Abstract
Background
Real‐time monitoring is used to the ends of postmarketing observational research on newly marketed drugs. We implemented a pilot near‐real‐time monitoring program on the test case of oral anticoagulants. Specifically, we evaluated the safety and effectiveness of direct oral anticoagulants compared to vitamin K antagonists in nonvalvular atrial fibrillation secondary prevention during 2013‐2015 in the Lazio Region, Italy.
Methods and Results
A cohort study was conducted using a sequential propensity‐score–matched new user parallel‐cohort design. Sequential analyses were performed using Cox models. Overall, 10 742 patients contributed to the analyses. Compared with vitamin K antagonists, direct oral anticoagulant use was associated with a reduction of all‐cause mortality (0.81; 95% confidence interval [CI] 0.66‐0.99), cardiovascular mortality (0.71; 95% CI 0.54‐0.93), myocardial infarction (0.67; 95% CI 0.43‐1.04), ischemic stroke (0.87; 95% CI 0.52‐1.45), hemorrhagic stroke (0.25; 95% CI 0.07‐0.88), and with a nonsignificant increase of gastrointestinal bleeding (1.26; 95% CI 0.69‐2.30).
Conclusions
The present pilot study is a cornerstone to develop real‐time monitoring for new drugs in our region.
Keywords: anticoagulant, comparative effectiveness, drug therapy, monitoring, pharmacoepidemiology, pilot, real‐world, surveillance
Subject Categories: Mortality/Survival, Quality and Outcomes, Complications, Secondary Prevention, Epidemiology
Clinical Perspective
What Is New?
This is the first pilot of a near‐real‐time monitoring program of newly marketed drugs in Italy.
Administrative health claims data were used to provide near‐real‐time evidence for the safety and effectiveness of newly marketed direct oral anticoagulants.
What Are the Clinical Implications?
In this study on secondary prevention in patients affected by nonvalvular atrial fibrillation, use of new direct oral anticoagulants compared with vitamin K antagonists was associated with a lower risk of all‐cause and cardiovascular mortality, hemorrhagic stroke, myocardial infarction, and ischemic stroke, although the risk of gastrointestinal bleeding was increased.
This pilot program lays the basis for the implementation of real‐time monitoring of new drugs in our region and elsewhere.
Introduction
Efficacy and safety of new drugs are typically evaluated in randomized controlled trials, but clinical trials may not always be sufficiently informative. Major limitations of randomized controlled trials are the small and selected study populations, the short observation time, and the well‐monitored adherence, all of which do not reflect real‐world conditions.1 Postmarketing observational studies are needed to complement the results of clinical trials.2
A standardized methodology has been implemented in the context of the Sentinel Program,3, 4 which allows monitoring of the safety and effectiveness of newly marketed drugs through aggregation of data from different data sources, as soon as the data become available, using standardized methods.5, 6, 7, 8, 9, 10, 11, 12 Postmarketing information is particularly useful for new drugs that have not shown a clear superiority versus the comparator drug in randomized controlled trials in the context of incremental licensing procedures, such as “adaptive licensing.”
Direct oral anticoagulants (DOACs, ie, dabigatran, rivaroxaban, apixaban) offer an alternative to vitamin K antagonists (VKAs, ie, warfarin, acenocoumarol) for the prevention of stroke or systemic embolism and all‐cause mortality in patients with nonvalvular atrial fibrillation (AF). The main advantages of using DOACs with respect to VKAs are that there is no need to monitor the international normalized ratio and that they show fewer interactions with food. On the other hand, some DOACs require renal function to be regularly monitored13 and are associated with higher costs.
A meta‐analysis, based on randomized controlled trials comparing individual DOACs with warfarin14, 15, 16 among nonvalvular AF patients,17 showed a significant reduction in the risk of total mortality and hemorrhagic stroke and an increased risk for gastrointestinal bleeding associated with the randomization to DOACs. Subsequently, several healthcare database analyses comparing individual DOACs versus warfarin or VKAs have been conducted to answer questions regarding their relative safety and effectiveness in routine care, but results have not been homogeneous among different studies.18, 19, 20, 21, 22, 23, 24, 25, 26, 27
In a context of rapidly accumulating postmarketing information, the establishment of a robust framework capable of generating valid, timely information on the safety and effectiveness of new medications to either support or limit evolving observed prescribing changes (Figure S1) is highly valuable. We were interested in the pilot implementation of a medication‐monitoring program and chose oral anticoagulants as a test case in response to a request by the regional healthcare government. This request was motivated by the current absence of effectiveness and safety information on these agents as used in routine care in Italy. The ultimate goal is the creation of a monitoring framework that could promptly provide Italian prescribers with relevant clinical information on the safety and effectiveness of newly marketed drugs.
Methods
Study Design
We conducted a sequential propensity score (PS)–matched new user parallel cohort design of DOAC versus VKA initiators and implemented a pilot near‐real‐time monitoring program in the Lazio Region in central Italy, leveraging population‐based healthcare data. This design has many key strengths,28 1 of which is to reduce channeling bias, which may be particularly pronounced in studies of newly marked drugs.
