Abstract
Background
Recent studies demonstrated a strong association between atrial fibrillation (AF) and epicardial fat around the left atrium (LA). We sought to assess whether epicardial fat volume around the LA is associated with AF, and to determine the additive value of LA‐epicardial fat measurements to LA structural remodeling for identifying patients with AF using 3‐dimensional multi‐echo Dixon fat–water separated cardiovascular magnetic resonance.
Methods and Results
A total of 105 subjects were studied: 53 patients with a history of AF and 52 age‐matched patients with other cardiovascular diseases but no history of AF. The 3‐dimensional multi‐echo Dixon fat‐water separated sequence was performed for LA‐epicardial fat measurements. AF patients had significantly greater LA‐epicardial fat (28.9±12.3 and 14.2±7.3 mL for AF and non‐AF, respectively; P<0.001) and LA volume (110.8±38.2 and 89.7±30.3 mL for AF and non‐AF, respectively; P=0.002). LA‐epicardial fat adjusted for LA volume was still higher in patients with AF compared with those without AF (P<0.001). LA‐epicardial fat and hypertension were independently associated with the risk of AF (odds ratio, 1.17; 95% confidence interval, 1.10%–1.25%, P<0.001, and odds ratio, 3.29; 95% confidence interval, 1.17%–9.27%, P=0.03, respectively). In multivariable logistic regression analysis adjusted for body surface area, LA‐epicardial fat remained significant and an increase per mL was associated with a 42% increase in the odds of AF presence (odds ratio, 1.42; 95% confidence interval, 1.23%–1.62%, P<0.001). Combined assessment of LA‐epicardial fat and LA volume provided greater discriminatory performance for detecting AF than LA volume alone (c‐statistic=0.88 and 0.74, respectively, DeLong test; P<0.001).
Conclusions
Cardiovascular magnetic resonance 3‐dimensional Dixon‐based LA‐epicardial fat volume is significantly increased in AF patients. LA‐epicardial fat measured by 3‐dimensional Dixon provides greater performance for detecting AF beyond LA structural remodeling.
Keywords: 3D Dixon, atrial fibrillation, cardiovascular magnetic resonance imaging, epicardial fat, left atrial volume index
Subject Categories: Atrial Fibrillation, Magnetic Resonance Imaging (MRI)
Clinical Perspective
What Is New?
Cardiovascular magnetic resonance 3‐dimensional Dixon‐based left atrium (LA)–epicardial fat volume is significantly increased in atrial fibrillation patients.
The integration of LA‐epicardial fat and LA volume provides greater discriminatory performance for identifying atrial fibrillation patients than LA volume alone.
What Are the Clinical Implications?
Three‐dimensional multi‐echo Dixon fat–water separated sequence provides accurate quantification of LA‐epicardial fat volume, while cine CMR does not allow for reliable separation of fat and water. Computed tomography has limitations because of its radiation exposure and need for an iodinated contrast agent.
Further studies are necessary to examine the utility of 3‐dimensional Dixon‐derived LA‐epicardial fat for predicting the development of atrial fibrillation.
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and has a significant impact on heart failure progression and an increased risk of thromboembolism, resulting in increased mortality and morbidity.1, 2, 3 Although left atrial (LA) enlargement is the major player involved in the development of AF,4, 5, 6, 7, 8 epicardial fat is also highly associated with AF.9, 10, 11, 12, 13 Epicardial fat serves as a local energy supply and an endocrine organ that produces a number of adipokines that can freely diffuse into the adjacent myocardium, and may be associated with myocardial inflammation14, 15, 16 and LA fibrosis of subepicardial fat infiltration.16 Given that epicardial fat extends into the adjacent myocardium,14, 16 there is an increasing interest in the identification of epicardial fat around the LA.11, 12
Cardiovascular magnetic resonance (CMR) allows noninvasive quantification of fat volume in the heart.17, 18 Fat–water separation sequences such as Dixon provide volumetric quantification of fat.19, 20, 21, 22, 23 Tereshchenko et al demonstrated that interatrial septum fat, measured in the interatrial septum using a fat–water separated sequence, was associated with AF risk.24 LA‐epicardial fat shows a quite nonuniform spatial distribution.25, 26 Batal et al demonstrated there is a substantial difference in the anatomical distribution of peri‐atrial fat thickness.11 A computed tomography (CT) study by Tsao et al found that most LA‐epicardial fat was located in the region surrounded by the superior vena cava, right pulmonary artery, and right‐sided roof of the LA.27 Therefore, accurate assessment of LA‐epicardial fat volume requires a volumetric assessment using 3‐dimensional (3D) imaging. Three‐dimensional fat–water separation sequences enable quantification of fat around the LA. In this study, we aim to utilize the 3D Dixon sequence to (1) quantify LA‐epicardial fat volume in patients with AF, (2) assess the impact of LA‐epicardial fat on LA structural remodeling in patients with and without AF, and (3) to assess the incremental value of LA‐epicardial fat measurement as a noninvasive anatomical marker of AF burden to LA enlargement in this population.
