared with transient WRF.
Table 9.
Effect of Persistent and Transient WRF on 1‐Year Follow‐up Mortality
| HR | 95% CI | P Value | ||
|---|---|---|---|---|
| Total population | ||||
| WRF vs no WRF | Unadjusted | 2.16 | 1.88 to 2.48 | <0.001 |
| Adjusted | 1.44 | 1.19 to 1.75 | <0.001 | |
| Transient WRF vs no WRF | Unadjusted | 1.98 | 1.71 to 2.30 | <0.001 |
| Adjusted | 1.35 | 1.09 to 1.67 | 0.006 | |
| Persistent WRF vs Transient WRF | Unadjusted | 1.27 | 1.09 to 1.48 | 0.003 |
| Adjusted | 1.17 | 0.95 to 1.44 | 0.150 | |
| HFrEF | ||||
| WRF vs no WRF | Unadjusted | 2.09 | 1.74 to 2.51 | <0.001 |
| Adjusted | 1.35 | 1.06 to 1.71 | 0.014 | |
| Transient WRF vs no WRF | Unadjusted | 1.88 | 1.53 to 2.30 | <0.001 |
| Adjusted | 1.32 | 1.02 to 1.71 | 0.038 | |
| Persistent WRF vs Transient WRF | Unadjusted | 1.33 | 1.09 to 1.64 | 0.006 |
| Adjusted | 1.14 | 0.89 to 1.46 | 0.290 | |
| HFpEF | ||||
| WRF vs no WRF | Unadjusted | 2.17 | 1.64 to 2.88 | <0.001 |
| Adjusted | 1.54 | 1.08 to 2.18 | 0.016 | |
| Transient WRF vs no WRF | Unadjusted | 2.06 | 1.52 to 2.80 | <0.001 |
| Adjusted | 1.50 | 1.03 to 2.20 | 0.034 | |
| Persistent WRF vsTransient WRF | Unadjusted | 1.19 | 0.84 to 1.69 | 0.322 |
| Adjusted | 1.14 | 0.75 to 1.72 | 0.538 | |
CI indicates confidence interval; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; WRF, worsening renal function.
Discussion
In the current study, we determined the predictors of WRF during admission and examined the prognostic value of WRF for in‐hospital and short‐ and long‐term outcomes according to the HF type (ie, HFrEF versus HFpEF) and severity of WRF (ie, transient versus persistent WRF). As expected, patients with more‐severe WRF had more cardiac risk factors at admission. Among the various predictors of WRF, CRF and usage of inotropes were the predictors with the largest odds ratio. Interestingly, although WRF was a significant risk factor in both HFpEF and HFrEF, the effect size on in‐hospital outcomes was 3‐fold larger in HFpEF. Finally, WRF was associated with short‐ and long‐term mortality in both HFrEF and HFpEF, and the mortality rate increased alongside the degree of WRF, from no WRF, to transient WRF, and then to persistent WRF. After adjustment, transient WRF was an independent risk factor for long‐term mortality, whereas persistent WRF had no additive risk compared to transient WRF.
Predictors of WRF in HFrEF and HFpEF
There exists a close neurohumoral interaction between the heart and kidney.5, 6 Moreover, HFrEF and HFpEF have distinct anatomical contributors to the differences in hemodynamic and neurohumoral effects; specifically, patients with HFrEF have higher BNP levels than do HFpEF patients in this study and others.11 In this study, prevalence of WRF was higher in patients with HFrEF than in those with HFpEF, and HFrEF was associated with a 1.19‐fold increased risk for WRF. A significant correlation between WRF and BNP levels was also found (data not shown).
Regarding the predictors of WRF, most predictors of WRF were similar in HFrEF and HFpEF; among them, CRF and inotropic usage were shown to have the greatest impact. Patients with preexisting renal dysfunction are more vulnerable to diverse insults, which is demonstrated by the increased prevalence of WRF and persistent WRF in patients with more‐decreased initial GFR. Furthermore, use of inotropic agents indicates a hemodynamic instability that may cause renal hypoperfusion.
