Abstract
Background
While it is well known that heart failure patients presenting to the emergency room (ER) have high short‐term mortality after discharge, the outcomes of patients with heart failure with repeated ER visits within a short time are not known. In this study, we aimed to determine whether clustering is associated with an increased risk of death.
Methods and Results
This is a retrospective, population‐based cohort study with an accrual window between 2003 and 2014 and maximal follow‐up up to and including March 31, 2015. Data were obtained from administrative databases from Ontario, Canada. Clustering was defined a priori as 3 or more ER visits within a 6‐month period. The main outcome of interest was time to death conditional on 6‐month survival. A total of 72 810 patients with an index hospitalization for acute heart failure were evaluated. ER clustering was observed in 15.1% of the population. Increased burden of comorbidities, primary rural residence, and lack of primary care provider were identified as factors associated with ER clustering. Age‐ and sex‐adjusted mortality for clustered patients was higher than for nonclustered (hazard ratio [HR] 1.51; 95% confidence interval, 1.47–1.55, P<0.0001). Adjusted mortality risk was also higher for patients with clustered ER visits (HR 1.42; 95% confidence interval 1.38–1.46; P<0.0001).
Conclusions
Clustering, as defined by 3 or more ER visits for any reason within 6 months of index heart failure hospitalization reflects a novel risk factor associated with increased mortality. Future research into the strategies to better manage complex patients with heart failure with recurrent ER visits are warranted.
Keywords: clustering, emergency room, recurrent event
Subject Categories: Cardiomyopathy, Heart Failure, Aging, Mortality/Survival, Quality and Outcomes
Clinical Perspective
What Is New?
Clustering was defined as 3 or more hospitalizations within a 6‐month period.
Clustering encompasses complex interactions among individuals, their biology, and the healthcare system.
The hazard of death was significantly higher for patients with clustered visits than for patients without clustered visits.
What Are the Clinical Implications?
Clustering better defines a subset of patients at increased risk of adverse outcomes.
Clustering is a simple yet robust risk stratification tool when assessing patients in the ER.
Patients with clustered visits represent a vulnerable population that might benefit from dedicated healthcare resources.
The management of patients with clustering ER visits should include a multidimensional understanding of the associated comorbidities.
Clustering rates can be used as a potential target of interventions for quality improvement initiatives in the field.
Heart failure (HF) is a chronic condition associated with significant mortality, morbidity, and high rates of healthcare utilization,1, 2 mostly attributable to emergent care and hospitalizations.3 As the number of patients with HF increases due to an aging population, rising prevalence of risk factors, and improved survival of acute cardiovascular events, older adults with HF have an increased burden of both cardiac and noncardiac comorbidities that complicate HF management, increase health utilization, and adversely impact outcomes.4 Consequently, significant research and quality improvement efforts have been directed at reducing the demand for acute services and improve overall outcomes.4, 5
One prominent area of HF quality improvement research has focused on understanding the patient and system factors that lead to emergency room (ER) visits and hospitalizations for acute decompensated heart failure (ADHF). Over the past decade, the number of patients managed in the ER for ADHF has increased substantially6 with a proportional rise in health system costs.7, 8 Importantly, one third of patients with HF frequently use the ER and account for over half of all ER visits for ADHF.9
More recently, there has been a heightened awareness in frequent users of the ER for ADHF, because of both the system costs associated with frequent use of acute services and the associated mortality burden shortly after discharge.10, 11 Previous research has examined ways to risk stratify patients with HF presenting to the ER, particularly to understand the risk of repeat ER visits, hospitalization, or death.12 One aspect of this patient population that has not been examined is the subset of patients with HF who present to the ER multiple times within a short time frame after discharge. In this study, we describe the patient and system factors associated with patients with HF who have a clustered set of ER visits, and compare patient outcomes to those patients with HF who presented to the ER but did not have clustered visits.
We hypothesize that patients with HF with clustered ER visits, defined as those patients seen in the ER more than 3 times in a 6‐month period, have higher mortality than those without clustered visits.
Methods
Due to Ontario privacy laws and Institute for Clinical Evaluative Sciences regulations, the data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. This study was approved by the Sunnybrook Research Ethics Board. Informed consent was waived.
