Abstract
Background
Chronic kidney disease (CKD) remains an independent predictor of cardiovascular morbidity and mortality. CKD complicates referral for percutaneous coronary intervention (PCI) in non–ST‐segment–elevation myocardial infarction (NSTEMI) patients because of the risk for acute kidney injury and the need for dialysis, with American College of Cardiology/American Heart Association guidelines underscoring the limited data on these patients.
Methods and Results
Using the National Inpatient Sample to analyze hospitalizations in the United States from 2004 to 2014, we sought to assess PCI utilization and in‐hospital outcomes in NSTEMI admissions with CKD. NSTEMI admissions were identified by International Classification of Diseases, Ninth Edition, Clinical Modification (ICD‐9‐CM) code 410.7. CKD admissions were identified by ICD‐9‐CM code 585. Propensity score–matched cohorts of patients with NSTEMI were matched for age, sex, comorbidities, race, median household income, primary payer status, and hospital characteristics. Of 4 488 795 hospitalizations for NSTEMI, 31% underwent PCI. Overall, 89% of admissions had no CKD. In addition, 32% of NSTEMI admissions with no CKD and 23%, 14%, and 22% with CKD stages 3, 4, and 5 underwent PCI, respectively. Hospitalized NSTEMI patients with CKD stages 4 and 5 had 41% and 20% less likelihood, respectively, of undergoing PCI compared with those with no CKD. Among hospitalized NSTEMI patients with no CKD or CKD stage 3, 4, or 5, PCI‐treated groups had 63%, 57%, 39%, and 59% lower likelihood, respectively, of all‐cause, in‐hospital mortality compared with propensity score–matched medically managed groups.
Conclusions
PCI use decreased among hospitalized NSTEMI patients as CKD severity increased, and all‐cause, in‐hospital mortality was greater for NSTEMI patients admitted with more severe CKD regardless of treatment strategy.
Keywords: acute coronary syndrome, chronic kidney disease
Subject Categories: Quality and Outcomes, Coronary Artery Disease
Clinical Perspective
What Is New?
Non–ST‐segment–elevation myocardial infarction patients treated with percutaneous coronary intervention have less likelihood of all‐cause, in‐hospital mortality compared with propensity score–matched medically managed groups across all chronic kidney disease subgroups.
What Are the Clinical Implications?
Although increasing severity of chronic kidney disease is associated with poor in‐hospital outcomes among patients with non–ST‐segment–elevation myocardial infarction, percutaneous coronary intervention likely reduces in‐hospital mortality among these patients across all chronic kidney disease stages compared with medical management only.
Introduction
Chronic kidney disease (CKD) is an independent predictor of poor cardiovascular outcomes.1, 2 Patients with CKD have accelerated atherosclerosis and an increased risk of myocardial infarction, with cardiovascular disease remaining the most common cause of death.3 Based on previous seminal studies,4, 5, 6 the 2014 American College of Cardiology/American Heart Association (ACC/AHA) and the 2015 European Society of Cardiology guidelines recommend an urgent invasive strategy in high‐risk patients presenting with non–ST‐segment–elevation myocardial infarction (NSTEMI).7, 8 However, CKD patients are often denied invasive coronary angiography and percutaneous coronary intervention (PCI) because of concerns about acute kidney injury accelerating their progression to dialysis.9, 10 In addition, patients with advanced CKD (stages 4 and 5) have been excluded routinely from most large randomized controlled trials (RCTs) of PCI in acute coronary syndrome.11, 12 This is especially relevant because even mildly abnormal renal function has been independently associated with adverse outcomes following NSTEMI.1, 2 RCTs of PCI are not feasible in this population given the lack of clinical equipoise and multicomorbidity, and thus we performed a national propensity score–matched analysis to better understand the role of PCI for CKD patients presenting with NSTEMI. The aims of the study were to examine national trends in PCI use among CKD patients hospitalized for NSTEMI and to assess all‐cause, in‐hospital mortality in propensity score–matched NSTEMI patients with CKD treated with either PCI or medical therapy. We hypothesized that PCI use among NSTEMI patients with CKD would be associated with lower all‐cause, in‐hospital mortality.
Methods
The data and study materials are publicly available, and the analytic methods will be made available to other researchers on request, by contacting the corresponding author, for purposes of reproducing the results or replicating the procedure. The full data set is available at the Healthcare Cost and Utilization Project and National Inpatient Sample (NIS).13
Data Sources and Study Population
This study involved a population‐based sample of patients with NSTEMI and CKD who were admitted to hospitals in 46 states from 2004 to 2014. The 2004–2014 NIS is a set of hospital inpatient databases collected by the Healthcare Cost and Utilization Project. The NIS is the largest publicly available, all‐payer, inpatient‐care database with discharge data from >1200 hospitals, a stratified sample of 20% of all US hospital discharges.13 These data include primary and secondary admission diagnoses, primary and secondary procedures, admission and discharge status, demographic information (eg, sex, age, race and ethnicity, ZIP code–derived median income, and length of stay), hospital region, teaching status, and bed size.14 The study was exempted from institutional review board approval, and the requirement for informed consent was waived because the database uses previously collected deidentified data.
Data Extraction and Study Design
Diagnoses and procedures were identified from the International Classification of Diseases, Ninth Edition, Clinical Modification (ICD‐9‐CM) diagnostic codes. Our sample included individuals who were admitted with a principal diagnosis of NSTEMI, identified by ICD‐9‐CM code 410.7. We excluded patients with age <18 years and missing information on age, sex, or mortality. We also excluded patients with CKD stage 1 and 2 (glomerular filtration rate >60 mL/min/1.73m2) because coding for CKD stages 1 and 2 in the hospital setting has been found to be insensitive.15 CKD patients and end‐stage renal disease (ESRD) patients were identified within the NSTEMI subset by ICD‐9‐CM code 585.X in the secondary diagnosis field. Patients on dialysis were identified by ICD‐9‐CM code 39.95 (hemodialysis) and 54.98 (peritoneal dialysis) in either the primary or secondary field. PCI was identified by ICD‐9‐CM code 36.06 or 36.07 in either the primary or secondary field. The study population was divided into 4 groups: (1) no CKD; (2) CKD stage 3; (3) CKD stage 4; and (4) CKD stage 5, ESRD, or dialysis (hemodialysis or peritoneal dialysis). Patients who went on dialysis because of acute kidney injury (AKI), ICD‐9‐CM code 584.X, were not included in group 4. In previous administrative databases, ICD‐9‐CM coding of chronic renal insufficiency has been shown to have sensitivity of 81.9%, specificity of 98.6%, positive predictive value of 71.2%, and negative predictive value of 99.2%.16 Baseline comorbidities were identified, and comorbidity index was used with methods described by Elixhauser et al.17
Statistical Analyses
Univariate and distributional analysis included measures of central tendency, kurtosis, and skew. Bivariate comparisons, such as those comparing the patient characteristics and in‐hospital mortality, were made using Pearson χ2 tests for dichotomous outcomes and t tests or Kruskal–Wallis tests for continuous outcomes. Hierarchical 2‐level logistic regression models with hospital identifier as a clustering effect were used to assess utilization of PCI in NSTEMI patients with CKD and in‐hospital outcomes with covariates including age, sex, comorbidities, race, median household income, primary payer status, and hospital characteristics (weekend or weekday admission, hospital bed size, hospital region, and hospital teaching status). Comorbidities included in the regression models were obesity, hypertension, diabetes mellitus, heart failure, chronic pulmonary disease, peripheral vascular disease, smoking, and hyperlipidemia. All analyses were weighted using NIS‐provided weights to create national estimates.