Source of Data
The Lazio Region healthcare assistance file collects demographic and residence information of all residents living in the Lazio Region and registered in the regional health service, accounting for ≈95% of the overall population. This database can be linked with other regional health information systems through an anonymous unique patient identifier, to capture the clinical history of this population. Specifically, information about mortality (date, place, and cause of death coded by International Classification of Diseases 9th Revision [ICD‐9] code) was retrieved from the regional Mortality Information System. Information regarding admissions to regional hospitals (eg, primary and secondary diagnoses and procedures recorded at discharge, coded according to ICD‐9‐CM [Clinical Modification]) was retrieved from the Hospital Information System. Information on specialist visits (eg, visits and exams, prescription codes, and prescription dates) was collected from the Outpatient Specialist Service Information System. Data about emergency room visits (ie, up to 5 diagnoses coded according to ICD‐9‐CM, patient severity [triage code], and some clinical parameters) were collected from the Healthcare Emergency Information System. Information on drugs reimbursed by the healthcare system and dispensed by public and private pharmacies or by hospital pharmacies at discharge (ie, the national drug register code, which is related to the international ATC [Anatomical Therapeutic Chemical Classification System], claim date, number of pills), was available from the Regional Drug Dispense Registry.
All Information Systems were updated to the end of 2015.
The present study is based on anonymized patient data available in the regional health information system, and the study protocol obtained consensus from the regional ethics committee. The data, analytic methods, and study materials have been and will be made available to other researchers for purposes of reproducing the results or replicating the procedure on request to the corresponding author.
Study Population
Inclusion/Exclusion Criteria
The study population consisted of sequential cohorts of DOAC or VKA new users aged 18 to 100 years between July 1, 2013 and December 31, 2015. In Italy, DOACs were authorized for nonvalvular AF treatment during 2013: the first was dabigatran on June 19, followed by rivaroxaban and apixaban later in September 2013 and January 2014. We considered a period of 11 days as the minimum time gap for physicians to begin to implement the extended indication. Moreover, this choice allowed us to easily divide the overall study period into 3‐month sequential interim periods.
Study participants were patients not prescribed with any oral anticoagulant drugs in the 6 months before the first drug claim for a DOAC or a VKA agent during the study period (index date). We only included drug initiators who were continuously enrolled in the regional healthcare assistance file throughout the 12 months preceding the index date and who had a diagnosis of AF (ICD‐9‐CM codes 427.31 or 427.32) registered in Hospital Information System or Healthcare Emergency Information System in the 12 months before the index date.
We excluded patients with mitral stenosis or mechanical heart valve in order to select only patients with nonvalvular AF. Patients undergoing dialysis or with a history of renal transplant were also excluded as severe renal impairment is a contraindication for DOAC prescription. Finally, patients with joint replacement were excluded to ensure that DOACs were used for the AF indication only. All exclusion criteria were assessed during the 12 months before the index date (code lists of exclusion criteria are reported in Table S1).
Exposure
We compared the overall group of DOACs marketed in Italy during the study period (dabigatran, rivaroxaban, apixaban) with VKAs (warfarin, acenocoumarol). Drugs were identified using ATC codes (rivaroxaban ATC B01AF01, apixabam ATC B01AF02, dabigatran ATC B01AE07, warfarin ATC B01AA03, acenocoumarol ATC B01AA07).
Because information on the exact number of days supplied is not available in the Regional Drug Dispense Registry, patients' drug use periods were calculated using the defined daily doses (DDD) metric as defined by the World Health Organization.29 For each prescription the total number of DDDs was translated into the number of days in which the patient was treated, counting 1 DDD per day and distributing all available DDDs to the days of follow‐up and allowing for the use of accumulated DDDs over time.
We allowed for a renewal grace time (a maximum number of days without any drug supply permitted between 2 consecutive drug claims of the same drug group) of 90 days and a final grace period (extension of the observation period after the last day of exposure) of 90 days.
The duration of the grace periods was chosen on the basis of the distribution of the mean difference between 2 consecutive drug claims observed in the study population and on the basis of a descriptive analysis for a sample of our VKA population for whom we obtained information regarding the individual prescribed doses.
Follow‐up and Outcomes
Follow‐up started on the day following the index date and ended at the occurrence of the first event among a study outcome, death, regional healthcare assistance disenrollment, discontinuation of the index drug treatment (defined as a gap greater than 90 days between the last day covered by a drug claim and the start of the subsequent drug claim of the same drug group; date of discontinuation was defined as the date of last day covered by DDD prescribed plus the grace period of 90 days), switch to the alternative drug group, and end of the study period (December 31, 2015), in an as‐treated approach.
The primary study outcome was mortality for any cause; secondary outcomes were cardiovascular mortality, acute myocardial infarction, ischemic and hemorrhagic stroke, and gastrointestinal bleeding (see Table S2 for outcome definitions). Each outcome was evaluated separately. If more than 1 study outcome occurred during the follow‐up time, we considered each of them in separate analyses. If patients experienced the same study outcome more than once, only the first outcome was considered.
Patient Characteristics
Patient characteristics were measured from the different health information systems during the year before the index date and included demographic information, comorbidities (eg, risk factors for bleeding, ischemic stroke), drug use (eg, oral cardiovascular agents, medications that increase bleeding risk, interacting medications), measures of health service utilization, a combined comorbidity score,30 CHA2DS2‐VASc and HAS‐BLED scores,31 adapted for administrative data, for a total of 90 potential confounders (see Table S3 for a complete list of patient characteristics and related ICD‐9‐CM and ATC codes).