Data Sources
The administrative databases linked in this study include the Canadian Institute for Health Information for hospital admissions and day surgery; the National Ambulatory Care Reporting System for all records of emergency room visits; the Ontario Health Insurance Plan claim database for outpatient physician visits; the Ontario Drug Benefit program for outpatient medication claims for those 65 years old and older; and the Registered Person Database providing basic demographic data on all individuals. Data were linked using unique encrypted patient identifiers and analyzed at the Institute for Clinical Evaluative Sciences in Toronto, Ontario.
Study Population
Because of the universal health coverage in Ontario, administrative data for hospital admissions, emergency visits, and physician outpatient services are complete and linkable through unique encrypted patient identifiers. Prescription claims are available for those over 65 years old through the Ontario Drug Benefit Database. Other linkage with the Registered Person Database provides demographic information such as birth/death dates, sex, neighborhood income quintile, and rurality.
We conducted a retrospective population‐based analysis of all ADHF patients admitted during the period April 1, 2003, to March 31, 2014. Patients with a main diagnosis of ADHF (International Classification of Diseases, Tenth Revision, [ICD‐10] code as “I50”) who were residents of Ontario, Canada, and aged between 18 and 105 years old were included in the cohort of interest. We excluded those patients with elective admission, invalid health card, length of hospital stay for acute ≤1 day, or whose death occurred before or during index hospital stay. To capture the first presentation for HF, patients who had a history of ADHF‐related hospitalizations within 5 years before index hospitalization were also excluded. For the purpose of cohort generation, if recurrent hospitalizations took place within 180 days of index hospital discharge, only the first hospitalization was used (index event). Figure 1 depicts the cohort flowchart, and Figure S1 shows the cohort derivation process.
Figure 1.
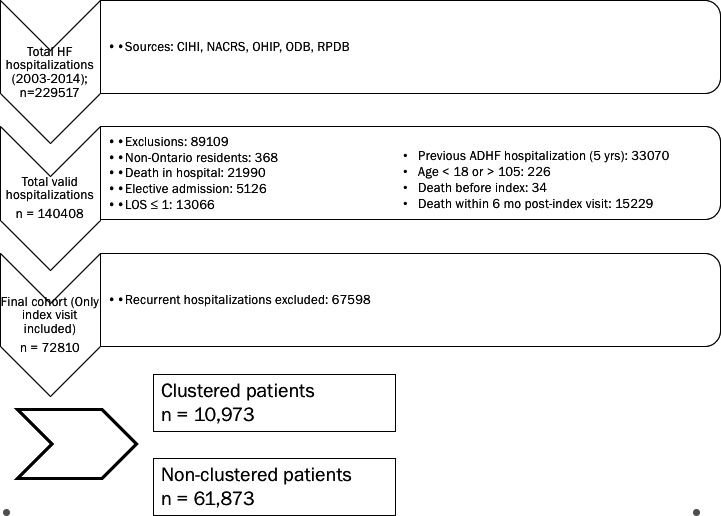
Cohort flowchart. ADHF indicates acute decompensated heart failure; CIHI, Canadian Institutes of health Information; NACRS, National Ambulatory Care Reporting System; ODB, Ontario Drug Benefit; RPDB, Registered Patients Database; OHIP, Ontario Health Insurance Plan.
Outcomes and Definitions
The outcome of interest was time to death conditional on 6‐month survival. Mortality was determined by linking to the Registered Person Database for all deaths until the end of follow‐up, March 31, 2015; survival time was calculated from 6 months after index discharge to day of death or end of follow‐up, whichever occurred first.
The main exposure was clustered ER visits, defined a priori as having at least 3 ER visits for any reason within 6 months of index hospital discharge. This was defined in previous research on ER utilization among patients with HF.9 Reasons for each of the clustered ER visits were also recorded and specified.
Hospital volume
Hospital age and sex–standardized admission rates were calculated as described previously.13 The institutions were divided into tertiles representing low, medium, and high admissions rates.
Statistical Analysis
Comparison of baseline characteristics between patients with clustered and nonclustered ER visits was carried out by Pearson's chi square test for categorical variables and ANOVA or Kruskal‐Wallis test for continuous variables. Kaplan‐Meier curves were plotted to compare time to death between these 2 groups using the log‐rank test.
The odds ratios of predictors of clustered ER visits were analyzed by univariate logistic regression, and the survival analysis was conducted by multivariable Cox proportional hazard models with adjustment for age, sex, and clustered ER visits along with 22 covariates in the Krumholz14 model, including cardiovascular and other comorbidities in the past 5 years before index hospitalizations.
All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC) in a UNIX environment. P ≤0.05 was considered significant.
