Abstract
Background
Hospital procedures have been associated with cognitive change in older patients. This study aimed to document the prevalence of mild cognitive impairment in individuals undergoing left heart catheterization (LHC) before the procedure and the incidence of cognitive decline to 3 months afterwards.
Methods and Results
We conducted a prospective, observational, clinical investigation of elderly participants undergoing elective LHC. Cognition was assessed using a battery of written tests and a computerized cognitive battery before the LHC and then at 3 months afterwards. The computerized tests were also administered at 24 hours (or discharge) and 7 days after LHC. A control group of 51 community participants was recruited to calculate cognitive decline using the Reliable Change Index. Of 437 participants, mild cognitive impairment was identified in 226 (51.7%) before the procedure. Computerized tests detected an incidence of cognitive decline of 10.0% at 24 hours and 7.5% at 7 days. At 3 months, written tests detected an incidence of cognitive decline of 13.1% and computerized tests detected an incidence of 8.5%. Cognitive decline at 3 months using written tests was associated with increasing age, whereas computerized tests showed cognitive decline was associated with baseline amnestic mild cognitive impairment, diabetes mellitus, and prior coronary stenting.
Conclusions
More than half the patients aged >60 years presenting for LHC have mild cognitive impairment. LHC is followed by cognitive decline in 8% to 13% of individuals at 3 months after the procedure. Subtle cognitive decline both before and after LHC is common and may have important clinical implications.
Clinical Trial Registration Information
URL: http://www.anzctr.org.au. Unique identifier: ACTRN12607000051448.
Keywords: catheterization, cognition, cognitive impairment, coronary angiography, left heart catheterization
Subject Categories: Angiography, Cognitive Impairment
Clinical Perspective
What Is New?
A total of 51.7% of individuals >60 years undergoing left heart catheterization were shown to have mild cognitive impairment before the procedure when tested with a battery of cognitive tests.
At 3 months after left heart catheterization, 8% to 13% of individuals experienced further cognitive decline.
What Are the Clinical Implications?
Mild cognitive impairment is common in elderly individuals undergoing left heart catheterization and exceeds prevalence in the general population, most likely because of the presence of cardiovascular disease.
We suggest that elderly patients presenting for left heart catheterization should have baseline cognitive assessment as a routine and that consideration of the risk of further cognitive decline should form part of the informed consent process.
Introduction
Left heart catheterization (LHC) for either diagnostic and/or therapeutic purposes is a common procedure. More than 1.3 million LHC procedures are performed every year in the United States, with a median patient age of 65 years.1 In Australia, >160 000 LHCs are performed every year.2 The older age in this group of patients and the presence of cardiovascular disease are important risk factors for preexisting cognitive decline. Although cognitive decline has been observed after prolonged left atrial ablation procedures,3 there is limited information using sensitive cognitive testing on the impact of LHC procedures on cognition in older adults. Cognitive decline in elderly people may be subtle and often requires objective neuropsychological testing to be identified. Petersen et al4 described mild cognitive impairment (MCI)5 as an entity in which the individual was neither demented nor impaired in activities of daily life, but in whom there was both a subjective complaint by the individual or an informant and objective evidence of cognitive decline. The prevalence of MCI ranges from 14% to 18% in adults aged ≥70 years.6 In older adults, MCI may reflect the early stages of neurodegenerative diseases, such as Alzheimer disease, but may also reflect cerebral vascular disease. Accordingly, cerebrovascular contributions in those with cardiovascular disease are likely to make those presenting for LHC prone to MCI.7 Rosengart et al have shown that individuals with coronary artery disease (CAD) scheduled for either coronary artery surgery or percutaneous interventions performed worse than healthy controls on a battery of neuropsychological tests.8 In another investigation, up to one third of patients presenting for LHC had Montreal Cognitive Assessment scores <26, indicating some degree of cognitive impairment.9 Although LHC is considered to be a minimally invasive procedure, if there is a high baseline incidence of MCI, then individuals presenting for LHC may be at risk for postprocedural cognitive decline. This holds true for orthopedic surgery, where patients with cognitive impairment at baseline were particularly at risk for further decline afterwards.10 To date, small studies have shown conflicting results after LHC at 3 months.9, 11 To investigate these issues, we established a large, prospective, observational study with the primary aim to formally document the prevalence of MCI in older adults scheduled for LHC and to identify cognitive outcomes up to 3 months after the procedure using sensitive testing tools. In addition, we explored the association of clinical management subsequent to LHC with cognitive outcome.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
This investigation was a prospective, observational, clinical study, in patients scheduled for LHC, with the primary aim to identify the prevalence of MCI and subsequent cognitive performance to 3 months. A secondary aim was to document the effect of subsequent clinical management on cognitive changes after LHC. All patients underwent baseline coronary angiography and left ventriculography. Subsequent management pathways were classified as follows: no further interventional therapy; minor intervention (including percutaneous coronary intervention); or major intervention (major noncardiac or cardiac surgery [valve replacement and/or coronary artery bypass graft]).
The study was registered with the Australian Clinical Trials Registry. Patients were recruited from 2 large tertiary hospitals between July 5, 2007 and July 22, 2012. Three‐month data from 168 of these patients have been previously published as part of a comparative article,12 whereas a subset of 56 patients was studied using transcranial Doppler to detect microemboli.13 Patients for elective LHC were recruited from waiting lists at St Vincent's Hospital or St Vincent's Private Hospital (Melbourne, Australia). Inclusion criteria were as follows: age ≥50 years; residence within sufficient proximity to enable cognitive testing at home; no history of neurological disease; and adequate English for neuropsychological testing. Exclusion criteria included evidence of poor cognitive function on the basis of a baseline Mini‐Mental State Examination score <26; a Clinical Dementia Rating score ≥1.0; physical limitation to neuropsychological testing (eg, blindness); significant comorbidities (eg, severe cardiac failure); and long‐term medication use that may confound cognitive testing (eg, benzodiazepines).