Methods
The data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Population
We prospectively studied 53 consecutive patients with a history of AF scheduled for pulmonary vein (PV) isolation and 52 age‐matched patients with no history of AF who underwent CMR for evaluation of suspected cardiovascular diseases and had LA enlargement (LA axial dimension >3.8 cm, LA 4‐chamber length >5.2 cm or LA 2‐chamber length >5.2 cm). Paroxysmal AF was defined as recurrent AF that terminates spontaneously within 7 days. Persistent AF was defined as AF that is sustained beyond 7 days or that lasts <7 days but necessitates pharmacological or electrical cardioversion. Long‐standing persistent AF was defined as AF of >1 year in duration in which cardioversion has either failed or not been attempted. Exclusion criteria included general contraindications for CMR (eg, pacemaker, claustrophobia, and AF with rapid ventricular response). The study protocol was approved by our institutional review board, and written informed consent forms were obtained from all subjects.
CMR Protocol
All CMR images were acquired with a 1.5‐T scanner (Achieva 1.5 T; Philips Medical Systems, Best, The Netherlands) equipped with a 5‐ or 32‐element cardiac‐surface coil. The CMR protocol included (1) evaluation of left/right ventricular (LV/RV) function using balanced steady‐state free precession cine CMR, (2) 3D multi‐echo Dixon fat–water separated sequence for evaluation of epicardial fat,20 (3) MR angiography for evaluation of PV anatomy (AF patients only), and (4) late gadolinium enhancement (LGE) for evaluation of scarring. Ten to 12 short‐axis stack images and 2‐ and 4‐chamber long‐axis images were acquired using a breath‐hold, ECG‐gated balanced steady‐state free precession (slice thickness, 8 mm; gap, 2 mm; in‐plane spatial resolution 2×2 mm2; 30‐ms temporal resolution).28 A 3D transaxial ECG‐ and respiratory navigator gated magnetization modified Dixon sequence was obtained with the following sequence: 5‐mm slice thickness, repetition time/echo time 1/echo time 2=5.3/1.7/3.5 ms, flip angle=15°, field of view =320×405×100 mm, acquisition matrix=212×266, spatial resolution=1.5×1.5×2.0 mm and SENSE factor=1.5 in both phase encoding directions, water fat shift=0.16 pixel, with arrhythmia rejection, T2 preparation=50 ms. Trigger delay was set to end‐diastole and the acquisition window was 96 to 176 ms. First‐pass angiography of the PV was obtained after administration of a bolus of 0.1 mmol/kg of Gd‐DTPA (Magnevist; Bayer Schering, Berlin, Germany) or Gd‐BOPTA (MultiHance; Bracco Imaging SpA, Milan, Italy). Twenty minutes later, short‐ and long‐axis inversion recovery LGE images were acquired with a 3D phase‐sensitive inversion recovery sequence (5‐mm slice thickness, repetition time/echo time=5.3/2.1 ms, flip angle=70°, field of view=320×320×125 mm, acquisition matrix=224×224×23, and spatial resolution=1.4×1.4×1.5 mm).
Image Analysis
CMR images were analyzed by a blinded investigator using a commercial workstation (Extend MR WorkSpace, version 2.3.6.3; Philips Healthcare). At end‐diastole and end‐systole, epi‐ and endocardial LV borders were manually traced in contiguous short‐axis cine images covering the apex to mitral valve plane to calculate end‐diastolic volume and end‐systolic volume, stroke volume, and ejection fraction. LV mass was calculated as the sum of the myocardial volume multiplied by the specific gravity (1.05 g/mL) of the myocardial tissue. Multiplanar reconstructions of the PV were generated and the PV cross‐sectional area was measured. For LA analysis, all parameters were measured at the LV diastole of the frame immediately before the mitral leaflet opening. LA volume was measured from the area‐length method using atrial length (from the back wall to the line across the hinge points of the mitral valve) and border excluding atrial appendage and PVs in the 2‐ and 4‐chamber views with the following formula: LA volume=(4‐chamber area)×(2‐chamber area)×0.85/shorter atrial length. Epicardial fat was visually defined as regions of high signal intensity between the myocardium and the pericardium. Based on the cine CMR images, we defined LA‐epicardial fat subtending the LA from the mitral annulus to the bifurcation of the pulmonary artery. Areas of fat were manually traced on consecutive transaxial images and multiplied by the slice thickness to calculate LA‐epicardial fat volume with a commercial workstation (OsiriX environment; Pixmeo, Geneva, Switzerland) (Figure 1). To evaluate inter‐ and intraobserver reproducibility, measurements of LA‐epicardial fat volume from a random sample of 10 AF patients were independently assessed by 2 observers (S.N., with 8 years of experience and M.N., with 6 years of experience), and 1 observer (S.N.) measured LA‐epicardial fat volume twice on 2 separate days with a washout period of at least 2 weeks. On LGE images, the presence or absence of LGE in the LV was visually assessed.
Figure 1.

Measurement of left atrial epicardial fat volume. Dixon image analysis for the measurement of left atrial epicardial fat volume (LA‐epicardial fat) at high level (A), high‐middle level (B), low‐middle level (C), and low level (D) in the LA. The dotted yellow lines show the caudal and cranial limits for the measurement of LA‐epicardial fat and the arrows point at the counter of the pericardial outline. A region of interest was placed on high signal intensity area inside pericardial outline around LA (C) for each transaxial image and total LA‐epicardial fat was calculated as the sum of epicardial fat multiplied by the slice thickness of 5 mm.