Results
Patients Characteristics
Between 2003 and 2014 a total of 229 517 hospitalizations for HF were identified, which corresponded to 142 443 unique patients; of these, 156 707 visits (68.3%) were excluded, Figure 1 depicts the cohort flow chart. After exclusion rules were applied, a total of 72 810 individuals remained alive 6 months after their incident HF admission, of these, 10 973 (15.1%) had clustered visits. The mean age of the cohort was 75.68 years (±12.37). Compared to nonclustered patients, patients with clustered ER visits tended to be slightly younger and more likely to be male. Clustered patients had higher rates of both cardiac and noncardiac comorbidities, and particularly had a higher rate of coronary artery disease, diabetes mellitus, chronic renal failure, peripheral vascular disease, and major psychiatric disorders than nonclustered patients. Patients who clustered were also more likely to have a rural residence (20.5% vs 13.6%; P<0.001) and less likely to have a primary care provider than nonclustered HF patients (53.6% vs 59.8%; P<0.001). Patients with clustered HF visits were also more likely to present to smaller hospitals (8.9% vs 4.7%; P<0.001), with low volumes of HF visits (6.6% vs 3.4%; P<0.001). Table 1 describes the remaining baseline characteristics of the cohort. Patients with clustered ER visits had a total of 49 280 visits to the ER, recurrent visits were attributable to HF (16.29%), other cardiovascular reason (5.27%), or a noncardiovascular reason (78.45%). Of the patients who did not cluster, the distribution of number of ER visits was as follows: 0 visits, 34 209 (55.3%); 1 visit, 18 167 (29.4%); 2 visits, 9461 (15.3%).
Table 1.
Demographics Stratified by Cluster ER Visits Within 6 Months After Index Hospitalization Discharge
| Overall (n=72 810) | Cluster (n=10 973) | Noncluster (n=61 837) | P Value | |
|---|---|---|---|---|
| Patient | ||||
| Age, y | 75.68±12.37 | 75.05±12.36 | 75.79±12.37 | <0.001 |
| Male | 36 228 (49.8%) | 5668 (51.7%) | 30 560 (49.4%) | <0.001 |
| Hypertension | 62 113 (85.3%) | 9638 (87.8%) | 52 475 (84.9%) | <0.001 |
| Diabetes mellitus | 33 606 (46.2%) | 5607 (51.1%) | 27 999 (45.3%) | <0.001 |
| Coronary artery disease | 30 442 (41.8%) | 5250 (47.8%) | 25 192 (40.7%) | <0.001 |
| Chronic kidney disease | 15 852 (21.8%) | 2943 (26.8%) | 12 909 (20.9%) | <0.001 |
| COPD | 28 244 (38.8%) | 5108 (46.6%) | 23 136 (37.4%) | <0.001 |
| Peripheral vascular disease | 5165 (7.1%) | 1036 (9.4%) | 4129 (6.7%) | <0.001 |
| Cancer (any) | 7340 (10.1%) | 1300 (11.8%) | 6040 (9.8%) | <0.001 |
| Major psychiatric disorder | 5352 (7.4%) | 1087 (9.9%) | 4265 (6.9%) | <0.001 |
| Charlson comorbidity index | 2.08±1.86 | 2.47±2 | 2.01±1.82 | <0.001 |
| Charlson ≥2 | 41 031 (56.3%) | 7074 (64.5%) | 33 975 (54.9%) | |
| PCP | 42 850 (58.9%) | 5883 (53.6%) | 36 967 (59.8%) | <0.001 |
| Rostered | ||||
| Not rostered | 29 960 (41.1%) | 5090 (46.4%) | 24 870 (40.2%) | <0.001 |
| Rostered | 29 364 (40.3%) | 3706 (33.8%) | 25 658 (41.5%) | |
| Virtually rostered | 13 486 (18.5%) | 2177 (19.8%) | 11 309 (18.3%) | |
| Previous year visit | ||||
| GP | 67 669 (92.9%) | 10 360 (94.4%) | 57 309 (92.7%) | <0.001 |
| Cardiologist | 23 442 (32.2%) | 3979 (36.3%) | 19 463 (31.5%) | |
| Rural postal code | 10 668 (14.7%) | 2251 (20.5%) | 8417 (13.6%) | <0.001 |
| Hospital | ||||
| Hospital (type) | <0.001 | |||
| Community | 52 347 (71.9%) | 7426 (67.7%) | 44 921 (72.6%) | |
| Small | 3901 (5.4%) | 978 (8.9%) | 2923 (4.7%) | |
| Teaching | 16 122 (22.1%) | 2518 (22.9%) | 13 604 (22%) | |
| Hospital (HF volume) | ||||
| High | 55 990 (76.9%) | 7721 (70.4%) | 48 269 (78.1%) | <0.001 |
| Low | 2821 (3.9%) | 727 (6.6%) | 2094 (3.4%) | |
| Medium | 13 999 (19.2%) | 2525 (23.0%) | 11 474 (18.6%) | |
| Mortality since index hospitalization | ||||
| 180 d | ||||
| 0 | 10 973 (100%) | 61 837 (100%) | n/a | |
| 365 d | ||||
| 0 | 9080 (82.7%) | 56 231 (90.9%) | <0.001 | |
| 1 | 1893 (17.3%) | 5606 (9.1%) | ||
COPD indicates chronic obstructive pulmonary disease; ER, emergency room; GP, general practitioner; HF, heart failure; PCP, primary care provider (denotes enrollment with a primary care provider program).