Statistical Analysis
Identification of Sequential PS‐Matched Cohorts
We started the monitoring program on July 1, 2013. After the first monitoring period comprising 6 months (July 2013 through December 2013), we used subsequent monitoring intervals of 3 months for cohort update. In each interval we identified new users of DOACs and VKAs on a periodic basis as data became available. In this pilot phase we identified 9 monitoring periods. In Italy healthcare data are collected for administrative purposes by the regional government, which then grants access to updates with a 6‐month delay. In this study we implemented a sequential analysis built on 3‐month windows to mimic an ideal situation characterized by 3‐month delays between data collection and analysis.
For each monitoring period, we estimated PS models on all eligible initiators during that interval, keeping matches from previous intervals fixed. PS was estimated in a logistic regression model as the probability of being prescribed with a DOAC versus a VKA conditional on the 90 potential confounders reported in Table S3. DOAC initiators were 1:1 PS‐matched to their nearest VKA initiators within a caliper of 0.05 on the PS scale.32 In each monitoring period, covariate balance between the 2 matched exposure groups was evaluated through absolute standardized differences; values below 0.1 were interpreted as evidence of good balance achievement.33
Sequential Analyses
To compare the risk of each outcome of interest between DOAC and VKA new users over time, at the end of each monitoring period we calculated cumulative PS‐adjusted hazard ratios (HRs) and their 95% confidence intervals (CIs) using Cox proportional hazard models stratified by matched set. The proportional hazards assumption was assessed using Schoenfeld residuals.
We decided a priori to continue the monitoring program throughout the entire study period July 2013 through December 2015, so we did not conduct sequential testing34, 35, 36 at each interim analysis to assess whether the accumulated evidence was sufficient to stop or to continue the monitoring.
To account for the fact that patients may be prescribed therapeutic doses other than the DDD or may not be perfectly adherent to daily drug therapy, we performed an intention‐to‐treat analysis, in which the follow‐up started on the day following the index date and ended at the occurrence of the first event among a study outcome of death, regional healthcare assistance disenrollment, 12 months of follow‐up, or end of study period (December 31, 2015), without considering index treatment discontinuation.
Implementation Details
In the first monitoring period (July 2013 through December 2013), all DOAC and VKA users with an index date in this period were enrolled, applying inclusion/exclusion criteria, and the information data related to 90 covariates (retrieved from different health information systems in the year before the index date) were used to build the PS. Then, DOAC and VKA users were matched 1:1, using the nearest‐neighbor method. The 2 matched cohorts were followed‐up from the day after the index date to the occurrence of the first event among study outcome, death, disenrollment, discontinuation, switching, and end of first monitoring period (December 31, 2013). At this point the first analysis was performed running a Cox proportional hazard model stratified by match set to estimate the HRs for the study outcomes. In the second monitoring period (January 2014 through March 2014), all DOAC and VKA users with an index date in this period were enrolled, and the information related to 90 covariates was used to build the PS and to match them 1:1. The 2 matched cohorts were followed up from the day after the index date to the occurrence of the first event among study outcome, death, disenrollment, discontinuation, switching, and end of second monitoring period (March 31, 2014). Meanwhile, follow‐up time for the DOAC and VKA users cohorts already matched in the first monitoring period were extended until the occurrence of the first event among study outcome, death, disenrollment, discontinuation, switching, and end of the second monitoring period. At this point the second analysis was performed running a crude Cox proportional hazard model to estimate the second updating study outcome HRs. This procedure was then used for the further monitoring periods, following the scheme proposed by Schneeweiss and colleagues.28
Analyses were performed using SAS 9.2 (SAS Institute Inc, Cary, NC) and Stata version 12 (Stata Corporation, College Station, TX).
Results
Study Population and Patient Characteristics
During the study period, DOAC use increased steadily, while VKA use sharply dropped until DOACs outweighed VKAs in September 2015 (Figure S1). Overall, 124 684 patients initiated an oral anticoagulant agent during the study period. After the application of the inclusion and exclusion criteria, the study population accounted for 19 201 patients overall, with the following distribution in each of the 9 periods: 4199 patients in the first period (19.7% DOACs), 2351 in the second (30.2% DOACs), 1901 in the third (35.6% DOACs), 1657 in the fourth (41.2% DOACs), 1817 in the fifth (48.4% DOACs), 1990 in the sixth (53.6% DOACs), 1959 in the seventh (55.3% DOACs), 1515 in the eighth (58.8% DOACs), 1815 in the ninth (63.5% DOACs) (Figure 1).
Figure 1.
