Abstract
Background
Medication adherence improves outcomes for patients with heart failure, but adherence rates remain low. We examined the association between earlier postdischarge follow‐up and medication adherence.
Methods and Results
We performed a retrospective cohort study of patients ≥65 years who were hospitalized for heart failure, covered by Medicare Part D, and discharged alive from April 2006 to October 2012 using the Get With The Guidelines‐Heart Failure Registry linked to Medicare claims. Patients were categorized into 4 groups by timing of first postdischarge follow‐up visit: ≤1, 1 to 2, 2 to 6, and >6 weeks. Medication adherence was defined by proportion of days covered of >80% at 90 days and 1‐year posthospital discharge to 5 guideline‐directed medical therapies (angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker, evidence‐based β‐blocker, aldosterone antagonist, hydralazine/isosorbide dinitrate, and anticoagulation for atrial fibrillation). Among 9878 patients with heart failure, 73% had left ventricular ejection fraction ≤40%, median age was 78 years (25th–75th percentile, 71–84), and 48% were male. Overall, 30% had a follow‐up appointment within 1‐week postdischarge and 25% >6 weeks. At 1 year, medication adherence was 53% for evidence‐based β‐blockers, 48% for angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, and 8% for hydralazine/isosorbide dinitrate. We found no significant association between timing of first follow‐up visit and medication adherence at 1 year (1.04, 0.92–1.17) when comparing follow‐up visits >6 weeks to the earliest ones.
Conclusions
Posthospital heart failure discharge, overall adherence to medical therapies in Medicare beneficiaries was low. Early follow‐up was not associated with increased medication adherence to guideline‐directed medical therapy in the short or long term.
Keywords: heart failure, medication adherence, postdischarge follow‐up
Subject Categories: Quality and Outcomes, Heart Failure
Clinical Perspective
What Is New?
Overall adherence to guideline‐directed medical therapies in a population of patients with heart failure who are Medicare beneficiaries is low.
Early follow‐up after a heart failure hospitalization is not associated with increased medication adherence.
What Are the Clinical Implications?
Previous studies may have been overly optimistic about the benefits of early follow‐up in and of itself, and the marginal benefits may not translate to all aspects of care, such as medication adherence.
There remains a limited understanding to delineate specific factors associated with increased medication adherence that can guide development of successful, implementable interventions.
Despite advances in knowledge of the pathophysiology of heart failure (HF) and an expanding array of evidence‐based, guideline‐directed treatment options, once patients develop HF, their rates of hospitalization and mortality have remained relatively unchanged.1 One potential contributing factor to the persistence of poor outcomes is challenges with medication adherence, which is a critical self‐care behavior for patients with HF. Previous data suggest that nonadherence to HF medications is associated with an increase in all‐cause mortality and cardiovascular hospitalizations.2 Medication adherence is especially important during the transition of care from hospital to home, given the high number of medication changes that occur during this vulnerable period.3 Current guidelines recommend that adherence be assessed before hospital discharge, at the first postdischarge visit, and in subsequent follow‐up visits.4
A key aspect of HF transitional care is early postdischarge follow‐up,5 though its impact on HF medication adherence has not been studied. In patients with a recent myocardial infarction, delayed outpatient follow‐up of more than 6 weeks postdischarge was found to be associated with worse short‐ and long‐term medication adherence; however, to the best of our knowledge, there are no data addressing this topic in patients with chronic conditions, such as HF.6 Our current study aimed to assess the potential relationship between timing of postdischarge follow‐up and medication adherence. We hypothesized that earlier posthospital discharge follow‐up was associated with increased rates of medication adherence.
Methods
Inpatient Data
The data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. The American Heart Association's Get With The Guidelines (GWTG) program is a quality improvement initiative that strives to improve the in‐hospital care of patients with heart disease through increased adherence to guidelines.7 Details regarding the GWTG program for coronary artery disease, stroke, and HF have been previously described.7, 8 GWTG‐HF is a HF disease‐specific registry that started in 2005 and consists of clinical data abstracted from hospitalized patients with a primary discharge diagnosis of HF. Participating hospitals submit data on eligible patients in compliance with the Joint Commission and Centers for Medicare & Medicaid Service standards through the use of an internet‐based patient management tool (Quintiles Real‐World & Late Research, Cambridge, MA).8, 9 Variables collected include demographic information, clinical characteristics, medical history, medications, in‐hospital treatments, in‐hospital outcomes, and discharge information including medications prescribed at discharge.10, 11
As part of the GWTG‐HF program, all participating institutions were required to comply with local regulatory and privacy guidelines and, if required, to obtain institutional review board approval. Since data were used primarily at the local site for quality improvement, sites were granted a waiver of informed consent under the common rule. Quintiles (Cambridge, MA) served as the data collection coordination center for the American Heart Association/American Stroke Association's GWTG program. The Duke Clinical Research Institute (Durham, NC) served as the data analysis center. The Duke University Medical Center institutional review board approved this study.
Outpatient Medication Data
We also used Medicare data including inpatient claim files, corresponding denominator files, carrier claims data, and Part D prescription drug data. Inpatient files contained admission and discharge dates, International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnosis and procedure codes, and beneficiary demographic information. Denominator files included encrypted identifiers, dates of birth, dates of death, and information regarding program eligibility and enrollment. Carrier claims data were used to identify first postdischarge outpatient visit. We assessed levels of adherence to HF medications in patients age 65 years and older by using Centers for Medicare & Medicaid Service Medicare Part D prescription fill data, which included the name of the drug, dosage, date dispensed, and number of days supplied. In order to identify GWTG‐HF Registry patients in Centers for Medicare & Medicaid Service Medicare Part D claims data, we used a combination of indirect identifiers to link the 2 data sources, as previously described.12
Study Population
From the linked data set, we included patients who were discharged alive from a HF hospitalization between April 1, 2006 and October 1, 2012 who were on at least 1 evidence‐based HF medication. In order to accurately determine the starting supply of medication upon discharge, we only included patients enrolled in Part D Medicare coverage at least 90 days before the date of discharge. We excluded patients who died during the hospitalization, who left against medical advice, or who were transferred to a different facility such as skilled nursing facility or hospice, since we did not have access to prescription records from those sites. We also excluded patients who died or lost Medicare coverage within 90 days of discharge and patients who had a follow‐up appointment on the same day as discharge similar to previous analyses.6 For patients with multiple eligible hospital admissions during the study period, only the first hospitalization was included in the analysis.