All participants gave written informed consent, and the study was approved by the St Vincent's Hospital Ethics Review Board (Human Research Ethics Committee no. 172/06).
Cognitive Testing and Classification
All study participants completed a conventional paper‐and‐pencil (written) test battery during home visits at baseline and at 3 months after LHC. The test battery was chosen to examine specific psychological domains and consisted of the following: the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) Auditory Verbal Learning Test (episodic memory); Trail Making Test Parts A and B (attention/executive function); Digit Symbol Substitution Test (attention psychomotor function); Controlled Oral Word Association Test (executive function); CERAD Semantic Fluency test, animals (verbal fluency); and the Grooved Pegboard test, dominant and nondominant hands (manual dexterity). All of these tests have been described elsewhere.14 Memory is believed to be the best indicator for future cognitive decline attributable to Alzheimer disease, whereas executive function is more likely to reflect vascular cognitive impairment, although there is much overlap between all the psychological domains.
Computerized tests are simple to administer, lend themselves to more repeated use, and can consequently be administered at more frequent time intervals.15 Participants were thus also administered a computerized cognitive test battery at baseline (during the week before LHC in conjunction with the written tests) and at 24 hours (or discharge if before this), 7 days, and 3 months after LHC (in conjunction with the written tests).
All testing (except the 24‐hour test) was done at home visits by the research team. In addition, the National Adult Reading Test was administered at baseline to estimate intelligence quotient,16 in addition to screening using the Mini‐Mental State Examination. Visual analogue scales for anxiety, depression, and fatigue (10‐cm line, where 0 is minimum and 10 is maximum) were administered at baseline and 3 months because these have been shown to affect cognitive function.
The computerized battery consisted of a subset of 4 tasks from the Cogstate battery (Melbourne, Australia). These were the maze (executive function) and the use of computerized playing cards to assess detection reaction time, choice reaction time, and one‐back test (working memory and psychomotor function).17 Reaction times <100 and >1000 ms were classified as abnormally fast or slow responses and excluded (as per Cogstate validation rules).15 The reaction time distributions for each test showed significant negative skew (ie, skew/ skew SD >1.96); therefore, these were normalized using a logarithmic base‐10 transformation. The mean of the log10 reaction time was calculated for each participant for each task. For the computerized battery, cognitive decline was defined using the Reliable Change Index (RCI; calculation described later) if an individual decreased by 1.5 SDs on ≥2 of the 5 tests to maintain the type I error at <5%.18
MCI was defined at baseline by the absence of dementia, preservation of normal activities of daily living (self‐report), and the presence of a subjective complaint in cognition by either the participant or informant (in response to the question, “Do you have a problem with thinking or memory in daily life?”), together with cognitive performance on any 1 written test >1.5 SDs below normative data. The normative data came from standard published sources and were adjusted for age. MCI was classified as amnestic MCI (aMCI; single domain or multidomain) or nonamnestic (single domain or multidomain), depending on which tests/cognitive domains were implicated.6 This recognizes that memory decline is believed to indicate deterioration attributable to Alzheimer disease in the early stages.
At 3 months, cognitive decline for written tests was calculated using the RCI.19 Calculation of RCI requires a control group to undergo the same tests at the same time intervals as study participants. We used a control group consisting of patients with osteoarthritis who were recruited from advertisements in appropriate newsletters and senior citizens centers.10 To calculate the RCI for tests, the difference in performance from the baseline to 3‐month assessment was computed for each test for each of the control subjects. The mean and SD of the distributions of difference scores for each test were then established. For each subject, assessment at 3 months for each test was used to calculate the RCI, as follows: RCI=(patient score−baseline score)−(practice effect estimated from controls)/(SD of difference scores estimated from control group). For each test, deterioration in RCI ≥1.96 was classified as abnormal decline in cognitive performance. The cutoff of RCI=−1.96 is the point beyond which 5% of the values from the normal sample population will fall (ie, P<0.05, 1 tailed). Patients who completed ≥2 tests of the neuropsychological batteries were included in the analyses for each battery of tests (written tests and computerized tests). Cognitive decline was defined only if an individual showed cognitive decline on ≥2 performance tests and/or a combined Z score ≤ −1.96 (defined as ∑ Z scores/SD ∑ Z scores in controls)
Clinical Management
As an indicator of atheroma burden, and noting the correlation between even simple scoring systems,20 diagnostic LHC outcomes were graded simply according to number of vessels with >50% narrowing: group 0, no significant CAD; group 1, grade 1 CAD (1 vessel affected); group 2, grade 2 CAD (2 vessels affected); and group 3, grade 3 CAD (3 vessels affected).
Oral temazepam (10 mg) and promethazine (25 mg) were administered 30 minutes before the procedure for premedication and sedation. No other sedatives were given. LHC was performed via a percutaneous femoral approach using local anesthetic at the puncture site, according to institutional protocol. Acetaminophen was used for postprocedural analgesia.
Blood was also collected at baseline for apolipoprotein E4 genotyping.
Statistical Analysis
We anticipated a prevalence of baseline MCI of 35% on the basis of a previous study of patients with cardiovascular disease who were scheduled for cardiac surgery.21 Assuming an overall incidence of further cognitive decline at 3 months of 13% (on the basis of patients undergoing cardiac surgery14) then to detect a 4:1 ratio of cognitive decline in patients with MCI versus those without MCI (on the basis of population data in which subjects with MCI convert to dementia at 4 to 5 times those without MCI6), we estimated that we would require 148 patients with MCI and 148 patients without MCI. To acquire this number, a total of 423 patients would be required to achieve a power of 80% and α of 0.05. Assuming a loss to follow‐up of 20%, we aimed to recruit 510 patients.