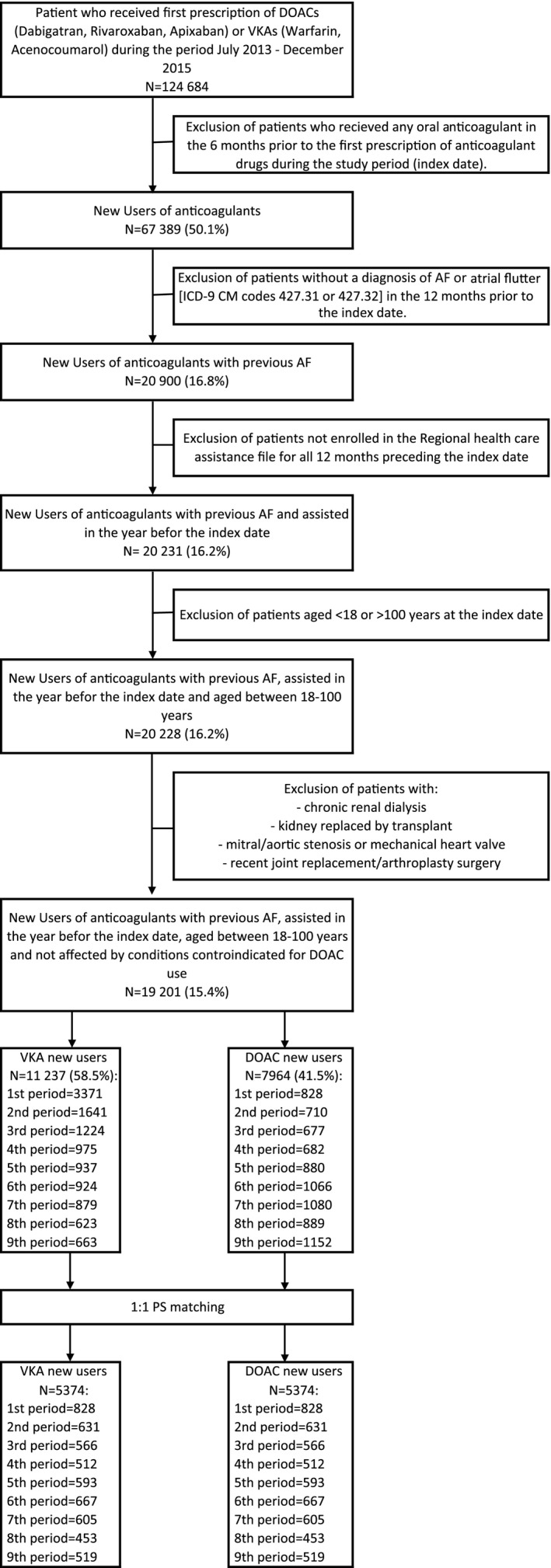
Cohort selection. AF indicates atrial fibrillation; DOAC, direct oral anticoagulants; ICD‐9‐CM, the International Classification of Diseases, 9th Revision, Clinical Modification; PS, propensity score; VKA, vitamin K antagonists.
Before PS matching, some covariates were unbalanced across most monitoring periods (data not shown). VKA patients were more likely to have a history of chronic kidney disease, percutaneous coronary intervention, acute myocardial infarction, and other cardiovascular diseases, whereas DOAC patients had a higher prevalence of prior ischemic stroke. VKA patients were also more likely to receive treatment with heparin and diuretics at baseline. After PS matching, all patient characteristics were well balanced, as assessed by absolute standardized differences lower than 0.1 (Table S4 reports patient characteristics and their balance between the 2 groups at the end of the ninth period before and after PS matching).
PS‐matched sequential cohorts steadily accumulated over time, starting with 1650 enrollees in the first monitoring period and reaching 10 742 in the ninth period.
Safety and Effectiveness Outcomes
For all outcomes of interest, with increasing numbers of enrollees, power and precision of the effect estimates increased over time (Figures 2, 3, 4, 5, 6 through 7).
Figure 2.
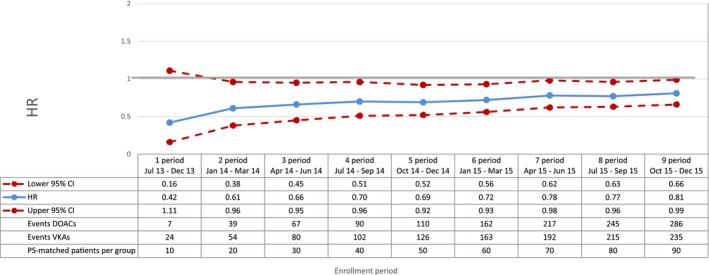
Mortality—sequential analysis of new users of DOACs vs VKAs—HR and 95% CI. CI indicates confidence interval; DOAC, direct oral anticoagulants; HR, hazard ratio; PS, propensity score; VKA, vitamin K antagonists.
Figure 3.
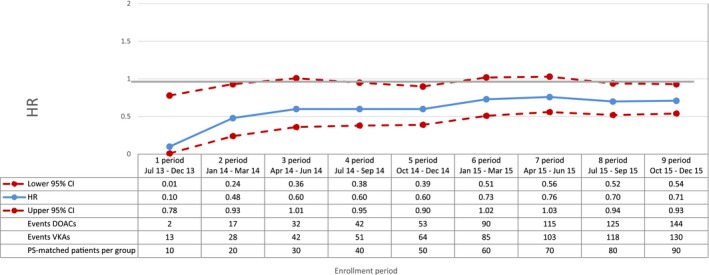
Cardiovascular mortality—sequential analysis of new users of DOACs vs VKAs—HR and 95% CI. CI indicates confidence interval; DOAC, direct oral anticoagulants; HR, hazard ratio; PS, propensity score; VKA, vitamin K antagonists.
Figure 4.
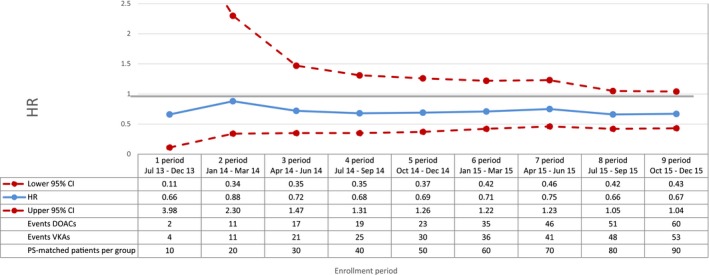
Acute myocardial infarction—sequential analysis of new users of DOACs vs VKAs—HR and 95% CI. CI indicates confidence interval; DOAC, direct oral anticoagulants; HR, hazard ratio; PS, propensity score; VKA, vitamin K antagonists.