Data Definitions
The first outpatient clinic visit was defined as the first postdischarge appointment after index HF hospitalization with a cardiologist, a primary care physician, internist, or advanced practice provider in a primary care setting as determined by Medicare carrier claims data. Medication adherence was determined through the use of Medicare Part D prescription drug claims data to calculate the proportion of days covered (PDC). Consistent with previous studies, a PDC >80% was considered adherent.13 Adherence was assessed at 90 days and at 1‐year postindex discharge for patients who were alive and enrolled in Medicare Part D at that time. We assessed medication adherence to guideline‐directed medical therapy for HF patients, which included angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker for patients with HF with reduced ejection fraction (HFrEF); evidence‐based β‐blockers for patients with HFrEF; aldosterone receptor antagonists for patients with HFrEF; hydralazine/isosorbide dinitrate for black patients with HFrEF; and anticoagulants such as warfarin, dabigatran, apixaban, and rivaroxaban in patients with atrial fibrillation. All patients had an indication and no contraindication for these therapies, per the GWTG‐HF Registry. For hydralazine/isosorbide dinitrate, we considered patients adherent only if they were taking both medications concurrently. The fixed‐dose combination form of the medication was split into its components, which were then treated as individual medications for the purposes of calculating PDC.
Statistical Analysis
Patients were divided into 4 groups based on the timing of outpatient postdischarge follow‐up appointment: ≤1 week, 1 to 2 weeks (8–14 days), 2 to 6 weeks (15–42 days), and >6 weeks (>42 days), which was similar to a prior analysis.6 The 4 different timing groups were treated as ordinal and categorical. Patient demographic characteristics, medical history, admission data, admission and discharge medications, and hospital characteristics were described and compared for all HF patients by timing of postdischarge appointment. Proportions were reported for categorical variables, and median and interquartile ranges were reported for continuous variables. The Cochran‐Mantel‐Haenszel χ2 test on rank‐based group mean scores was used to compare binary or nominal categorical variables; the χ2 test on 1‐df rank correlation was used to compare continuous variables or ordinal categorical variables. Variable missing rates are reported for reference in Table S1.
We reported the proportion of patients who were adherent to each HF medication at 90 days and 1 year by groups based on the timing of postdischarge follow‐up visit. We also stratified this analysis by first visit with a cardiologist or noncardiologist. We then compared the medication adherence of each follow‐up group referenced to the earliest follow‐up group of ≤1 week. By treating each individual medication as an opportunity for adherence, we used mixed‐effects logistic regression models for composite adherence to assess the adjusted association. As a result, each patient could be included in the model up to 5 times depending on the number of medications prescribed at discharge. Within‐hospital clustering was accounted for using hospital random intercepts, and correlation between repeated opportunities on the same patients was modeled assuming a compound symmetric residual covariance structure. Covariates utilized for adjustment included age, sex, race, socioeconomic status (household income, house value, high school degree, college degree), medical history (anemia, ischemic cause, cerebrovascular accident/transient ischemic attack, diabetes mellitus [insulin‐ and non–insulin treated], hyperlipidemia, hypertension, chronic obstructive pulmonary disease or asthma, peripheral vascular disease, renal insufficiency, smoking, implantable cardioverter defibrillator, cardiac resynchronization therapy pacemaker, cardiac resynchronization therapy defibrillator), examination findings and laboratory values (heart rate, systolic blood pressure, sodium, blood urea nitrogen, ejection fraction groups), discharge year (in 0.25 increment for quarter), length of stay from index hospitalization, whether the patient was transferred‐in for index hospitalization, number of medication classes indicated at index discharge, and hospital characteristics (geographic region, teaching status, number of beds, rural location, heart transplant site). The analysis was repeated for each individual HF medication.
Medication adherence was also analyzed in subgroups of age (≥75 years versus <75 years), sex (male versus female), race (white versus nonwhite), and by number of new medications initiated at discharge (0, 1, 2, ≥3). A separate analysis was performed in HFrEF patients, defined as left ventricular ejection fraction ≤40% or a qualitative ejection fraction description of “moderate/severe dysfunction.” All tests were 2‐sided, and a P value <0.05 was considered statistically significant. All analyses were performed with SAS software, version 9.4 (SAS Institute, Inc, Cary, NC).
Results
The final study population consisted of 9878 HF patients from 300 hospitals for the 90‐day follow‐up cohort. After excluding patients who died or lost Part D eligibility within 1 year postdischarge, the 1‐year follow‐up cohort consisted of 6615 patients with HF from 265 hospitals (Figure). Of the 9878 total patients with HF in the analysis, 73% were categorized as patients with HFrEF. The median age was 78 years (25th–75th percentile, 71–84 years), 48% of the population was male, and 75% were white. Overall, 30% of the population had a follow‐up appointment within the first week after discharge, 21% had their first follow‐up visit 1 to 2 weeks postdischarge, 24% had their visit 2 to 6 weeks postdischarge, and 25% did not follow up with a provider until >6 weeks post–hospital discharge. Among the 9878 patients in the study population, 40% had their first follow‐up appointment with a cardiologist.
Figure 1.
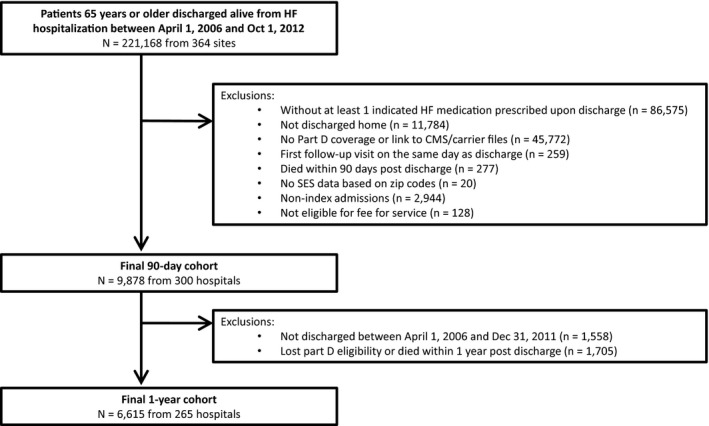
Derivation of the 90‐day and 1‐year study populations. This figure displays the derivation of our 90‐day and 1‐year study populations from all patients 65 years or older who were discharged alive from hospitalization for heart failure between April 1, 2006 and October 1, 2012. CMS indicates Center for Medicare and Medicaid Services; HF, heart failure; SES, socioeconomic status.
Table 1 shows the baseline and hospital characteristics of the population of HF patients analyzed stratified by timing of first follow‐up appointment. Patients who had their first postdischarge follow‐up appointment at a later time were more likely to be younger, black, have a lower median home value, and be patients with HFrEF compared with patients who had an early follow‐up (all P<0.001). Those with a later first follow‐up visit were also more likely to have a medical history of dialysis and smoking and less likely to have a history of atrial fibrillation when compared with patients who had an earlier follow‐up (all P<0.001). They were also more likely to be discharged from a teaching hospital or a hospital with a greater number of beds when compared with those who had their first follow‐up appointment at an earlier time (all P<0.001).
Table 1.