To control for imbalances in patient characteristics and institutional characteristics between treatment groups, we constructed 4 separate match cohorts for all CKD subgroups. We used the same variables included in the above‐mentioned hierarchical 2‐level logistic regression models to predict likelihood (propensity) of receiving PCI (using separate multivariable logistic regression). Patients with the nearest propensity scores in 2 treatment groups (medical management only versus PCI) were matched using a 1:1 and 1:2 scheme without replacement using the Greedy method. Maximum propensity difference (caliper width) allowed was 0.05. Patients without matched observation were excluded. Simple logistic regression was used to generate odds ratios (ORs) for propensity score–matched cohorts. C index and Hosmer–Lemeshow goodness‐of‐fit tests were used to assess appropriateness of propensity score models.18
Outcomes
The primary outcome measured, in‐hospital mortality, was assessed using hierarchical 2‐level logistic regression and propensity score matching. Secondary outcomes assessed included rates of bleeding requiring transfusion, in‐hospital death due to bleeding, and likelihood of undergoing PCI.
Results
Baseline Patient Characteristics
Of 4 488 795 patients hospitalized for NSTEMI, 1 373 118 (31%) underwent PCI (Table 1). Among all NSTEMI patients, 89% had no CKD (n=3 998 151), and 4.6% (n=207 351), 2.0% (n=88 179), and 4.3% (n=195 113) of NSTEMI patients had CKD stages 3, 4, and 5, respectively. Overall, 32% of NSTEMI patients with no CKD and 23%, 14%, and 22% with CKD stages 3, 4, and 5, respectively, underwent PCI. Figure 1 demonstrates adjusted all‐cause, in‐hospital mortality rates by treatment approach among NSTEMI patients with various stages of CKD severity, using propensity score–matched groups. In‐hospital mortality was 1.2% among NSTEMI patients with no CKD who underwent PCI and 3.2% among those who did not undergo PCI (P<0.001). As CKD severity increased, in‐hospital mortality increased among NSTEMI patients treated with revascularization and medical management. NSTEMI patients with CKD stage 5 who underwent PCI had in‐hospital mortality of 3.9% compared with 9.0% if they did not undergo PCI (P<0.001).
Table 1.
Clinical and Demographic Characteristics of NSTEMI Admissions
| Characteristic | Overall | No CKD | CKD Stage 3 | CKD Stage 4 | CKD Stage 5/ESRD/Dialysis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N=4 488 795 | n=3 998 151 | n=207 351 | n=88 179 | n=195 113 | |||||||||||
| No PCI | PCI | P Value | No PCI | PCI | P Value | No PCI | PCI | P Value | No PCI | PCI | P Value | No PCI | PCI | P Value | |
| Admissions, n | 3 115 677 | 1 373 118 | 2 726 812 | 1 271 339 | 160 282 | 47 069 | 75 446 | 12 733 | 153 137 | 41 976 | |||||
| Age, y, % | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||||||||
| 18–34 | 0.7 | 0.7 | 0.8 | 0.7 | 0.2 | 0.1 | 0.2 | 0.2 | 0.8 | 0.8 | |||||
| 35–49 | 7 | 13 | 7.4 | 14 | 2.2 | 2.9 | 2.3 | 4.4 | 7.2 | 8.6 | |||||
| 50–64 | 24 | 37 | 25 | 38 | 14 | 19 | 14 | 18 | 30 | 36 | |||||
| 65–79 | 35 | 35 | 35 | 34 | 38 | 47 | 36 | 45 | 42 | 42 | |||||
| ≥80 | 33 | 15 | 33 | 14 | 46 | 31 | 48 | 32 | 20 | 12 | |||||
| Sex, % | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||||||||
| Male | 54 | 65 | 54 | 66 | 58 | 63 | 54 | 58 | 56 | 58 | |||||
| Female | 46 | 35 | 46 | 34 | 42 | 37 | 46 | 42 | 44 | 42 | |||||
| Race, % | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||||||||
| White | 63 | 64 | 64 | 65 | 68 | 68 | 65 | 65 | 45 | 46 | |||||
| Black/Hispanic/Asian | 18 | 15 | 17 | 14 | 20 | 17 | 21 | 19 | 39 | 37 | |||||
| Other | 2.9 | 3.6 | 2.9 | 3.5 | 2.7 | 3.2 | 2.6 | 3 | 3.5 | 5.1 | |||||
| Missing | 16 | 17 | 16 | 18 | 10 | 12 | 12 | 13 | 13 | 12 | |||||
| Comorbidities, % | |||||||||||||||
| Obesity | 11 | 14 | <0.0001 | 11 | 14 | <0.0001 | 16 | 20 | <0.0001 | 13 | 17 | <0.0001 | 10 | 13 | <0.0001 |
| Hypertension | 69 | 71 | <0.0001 | 68 | 70 | <0.0001 | 83 | 87 | <0.0001 | 78 | 83 | <0.0001 | 86 | 90 | <0.0001 |
| Diabetes mellitus | 39 | 35 | <0.0001 | 36 | 33 | <0.0001 | 53 | 57 | <0.0001 | 57 | 62 | <0.0001 | 62 | 68 | <0.0001 |
| Heart failure | 41 | 20 | <0.0001 | 38 | 17 | <0.0001 | 62 | 47 | <0.0001 | 70 | 59 | <0.0001 | 59 | 52 | <0.0001 |
| Chronic pulmonary disease | 25 | 18 | <0.0001 | 25 | 17 | <0.0001 | 28 | 25 | <0.0001 | 27 | 25 | <0.0001 | 22 | 21 | <0.0001 |
| Peripheral vascular disease | 13 | 10 | <0.0001 | 12 | 9.4 | <0.0001 | 22 | 23 | 0.0305 | 22 | 26 | <0.0001 | 23 | 24 | 0.0061 |
| Smoking | 16 | 27 | <0.0001 | 17 | 28 | <0.0001 | 9 | 12 | <0.0001 | 7 | 8.8 | <0.0001 | 8 | 8.9 | <0.0001 |
| Hyperlipidemia | 50 | 67 | <0.0001 | 50 | 67 | <0.0001 | 59 | 72 | <0.0001 | 53 | 63 | <0.0001 | 42 | 53 | <0.0001 |
CKD indicates chronic kidney disease; ESRD, end‐stage renal disease; NSTEMI, non–ST‐segment–elevation myocardial infarction; PCI, percutaneous coronary intervention.
