Abstract
Background
Acute kidney injury is a frequent complication after cardiac surgery and is associated with adverse outcomes. Although short‐term calorie restriction (CR) has proven protective in rodent models of acute kidney injury, similar effects have not yet been demonstrated in humans.
Methods and Results
CR_KCH (Effect of a Preoperative Calorie Restriction on Renal Function After Cardiac Surgery) is a randomized controlled trial in patients scheduled for cardiac surgery. Patients were randomly assigned to receive either a formula diet containing 60% of the daily energy requirement (CR group) or ad libitum food (control group) for 7 days before surgery. In total, 82 patients were enrolled between April 16, 2012, and February 5, 2015. There was no between‐group difference in the primary end point of median serum creatinine increment after 24 hours (control group: 0.0 mg/dL [−0.1 – (+0.2) mg/dL]; CR group: 0.0 mg/dL [−0.2 – (+0.2) mg/dL]; P=0.39). CR prevented a rise in median creatinine at 48 hours (control group: +0.1 mg/dL [0.0 – 0.3 mg/dL]; CR group: −0.1 mg/dL [−0.2 – (+0.1) mg/dL]; P=0.03), with most pronounced effects observed in male patients and patients with a body mass index >25. This benefit persisted until discharge: Median creatinine decreased by 0.1 mg/dL (−0.2 – 0.0 mg/dL) in the CR group, whereas it increased by 0.1 mg/dL (0.0 – 0.3 mg/dL; P=0.0006) in the control group. Incidence of acute kidney injury was reduced by 5.8% (41.7% in the CR group compared with 47.5% in the control group). Safety‐related events did not differ between groups.
Conclusions
Despite disappointing results with respect to creatinine rise within the first 24 hours, the benefits observed at later time points and the subgroup analyses suggest the protective potential of short‐term CR in patients at risk for acute kidney injury, warranting further investigation.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01534364.
Keywords: acute kidney injury, calorie restriction, cardiac surgery, dietary restriction, preconditioning
Subject Categories: Nephrology and Kidney, Translational Studies, Ischemia, Clinical Studies, Diet and Nutrition
Clinical Perspective
What Is New?
In a 82‐patient study, 7‐day calorie restriction was not successful in preventing a rise in median creatinine at 24 hours, but it ameliorated renal failure at 48 hours after cardiac surgery, suggesting a protective potential of short‐term calorie restriction.
This study provides first evidence in humans that preoperative calorie restriction is safe and feasible in cardiac surgery.
This study is the first controlled and randomized clinical trial assessing the potential benefit of calorie restriction for organ protection.
What Are the Clinical Implications?
Dietary restriction may provide a novel and promising approach for inducing organ protection.
The results of this study warrant further investigation to assess the value of preconditioning strategies using nutritional interventions.
Introduction
With incidence of up to 40%, acute kidney injury (AKI) is a frequently encountered complication after cardiac surgery.1 Even mild cases are associated with longer hospital stay, higher morbidity, and increased short‐ and long‐term mortality compared with those without AKI.2, 3 Despite a multitude of clinical trials using pharmacological agents, the quest for an effective renoprotective treatment has been unsuccessful so far.4 Promising and innovative nonpharmacological strategies, including remote ischemic preconditioning (RIPC), have emerged recently; however, the results of several trials investigating RIPC in diverse clinical settings are equivocal.5, 6, 7, 8
Short‐term dietary restriction has been shown to induce robust cellular stress resistance in various species, leading to reduced organ damage susceptibility. Mitchell and coworkers showed that a reduction in daily calorie intake by 30% over short time periods prevented AKI in a murine ischemia–reperfusion injury model.9 The astonishingly potent effect of calorie restriction (CR) on AKI was confirmed by a more recent study from our group.10 Similar results have been reported in other models of renal injury (eg, cisplatinum‐induced AKI11) and in other organ systems.12 Although a short‐term diet has been proven to be feasible and safe in patients undergoing living‐donor kidney transplantation,13, 14 no prospective trials have been undertaken to investigate the possible impact of moderate CR as an organ‐protective measure. We performed a prospective, single‐center, randomized, open‐label clinical trial to test the hypothesis that 1 week of a 40% reduction of calorie intake prevents AKI in at‐risk patients undergoing cardiac surgery.
Methods
The data, analytic methods, and study materials will not be made available permanently to other researchers for purposes of reproducing the results or replicating the procedure; however, all data, analytic methods, and study materials will be provided on request.
Study Design and Participants
This pilot trial was designed and conducted as a randomized, open‐label, single‐center study at the University Hospital Cologne. Approval was obtained from the institutional review board of the University Hospital Cologne. Adult patients (aged >18 years) scheduled for elective cardiac surgery involving cardiopulmonary bypass who carried at least one of the following risk factors for developing AKI15 were enrolled after having obtained written informed consent: age >70 years, chronic kidney disease, diabetes mellitus, congestive heart failure of New York Heart Association class 3 or 4, reduced left ventricular ejection fraction (<50%), peripheral vascular disease, planned combined coronary artery bypass grafting and valve surgery, previous cardiac surgery, and chronic obstructive pulmonary disease. Exclusion criteria were end‐stage renal disease, kidney transplantation, pregnancy, weight loss >1 kg within 2 weeks before enrollment unless due to diuretic medication, body mass index (calculated as kg/m2) of <18.5 or >35, evidence of malignancy or uncontrolled infection, or the inability to give informed consent. The study was conducted in accordance with the Declaration of Helsinki and the good clinical practice guidelines by the International Conference on Harmonization. The study protocol is provided in Data S1.
Randomization
At 8 to 12 days before the scheduled day of surgery, eligible patients were invited for a screening visit. Malnourishment was ruled out by careful physical examination, assessment of body weight and body composition using a bioimpedance scale (Tanita BC‐418 segmental body composition analyzer), and confirmation of normal serum albumin. After having given written informed consent, patients were randomly assigned using a 1:1 ratio to either receive a calorie‐restricted diet (CR group) or an ad libitum diet (control group). Randomization codes were generated by the Institute of Medical Statistics and Computational Biology (University of Cologne, Germany) and provided to the study center in sealed, consecutively numbered, opaque envelopes.