Healthcare Utilization
Readmission rates within the 6‐month post–index visit between clustered (37.48%) and nonclustered groups (10.67%) were significantly different (P<0.0001). Over 90% of patients with HF in both the clustered and nonclustered groups visited with their primary care provider within a year before the index hospitalization (94.4% vs 92.7%; P<0.001). The median number of general practitioner visits within the year was slightly higher for clustered patients compared to nonclustered patients (9 vs 8; P<0.001); a greater proportion of clustered patients were not rostered to a specific family health organization (46.4% vs 40.2%; P<0.001). Additionally, a higher proportion of clustered patients saw a cardiologist within the year before the index hospitalization (36.3% vs 31.5%, P<0.001).
Clustered patients had higher use of HF therapies, including angiotensin‐converting enzyme inhibitors/angiotensin II receptor blockers (72.7% vs 68.5%; P<0.001), beta blockers (72.8% vs 67.1%, P<0.001), mineralocorticocoid receptor antagonists (10.2 vs 7.9%; P<0.001), as well as diuretics (72.8% vs 67.1%; P<0.001); see Table S1.
Overall, 4783 (43.6%) patients had non–HF‐related presentations to the ER in the 6 months after index presentation. Noncardiovascular visits accounted for more than two thirds of ER presentations when clustering was present during the study window.
Predictors of Clustering
Table 2 shows the multivariate analysis of patient and hospital predictors of clustered patients. Patient comorbidity, as expressed by the Charlson comorbidity index, was a significant predictor of clustering. Rural residence (odds ratio [OR], 1.277; 95% confidence interval [CI], 1.193–1.367; P<0.0001), presenting to a small hospital (OR, 1.266; 95% CI, 1.135–1.412; P<0.0001), and hospitals that see low volumes of HF patients (OR, 1.584; 95% CI, 1.392–1.803; P<0.0001) were system‐level factors that predicted clustering of patients with HF.
Table 2.
Clustered ER Visits by Multivariate Logistic Regression
| Variable | Value/Comparison | OR | CI | P Value |
|---|---|---|---|---|
| Age group, y | ||||
| <50 | ||||
| 50–64 | vs <50 | 0.774 | 0.686–0.872 | <0.0001 |
| 65–74 | vs <50 | 0.808 | 0.720–0.907 | 0.0003 |
| ≥75 | vs <50 | 0.734 | 0.657–0.820 | <0.0001 |
| Sex | M vs F | 1.028 | 0.986–1.072 | <0.1881 |
| Charlson index | 1 vs 0 | 1.166 | 1.089–1.247 | <0.0001 |
| 2 vs 0 | 1.337 | 1.253–1.428 | <0.0001 | |
| 3 vs 0 | 1.615 | 1.507–1.731 | <0.0001 | |
| 4 vs 0 | 1.796 | 1.660–1.943 | <0.0001 | |
| 5+ vs 0 | 2.122 | 1.971–2.285 | <0.0001 | |
| Rural postal code | Y vs N | 1.277 | 1.193–1.367 | <0.0001 |
| PCP | Y vs N | 0.796 | 0.764–0.831 | <0.0001 |
| Hospital type | Small vs community | 1.266 | 1.135–1.412 | <0.0001 |
| Teaching vs community | 1.215 | 1.153–1.279 | <0.0001 | |
| Hospital (HF volume) | Low vs high | 1.584 | 1.392–1.803 | <0.0001 |
| Medium vs high | 1.309 | 1.233–1.389 | <0.0001 | |
CI indicates confidence interval; ER, emergency room; HF, heart failure; OR, odds ratio; PCP, primary care provider.