Baseline Patient and Hospital Characteristics in Patients With HF by Timing of First Follow‐Up
| Variable | Overall | ≤1 Wk | 1 to 2 Wks | 2 to 6 Wks | >6 Wks | P Value |
|---|---|---|---|---|---|---|
| N=9878 | N=2943 | N=2084 | N=2383 | N=2468 | ||
| Demographics | ||||||
| Age, ya | 78 (71–84) | 79 (72–84) | 79 (72–84) | 77 (71–84) | 76 (70–83) | <0.0001 |
| Female | 51.90 | 50.63 | 53.74 | 53.34 | 50.49 | 0.9556 |
| Race | <0.0001 | |||||
| White | 75.18 | 77.81 | 77.55 | 74.81 | 70.44 | |
| Black | 13.48 | 10.73 | 11.72 | 13.80 | 17.88 | |
| Hispanic (any race) | 6.98 | 6.76 | 6.41 | 7.28 | 7.43 | |
| Asian | 1.63 | 2.44 | 1.59 | 1.29 | 1.03 | |
| Other | 2.74 | 2.26 | 2.73 | 2.83 | 3.22 | |
| Household income, ×$1000a | 51 (45–60) | 51 (45–60) | 51 (45–60) | 51 (44–59) | 51 (45–60) | 0.0024 |
| Home value, ×$1000a | 164 (123–254) | 174 (127–276) | 166 (123–254) | 157 (120–238) | 162 (118–250) | <0.0001 |
| High school degreea | 86 (82–90) | 86 (82–90) | 87 (83–90) | 86 (82–90) | 85 (82–89) | <0.0001 |
| College degreea | 26 (19–31) | 26 (20–31) | 27 (19–31) | 26 (18–31) | 25 (18–29) | <0.0001 |
| Medical history | ||||||
| Atrial flutter/fibrillation | 51.98 | 58.08 | 54.43 | 50.00 | 44.43 | <0.0001 |
| COPD or asthma | 27.92 | 25.77 | 28.15 | 29.11 | 29.19 | 0.0035 |
| Diabetes mellitus | 40.14 | 39.47 | 39.17 | 40.94 | 41.02 | 0.1620 |
| Hyperlipidemia | 52.10 | 53.49 | 52.67 | 51.38 | 50.62 | 0.0276 |
| Hypertension | 76.43 | 75.98 | 76.69 | 76.79 | 76.41 | 0.6730 |
| Peripheral vascular disease | 12.60 | 12.53 | 12.44 | 12.77 | 12.66 | 0.8225 |
| Prior MI | 24.37 | 22.06 | 24.02 | 24.96 | 26.90 | <0.0001 |
| CVA/TIA | 15.03 | 14.13 | 15.26 | 14.69 | 16.27 | 0.0582 |
| ICD only | 12.98 | 12.95 | 12.13 | 13.44 | 13.30 | 0.5089 |
| Heart failure (prior diagnosis) | 68.09 | 66.83 | 67.12 | 69.51 | 69.09 | 0.0312 |
| Anemia | 15.12 | 15.48 | 15.51 | 14.82 | 14.64 | 0.3324 |
| Pacemaker | 19.16 | 19.22 | 20.04 | 18.57 | 18.90 | 0.5577 |
| Dialysis, chronic | 2.87 | 1.64 | 1.66 | 3.97 | 4.35 | <0.0001 |
| Renal insufficiency, chronic | 17.73 | 18.01 | 17.72 | 18.12 | 17.00 | 0.4444 |
| Depression | 8.33 | 8.58 | 7.40 | 8.93 | 8.27 | 0.9457 |
| CRT‐P (pacing only) | 1.05 | 1.14 | 1.06 | 1.16 | 0.82 | 0.3467 |
| CRT‐D (with ICD) | 4.84 | 5.73 | 5.14 | 6.07 | 2.32 | <0.0001 |
| Ischemic causeb | 62.98 | 62.49 | 62.69 | 63.75 | 63.07 | 0.5209 |
| Smoking | 10.79 | 8.69 | 9.03 | 11.87 | 13.73 | <0.0001 |
| Vitals on admission | ||||||
| Heart rate, bpma | 80 (70–95) | 80 (70–95) | 81 (70–96) | 80 (70–95) | 80 (70–95) | 0.5991 |
| SBP, mm Hga | 137 (120–156) | 136 (120–156) | 137 (120–156) | 138 (120–156) | 138 (121–156) | 0.0820 |
| BMIa | 26.8 (23.1–31.4) | 26.8 (23.1–31.3) | 26.9 (23.2–31.4) | 26.9 (23.2–31.2) | 26.7 (22.8–31.5) | 0.3732 |
| LVEF groups | <0.0001 | |||||
| HFrEF, reduced | 72.75 | 68.07 | 69.82 | 74.78 | 78.86 | |
| Lab measures | ||||||
| Serum sodium, mEq/La | 138 (136–141) | 138 (135–140) | 138 (136–141) | 138 (136–141) | 138 (136–141) | 0.0012 |
| Serum creatinine, mg/dLa | 1.3 (1–1.7) | 1.3 (1–1.7) | 1.2 (1–1.6) | 1.3 (1–1.7) | 1.3 (1–1.7) | 0.0954 |
| BUN, mg/dLa | 24 (17–34) | 24 (18–34) | 24 (17–34) | 24 (17–34) | 23 (17–33) | 0.0864 |
| LOS, da | 4 (2–6) | 4 (2–6) | 4 (2–6) | 4 (2–6) | 4 (2–5) | 0.0242 |
| Transferred‐in | 6.21 | 5.41 | 5.83 | 6.87 | 6.81 | 0.0160 |
| Hospital characteristics | ||||||
| Number of bedsa | 400 (258–556) | 372 (230–555) | 383 (240–555) | 404 (266–559) | 425 (283–556) | <0.0001 |
| Geographic region | <0.0001 | |||||
| West | 10.01 | 11.89 | 9.45 | 8.27 | 9.93 | |
| South | 33.32 | 29.49 | 31.43 | 36.51 | 36.39 | |
| Midwest | 22.81 | 22.09 | 23.51 | 23.58 | 22.33 | |
| Northeast | 33.86 | 36.53 | 35.60 | 31.64 | 31.36 | |
| Rural location | 5.52 | 5.64 | 6.67 | 5.08 | 4.82 | 0.0721 |
| Teaching status | 61.28 | 58.68 | 57.63 | 61.72 | 67.03 | <0.0001 |
| Heart transplant hospital | 11.72 | 11.23 | 11.36 | 12.86 | 11.51 | 0.4423 |
BMI indicates body mass index; bpm, beats per minute; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; CRT‐D, cardiac resynchronization therapy defibrillator; CRT‐P, cardiac resynchronization therapy pacemaker; CVA, cerebrovascular accident; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; ICD, implantable cardioverter defibrillator; LOS, length of stay; LVEF, left ventricular ejection fraction; MI, myocardial infarction; SBP, systolic blood pressure; TIA, transient ischemic attack.
Continuous variables presented as median (interquartile range). All other variables are categorical and presented as proportions.