Procedures
Patients in the CR group were provided with a formula diet (Fresubin energy fiber drink) that contained all necessary macro‐ and micronutrients; however, the amount was limited to provide only 60% of the daily energy expenditure (DEE), as calculated using the Mifflin–St. Jeor equation and individually assessed activity factors. Participants were instructed not to consume extra food or calorie‐containing beverages like alcohol, fruit juices, or soft drinks. The diet commenced on preoperative day −7 and was maintained until day −1. On the day of surgery, patients were maintained in a fasting state until surgery. In the control group, patients were allowed to ingest food at their own discretion and were encouraged to stick to their normal eating habits, thereby ensuring nonrestricted calorie intake. All participants in both groups were provided with diaries and reported their food consumption on a daily basis. In addition, regular phone calls ascertained patients' well‐being and monitored adherence to the designated protocol. Reassessment of all participants was performed on the day of admittance (day −1). All blood and urine samples at baseline (ie, immediately before surgery) and within the postoperative 48‐hour time frame were obtained at prespecified time points; laboratory parameters beyond day 2 after surgery as well as type of surgery, cardiopulmonary bypass time, and aortic cross‐clamp time were extracted from the medical records. Baseline demographic and clinical data including comorbidities and medication were assessed at the screening visit (between days −12 and −8). Anthropometric data (eg, body weight, body composition) were recorded at the screening visit and at hospital admittance (day −1). All participants were instructed to collect their urine over a 24‐hour period at day −2, and estimation of the daily protein intake was performed by assessing urinary urea nitrogen appearance in this 24‐hour sample and the equation proposed by Maroni et al.16 After surgery, hourly urine output was recorded as long as an indwelling urinary catheter was in place (at least 24 hours). All patients were followed up until their hospital discharge.
All patients received sevoflurane for anesthesia. Surgical procedures were carried out according to the local standard and were not influenced by the study protocol. Other putative measures of organ protection (eg, RIPC) were not performed throughout the study.
Outcomes
Rise in serum creatinine from baseline to 24 hours after cross‐clamping was analyzed as the primary end point. As a point estimate of the effect size, we calculated the change of serum creatinine in the CR group minus the change of serum creatinine in the control group. Secondary end points were rise of serum creatinine from baseline to 48 hours after cross‐clamping; maximum rise of serum creatinine from baseline within 48 hours after cross‐clamping; rise in urinary NGAL (neutrophil gelatinase–associated lipocalin) from baseline to 8 hours after cross‐clamping; occurrence of AKI, as defined by Kidney Disease: Improving Global Outcomes (KDIGO) criteria,17 within 72 hours after surgery; change of serum creatinine from baseline to discharge; need for renal replacement therapy; length of hospital stay; in‐hospital mortality; incidence of perioperative myocardial infarction, stroke, and atrial fibrillation; and evolution of the following biochemical parameters from baseline to 24 hours after cross‐clamping: C‐reactive protein, white blood cell count, creatinine kinase, troponin T, lactate dehydrogenase, NT‐proBNP (N‐terminal pro–brain natriuretic peptide), and lactate. The decision to initiate renal replacement therapy was made exclusively by the treating physician and was not influenced by study personnel. Safety‐related events were captured throughout the study. All events that were judged by the investigator as having reasonable causal relation to the provided diet were considered to be treatment‐related adverse events.
Statistical Analyses
Based on published data,8, 18 we estimated the mean increase of serum creatinine from baseline to 24 hours after cross‐clamping to be 0.4 mg/dL (SD 0.25 mg/dL). A difference in the change of 0.2 mg/dL in serum creatinine in this time frame between the 2 groups was considered to be clinically significant. Assuming a 2‐tailed type I error of 5% and a dropout rate of 20%, we calculated a sample size of 41 for each group to give 80% power. Primary analysis was done with the intention‐to‐treat approach, excluding patients without serum creatinine values at baseline or at 24 hours. All results are given as median (interquartile range [IQR]) unless stated otherwise.
Subgroup analyses were performed with regard to sex, age, body mass index, history of diabetes mellitus, ischemia time, type of surgery (valve and/or bypass), and chronic kidney disease stage. Groups were compared using the χ2 test with categorical data sets or the Mann–Whitney U test with numerical data sets. No race‐ or ethnicity‐specific analyses were performed because all included patients were white. All statistical testing was 2‐sided, and P<0.05 was considered significant. Analysis software used was SAS 9.3 (SAS Institute) and IBM SPSS Statistics version 23.
Results
Patients were enrolled between April 16, 2012, and February 5, 2015. Overall, 278 potentially eligible patients were identified and contacted. Of these, 194 declined participation; 84 were enrolled; and, finally, 82 patients (41 in each group) were randomized. In total, 36 patients in the CR group and 40 patients in the control group were included in the intention‐to‐treat analysis (Figure 1). Patient demographics and clinical characteristics are shown in Table 1.
Figure 1.
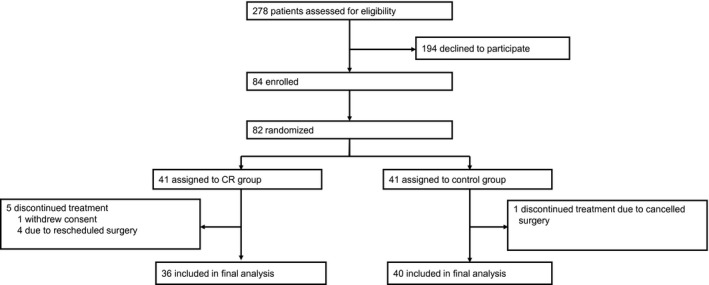
Patient flow and randomization. CR indicates calorie reduction.
Table 1.