Figure 5.
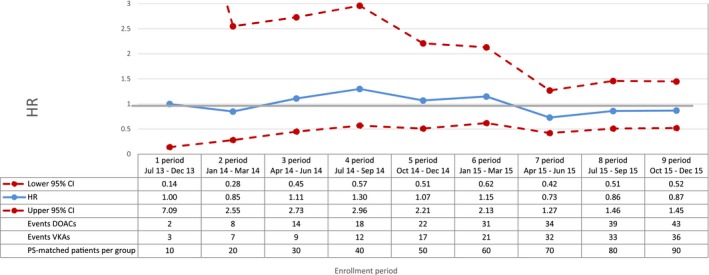
Ischemic stroke—sequential analysis of new users of DOACs vs VKAs—HR and 95% CI. CI indicates confidence interval; DOAC, direct oral anticoagulants; HR, hazard ratio; PS, propensity score; VKA, vitamin K antagonists.
Figure 6.
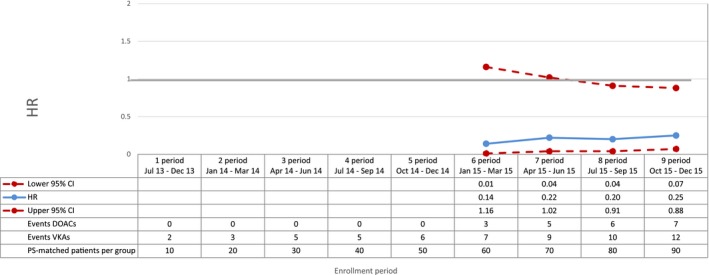
Hemorrhagic stroke—sequential analysis of new users of DOACs vs VKAs—HR and 95% CI. CI indicates confidence interval; DOAC, direct oral anticoagulants; HR, hazard ratio; PS, propensity score; VKA, vitamin K antagonists.
Figure 7.
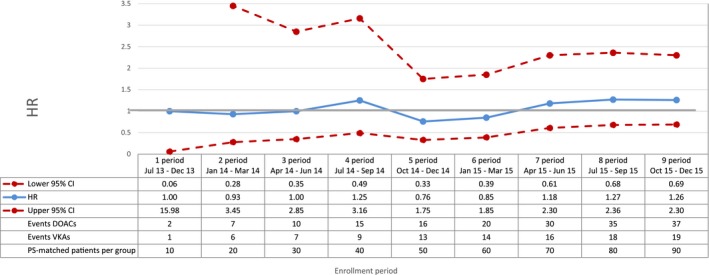
Gastrointestinal bleeding—sequential analysis of new users of DOACs vs VKAs—HR and 95% CI. CI indicates confidence interval; DOAC, direct oral anticoagulants; HR, hazard ratio; PS, propensity score; VKA, vitamin K antagonists.
Compared with VKAs, DOACs were associated with a decrease in the risk of total mortality, with a broad confidence interval in the first period (HR 0.42; 95% CI 0.16‐1.11) and a more precise estimate at the end of the study period (HR 0.81; 95% CI 0.66‐0.99) (Figure 2). DOAC use was also associated with a 29% reduction in the risk of cardiovascular mortality (HR 0.71; 95% CI 0.54‐0.93, by the end of the study period) compared with VKA use (Figure 3). By the end of the study period, we observed a decrease in risk of acute myocardial infarction associated with the use of DOACs (HR 0.67; 95% CI 0.43‐1.04), although effect estimates were imprecise due to the low number of events (Figure 4). DOAC use was also associated with a nonsignificant reduction in the risk of ischemic stroke (HR 0.87; 95% CI 0.52‐1.45) and with a meaningful but imprecise reduction in the risk of hemorrhagic stroke (HR 0.25; 95% CI 0.07‐0.88) and ischemic stroke (HR 0.87; 95% CI 0.52‐1.45) (Figures 5 and 6). Finally, we observed a nonsignificant excess in the risk of gastrointestinal bleeding among DOAC initiators compared with patients initiating VKAs (HR 1.26; 95% CI 0.69‐2.30) (Figure 7).
Results from the intention‐to‐treat analysis mostly confirmed the main findings (Table S5).
Discussion
In this pilot implementation of a near‐real‐time monitoring program in Italy, patients with nonvalvular AF initiating DOACs had a significant reduction in the risk of all‐cause and cardiovascular mortality and in the risk of hemorrhagic stroke compared with VKA initiators with AF. DOACs were also associated with a slightly decreased risk of myocardial infarction and ischemic stroke and with a nonsignificant increased risk of gastrointestinal bleeding. The different outcomes were analyzed independently from each other, and competing risks were not considered.
Our findings are in line with results of 3 meta‐analyses of randomized clinical trials comparing DOACs versus VKAs.17, 37, 38 Specifically, the reduced risk among DOAC users to experience all‐cause mortality, hemorrhagic stroke, and ischemic outcomes is comparable across studies. Also, our nonsignificant finding of an increased risk of gastrointestinal bleeding is confirmed by 2 of the meta‐analyses.17, 37 Similarly, our results are in line with findings from previous observational studies18, 19, 20, 21, 23, 24, 25 that compared single DOACs versus warfarin.