Unadjusted and Adjusted Outcomes
In patients who survived 6 months past the index ER visit, mortality was significantly higher in those with clustering compared to no clustering (Figure 2). The unadjusted hazard of death was significantly higher for patients with clustered visits (hazard ratio [HR] 1.43; 1.4–1.47; P<0.0001). The age‐ and sex‐adjusted hazard of death for clustered patients compared to nonclustered was significantly higher (HR, 1.51; 95% CI, 1.47–1.55; P<0.0001). Table 3 shows the adjusted mortality using the Krumholz model. The mortality for clustered patients was significantly higher for clustered vs nonclustered patients with HF (HR, 1.39; 95% CI, 1.36–1.43; P<0.0001).
Figure 2.
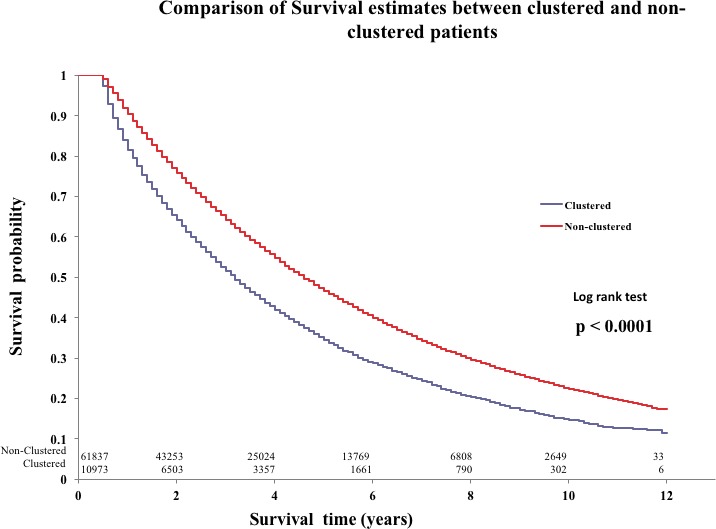
Comparison of survival estimates between clustered and nonclustered patients.
Table 3.
Survival Analysis for Time to Death With Age, Sex, Clustering Adjusted for Krumholz Model Covariates
| Variable | Value/Comparison | HR | CI | P Value |
|---|---|---|---|---|
| Cluster | 1 vs 0 | 1.39 | 1.36–1.43 | <0.0001 |
| Age group, y | ||||
| <50 | ||||
| 50–64 | vs <50 | 1.76 | 1.60–1. 92 | <0.0001 |
| 65–74 | vs <50 | 2.78 | 2.54–3.03 | <0.0001 |
| ≥75 | vs <50 | 5.23 | 4.79–5.70 | <0.0001 |
| Sex | M vs F | 1.14 | 1.12–1.16 | <0.0001 |
| Krumholz model covariates | ||||
| CABG | 0.65 | 0.61–0.69 | <0.0001 | |
| PTCA | 0.81 | 0.77–0.85 | <0.0001 | |
| Heart failure | 1.01 | 0.98–1.04 | 0.6598 | |
| MI | 1.13 | 1.10–1.16 | <0.0001 | |
| Unstable angina | 1.02 | 0.98–1.05 | 0.347 | |
| Atherosclerosis | 0.96 | 0.94–0.98 | 0.0003 | |
| Cardiopulmonary‐respiratory failure and shock | 0.99 | 0.95–1.03 | 0.589 | |
| Valvular heart disease | 1.08 | 1.05–1.11 | <0.0001 | |
| Hypertension | 1.02 | 0.99–1.05 | 0.1851 | |
| Stroke | 1.16 | 1.11–1.21 | <0.0001 | |
| Renal failure | 1.28 | 1.25–1.31 | <0.0001 | |
| COPD | 1.23 | 1.21–1.26 | <0.0001 | |
| Pneumonia | 1.18 | 1.15–1.20 | <0.0001 | |
| Diabetes mellitus | 1.10 | 1.08–1.13 | <0.0001 | |
| Protein‐calorie malnutrition | 1.19 | 1.10–1.27 | <0.0001 | |
| Dementia | 1.41 | 1.37–1.45 | <0.0001 | |
| Hemiplegia, paraplegia, paralysis, functional disability | 1.00 | 0.93–1.07 | 0.9688 | |
| PVD | 1.18 | 1.15–1.22 | <0.0001 | |
| Metastatic cancer | 1.91 | 1.81–2.02 | <0.0001 | |
| Trauma | 1.17 | 1.14–1.21 | <0.0001 | |
| Major psychiatric disorders | 1.08 | 1.04–1.12 | <0.0001 | |
| Liver disease | 1.20 | 1.11–1.29 | <0.0001 | |
CABG indicates coronary artery bypass graft; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; MI, myocardial infarction; PTCA, percutaneous transluminal coronary angioplasty; PVD, peripheral vascular disease.