Ischemic cause includes medical history of coronary artery disease, prior myocardial infarction or prior revascularization (percutaneous coronary intervention or bypass surgery).
Table 2 shows use of HF medications at discharge. Overall rates of prescription of aldosterone antagonists and hydralazine/isosorbide dinitrate in indicated patients were low, with 27% of indicated patients receiving prescribed aldosterone antagonists at discharge and 25% of indicated patients for hydralazine/isosorbide dinitrate. We observed that rates of medication adherence were low overall. At 90 days and 1 year, the overall rates of adherence as defined by PDC >80% were as follows: 58% and 52%, respectively, to evidence‐based β‐blockers, 55% and 48% to angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, 48% and 36% to aldosterone antagonists, 8.9% and 7.8% to hydralazine/isosorbide dinitrate, and 46% and 40% to anticoagulants. Table 3 shows the medication adherence rates in patients with HF by timing of the first follow‐up appointment. We did not detect a difference in adherence rates between patients in the different follow‐up categories at 90 days. At 1 year, the adjusted composite medication adherence rates indicated that adherence was higher in patients who had a 1‐ to 2‐week follow‐up appointment compared with those who visited their provider within the first week (odds ratio [OR] 1.18, P=0.01). Lack of a clear association between adherence rates and patients with different timing of postdischarge follow‐up appointments persisted when analysis was performed with patients stratified by first visit to a cardiologist or noncardiologist.
Table 2.
Discharge Medications in Patients With HF by Timing of First Follow‐Up
| Medications | Overall | ≤1 Wk | 1 to 2 Wks | 2 to 6 Wks | >6 Wks | P Value |
|---|---|---|---|---|---|---|
| Before admission (among indicated patients at discharge according to HF measures) | ||||||
| β‐Blockers | 2973 (63.1) | 798 (62.7) | 570 (62.0) | 713 (61.3) | 892 (65.8) | 0.1133 |
| ACEi/ARB | 2755 (49.4) | 730 (47.5) | 555 (50.8) | 649 (46.8) | 821 (52.8) | 0.0181 |
| Aldosterone antagonist | 652 (14.0) | 163 (13.0) | 134 (14.4) | 172 (15.2) | 183 (13.6) | 0.5999 |
| Hydralazine nitrate | 198 (19.2) | 55 (23.7) | 25 (14.3) | 54 (20.0) | 64 (18.1) | 0.3052 |
| Anticoagulation therapy | 2892 (69.2) | 923 (69.1) | 637 (70.7) | 669 (69.4) | 663 (67.7) | 0.4741 |
| Prescribed at discharge (among indicated patients at discharge according to HF measures) | ||||||
| Evidence‐based specific β‐blockers | 5065 (84.0) | 1435 (84.9) | 1014 (83.7) | 1266 (84.5) | 1350 (82.7) | 0.1262 |
| ACEi/ARB at discharge | 5273 (94.6) | 1450 (94.3) | 1035 (94.7) | 1313 (94.7) | 1475 (94.9) | 0.4531 |
| Aldosterone antagonist at discharge | 1664 (27.1) | 475 (28.0) | 380 (30.7) | 424 (28.1) | 385 (22.6) | 0.0002 |
| Hydralazine nitrate at discharge | 259 (25.1) | 63 (27.2) | 49 (28.0) | 61 (22.6) | 86 (24.4) | 0.3205 |
| Anticoagulation for afib/flutter | 4600 (88.3) | 1593 (91.3) | 1031 (90.0) | 1037 (87.1) | 939 (83.0) | <0.0001 |
| Prescribed as a new medication at dischargea (among indicated patients who were not on that medication before admission) | ||||||
| Evidence‐based specific β‐blockers | 2570 (84.0) | 752 (84.3) | 529 (82.4) | 673 (85.7) | 616 (83.1) | 0.9189 |
| ACEi/ARB at discharge | 2573 (91.3) | 736 (91.1) | 489 (90.9) | 678 (91.9) | 670 (91.4) | 0.6993 |
| Aldosterone antagonist at discharge | 1100 (20.0) | 333 (21.7) | 261 (23.6) | 270 (20.2) | 236 (15.5) | <0.0001 |
| Hydralazine nitrate at discharge | 141 (16.9) | 33 (18.6) | 32 (21.3) | 29 (13.4) | 47 (16.3) | 0.2726 |
| Anticoagulation for afib/flutter | 1760 (75.9) | 682 (83.0) | 404 (79.5) | 384 (73.7) | 290 (61.8) | <0.0001 |
Reported as number of patients (%). ACEi indicates angiotensin‐converting enzyme inhibitor; Afib, atrial fibrillation; afib/flutter, atrial fibrillation/atrial flutter; ARB, angiotensin receptor blocker; HF, heart failure.
New medication at discharge: a medication prescribed to an indicated patient at discharge, where the patient was not on that medication before admission.
Table 3.
Medication Adherence in Patients With HF by Timing of First Follow‐Up
| Adherence, n (%) | PDC >80% | Composite Medication Adherence | |||||
|---|---|---|---|---|---|---|---|
| Evidence‐Based β‐Blocker | ACEi/ARB | Aldosterone Antagonist | Hydralazine Nitrate | Anticoagulants | Adjusted ORa (95% CI) | P Value | |
| At 90 d | 5065 | 5273 | 1664 | 259 | 4600 | ||
| ≤1 wk | 836 (58.3) | 781 (53.9) | 240 (50.5) | 8 (12.7) | 702 (44.1) | Reference | |
| 1–2 wks | 586 (57.8) | 586 (56.6) | 187 (49.2) | 6 (12.2) | 494 (47.9) | 1.08 (0.98–1.19) | 0.1419 |
| 2–6 wks | 734 (58.0) | 723 (55.1) | 196 (46.2) | 2 (3.3) | 503 (48.5) | 1.06 (0.97–1.17) | 0.2111 |
| >6 wks | 754 (55.9) | 789 (53.5) | 182 (47.3) | 7 (8.1) | 412 (43.9) | 0.97 (0.88–1.07) | 0.5346 |
| P value | 0.2318 | 0.6863 | 0.2265 | 0.2211 | 0.5188 | ||
| At 1 y | 3379 | 3651 | 1083 | 179 | 3018 | ||
| ≤1 wk | 467 (52.9) | 440 (47.6) | 114 (39.0) | 4 (9.5) | 363 (36.6) | Reference | |
| 1–2 wks | 345 (54.4) | 339 (50.3) | 95 (41.3) | 3 (9.1) | 284 (43.7) | 1.18 (1.04–1.34) | 0.0102 |
| 2–6 wks | 423 (52.3) | 435 (49.8) | 82 (31.9) | 1 (2.5) | 271 (42.4) | 1.11 (0.98–1.25) | 0.0976 |
| >6 wks | 548 (52.0) | 543 (46.1) | 99 (32.6) | 6 (9.4) | 285 (38.7) | 1.04 (0.92–1.17) | 0.5165 |
| P value | 0.5217 | 0.3658 | 0.0301 | 0.9265 | 0.2447 | ||
ORs for each of the follow‐up groups were calculated using the adherence at ≤1 wk as a reference. OR >1 indicates better adherence when compared with the ≤1‐week follow‐up group and OR <1 indicate worse adherence when compared with the ≤1‐week follow‐up group. ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CI, confidence interval; HF, heart failure; OR, odds ratio; PDC, proportion of days covered.
