Abstract
Background
Prior studies have shown that survivors of acute myocardial infarction (AMI) complicated by cardiogenic shock are likely to have increased risk of readmissions in the early post‐discharge period. However, the contemporary prevalence, reasons, and predictors of 30‐day readmissions are not well known.
Methods and Results
Hospitalizations for a primary diagnosis of AMI complicated by cardiogenic shock, and discharged alive, were identified in the 2013 and 2014 Nationwide Readmissions Databases. Prevalence and reasons for 30‐day unplanned readmissions were investigated. A hierarchical logistic regression model was used to identify independent predictors of 30‐day readmissions. Among 1 116 933 patient hospitalizations with AMI, 39 807 (3.6%) had cardiogenic shock and were discharged alive. Their 30‐day readmission rate was 18.6%, with a median time for readmission 10 days post discharge. Predictors of readmission included: non–ST‐segment elevation myocardial infarction, female sex, low‐income status, nonprivate insurance, chronic renal failure, long‐term ventricular assist device or intra‐aortic balloon placement, and tachyarrhythmia. The majority of readmissions were attributable to cardiac‐related causes (52%); heart failure being the most frequent cardiac cause (39% of all cardiac causes). Noncardiac‐related readmissions included infections (14.9%), bleeding (5.3%), and respiratory causes (4.9%). The median cost per readmission was $9473 US dollars ($5037–20 199).
Conclusions
Among survivors of AMI complicated by cardiogenic shock who were discharged from hospital, almost 1 in 5 are readmitted at 30 days, mainly because of cardiac reasons such as heart failure and new AMI. The risk of readmission was associated with certain baseline patient/hospital characteristics.
Keywords: cardiogenic shock, heart failure, myocardial infarction, readmission
Subject Categories: Myocardial Infarction, Quality and Outcomes
Clinical Perspective
What Is New?
Rates of early readmission after acute myocardial infarction complicated by cardiogenic shock remains high, with ≈1 of 5 patients readmitted within 30 days of discharge.
Common causes of 30‐day readmission are cardiovascular, with the majority of readmissions related to heart failure exacerbation and new myocardial infarction.
Certain baseline patient characteristics, in‐hospital complications, and hospital characteristics independently predicted 30‐day hospital readmissions rates.
What Are the Clinical Implications?
Future studies are required to evaluate the effect of targeting various predictors of 30‐day readmissions in patients with acute myocardial infarction complicated by cardiogenic shock on readmission rates.
Assessment of the impact of a multidisciplinary team approach for patients with acute myocardial infarction complicated by cardiogenic shock is warranted given the evidence of a strong correlation between noncardiac comorbidities, such as diabetes mellitus and chronic kidney disease, and 30‐day readmissions rates.
Introduction
Cardiogenic shock complicating acute myocardial infarction (AMI) portends a poor prognosis. In the era before coronary revascularization, this setting was often considered fatal. For example, in the GUSTO‐I (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) study1 and the SHOCK (Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock) registry,2 only 40% of patients survived the hospitalization. With increased prevention measures and adoption of revascularization, in‐hospital survival rates improved. In an analysis from the National Registry of Myocardial Infarction, in‐hospital survival was ≈59% in 2004.3 A more recent analysis of the NCDR‐ACTION registry for 2007–2011 suggested that 2 of 3 such patients survived the index hospitalization.4 Despite advances in anticoagulation and mechanical support devices over the past few years, the in‐hospital mortality rate has been ≈30% in recent years.5, 6 With more patients surviving their index hospitalization, data regarding short‐ and longer‐term prognosis of this high‐risk population have been limited. An analysis from the NCDR‐ACTION registry suggested that these patients have a higher adjusted risk of mortality and/or hospitalization at 60 days but not at 1 year.7 However, the information in this area is limited and the underlying reasons for readmissions are unclear. To better address this knowledge gap, we aimed to determine the prevalence, predictors, and reasons for readmission among AMI hospitalizations complicated by cardiogenic shock using “real‐world” contemporary data from the Nationwide Readmissions Databases (NRD).
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. Institutional review board approval was not required given the public nature of the NRD database, with the absence of any personal identifying information.
Data Source
Data were obtained from the 2013 to 2014 NRD. The NRD is part of the all‐payer databases developed by the Agency for Healthcare Research and Quality for the Healthcare Cost and Utilization Project (HCUP). The NRD is created from the State Inpatient Databases and represents ≈50% of all US hospitalizations. Unweighted, the NRD contains data from ≈17 million discharges per year and weighted it estimates roughly 36 million discharges, excluding rehabilitation and long‐term acute care facilities. Discharge weights are provided to estimate national estimates. The NRD contains verified patient linkage numbers that can be used to track a patient across hospitals within a state. However, the patient linkage numbers do not track the same person from 1 year into another.
Study Population
The NRD was used to identify patients hospitalized with a primary diagnosis of ST‐segment elevation myocardial infarction (STEMI) (International Classification of Diseases, Ninth Edition, Clinical Modification [ICD‐CM‐9] codes 410.1x, 410.2x, 410.3x, 410.4x, 410.5x, 410.6x, 410.8x, and 410.9x) or non–ST‐segment elevation myocardial infarction (NSTEMI) (ICD‐CM‐9 code 410.7x) during the years 2013 and 2014. We included only those with a primary diagnosis of STEMI or NSTEMI during the index hospitalization since this represents the primary reason for the hospitalization, in an attempt to exclude hospitalizations with type 2 AMI.8, 9 Cardiogenic shock was identified using the ICD‐CM‐9 code 785.51. Hospitalization records were excluded if: (1) patients were younger than 18 years; (2) the patient died during the index hospitalization; or (3) the discharge month was December since 30‐day readmission data would be lacking (Figure 1).
Figure 1.
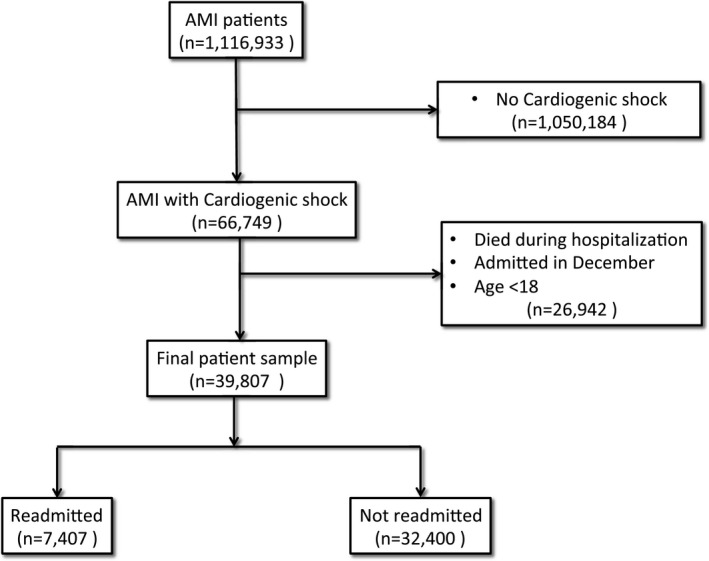
Study flow chart diagram. AMI indicates acute myocardial infarction.