Figure 1.
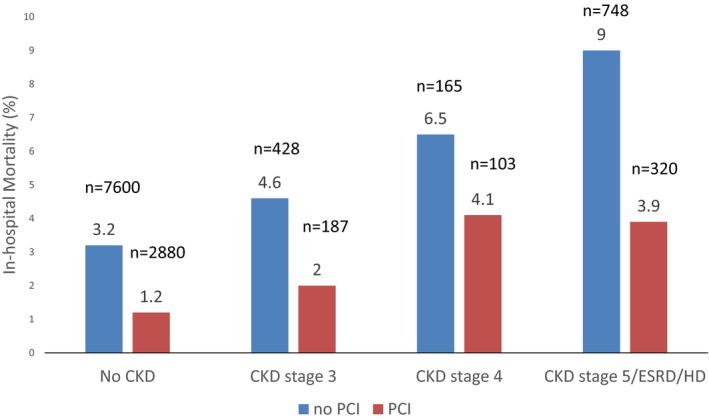
All‐cause, in‐hospital mortality of non–ST‐segment–elevation myocardial infarction patients by CKD status in propensity score‐–matched groups. CKD indicates chronic kidney disease; ESRD, end‐stage renal disease; HD, hemodialysis; PCI, percutaneous coronary intervention.
Clinical and demographic differences as well as discharge characteristics in NSTEMI patients across CKD stages are shown in Tables 1 and 2. Prevalence of hypertension, diabetes mellitus, heart failure, and peripheral vascular disease significantly increased as CKD severity increased. As CKD stage increased, NSTEMI patients who underwent PCI were significantly more likely to be discharged to a facility as opposed to home, with 16% of NSTEMI patients with CKD stage 5 versus 5.3% of NSTEMI patients with no CKD being discharged to a facility after PCI. Furthermore, as CKD stage increased, NSTEMI patients who underwent PCI or medical management had significant greater cost of hospitalization and longer length of stay.
Table 2.
Hospital and Discharge Characteristics of NSTEMI Admissions
| Overall | No CKD | CKD Stage 3 | CKD Stage 4 | CKD Stage 5/ESRD/Dialysis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No PCI | PCI | P Value | No PCI | PCI | P Value | No PCI | PCI | P Value | No PCI | PCI | P Value | No PCI | PCI | P Value | |
| <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||||||
| Discharge to home, % | 58 | 92 | 58 | 93 | 58 | 84 | 58 | 78 | 55 | 79 | |||||
| Discharge to facility, % | 36 | 6.1 | 36 | 5.3 | 36 | 14 | 34 | 18 | 34 | 16 | |||||
| In‐hospital mortality, % | 5.5 | 1.3 | <0.0001 | 5.2 | 1.2 | <0.0001 | 5.4 | 2.1 | <0.0001 | 7.2 | 4.04 | <0.0001 | 9.5 | 3.9 | <0.0001 |
| Median length of stay, d | 4 | 3 | <0.0001 | 4 | 3 | <0.0001 | 5 | 4 | <0.0001 | 5 | 6 | <0.0001 | 5 | 5 | <0.0001 |
| Mean length of stay, d | 5.6 | 3.7 | <0.0001 | 5.4 | 3.5 | <0.0001 | 6.4 | 5.6 | <0.0001 | 6.8 | 7.7 | <0.0001 | 7.9 | 6.7 | <0.0001 |
| Median cost of care ($) | 10 168 | 19 309 | <0.0001 | 9959 | 19 047 | <0.0001 | 10 901 | 21 920 | <0.0001 | 10 816 | 24 977 | <0.0001 | 13 583 | 25 421 | <0.0001 |
| Mean cost of care ($) | 17 478 | 22 751 | <0.0001 | 17 087 | 22 243 | <0.0001 | 18 402 | 26 454 | <0.0001 | 16 915 | 30 348 | <0.0001 | 23 847 | 31 803 | <0.0001 |
CKD indicates chronic kidney disease; ESRD, end‐stage renal disease; NSTEMI, non–ST‐segment–elevation myocardial infarction; PCI, percutaneous coronary intervention.
In‐Hospital Outcomes Among NSTEMI Patients
Table 3 demonstrates the effect of CKD on all‐cause, in‐hospital mortality; rates of bleeding requiring transfusion; and all‐cause, in‐hospital death due to bleeding among NSTEMI patients after multivariate adjustment for age, sex, race, comorbidities, median household income, primary payer status, and hospital characteristics (P<0.0001). NSTEMI admission with severe CKD (stage ≥4) was associated with greater all‐cause, in‐hospital death compared with NSTEMI admission with no CKD; specifically, NSTEMI patients with CKD stage 5 had a 2.06 times higher likelihood of in‐hospital death compared with NSTEMI patients with no CKD (OR: 2.06; 95% CI, 1.97–2.15; P<0.0001). NSTEMI patients admitted with CKD stage ≥3 had greater rates of bleeding requiring transfusion compared with those admitted with no CKD. NSTEMI patients admitted with CKD stage 5 had a 1.59 times higher likelihood of bleeding requiring transfusion compared with those admitted with no CKD (OR: 1.59; 95% CI, 1.48–1.71; P<0.0001). However, the impact of bleeding on in‐hospital death was significant only among NSTEMI admissions with CKD stage 5 (OR: 1.97; 95% CI, 1.75–2.23; P<0.0001).
Table 3.