Patient Demographics and Clinical Characteristics
| CR Group (n=36) | Control Group (n=40) | |
|---|---|---|
| Age (y), median (IQR) | 72 (63–76) | 75 (70–77) |
| Male, n (%) | 29 (80.6) | 31 (77.5) |
| Weight at screening (kg), median (IQR) | 84.6 (72.1–91.4) | 79.1 (75.1–92.7) |
| BMI at screening (kg/m2), median (IQR) | 26.9 (24.7–30.7) | 26.7 (25.2–30.3) |
| Creatinine at screening (mg/dL), median (IQR) | 1.1 (0.9–1.3) | 1.1 (1.0–1.3) |
| Creatinine day −1 (mg/dL), median (IQR) | 1.2 (1.0–1.6) | 1.1 (0.9–1.3) |
| Creatinine day 0 (mg/dL), median (IQR) | 1.1 (0.9–1.4) | 1.1 (0.9–1.3) |
| Cleveland Clinic Foundation score, median (IQR) | 2 (2–4) | 3 (2–4) |
| Medical history, n (%) | ||
| Chronic kidney disease | 15 (41.7) | 15 (37.5) |
| Peripheral arterial disease | 4 (11.1) | 7 (17.5) |
| Congestive heart failure | 8 (22.2) | 6 (15.0) |
| Previous heart surgery | 3 (8.3) | 1 (2.5) |
| Coronary artery disease | 30 (83.3) | 25 (62.5) |
| Left main stem disease | 6 (16.7) | 4 (10.0) |
| COPD | 5 (13.9) | 6 (15.0) |
| Hypertension | 33 (91.7) | 33 (82.5) |
| Diabetes mellitus | 13 (36.1) | 20 (50.0) |
| Medication, n (%) | ||
| ACEI | 29 (80.6) | 31 (77.5) |
| Aldosterone antagonist | 5 (13.9) | 9 (22.5) |
| Beta blocker | 33 (91.7) | 31 (77.5) |
| Calcium antagonist | 11 (30.6) | 5 (12.5) |
| Lipid‐lowering drugs | 31 (86.1) | 26 (65.0) |
| Antiplatelet therapy | 26 (72.2) | 26 (65.0) |
| Diuretics | 18 (50) | 31 (77.5) |
| Cross‐clamp time (min), median (IQR) | 59.0 (52–82) | 58.5 (45–80) |
| Bypass time (min), median (IQR) | 99.0 (78–123) | 92.0 (68–122) |
| Type of operation, n (%) | ||
| CABG | 14 (38.9) | 13 (32.5) |
| Valve | 9 (25.0) | 14 (35.0) |
| Combined or other | 13 (36.1) | 13 (32.5) |
ACEI indicates angiotensin‐converting enzyme inhibitor; BMI, body mass index; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; CR calorie restriction; IQR, interquartile range.
According to the patients' diary recordings and regular personal interviews, the median daily calorie intake in the CR group was 1313 kcal (IQR: 1224–1412 kcal), which is equivalent to 60% (1.5%) of the calculated DEE. Calculation of additionally ingested calories was accurate for the CR group because patients added only minor amounts of food or countable pieces (eg, fruit, piece of cake); in contrast, precise estimation of true calorie uptake in the control group was hampered by missing or uncertain reports of consumed food quantities. However, all patients in the control group reported that they had not changed their eating habits and had not conducted a diet on their own. To further assess compliance with dietary protocols, we analyzed weight changes from the time of screening to day −1 in both groups. Median weight loss in the control group was 0.1 kg (−0.8 – 0.8 kg), whereas patients in the CR group lost 3.0 kg (−3.9 – (−2.2) kg; P<0.0001). In the latter group, the marked weight change was largely accounted for by a high loss of total body water (−2.4 kg [−3.8 – (−1.9) kg]) compared with the control group (+0.1 kg [−1.4 − 0.9 kg]; P<0.0001). The calculated loss of dry body weight (ie, excluding changes in water balance) was −0.6 kg (−1.3 − 0.5 kg) in the CR group—which is in line with what can be expected with 1 week of a 40% CR—and +0.2 kg (−1.0 − 1.8 kg) in the control group (P=0.06), indicating a true dietary between‐group difference with respect to calorie intake. The estimated daily protein intake according to the equation proposed by Maroni was not different in both groups, with 0.8 g/kg (0.7 – 1.0 g/kg) in the CR group and 0.9 g/kg (0.7 – 1.0 g/kg) in the control group (P=0.67), respectively. It is likely, however, that the calculation based on urinary urea nitrogen appearance is an overestimation of the true protein intake in the CR group because of the catabolic breakdown of endogenous proteins. The protein content of the actually ingested amount of the formula diet was 0.6 g/kg (0.6 – 0.7 g/kg). Consequently, there is evidence of a protein restriction in the CR group compared with the control group; however, this difference is likely to be considerably smaller than 40%.
No difference was noted in median serum creatinine at the screening visit, with 1.1 mg/dL (0.9–1.3 mg/dL) in the CR group and 1.1 mg/dL (1.0–1.3 mg/dL) in the control group (Table 1). However, there was a 0.1‐mg/dL increase in creatinine between the screening visit and day −1 to 1.2 mg/dL (1.0–1.6 mg/dL) in the CR group, and this incremental change was highly significant (P=0.0001) compared with the control group, in which serum creatinine was not altered (1.1 mg/dL [0.9–1.3 mg/dL]). Influences of the diet on anthropometric characteristics and biochemical parameters are summarized in Table 2.
Table 2.
Anthropometric Characteristics and Biochemical Parameters
| CR Group (n=36) | Control Group (n=40) | P Value | |
|---|---|---|---|
| Δ Weight screening to day −1, kg | −3.0 (−3.9 to −2.2) | −0.1 (−0.8 to 0.8) | <0.0001 |
| Δ Dry weight screening to day −1, kg | −0.6 (−1.3 to 0.5) | 0.2 (−1.0 to 1.8) | 0.057 |
| Δ Body water screening to day −1, kg | −2.4 (−3.8 to −1.9) | −0.1 (−1.4 to 0.9) | <0.0001 |
| Δ Creatinine screening to day −1, mg/dL | 0.0 (−0.1 to 0.1) | 0.1 (0.1–0.3) | 0.0001 |
| Calculated daily energy expenditure (DEE), kcal | 2160 (1939–2334) | 2153 (1909–2343) | 0.75 |
| Reported mean daily calorie intake during CR (kcal) | 1313 (1224–1412) | ··· | ··· |
| Reported mean daily calorie intake during CR (% of DEE) | 60 (60–62) | ··· | ··· |
| Calculated daily protein intake during CR, g/kg | 0.6 (0.56–0.66) | ··· | ··· |
| Daily protein intake calculated from urinary urea nitrogen appearance, g/kg | 0.8 (0.7–1.0) | 0.9 (0.7–1.0) | 0.67 |
Values presented as median (interquartile range). CR indicates calorie restriction; DEE, daily energy expenditure.
With a median change of serum creatinine from baseline to 24 hours after cross‐clamping of 0.0 mg/dL (−0.1 – (+0.2) mg/dL) for control group versus 0.0 mg/dL (−0.2 – (+0.2) mg/dL) for CR group (P=0.39) and an effect size of 0.04 mg/dL (95% CI, −0.14 to 0.21), there was no difference in the primary end point (Figure 2).
Figure 2.