Group comparisons were made using unpaired t tests for continuous variables, the Spearman ranked correlation coefficient or Wilcoxon rank‐sum test for ranked data, and χ2 or Fisher's exact test for dichotomous data. All hypothesis testing was 2 tailed. Associations were determined using univariable analysis and multivariable logistic regression, with a probability value of P<0.2 set for entry into the multivariable regression models. Tests were performed using STATA, version 14.0 (StataCorp, College Station, TX). A probability value of P<0.05 indicated statistical significance.
Results
Review of patient recruitment in 2012 showed that the baseline MCI rate was >50%, such that the then recruited 455 patients exceeded the number with MCIs to meet our power requirement of n=148 participants with MCI; recruitment was, therefore, stopped. After exclusions following baseline testing, 437 patients had a completed angiogram, with 390 completing 3‐month follow‐up. The trial profile is shown in the Figure. Fifty‐one controls were used to calculate RCI.10 Patient and control baseline characteristics are shown in Table 1. Controls were older, with more women, and were shorter and weighed less than study participants; they had less diabetes mellitus, a lower prevalence of smoking, less hypercholesterolemia, and higher Mini‐Mental State Examination scores. Baseline cognitive test scores are shown in Table 2. Controls performed significantly better than patients on CERAD Auditory Verbal Learning Test recall (both immediate and delayed), Controlled Oral Word Association Test, and CERAD semantic fluency.
Figure 1.
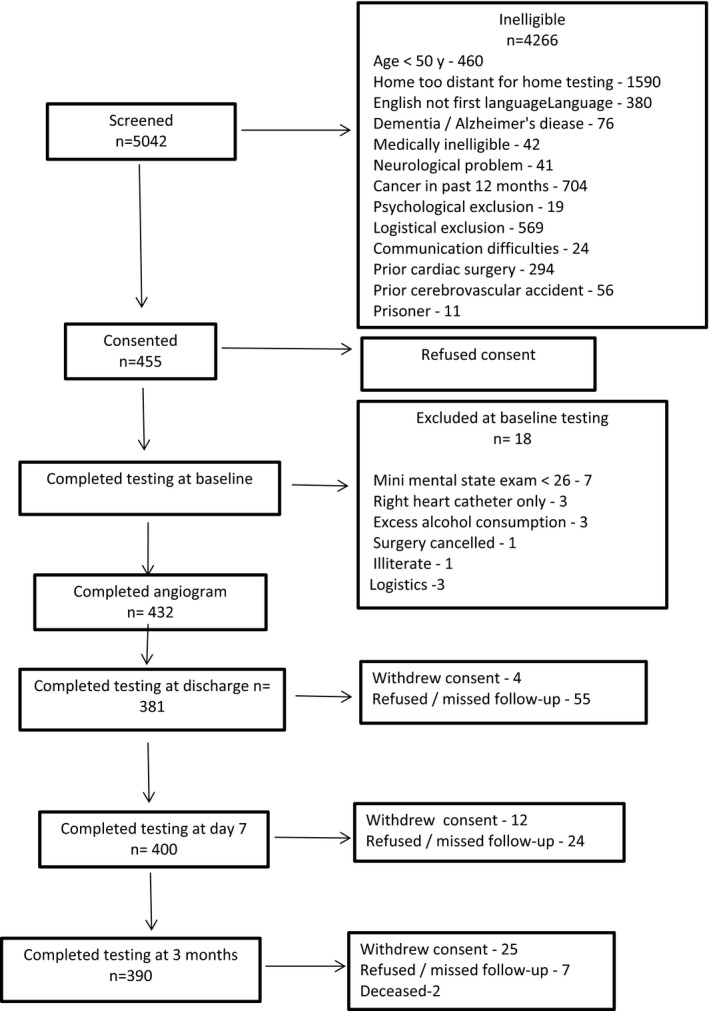
Flow diagram.
Table 1.
Baseline Characteristics
| Characteristics | Left Heart Catheterization (n=437) | Controls (n=51) | P Value |
|---|---|---|---|
| Age, y | 65.5±8.7 | 72.0±7.2 | <0.01 |
| Sex ratio, male/female | 267:170 | 13:38 | <0.01 |
| Height, cm | 169.7±10.3 | 166.0±8.6 | 0.02 |
| Weight, kg | 84.4±18.1 | 75.3±17.3 | <0.01 |
| BMI, kg/m2 | 29.1±5.9 | 27.2±5.0 | 0.03 |
| Diabetes mellitus | 112 (26) | 6 (12) | 0.03 |
| Hypertension | 285 (66) | 34 (67) | 0.89 |
| Peripheral vascular disease | 41 (10) | 3 (6) | 0.33 |
| History of myocardial infarct | 94 (22) | 7 (14) | 0.18 |
| Prior coronary stenting | 84 (19) | 0 | <0.01 |
| History of smoking | 286 (66) | 20 (39) | <0.01 |
| Hypercholesterolemia | 296 (69) | 26 (51) | 0.01 |
| Estimated IQ | 108.3±10.8 | 115.4±9.0 | <0.01 |
| MMSE score | 28.1±1.2 | 28.5±1.2 | 0.02 |
| Medications | |||
| Statins | 260 (60) | 23 (46) | 0.06 |
| β‐Blockers | 166 (38) | 6 (12) | <0.01 |
| ACE inhibitors | 133 (31) | 11 (22) | 0.20 |
Data are presented as mean±SD or number (percentage). ACE indicates angiotensin‐converting enzyme; BMI, body mass index; IQ, intelligence quotient; and MMSE, Mini‐Mental State Examination.