The adjusted ORs were calculated by considering adherence to all medications. Covariates utilized for adjustment included age, sex, race, socioeconomic status (household income, house value, high school degree, college degree), medical history (anemia, ischemic cause, cerebrovascular accident/transient ischemic attack, diabetes mellitus [insulin and noninsulin treated], hyperlipidemia, hypertension, chronic obstructive pulmonary disease or asthma, peripheral vascular disease, renal insufficiency, smoking, implantable cardioverter defibrillator, cardiac resynchronization therapy pacemaker, cardiac resynchronization therapy defibrillator), examination findings and laboratory values (heart rate, systolic blood pressure, sodium, blood urea nitrogen, ejection fraction groups), discharge year (in 0.25 increment for quarter), length of stay from index hospitalization, whether the patient was transferred‐in for index hospitalization, number of medication classes indicated at index discharge, and hospital characteristics (geographic region, teaching status, number of beds, rural location, heart transplant site).
We report the adherence to individual medication groups in Table 4. We observed no significant association between timing of follow‐up and individual medication adherence in evidence‐based β‐blockers, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, aldosterone antagonist, and hydralazine/isosorbide dinitrate medication groups. In patients taking anticoagulants, the adjusted results at 90 days and 1 year demonstrate that patients who had a follow‐up visit between 1 and 6 weeks had better medication adherence than in patients who followed up within 1 week (at 90 days, OR 1.18, P=0.05 for 1–2‐week follow‐up, OR 1.24, P=0.01 for 2–6‐week follow‐up; at 1 year, OR 1.41, P=0.001 for 1–2‐week follow‐up, and OR 1.33, P=0.001 for 2–6‐week follow‐up).
Table 4.
Medication Adherence by Individual Medication and Timing of Postdischarge Follow‐Up
| Medications | Timing of First Follow‐Up | At 90 D | At 1 Y | ||
|---|---|---|---|---|---|
| Adjusted ORa (95% CI) | P Value | Adjusted ORa (95% CI) | P Value | ||
| Evidence‐based β‐blocker | ≤1 wk | Reference | Reference | ||
| 1–2 wks | 0.98 (0.83–1.16) | 0.8540 | 1.05 (0.85–1.29) | 0.6631 | |
| 2–6 wks | 0.99 (0.84–1.15) | 0.8547 | 0.99 (0.81–1.20) | 0.8963 | |
| >6 wks | 0.90 (0.77–1.06) | 0.2176 | 0.98 (0.81–1.19) | 0.8354 | |
| ACEi/ARB | ≤1 wk | Reference | Reference | ||
| 1–2 wks | 1.12 (0.95–1.32) | 0.1748 | 1.12 (0.92–1.38) | 0.2629 | |
| 2–6 wks | 1.06 (0.91–1.24) | 0.4464 | 1.13 (0.93–1.37) | 0.2098 | |
| >6 wks | 0.97 (0.84–1.14) | 0.7403 | 0.96 (0.80–1.16) | 0.7019 | |
| Aldosterone antagonist | ≤1 wk | Reference | Reference | ||
| 1–2 wks | 0.96 (0.72–1.27) | 0.7553 | 1.11 (0.75–1.63) | 0.6018 | |
| 2–6 wks | 0.86 (0.65–1.13) | 0.2746 | 0.78 (0.53–1.14) | 0.1964 | |
| >6 wks | 0.90 (0.67–1.20) | 0.4763 | 0.86 (0.59–1.25) | 0.4287 | |
| Hydralazine nitrate | ≤1 wk | Reference | Reference | ||
| 1–2 wks | 1.05 (0.30–3.69) | 0.9416 | 2.99 (0.51–17.67) | 0.2263 | |
| 2–6 wks | 0.49 (0.13–1.80) | 0.2830 | 0.37 (0.07–2.03) | 0.2492 | |
| >6 wks | 0.79 (0.25–2.50) | 0.6853 | 0.93 (0.18–4.88) | 0.9296 | |
| Anticoagulants | ≤1 wk | Reference | Reference | ||
| 1–2 wks | 1.18 (1.00–1.39) | 0.0450 | 1.41 (1.15–1.74) | 0.0012 | |
| 2–6 wks | 1.24 (1.05–1.46) | 0.0097 | 1.33 (1.07–1.64) | 0.0087 | |
| >6 wks | 1.04 (0.88–1.24) | 0.6490 | 1.17 (0.95–1.45) | 0.1352 | |
OR for each of the follow‐up groups were calculated using the adherence at ≤1 wk as a reference for each category of medication. OR >1 indicates better adherence when compared with the ≤1‐wk follow‐up group and OR <1 indicates worse adherence when compared with the ≤1‐wk follow‐up group. ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CI, confidence interval; OR, odds ratio.
The adjusted ORs were calculated by considering adherence to each individual medication. All covariates utilized for adjustment can be found in the footnote of Table 4.
In subgroup analysis by age, sex, and race as shown in Table 5, we observed that adherence rates at 1 year postdischarge from index hospitalization were higher in patients who had follow‐up appointments later when compared with patients who had follow‐up appointments within the first week among those 75 and older and in females (in patients 75 years and older at 1 year, OR 1.30, P=0.001 for 1–2‐week follow‐up, OR 1.21, P=0.02 for 2–6‐week follow‐up, OR 1.16, P=0.05 for >6‐week follow‐up; in females at 1 year, OR 1.23, P=0.02 for 1–2‐week follow‐up, OR 1.23, P=0.02 for 2–6‐week follow‐up). There were no significant associations between timing of follow‐up appointment and medication adherence when stratified by race. Similarly, as shown in Table 6, we found no significant association in our exploratory analysis of medication adherence at 90 days and 1 year by timing of follow‐up when stratified by number of new medications initiated at discharge.
Table 5.