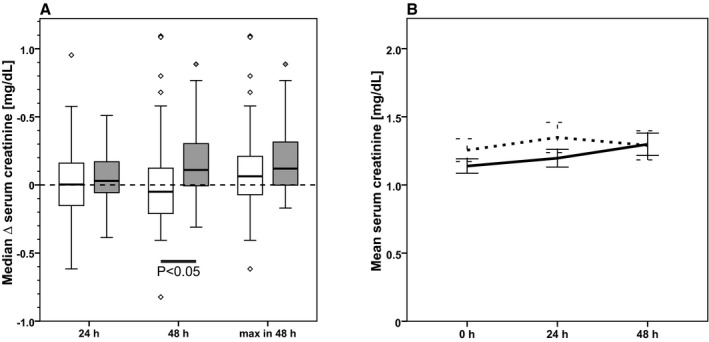
Intention‐to‐treat analysis. Evolution of serum creatinine from baseline to 24 hours after cross‐clamping (primary end point) and from baseline to 48 hours after cross‐clamping and maximum increase of serum creatinine within 48 hours after cross‐clamping in CR patients (white boxes, dashed line; n=36) and control patients (gray boxes, solid line; n=40). A, Box plots showing the change of serum creatinine from baseline to specified time points. B, Development of mean (±SEM) serum creatinine from baseline (0 hour) to 48 hours after cross‐clamping.
In accordance with the KDIGO recommendations for diagnosing AKI, we assessed the rise in serum creatinine within the first 48 hours after cross‐clamping. Within this time frame, the serum creatinine decreased by 0.1 mg/dL (−0.2 – (+0.1) mg/dL) in the CR group and increased by 0.1 mg/dL (0.0 – 0.3 mg/dL) in the control group (P=0.03; Figure 2). Of note, in the CR group, the maximum increment was detected in the time period between baseline and 24 hours, whereas serum creatinine levels were stable or slightly decreasing between 24 and 48 hours. In contrast, in the control group, we found a steady increase of serum creatinine over the entire 48‐hour interval. Subgroup analyses showed that the significant beneficial effect of CR on change of creatinine from baseline to 48 hours shown for the entire group was largely attributable to participants who were male (P=0.0047), who had a body mass index >25 (P=0.023), and who had chronic kidney disease stage 1 (P=0.0253; Figure 3), whereas age, history of diabetes mellitus, ischemia time, and type of surgery had no influence. Furthermore, there was a highly significant between‐group difference in creatinine evolution between baseline and discharge, with a decrease of 0.1 mg/dL (−0.2 – 0.0 mg/dL) in the CR group and an increase of 0.1 mg/dL (0.0 – 0.3 mg/dL) in the control group (P=0.0006).
Figure 3.
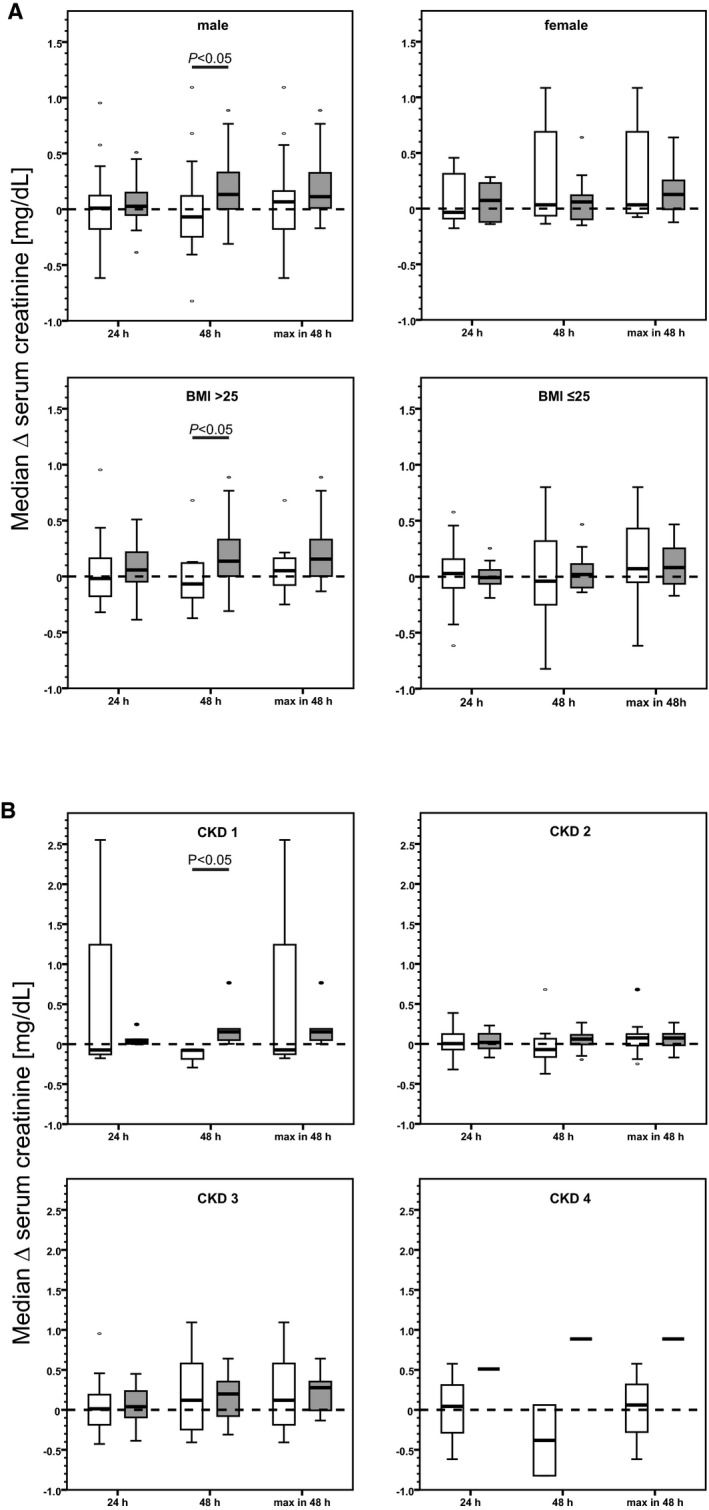
Subgroup analyses showing impact of sex (A, upper panel), body mass index (BMI; A, lower panel) and chronic kidney disease (CKD) stages (B). A, Male participants: calorie reduction (CR) group (white boxes), 29 of 36 participants; control group (gray boxes), 31 of 40 participants. BMI >25: CR group, 22 of 36 participants; control group, 30 of 40 participants. B, CKD 1: CR group (white boxes), n=3; control group (gray boxes), n=5. CKD 2: CR group, n=15; control group, n=13. CKD 3: CR group, n=13; control group, n=20. CKD 4: CR group, n=2; control group, n=1. Box plots showing the change of serum creatinine from baseline to specified time points.