Table 2.
Baseline Test Scores
| Written Tests | Left Heart Catheterization (n=437) | Controls (n=51) | P Value |
|---|---|---|---|
| MMSE | 28.07 (1.24) | 28.49 (1.19) | 0.02 |
| Estimated IQ | 108.31 (10.83) | 115.40 (8.99) | <0.01 |
| CERAD AVLT, n | 17.48 (3.61) | 18.66 (3.93) | 0.03 |
| TMTA, s | 48.49 (22.25) | 43.12 (14.85) | 0.09 |
| TMTB, s | 113.46 (59.82) | 97.73 (55.41) | 0.07 |
| DSST, n | 38.02 (11.56) | 41.14 (11.26) | 0.07 |
| COWAT, n | 32.98 (11.48) | 42.90 (13.19) | <0.01 |
| CERAD semantic fluency (animals), n | 17.82 (4.78) | 19.92 (4.79) | <0.01 |
| GPD, s | 95.89 (37.47) | 89.55 (23.52) | 0.25 |
| GPND, s | 105.40 (41.84) | 98.49 (25.55) | 0.26 |
| CERAD AVLT recall | 4.18 (2.17) | 5.24 (2.01) | <0.01 |
| Visual Analogue Scale, cm | |||
| Anxiety | 35.34 (25.60) | 21.76 (21.90) | <0.01 |
| Depression | 22.21 (23.62) | 13.31 (16.24) | <0.01 |
| Fatigue | 40.59 (27.60) | 29.71 (22.75) | <0.01 |
| Simple reaction time, ms | 2.58 (0.13) | 2.56 (0.13) | 0.30 |
| Complex reaction time, ms | 2.75 (0.08) | 2.77 (0.08) | 0.50 |
| One‐back test, ms | 3.10 (0.13) | 3.10 (0.12) | 0.85 |
| Maze, n | |||
| Total | 69.91 (27.72) | 74.84 (34.43) | 0.25 |
| Errors | 9.87 (5.49) | 10.86 (6.07) | 0.23 |
For written tests, results are number correct (higher score better), time taken (in seconds; lower score better), or Visual Analogue Scale scores (in cm). Computer tests (n=434) include simple and complex reaction times, one‐back test, and maze tasks. All data are presented as mean (SD). CERAD AVLT indicates Consortium to Establish a Registry for Alzheimer's Disease–Auditory Verbal Learning Test (immediate and delayed recall); COWAT, Controlled Oral Word Association Test; DSST, Digit Symbol Substitution Test; GPD, Grooved Peg Board Test, Dominant; GPND, Grooved Peg Board Test, Nondominant; IQ, intelligence quotient; MMSE, Mini‐Mental State Examination; TMTA, Trail Making Test Part A; and TMTB, Trail Making Test Part B.
Up to 3 months after LHC, 322 patients (74%) were managed medically or required no treatment, 71 (16%) underwent a minor unrelated medical procedure (n=21) or subsequent percutaneous coronary intervention (n=50), and 44 (10%) underwent major unrelated medical procedures (n=10) or cardiac surgery (n=34 [n=11 valve only; n=22 coronary artery bypass graft {CABG} or CABG ± valve; n=1 pericardectomy]).
Using the written tests, MCI was classified preoperatively in 226 of 437 patients (51.7%), 176 of whom were classified as aMCI. Of these patients, 76 had single‐domain aMCI and 100 had multidomain aMCI. Executive function was decreased in 123 of 431 patients (28.5%), of whom 89 also had memory impairment. Baseline MCI (any) was not associated with age (t=−0.25, P=0.81), estimated intelligence quotient (t=−0.24, P=0.81), or CAD grade (χ2=2.20, P=0.53).
Cognitive testing (computerized) at the time of hospital discharge (or 24 hours) and also at 7 days after procedure showed no group‐level difference compared with controls (Table 3). There was also no group‐level difference between baseline and 3‐month computerized testing for either LHC participants or controls. Individual patient analysis indicated cognitive decline (RCI) in 38 or 380 patients (10.0%) at discharge and 30 of 401 patients (7.5%) at 7 days using computerized tests.
Table 3.
Computer Test Score Results
| Computer Test | Left Heart Catheterization | Controls | P Value |
|---|---|---|---|
| 24 h or Discharge | |||
| Simple reaction time, ms | 2.57 (0.25) | 2.57 (0.13) | 0.98 |
| Complex reaction time, ms | 2.74 (0.26) | 2.76 (0.08) | 0.66 |
| One‐back test, ms | 3.06 (0.34) | 3.08 (0.11) | 0.64 |
| Maze, n | |||
| Total | 67.99 (27.92) | 61.62 (23.83) | 0.12 |
| Errors | 10.76 (6.08) | 10.42 (4.77) | 0.71 |
| 7 d | |||
| Simple reaction time, ms | 2.58 (0.17) | 2.57 (0.13) | 0.85 |
| Complex reaction time, ms | 2.74 (0.16) | 2.76 (0.08) | 0.57 |
| One‐back test, ms | 3.07 (0.12) | 3.08 (0.11) | 0.71 |
| Maze, n | |||
| Total | 61.34 (23.80) | 61.62 (23.83) | 0.94 |
| Errors | 9.58 (4.95) | 10.42 (4.77) | 0.27 |
Data are presented as mean (SD). At 24 hours or discharge, n=380 for left heart catheterization and n=51 for control; at 7 days, n=401 for left heart catheterization and n=51 for control.