Statistical Analysis
Continuous variables are expressed as mean± SD and compared using an unpaired Student t test or Mann–Whitney nonparametric test if not normally distributed. Categorical variables were expressed as counts and percentages, and compared using a χ2 test. One‐way ANOVA with Bonferroni adjustment for multiple comparisons was applied after the overall 3 group comparisons. Skewed distributions were logarithmically transformed before regression analysis, and a regression analysis was performed to investigate possible associations of LA‐epicardial fat and LA size. An analysis of covariance was used to test for equality of the regression slopes between patients with and without AF. Univariable logistic regression models were used to assess the association between each variable and AF. For multivariable modeling, we applied the rule of thumb of having between 5 and 10 outcomes per predictor, and included the 5 most significant variables and 1 covariate (male sex). Results are reported as odds ratio with 95% confidence intervals (CI). The area under the receiver operating characteristic was calculated and compared for all diagnostic testing strategies for AF. For clinical applicability purposes, we created a logistic regression model with the previously established clinical characteristics (age, male sex, body mass index, hypertension, and LA volume). Subsequently, we integrated LA‐epicardial fat to the clinical model to assess whether AF discrimination improved. All tests were 2 sided and a P<0.05 was considered statistically significant. Intra‐ and interobserver reliability of LA‐epicardial fat volume measurements were assessed with the intraclass correlation coefficient. All analyses were performed using SPSS (version 19.0; International Business Machines Inc, Chicago, IL), MedCalc for Windows (version 14.8.1; MedCalc Software, Ostend, Belgium), and R version 3.2.3 (R Project for Statistical Computing).
Results
The patient clinical characteristics are summarized in Table 1. The mean age of the total population was 54 years; 64% of patients were male. The mean body mass index and body surface area (BSA) were 27.8±5.5 and 1.98±0.26, respectively. However, the age of patients with and without AF was similar (P=NS). AF patients were more likely to have higher BSA (P=0.008) and hypertension (P=0.03). A trend toward greater use of statins and β‐blockers in AF patients was also observed (P=0.06 and 0.14, respectively).
Table 1.
Patients’ Characteristics
| Characteristics | Non AF Patients (n=52) | AF Patients (n=53) | P Value |
|---|---|---|---|
| Age, y | 58±11 | 58±11 | 0.91 |
| Male sex, n (%) | 34 (65) | 39 (74) | 0.36 |
| BMI, kg/m2 | 27.7±5.7 | 28.4±5.1 | 0.50 |
| BSA, m2 | 1.93±0.27 | 2.07±0.24 | 0.008 |
| Heart rate, beats/min | 66±11 | 66±20 | 0.91 |
| Systolic blood pressure, mm Hg | 130±17 | 127±17 | 0.42 |
| Diastolic blood pressure, mm Hg | 75±11 | 75±12 | 0.96 |
| Hypertension (%) | 15 (29) | 26 (49) | 0.03 |
| Diabetes mellitus (%) | 5 (10) | 6 (11) | 0.78 |
| Dyslipidemia (%) | 11 (21) | 20 (38) | 0.06 |
| Current smoking (%) | 3 (6) | 5 (9) | 0.37 |
| Obstructive sleep apnea (%) | 4 (8) | 7 (13) | 0.36 |
| NYHA ≥ III (%) | 5 (10) | 2 (4) | 0.21 |
| CKD stage III | 5 (10) | 5 (9) | 0.62 |
| Medication use, n (%) | |||
| ACEI or ARB | 20 (39) | 25 (47) | 0.37 |
| β‐Blocker | 21 (40) | 29 (55) | 0.14 |
| Calcium‐channel blocker | 8 (15) | 7 (13) | 0.75 |
| Statin | 10 (19) | 19 (36) | 0.06 |
| Diuretics | 9 (17) | 8 (15) | 0.76 |
| AF type | |||
| Paroxysmal | ··· | 39 (74) | |
| Persistent | ··· | 10 (19) | |
| Long‐standing persistent | ··· | 4 (7) | |
Values are mean±SD or n (%). ACEI indicates angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blockers; BMI, body mass index; BSA, body surface area; CKD, chronic kidney disease; NYHA, New York Heart Association.
Table 2 summarizes CMR findings between the 2 groups. Patients with AF had significantly greater LA volumes as well as LA 2 chamber and 4 chamber areas (P<0.05). LA‐epicardial fat was significantly higher in patients with AF compared with patients without AF (P<0.001). In 52 patients without AF, the mean BSA‐adjusted LA‐epicardial fat was 7.3±3.5 mL/m2, which was significantly lower than that in AF patients (P<0.001) (Figure 2).
Table 2.