Patient and Hospital Characteristics
Baseline patient characteristics included demographics (age, sex, median household income by ZIP code, and primary expected payer), weekend versus weekday admission, and other relevant comorbidities (eg, smoking, dyslipidemia, atrial fibrillation, known coronary artery disease, family history of coronary artery disease, prior myocardial infarction, prior percutaneous coronary intervention, and prior coronary artery bypass grafting) were identified using the corresponding ICD‐CM‐9 codes (Table 1). Hospital‐related characteristics such as bed size (small, medium, and large), location (urban versus rural), and teaching status were also identified.
Table 1.
ICD‐9 Codes of All Covariates Used in the Study
| Variable | ICD‐9 Code |
|---|---|
| Family history of CAD | V17.3 |
| Prior MI | 412 |
| Prior PCI | V45.82 |
| Prior CABG | V45.81 |
| Prior stroke/TIA | V12.54 |
| Carotid artery disease | 433.10 |
| Smoking history | V15.82, 305.1 |
| Dyslipidemia | 53 |
| Acute renal failure | 584.5 to 584.9 |
| Acute ischemic stroke | 433.01, 433.11, 433.21, 433.31, 433.81, 433.91, 434.01, 434.11, 434.91, 435.x, 436 |
| Intracranial hemorrhage | 430, 431, 432.x |
| Gastrointestinal bleeding | 578.0, 578.1, 578.9 |
| Pneumonia | 486, 481, 482.8, 482.3 |
| DVT/PE | 451.1, 451.2, 451.81, 451.9, 453.1, 453.2, 453.8, 453.9, 415.1 |
| Sepsis | 995.91, 996.64, 038x, 995.92, 998.59, and 999.3 |
| Atrial fibrillation | 427.31 |
| Ventricular tachycardia | 427.1 |
| Ventricular fibrillation | 427.41 |
| PCI | 00.66, 36.01, 36.06, 36.07, and 36.09 |
| Intubation/mechanical ventilation | 96.01 to 96.05, 96.7x |
| Intra‐aortic balloon pump placement | 37.61 |
| Short‐term ventricular assist device | 37.68, 37.60, 37.62, or 37.65 |
| Long‐term ventricular assist device placement | 37.66, 37.52 |
| Pacemaker/defibrillator placement | 37.80–83, 00.51, 00.50, 37.94, 37.96 |
Other variables not reported were collected using Elixhauser Comorbidity Software (https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp). CABG indicates coronary artery bypass grafting; CAD, coronary artery disease; DVT/PE, deep venous thrombosis/pulmonary embolization; ICD‐9, International Classification of Diseases, Ninth Edition; MI, myocardial infarction; PCI, percutaneous coronary intervention; TIA, transient ischemic attack.
Outcome Measure
The primary outcome of interest for this study was 30‐day all‐cause unplanned hospital readmission. Readmissions were captured according to methodology recommended by the HCUP.10 If a record had >1 readmission within 30 days of discharge, only the first readmission was included. Transfer to another hospital was not considered as a readmission. Time to readmission was calculated as the number of days between hospital discharge after index hospitalization and the initial day of hospital readmission. The primary diagnosis for each readmission record was reviewed individually (independently by 2 authors masked to each other's findings, and reconciliation of the differences between both authors was done by a third author) and were further categorized into cardiac and noncardiac related. Cardiac causes included heart failure, reinfarction (ie, STEMI or NSTEMI), arrhythmias, conduction disorders, chest pain, hypertension, hypotension, syncope, pericarditis, coronary artery disease, and others. Noncardiac causes included respiratory, infectious, bleeding, renal, gastrointestinal, trauma, hematological, neoplasms, endocrine/metabolic, neurological (including transient ischemic attack/stroke), psychiatric, and others. The median length of stay and median hospital costs were also calculated.
Statistical Analysis
Categorical variables are expressed using frequencies and compared using Pearson chi‐square test. Nonskewed continuous variables are expressed using mean±SD and compared using Student t test. Skewed continuous variables are expressed using median with 25th to 75th percentile range and compared using the Wilcoxon rank‐sum test. Weighted estimates were calculated by survey analysis methods (svy command in Stata) using the variable “DISCWT” provided by NRD as a weight variable and “HOSP_NRD” as the clustering variable as recommended by the AHRQ. This was performed to ensure accurate national representative estimates of the US population of hospitalized patients. The HOSP_NRD variable is a HCUP hospital identifier specifically created for the NRD. The purpose of this variable is to accurately identify inpatient records that are associated with the same hospital. HOSP_NRD does not link to other HCUP databases or to external databases.10 Independent predictors of 30‐day readmission were identified with a 2‐level hierarchical multivariable logistic regression model (multilevel mixed‐effects logistic regression model “melogit” in Stata) to account for in‐between hospital variations and hospital clustering effect using the hospital cluster variable “HOSP_NRD” as a second‐level variable. A total of 63 covariates were included in the first‐level model: they included baseline demographics, chronic comorbidities, hospital characteristics, in‐hospital procedures (eg, percutaneous coronary intervention), and in‐hospital complications. Statistical analyses were conducted using Stata software version 14 (StataCorp). A 2‐sided value of P<0.05 was set for statistical significance. Categorical variables are presented as frequencies and percentages and continuous variables as mean±SD or median and 25th to 75th percentile range.
Results
Among 1 116 933 hospitalizations with a primary diagnosis of AMI in the NRD database years 2013 and 2014 (335 548 with STEMI, and 781 385 with NSTEMI), 66 749 (6.0%) developed cardiogenic shock. Of those, 39 807 (59.6%) were discharged alive from January until November, representing the final cohort included in our analysis (Figure 1).
Prevalence and Predictors of Readmission
Of 39 807 hospitalizations with AMI and cardiogenic shock, a total of 7407 (18.6%) were readmitted after 30 days, with a median time for readmission of 10 days (4–18 days) after discharge (Figure 2). Meanwhile, 131 043 AMI hospitalizations without cardiogenic shock were readmitted of 921 004 index hospitalizations discharged alive at the same period (14.2% versus 18.6%, P<0.0001). Table 2 summarizes the baseline characteristics for the cohort. Table 3 summarizes the hospital characteristics and Table 4 reports the in‐hospital procedures and complications in each group.
Figure 2.