At 3 months, group analysis showed no difference from controls in computerized tests (Table 4); however, written test performance was more impaired in patients than controls in CERAD Auditory Verbal Learning Test delayed recall, Controlled Oral Word Association Test, and CERAD semantic fluency (as for baseline) and also in the Digit Symbol Substitution Test and Trail Making Test Parts A and B (Table 5). At this testing time, there were no differences in anxiety, depression, or fatigue compared with controls.
Table 4.
Computer Test Score Results at 3 Months
| Computer Test | Left Heart Catheterization (n=389) | Controls (n=51) | P Value |
|---|---|---|---|
| Simple reaction time, ms | 2.59 (0.11) | 2.57 (0.12) | 0.35 |
| Complex reaction time, ms | 2.75 (0.07) | 2.75 (0.07) | 0.93 |
| One‐back test, ms | 3.06 (0.11) | 3.08 (0.10) | 0.11 |
| Maze, n | |||
| Total | 58.76 (21.92) | 63.69 (26.21) | 0.15 |
| Errors | 8.43 (4.83) | 9.55 (4.11) | 0.13 |
Test results are either time (in milliseconds) or number correct. All data are presented as mean (SD).
Table 5.
Neuropsychological Test Score Results at 3 Months
| Neuropsychological Test | Left Heart Catheterization (n=390) | Controls (n=51) | P Value |
|---|---|---|---|
| CERAD AVLT, n | 18.48 (3.85) | 19.56 (3.23) | 0.06 |
| TMTA, s | 47.88 (26.76) | 38.29 (12.31) | 0.01 |
| TMTB, s | 107.48 (57.66) | 83.50 (41.37) | <0.01 |
| DSST, n | 39.69 (11.62) | 46.06 (9.63) | <0.01 |
| COWAT, n | 34.77 (11.94) | 44.45 (14.67) | <0.01 |
| CERAD semantic fluency (animals), n | 17.57 (4.55) | 21.08 (5.09) | <0.01 |
| GPD, s | 92.50 (32.95) | 85.22 (16.70) | 0.14 |
| GPND, s | 102.17 (44.02) | 95.54 (21.83) | 0.32 |
| CERAD AVLT recall, n | 4.80 (2.12) | 5.69 (1.89) | <0.01 |
| Visual Analogue Scale, cm | |||
| Depression | 20.5 (22.66) | 19.98 (23.63) | 0.92 |
| Anxiety | 26.27 (24.00) | 26.19 (22.87) | 0.98 |
| Fatigue | 35.89 (26.24) | 32.69 (26.17) | 0.43 |
Test results are number correct, time taken (in milliseconds), or Visual Analogue Scale score (cm). All data are presented as mean (SD). CERAD AVLT indicates Consortium to Establish a Registry for Alzheimer's Disease–Auditory Verbal Learning Test (immediate and delayed recall); COWAT, Controlled Oral Word Association Test; DSST, Digit Symbol Substitution Test; GPD, Grooved Peg Board Test, Dominant; GPND, Grooved Peg Board Test, Nondominant; TMTA, Trail Making Test Part A; and TMTB, Trail Making Test Part B.
At 3 months, individual analysis of written tests identified cognitive decline in 51 of 390 patients (13.1%), whereas computer test results showed cognitive decline in 33 of 389 patients (8.5%) at 3 months (Table 6). MCI was identified in 156 of 389 patients (40%), of whom 104 were classified as having MCI.
Table 6.
Incidence of Cognitive Decline at 3 Months
| Procedure to 3 Months | All Patients (n=437) | Cognitive Decline (Written Tests) (n=390) | Cognitive Decline (Computerized Tests) (n=389) |
|---|---|---|---|
| No procedures |
322 (74) [69–78] |
34/283 (12.0) [8–16] |
26/283 (9.2) [6–13] |
| Minor procedure (71 unrelated procedures and 50 PCIs) |
71 (16) [13–20] |
10/66 (15) [8–26] |
5/65 (7.7) [2–17] |
| Major procedure (unrelated) |
10 (2.3) [1–4] |
1/10 (10) [0–44] |
0/10 (0) [0–30] |
| Major procedure (cardiac) (CABG surgery, n=22) |
34 (7.8) [5–11] |
6/31 (19.3) [8–38] |
2/31 (6.4) [0–21] |
| Total | 437 |
51/390 (13.1) [10–17] |
33/389 (8.5) [6–11] |
Test results are number/total (percentage) [95% confidence interval]. CABG indicates coronary artery bypass graft; and PCI, percutaneous coronary intervention.
Subsequent procedural interventions, major or minor, to 3 months were not associated with cognitive decline using either written tests (odds ratio [OR], 1.24 [95% confidence interval [CI], 0.93–1.66]; P=0.15) or computerized tests (OR, 0.80 [95% CI, 0.50–1.28]; P=0.35) (Table 6). However, patients who had CABG surgery (n=22) in the intervening period showed a 22.7% incidence of cognitive decline at 3 months.
Multivariable analysis of written test results showed age to be the only significant predictor of cognitive decline at 3 months (OR, 1.06 [95% CI, 1.02–1.10]; P<0.01) (Table 7). When considering the computerized test results, multivariable analysis demonstrated cognitive decline at 3 months (Table 8) was associated with diabetes mellitus (OR, 2.31 [95% CI, 1.09–4.90]; P=0.03), prior coronary stenting (OR, 2.73 [95% CI, 1.22–6.10]; P=0.01), and multidomain aMCI at baseline (OR, 2.43 [95% CI, 1.11–5.32]; P=0.03). Cognitive decline with computerized tests at 3 months was also associated, on multivariable analysis, with apolipoprotein E4 genotype positive (OR, 2.90 [95% CI, 1.25–6.70]; P=0.01) and body mass index (OR, 0.89 [95% CI, 0.83–0.96]; P<0.01), but these were removed to avoid overfitting of the model (an event rate at 3 months of n=33).