Urinary NGAL 8 hours after cross‐clamping and all other biochemical parameters at 24 hours after surgery were not different in the 2 groups (Table 3). The incidence of AKI according to the KDIGO criteria was lower in the CR group (41.7%) than in the control group (47.5%); however, this difference was not statistically significant (Table 4). No differences were observed with respect to the rate of renal replacement therapy, death, length of stay, and incidence of perioperative myocardial infarction, stroke, or atrial fibrillation.
Table 3.
Secondary End Points: Biochemical Parameters
| CR Group (n=36) | Control Group (n=40) | P Value | |
|---|---|---|---|
| CK, day 0, U/L | 98 (64–145) | 83 (55–129) | 0.28 |
| Δ CK, day 0 to 24 h after cross‐clamping, U/L | 596 (440–779) | 539 (347–839) | 0.39 |
| LDH, day 0, U/L | 207 (185–238) | 220 (194–230) | 0.46 |
| Δ LDH, day 0 to 24 h after cross‐clamping, ng/L | 107 (65–145) | 100 (37–172) | 0.92 |
| NT‐proBNP, day 0, ng/L | 576 (268–1782) | 827 (438–1708) | 0.25 |
| Δ NT‐proBNP, day 0 to 24 h after cross‐clamping, ng/L | 924 (340–4359) | 381 (−304 to 2295) | 0.27 |
| CRP, day 0, mg/L | 3.0 (3.0–3.2) | 3.0 (3.0–4.6) | 0.44 |
| Δ CRP, day 0 to 24 h after cross‐clamping, mg/L | 104 (82–130) | 97 (73–124) | 0.51 |
| WBC, day 0, ×1E9/L | 6.2 (4.9–6.8) | 6.4 (4.9–7.4) | 0.57 |
| Δ WBC, day 0 to 24 h after cross‐clamping, ×109/L | 3.2 (1.5–5.2) | 3.8 (1.9–5.5) | 0.39 |
| Lactate, day 0, mmol/L | 1.1 (1.0–1.6) | 1.2 (1.0–1.6) | 0.85 |
| Δ Lactate, day 0 to 24 h after cross‐clamping, mmol/L | 0.4 (0.0–0.6) | 0.5 (0.1–1.5) | 0.27 |
| NSE, day 0, μmol/L | 23.8 (20.1–28.3) | 22.9 (19.8–29.5) | 0.66 |
| Δ NSE, day 0 to 24 h after cross‐clamping, μmol/L | 5.7 (−1.6 to 12.5) | 10.9 (0.5–19.4) | 0.26 |
| Troponin T, day 0, μg/L | 0.013 (0.010–0.024) | 0.019 (0.012–0.035) | 0.045 |
| Δ Troponin T, day 0 to 24 h after cross‐clamping, μg/L | 0.558 (0.201–1.065) | 0.458 (0.343–0.790) | 0.85 |
| NGAL in urine, day 0, μmol/L | 17.8 (10.0–32.9) | 13.8 (10.0–26.3) | 0.41 |
| Δ NGAL in urine, day 0 to 8 h after cross‐clamping, μmol/L | 17.4 (−3.9 to 48.4) | 9.6 (−2.5 to 30.4) | 0.56 |
| Creatinine at discharge, mg/dL | 1.0 (0.9–1.3) | 1.2 (1.0–1.4) | 0.24 |
| Δ Creatinine, day 0 to discharge, mg/dL | −0.08 (−0.18 to 0.04) | 0.07 (−0.02 to 0.26) | 0.0006 |
Parameters measured in serum unless otherwise indicated. Day 0 indicates baseline value before surgery. Values presented as median (interquartile range). CK indicates creatine kinase; CR, calorie restriction; CRP, C‐reactive protein; LDH, lactate dehydrogenase; NGAL, neutrophil gelatinase–associated lipocalin; NSE, neuron‐specific enolase; NT‐proBNP, N‐terminal pro‐b‐type natriuretic peptide; WBC, white blood cell count.
Table 4.
Secondary End Points: Clinical Parameters
| CR Group (n=36) | Control Group (n=40) | P Value | |
|---|---|---|---|
| AKI, n, (%) | 15 (41.7) | 19 (47.5) | 0.60 |
| KDIGO stage 1, n, (%) | 7 (19.4) | 13 (32.5) | |
| KDIGO stage 2, n, (%) | 6 (16.7) | 5 (12.5) | |
| KDIGO stage 3, n, (%) | 2 (5.6) | 1 (2.5) | |
| RRT, n, (%) | 2 (5.6) | 0 (0.0) | 0.13 |
| Cumulative hours of urine output <0.5 mL/kg/h, mean (SD) | |||
| 0–24 h after cross‐clamping (h) | 2.1 (3.7) | 2.3 (2.9) | 0.72 |
| 24–48 h after cross‐clamping (h) | 1.1 (2.3) | 1.8 (3.3) | 0.36 |
| 48–72 h after cross‐clamping (h) | 0.2 (0.6) | 0.9 (1.7) | 0.04 |
| Length of stay (d), median (IQR) | 10 (9–11.5) | 10 (8–12) | 0.68 |
| Length of stay on ICU (h), median (IQR) | 38.5 (23.5–78.5) | 39.5 (22–93) | 0.63 |
| Catecholamine administration (h), median (IQR) | 16 (9.0–32.0) | 16.5 (8.5–34.5) | 0.79 |
| Mechanical ventilation (h), median (IQR) | 14.5 (12.0–25.5) | 17.5 (13.0–24.5) | 0.55 |
| New onset of atrial fibrillation, n, (%) | 3 (8) | 0 (0) | 0.06 |
| Nonfatal perioperative myocardial infarction, n, (%) | 0 (0) | 0 (0) | NA |
| Nonfatal perioperative stroke, n, (%) | 0 (0) | 0 (0) | NA |
| Death, n, (%) | 2 (5.6) | 2 (5.0) | 0.91 |
AKI indicates acute kidney injury; CR, calorie restriction; ICU, intensive care unit; IQR, interquartile range; KDIGO, Kidney Disease: Improving Global Outcomes; NA, not assessed; RRT, renal replacement therapy.