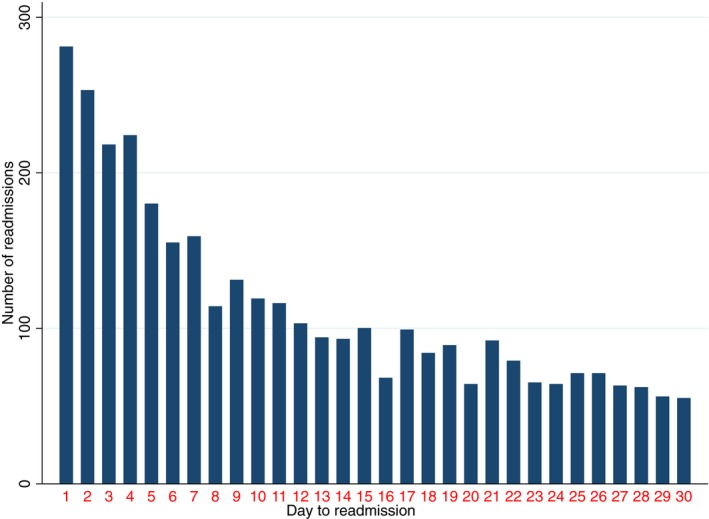
Frequencies of readmission according to the number of days after discharge in the acute myocardial infarction with cardiogenic shock hospitalization cohort.
Table 2.
Baseline Characteristics of the Study Cohort Stratified by Readmission Status
| Variable | Overall, % | No Readmission, % | Readmission, % | P Value |
|---|---|---|---|---|
| Patients, No. | 100 (39 807) | 81.4 (32 400) | 18.6 (7407) | |
| Primary diagnosis | ||||
| STEMI | 56.9 | 58.1 | 51.7 | <0.0001 |
| Patient demographics | ||||
| Age, mean (95% CI), y | 66.5 (66.3–66.8) | 66.2 (65.9–66.5) | 67.8 (67.3–68.4) | <0.0001 |
| Female | 33.2 | 32.4 | 37.0 | <0.0001 |
| Weekend admission | 26.8 | 26.7 | 27.4 | 0.458 |
| Family history of CAD | 15.4 | 16.3 | 11.8 | <0.0001 |
| Prior MI | 17.8 | 18.1 | 16.7 | 0.078 |
| Prior PCI | 20.2 | 20.5 | 18.7 | 0.028 |
| Prior CABG | 13.6 | 13.9 | 12.3 | 0.022 |
| Prior stroke | 13.6 | 14.0 | 11.8 | 0.002 |
| Carotid artery disease | 11.7 | 12.1 | 9.7 | <0.0001 |
| Smoking history | 42.4 | 43.4 | 37.9 | <0.0001 |
| Dyslipidemia | 9.6 | 10.1 | 7.5 | <0.0001 |
| Median home income | 0.089 | |||
| 1st to 25th percentile | 27.6 | 27.2 | 29.6 | |
| 26th to 50th percentile | 28.5 | 28.7 | 27.6 | |
| 51st to 75th percentile | 24.6 | 24.7 | 24.2 | |
| 75th to 100th percentile | 19.3 | 19.4 | 18.6 | |
| Expected payer | <0.0001 | |||
| Medicare | 57.6 | 55.8 | 65.1 | |
| Medicaid | 8.6 | 8.4 | 9.5 | |
| Private | 24.3 | 25.8 | 18.0 | |
| Self | 5.4 | 5.7 | 4.2 | |
| No charge | 0.7 | 0.7 | 0.6 | |
| Other | 3.4 | 3.6 | 2.5 | |
| Chronic comorbidities | ||||
| AIDS | 0.1 | 0.1 | 0.1 | 0.831 |
| Alcohol abuse | 4.9 | 5.0 | 4.4 | 0.207 |
| Anemia | 22.7 | 21.4 | 28.3 | <0.0001 |
| Collagen and rheumatologic disease | 2.4 | 2.2 | 3.0 | 0.033 |
| Chronic blood loss | 1.2 | 1.1 | 1.5 | 0.157 |
| CHF | 4.2 | 4.1 | 4.9 | 0.068 |
| COPD | 23.1 | 22.3 | 26.2 | <0.0001 |
| Coagulopathy | 16.7 | 16.4 | 18.1 | 0.018 |
| Depression | 7.1 | 6.9 | 7.9 | 0.043 |
| DM uncomplicated | 30.2 | 29.7 | 32.6 | <0.0001 |
| DM complicated | 8.6 | 7.6 | 12.7 | <0.0001 |
| Drug abuse | 3.0 | 3.0 | 3.4 | 0.296 |
| Hypertension | 64.9 | 64.5 | 66.7 | 0.025 |
| Hypothyroidism | 9.9 | 9.6 | 11.2 | 0.017 |
| Chronic liver disease | 1.8 | 1.7 | 2.2 | 0.064 |
| Lymphoma | 0.6 | 0.6 | 0.7 | 0.361 |
| Fluid and electrolytes disturbances | 46.1 | 45.0 | 50.7 | <0.0001 |
| Metastatic cancer | 0.8 | 0.7 | 1.2 | 0.007 |
| Neurological disorders | 7.1 | 7.2 | 6.6 | 0.275 |
| Obesity | 16.7 | 16.8 | 16.5 | 0.690 |
| Paralysis | 2.5 | 2.5 | 2.7 | 0.491 |
| Peripheral vascular disease | 14.8 | 13.7 | 19.5 | <0.0001 |
| Psychosis | 3.0 | 2.7 | 4.0 | 0.001 |
| Pulmonary circulation disorders | 0.7 | 0.6 | 0.8 | 0.363 |
| Chronic renal failure | 22.7 | 21.0 | 30.1 | <0.0001 |
| Peptic ulcer disease | ··· | ··· | ··· | |
| Valvular heart disease | 1.4 | 1.3 | 1.5 | 0.340 |
| Weight loss | 8.5 | 8.1 | 10.1 | 0.001 |
CABG indicates coronary artery bypass grafting; CAD, coronary artery disease; CHF, congestive heart failure; CI, confidence interval; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; MI, myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐segment elevation myocardial infarction.
Table 3.
Hospital Characteristics of the Cohort Stratified by Readmission Status
| Variable | Overall, % | No Readmission, % | Readmission, % | P Value |
|---|---|---|---|---|
| Peri‐hospital population size | <0.0001 | |||
| Central metropolitan >1 million | 23.8 | 23.0 | 27.3 | |
| Fringe metropolitan >1 million | 24.7 | 24.5 | 25.8 | |
| Metropolitan 250 000 to 999 999 population | 20.5 | 20.8 | 19.2 | |
| Metropolitan 50 000 to 249 999 population | 10.3 | 10.3 | 10.2 | |
| Micropolitan | 11.5 | 12.0 | 9.7 | |
| Nonmetropolitan or micropolitan | 9.1 | 9.4 | 7.8 | |
| Hospital bed size | 0.11 | |||
| Small bed size | 6.9 | 7.1 | 6.3 | |
| Medium bed size | 21.7 | 22.0 | 20.7 | |
| Large bed size | 71.4 | 71.0 | 73.0 | |
| Hospital ownership | 0.03 | |||
| Government nonfederal | 10.0 | 9.8 | 11.1 | |
| Private nonprofit | 76.3 | 76.7 | 74.8 | |
| Private investment | 13.6 | 13.5 | 14.1 | |
| Hospital teaching status | 0.61 | |||
| Urban nonteaching | 31.9 | 31.8 | 32.3 | |
| Urban teaching | 63.2 | 63.2 | 63.2 | |
| Rural nonteaching | 4.9 | 5.0 | 4.5 |
Table 4.