Patients’ CMR Characteristics
| Characteristics | Non AF Patients (n=52) | AF Patients (n=53) | P Value |
|---|---|---|---|
| LV EDV, mL | 173.7±53.4 | 169.4±49.3 | 0.67 |
| LV EDVI, mL/m2 | 90.2±26.5 | 81.1±19.0 | 0.048 |
| LV ESV, mL | 80.3±44.3 | 73.8±34.5 | 0.40 |
| LV EF, % | 56.2±12.2 | 57.8±8.4 | 0.44 |
| LV mass, g | 108.7±39.5 | 105.9±33.6 | 0.69 |
| LV mass index, g/m2 | 55.9±17.3 | 50.5±12.9 | 0.08 |
| LV LGE, n (%) | 12 (23) | 1 (2) | 0.001 |
| RV EDV, mL | 154.6±40.5 | 169.9±46.3 | 0.08 |
| RV EDVI, mL/m2 | 79.6±18.3 | 81.5±17.4 | 0.59 |
| RV ESV, mL | 66.1±26.1 | 75.2±28.0 | 0.09 |
| RV EF, % | 58.1±9.6 | 56.3±8.6 | 0.34 |
| LA dimension (axial), cm | 3.8±0.8 | 3.9±0.8 | 0.63 |
| LA length (4 chamber), cm | 5.7±0.8 | 5.7±1.0 | 0.95 |
| LA length (2 chamber), cm | 5.3±1.0 | 5.4±1.0 | 0.47 |
| LA area (4 chamber), cm2 | 23.9±6.0 | 27.2±7.0 | 0.02 |
| LA area (2 chamber), cm2 | 23.4±5.1 | 26.1±6.3 | 0.03 |
| LA volume, mL | 89.7±30.3 | 110.8±38.2 | 0.002 |
| LA volume index, mL/m2 | 46.8±16.1 | 53.2±15.6 | 0.04 |
| LA epicardial fat, mL | 14.2±7.3 | 28.9±12.3 | <0.001 |
| LA epicardial fat index, mL/m2 | 7.3±3.5 | 13.9±5.6 | <0.001 |
| LA epicardial fat/LA volume | 0.16±0.08 | 0.27±0.12 | <0.001 |
| Left superior PV CSA, mm2 | ··· | 198.0 (154.0–248.0) | |
| Left inferior PV CSA, mm2 | ··· | 154.0 (117.5–189.0) | |
| Right superior PV CSA, mm2 | ··· | 326.0 (255.0–477.0) | |
| Right inferior PV CSA, mm2 | ··· | 215.0 (182.0–292.0) | |
| Right middle PV CSA, mm2 | ··· | 100.5 (50.0–151.0) | |
| Total PVs CSA, mm2 | ··· | 869.5 (711.0–1081.0) | |
| RA length (4 chamber), cm | 5.5±0.7 | 5.9±0.8 | 0.02 |
Values are mean±SD, n (%), or median (interquartile range). AF indicates atrial fibrillation; CMR, cardiovascular magnetic resonance; CSA, cross‐sectional area; EDV, end‐diastolic volume; EDVI, end‐diastolic volume index; EF, ejection fraction; ESV, end‐systolic volume; LA, left atrial; LGE, late gadolinium enhancement; LV, left ventricular; PV, pulmonary vein; RA, right atrial; RV, right ventricular.
Figure 2.

Individual BSA‐adjusted left atrial epicardial fat volume. Body surface area adjusted left atrial epicardial fat volume (BSA‐adjusted LA‐epicardial fat) was significantly elevated in AF patients in comparison to patients without AF (P<0.001). AF indicates atrial fibrillation.
In the subgroup of AF patients, there was a trend toward long‐standing persistent AF being associated with higher BSA‐adjusted LA‐epicardial fat (19.2±7.3, 13.2±5.6, and 13.5±5.3 mL/m2 for long‐standing persistent, persistent, and paroxysmal AF, respectively; P=0.16 and 0.20, respectively). LA‐epicardial fat was not associated with the total PV cross‐sectional area. The intraclass correlation coefficients for interobserver and intraobserver measurements of LA‐epicardial fat volume were 0.87 (95% CI, 0.56%–0.97%) and 0.95 (95% CI, 0.81%–0.99%), respectively. Higher prevalence of LV LGE in patients without AF was statistically significant (P=0.001).
Association Between LA Volume and LA‐Epicardial Fat According to the Presence or Absence of AF
LA‐epicardial fat moderately and positively correlated with LA volume in the whole population (r=0.47, P<0.001). Figure 3 shows the regression analysis of LA‐epicardial fat plotted against the LA volume in the patient subgroup with and without AF. In both groups, the LA‐epicardial fat correlates with the LA volume (AF group, r=0.48, P<0.001 and non‐AF group, r=0.39, P=0.004). However, the line for the AF group lies significantly above the line for the non‐AF group (P<0.001), indicating that patients with AF have significantly higher LA‐epicardial fat for an equivalent LA volume. Even after adjusting for BSA, the difference in the regression analysis between the line for the AF group and the line for the non‐AF group remained statistically significant (P<0.001).
Figure 3.

Correlation of LA‐epicardial fat and LA volume. The regression line for the AF group lies significantly above the line for the non‐AF group (P<0.001), although the LA‐epicardial fat correlates with the LA volume in both groups (AF group [pink plots]; r=0.48, P<0.001 and non‐AF group [green plots]; r=0.39, P=0.004). AF indicates atrial fibrillation; LA, left atrial.