WRF as a Risk Factor in HF
Defined as reduction in glomerular filtration, WRF has been associated with adverse outcomes in patients with HF in previous studies. Pimentel et al reported that patients with WRF had a significantly increased risk of all‐cause death and hospital admission in chronic HF patients.14 Damman et al showed that WRF was associated with lower survival and more morbidity in acute HF patients, from the COACH (Coordinating Study Evaluating Outcome of Advising and Counseling in Heart Failure) study.15 Additionally, a meta‐analysis by Damman et al including 45 000 patients from 28 studies showed that WRF was associated with mortality whereas CRF, hypertension, diabetes mellitus, age, and diuretic use were significant predictors for occurrence of WRF.4 Although these studies show similar results, the precise mechanism by which WRF exerts adverse prognostic effects remains unclear. Testani et al explained that several hypothetical mechanisms, including inflammation, oxidative stress, or induction of apoptosis by uremic toxins, may partially contribute to the adverse effect of WRF.16 However, this study also claimed that it is still uncertain whether WRF is a mediator or marker for adverse outcomes.
In this study, magnitude of effect size for WRF decreased from 3.18, to 1.66, to 1.39 for adverse in‐hospital outcomes, and 3‐ and 12‐month mortality, respectively. Because we defined WRF as an in‐hospital event, this decrease seems understandable. Although the exact mechanisms are still not well defined, there are a couple of proposed explanations for the role of WRF as a predictor of adverse outcomes. Renal dysfunction may reflect impaired hemodynamic status that is related to severity of the underlying HF, reflected by the high HR for in‐hospital outcomes.17 Furthermore, renal dysfunction might be a marker of general vascular disease, such as severity of atherosclerosis in both the kidney and heart, which explains the long‐term outcomes with a smaller impact size.18
Impact of WRF on Outcomes in HFrEF Versus HFpEF
Whereas WRF was an independent risk factor for all analyzed outcomes, the effect size was larger in HFpEF; particularly for in‐hospital outcomes, effect size was 3‐fold larger in HFpEF. No current studies have compared the effect size of WRF in HF subtypes, whereas a previous study showed that WRF was a predictor of adverse events in HFpEF.19
Regarding the distinct pathophysiology of HFpEF and HFrEF, it is known that unfavorable volume distribution is a more‐frequent cause of acute decompensation in HFpEF, rather than absolute volume overload.20 Therefore, WRF in HFpEF may reflect a relative hypovolemic status with impaired fluid refilling from the extravascular space to the plasma, which can aggravate both renal and cardiac function. In contrast, patients with HFrEF experience absolute volume overload. WRF in HFrEF may develop during decongestion therapy with diuretics, which can temporarily reduce low effective circulating volume. This could account for the higher proportion of transient WRF in the HFrEF group in our population. Moreover, WRF in HFpEF could be more strongly associated with adverse outcomes compared with HFrEF. However, this can only partially explain the larger effect of WRF in HFpEF, attributed to the complex pathophysiology of WRF.
The other independent predictors of mortality in the HFrEF subgroup were closely identical to those of the total HF population, whereas the HFpEF subgroup showed a smaller number of predictors. HFrEF seemed to be more multifactorial compared with HFpEF, and WRF seemed to play a larger role in HFpEF.
Two Types of WRF: Transient Versus Persistent WRF
From our study, severity of WRF increased as renal function at admission worsened, with more patients with persistent WRF among those with lower GFR. Transient WRF occurs in those with derangements in hemodynamics and neurohormones during acute HF, which may include interaction with diuretics or other theoretically reversible factors. On the other hand, persistent WRF may occur from insults related to coexisting comorbidities and aspects of the HF disease process itself that are irreversible.21
The question of the effect of transient WRF remains controversial. Krishnamoorthy et al showed that transient WRF was associated with a higher risk of 90‐day mortality,22 whereas Aronson et al23 reported that patients hospitalized for HF with transient WRF did not have significantly increased mortality at 6 months postdischarge. In our study, in terms of postdischarge short‐ and long‐term outcomes, transient WRF was an equivalent risk factor to persistent WRF. This discrepancy may be partially attributed to the various definitions of WRF and the outcomes of each study. Because we defined transient WRF as the recovery of renal function during hospitalization,24 those with a slower recovery (ie, those who show recovered renal function at outpatient follow‐up) could have been categorized into the persistent WRF group, thereby diluting the pure effect of persistent WRF. Further studies using universal definitions are mandatory to investigate the effect of transient WRF on outcomes in patients with acute HF.