On the basis of the patients' diary recordings, we identified those patients who had meticulously followed the assigned dietary protocol: In the CR group, ≤60% of the calculated DEE was consumed (n=13); in the control group, ≥80% of the calculated DEE was consumed (n=11). These individuals were included in the per‐protocol analysis. As in the primary analysis, no statistically significant differences were found with regard to any of the predefined outcome parameters between the 2 groups in the per‐protocol analysis; however, with n=13 in the CR arm and n=11 in the control arm, the group size was very small. As in the overall analysis, there was an increase in median serum creatinine in the CR group between the screening visit and day −1, from 0.9 mg/dL (0.7 – 1.3 mg/dL) to 1.3 mg/dL (0.8 – 1.5 mg/dL), but no change in the control group (screening: 1.2 mg/dL [0.9 – 1.4 mg/dL]; at day −1: 1.2 mg/dL [0.9 – 1.5 mg/dL]; P=0.013). Of note, over the first 48 hours after cross‐clamping, mean serum creatinine declined steadily in the CR group, whereas there was a steady increase in the control group (Figure 4).
Figure 4.
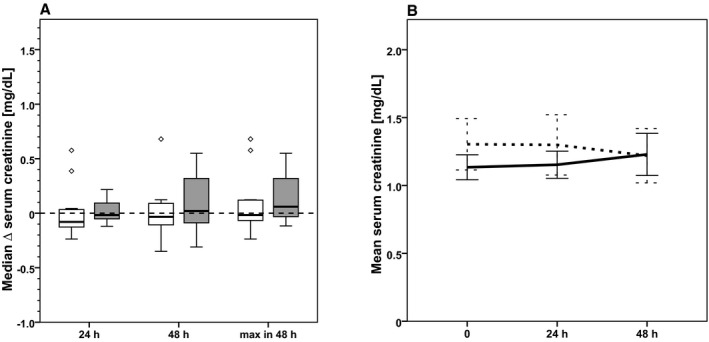
Per‐protocol analysis. Evolution of serum creatinine from baseline to 24 hours after cross‐clamping (primary end point) and from baseline to 48 hours after cross‐clamping and the maximum increase of serum creatinine within 48 hours after cross‐clamping in CR patients (white boxes, dashed line, n=13) and control patients (gray boxes, solid line, n=11). A, Box plots showing the change of serum creatinine from baseline to specified time points. B, Development of mean (±SEM) serum creatinine from baseline (0 hour) to 48 hours after cross‐clamping.
Patients were contacted by phone every second day between days −7 and −1, and their general condition and possible safety‐related events were recorded. Throughout the diet, 45% of the patients described their general condition as good, 48% as fair, and 7% as poor. Expectedly, an increased sensation of appetite was reported in the CR group; however, only 9% of patients classified this sensation as severe, and 34% rated it as moderate; the majority (57%) had no or only minor complaints. Adverse events were assessed throughout the hospital stay. Two deaths were recorded in each group and were not deemed treatment‐related: 1 case of acute respiratory distress syndrome and 1 case of carotid artery occlusion were recorded in the control group, and 1 case of coronary bypass occlusion and 1 case of heart failure due to paravalvular leakage were recorded in the CR group. Regarding possible treatment‐related events, 3 patients in the CR group and none in the control group experienced perioperative atrial fibrillation (P=0.06).
Discussion
In this study, we found that 7‐day preoperative CR to 60% of the DEE in patients at risk for postsurgery AKI had no impact on the increase of serum creatinine at 24 hours after cardiac surgery but showed a significant favorable effect on creatinine kinetics at 48 hours and at later time points.
It has been known since the late 1930s that long‐term dietary restriction—defined as CR without causing signs of malnutrition—leads to extension of life span by ≈20% in rats, and this finding was repeatedly confirmed in many species, from yeast to primates.19, 20, 21 Although the underlying mechanisms are not yet fully elucidated, the current concepts suggest enhanced cellular stress resistance as the major driver of this phenomenon.22, 23 Possible underlying mechanisms include enhanced autophagy, mitochondrial reactive oxygen species handling, induction of AMPK (AMP‐activated protein kinase) signaling, inhibition of mTOR (mammalian target or rapamycin) signaling, altered hypoxia signaling, enhanced DNA damage repair, and modulation of the immune system.20 The impact of a short‐term diet on promoting stress resistance in the acute setting has been investigated in more detail only recently.24 With respect to the kidney, Mitchell et al published a proof‐of‐principle study in a murine renal ischemia–reperfusion injury model in which short‐term CR completely prevented death from AKI and dramatically ameliorated renal failure.9 Thus, as an analogy to ischemic preconditioning, dietary preconditioning using moderate short‐term CR could be exploited as an effective, easy‐to‐use, and inexpensive procedure to prevent anticipated kidney injury. Moreover, although the beneficial effect of ischemic preconditioning on cardiac recovery in a model of myocardial infarction was lost with age25—a possible explanation for the disappointing findings of 2 recent clinical trials investigating the impact of RIPC on cardiac outcomes in patients with an average age of 70 years—dietary preconditioning apparently is independent of age, at least in animal models. Of note, ischemic preconditioning–induced organ protection can be restored even in old animals if they are pretreated with CR.26 Despite the simplicity and clinical feasibility of a short‐term dietary preconditioning protocol, there is a complete lack of clinical studies investigating the value of dietary preconditioning for organ protection. In a recent trial that analyzed the effect of a calorie‐restricted enteral feeding protocol in critically ill patients on mortality, the only significant difference compared with standard enteral feeding was a lower incidence rate of renal replacement therapy, indicating that dietary restriction might be beneficial if used not only before but also after the onset of injury.27 We thus set out to conduct this single‐center, randomized, controlled, open‐label, clinical, pilot study in AKI‐prone patients scheduled for cardiac surgery.
Although the results regarding the primary outcome did not meet the expectations in the intention‐to‐treat analysis, there was a significant between‐group difference when looking at serum creatinine change at 48 hours, the time frame used in the KDIGO AKI classification, and at discharge. Of note, analysis of the timely evolution of kidney function revealed that serum creatinine levels in the CR group were restored quickly after day 1, whereas there was a steady increase in creatinine over the first 2 days in the control group. Moreover, with 41.7% versus 47.5%, respectively, the AKI incidence was considerably lower in the CR group than in the control group. Subgroup analyses suggest that the protective effect might be most prominent in men and in obese patients, a finding that is in line with observations made in animal experiments28 and in the clinical setting.29, 30 Of note, serum creatinine decreased steadily until the day of discharge in the CR group but increased in the control group with a net difference of 0.15 mg/dL. These findings indicate that a prophylactic short‐term diet might indeed have a beneficial effect on renal recovery and restoration of kidney function in patients undergoing cardiac surgery.