Adjusted ORs of Medication Adherence at 90 Days and 1 Year in Subgroups
| Subgroups | Timing of First Follow‐Up | At 90 D | At 1 Y | ||
|---|---|---|---|---|---|
| Adjusted ORa (95% CI) | P Value | Adjusted ORa (95% CI) | P Value | ||
| Age (y) | |||||
| ≥75 | ≤1 wk | Reference | Reference | ||
| 1–2 wks | 1.15 (1.02–1.30) | 0.0261 | 1.30 (1.11–1.52) | 0.0012 | |
| 2–6 wks | 1.08 (0.95–1.22) | 0.2326 | 1.21 (1.04–1.42) | 0.0151 | |
| >6 wks | 1.00 (0.88–1.13) | 0.9888 | 1.16 (1.00–1.35) | 0.0472 | |
| <75 | ≤1 wk | Reference | Reference | ||
| 1–2 wks | 0.96 (0.81–1.13) | 0.5939 | 1.00 (0.81–1.23) | 0.9822 | |
| 2–6 wks | 1.03 (0.88–1.21) | 0.6823 | 0.95 (0.79–1.15) | 0.6123 | |
| >6 wks | 0.92 (0.79–1.07) | 0.2838 | 0.87 (0.72–1.04) | 0.1332 | |
| Sex | |||||
| Female | ≤1 wk | Reference | Reference | ||
| 1–2 wks | 1.12 (0.98–1.29) | 0.1073 | 1.23 (1.03–1.47) | 0.0219 | |
| 2–6 wks | 1.19 (1.04–1.37) | 0.0114 | 1.23 (1.04–1.46) | 0.0178 | |
| >6 wks | 1.04 (0.91–1.20) | 0.5739 | 1.13 (0.96–1.33) | 0.1539 | |
| Male | ≤1 wk | Reference | Reference | ||
| 1–2 wks | 1.04 (0.90–1.20) | 0.5904 | 1.14 (0.95–1.36) | 0.1601 | |
| 2–6 wks | 0.95 (0.83–1.09) | 0.4462 | 1.00 (0.84–1.19) | 0.9825 | |
| >6 wks | 0.91 (0.79–1.04) | 0.1563 | 0.96 (0.82–1.13) | 0.6379 | |
| Race | |||||
| White | ≤1 wk | Reference | Reference | ||
| 1–2 wks | 1.05 (0.94–1.18) | 0.4080 | 1.15 (1.00–1.33) | 0.0548 | |
| 2–6 wks | 1.09 (0.97–1.22) | 0.1321 | 1.11 (0.96–1.27) | 0.1541 | |
| >6 wks | 1.01 (0.90–1.13) | 0.9038 | 1.06 (0.92–1.21) | 0.4299 | |
| Other | ≤1 wk | Reference | Reference | ||
| 1–2 wks | 1.16 (0.95–1.42) | 0.1361 | 1.27 (0.98–1.64) | 0.0671 | |
| 2–6 wks | 0.99 (0.82–1.19) | 0.9004 | 1.11 (0.87–1.42) | 0.3816 | |
| >6 wks | 0.88 (0.73–1.05) | 0.1521 | 1.00 (0.80–1.25) | 0.9804 | |
OR for each of the follow‐up groups were calculated using the adherence at ≤1 wk as a reference. OR >1 indicates better adherence when compared with the ≤1‐wk follow‐up group and OR <1 indicate worse adherence when compared with the ≤1‐wk follow‐up group. CI indicates confidence interval; OR, odds ratio.
The adjusted ORs were calculated by considering composite adherence to all medications. All covariates utilized for adjustment can be found in the footnote of Table 4.
Table 6.
Medication Adherence at 90 Days and 1 Year Stratified by Number of New Medications at Discharge
| Number of New Medications | Timing of First Follow‐Up | At 90 D | At 1 Y | ||
|---|---|---|---|---|---|
| Adjusted ORa (95% CI) | P Value | Adjusted ORa (95% CI) | P Value | ||
| 0 | ≤1 wk | Reference | Reference | ||
| 1–2 wks | 0.97 (0.76–1.24) | 0.8167 | 1.34 (0.99–1.81) | 0.0561 | |
| 2–6 wks | 0.80 (0.63–1.00) | 0.0531 | 0.99 (0.74–1.31) | 0.9208 | |
| >6 wks | 0.89 (0.71–1.10) | 0.2779 | 1.15 (0.89–1.49) | 0.2706 | |
| 1 | ≤1 wk | Reference | Reference | ||
| 1–2 wks | 1.10 (0.93–1.30) | 0.2505 | 1.24 (1.00–1.53) | 0.0500 | |
| 2–6 wks | 1.16 (0.98–1.37) | 0.0762 | 1.19 (0.97–1.46) | 0.0967 | |
| >6 wks | 0.95 (0.80–1.12) | 0.5135 | 1.07 (0.88–1.31) | 0.4973 | |
| 2 | ≤1 wk | Reference | Reference | ||
| 1–2 wks | 1.20 (0.94–1.53) | 0.1498 | 1.08 (0.79–1.48) | 0.6356 | |
| 2–6 wks | 0.96 (0.77–1.21) | 0.7540 | 0.89 (0.66–1.19) | 0.4260 | |
| >6 wks | 0.88 (0.70–1.12) | 0.3018 | 0.81 (0.61–1.07) | 0.1301 | |
| 3 or more | ≤1 wk | Reference | Reference | ||
| 1–2 wks | 1.15 (0.82–1.62) | 0.4243 | 0.95 (0.60–1.51) | 0.8254 | |
| 2–6 wks | 1.09 (0.78–1.51) | 0.6209 | 1.08 (0.69–1.71) | 0.7268 | |
| >6 wks | 1.06 (0.74–1.54) | 0.7440 | 0.80 (0.50–1.29) | 0.3705 | |
OR for each of the follow‐up groups were calculated using the adherence at ≤1 wk as a reference. OR >1 indicates better adherence when compared with the ≤1‐wk follow‐up group and OR <1 indicate worse adherence when compared with the ≤1‐wk follow‐up group. CI indicates confidence interval; OR, odds ratio.
The adjusted ORs were calculated by considering composite adherence to all medications. All covariates utilized for adjustment can be found in the footnote of Table 4.
Discussion
In this study of a nationwide sample of hospitalized patients with HF with Medicare Part D coverage at GWTG‐HF fully participating hospitals, we aimed to explore the association between timing of postdischarge follow‐up appointment and short‐ and long‐term medication adherence. We found that contrary to our hypothesis, no association exists between timing of postdischarge follow‐up appointment and medication adherence. In addition, when we assessed medication adherence to guideline‐directed medical therapy as defined by a PDC of >80%, we observed overall low rates at 90 days and at 1 year of 42% and 35%, respectively, for all of the medications considered in our study.
Previous studies provide ample evidence to support the importance of medication adherence. A report by the World Health Organization suggested that “Increasing the effectiveness of adherence interventions may have a far greater impact on the health of the population than any improvement in specific medical treatments.”14 Specifically, for patients with HF, several studies have found that medication nonadherence has been associated with increased risk of mortality and hospitalizations, shorter event‐free survival, and increased risk of readmissions.2, 14, 15, 16, 17 The importance of medication adherence in improving outcomes for patients with HF necessitates the identification of factors that contribute to short‐ and long‐term medication adherence. One review examined aspects of broad domains that affect adherence as put forth by the World Health Organization, including factors related to socioeconomics, the health system, the condition itself and treatment thereof, and the patient.18, 19 Socioeconomic status, level of education, patient–provider relationship, the fluctuating acute and chronic nature of HF, and patient knowledge of the condition were some of the HF‐related factors that were identified.19 Nevertheless, these areas are complex and intertwined, and there remains a limited understanding to delineate specific factors associated with increased medication adherence that can guide development of successful, implementable interventions.