In‐Hospital Procedures and Complications of the Cohort Stratified by Readmission Status
| Variable | Overall, % | No Readmission, % | Readmission, % | P Value |
|---|---|---|---|---|
| In‐hospital procedures | ||||
| PCI | 59.3 | 60.0 | 56.3 | 0.0002 |
| Long‐term VAD | 0.5 | 0.4 | 0.7 | 0.008 |
| Short‐term VAD | 3.4 | 3.4 | 3.6 | 0.543 |
| IABP | 39.6 | 39.1 | 41.8 | 0.009 |
| Pacemaker/defibrillator | 3.9 | 3.9 | 4.0 | 0.783 |
| Intubation/mechanical ventilation | 34.8 | 34.2 | 37.6 | 0.001 |
| In‐hospital complications | ||||
| Acute renal failure | 45.9 | 45.1 | 49.5 | <0.0001 |
| Pneumonia | 20.9 | 21.0 | 20.5 | 0.565 |
| GIB | 13.4 | 13.9 | 11.3 | <0.0001 |
| ICH | 10.0 | 10.6 | 7.7 | <0.0001 |
| Acute ischemic stroke/TIA | 12.7 | 13.1 | 11.0 | 0.001 |
| DVT/PE | 9.8 | 10.3 | 7.6 | <0.0001 |
| Sepsis | 17.9 | 17.9 | 17.7 | 0.806 |
| Atrial fibrillation | 33.3 | 32.9 | 34.8 | 0.040 |
| Ventricular tachycardia | 25.5 | 25.7 | 24.8 | 0.338 |
| Ventricular fibrillation | 23.3 | 24.0 | 20.1 | <0.0001 |
DVT/PE indicates deep venous thrombosis/pulmonary embolism; GIB, gastrointestinal bleeding; IABP, intra‐aortic balloon pump; ICH, intracranial hemorrhage; PCI, percutaneous coronary intervention; TIA, transient ischemic attack; VAD, ventricular assist device.
Multivariate analysis identified the following patient characteristics to be associated with 30‐day readmission: female sex (odds ratio [OR], 1.12; 95% confidence interval [CI], 1.02–1.23, P=0.022), anemia (OR, 1.15; 95% CI, 1.04–1.29 [P=0.009]), chronic obstructive pulmonary disease (OR, 1.16; 95% CI, 1.05–1.29 [P=0.003]), diabetes mellitus without (OR, 1.15; 95% CI, 1.05–1.26 [P=0.004]) or with (OR, 1.44; 95% CI, 1.24–1.68 [P<0.0001]) complications, peripheral vascular disease (OR, 1.29; 95% CI, 1.14–1.45 [P<0.0001]), and chronic renal failure (OR, 1.25; 95% CI, 1.11–1.40 [P<0.0001]). The clinical presentation of STEMI versus NSTEMI was a predictor of marginal significance, suggesting a lower incidence of 30‐day readmission among patients with STEMI (OR, 0.91; 95% CI, 0.82–1.00 [P=0.042]). Placement of a long‐term ventricular assist device or intra‐aortic balloon pump was associated with higher 30‐day readmission rates (Table 4). Among the in‐hospital complications evaluated, ventricular tachycardia and atrial fibrillation were associated with higher readmission rates (Table 4). Meanwhile, high median home income (OR, 0.84; 95% CI, 0.73–0.96 [P=0.010]) and non‐Medicare/Medicaid payers and hospitals located in low‐population areas were all associated with lower 30‐day readmissions (Table 5).
Table 5.
Predictors of 30‐Day Readmission Following AMI and Cardiogenic Shock
| Variable | Odds Ratio | 95% CI | P Value | |
|---|---|---|---|---|
| Primary diagnosis | ||||
| STEMI | 0.91 | 0.82 | 1.00 | 0.042 |
| Patient demographics | ||||
| Age >80 y | 0.97 | 0.86 | 1.09 | 0.589 |
| Female sex | 1.12 | 1.02 | 1.23 | 0.022 |
| Weekend admission | 1.04 | 0.95 | 1.15 | 0.393 |
| Family history of CAD | 0.82 | 0.67 | 1.00 | 0.053 |
| Prior MI | 1.06 | 0.90 | 1.24 | 0.493 |
| Prior PCI | 1.03 | 0.89 | 1.19 | 0.733 |
| Prior CABG | 1.10 | 0.90 | 1.33 | 0.346 |
| Prior stroke/TIA | 0.93 | 0.75 | 1.14 | 0.463 |
| Carotid artery disease | 0.92 | 0.67 | 1.26 | 0.597 |
| Smoking history | 0.90 | 0.81 | 0.99 | 0.031 |
| Dyslipidemia | 2.40 | 0.49 | 11.69 | 0.279 |
| Median home income (reference: 1st to 25th percentile) | ||||
| 26th to 50th percentile | 0.89 | 0.80 | 1.00 | 0.044 |
| 51st to 75th percentile | 0.88 | 0.78 | 1.01 | 0.066 |
| 75th to 100th percentile | 0.84 | 0.73 | 0.96 | 0.010 |
| Expected payer (reference: Medicare) | ||||
| Medicaid | 0.99 | 0.85 | 1.16 | 0.920 |
| Private | 0.70 | 0.62 | 0.79 | <0.0001 |
| Self | 0.76 | 0.61 | 0.93 | 0.009 |
| No charge | 0.94 | 0.58 | 1.52 | 0.797 |
| Other | 0.69 | 0.54 | 0.88 | 0.004 |
| Chronic comorbidities | ||||
| AIDS | 1.06 | 0.40 | 2.81 | 0.911 |
| Alcohol abuse | 0.91 | 0.74 | 1.13 | 0.404 |
| Anemia | 1.15 | 1.04 | 1.29 | 0.009 |
| Collagen vascular disease | 1.31 | 0.97 | 1.76 | 0.074 |
| Chronic blood loss | 1.12 | 0.77 | 1.62 | 0.545 |
| CHF | 0.95 | 0.76 | 1.18 | 0.635 |
| Coagulopathy | 0.95 | 0.85 | 1.07 | 0.385 |
| COPD | 1.16 | 1.05 | 1.29 | 0.003 |