In‐Hospital Outcomes of NSTEMI Admissions With Various CKD Stages
| In‐Hospital Mortality | Bleeding Requiring Transfusion | In‐Hospital Death Due to Bleeding | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| No CKD | Reference | Reference | Reference | |||
| CKD stage 3 | 0.92 (0.87–0.97) | 0.001 | 1.20 (1.12–1.30) | <0.001 | 0.89 (0.76–1.05) | 0.170 |
| CKD stage 4 | 1.13 (1.06–1.21) | <0.001 | 1.48 (1.35–1.63) | <0.001 | 1.05 (0.86–1.30) | 0.629 |
| CKD stage 5/ESRD/dialysis | 2.06 (1.97–2.15) | <0.001 | 1.59 (1.48–1.71) | <0.001 | 1.97 (1.75–2.23) | <0.001 |
Multivariable logistic regression adjusted for age, sex, race, mode of therapy (medical management, placement of bare metal stent, or placement of drug‐eluting stent), comorbidities (obesity, hypertension, diabetes mellitus, heart failure, chronic pulmonary disease, peripheral vascular disease, smoking, and hyperlipidemia), median household income, primary payer insurance status, admission type (elective vs non elective), admission day (weekend or weekday), hospital bed size, hospital region, and hospital teaching status. CI indicates confidence interval; CKD, chronic kidney disease; ESRD, end‐stage renal disease; NSTEMI, non–ST‐segment–elevation myocardial infarction; OR, odds ratio.
Management Characteristics
Table 4 demonstrates the adjusted likelihood of NSTEMI patients undergoing placement of a bare metal or drug‐eluting stent. After multivariate logistic regression, NSTEMI patients with CKD stages 3, 4, and 5 had 10%, 41%, and 20% less likelihood, respectively, of undergoing PCI compared with NSTEMI patients with no CKD (P<0.001).
Table 4.
Likelihood of Undergoing PCI Among NSTEMI Admissions With Various CKD Stages
| PCI | BMS | DES | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| No CKD | Reference | Reference | Reference | |||
| CKD stage 3 | 0.90 (0.86–0.93) | <0.001 | 0.97 (0.92–1.03) | 0.3635 | 0.86 (0.83–0.90) | <0.001 |
| CKD stage 4 | 0.59 (0.56–0.62) | <0.001 | 0.64 (0.59–0.70) | <0.001 | 0.57 (0.53–0.60) | <0.001 |
| CKD stage 5/ESRD/dialysis | 0.80 (0.78–0.83) | <0.001 | 0.87 (0.82–0.91) | <0.001 | 0.78 (0.75–0.81) | <0.001 |
Multivariable logistic regression adjusted for age, sex, race, comorbidities (obesity, hypertension, diabetes mellitus, heart failure, chronic pulmonary disease, peripheral vascular disease, smoking, and hyperlipidemia), median household income, primary payer insurance status, admission type (elective vs non elective), admission day (weekend or weekday), hospital bed size, hospital region, and hospital teaching status. BMS indicates bare metal stent; CI, confidence interval; CKD, chronic kidney disease; DES, drug‐eluting stent; ESRD, end‐stage renal disease; NSTEMI, non–ST‐segment–elevation myocardial infarction; OR, odds ratio; PCI, percutaneous coronary intervention.
All‐Cause, In‐Hospital Mortality of NSTEMI Patients by Treatment Strategy
Table 5 illustrates the impact of CKD severity on all‐cause, in‐hospital mortality among NSTEMI patients who underwent medical management only or revascularization with PCI after multivariate logistic regression. NSTEMI patients with more severe CKD had increased in‐hospital mortality regardless of treatment approach compared with NSTEMI patients with no CKD. NSTEMI patients with CKD stage 5 who were medically managed had 2.04 times higher likelihood of in‐hospital mortality compared with NSTEMI patients with no CKD (OR: 2.04; 95% CI, 1.95–2.13; P<0.0001). NSTEMI patients with CKD stage 5 who were treated with a bare metal or drug‐eluting stent had 2.10 or 2.02 times higher likelihood, respectively, of in‐hospital mortality compared with NSTEMI admissions with no CKD (base metal stent: OR: 2.10; 95% CI, 1.70–2.59; P<0.001; drug‐eluting stent: OR 2.02; 95% CI 1.71–2.39, P<0.001).
Table 5.
All‐Cause, In‐Hospital Mortality of NSTEMI Admissions With Various CKD Stages
| Medical Management | BMS | DES | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| No CKD | Reference | Reference | Reference | |||
| CKD stage 3 | 0.91 (0.86–0.96) | <0.001 | 1.07 (0.85–1.35) | 0.590 | 0.92 (0.74–1.13) | 0.432 |
| CKD stage 4 | 1.10 (1.03–1.18) | 0.008 | 1.45 (1.00–2.09) | 0.050 | 1.73 (1.34–2.23) | <0.001 |
| CKD stage 5/ESRD/dialysis | 2.04 (1.95–2.13) | <0.001 | 2.10 (1.70–2.59) | <0.001 | 2.02 (1.71–2.39) | <0.001 |
Multivariable logistic regression adjusted for age, sex, race, comorbidities (obesity, hypertension, diabetes mellitus, heart failure, chronic pulmonary disease, peripheral vascular disease, smoking, and hyperlipidemia), median household income, primary payer insurance status, admission type (elective vs non elective), admission day (weekend or weekday), hospital bed size, hospital region, and hospital teaching status. BMS indicates bare metal stent; CI, confidence interval; CKD, chronic kidney disease; DES, drug‐eluting stent; ESRD, end‐stage renal disease; NSTEMI, non–ST‐segment–elevation myocardial infarction; OR, odds ratio.
All‐Cause, In‐Hospital Mortality in a Propensity Score–Matched Cohort
Table 6 demonstrates all‐cause, in‐hospital mortality in NSTEMI patients among different stages of CKD using propensity score–matched pairs (1:1) of PCI and medically treated patients. Among NSTEMI patients who underwent placement of a bare metal or drug‐eluting stent, in‐hospital mortality decreased in NSTEMI patients regardless of baseline level of CKD. Specifically, among NSTEMI patients with no CKD and with CKD stage 3, 4, or 5, PCI‐treated groups had 63% (P<0.0001) and 57% (P<0.0001), 39% (P<0.0001), or 59% (P<0.0001) lower likelihood, respectively, of all‐cause, in‐hospital mortality compared with propensity score–matched medically managed groups.
Table 6.