At the time we started monitoring, evidence on the comparative effectiveness of DOACs versus VKAs was still not conclusive, especially regarding the real‐world setting. Therefore, our regional health policy makers committed to this study. As mentioned above, we believe this is still a relevant clinical question in the context of local settings where specific patterns of use of medications may play an important role toward their overall safety and effectiveness. This relevant question is embedded within the first pilot implementation of a monitoring framework in Italy and, to our knowledge, in Europe. This system could be used to promptly monitor new drugs nationwide with the ultimate goal to provide stakeholders with information for rapid decision making.
In this pilot monitoring program the sequential accrual of the data was simulated to conduct sequential analyses. As new medications enter the market, this monitoring framework will promptly provide Italian prescribers with relevant clinical information on the safety and effectiveness of new agents in “near”‐real‐time, which comes from the fact that there is generally a lag between when the drug is delivered to a patient and when the data become available for analysis.28, 39 This occurs in temporal updates, which we refer to as “monitoring periods” in the current article. This is a peculiarity of claims data in general and, thus, of postmarketing surveillance programs based on claims data, including the US Sentinel program. In Italy, healthcare data are collected for administrative purposes by the regional government, which then grants access to updates with a 6‐month delay. In this study we implemented a sequential analysis built on 3‐month windows to mimic an ideal situation characterized by 3‐month delays between data collection and analysis. The usefulness of a real‐time monitoring system as demonstrated by this pilot study may drive the process of accelerating data access in Italy.
As in the majority of observational studies based on administrative databases, confounding is a challenge. We tried to rule out measurable confounding as much as possible using specific techniques in the design and in the analysis. To this end, we excluded patients with hospital and/or specialist care codes for chronic dialysis and those with kidney replaced by transplant (Table S1). In the propensity score we accounted for over 90 potential confounders, which included chronic kidney disease, percutaneous coronary intervention, and the use of antiplatelets (Table S3).
In studying newly authorized drugs, confounding by indication is a potential risk. In a monitoring program it is fundamental to account for the potential temporal changes in prescribing patterns. As shown in Figure S1, prescribing patterns of DOACs and VKAs rapidly changed over time: in the first month after authorization, DOACs accounted for about 10% of newly prescribed anticoagulants in AF patients, whereas at the end of our observation period, DOACs had become the first anticoagulant choice. To account for these rapid changes, we PS‐matched patients within 3‐month monitoring periods.
Another critical issue may come from socioeconomic differences in access to treatment and risk of the outcome, but a previous investigation on secondary prevention after myocardial infarction in a similar population in the same region showed that in our healthcare system, where chronic drug treatment is equally accessible to all residents, this is not an issue.40
A strength of our population‐based observational study is that we were able to enroll all patients treated with the study drugs in a real‐world setting, independently of older ages, comedications, comorbidities, and so forth. Consequently, our population is older and sicker than those included in clinical trials and is representative of patients actually treated. In order to guarantee internal validity, we applied some exclusion criteria, such as renal disorders, and therefore, our results may not be transferrable to special populations such as patients with chronic kidney disease.
Our study has several limitations, one of which is the risk of residual confounding. We accounted for 90 potential confounders available in our data, but we did not have any detailed clinical information, which might play an important role. In particular, we built proxies of CHA2DS2‐VASc and HAS‐BLED scores, but as values of creatinine clearance were not available, we used the number of creatinine tests instead. Moreover, our data lack important sociodemographic information such as body mass index, smoking, and socioeconomic status. For a subset of the study population, receiving care at an anticoagulant center of the Lazio Region, some clinical variables recorded during ambulatory visits, which are not captured in administrative databases (such as type and dosage of anticoagulant drugs, exact HAS‐BLED and CHA2DS2‐VASc score, international normalized ratio value, creatinine clearance, and others), will become available for subsequent monitoring periods. This information will allow us to evaluate the balance of these potential unmeasured confounders between exposure groups within this subset and to possibly use that balance for adjustment purposes.
Another limitation of this study was the adherence calculation using the DDD to approximate the days supplied, especially for VKAs, as physicians frequently need to adapt individual prescribed doses according to periodic international normalized ratio measurements, and our data provide neither individual doses nor results of the international normalized ratio measurements. We addressed this limitation by applying a grace period of 90 days in the main analysis and by performing sensitivity analyses with an intention‐to‐treat approach, which produced consistent results to the main findings.
Weaknesses related to study power, unmeasured confounding, and generalizability will be addressed in a next step, extending the study population to other Italian regions and performing external adjustment using detailed clinical information available for a subsample of the Lazio cohort. A larger sample size will also allow for comparing single DOACs versus single VKAs and performing intraclass comparisons among individual DOAC agents to test the potential differences in safety and effectiveness among the different DOACs highlighted previously.22
Conclusions
The present study describes the pilot implementation of a monitoring program for newly marketed medications in the Lazio region and demonstrates the feasibility of such a framework to produce timely and valid evidence on the comparative safety and effectiveness of new drugs. In Italy, all healthcare–related data are routinely collected for administrative purposes, and the access does not imply any extra costs. Using these data for postmarketing surveillance is actually an added value, which requests an investment in human resources but not in data acquisition. Thus, a system based on routinely collected data is much more cost‐effective than any active data collection for monitoring purposes. Although active pharmacovigilance is based on cases reported by healthcare providers and thus depends on their awareness and willingness to actively feed the system, a system based on routine data can identify a much larger range of outcomes. A fully developed monitoring system will be a useful instrument for clinicians and healthcare decision makers, defining the net incremental value of new agents.
Sources of Funding
This work was supported by a grant from the regional Pharmacovigilance call 2014 with grants from the Italian Medicine Agency.
Disclosures
None.