Given the dramatic impact of short‐term dietary restriction in animal studies, the question arises of why such small effects were observed in this trial. Several protocol‐derived factors may have mitigated the protective effect of CR. First, it is unknown how long a diet must be applied and how much caloric content has to be restricted to elicit a protective effect in humans. To this end, the 7‐day diet used in this pilot trial might well have been inappropriate. Moreover, we calculated DEE by using the widely accepted Mifflin–St. Jeor equation. Whether this equation (or any alternative published equation) is applicable to often very sick patients awaiting cardiac surgery is at least questionable; probably the yielded numbers overestimate the true energy demand or at least do not mirror the actually ingested amount of calories in this population. Consequently, the true calorie intake in the control group was probably lower than expected from the estimation, and the difference between the 2 groups may have been lower than anticipated. Potential unintended CR in the control group is a key confounder in CR studies, as was shown by the divergent results of the primate trials on this subject.19 In turn, although there was an excess net loss of dry body mass of 0.8 kg in the CR group over the control group, indicating a true dietary effect, the target of 40% CR compared with the control group was probably not achieved. Second, with respect to the composition of the diet itself, more recent work suggested that the reduction of protein intake, rather than the limitation of energy supply, leads to increased cellular stress resistance, which in turn promotes an organ‐protective effect.31, 32 In our trial, however, the daily protein intake was not markedly different in both groups. Third, although we enrolled only patients with at least 1 risk factor for developing postsurgery AKI, calculation using the Cleveland Clinic Foundation score33 revealed that our patients carried only a low to intermediate risk (3 points [2–4 points] in the control and 2 points [2–4 points] in the CR group [P=0.9]). In 2 adequately powered studies investigating the organ‐protective impact of RIPC in patients who were not preselected for an additional AKI risk—the ERICCA trial (Effect of Remote Ischemic Preconditioning on Clinical Outcomes in Patients Undergoing Coronary Artery Bypass Graft Surgery)5 and the RIPHeart trial (Remote Ischemic Preconditioning for Heart Surgery)6—no benefit was observed for any of the clinical end points, including AKI incidence. In contrast, in another study focusing on AKI after cardiac surgery as the primary end point in patients with a Cleveland Clinic Foundation score >6, Zarbock et al reported a highly significant protective effect of RIPC.7 Similarly, we might speculate that preconditioning protocols in general are most likely to be effective in high‐risk patients.
Despite identical levels at the time of screening in both groups (1.1 mg/dL), mean serum creatinine increased by 0.2 mg/dL (0.2 mg/dL) after 1 week of CR but did not change (0.0 mg/dL [0.2 mg/dL]) in the control group (P=0.0001). Why this occurred is not clear. The statistically significant (P<0.0001) median loss of 2.4 kg of body water in the CR group (versus a 0.1‐kg gain in the control group)—most likely related to a lower sodium intake compared with the normal Western diet—may have led to a clinically relevant volume deficit that ultimately rendered the kidney even more susceptible to further damage. Consequently, the observed favorable effect of CR on creatinine kinetics would represent an underestimation of the true beneficial potential of CR.
We also analyzed biomarkers of functional and/or structural integrity of organs other than the kidney (ie, troponin T, creatinine kinase, NT‐proBNP, and neuron‐specific enolase), as well as general markers of inflammation (C‐reactive protein, white blood cell count), systemic ischemia (lactate), and cellular integrity (lactate dehydrogenase) at 24 hours after cross‐clamping. CR had no impact on any of these biomarkers, suggesting that moderately reduced energy supply before surgery is safe and does not interfere with short‐term systemic recovery.
Finally, our study has several methodological limitations. The observed creatinine rise within the first 24 hours after surgery was 0.1 mg/dL and thus well below what we expected; therefore, the calculated group size in this pilot trial has to be considered too small to come to a clear estimate of the protective potential of CR. Furthermore, because a reduction in calorie intake was readily noticed by study participants, randomization in a blinded fashion was not possible. Moreover, accurate supervision of calorie intake, especially in the control group, was hampered by the ambulatory design of the trial.
In conclusion, the findings of this trial do not reflect the remarkable effects of CR seen in animal experiments; however, the intervention itself is safe and feasible, and the analysis of secondary end points revealed small but promising beneficial signals. Given the mounting experimental evidence that dietary interventions are powerful tools for promoting enhanced cellular stress resistance with far‐ranging impact for disease prevention and treatment, further clinical investigations are warranted.
Sources of Funding
This study was supported by Fresenius Kabi, Bad Homburg, Germany.
Disclosures
None.
Supporting information
Data S1. Study protocol.
Acknowledgments
We thank Cornelia Böhme for her help with recruitment and data collection.
(J Am Heart Assoc. 2018;7:e008181 DOI: 10.1161/JAHA.117.008181.)29535139
References
- 1. Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1:19–32. [DOI] [PubMed] [Google Scholar]
- 2. Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, Bihorac A. Acute kidney injury is associated with increased long‐term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–2453. [DOI] [PubMed] [Google Scholar]
- 3. Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, Hiesmayr M. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15:1597–1605. [DOI] [PubMed] [Google Scholar]
- 4. Landoni G, Bove T, Szekely A, Comis M, Rodseth RN, Pasero D, Ponschab M, Mucchetti M, Bove T, Azzolini ML, Caramelli F, Paternoster G, Pala G, Cabrini L, Amitrano D, Borghi G, Capasso A, Cariello C, Carpanese A, Feltracco P, Gottin L, Lobreglio R, Mattioli L, Monaco F, Morgese F, Musu M, Pasin L, Pisano A, Roasio A, Russo G, Slaviero G, Villari N, Vittorio A, Zucchetti M, Guarracino F, Morelli A, De Santis V, Del Sarto PA, Corcione A, Ranieri M, Finco G, Zangrillo A, Bellomo R. Reducing mortality in acute kidney injury patients: systematic review and international web‐based survey. J Cardiothorac Vasc Anesth. 2013;27:1384–1398. [DOI] [PubMed] [Google Scholar]