Impact of PCI on All‐Cause, In‐Hospital Mortality Among NSTEMI Admissions
| CKD Stage | Matched Pairs, n | Medical Management | PCI | ||
|---|---|---|---|---|---|
| OR (95% CI) | P Value | AUC | |||
| No CKD | 232 786 | Reference | 0.37 (0.36–0.39) | <0.001 | 0.72 |
| CKD stage 3 | 9355 | Reference | 0.43 (0.36–0.51) | <0.001 | 0.67 |
| CKD stage 4 | 2525 | Reference | 0.61 (0.48–0.79) | <0.001 | 0.66 |
| CKD stage 5/ESRD/dialysis | 8300 | Reference | 0.41 (0.36–0.47) | <0.001 | 0.64 |
Propensity model adjusted for age, sex, race, comorbidities (obesity, hypertension, diabetes mellitus, heart failure, chronic pulmonary disease, peripheral vascular disease, smoking, and hyperlipidemia), median household income, primary payer insurance status, admission type (elective vs non elective), admission day (weekend or weekday), hospital bed size, hospital region, and hospital teaching status. AUC indicates area under the curve; CI, confidence interval; CKD, chronic kidney disease; ESRD, end‐stage renal disease; NSTEMI, non–ST‐segment–elevation myocardial infarction; OR, odds ratio; PCI, percutaneous coronary intervention.
To ensure proper balance between the propensity score–matched patients admitted for NSTEMI who did and did not undergo PCI, admission characteristics are provided for each CKD subgroup: no CKD (Table S1); CKD stage 3 (Table S2); CKD stage 4 (Table S3); and CKD stage 5, ESRD, or dialysis (Table S4). We noted no significant admission differences among the propensity score–matched groups in any CKD subgroup.
In addition, we performed ad hoc multivariate analysis to assess the interaction between PCI and CKD status on all‐cause, in‐hospital mortality (Table S5). We noted an interaction between PCI and CKD status. There was a declining reduction in mortality with PCI as CKD severity increased.
We also performed a propensity score match using a ratio of 1:2 (1 case to 2 controls). Using this method, we observed similar results regarding all‐cause, in‐hospital mortality in NSTEMI patients among different stages of CKD treated with PCI versus medical management (Table S6).
AKI Necessitating Hemodialysis
Figure 2 illustrates the incidence of AKI necessitating dialysis based on CKD stage using a propensity score–matched model. There was a significantly greater incidence of AKI requiring dialysis among NSTEMI patients admitted with CKD stage 4 who underwent PCI compared with the medically managed propensity score–matched group (7.28% versus 4.3%, P<0.001).
Figure 2.
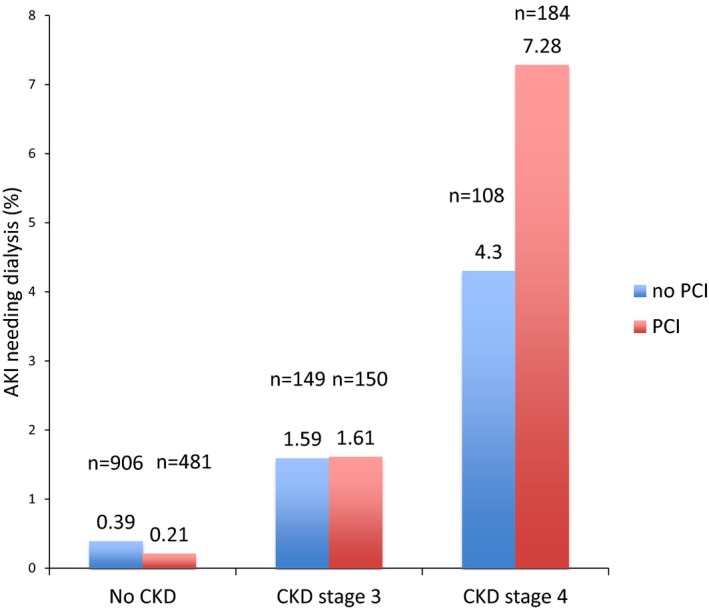
AKI needing dialysis based on CKD stage. AKI indicates acute kidney injury; CKD, chronic kidney disease; PCI, percutaneous coronary intervention.
Discussion
Using the NIS database—the largest all‐payer, inpatient‐care database in the United States, representative of >95% of the population—we demonstrated the following results among NSTEMI patients from 2004 to 2014: (1) Increasing CKD severity was associated with increased rates of all‐cause, in‐hospital mortality and decreased utilization of PCI; (2) increasing CKD severity was associated with increasing bleeding requiring transfusion and in‐hospital death due to bleeding; and (3) patients treated with PCI had less likelihood of all‐cause, in‐hospital mortality compared with propensity score–matched medically managed groups across all CKD subgroups.
Current reports anticipate that the United States will experience a considerable shift in its age structure, with the proportion of people aged >65 years expected to grow faster than any other age group and to increase by >60% in the next 15 years.19 Furthermore, the incidence of acute myocardial infarction increases sharply with age. Whereas unstable angina, NSTEMI, and STEMI are often viewed as increasingly severe points along a disease spectrum, incidence rates of STEMI have declined significantly while NSTEMI rates have increased partly related to the aging population with multiple comorbidities.20 Among the numerous risk factors for acute coronary syndrome, renal dysfunction has been found to be an independent risk factor for cardiovascular disease and is observed in 40% of patients with NSTEMI.21, 22
There is a relative lack of representation of NSTEMI patients with advanced stages of CKD (stages 4 and 5) in large clinical trials. Observational studies have found that CKD is associated with poor in‐hospital, short‐term, and long‐term outcomes among patients with NSTEMI.23, 24, 25 In addition, some observational trials have shown that the outcomes of undergoing PCI are worse in patients with CKD compared with patients with normal renal function.26, 27, 28 However, the benefit of early aggressive reperfusion therapy in CKD patients remains uncertain because these patients have traditionally been excluded from major clinical trials, with no dedicated RCTs of PCI versus medical therapy in this important subgroup. This situation is reflected in the current 2014 ACC/AHA and 2015 European Society of Cardiology guidelines, which state that there are “insufficient data on the benefit‐to‐risk ratio of an invasive strategy in patients with NSTEMI and advanced CKD (stages 4 and 5).” Well‐matched retrospective analyses provide the most contemporary evidence for this population in the absence of RCTs.