Supporting information
Table S1. Exclusion Criteria
Table S2. Outcomes of Interest
Table S3. Potential Confounders Included in the PS: (1) Potential Confounders Included in the PS and (2) ATC Codes for Medications
Table S4. Baseline Characteristics for the Overall Population Before PS Matching and for the Sequential Cohort After PS Matching in Each Monitoring Period
Table S5. Sequential Analysis of Study Outcomes: Intention‐to‐Treat Analysis
Figure S1. New users of anticoagulant drugs: time trend.
(J Am Heart Assoc. 2018;7:e008034 DOI: 10.1161/JAHA.117.008034.)29525786
References
- 1. FDA communication. Available at: https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm590808.htm. Accessed July 1, 2016.
- 2. Eichler HG, Oye K, Baird LG, Abadie E, Brown J, Drum CL, Ferguson J, Garner S, Honig P, Hukkelhoven M, Lim JC, Lim R, Lumpkin MM, Neil G, O'Rourke B, Pezalla E, Shoda D, Seyfert‐Margolis V, Sigal EV, Sobotka J, Tan D, Unger TF, Hirsch G. Adaptive licensing: taking the next step in the evolution of drug approval. Clin Pharmacol Ther. 2012;91:426–437. [DOI] [PubMed] [Google Scholar]
- 3. Platt R, Wilson M, Chan KA, Benner JS, Marchibroda J, McClellan M. The new Sentinel Network—improving the evidence of medical‐product safety. N Engl J Med. 2009;361:645–647. [DOI] [PubMed] [Google Scholar]
- 4. Behrman RE, Benner JS, Brown JS, McClellan M, Woodcock J, Platt R. Developing the Sentinel System—a national resource for evidence development. N Engl J Med. 2011;364:498–499. [DOI] [PubMed] [Google Scholar]
- 5. Gagne JJ, Rassen JA, Walker AM, Glynn RJ, Schneeweiss S. Active safety monitoring of new medical products using electronic healthcare data: selecting alerting rules. Epidemiology. 2012;23:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rassen JA, Avorn J, Schneeweiss S. Multivariate‐adjusted pharmacoepidemiologic analyses of confidential information pooled from multiple health care utilization databases. Pharmacoepidemiol Drug Saf. 2010;19:848–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown JS, Lane K, Moore K, Platt R. Defining and Evaluating Possible Database Models to Implement the FDA Sentinel Initiative; U.S. Food and Drug Administration: FDA‐2009‐N‐0192‐0005. 2009. Available at: https://www.pharmamedtechbi.com/~/media/Images/Publications/Archive/The%20Pink%20Sheet/71/020/00710200015/sentinel_database_models_05_09.pdf. Accessed July 1, 2016.
- 8. Nelson JC, Cook AJ, Yu O, Dominguez C, Zhao S, Greene SK, Fireman BH, Jacobsen SJ, Weintraub ES, Jackson LA. Challenges in the design and analysis of sequentially monitored postmarket safety surveillance evaluations using electronic observational health care data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):62–71. [DOI] [PubMed] [Google Scholar]
- 9. Avery TR, Kulldorff M, Vilk Y, Li L, Cheetham TC, Dublin S, Davis RL, Liu L, Herrinton L, Brown JS. Near real‐time adverse drug reaction surveillance within population‐based health networks: methodology considerations for data accrual. Pharmacoepidemiol Drug Saf. 2013;22:488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Curtis LH, Weiner MG, Boudreau DM, Cooper WO, Daniel GW, Nair VP, Raebel MA, Beaulieu NU, Rosofsky R, Woodworth TS, Brown JS. Design considerations, architecture, and use of the Mini‐Sentinel distributed data system. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):23–31. [DOI] [PubMed] [Google Scholar]
- 11. Schneeweiss S. A basic study design for expedited safety signal evaluation based on electronic healthcare data. Pharmacoepidemiol Drug Saf. 2010;19:858–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gagne JJ, Wang SV, Rassen JA, Schneeweiss S. A modular, prospective, semi‐automated drug safety monitoring system for use in a distributed data environment. Pharmacoepidemiol Drug Saf. 2014;23:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heidbuchel H, Verhamme P, Alings M, Antz M, Diener HC, Hacke W, Oldgren J, Sinnaeve P, Camm AJ, Kirchhof P. Updated European Heart Rhythm Association practical guide on the use of non‐vitamin‐K antagonist anticoagulants in patients with non‐valvular atrial fibrillation: executive summary. Eur Heart J. 2017;38:2137–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Dariu H, Diener HC, Joyner CD, Wallentin L; the RE‐LY Steering Committee and Investigators . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 15. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KAA, Califf RM; the ROCKET AF Steering Committee, for the ROCKET AF Investigators . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 16. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez‐Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; ARISTOTLE Committees and Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 17. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 18. Graham DJ, Reichman ME, Wernecke M, Zhang R, Southworth MR, Levenson M, Sheu TC, Mott K, Goulding MR, Houstoun M, MaCurdy TE, Worrall C, Kelman JA. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation. 2015;131:157–164. [DOI] [PubMed] [Google Scholar]