Table 7.
Univariable and Multivariable Analysis of Predictors of Cognitive Decline at 3 Months Using Written Test Battery
| Variable | Univariable Test Statistic | Univariable P Value | OR (95% CI) | P Value |
|---|---|---|---|---|
| Age | t=−3.22 | <0.01 | 1.06 (1.02–1.10) | <0.01 |
| Estimated IQ | χ2=0.11 | 0.91 | 0.99 (0.96–1.02) | 0.57 |
| Procedure to 3 mo | Z score=−1.13 | 0.26 | 1.09 (0.86–1.38) | 0.49 |
| CAD gradea | Z score=−0.32 | 0.75 | 3.73 (0.83–16.69) | 0.08 |
CAD indicates coronary artery disease; CI, confidence interval; IQ, intelligence quotient; and OR, odds ratio.
Grade 0 indicates no significant coronary artery disease; grade 1, 1 vessel affected; grade 2, 2 vessels affected; and grade 3, 3 vessels affected.
Table 8.
Univariable and Multivariable Analysis of Predictors of POCD at 3 Months Using Computerized Test Battery
| Predictor | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| Test Statistic | P Value | OR (95% CI) | P Value | |
| Agea | t=−2.28 | 0.023 | ··· | ··· |
| Estimated IQa | χ2=0.05 | 0.958 | ··· | ··· |
| Diabetes mellitus | χ2=5.53 | 0.019 | 2.31 (1.09–4.90) | 0.03 |
| Prior stent | χ2=4.58 | 0.032 | 2.73 (1.22–6.10) | 0.01 |
| aMCI multidomain | χ2=3.89 | 0.049 | 2.43 (1.11–5.32) | 0.03 |
| BMIb | t=2.29 | 0.023 | 0.89 (0.83–0.96) | <0.01 |
| ApoE4 positiveb | χ2=2.85 | 0.091 | 2.84 (1.23–6.56) | 0.01 |
aMCI indicates amnestic mild cognitive impairment; ApoE4, apolipoprotein E4; BMI, body mass index; CI, confidence interval; IQ, intelligence quotient; OR, odds ratio; and POCD, postoperative cognitive dysfunction.
Age and estimated IQ are not significant on multivariable analysis and are removed from the model.
These variables removed from the model to avoid overfit.
There were no major complications (stroke or death) after LHC in our study patients.
Discussion
The main findings of this investigation are that >50% of individuals presenting for elective LHC meet clinical criteria for MCI and that up to 13% of patients have further cognitive impairment at 3 months. Although the mean age of patients in this study was 65 years, the prevalence of MCI at baseline exceeds the population prevalence of 14% to 18% in those aged ≥70 years. MCI is considered to be an early indication of future dementia, and in population studies, it progresses to dementia at an annual rate of 6% to 10% per year compared with 1% to 2% per year for those without MCI.6 The most plausible explanation for the high prevalence of MCI in this study is an association with the cardiovascular disease that brought these individuals to LHC, in addition to the age‐related risk.
Vascular cognitive impairment describes cognitive changes related to vascular causes and encompasses any degree of cognitive decline caused by, or associated with, vascular disease or its risk factors. Vascular cognitive impairment, no dementia, refers to the early stages, analogous to MCI.22 Indeed, the distinction between MCI and vascular cognitive impairment, no dementia, can become blurred.23 The combination of 2 common pathological conditions (Alzheimer disease and vascular cognitive impairment) is often present and is known as mixed dementia.24 The high prevalence of MCI observed in this study is likely attributable to the presence of vascular cognitive impairment or mixed dementia cause in patients undergoing elective LHC. The implication is that many of these patients will progress to dementia at a rate that exceeds population norms. This is consistent with a recently published finding that the prevalence of dementia at 7.5 years after cardiac surgery is >3 times the prevalence in the general population and may be because of vascular disease.25
The second major finding of this study is that 8.5% (computerized tests) to 13.1% (written tests) of individuals showed a measurable decline in cognition at 3 months after LHC. The difference in incidence between the 2 modes of cognitive testing may be accounted for, to some extent, by the fact that the 2 modalities interrogate slightly different cognitive domains. We did not identify an association with prior MCI or cognitive decline at 3 months using written tests; however, the possible influence of widespread or longer duration of vascular disease is supported by the association of cognitive decline at 3 months using computerized tests with prior coronary stenting and diabetes mellitus.
Microembolism has long been identified during LHC, being both gaseous and particulate. However, studies on cognitive outcomes and transcranial Doppler have failed to identify any consistent association between embolic load and cognitive impairment.9, 13
A limitation of this study is that the control group, recruited from the community with osteoarthritis, may have had features, such as depression, that distinguished them from completely fit individuals. As it was, the control group was older and differed in several baseline characteristics from the study group. They had a higher intelligence quotient and performed better on baseline testing. Nevertheless, they represent a group without overt cardiovascular disease against which the impact of cardiovascular disease can be highlighted, and the control group still enables adjustment for retesting and time‐related changes. The attribution of cognitive decline is an individual outcome, on the basis of performance in several tests compared with controls. As such, pooling results as group comparisons is less meaningful than identifying the proportion of individuals affected. The differences in the results of written and computer testing reflect the different attributes of these tools.26 For this reason, we have reported the results of both methods of assessment.