Discussion
Patients with HF who frequently use acute care services are known to have a poor prognosis and have thus become a focus for quality improvement initiatives designed specifically to reduce HF readmissions.15 Real‐world patients with HF are afflicted by multiple comorbidities,16 including many noncardiac comorbidities that negatively impact outcomes. While it is well known that patients with HF presenting to the ER have high short‐term mortality after discharge,11 the outcomes of patients with HF with repeated ER visits within a short period are not known. In this large, population‐based study, we found patients with HF who presented to the ER more than 3 times in a 6‐month period had a higher burden of both cardiac and noncardiac comorbidities and had a significantly higher mortality rate than patients with nonclustered visits for HF, even when adjusting for patient comorbidities.
This study looked at a highly select subset of the population, those with a previous HF hospitalization in the preceding 5 years or rehospitalization within 6 months after index visit were intentionally excluded in order to avoid the confounding effect of recurrent hospitalizations on mortality risk.17 In our cohort, this group still represented ≈30% of hospitalizations.
Our results build on previous work, suggesting that patients with HF presenting to the ER are high risk.17, 18, 19 Prior work has shown that hospitalized patients with HF had high mortality and morbidity and consumed significantly more healthcare resources,20 with the main driver being repeated ER visits and hospitalizations. Despite the known risk in this cohort of patients, about one third of patients with HF are discharged from the hospital, with a wide degree of variation in the admission rate of patients with HF.8, 13 The impact of multiple ER visits and admissions is significant. Patients discharged from the ER who had multiple prior admissions for HF had an increased risk of mortality, which increased independently with each event.21 Our study adds to the literature, as we have identified a subset of patients who have multiple ER visits within a short period of time that have a significantly higher mortality rate. Clustering of ER visits may represent an additional risk factor for clinicians to be mindful of, when making management decisions in the ER, independent of the reason for the ER visit.
Several important considerations are worth mentioning. Not surprisingly, the clustered patients exhibited a significantly higher proportion of comorbidities than nonclustered patients, including noncardiac conditions and mental health disorders. In addition, most ER visits made by patients with HF resulted from noncardiac conditions, suggesting that the burden of comorbidities may have led to increased ER use, but also that outcomes were worse irrespective of the reason for the ER visit. Clearly, addressing HF alone is not sufficient to address this issue, and understanding the reasons underlying the phenomenon of recurrent ER visits is key in implementing interventions for this high‐risk population.
Moreover, clustered patients were more commonly seen at smaller and more rural hospitals. While one may conclude that higher ER use could be a manifestation of healthcare system failure that reflects poor access to primary care in these smaller communities, it is important to highlight that clustered patients were more often seen by a general practitioner and specialist in the year preceding their index event; furthermore, 94.6% of patients with clustered visits versus 92% of those without clustered visits had at least one visit to a general practitioner following index presentation. Alhough this is not proof of an appropriate primary care network, it does reflect the complexities around the care of patients with multiple ER visits where patient‐ and system‐related factors play a role in shaping outcomes for patients with HF and multiple comorbidities. Prior literature has also shown that rural hospitals are often used to accessing primary care for patients with coronary disease with no difference in outcomes, however, in this case, mortality was higher for patients with HF.22 Further research into the drivers of ER utilization for patients with HF in these communities is warranted to understand if repeated ER visits indicate a gap in quality of care.
Given the poor outcomes associated with repeat ER visits and readmissions for HF, better strategies of risk stratification in the ER have been developed.4, 23 One of these, the Emergency Heart Failure Mortality Risk Grade, combines multiple point‐of‐care clinical parameters to guide admission decisions in the ER. This risk score has now been turned into an online calculator to assist ER physicians in assessing risk for a single HF visit.12 From the clinical perspective, identification of clustering through history taking is a simple and reliable way to risk stratify patients with HF as potentially high risk. Identifying clustering could be a useful addition to current HF risk calculators by emergency physicians, and strategies to better assess and manage these complex patients in the community have the potential to significantly improve outcomes. At the systems level, identification of population at risk can lead to important redesign of healthcare systems directed at allocating resources where they are needed most.