Combined Assessment of LA‐Epicardial Fat, LA Volume, and Clinical Parameters for Detecting AF
Univariable and multivariable logistic regression analyses of clinical and CMR parameters for AF are summarized in Table 3. Although BSA, hypertension, absence of LV LGE, increased LA volume, and LA‐epicardial fat were each univariately associated with AF, increased LA‐epicardial fat and hypertension were independently associated with AF (odds ratio, 1.17; 95% CI, 1.10%–1.25%, P<0.001, and odds ratio, 3.29; 95% CI, 1.17%–9.27%, P=0.024, respectively). In a multivariable analysis adjusting for BSA, LA‐epicardial fat remained significant and each mL increase was associated with a 42% increase in the odds of AF presence (odds ratio, 1.42; 95% CI, 1.23%–1.62%, P<0.001). Although increased LA volume was an important predictor of AF, LA volume was no longer significant in multivariable analysis. LA volume assessment of cine CMR had a sensitivity of 74%, a specificity of 63%, a negative predictive value of 76%, and a positive predictive value of 59% with an area under the receiver operating characteristic (area under the curve) of 0.74 (95% CI, 0.65%–0.82%) for AF (Figure 4A). Given the cut‐off LA‐epicardial fat value of 18 mL that was determined by receiver operating characteristic curve analysis in presenting AF, integration of LA‐epicardial fat and LA volume assessment provided better discriminatory performance with a sensitivity of 81% and a specificity of 85% with an area under the curve of 0.88 (95% CI, 0.82%–0.94%, DeLong test; P<0.001). When LA‐epicardial fat was combined with the clinical model (including age, male sex, body mass index, hypertension, and LA volume), there was a greater c‐statistic than in the clinical model alone (c‐statistic=0.90 and 0.80, respectively, DeLong test; P=0.002) (Figure 4A and 4B). There was a similar trend between the LA‐epicardial fat and LA volume indices and the clinical model (Figure 4C and 4D).
Table 3.
Univariable and Multivariable Analysis for the Association With AF
| Characteristics | Univariable Analysis | Multivariable Analysis Model 1 | Multivariable Analysis Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Age, y | 1.00 | 0.97–1.04 | 0.91 | ||||||
| Male | 1.48 | 0.64–3.40 | 0.36 | 0.19 | 0.04–0.91 | 0.037 | 0.18 | 0.04–0.89 | 0.036 |
| BMI | 1.03 | 0.95–1.10 | 0.50 | ||||||
| BSA, per 0.1 increase | 1.24 | 1.06–1.46 | 0.009 | 1.13 | 0.84–1.52 | 0.40 | 1.32 | 0.99–1.78 | 0.063 |
| Hypertension | 2.38 | 1.06–5.32 | 0.035 | 3.29 | 1.17–9.27 | 0.024 | 2.99 | 1.08–8.29 | 0.035 |
| Diabetes mellitus | 1.20 | 0.34–4.20 | 0.78 | ||||||
| Dyslipidemia | 2.26 | 0.95–5.38 | 0.065 | 1.88 | 0.49–7.16 | 0.35 | 1.91 | 0.50–7.29 | 0.34 |
| Obstructive sleep apnea | 1.83 | 0.50–6.66 | 0.36 | ||||||
| NYHA ≥ III | 0.37 | 0.07–1.99 | 0.25 | ||||||
| CKD stage III | 0.98 | 0.27–3.60 | 0.98 | ||||||
| CMR parameters | |||||||||
| LV EDVI | 0.98 | 0.97–1.00 | 0.052 | ||||||
| LV ESV | 1.00 | 0.99–1.01 | 0.40 | ||||||
| LV EF, per 1% decrement | 0.99 | 0.95–1.02 | 0.43 | ||||||
| LV mass index | 0.98 | 0.95–1.00 | 0.083 | ||||||
| LV LGE | 0.06 | 0.01–0.51 | 0.010 | ||||||
| RV EDVI | 1.01 | 0.98–1.03 | 0.58 | ||||||
| RV ESV | 1.01 | 1.00–1.03 | 0.095 | ||||||
| RV EF, per 1% decrement | 1.02 | 0.98–1.07 | 0.34 | ||||||
| LA volume | 1.02 | 1.01–1.03 | 0.004 | 1.00 | 0.98–1.02 | 0.96 | 1.00 | 0.98–1.02 | 0.89 |
| LA volume index | 1.03 | 1.00–1.05 | 0.045 | ||||||
| LA epicardial fat | 1.17 | 1.10–1.24 | <0.001 | 1.17 | 1.10–1.25 | <0.001 | |||
| LA epicardial fat index | 1.40 | 1.23–1.60 | <0.001 | 1.42 | 1.23–1.62 | <0.001 | |||
AF indicates atrial fibrillation; BMI, body mass index; BSA, body surface area; CI, confidence interval; CKD, chronic kidney disease; CMR, cardiovascular magnetic resonance; EDVI, end‐diastolic volume index; EF, ejection fraction; ESV, end‐systolic volume; LA, left atrial; LGE, late gadolinium enhancement; LV, left ventricular; NYHA, New York Heart Association; RV, right ventricular.
Figure 4.
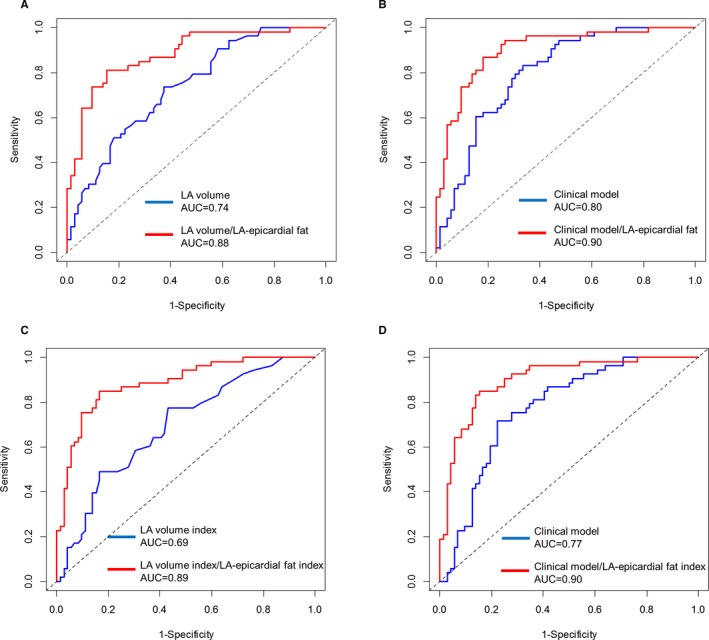
Comparison of the ROC curves for LA volume and LA‐epicardial fat for AF. Receiver operating characteristic (ROC) curve and corresponding area under the curve (AUC) describing the discriminatory performance of integration of the left atrial epicardial fat volume (LA‐epicardial fat) (red line) and LA volume (blue line) (A). Integration with the clinical model (blue line) (B) to identify AF. Integration of LA‐epicardial fat index (red line) and LA volume index alone (blue line) and clinical model (blue line) is shown in (C and D), respectively. AF indicates atrial fibrillation; LA, left atrial.