| Depression | 1.09 | 0.93 | 1.27 | 0.307 |
| DM without complications | 1.15 | 1.05 | 1.26 | 0.004 |
| DM with complications | 1.44 | 1.24 | 1.68 | <0.0001 |
| Drug abuse | 1.17 | 0.90 | 1.53 | 0.234 |
| Hypertension | 0.96 | 0.87 | 1.06 | 0.411 |
| Hypothyroidism | 1.05 | 0.92 | 1.20 | 0.456 |
| Chronic liver disease | 1.18 | 0.92 | 1.53 | 0.196 |
| Lymphoma | 1.07 | 0.64 | 1.77 | 0.806 |
| Fluid and electrolytes disturbance | 1.07 | 0.98 | 1.17 | 0.125 |
| Metastatic cancer | 1.62 | 1.06 | 2.46 | 0.024 |
| Neurological disorders | 0.82 | 0.69 | 0.98 | 0.026 |
| Obesity | 0.90 | 0.79 | 1.02 | 0.091 |
| Paraplegia | 0.86 | 0.65 | 1.14 | 0.290 |
| PVD | 1.29 | 1.14 | 1.45 | <0.0001 |
| Psychosis | 1.38 | 1.07 | 1.77 | 0.013 |
| Pulmonary vascular disease | 1.05 | 0.58 | 1.88 | 0.873 |
| Chronic renal failure | 1.25 | 1.11 | 1.40 | <0.0001 |
| Tumors | 1.25 | 0.91 | 1.71 | 0.176 |
| Peptic ulcer | 3.34 | 0.49 | 22.67 | 0.216 |
| Weight loss | 1.02 | 0.87 | 1.18 | 0.838 |
| Valvular heart disease | 0.91 | 0.62 | 1.35 | 0.653 |
| Procedures | ||||
| PCI | 1.03 | 0.94 | 1.12 | 0.590 |
| Long‐term VAD | 1.78 | 1.16 | 2.71 | 0.008 |
| Short‐term VAD | 1.13 | 0.90 | 1.42 | 0.291 |
| IABP | 1.17 | 1.06 | 1.29 | 0.001 |
| Pacemaker/defibrillator placement | 0.91 | 0.73 | 1.13 | 0.405 |
| Intubation/mechanical ventilation | 1.05 | 0.94 | 1.16 | 0.390 |
| In‐hospital complications | ||||
| Acute renal failure | 1.04 | 0.94 | 1.14 | 0.467 |
| Acute ischemic stroke/TIA | 1.12 | 0.87 | 1.44 | 0.386 |
| ICH | 0.57 | 0.24 | 1.33 | 0.193 |
| GIB | 0.87 | 0.70 | 1.07 | 0.190 |
| Pneumonia | 1.02 | 0.89 | 1.17 | 0.761 |
| DVT/PE | 0.55 | 0.17 | 1.75 | 0.310 |
| Sepsis | 1.16 | 1.00 | 1.35 | 0.051 |
| Atrial fibrillation | 1.12 | 1.01 | 1.23 | 0.024 |
| Ventricular tachycardia | 1.16 | 1.03 | 1.31 | 0.017 |
| Ventricular fibrillation | 0.98 | 0.85 | 1.12 | 0.748 |
| Hospital characteristics | ||||
| Population size (reference: large metropolitan >1 million population) | ||||
| Fringe metropolitan >1 million | 0.95 | 0.84 | 1.07 | 0.408 |
| Metropolitan 250 000–999 999 population | 0.83 | 0.74 | 0.94 | 0.004 |
| Metropolitan 50 000–249 999 population | 0.91 | 0.78 | 1.05 | 0.190 |
| Micropolitan | 0.67 | 0.57 | 0.79 | <0.0001 |
| Nonmetropolitan or micropolitan | 0.66 | 0.54 | 0.80 | <0.0001 |
| Hospital bed size (reference is small bed size) | ||||
| Medium bed size | 1.06 | 0.87 | 1.30 | 0.544 |
| Large bed size | 1.09 | 0.91 | 1.31 | 0.352 |
| Hospital ownership (reference is government hospitals) | ||||
| Private nonprofit | 0.83 | 0.73 | 0.94 | 0.004 |
| Private investment | 0.87 | 0.74 | 1.03 | 0.104 |
| Hospital teaching status (reference is urban nonteaching) | ||||
| Urban teaching | 0.97 | 0.88 | 1.07 | 0.514 |
| Rural nonteaching | 1.07 | 0.82 | 1.40 | 0.628 |
AMI indicates acute myocardial infarction; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CHF, congestive heart failure; CI, confidence interval; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; DVT/PE, deep venous thrombosis/pulmonary embolization; GIB, gastrointestinal bleeding; IABP, intra‐aortic balloon pump; ICH, intracranial hemorrhage; MI, myocardial infarction; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; STEMI, ST‐segment elevation myocardial infarction; TIA, transient ischemic attack; VAD, ventricular assist device.
Reasons for 30‐Day Readmissions
The most frequent reasons for readmission were cardiac (51.5%), infectious (14.8%), and bleeding (5.3%) (Figure 3). The most frequent cardiac reason for readmission was heart failure (39%) followed by reinfarction (14.4%) and coronary artery disease (13.3%) (Figure 4). The noncardiac‐related causes (48.5%) included infections (14.8%) (Figure 5), bleeding (5.3%) (gastrointestinal bleeding contributed to 58% of all bleeding causes) (Figure 6), respiratory (4.9%), iatrogenic (3.7%) (device‐related complications contributed to 60.3% of all iatrogenic causes), renal (2.8%), gastrointestinal (2.7%), metabolic/endocrine (2.5%), neurological (2.4%), vascular (1.1%), and others (Figure 3). Among those readmitted within 30 days, the in‐hospital mortality rate was 7.6%.
Figure 3.
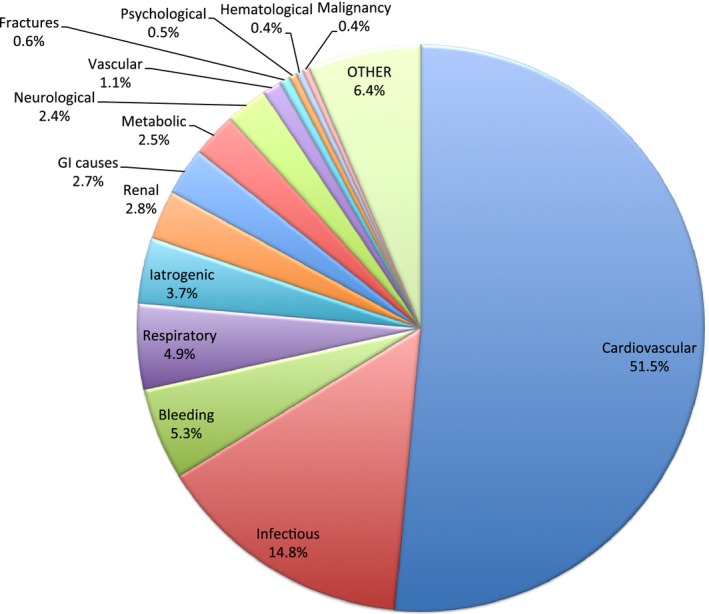
Overall causes of 30‐day readmission. GI indicates gastrointestinal.