Our study should be interpreted in the context of some notable limitations: First and foremost, the definition of “clustering” was based on clinical experience and prior published research where it was felt that this definition would capture patients at real risk and at the same time avoiding “statistical noise” if fewer number of visits had been chosen.9 As a result, this is a highly select population representing only 30% of all HF hospitalizations in our cohort and as such limits generalizability. Importantly, however, we were able to demonstrate an independent effect of clustering on mortality risk and a potential target for intervention.
Our database lacked granularity in important clinical variables such as vital sign variables, biomarkers, or ejection fraction, which are known to influence risk stratification. Although we examined the role of several patient characteristics, as well as socioeconomic and access‐related factors, other unmeasured variables may have influenced the results of our analysis. The drivers for increased ER visits may vary between jurisdictions and reflect local circumstances. The results and conclusions from this study are relevant to the Canadian context, where universal health care is the norm, but may not be applicable in other settings. On the other hand, the homogeneity of the population in a contemporary cohort of patients as well as robust statistical methods and adjustment for a well‐established risk adjustment model are important strengths in this study. The findings of this study add to the armamentarium of risk prediction tools in HF and enable further research in this field.
In conclusion, clustering, a novel concept described here as patients who present 3 or more times to the ER within 6 months of an index heart failure hospitalization, was associated with a higher hazard of death. Future research into the strategies to better manage patients with complex HF with recurrent ER visits is warranted.
Sources of Funding
The project was funded through an Innovation in Quality Award from the University of Toronto Division of Cardiology. Dr Lee is supported by a midcareer investigator award from the Heart and Stroke Foundation and the Ted Rogers Chair in Heart Function Outcomes. The Institute for Clinical Evaluative Sciences is supported in part by a grant from the Ontario Ministry of Health and Long Term Care. The opinions, results, and conclusions are those of the authors and no endorsement by the Ministry of Health and Long‐Term Care or by the Institute for Clinical Evaluative Sciences is intended or should be inferred. Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information. However, the analyses, conclusions, opinions, and statements expressed herein are those of the author, and not necessarily those of the Canadian Institute for Health Information.
Disclosures
None.
Supporting information
Table S1. Medications for Individuals >65years old (Within 6 Months of Index Hospitalization Discharge)
Figure S1. Cohort time frame definitions.
(J Am Heart Assoc. 2018;7:e007569 DOI: 10.1161/JAHA.117.007569.)
References
- 1. Lloyd‐Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel‐Smoller S, Wong N, Wylie‐Rosett J, Hong Y. Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. [DOI] [PubMed] [Google Scholar]
- 2. Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–115. [DOI] [PubMed] [Google Scholar]
- 3. Ryden‐Bergsten T, Andersson F. The health care costs of heart failure in Sweden. J Intern Med. 1999;246:275–284. [DOI] [PubMed] [Google Scholar]
- 4. Lee DS, Stitt A, Austin PC, Stukel TA, Schull MJ, Chong A, Newton GE, Lee JS, Tu JV. Prediction of heart failure mortality in emergent care: a cohort study. Ann Intern Med. 2012;156:767–775. [DOI] [PubMed] [Google Scholar]
- 5. Wijeysundera HC, Trubiani G, Wang X, Mitsakakis N, Austin PC, Ko DT, Lee DS, Tu JV, Krahn M. A population‐based study to evaluate the effectiveness of multidisciplinary heart failure clinics and identify important service components. Circ Heart Fail. 2013;6:68–75. [DOI] [PubMed] [Google Scholar]
- 6. Hugli O, Braun JE, Kim S, Pelletier AJ, Camargo CA Jr. United States emergency department visits for acute decompensated heart failure, 1992 to 2001. Am J Cardiol. 2005;96:1537–1542. [DOI] [PubMed] [Google Scholar]