Discussion
In the prospective study of LA‐epicardial fat using the 3D Dixon method, we demonstrate that (1) LA‐epicardial fat is significantly increased in patients with AF compared with those with other cardiovascular diseases; (2) LA‐epicardial fat correlated with LA volume, but the regression analysis of LA‐epicardial fat plotted against the LA volume revealed a significant difference between the line for the AF group and the line for non‐AF; and (3) multiparametric CMR combining cine CMR‐derived LA volume and LA‐epicardial fat measured by 3D Dixon provided a greater area under the curve to detect AF. These results suggest that the effect of LA‐epicardial fat on LA structural remodeling may outweigh other pathogenic effects in AF patients.
Homsi et al used a 3D Dixon method in a fat–muscle phantom study and showed an excellent correlation between cardiac fat volumes measured using 3D Dixon and true fat volumes.20 In addition, Dixon image quality has been proven sufficient for detecting pericardial outlines, indicating its capability to accurately quantify epicardial fat. In a study by Wong et al, cine CMR‐derived pericardial fat and LA volume were assessed.29 However, this method does not allow for a reliable separation of fat and water, and the pericardial fluid also registers a high signal. The high spatial resolution of CT permits accurate measurements of epicardial fat volume; however, CT has limitations because of its radiation exposure and need for an iodinated contrast agent.
Pericardial fat has been shown to be associated with LA size.13, 29 It is increasingly appreciated that epicardial fat is a metabolically active tissue, a rich source of adipokines, and LA‐epicardial fat may be more pathogenically relevant to AF occurrence. We observed that LA‐epicardial fat volume was associated with LA volume. This is supported by the CT results of Batal et al that posterior LA‐epicardial fat pad thickness was related to the LA area.11 However, in their study, epicardial fat and LA size were not volumetrically quantified. The present study overcomes these limitations and provides a robust measurement of LA structural remodeling as well as volumetric epicardial fat quantification. Furthermore, we found a substantial difference in the regression analysis of LA‐epicardial fat in relation to LA volume between patients with and without AF. Our results support the theory that LA‐epicardial fat may be pathogenic and an important therapeutic target for reducing AF burden.
In the current study, there was a trend toward higher prevalence of dyslipidemia in AF patients. However, dyslipidemia was not significant, although it was included as a potential confounder for multivariable modeling. In addition, there were no significant differences in LA‐epicardial fat and BSA‐adjusted LA‐epicardial fat between patients with and without dyslipidemia.
Investigators have attempted to provide better risk stratification to predict the incident and recurrent AF. Marrouche et al assessed LA tissue fibrosis on LGE images and demonstrated that LGE atrial fibrosis predicted AF recurrence in a multicenter setting.30 We have also previously reported the association between LA LGE fibrosis and AF recurrence.31 However, LA LGE imaging remains technically challenging because of the thin‐walled LA and the confounding blood pool signal. Studies suggest that the secretome in peri‐atrial epicardial fat can induce atrial fibrosis and precede development of AF; therefore, assessment of peri‐atrial epicardial fat in a population that is at risk of AF is of great interest. Our results demonstrate that combined assessment of cine and noncontrast 3D Dixon provides better risk stratification to identify AF patients beyond both the clinical model and LA volume alone. This is supported by a CT study by van Rosendael et al, who reported that posterior LA fat measurement was useful for identifying AF patients.32 Thus, the integration of LA‐epicardial fat measurement may identify high‐risk populations of AF‐prone patients.27
Our study has several limitations. It is a single‐center and observational study with a relatively small sample size. The results in the current study depend heavily on the selected non‐AF group, but the difference between the LA‐epicardial fat levels is very large compared with random samples, with the means separated by about 1 individual SD. We applied the T2 preparation for 3D multi‐echo Dixon to obtain better image quality. However, the relaxation effects because of T2 preparation might have an influence on the fat volume results. More patients in the group with AF used statins, which may be regarded as a confounder.33 However, even with statins, these patients had higher LA‐epicardial fat compared with patients without AF.
In conclusion, LA‐epicardial fat is significantly increased for an equivalent LA volume in patients with AF. The integration of LA‐epicardial fat to LA volume alone as well as to the clinical model (including age, male sex, body mass index, hypertension, and LA volume) provides greater discriminatory performance for identifying AF patients. Further studies are necessary to examine the utility of 3D Dixon‐derived LA‐epicardial fat for predicting the development of AF.