Using propensity score matching to control for patient and hospital comorbidities, our results demonstrate the benefit of PCI regarding in‐hospital mortality, even among NSTEMI patients with advanced CKD. Our findings expand those in the SWEDEHEART (Swedish Web‐System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies) study, which revealed that an early invasive strategy was associated with improved short‐term survival in NSTEMI patients with mild–moderate renal insufficiency. However, this benefit declined with lower renal function and was not clear in those with renal failure or who were on dialysis.9 Previous subgroup analysis of 5 RCTs, called VINO (Value of First Day Coronary Angiography/Angioplasty In Evolving Non ST‐Segment Elevation Myocardial Infarction), FRISC II (The Framingham and Fast Revascularization During Instability in Coronary Artery Disease), TIMI IIIB (Thrombolysis in myocardial infarction IIIB clinical trial), ICTUS (Invasive vs Conservative Treatment in Unstable Coronary Syndromes), and TACTICS‐TIMI 18 (Treat Angina with Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy‐Thrombolysis in Myocardial Infarction 18), TIMI IIIB (Thrombolysis in myocardial infarction IIIB clinical trial), ICTUS (Invasive vs Conservative Treatment in Unstable Coronary Syndromes), and TACTICS‐TIMI 18 (Treat Angina with Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy‐Thrombolysis in Myocardial Infarction 18), compared the outcomes of an early invasive versus conservative approach in patients presenting with NSTEMI. Of the 7481 randomly assigned patients, only 267 patients had CKD stage 4 or 5, and the majority of patients were from the TIMI IIIB trial, the oldest trial included in the pooled analysis.29 The study revealed that an early invasive strategy was associated with significant reduction in rehospitalizations but nonsignificant reduction in all‐cause mortality. It is possible that pooled analysis of the VINO, FRISC II, ICTUS, TIMI IIIB, and TACTICS‐TIMI 18 studies was underpowered to detect significant reductions in mortality in NSTEMI patients with advanced stages of CKD undergoing PCI. This is exemplified by the fact that a relatively small number of trials were included in the pooled analysis, with only a modest number of patients with CKD stage ≥3 and a low number of fatalities. Furthermore, heterogeneity of the trials that were included in this pooled analysis also may have contributed to the lack of statistically significant benefit.
Previous studies have also demonstrated baseline renal function is a strong independent predictor of in‐hospital mortality after NSTEMI treated with early revascularization.30, 31, 32 Our study results expand on these findings because we found that baseline renal function is not only a significant independent predictor of in‐hospital death among patients who underwent revascularization but also a significant independent predictor of in‐hospital mortality among NSTEMI patients who underwent medical management only. Adjusted analysis showed that NSTEMI patients with CKD stage 5, ESRD, or dialysis who underwent medical management had significantly higher in‐hospital mortality only compared with those with no CKD. This finding may be partially explained by the greater comorbidity burden we noted as renal function worsened among NSTEMI patients. NSTEMI patients with severe renal impairment are less likely to be given standard medical therapy including aspirin, beta blockers, and angiotensin‐converting enzyme inhibitors, even among those considered “ideal” candidates.33 Recent data from the ACTION registry demonstrated lower use of evidence‐based therapies, in‐hospital procedures, and cardioprotective medications as well as frequent overdosing of medications among NSTEMI patients with severe CKD.22 Our findings are in line with these results: We found that increasing CKD severity was associated with significantly decreased utilization of bare metal or drug‐eluting stents and increased bleeding risk. Patients with CKD stages 4 and 5 had the lowest utilization of PCI for NSTEMI treatment. This may be partially explained by fear of increased risk of contrast‐induced nephropathy transitioning to dialysis.25, 34, 35, 36 Using propensity score–matched NSTEMI admissions, our results demonstrated that the incidence of AKI requiring hemodialysis was significantly higher among NSTEMI patients admitted with CKD stage 4 who underwent PCI compared with those who did not; however, our study database did not allow us to define the timing of AKI relative to when PCI was performed.
Shroff et al have shown that among patients presenting with acute coronary syndrome, the likelihood of in‐hospital bleeding and mortality for patients with advanced CKD was 62% and 44% higher, respectively, compared with non‐CKD patients (P<0.001).37 Advanced CKD was defined as creatinine ≥2.5 mg/dL. Similarly, our study found the likelihood of bleeding requiring transfusion was 20%, 48%, and 59% higher among NSTEMI patients with CKD stages 3, 4, and 5 (or with ESRD or on hemodialysis or peritoneal dialysis), respectively, compared with patients with no CKD. However, we found that in‐hospital death due to bleeding was significantly greater only among NSTEMI patients with CKD stage 5, ESRD, or hemodialysis; these patients had 1.97 times greater in‐hospital mortality compared with those with no CKD. Differences in our results may be partially explained by differences in patient groups—Shroff et al identified acute coronary syndrome patients according to advanced CKD, non‐CKD, and use of dialysis.
Our study must be interpreted in light of its limitations. We could not account for the various factors that influence the decision to manage a patient medically versus invasively, specifically, individual patient preference, cardiovascular risk, and comorbidities. Consequently, no causal relationship could be determined between in‐hospital outcomes and medical management or PCI. Furthermore, there is likely selection bias in terms of which patients received PCI versus medical management, and that bias could not be accounted for using this database. Because of the constraints of the database, we could not identify patients who underwent angiography and then underwent surgical intervention. In addition, there is a possibility of coding errors, although we do not suspect these errors to affect certain study subgroups more than others. We were also unable to obtain information regarding the amount of contrast used or the rates of contrast nephropathy in patients who underwent PCI, and we did not have information on the types of medications used in patients who did or did not undergo PCI. We also did not have detailed clinical and laboratory data to detect the presence of kidney damage, such as degree of albuminuria, urinary sediment abnormalities, and pathologic histological abnormalities. Moreover, propensity score matching may not have made the groups alike regarding important unmeasured confounders.
There is a high likelihood of differential misclassification in our study. Previous research has shown that clinicians are more likely to code for severe CKD than mild CKD, especially given the high prevalence of multicomorbidity among CKD patients.15 Consequently, there is limited sensitivity of ICD‐9‐CM coding for mild CKD. To mitigate this misclassification, we did not include NSTEMI patients with CKD stages 1 and 2; however, there is still a high possibility of this effect among our study groups. Although speculative, this approach may have resulted in less prevalence and accuracy of in‐hospital outcomes among NSTEMI patients with CKD stages 3 and 4 compared with NSTEMI patients with CKD stage 5 or ESRD.