- 7. Weintraub NL, Collins SP, Pang PS, Levy PD, Anderson AS, Arslanian‐Engoren C, Gibler WB, McCord JK, Parshall MB, Francis GS, Gheorghiade M; on behalf of the American Heart Association Council on Clinical Cardiology and Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation . Acute heart failure syndromes: emergency department presentation, treatment and disposition: current approaches and future aims: a scientific statement from the American Heart Association. Circulation. 2010;122:1975–1996. [DOI] [PubMed] [Google Scholar]
- 8. Ezekowitz JA, Bakal JA, Kaul P, Westerhout CM, Armstrong PW. Acute heart failure in the emergency department: short and long‐term outcomes of elderly patients with heart failure. Eur J Heart Fail. 2008;10:308–314. [DOI] [PubMed] [Google Scholar]
- 9. Hasegawa K, Tsugawa Y, Camargo CA, Brown DFM. Frequent utilization of the emergency department for acute heart failure syndrome: a population‐based study. Circ Cardiovasc Qual Outcomes. 2014;7:735–742. [DOI] [PubMed] [Google Scholar]
- 10. Obi EN, Swindle JP, Turner SJ, Russo PA, Altan A. Health care costs among patients with heart failure: a comparison of costs between matched decedent and survivor cohorts. Adv Ther. 2017;34:261–276. [DOI] [PubMed] [Google Scholar]
- 11. Lee DS, Schull MJ, Alter DA, Austin PC, Laupacis A, Chong A, Tu JV, Stukel TA. Early deaths in patients with heart failure discharged from the emergency department: a population‐based analysis. Circ Heart Fail. 2010;3:228–235. [DOI] [PubMed] [Google Scholar]
- 12. Web calculator. Available at: http://www.ccort.ca. Accessed October 2017.
- 13. Bhatia RS, Austin PC, Stukel TA, Schull MJ, Chong A, Tu JV, Lee DS. Outcomes in patients with heart failure treated in hospitals with varying admission rates: population‐based cohort study. BMJ Qual Saf. 2014;23:981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krumholz HM, Wang Y, Mattera JA, Wang Y, Han LF, Ingber MJ, Roman S, Normand SLT. An administrative claims model suitable for profiling hospital performance based on 30‐day mortality rates among patients with heart failure. Circulation. 2006;113:1693–1701. [DOI] [PubMed] [Google Scholar]
- 15. Desai AS, Stevenson LW. Re‐hospitalization for heart failure: predict or prevent? Circulation. 2012;126:501–506. [DOI] [PubMed] [Google Scholar]
- 16. Angermann CE. Comorbidities in heart failure: a key issue. Eur J Heart Fail. 2009;8(suppl):i5–i10. [Google Scholar]
- 17. Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154:260–266. [DOI] [PubMed] [Google Scholar]
- 18. O'Connor CM, Abraham WT, Albert NM, Clare R, Gattis Stough W, Gheorghiade M, Greenberg BH, Yancy CW, Young JB, Fonarow GC. Predictors of mortality after discharge in patients hospitalized with heart failure: an analysis from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF). Am Heart J. 2008;156:662–673. [DOI] [PubMed] [Google Scholar]
- 19. Lee DS, Gona P, Albano I, Larson MG, Benjamin EJ, Levy D, Kannel WB, Vasan RS. A systematic assessment of causes of death after heart failure onset in the community: impact of age at death, time period, and left ventricular systolic dysfunction. Circ Heart Fail. 2011;4:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bharmal M, Gemmen E, Zyczynski T, Linnstaedt A, Kenny D, Marelli C. Resource utilization, charges and mortality following hospital inpatient admission for congestive heart failure among the elderly in the US. J Med Econ. 2008;11:397–414. [DOI] [PubMed] [Google Scholar]
- 21. Lee DS, Austin PC, Stukel TA, Alter DA, Chong A, Parker JD, Tu JV. “Dose‐dependent” impact of recurrent cardiac events on mortality in patients with heart failure. Am J Med. 2009;122:162–169. [DOI] [PubMed] [Google Scholar]
- 22. Tran C, Wijeysundera HC, Qui F, Tu JV, Bhatia S. Comparing the ambulatory care and outcomes for rural and urban patients with chronic ischemic heart disease: a population‐based cohort study. Circ Cardiovasc Qual Outcomes. 2014;7:835–843. [DOI] [PubMed] [Google Scholar]
- 23. Hsiao J, Motta M, Wyer P. Validating the acute heart failure index for patients presenting to the emergency department with decompensated heart failure. Emerg Med J. 2012;29:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Medications for Individuals >65years old (Within 6 Months of Index Hospitalization Discharge)
Figure S1. Cohort time frame definitions.