Sources of Funding
Nakamori receives scholarship support from Mie University Foundation International; R. Nezafat receives grant support from National Institutes of Health 1R21HL127650, 1R01HL129185, and American Heart Association 15EIA22710040.
Disclosures
None.
Acknowledgments
We thank Patrick Pierce, RT, Beth Goddu, RT, and Sophie Berg, RN for the performance of CMR studies, and Jennifer Rodriguez for the editorial assistance.
(J Am Heart Assoc. 2018;7:e008232 DOI: 10.1161/JAHA.117.008232.)29572324
References
- 1. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 2. Chugh SS, Blackshear JL, Shen WK, Hammill SC, Gersh BJ. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol. 2001;37:371–378. [DOI] [PubMed] [Google Scholar]
- 3. Patel NJ, Deshmukh A, Pant S, Singh V, Patel N, Arora S, Shah N, Chothani A, Savani GT, Mehta K, Parikh V, Rathod A, Badheka AO, Lafferty J, Kowalski M, Mehta JL, Mitrani RD, Viles‐Gonzalez JF, Paydak H. Contemporary trends of hospitalization for atrial fibrillation in the United States, 2000 through 2010 implications for healthcare planning. Circulation. 2014;129:2371–2379. [DOI] [PubMed] [Google Scholar]
- 4. Osranek M, Fatema K, Qaddoura F, Al‐Saileek A, Barnes ME, Bailey KR, Gersh BJ, Tsang TSM, Zehr KJ, Seward JB. Left atrial volume predicts the risk of atrial fibrillation after cardiac surgery. A prospective study. J Am Coll Cardiol. 2006;48:779–786. [DOI] [PubMed] [Google Scholar]
- 5. Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TSM. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357–2363. [DOI] [PubMed] [Google Scholar]
- 6. Cozma D, Popescu BA, Lighezan D, Lucian P, Mornos C, Ginghina C, Dragulescu SI. Left atrial remodeling: assessment of size and shape to detect vulnerability to atrial fibrillation. Pacing Clin Electrophysiol. 2007;30:147–150. [DOI] [PubMed] [Google Scholar]
- 7. Casaclang‐Verzosa G, Gersh BJ, Tsang TSM. Structural and functional remodeling of the left atrium. Clinical and therapeutic implications for atrial fibrillation. J Am Coll Cardiol. 2008;51:1–11. [DOI] [PubMed] [Google Scholar]
- 8. Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol. 2014;63:493–505. [DOI] [PubMed] [Google Scholar]
- 9. Fox CS, Gona P, Hoffmann U, Porter SA, Salton CJ, Massaro JM, Levy D, Larson MG, D'Agostino RB, O'Donnell CJ, Manning WJ. Pericardial fat, intrathoracic fat, and measures of left ventricular structure and function: the Framingham Heart Study. Circulation. 2009;119:1586–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thanassoulis G, Massaro JM, O'Donnell CJ, Hoffmann U, Levy D, Ellinor PT, Wang TJ, Schnabel RB, Vasan RS, Fox CS, Benjamin EJ. Pericardial fat is associated with prevalent atrial fibrillation: the Framingham Heart Study. Circ Arrhythm Electrophysiol. 2010;3:345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Batal O, Schoenhagen P, Shao M, Ayyad AE, Van Wagoner DR, Halliburton SS, Tchou PJ, Chung MK. Left atrial epicardial adiposity and atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wong CX, Sun MT, Odutayo A, Emdin CA, Mahajan R, Lau DH, Pathak RK, Wong DT, Selvanayagam JB, Sanders P, Clarke R. Associations of epicardial, abdominal, and overall adiposity with atrial fibrillation. Circ Arrhythm Electrophysiol. 2016;9:e004378. [DOI] [PubMed] [Google Scholar]
- 13. Al Chekakie MO, Welles CC, Metoyer R, Ibrahim A, Shapira AR, Cytron J, Santucci P, Wilber DJ, Akar JG. Pericardial fat is independently associated with human atrial fibrillation. J Am Coll Cardiol. 2010;56:784–788. [DOI] [PubMed] [Google Scholar]
- 14. Tansey DK, Aly Z, Sheppard MN. Fat in the right ventricle of the normal heart. Histopathology. 2005;46:98–104. [DOI] [PubMed] [Google Scholar]
- 15. Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, O'Donnell CJ, Fox CS. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community‐based sample the Framingham Heart Study. Circulation. 2008;117:605–613. [DOI] [PubMed] [Google Scholar]
- 16. Haemers P. Atrial fibrillation is associated with the fibrotic remodeling of adipose tissue in the subepicardium of sheep and human atria. Eur Heart J. 2017;38:53–61. [DOI] [PubMed] [Google Scholar]
- 17. Haacke EM, Brown RW, Thompson MR, Venkatesan R. Magnetic Resonance Imaging: Physical Principles and Sequence Design. New York: John Wiley & Sons; 1999:421–449. [Google Scholar]
- 18. Stark DD, Bradley WG Jr. Magnetic Resonance Imaging. St. Louis: Mosby Inc.; 1999:159–179. [Google Scholar]
- 19. Ma J. Dixon techniques for water and fat imaging. J Magn Reson Imaging. 2008;28:543–558. [DOI] [PubMed] [Google Scholar]