The risks and complications associated with LHC have been well described. Major adverse cardiac and cerebrovascular events (stroke) occur in <2% of patients for diagnostic LHC, with mortality <0.8%.27 We identified no strokes and no procedural deaths. Although such complications may be rare, all patients are advised of these risks as part of the informed consent process because they are material, despite the low incidence. Cognitive decline after LHC may not be as catastrophic as other adverse events, but it is nonetheless likely to be of significance to all individuals because cognition is highly valued. Cognitive decline should, thus, be recognized as a relevant and not‐uncommon complication that may follow LHC, which, even if mild, may have the potential to impinge on the everyday activities of the individual.28 For this reason, it would seem appropriate that cognitive decline should be identified as a common adverse event after LHC within the informed consent process.
CABG surgery has been implicated as a major cause of cognitive decline in individuals with CAD. Known as postoperative cognitive dysfunction (POCD), the reported incidence has varied widely, ranging from 6.6%29 to 79%,30 and is influenced by time of testing after the procedure, among other factors. Our findings of 8% to 13% incidence of cognitive decline at 3 months after LHC suggest that the studies investigating POCD after CABG surgery may represent even more decline than previously considered, if the postangiography level is already a point from which some patients decline even further after CABG surgery. Indeed, the few patients in this study having CABG surgery before testing at 3 months showed a 22.7% incidence of POCD. Virtually all CABG surgery patients have undergone LHC before surgery, yet the effects of LHC on individual baseline cognitive testing before cardiac surgery have not been considered in existing studies. It can only be assumed that many of the patients studied for POCD after CABG surgery may not only have had MCI, but had already experienced cognitive decline subsequent to the LHC and before their cardiac surgery. Such a decline would lead to a lower cognitive baseline that is likely to underestimate the actual incidence of POCD from preangiography to postcardiac surgery. This finding warrants further investigation.
In addition to studies that have focused on cardiac surgery alone, 1 study has sought to compare the cognitive change measured after cardiac surgery with controls who have not undergone cardiac surgery but have CAD. However, even in this scenario, the controls had all undergone prior coronary angiography to diagnose CAD.31 Other studies comparing cognitive decline after cardiac surgery with that after percutaneous interventions have also failed to take into account the effect of the coronary angiography before the percutaneous coronary intervention.8, 32
In summary, we have found a high prevalence of MCI in individuals for elective LHC. Furthermore, LHC was followed by cognitive decline in 8% to 13% of individuals at 3 months after the procedure, which is similar to the incidence after both noncardiac and cardiac surgery.12 Using a computerized test battery, baseline aMCI, prior coronary stenting, and diabetes mellitus were independent predictors of decline at 3 months postoperatively, implicating vascular disease in this decline. This suggests that comorbid disease, in particular cardiovascular disease, together with processes of the intervention are affecting the cognition of the elderly patients undergoing LHC. We suggest that elderly patients presenting for LHC should have baseline cognitive assessment as a routine and that consideration of the risk of further cognitive decline should form part of the informed consent process. This issue will assume even more importance in the future as the population ages.
Sources of Funding
This work was supported by Australian National Health and Medical Research Council, Canberra, Australian Capital Territory, Australia (project grant 45462).
Disclosures
Maruff is an employee at Cogstate P/L. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2018;7:e008004 DOI: 10.1161/JAHA.117.008004.)29525780
References
- 1. Dehmer GJ, Weaver D, Roe MT, Milford‐Beland S, Fitzgerald S, Hermann A, Messenger J, Moussa I, Garratt K, Rumsfeld J, Brindis RG. A contemporary view of diagnostic cardiac catheterization and percutaneous coronary intervention in the United States: a report from the CathPCI Registry of the National Cardiovascular Data Registry, 2010 through June 2011. J Am Coll Cardiol. 2012;60:2017–2031. [DOI] [PubMed] [Google Scholar]
- 2. Hospital Care for Cardiovascular Disease. Welfare Australian Institute of Health and Welfare ed. Canberra, Australia: Australian Government; 2002. https://www.aihw.gov.au/reports/heart-stroke-vascular-disease/cardiovascular-health-compendium/contents/hospital-care-for-cardiovascular-disease. Accessed March 1, 2017. [Google Scholar]
- 3. Medi C, Evered L, Silbert B, The A, Halloran K, Morton J, Kistler P, Kalman J. Subtle post‐procedural cognitive dysfunction following atrial fibrillation ablation. J Am Coll Cardiol. 2013;62:531–539. [DOI] [PubMed] [Google Scholar]
- 4. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. [DOI] [PubMed] [Google Scholar]
- 5. Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. [DOI] [PubMed] [Google Scholar]
- 6. Petersen RC, Roberts RO, Knopman DS, Boeve BF, Geda YE, Ivnik RJ, Smith GE, Jack CR Jr. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66:1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosengart TK, Sweet J, Finnin EB, Wolfe P, Cashy J, Hahn E, Marymont J, Sanborn T. Neurocognitive functioning in patients undergoing coronary artery bypass graft surgery or percutaneous coronary intervention: evidence of impairment before intervention compared with normal controls. Ann Thorac Surg. 2005;80:1327–1334. [DOI] [PubMed] [Google Scholar]