- 19. Abraham NS, Singh S, Alexander GC, Heien H, Haas LR, Crown W, Shah ND. Comparative risk of gastrointestinal bleeding with dabigatran, rivaroxaban, and warfarin: population based cohort study. BMJ. 2015;350:h1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maura G, Blotière PO, Bouillon K, Billionnet C, Ricordeau P, Alla F, Zureik M. Comparison of the short‐term risk of bleeding and arterial thromboembolic events in nonvalvular atrial fibrillation patients newly treated with dabigatran or rivaroxaban versus vitamin K antagonists: a French nationwide propensity‐matched cohort study. Circulation. 2015;132:1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seeger JD, Bykov K, Bartels DB, Huybrechts K, Zint K, Schneeweiss S. Safety and effectiveness of dabigatran and warfarin in routine care of patients with atrial fibrillation. Thromb Haemost. 2015;114:1277–1289. [DOI] [PubMed] [Google Scholar]
- 22. Larsen TB, Skjøth F, Nielsen PB, Kjældgaard JN, Lip GY. Comparative effectiveness and safety of non‐vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chan YH, Kuo CT, Yeh YH, Chang SH, Wu LS, Lee HF, Tu HT, See LC. Thromboembolic, bleeding, and mortality risks of rivaroxaban and dabigatran in Asians with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2016;68:1389–1401. [DOI] [PubMed] [Google Scholar]
- 24. Yao X, Abraham NS, Sangaralingham LR, Bellolio MF, McBane RD, Shah ND, Noseworthy PA. Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation. J Am Heart Assoc. 2016;5:e003725 DOI: 10.1161/JAHA.116.003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hernandez I, Baik SH, Piñera A, Zhang Y. Risk of bleeding with dabigatran in atrial fibrillation. JAMA Intern Med. 2015;175:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mahtani KR, Heneghan C. Novel oral anticoagulants for atrial fibrillation. BMJ. 2016;354:i5187. [DOI] [PubMed] [Google Scholar]
- 27. Mumoli N, Mastroiacovo D, Tamborini‐Permunian E, Vitale J, Giorgi‐Pierfranceschi M, Cei M, Dentali F. Dabigatran in nonvalvular atrial fibrillation: from clinical trials to real‐life experience. J Cardiovasc Med. 2017;18:467–477. [DOI] [PubMed] [Google Scholar]
- 28. Schneeweiss S, Gagne JJ, Glynn RJ, Ruhl M, Rassen JA. Assessing the comparative effectiveness of newly marketed medications: methodological challenges and implications for drug development. Clin Pharmacol Ther. 2011;90:777–790. [DOI] [PubMed] [Google Scholar]
- 29. WHO . DDD/ATC system. Available at: http://www.whocc.no/atc_ddd_index/. Accessed July 1, 2016.
- 30. Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen PC, Lip GY, Yeh G, Lin HJ, Chien KL. Risk of bleeding and stroke with oral anticoagulation and antiplatelet therapy in patients with atrial fibrillation in Taiwan: a nationwide cohort study. PLoS One. 2015;10:e0125257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rassen JA, Doherty M, Huang W, Schneeweiss S. Pharmacoepidemiology toolbox. Boston, MA: Available at: http://www.hdpharmacoepi.org. Accessed July 1, 2016. [Google Scholar]
- 33. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. De Mets DL, Lan KK. Interim analysis: the alpha spending function approach. Stat Med. 1994;13:1341–1352; discussion 1353‐1356. [DOI] [PubMed] [Google Scholar]
- 35. Yuan Y. Group Sequential Analysis Using the New SEQDESIGN and SEQTEST Procedures. Available at: https://support.sas.com/resources/papers/proceedings09/311-2009.pdf. Accessed July 1, 2016.
- 36. Nelson JC, Boudreau D, Wellman R, Yu O, Cook AJ, Maro J, Ouellet‐Hellstrom R, Floyd J, Heckbert SR, Pinheiro S, Reichman M, Shoaibi A. Mini‐sentinel methods. Improving sequential safety surveillance planning methods for routine assessments that use regression adjustment or weighting to control confounding. Available at: https://www.sentinelinitiative.org/sites/default/files/Methods/Mini-Sentinel_Methods_Improving-Sequential-Safety-Surveillance_Report.pdf. Accessed July 1, 2016.
- 37. Baker WL, Phung OJ. Systematic review and adjusted indirect comparison meta‐analysis of oral anticoagulants in atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2012;5:711–719. [DOI] [PubMed] [Google Scholar]
- 38. Dentali F, Riva N, Crowther M, Turpie AG, Lip GY, Ageno W. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta‐analysis of the literature. Circulation. 2012;126:2381–2391. [DOI] [PubMed] [Google Scholar]
- 39. Hartzema AG, Racoosin JA, MaCurdy TE, Gibbs JM, Kelman JA. Utilizing Medicare claims data for real‐time drug safety evaluations: is it feasible? Pharmacoepidemiol Drug Saf. 2011;20:684–688. [DOI] [PubMed] [Google Scholar]
- 40. Kirchmayer U, Agabiti N, Belleudi V, Davoli M, Fusco D, Stafoggia M, Arcà M, Barone AP, Perucci CA. Socio‐demographic differences in adherence to evidence‐based drug therapy after hospital discharge from acute myocardial infarction: a population‐based cohort study in Rome, Italy. J Clin Pharm Ther. 2012;37:37–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Exclusion Criteria
Table S2. Outcomes of Interest
Table S3. Potential Confounders Included in the PS: (1) Potential Confounders Included in the PS and (2) ATC Codes for Medications
Table S4. Baseline Characteristics for the Overall Population Before PS Matching and for the Sequential Cohort After PS Matching in Each Monitoring Period
Table S5. Sequential Analysis of Study Outcomes: Intention‐to‐Treat Analysis
Figure S1. New users of anticoagulant drugs: time trend.