Figure 4.
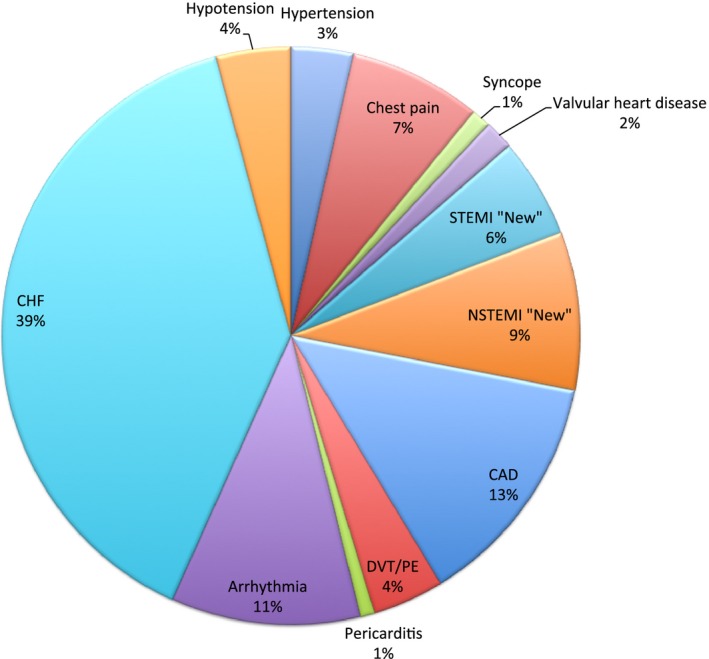
Cardiac causes of 30‐day readmission. CAD indicates coronary artery disease; CHF, congestive heart failure; DVT/PE, deep venous thrombosis/pulmonary embolism; NSTEMI, non–ST‐segment elevation myocardial infarction; STEMI, ST‐segment elevation myocardial infarction (Percentage is out of all cardiac 30‐day readmissions).
Figure 5.
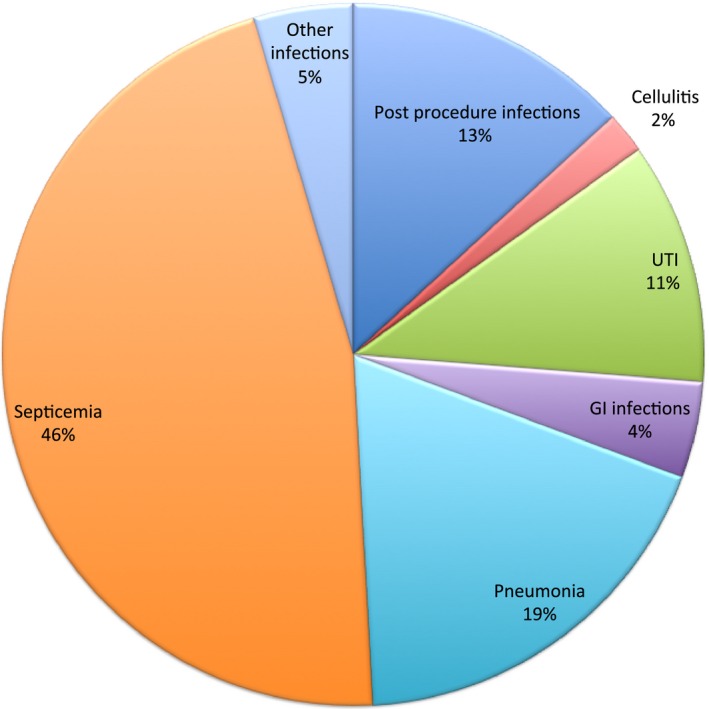
Infectious causes of 30‐day readmission. GI indicates gastrointestinal; UTI, urinary tract infection (Percentage is out of all infectious causes of 30‐day readmissions).
Figure 6.
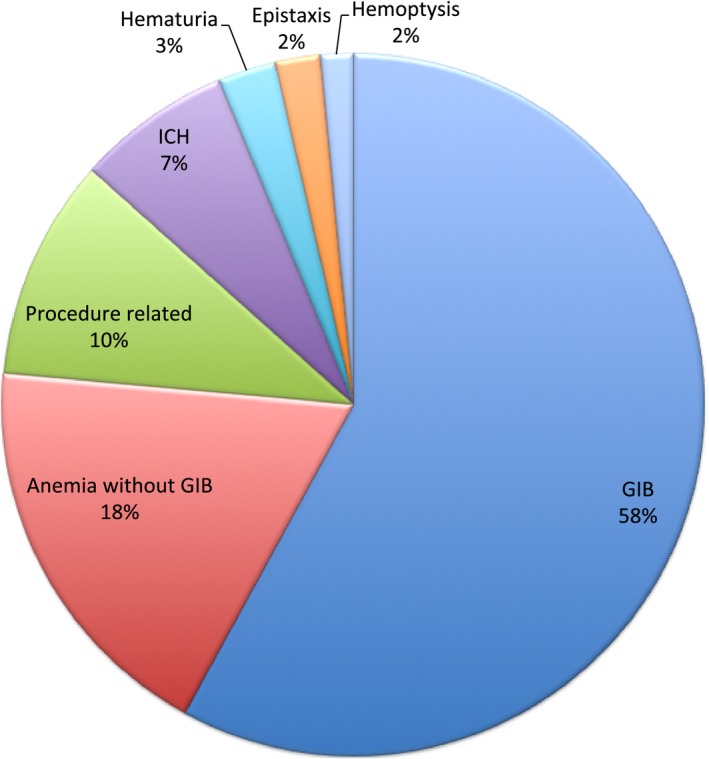
Bleeding causes of 30‐day readmission. GIB indicates gastrointestinal bleeding; ICH, intracranial hemorrhage (Percentage is out of all bleeding causes of 30‐day readmissions).
Median Length of Stay and Hospital Costs of 30‐Day Readmissions
The median length of stay in the readmitted cohort was 4 days (2–8 days) with total hospital costs of $129 000 000 US dollars (95% CI, $117 000 000–$141 000 000). The median hospital cost per one 30‐day readmission was $9473 US dollars ($5037–20 199).