- 5. Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, Knight R, Kunst G, Laing C, Nicholas J, Pepper J, Robertson S, Xenou M, Clayton T, Yellon DM; Investigators ET . Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med. 2015;373:1408–1417. [DOI] [PubMed] [Google Scholar]
- 6. Meybohm P, Bein B, Brosteanu O, Cremer J, Gruenewald M, Stoppe C, Coburn M, Schaelte G, Boning A, Niemann B, Roesner J, Kletzin F, Strouhal U, Reyher C, Laufenberg‐Feldmann R, Ferner M, Brandes IF, Bauer M, Stehr SN, Kortgen A, Wittmann M, Baumgarten G, Meyer‐Treschan T, Kienbaum P, Heringlake M, Schon J, Sander M, Treskatsch S, Smul T, Wolwender E, Schilling T, Fuernau G, Hasenclever D, Zacharowski K; Collaborators RIS . A multicenter trial of remote ischemic preconditioning for heart surgery. N Engl J Med. 2015;373:1397–1407. [DOI] [PubMed] [Google Scholar]
- 7. Zarbock A, Schmidt C, Van Aken H, Wempe C, Martens S, Zahn PK, Wolf B, Goebel U, Schwer CI, Rosenberger P, Haeberle H, Gorlich D, Kellum JA, Meersch M; Renal RI . Effect of remote ischemic preconditioning on kidney injury among high‐risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA. 2015;313:2133–2141. [DOI] [PubMed] [Google Scholar]
- 8. Zimmerman RF, Ezeanuna PU, Kane JC, Cleland CD, Kempananjappa TJ, Lucas FL, Kramer RS. Ischemic preconditioning at a remote site prevents acute kidney injury in patients following cardiac surgery. Kidney Int. 2011;80:861–867. [DOI] [PubMed] [Google Scholar]
- 9. Mitchell JR, Verweij M, Brand K, van de Ven M, Goemaere N, van den Engel S, Chu T, Forrer F, Muller C, de Jong M, van IJcken W, IJzermans JN, Hoeijmakers JH, de Bruin RW. Short‐term dietary restriction and fasting precondition against ischemia reperfusion injury in mice. Aging Cell. 2010;9:40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnsen M, Spath MR, Denzel MS, Gobel H, Kubacki T, Hoyer KJ, Hinze Y, Benzing T, Schermer B, Antebi A, Burst V, Muller RU. Oral supplementation of glucosamine fails to alleviate acute kidney injury in renal ischemia‐reperfusion damage. PLoS One. 2016;11:e0161315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ning YC, Cai GY, Zhuo L, Gao JJ, Dong D, Cui SY, Shi SZ, Feng Z, Zhang L, Sun XF, Chen XM. Beneficial effects of short‐term calorie restriction against cisplatin‐induced acute renal injury in aged rats. Nephron Exp Nephrol. 2013;124:19–27. [DOI] [PubMed] [Google Scholar]
- 12. Noyan H, El‐Mounayri O, Isserlin R, Arab S, Momen A, Cheng HS, Wu J, Afroze T, Li RK, Fish JE, Bader GD, Husain M. Cardioprotective signature of short‐term caloric restriction. PLoS One. 2015;10:e0130658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jongbloed F, de Bruin RW, Klaassen RA, Beekhof P, van Steeg H, Dor FJ, van der Harst E, Dolle ME, IJzermans JN. Short‐term preoperative calorie and protein restriction is feasible in healthy kidney donors and morbidly obese patients scheduled for surgery. Nutrients. 2016;8:E306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Ginhoven TM, de Bruin RW, Timmermans M, Mitchell JR, Hoeijmakers JH, Ijzermans JN. Pre‐operative dietary restriction is feasible in live‐kidney donors. Clin Transplant. 2011;25:486–494. [DOI] [PubMed] [Google Scholar]
- 15. Josephs SA, Thakar CV. Perioperative risk assessment, prevention, and treatment of acute kidney injury. Int Anesthesiol Clin. 2009;47:89–105. [DOI] [PubMed] [Google Scholar]
- 16. Maroni BJ, Steinman TI, Mitch WE. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int. 1985;27:58–65. [DOI] [PubMed] [Google Scholar]
- 17. KDIGO AKI Work Group: KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 18. Park M, Coca SG, Nigwekar SU, Garg AX, Garwood S, Parikh CR. Prevention and treatment of acute kidney injury in patients undergoing cardiac surgery: a systematic review. Am J Nephrol. 2010;31:408–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age‐related and all‐cause mortality in rhesus monkeys. Nat Commun. 2014;5:3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328:321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935. Nutrition. 1989;5:155–171; discussion 172 [PubMed] [Google Scholar]
- 22. Fontana L, Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell. 2015;161:106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Longo VD, Panda S. Fasting, circadian rhythms, and time‐restricted feeding in healthy lifespan. Cell Metab. 2016;23:1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brandhorst S, Harputlugil E, Mitchell JR, Longo VD. Protective effects of short‐term dietary restriction in surgical stress and chemotherapy. Ageing Res Rev. 2017;39:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jahangir A, Sagar S, Terzic A. Aging and cardioprotection. J Appl Physiol (1985). 2007;103:2120–2128. [DOI] [PubMed] [Google Scholar]
- 26. Abete P, Cacciatore F, Testa G, Della‐Morte D, Galizia G, de Santis D, Calabrese C, Cioppa A, Ferrara N, Rengo F. Ischemic preconditioning in the aging heart: from bench to bedside. Ageing Res Rev. 2010;9:153–162. [DOI] [PubMed] [Google Scholar]
- 27. Arabi YM, Aldawood AS, Haddad SH, Al‐Dorzi HM, Tamim HM, Jones G, Mehta S, McIntyre L, Solaiman O, Sakkijha MH, Sadat M, Afesh L; Permi TTG . Permissive underfeeding or standard enteral feeding in critically ill adults. N Engl J Med. 2015;372:2398–2408. [DOI] [PubMed] [Google Scholar]
- 28. Kang KP, Lee JE, Lee AS, Jung YJ, Kim D, Lee S, Hwang HP, Kim W, Park SK. Effect of gender differences on the regulation of renal ischemia‐reperfusion‐induced inflammation in mice. Mol Med Rep. 2014;9:2061–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aufhauser DD Jr, Wang Z, Murken DR, Bhatti TR, Wang Y, Ge G, Redfield RR III, Abt PL, Wang L, Svoronos N, Thomasson A, Reese PP, Hancock WW, Levine MH. Improved renal ischemia tolerance in females influences kidney transplantation outcomes. J Clin Invest. 2016;126:1968–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY. Temporal changes in incidence of dialysis‐requiring AKI. J Am Soc Nephrol. 2013;24:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hine C, Mitchell JR. Calorie restriction and methionine restriction in control of endogenous hydrogen sulfide production by the transsulfuration pathway. Exp Gerontol. 2015;68:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Robertson LT, Trevino‐Villarreal JH, Mejia P, Grondin Y, Harputlugil E, Hine C, Vargas D, Zheng H, Ozaki CK, Kristal BS, Simpson SJ, Mitchell JR. Protein and calorie restriction contribute additively to protection from renal ischemia reperfusion injury partly via leptin reduction in male mice. J Nutr. 2015;145:1717–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16:162–168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Study protocol.