Several reviews have found only modest success of the current interventions for improving medication adherence in patients with chronic diseases. In addition, these interventions are labor‐intensive, complex, and inconsistently effective, most commonly addressing education, integration of care, behavior change, and self‐monitoring.15, 20, 21, 22 For example, a randomized controlled trial of a pharmacist‐led intervention involved multiple contacts with the patient over the course of 9 months for education, prescription refills, and monitoring.23 Despite an 11% improvement in medication adherence in the intervention arm when compared with the usual‐care group, the effect dissipated at the conclusion of the program and such an intervention may not be readily scalable.
One essential intervention that has been shown to improve short‐ and long‐term medication adherence and outcomes is in‐hospital initiation of medical therapies for HF.24, 25 Consequently, the transition from hospital to home for a discharged patient with HF is an especially challenging time, given the changes in medications that occur and because guidelines currently recommend a follow‐up visit within 7 to 14 days postdischarge from a HF hospitalization.4, 26, 27 A study by Faridi et al in a population of patients with a recent acute myocardial infarction found that delayed outpatient follow‐up >6 weeks postdischarge correlated with worse short‐ and long‐term medication adherence. In contrast to these findings, we observed no association between early follow‐up and increased medication adherence in a population of patients with HF, even when analyzed by subgroups of age, sex, and race.
Possible reasons for the lack of association found in a population of patients with HF may be explained by the chronic nature of HF when compared with a cohort experiencing a recent acute myocardial infarction. Overall, 68% of patients in our study had a previous diagnosis of HF, and a similarly large number were already prescribed HF medications before their index hospitalization. As a result, when compared with a follow‐up visit for a recent acute myocardial infarction, the follow‐up visit for patients with chronic HF may not play as large a role in improving medication adherence rates. For example, populations in which early follow‐up may play an important role in encouraging better medication adherence include younger patients with HF, those without Medicare coverage, and those who quickly become asymptomatic following hospitalization; none of these are the case with our study population, but all require additional study. Furthermore, prior studies may have been overly optimistic about the benefits of early follow‐up in and of itself and the marginal benefits may not translate to all aspects of care, such as medication adherence. In a study by Hernandez et al, the association seen with early follow‐up as defined by a follow‐up visit within 7 days postdischarge and 30‐day rehospitalization only occurred in a single quartile of hospitals.5 Similarly, in another retrospective analysis, a follow‐up visit with a provider within 14 days postdischarge was not associated with a statistically significantly lower risk of death or rehospitalization if the follow‐up visit occurred with an unfamiliar physician when compared with a familiar physician.28
The data from this study demonstrate that overall adherence to guideline‐directed medical therapy remains low in patients with HF and is an issue that should not be overlooked. Overall rates of adherence as measured by PDC at 1 year after discharge were 53% for evidence‐based β‐blockers, 48% for angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, 36% for aldosterone antagonists, 8% for hydralazine/isosorbide dinitrate, and 40% for anticoagulants. Future studies should continue to focus on better characterizing adherence in patients with HF, identification of factors associated with medication adherence, designing interventions that can effectively improve adherence in patients with HF, and integration of those interventions into clinical practice.
Limitations
In addition to its retrospective nature, this study has some limitations. First, many different factors affect both medication adherence and timing of first outpatient follow‐up appointment. We adjusted for many of these factors, but residual measured and unmeasured confounding may have persisted. Second, our study was restricted to Medicare beneficiaries 65 years and older with Part D coverage, which is necessary in order to have access to medication claims data. As a result, although this is a nationwide sample, the results of this study may not be generalizable to all patients with HF. Third, limitations emerge regarding our method of measuring medication adherence. PDC utilizes prescription fills as a surrogate measure for adherence; therefore, we only account for medication fills and cannot determine whether patients actually took the medications. In addition, the Part D administrative data we used do not allow us to distinguish between nonadherence and appropriate discontinuation of the medication. Finally, our study population included patients hospitalized between 2006 and 2012, which limits our ability to detect the use of novel oral anticoagulants such as dabigatran, apixaban, and rivaroxaban, which were released during or after this time period; use of these medications could change the rate of adherence to anticoagulants in patients with HF. Routine clinic visits for international normalized ratio checks in patients on warfarin may also be an additional confounding factor in the association between timing of postdischarge follow‐up appointment and medication adherence.
Conclusion
In our analysis of an older HF population with Medicare Part D coverage, we found that overall rates of short‐ and long‐term medication adherence are low. Furthermore, we observed that improved transition of care post–hospital discharge by way of early follow‐up appointment was not associated with increased medication adherence. Future studies should focus on identifying factors and implementing interventions that improve adherence in patients with HF.
Sources of Funding
This project was supported by the GWTG Young Investigator Database Research Seed Grant. The Get With The Guidelines®–Heart Failure (GWTG‐HF) program is provided by the American Heart Association. GWTG‐HF is sponsored, in part, by Amgen Cardiovascular and has been funded in the past through support from Medtronic, GlaxoSmithKline, Ortho‐McNeil, and the American Heart Association Pharmaceutical Roundtable.
Disclosures
DeVore reports receiving research support from the American Heart Association, Amgen, and Novartis and consulting with Novartis. Fonarow reports consulting for Amgen, Janssen, Novartis, Medtronic, and St Jude Medical, and serving on the steering committee for GWTG. Allen reports receiving research support from the National Institutes of Health, the Patient‐Centered Outcomes Research Institute, and the American Heart Association; and serving as a consultant to Novartis and Janssen. Hernandez has received research support from the American Heart Association, Aires Pharmaceuticals, Amgen, AstraZeneca, Bristol‐Myers Squibb, Genentech, GlaxoSmithKline, Merck & Co., NHLBI, Novartis, PCORI, Portola Pharmaceuticals, and Sanofi. Dr Hernandez has also served as a consultant to Amgen, AstraZeneca, Bayer, Boehringer Engelheim, Boston Scientific, MyoKardia, Novartis, Pfizer, Pluristem Therapeutics, Sanofi, and Sensible. The remaining authors have no disclosures to report.
Supporting information
Table S1. Variable Missing Rates and Imputation Methods for Model Covariates.
Acknowledgments
The authors would like to thank Erin Campbell, MS, for her editorial contributions to the article. Campbell did not receive compensation for her contributions, apart from her employment at the institution where this study was conducted.
(J Am Heart Assoc. 2018;7:e007998 DOI: 10.1161/JAHA.117.007998.)
An abstract of these data was presented at the American Heart Association Scientific Sessions, November 11 to 15, 2017 in Anaheim, CA.