Conclusion
Using the largest publicly available, in‐hospital database in the United States, our results indicate that use of PCI decreases among NSTEMI patients as CKD severity increases and that all‐cause, in‐hospital mortality is greater in NSTEMI patients with more severe CKD regardless of treatment strategy. Patients with CKD presenting with NSTEMI appear to benefit from PCI compared with medical therapy. Prospective studies and RCTs are warranted to substantiate these findings and to assess the best revascularization strategies for this highly vulnerable population.
Disclosures
None.
Supporting information
Table S1. Propensity Demographics for Non–ST‐Segment–Elevation Myocardial Infarction Admissions With No Chronic Kidney Disease
Table S2. Propensity Demographics for Non–ST‐Segment–Elevation Myocardial Infarction Admissions With Chronic Kidney Disease Stage 3
Table S3. Propensity Demographics for Non–ST‐Segment–Elevation Myocardial Infarction Admissions With Chronic Kidney Disease Stage 4
Table S4. Propensity Demographics for Non–ST‐Segment–Elevation Myocardial Infarction Admissions With Chronic Kidney Disease Stage 5
Table S5. Multivariate Analysis to Assess Interaction Between Chronic Kidney Disease and Percutaneous Coronary Intervention on All‐Cause, In‐Hospital Mortality
Table S6. Impact of Percutaneous Coronary Intervention on All‐Cause in Hospital Mortality on Non–ST‐Segment–Elevation Myocardial Infarction Admissions
(J Am Heart Assoc. 2018;7:e007920 DOI: 10.1161/JAHA.117.007920.)29525779
References
- 1. Mehran R, Nikolsky E, Lansky AJ, Kirtane AJ, Kim YH, Feit F, Manoukian S, Moses JW, Ebrahimi R, Ohman EM, White HD, Pocock SJ, Dangas GD, Stone GW. Impact of chronic kidney disease on early (30‐day) and late (1‐year) outcomes of patients with acute coronary syndromes treated with alternative antithrombotic treatment strategies: an ACUITY (Acute Catheterization and Urgent Intervention Triage strategY) substudy. JACC Cardiovasc Interv. 2009;2:748–757. [DOI] [PubMed] [Google Scholar]
- 2. Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, White HD, Nordlander R, Maggioni A, Dickstein K, Zelenkofske S, Leimberger JD, Califf RM, Pfeffer MA. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. [DOI] [PubMed] [Google Scholar]
- 3. Herzog CA, Asinger RW, Berger AK, Charytan DM, Diez J, Hart RG, Eckardt KU, Kasiske BL, McCullough PA, Passman RS, DeLoach SS, Pun PH, Ritz E. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011;80:572–586. [DOI] [PubMed] [Google Scholar]
- 4. Damman P, Hirsch A, Windhausen F, Tijssen JG, de Winter RJ. 5‐year clinical outcomes in the ICTUS (Invasive versus Conservative Treatment in Unstable coronary Syndromes) trial a randomized comparison of an early invasive versus selective invasive management in patients with non‐ST‐segment elevation acute coronary syndrome. J Am Coll Cardiol. 2010;55:858–864. [DOI] [PubMed] [Google Scholar]
- 5. de Winter RJ, Windhausen F, Cornel JH, Dunselman PH, Janus CL, Bendermacher PE, Michels HR, Sanders GT, Tijssen JG, Verheugt FW. Early invasive versus selectively invasive management for acute coronary syndromes. N Engl J Med. 2005;353:1095–1104. [DOI] [PubMed] [Google Scholar]
- 6. Fox KA, Poole‐Wilson PA, Henderson RA, Clayton TC, Chamberlain DA, Shaw TR, Wheatley DJ, Pocock SJ. Interventional versus conservative treatment for patients with unstable angina or non‐ST‐elevation myocardial infarction: the British Heart Foundation RITA 3 randomised trial. Randomized Intervention Trial of unstable Angina. Lancet. 2002;360:743–751. [DOI] [PubMed] [Google Scholar]
- 7. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ. 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2354–2394. [DOI] [PubMed] [Google Scholar]
- 8. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S, Baumgartner H, Gaemperli O, Achenbach S, Agewall S, Badimon L, Baigent C, Bueno H, Bugiardini R, Carerj S, Casselman F, Cuisset T, Erol C, Fitzsimons D, Halle M, Hamm C, Hildick‐Smith D, Huber K, Iliodromitis E, James S, Lewis BS, Lip GY, Piepoli MF, Richter D, Rosemann T, Sechtem U, Steg PG, Vrints C, Luis Zamorano J. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST‐Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 9. Szummer K, Lundman P, Jacobson SH, Schon S, Lindback J, Stenestrand U, Wallentin L, Jernberg T. Influence of renal function on the effects of early revascularization in non‐ST‐elevation myocardial infarction: data from the Swedish Web‐System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART). Circulation. 2009;120:851–858. [DOI] [PubMed] [Google Scholar]
- 10. Nicola R, Shaqdan KW, Aran K, Mansouri M, Singh A, Abujudeh HH. Contrast‐induced nephropathy: identifying the risks, choosing the right agent, and reviewing effective prevention and management methods. Curr Probl Diagn Radiol. 2015;44:501–504. [DOI] [PubMed] [Google Scholar]
- 11. Cannon CP, Weintraub WS, Demopoulos LA, Vicari R, Frey MJ, Lakkis N, Neumann FJ, Robertson DH, DeLucca PT, DiBattiste PM, Gibson CM, Braunwald E. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med. 2001;344:1879–1887. [DOI] [PubMed] [Google Scholar]
- 12. Lagerqvist B, Husted S, Kontny F, Stahle E, Swahn E, Wallentin L. 5‐year outcomes in the FRISC‐II randomised trial of an invasive versus a non‐invasive strategy in non‐ST‐elevation acute coronary syndrome: a follow‐up study. Lancet. 2006;368:998–1004. [DOI] [PubMed] [Google Scholar]
- 13. HCUP Databases. Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality; 2017. [PubMed] [Google Scholar]
- 14. NIS Description of Data Elements. Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality; 2017. [Google Scholar]
- 15. Ronksley PE, Tonelli M, Quan H, Manns BJ, James MT, Clement FM, Samuel S, Quinn RR, Ravani P, Brar SS, Hemmelgarn BR. Validating a case definition for chronic kidney disease using administrative data. Nephrol Dial Transplant. 2012;27:1826–1831. [DOI] [PubMed] [Google Scholar]
- 16. Quan H, Li B, Saunders LD, Parsons GA, Nilsson CI, Alibhai A, Ghali WA. Assessing validity of ICD‐9‐CM and ICD‐10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43:1424–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 18. Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2014;33:1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wan H, Goodkind D, Kowal P. An aging world: 2015. 2016:3.