Study Limitations
There are several limitations in this study. First, although we applied the widely used definition of WRF, this definition has intrinsic limits. Increase in creatinine cannot directly reflect GFR, and it also depends strongly on the renal function at admission. Additionally, regarding that we defined transient and persistent WRF by the creatinine level during admission, some patients might have been misclassified, according to the baseline creatinine level of an individual. Furthermore, according to the time point of the initial laboratory test, the initial creatinine level might not have been the actual baseline level, and therefore some patients with WRF may have been missed. Second, given that this study was based on a registry cohort, it is subject to various biases. Although our study results prove the strong association between WRF and poor clinical outcomes, the observational nature of our study also limits the interpretation of the causal relationship between WRF and clinical outcomes.
Conclusions
WRF was present in 55% of patients in the KorAHF registry. Incidence of WRF increased as renal function at admission decreased and was higher in patients with HFrEF than in those with HFpEF. Predictors of WRF were similar in both HF types. WRF was a prognostic factor of adverse in‐hospital and long‐term outcomes, with a larger effect size in HFpEF compared with HFrEF. Regardless of the degree of WRF, transient and persistent WRF seem to be equivalent risk factors.
Sources of Funding
This work was supported by Research of Korea Centers for Disease Control and Prevention (2010‐E63003‐00, 2011‐E63002‐00, 2012‐E63005‐00, 2013‐E63003‐00, 2013‐E63003‐01, 2013‐E63003‐02, and 2016‐ER6303‐00).
Disclosures
None.
(J Am Heart Assoc. 2018;7:e007910 DOI: 10.1161/JAHA.117.007910.)29535141
References
- 1. de Silva R, Nikitin NP, Witte KK, Rigby AS, Goode K, Bhandari S, Clark AL, Cleland JG. Incidence of renal dysfunction over 6 months in patients with chronic heart failure due to left ventricular systolic dysfunction: contributing factors and relationship to prognosis. Eur Heart J. 2006;27:569–581. [DOI] [PubMed] [Google Scholar]
- 2. Damman K, Navis G, Voors AA, Asselbergs FW, Smilde TD, Cleland JG, van Veldhuisen DJ, Hillege HL. Worsening renal function and prognosis in heart failure: systematic review and meta‐analysis. J Card Fail. 2007;13:599–608. [DOI] [PubMed] [Google Scholar]
- 3. Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin‐converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail. 2011;4:685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Damman K, Valente MA, Voors AA, O'Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta‐analysis. Eur Heart J. 2014;35:455–469. [DOI] [PubMed] [Google Scholar]
- 5. Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation. 2010;121:2592–2600. [DOI] [PubMed] [Google Scholar]
- 6. Braam B, Joles JA, Danishwar AH, Gaillard CA. Cardiorenal syndrome—current understanding and future perspectives. Nat Rev Nephrol. 2014;10:48–55. [DOI] [PubMed] [Google Scholar]
- 7. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 8. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. [DOI] [PubMed] [Google Scholar]
- 9. Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite‐Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. [DOI] [PubMed] [Google Scholar]
- 10. Maisel AS, McCord J, Nowak RM, Hollander JE, Wu AH, Duc P, Omland T, Storrow AB, Krishnaswamy P, Abraham WT, Clopton P, Steg G, Aumont MC, Westheim A, Knudsen CW, Perez A, Kamin R, Kazanegra R, Herrmann HC, McCullough PA; Breathing Not Properly Multinational Study Investigators . Bedside B‐Type natriuretic peptide in the emergency diagnosis of heart failure with reduced or preserved ejection fraction. Results from the Breathing Not Properly Multinational Study. J Am Coll Cardiol. 2003;41:2010–2017. [DOI] [PubMed] [Google Scholar]