Discussion
In this observational analysis of a large, real‐world cohort of hospitalizations with AMI complicated by cardiogenic shock, we identified 30‐day readmission in almost 1 of 5 hospitalizations. The median time to readmission was 10 days. The risk of readmission at 30 days was marginally lower among patients with STEMI compared with NSTEMI. Female sex, low socioeconomic status, Medicaid insurance, mechanical support device placement (ie, intra‐aortic balloon pump and long‐term ventricular support devices), and atrial fibrillation, as well as ventricular tachycardia were all predictors of readmission at 30 days. To the best of our knowledge, this is the first study to evaluate the causes of readmission in patients hospitalized with AMI complicated by cardiogenic shock. In this cohort, cardiac‐related causes accounted for a majority of the readmissions, with heart failure and reinfarction being the most frequent reasons. The most frequent causes for noncardiac‐related admissions included infections, bleeding, and respiratory causes.
In recent years, the number of patients with AMI surviving cardiogenic shock to be discharged from the hospital has been increasing. However, this subset of patients represents a cohort that is more vulnerable for early readmissions. This is important for patients, payers, healthcare delivery systems, and advocacy groups to increase support for research in this area. Thirty‐day readmission has been used as a performance metric by the Centers for Medicare & Medicaid Services to assess the quality of hospital care and to penalize hospitals with higher‐than‐expected readmission rates for certain conditions such as AMI, heart failure, and pneumonia. In our study, the 30‐day readmission rate for AMI complicated with cardiogenic shock was 18.6%, which is slightly lower than hospitalizations with a principal diagnosis of congestive heart failure (23.5%) or chronic obstructive pulmonary disease (20.0%); however, it is considerably higher than those with a principal diagnosis of AMI (without cardiogenic shock) (14.2%), pneumonia (15.5%) or any other cause combined (12.2%).11 Several studies in the revascularization era indicate that post‐discharge AMI outcomes are worse in the setting of prior cardiogenic shock. However, this effect appeared to be time‐dependent and is clustered early on.7, 12 In fact, the mortality rates of these patients are comparable to those with AMI without cardiogenic shock at 5 years.13 In this study, the median time to readmission was 10 days (range 8–14 days), which is consistent with Medicare data that found the median time of readmission after AMI was 10 days.14 Therefore, improved prevention and interventions early in the course of management are likely important to improve outcomes in the short‐term period in these patients. Besides the morbidity and health resources consumption resulting from readmissions, our analysis suggests that readmissions after an AMI with cardiogenic shock are associated with a significant economic burden (ie, total cost ≈$65 million US dollars per year).
We identified certain patient‐related characteristics associated with higher risk of 30‐day readmission such as female sex, diabetes mellitus, anemia peripheral vascular disease, and chronic renal failure, as well as presentation with NSTEMI. These characteristics are consistent with other studies that have evaluated 30‐day readmission after AMI without cardiogenic shock. In a population‐based analysis of 3010 patients with AMI, diabetes mellitus, chronic obstructive pulmonary disease, and anemia were predictors of 30‐day readmissions, but the type of AMI was not.15 Thus, a multidisciplinary team approach (including a cardiologist, general internist, endocrinologist, nephrologist, and hematologist) might be a valid option for managing patients with AMI and cardiogenic shock. This approach could ultimately help in preventing unplanned noncardiac readmissions at the early post‐discharge period.
A previous analysis of the NRD evaluated readmission rates after AMI complicated by cardiogenic shock, stratified by sex, suggested that women have a higher risk of 30‐day readmission after AMI versus men.16 Unfortunately, it was not possible to assess other factors suggested to correlate with 30‐day readmission after AMI in general, such as complications of angiography/revascularization,15 as well as medication adherence17 and health literacy18 in our study since these variables are not available in the NRD. Potentially arranging for early post‐discharge physician follow‐up for those who are at increased risk of readmission could help mitigate this risk. Data from the Medicare population have suggested that earlier post‐discharge follow‐up after NSTEMI is linked with lower risk of 30‐day readmissions.19 We also identified that certain hospital‐related characteristics, such as those seen in low‐population areas, were linked to lower 30‐day readmission after AMI and cardiogenic shock. An analysis of the Medicare data shows some regional variations across hospitals in 30‐day readmission rates after AMI,20 which suggests that further improvements are also needed at the hospital levels.
Study Strengths and Limitations
The strength of this study includes the large number of hospitalizations that are included from real‐world data. By using multivariate hierarchal logistic regression, we attempted to account for any potential clustering effect between the hospitals. However, our study has several limitations. First, this is an observational, nonrandomized analysis. Although we adjusted for multiple confounding variables, the risk of unmeasured confounding could not be completely excluded. Second, the NRD lacks data regarding the access site (radial versus femoral), as well as the stent type, and important medications (ie, anticoagulants and antiplatelet therapy), which have been implicated in affecting the outcomes of patients with cardiogenic shock. Further, data regarding complete revascularization that are known to impact the outcomes in AMI and shock were not available.21, 22 Third, readmissions cannot be tracked across different states or across calendar years. Thus, our numbers are likely to represent underestimates. Fourth, the NRD represents an administrative database, thus it is subject to known limitations of such data sources (eg, coding errors and bias). Last, given the large sample size of the current study and the large number of variables included in the adjustment model, the statistical significance of some of the predictor variables with CIs approaching 1 could be caused by chance (ie, type I error). Thus, we strongly believe that the statistical significance of such predictors should be balanced with the magnitude of effect, the quality of the study, and findings shown in other studies.
Conclusions
AMI complicated with cardiogenic shock is associated with high rates of readmissions at 30 days, mainly attributable to cardiac reasons such as heart failure and new AMI. The risk of readmission is associated with certain baseline patient/hospital characteristics and in‐hospital procedures/complications.
Sources of Funding
Dr. Pepine receives support from the Gatorade Trust through funds distributed by the University of Florida, Department of Medicine; NIH NCATS–University of Florida Clinical and Translational Science UL1TR001427; and PCORnet‐OneFlorida Clinical Research Consortium CDRN‐1501‐26692.
Disclosures
None.
(J Am Heart Assoc. 2018;7:e008235 DOI: 10.1161/JAHA.117.008235.)29572325
This article was handled independently by Ajay Gupta, MD, MRCP, PhD, as a guest editor.