- 20. McManus DD, Gore J, Yarzebski J, Spencer F, Lessard D, Goldberg RJ. Recent trends in the incidence, treatment, and outcomes of patients with STEMI and NSTEMI. Am J Med. 2011;124:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wong JA, Goodman SG, Yan RT, Wald R, Bagnall AJ, Welsh RC, Wong GC, Kornder J, Eagle KA, Steg PG, Yan AT. Temporal management patterns and outcomes of non‐ST elevation acute coronary syndromes in patients with kidney dysfunction. Eur Heart J. 2009;30:549–557. [DOI] [PubMed] [Google Scholar]
- 22. Fox CS, Muntner P, Chen AY, Alexander KP, Roe MT, Cannon CP, Saucedo JF, Kontos MC, Wiviott SD. Use of evidence‐based therapies in short‐term outcomes of ST‐segment elevation myocardial infarction and non‐ST‐segment elevation myocardial infarction in patients with chronic kidney disease: a report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation. 2010;121:357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. James SK, Lindahl B, Siegbahn A, Stridsberg M, Venge P, Armstrong P, Barnathan ES, Califf R, Topol EJ, Simoons ML, Wallentin L. N‐terminal pro‐brain natriuretic peptide and other risk markers for the separate prediction of mortality and subsequent myocardial infarction in patients with unstable coronary artery disease: a Global Utilization of Strategies To Open occluded arteries (GUSTO)‐IV substudy. Circulation. 2003;108:275–281. [DOI] [PubMed] [Google Scholar]
- 24. Januzzi JL, Cannon CP, DiBattiste PM, Murphy S, Weintraub W, Braunwald E. Effects of renal insufficiency on early invasive management in patients with acute coronary syndromes (The TACTICS‐TIMI 18 Trial). Am J Cardiol. 2002;90:1246–1249. [DOI] [PubMed] [Google Scholar]
- 25. Santopinto JJ, Fox KA, Goldberg RJ, Budaj A, Pinero G, Avezum A, Gulba D, Esteban J, Gore JM, Johnson J, Gurfinkel EP. Creatinine clearance and adverse hospital outcomes in patients with acute coronary syndromes: findings from the global registry of acute coronary events (GRACE). Heart. 2003;89:1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marso SP, Gimple LW, Philbrick JT, DiMarco JP. Effectiveness of percutaneous coronary interventions to prevent recurrent coronary events in patients on chronic hemodialysis. Am J Cardiol. 1998;82:378–380. [DOI] [PubMed] [Google Scholar]
- 27. Kahn JK, Rutherford BD, McConahay DR, Johnson WL, Giorgi LV, Hartzler GO. Short‐ and long‐term outcome of percutaneous transluminal coronary angioplasty in chronic dialysis patients. Am Heart J. 1990;119:484–489. [DOI] [PubMed] [Google Scholar]
- 28. Ahmed WH, Shubrooks SJ, Gibson CM, Baim DS, Bittl JA. Complications and long‐term outcome after percutaneous coronary angioplasty in chronic hemodialysis patients. Am Heart J. 1994;128:252–255. [DOI] [PubMed] [Google Scholar]
- 29. Charytan DM, Wallentin L, Lagerqvist B, Spacek R, De Winter RJ, Stern NM, Braunwald E, Cannon CP, Choudhry NK. Early angiography in patients with chronic kidney disease: a collaborative systematic review. Clin J Am Soc Nephrol. 2009;4:1032–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mueller C, Neumann FJ, Perruchoud AP, Buettner HJ. Renal function and long term mortality after unstable angina/non‐ST segment elevation myocardial infarction treated very early and predominantly with percutaneous coronary intervention. Heart. 2004;90:902–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parikh PB, Jeremias A, Naidu SS, Brener SJ, Lima F, Shlofmitz RA, Pappas T, Marzo KP, Gruberg L. Impact of severity of renal dysfunction on determinants of in‐hospital mortality among patients undergoing percutaneous coronary intervention. Catheter Cardiovasc Interv. 2012;80:352–357. [DOI] [PubMed] [Google Scholar]
- 32. Hanna EB, Chen AY, Roe MT, Wiviott SD, Fox CS, Saucedo JF. Characteristics and in‐hospital outcomes of patients with non‐ST‐segment elevation myocardial infarction and chronic kidney disease undergoing percutaneous coronary intervention. JACC Cardiovasc Interv. 2011;4:1002–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Berger AK, Duval S, Krumholz HM. Aspirin, beta‐blocker, and angiotensin‐converting enzyme inhibitor therapy in patients with end‐stage renal disease and an acute myocardial infarction. J Am Coll Cardiol. 2003;42:201–208. [DOI] [PubMed] [Google Scholar]
- 34. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42:1050–1065. [DOI] [PubMed] [Google Scholar]
- 35. Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, Singh M, Bell MR, Barsness GW, Mathew V, Garratt KN, Holmes DR Jr. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–2264. [DOI] [PubMed] [Google Scholar]
- 36. Marenzi G, Lauri G, Assanelli E, Campodonico J, De Metrio M, Marana I, Grazi M, Veglia F, Bartorelli AL. Contrast‐induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2004;44:1780–1785. [DOI] [PubMed] [Google Scholar]
- 37. Shroff GR, Frederick PD, Herzog CA. Renal failure and acute myocardial infarction: clinical characteristics in patients with advanced chronic kidney disease, on dialysis, and without chronic kidney disease. A collaborative project of the United States Renal Data System/National Institutes of Health and the National Registry of Myocardial Infarction. Am Heart J. 2012;163:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Propensity Demographics for Non–ST‐Segment–Elevation Myocardial Infarction Admissions With No Chronic Kidney Disease
Table S2. Propensity Demographics for Non–ST‐Segment–Elevation Myocardial Infarction Admissions With Chronic Kidney Disease Stage 3
Table S3. Propensity Demographics for Non–ST‐Segment–Elevation Myocardial Infarction Admissions With Chronic Kidney Disease Stage 4
Table S4. Propensity Demographics for Non–ST‐Segment–Elevation Myocardial Infarction Admissions With Chronic Kidney Disease Stage 5
Table S5. Multivariate Analysis to Assess Interaction Between Chronic Kidney Disease and Percutaneous Coronary Intervention on All‐Cause, In‐Hospital Mortality
Table S6. Impact of Percutaneous Coronary Intervention on All‐Cause in Hospital Mortality on Non–ST‐Segment–Elevation Myocardial Infarction Admissions