References
- 1. Writing Committee Members , Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos G, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2016;134:e282–e293. [DOI] [PubMed] [Google Scholar]
- 2. Fitzgerald AA, Powers JD, Ho PM, Maddox TM, Peterson PN, Allen LA, Masoudi FA, Magid DJ, Havranek EP. Impact of medication nonadherence on hospitalizations and mortality in heart failure. J Card Fail. 2011;17:664–669. [DOI] [PubMed] [Google Scholar]
- 3. Allen LA, Fonarow GC, Liang L, Schulte PJ, Masoudi FA, Rumsfeld JS, Ho PM, Eapen ZJ, Hernandez AF, Heidenreich PA, Bhatt DL, Peterson ED, Krumholz HM; American Heart Association's Get With The Guidelines Heart Failure (GWTG‐HF) Investigators . Medication initiation burden required to comply with heart failure guideline recommendations and hospital quality measures. Circulation. 2015;132:1347–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Writing Committee Members , Yancy C, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240–e327. [DOI] [PubMed] [Google Scholar]
- 5. Hernandez AF, Greiner MA, Fonarow GC, Hammill GB, Heidenreich PA, Yancy CW, Peterson ED, Curtis LH. Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303:1716–1722. [DOI] [PubMed] [Google Scholar]
- 6. Faridi KF, Peterson ED, McCoy LA, Thomas L, Enriquez J, Wang TY. Timing of first postdischarge follow‐up and medication adherence after acute myocardial infarction. JAMA Cardiol. 2016;1:147–155. [DOI] [PubMed] [Google Scholar]
- 7. Smaha LA; American Heart Association . The American Heart Association Get With The Guidelines Program. Am Heart J. 2004;148:S46–S48. [DOI] [PubMed] [Google Scholar]
- 8. Hong Y, LaBresh KA. Overview of the American Heart Association “Get with the Guidelines” programs: coronary heart disease, stroke, and heart failure. Crit Pathw Cardiol. 2006;5:179–186. [DOI] [PubMed] [Google Scholar]
- 9. Ellrodt AG, Fonarow GC, Schwamm LH, Albert N, Bhatt DL, Cannon CP, Hernandez AF, Hlatky MA, Luepker RV, Peterson PN, Reeves M, Smith EE. Synthesizing lessons learned from get with the guidelines. Circulation. 2013;128:2447–2460. [DOI] [PubMed] [Google Scholar]
- 10. Shah B, Hernandez AF, Liang L, Al‐Khatib SM, Yancy CW, Fonarow GC, Peterson ED; Get With The Guidelines Steering Committee . Hospital variation and characteristics of implantable cardioverter‐defibrillator use in patients with heart failure: data from the GWTG‐HF (Get With The Guidelines‐Heart Failure) registry. J Am Coll Cardiol. 2009;53:416–422. [DOI] [PubMed] [Google Scholar]
- 11. DeVore AD, Cox M, Eapen ZJ, Yancy CW, Bhatt DL, Heidenreich PA, Peterson ED, Fonarow GC, Hernandez AF. Temporal trends and variation in early scheduled follow‐up after a hospitalization for heart failure: findings from Get With The Guidelines‐Heart Failure. Circ Heart Fail. 2016;9:e002344. [DOI] [PubMed] [Google Scholar]
- 12. Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choudhry NK, Shrank WH, Levin RL, Lee JL, Jan SA, Brookhart MA, Solomon DH. Measuring concurrent adherence to multiple related medications. Am J Manag Care. 2009;15:457–464. [PMC free article] [PubMed] [Google Scholar]
- 14. McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol. 2004;44:810–819. [DOI] [PubMed] [Google Scholar]
- 15. Ruppar TM, Cooper PS, Mehr DR, Delgado JM, Dunbar‐Jacob JM. Medication adherence interventions improve heart failure mortality and readmission rates: systematic review and meta‐analysis of controlled trials. J Am Heart Assoc. 2016;5:e002606 DOI: 10.1161/JAHA.115.002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Granger BB, Swedberg K, Ekman I, Granger CB, Olofsson B, McMurray JJ, Yusuf S, Michelson EL, Pfeffer MA; CHARM Investigators . Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: double‐blind, randomised, controlled clinical trial. Lancet. 2005;366:2005–2011. [DOI] [PubMed] [Google Scholar]
- 17. Wu JR, Moser DK, De Jong MJ, Rayens MK, Chung ML, Riegel B, Lennie TA. Defining an evidence‐based cutpoint for medication adherence in heart failure. Am Heart J. 2009;157:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Health Organization (WHO) , Sabete E, ed. Adherence to Long‐Term Therapies: Evidence for Action. Geneva: World Health Organization; 2003. WHO web site. Available at: http://www.who.int/chp/knowledge/publications/adherence_introduction.pdf. Updated 2003. Accessed August 4, 2017. [Google Scholar]
- 19. Leventhal MJ, Riegel B, Carlson B, De Geest S. Negotiating compliance in heart failure: remaining issues and questions. Eur J Cardiovasc Nurs. 2005;4:298–307. [DOI] [PubMed] [Google Scholar]
- 20. Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;2:CD000011. [DOI] [PubMed] [Google Scholar]
- 21. McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA. 2002;288:2868–2879. [DOI] [PubMed] [Google Scholar]
- 22. Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med. 2007;167:540–549. [DOI] [PubMed] [Google Scholar]
- 23. Murray MD, Young J, Hoke S, Tu W, Weiner M, Morrow D, Stroupe KT, Wu J, Clark D, Smith F, Gradus‐Pizlo I, Weinberger M, Brater DC. Pharmacist intervention to improve medication adherence in heart failure: a randomized trial. Ann Intern Med. 2007;146:714–725. [DOI] [PubMed] [Google Scholar]
- 24. Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O'Connor CM, Pieper K, Sun JL, Yancy C, Young JB; OPTIMIZE‐HF Investigators and Hospitals . Association between performance measures and clinical outcomes for patients hospitalized with heart failure. JAMA. 2007;297:61–70. [DOI] [PubMed] [Google Scholar]
- 25. Gattis WA, O'Connor CM, Gallup DS, Hasselblad V, Gheorghiade M; IMPACT‐HF Investigators and Coordinators . Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT‐HF) trial. J Am Coll Cardiol. 2004;43:1534–1541. [DOI] [PubMed] [Google Scholar]
- 26. Leendertse AJ, Egberts AC, Stoker LJ, van den Bemt PM; HARM Study Group . Frequency of and risk factors for preventable medication‐related hospital admissions in the Netherlands. Arch Intern Med. 2008;168:1890–1896. [DOI] [PubMed] [Google Scholar]
- 27. Krumholz HM. Post‐hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McAlister FA, Youngson E, Kaul P, Ezekowitz JA. Early follow‐up after a heart failure exacerbation: the importance of continuity. Circ Heart Fail. 2016;9:e003194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Variable Missing Rates and Imputation Methods for Model Covariates.