- 9. Jurga J, Tornvall P, Dey L, van der Linden J, Sarkar N, von Euler M. Does coronary angiography and percutaneous coronary intervention affect cognitive function? Am J Cardiol. 2016;118:1437–1441. [DOI] [PubMed] [Google Scholar]
- 10. Silbert B, Evered L, Scott DA, McMahon S, Choong P, Ames D, Maruff P, Jamrozik K. Preexisting cognitive impairment is associated with postoperative cognitive dysfunction after hip joint replacement surgery. Anesthesiology. 2015;122:1224–1234. [DOI] [PubMed] [Google Scholar]
- 11. Devapalasundarum A, Silbert B, Evered L, Scott D, MacIsaac A, Maruff P. Cognitive function in patients undergoing coronary angiography. Heart Asia. 2010;2:75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Evered L, Scott DA, Silbert B, Maruff P. Postoperative cognitive dysfunction is independent of type of surgery and anesthetic. Anesth Analg. 2011;112:1179–1185. [DOI] [PubMed] [Google Scholar]
- 13. Scott DA, Evered LA, Gerraty RP, MacIsaac A, Lai‐Kwon J, Silbert BS. Cognitive dysfunction follows left heart catheterisation but is not related to microembolic count. Int J Cardiol. 2014;175:67–71. [DOI] [PubMed] [Google Scholar]
- 14. Silbert B, Scott DA, Evered LA, Lewis MS, Kalpokas M, Maruff P, Myles PS, Jamrozik K. A comparison of the effect of high‐ and low‐dose fentanyl on the incidence of postoperative cognitive dysfunction after coronary artery bypass surgery in the elderly. Anesthesiology. 2006;104:1137–1145. [DOI] [PubMed] [Google Scholar]
- 15. Silbert BS, Maruff P, Evered LA, Scott DA, Kalpokas M, Martin KJ, Lewis MS, Myles PS. Detection of cognitive decline after coronary surgery: a comparison of computerized and conventional tests. Br J Anaesth. 2004;92:814–820. [DOI] [PubMed] [Google Scholar]
- 16. Nelson H. National Adult Reading Test (NART) for the Assessment of Premorbid Intelligence in Patients With Dementia: Test Manual. Windsor, England: Psychological Corporation; 1992. [Google Scholar]
- 17. Maruff P, Lim YY, Darby D, Ellis KA, Pietrzak RH, Snyder PJ, Bush AI, Szoeke C, Schembri A, Ames D, Masters CL; for the AIBL Research Group . Clinical utility of the cogstate brief battery in identifying cognitive impairment in mild cognitive impairment and Alzheimer's disease. BMC Psychol. 2013;1:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ingraham L, Aiken C. An empirical approach to determine criteria for abnormality in test batteries with multiple results. Neuropsychology. 1996;10:120–124. [Google Scholar]
- 19. Lewis M, Maruff P, Silbert B. Statistical and conceptual issues in defining post‐operative cognitive dysfunction. Neurosci Biobehav Rev. 2004;28:433–440. [DOI] [PubMed] [Google Scholar]
- 20. Neeland IJ, Patel RS, Eshtehardi P, Dhawan S, McDaniel MC, Rab ST, Vaccarino V, Zafari AM, Samady H, Quyyumi AA. Coronary angiographic scoring systems: an evaluation of their equivalence and validity. Am Heart J. 2012;164:547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Silbert BS, Scott DA, Evered LA, Lewis MS, Maruff PT. Preexisting cognitive impairment in patients scheduled for elective coronary artery bypass graft surgery. Anesth Analg. 2007;104:1023–1028. [DOI] [PubMed] [Google Scholar]
- 22. Stephan BC, Matthews FE, Khaw KT, Dufouil C, Brayne C. Beyond mild cognitive impairment: vascular cognitive impairment, no dementia (VCIND). Alzheimers Res Ther. 2009;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ebly EM, Hogan DB, Parhad IM. Cognitive impairment in the nondemented elderly: results from the Canadian Study of Health and Aging. Arch Neurol. 1995;52:612–619. [DOI] [PubMed] [Google Scholar]
- 24. Zekry D, Hauw JJ, Gold G. Mixed dementia: epidemiology, diagnosis, and treatment. J Am Geriatr Soc. 2002;50:1431–1438. [DOI] [PubMed] [Google Scholar]
- 25. Evered LA, Silbert BS, Scott DA, Maruff P, Ames D. Prevalence of dementia 7.5 years after coronary artery bypass graft surgery. Anesthesiology. 2016;125:62–71. [DOI] [PubMed] [Google Scholar]
- 26. Radtke FM, Franck M, Papkalla N, Herbig TS, Weiss‐Gerlach E, Kleinwaechter R, Wernecke KD, Spies CD. Postoperative cognitive dysfunction: computerized and conventional tests showed only moderate inter‐rater reliability. J Anesth. 2010;24:518–525. [DOI] [PubMed] [Google Scholar]
- 27. Tavakol M, Ashraf S, Brener SJ. Risks and complications of coronary angiography: a comprehensive review. Glob J Health Sci. 2012;4:65–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS. Long‐term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110:548–555. [DOI] [PubMed] [Google Scholar]
- 29. McLean RF, Wong BI, Naylor CD, Snow WG, Harrington EM, Gawel M, Fremes SE. Cardiopulmonary bypass, temperature, and central nervous system dysfunction. Circulation. 1994;90:II250–II255. [PubMed] [Google Scholar]
- 30. Shaw PJ, Bates D, Cartlidge NE, French JM, Heaviside D, Julian DG, Shaw DA. Neurologic and neuropsychological morbidity following major surgery: comparison of coronary artery bypass and peripheral vascular surgery. Stroke. 1987;18:700–707. [DOI] [PubMed] [Google Scholar]
- 31. Selnes OA, Grega MA, Borowicz LM Jr, Royall RM, McKhann GM, Baumgartner WA. Cognitive changes with coronary artery disease: a prospective study of coronary artery bypass graft patients and nonsurgical controls. Ann Thorac Surg. 2003;75:1377–1384. [DOI] [PubMed] [Google Scholar]
- 32. Wahrborg P, Booth JE, Clayton T, Nugara F, Pepper J, Weintraub WS, Sigwart U, Stables RH. Neuropsychological outcome after percutaneous coronary intervention or coronary artery bypass grafting: results from the Stent or Surgery (SoS) Trial. Circulation. 2004;110:3411–3417. [DOI] [PubMed] [Google Scholar]