- 20. Homsi R, Meier‐Schroers M, Gieseke J, Dabir D, Luetkens JA, Kuetting DL, Naehle CP, Marx C, Schild HH, Thomas DK, Sprinkart AM. 3D‐Dixon MRI based volumetry of peri‐ and epicardial fat. Int J Cardiovasc Imaging. 2016;32:291–299. [DOI] [PubMed] [Google Scholar]
- 21. Farrelly C, Shah S, Davarpanah A, Keeling AN, Carr JC. ECG‐gated multiecho Dixon fat‐water separation in cardiac MRI: advantages over conventional fat‐saturated imaging. AJR Am J Roentgenol. 2012;199:W74–W83. [DOI] [PubMed] [Google Scholar]
- 22. Homsi R, Sprinkart AM, Gieseke J, Yuecel S, Meier‐Schroers M, Luetkens J, Dabir D, Kuetting D, Marx C, Nadal J, Schild HH, Thomas D. 3D‐Dixon cardiac magnetic resonance detects an increased epicardial fat volume in hypertensive men with myocardial infarction. Eur J Radiol. 2016;85:936–942. [DOI] [PubMed] [Google Scholar]
- 23. Homsi R, Luetkens JA, Skowasch D, Pizarro C, Sprinkart AM, Gieseke J, Meyer Zur Heide Gen Meyer‐Arend J, Schild HH, Naehle CP. Left ventricular myocardial fibrosis, atrophy, and impaired contractility in patients with pulmonary arterial hypertension and a preserved left ventricular function: a cardiac magnetic resonance study. J Thorac Imaging. 2017;32:36–42. [DOI] [PubMed] [Google Scholar]
- 24. Tereshchenko LG, Rizzi P, Mewton N, Volpe GJ, Murthy S, Strauss DG, Liu CY, Marchlinski FE, Spooner P, Berger RD, Kellman P, Lima JA. Infiltrated atrial fat characterizes underlying atrial fibrillation substrate in patients at risk as defined by the ARIC atrial fibrillation risk score. Int J Cardiol. 2014;172:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lemola K, Sneider M, Desjardins B, Case I, Han J, Good E, Tamirisa K, Tsemo A, Chugh A, Bogun F, Pelosi F Jr, Kazerooni E, Morady F, Oral H. Computed tomographic analysis of the anatomy of the left atrium and the esophagus: implications for left atrial catheter ablation. Circulation. 2004;110:3655–3660. [DOI] [PubMed] [Google Scholar]
- 26. Sánchez‐Quintana D, Cabrera JA, Climent V, Farré J, Mendonça MC, Ho SY. Anatomic relations between the esophagus and left atrium and relevance for ablation of atrial fibrillation. Circulation. 2005;112:1400–1405. [DOI] [PubMed] [Google Scholar]
- 27. Tsao HM, Hu WC, Wu MH, Tai CT, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Wu TJ, Sheu MH, Chang CY, Chen SA. Quantitative analysis of quantity and distribution of epicardial adipose tissue surrounding the left atrium in patients with atrial fibrillation and effect of recurrence after ablation. Am J Cardiol. 2011;107:1498–1503. [DOI] [PubMed] [Google Scholar]
- 28. Yeon SB, Salton CJ, Gona P, Chuang ML, Blease SJ, Han Y, Tsao CW, Danias PG, Levy D, O'Donnell CJ, Manning WJ. Impact of age, sex, and indexation method on MR left ventricular reference values in the Framingham Heart Study offspring cohort. J Magn Reson Imaging. 2015;41:1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wong CX, Abed HS, Molaee P, Nelson AJ, Brooks AG, Sharma G, Leong DP, Lau DH, Middeldorp ME, Roberts‐Thomson KC, Wittert GA, Abhayaratna WP, Worthley SG, Sanders P. Pericardial fat is associated with atrial fibrillation severity and ablation outcome. J Am Coll Cardiol. 2011;57:1745–1751. [DOI] [PubMed] [Google Scholar]
- 30. Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, Kholmovski E, Burgon N, Hu N, Mont L, Deneke T, Duytschaever M, Neumann T, Mansour M, Mahnkopf C, Herweg B, Daoud E, Wissner E, Bansmann P, Brachmann J. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. 2014;311:498–506. [DOI] [PubMed] [Google Scholar]
- 31. Peters DC, Wylie JV, Hauser TH, Nezafat R, Han Y, Woo JJ, Taclas J, Kissinger KV, Goddu B, Josephson ME, Manning WJ. Recurrence of atrial fibrillation correlates with the extent of post‐procedural late gadolinium enhancement: a pilot study. JACC Cardiovasc Imaging. 2009;2:308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Rosendael AR, Dimitriu‐Leen AC, van Rosendael PJ, Leung M, Smit JM, Saraste A, Knuuti J, van der Geest RJ, van der Arend BWH, van Zwet EW, Scholte AJ, Delgado V, Bax JJ. Association between posterior left atrial adipose tissue mass and atrial fibrillation. Circ Arrhythm Electrophysiol. 2017;10:e004614. [DOI] [PubMed] [Google Scholar]
- 33. Park JH, Park YS, Kim YJ, Lee IS, Kim JH, Lee JH, Choi SW, Jeong JO, Seong IW. Effects of statins on the epicardial fat thickness in patients with coronary artery stenosis underwent percutaneous coronary intervention: comparison of atorvastatin with simvastatin/ezetimibe. J Cardiovasc Ultrasound. 2010;18:121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]