- 11. Kang SH, Park JJ, Choi DJ, Yoon CH, Oh IY, Kang SM, Yoo BS, Jeon ES, Kim JJ, Cho MC, Chae SC, Ryu KH, Oh BH; KorHF Investigators. Prognostic value of NT‐proBNP in heart failure with preserved versus reduced EF. Heart. 2015;101:1881–1888. [DOI] [PubMed] [Google Scholar]
- 12. Youn JC, Han S, Ryu KH. Temporal trends of hospitalized patients with heart failure in Korea. Korean Circ J. 2017;47:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herout PM, Harshaw Q, Phatak H, Saka G, McNeill A, Wu D, Sazonov V, DeSagun R, Shirani J. Impact of worsening renal function during hospital admission on resource utilization in patients with heart failure. Am J Cardiol. 2010;106:1139–1145. [DOI] [PubMed] [Google Scholar]
- 14. Pimentel R, Couto M, Laszczynska O, Frioes F, Bettencourt P, Azevedo A. Prognostic value of worsening renal function in outpatients with chronic heart failure. Eur J Intern Med. 2014;25:662–668. [DOI] [PubMed] [Google Scholar]
- 15. Damman K, Jaarsma T, Voors AA, Navis G, Hillege HL, vanVeldhuisen DJ ; COACH investigators . Both in‐ and out‐hospital worsening of renal function predict outcome in patients with heart failure: results from the Coordinating Study Evaluating Outcome of Advising and Counseling in Heart Failure (COACH). Eur J Heart Fail. 2009;11:847–854. [DOI] [PubMed] [Google Scholar]
- 16. Testani JM, Brisco‐Bacik MA. Worsening renal function and mortality in heart failure: causality or confounding? Circ Heart Fail. 2017;10: e003835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leithe ME, Margorien RD, Hermiller JB, Unverferth DV, Leier CV. Relationship between central hemodynamics and regional blood flow in normal subjects and in patients with congestive heart failure. Circulation. 1984;69:57–64. [DOI] [PubMed] [Google Scholar]
- 18. Garg AX, Clark WF, Haynes RB, House AA. Moderate renal insufficiency and the risk of cardiovascular mortality: results from the NHANES I. Kidney Int. 2002;61:1486–1494. [DOI] [PubMed] [Google Scholar]
- 19. Damman K, Perez AC, Anand IS, Komajda M, McKelvie RS, Zile MR, Massie B, Carson PE, McMurray JJ. Worsening renal function and outcome in heart failure patients with preserved ejection fraction and the impact of angiotensin receptor blocker treatment. J Am Coll Cardiol. 2014;64:1106–1113. [DOI] [PubMed] [Google Scholar]
- 20. Takei M, Kohsaka S, Shiraishi Y, Goda A, Izumi Y, Yagawa M, Mizuno A, Sawano M, Inohara T, Kohno T, Fukuda K, Yoshikawa T; West Tokyo Heart Failure Registry Investigators . Effect of estimated plasma volume reduction on renal function for acute heart failure differs between patients with preserved and reduced ejection fraction. Circ Heart Fail. 2015;8:527–532. [DOI] [PubMed] [Google Scholar]
- 21. Lanfear DE, Peterson EL, Campbell J, Phatak H, Wu D, Wells K, Spertus JA, Williams LK. Relation of worsened renal function during hospitalization for heart failure to long‐term outcomes and rehospitalization. Am J Cardiol. 2011;107:74–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krishnamoorthy A, Greiner MA, Sharma PP, DeVore AD, Johnson KW, Fonarow GC, Curtis LH, Hernandez AF. Transient and persistent worsening renal function during hospitalization for acute heart failure. Am Heart J. 2014;168:891–900. [DOI] [PubMed] [Google Scholar]
- 23. Aronson D, Burger AJ. The relationship between transient and persistent worsening renal function and mortality in patients with acute decompensated heart failure. J Card Fail. 2010;16:541–547. [DOI] [PubMed] [Google Scholar]
- 24. Logeart D, Tabet JY, Hittinger L, Thabut G, Jourdain P, Maison P, Tartiere JM, Solal AC. Transient worsening of renal function during hospitalization for acute heart failure alters outcome. Int J Cardiol. 2008;127:228–232. [DOI] [PubMed] [Google Scholar]