References
- 1. Holmes DR Jr, Bates ER, Kleiman NS, Sadowski Z, Horgan JH, Morris DC, Califf RM, Berger PB, Topol EJ. Contemporary reperfusion therapy for cardiogenic shock: the GUSTO‐I trial experience. The GUSTO‐I Investigators. Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries. J Am Coll Cardiol. 1995;26:668–674. [DOI] [PubMed] [Google Scholar]
- 2. Hochman JS, Buller CE, Sleeper LA, Boland J, Dzavik V, Sanborn TA, Godfrey E, White HD, Lim J, LeJemtel T. Cardiogenic shock complicating acute myocardial infarction–etiologies, management and outcome: a report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries for cardiogenic shocK? J Am Coll Cardiol. 2000;36:1063–1070. [DOI] [PubMed] [Google Scholar]
- 3. Babaev A, Frederick PD, Pasta DJ, Every N, Sichrovsky T, Hochman JS. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA. 2005;294:448–454. [DOI] [PubMed] [Google Scholar]
- 4. Anderson ML, Peterson ED, Peng SA, Wang TY, Ohman EM, Bhatt DL, Saucedo JF, Roe MT. Differences in the profile, treatment, and prognosis of patients with cardiogenic shock by myocardial infarction classification: a report from NCDR. Circ Cardiovasc Qual Outcomes. 2013;6:708–715. [DOI] [PubMed] [Google Scholar]
- 5. Wayangankar SA, Bangalore S, McCoy LA, Jneid H, Latif F, Karrowni W, Charitakis K, Feldman DN, Dakik HA, Mauri L, Peterson ED, Messenger J, Roe M, Mukherjee D, Klein A. Temporal trends and outcomes of patients undergoing percutaneous coronary interventions for cardiogenic shock in the setting of acute myocardial infarction: a report from the CathPCI Registry. JACC Cardiovasc Interv. 2016;9:341–351. [DOI] [PubMed] [Google Scholar]
- 6. Goldberg RJ, Makam RC, Yarzebski J, McManus DD, Lessard D, Gore JM. Decade‐long trends (2001–2011) in the incidence and hospital death rates associated with the in‐hospital development of cardiogenic shock after acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2016;9:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shah RU, de Lemos JA, Wang TY, Chen AY, Thomas L, Sutton NR, Fang JC, Scirica BM, Henry TD, Granger CB. Post‐hospital outcomes of patients with acute myocardial infarction with cardiogenic shock: findings from the NCDR. J Am Coll Cardiol. 2016;67:739–747. [DOI] [PubMed] [Google Scholar]
- 8. Elgendy IY, Mahmoud AN, Mansoor H, Bavry AA. Early invasive versus initial conservative strategies for women with non‐ST‐elevation acute coronary syndromes: a nationwide analysis. Am J Med. 2017;130:1059–1067. [DOI] [PubMed] [Google Scholar]
- 9. Mahmoud AN, Elgendy IY, Mansoor H, Wen X, Mojadidi MK, Bavry AA, Anderson RD. Early invasive strategy and in‐hospital survival among diabetics with non‐ST‐elevation acute coronary syndromes: a contemporary national insight. J Am Heart Assoc. 2017;6:e005369 DOI: 10.1161/JAHA.116.005369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. US Agency for Healthcare Research and Quality . Overview of Key Readmission Measures and Methods. 2012. Available at: https://www.hcup-us.ahrq.gov/reports/methods/methods.jsp. Accessed August 17, 2017.
- 11. Fingar K, Washington R. Trends in Hospital Readmissions for Four High‐Volume Conditions, 2009‐2013. HCUP Statistical Brief #196. November 2015. Rockville, MD: Agency for Healthcare Research and Quality; Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb196-Readmissions-Trends-High-Volume-Conditions.pdf. Accessed August 17, 2017. [PubMed] [Google Scholar]
- 12. Bagai A, Chen AY, Wang TY, Alexander KP, Thomas L, Ohman EM, Hochman JS, Peterson ED, Roe MT. Long‐term outcomes among older patients with non‐ST‐segment elevation myocardial infarction complicated by cardiogenic shock. Am Heart J. 2013;166:298–305. [DOI] [PubMed] [Google Scholar]
- 13. Aissaoui N, Puymirat E, Simon T, Bonnefoy‐Cudraz E, Angoulvant D, Schiele F, Benamer H, Quandalle P, Prunier F, Durand E, Berard L, Blanchard D, Danchin N. Long‐term outcome in early survivors of cardiogenic shock at the acute stage of myocardial infarction: a landmark analysis from the French registry of Acute ST‐elevation and non‐ST‐elevation Myocardial Infarction (FAST‐MI) Registry. Crit Care. 2014;18:516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, Barreto‐Filho JA, Kim N, Bernheim SM, Suter LG, Drye EE, Krumholz HM. Diagnoses and timing of 30‐day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dunlay SM, Weston SA, Killian JM, Bell MR, Jaffe AS, Roger VL. Thirty‐day rehospitalizations after acute myocardial infarction: a cohort study. Ann Intern Med. 2012;157:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mahmoud AN, Elgendy IY. Gender impact on 30‐day readmissions after hospitalization with acute myocardial infarction complicated by cardiogenic shock (from the 2013–2014 National Readmissions Database). Am J Cardiol. 2017;121:523–528. [DOI] [PubMed] [Google Scholar]
- 17. Zhang Y, Kaplan CM, Baik SH, Chang CC, Lave JR. Medication adherence and readmission after myocardial infarction in the Medicare population. Am J Manag Care. 2014;20:e498–e505. [PMC free article] [PubMed] [Google Scholar]
- 18. Bailey SC, Fang G, Annis IE, O'Conor R, Paasche‐Orlow MK, Wolf MS. Health literacy and 30‐day hospital readmission after acute myocardial infarction. BMJ Open. 2015;5:e006975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hess CN, Shah BR, Peng SA, Thomas L, Roe MT, Peterson ED. Association of early physician follow‐up and 30‐day readmission after non‐ST‐segment‐elevation myocardial infarction among older patients. Circulation. 2013;128:1206–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krumholz HM, Merrill AR, Schone EM, Schreiner GC, Chen J, Bradley EH, Wang Y, Wang Y, Lin Z, Straube BM, Rapp MT, Normand SL, Drye EE. Patterns of hospital performance in acute myocardial infarction and heart failure 30‐day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2:407–413. [DOI] [PubMed] [Google Scholar]
- 21. Thiele H, Akin I, Sandri M, Fuernau G, de Waha S, Meyer‐Saraei R, Nordbeck P, Geisler T, Landmesser U, Skurk C, Fach A, Lapp H, Piek JJ, Noc M, Goslar T, Felix SB, Maier LS, Stepinska J, Oldroyd K, Serpytis P, Montalescot G, Barthelemy O, Huber K, Windecker S, Savonitto S, Torremante P, Vrints C, Schneider S, Desch S, Zeymer U; CULPRIT‐SHOCK Investigators . PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017;377:2419–2432. [DOI] [PubMed] [Google Scholar]
- 22. Elgendy IY, Mahmoud AN, Kumbhani DJ, Bhatt DL, Bavry AA. Complete or culprit‐only revascularization for patients with multivessel coronary artery disease undergoing percutaneous coronary intervention: a pairwise and network meta‐analysis of randomized trials. JACC Cardiovasc Interv. 2017;10:315–324. [DOI] [PubMed] [Google Scholar]


