Abstract
Background
Remote ischemic preconditioning (RIPC) has been suggested to protect against certain forms of organ injury after cardiac surgery. Previously, we reported the main results of RIPHeart (Remote Ischemic Preconditioning for Heart Surgery) Study, a multicenter trial randomizing 1403 cardiac surgery patients receiving either RIPC or sham‐RIPC.
Methods and Results
In this follow‐up paper, we present 1‐year follow‐up of the composite primary end point and its individual components (all‐cause mortality, myocardial infarction, stroke and acute renal failure), in a sub‐group of patients, intraoperative myocardial dysfunction assessed by transesophageal echocardiography and the incidence of postoperative neurocognitive dysfunction 5 to 7 days and 3 months after surgery. RIPC neither showed any beneficial effect on the 1‐year composite primary end point (RIPC versus sham‐RIPC 16.4% versus 16.9%) and its individual components (all‐cause mortality [3.4% versus 2.5%], myocardial infarction [7.0% versus 9.4%], stroke [2.2% versus 3.1%], acute renal failure [7.0% versus 5.7%]) nor improved intraoperative myocardial dysfunction or incidence of postoperative neurocognitive dysfunction 5 to 7 days (67 [47.5%] versus 71 [53.8%] patients) and 3 months after surgery (17 [27.9%] versus 18 [27.7%] patients), respectively.
Conclusions
Similar to our main study, RIPC had no effect on intraoperative myocardial dysfunction, neurocognitive function and long‐term outcome in cardiac surgery patients undergoing propofol anesthesia.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT01067703.
Keywords: cardio‐vascular surgery, ischemia/reperfusion injury, remote ischemic preconditioning
Subject Categories: Clinical Studies, Cardiovascular Surgery, Ischemia
Clinical Perspective
What Is New?
Little evidence exists about remote ischemic preconditioning's potential effect on mid to long‐term clinical outcome.
Here, we show that remote ischemic preconditioning may not have beneficial effects on intraoperative myocardial dysfunction, neurocognitive dysfunction or 1‐year follow‐up on mortality, myocardial infarction, stroke, and acute renal failure.
What Are the Clinical Implications?
These results suggest that remote ischemic preconditioning may have no effect on clinical outcomes in cardiac surgery patients.
Repeated short episodes of ischemia and reperfusion of non‐vital tissues have been shown to reduce organ injury of remote vital organs in patients undergoing cardiac surgery, a phenomenon known as remote ischemic preconditioning (RIPC).1, 2 However, the results of existing studies are inconsistent. While most of previous studies including our RIPHeart (Remote Ischemic Preconditioning for Heart Surgery) Study3 primarily focused on short term outcomes,4, 5, 6, 7, 8 few evidence exists about RIPC`s potential effect on mid‐ to long‐term outcomes.9
Here, we present the 1 year follow‐up data, and in a sub‐cohort of patients, myocardial dysfunction assessed by transesophageal echocardiography and the incidence of postoperative neurocognitive dysfunction (POCD) 5 to 7 days and 3 months after surgery.
Methods
The data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Design
In this prospective, randomized, double blind, multicenter, parallel group controlled study adult cardiac surgical patients were screened between January 2011 to May 2014. In total 1403 patients underwent randomization. The study included patients (aged ≥18 years, female and male) scheduled for any elective cardio‐vascular surgery requiring cardiopulmonary bypass, after written informed consent. Key exclusion criteria were related to specific surgical procedures (eg, off‐pump heart surgery, urgent surgery) and to patients with severe organ dysfunction (eg, ejection fraction <30%, severe renal failure). The ethics committees of the University of Kiel, Germany, and all the other participating centers approved of the study protocol, patient information, and informed consent was received from all patients. Further details and the main primary end point have previously been published.3, 10
RIPC was induced by 4 × 5 minutes cycles of ischemia/reperfusion using a manual blood pressure cuff after induction of propofol‐based anesthesia. A dummy‐arm with a second blood pressure cuff was used and applied in accordance to the RIPC protocol to the sham‐RIPC group.
Long‐Term Follow‐Up
The assessment of 1‐year follow‐up was a predefined secondary analysis of a binary composite complication rate defined by all‐cause mortality, non‐fatal myocardial infarction, any new stroke, and/or acute renal failure. In volunteering centers, intraoperative myocardial dysfunction and the incidence of postoperative neurocognitive dysfunction were assessed in a non‐selected sub‐cohort of patients.
Intraoperative Myocardial Performance
Myocardial dysfunction was assessed by transesophageal echocardiography before and 30 minutes after cardiopulmonary bypass including fractional shortening (by Teichholz), left ventricular end‐diastolic volume (by Simpson), left ventricular ejection fraction (by Simpson and by Teichholz), left ventricular ejection time, maximal speed in left ventricular outflow tract Vmax, deceleration time (DTEarly), earlymax/atrialmax ratio (E/A ratio), left ventricular end‐systolic volume (by Simpson), mitral close‐to‐open time (MCO, end A to end E).
Neurocognitive Assessment
POCD was evaluated by a comprehensive neurocognitive test battery, as we have previously used in a pilot study.5 POCD was assessed 1 day before, 5 to 7 days and 3 months after surgery in accordance with the “Statement of Consensus on the Assessment of Neurobehavioral Outcome After Cardiac Surgery” for cardio surgical patients.11, 12 First, Mini–Mental State Examination (MMSE) test was performed, and detailed POCD analysis was only continued in patients with MMSE ≥24 points. POCD sub‐tests included the four main domains: memory, motor skills, attention and executive function. Memory included the Rey's Auditory Verbal Learning Test (RAVLT: RAVLT 1‐3, RAVLT LT), motor skills the Purdue Pegboard Test (PBT: dominant and non‐dominant), attention the Stroop Color Word Interference Test (STROOP: STROOP I, STROOP II, STROOP III), the Trail Making Test (TMT), Digit Span Test (Digit span: forward and backwards) and the Digit Symbol Substitution Test (DSST), and executive function the Verbal Fluency Test (VFT: semantic and phonetic).
Statistical Analysis
One‐year estimates with 95% confidence interval for the composite end point and its components were derived from respective time to event or cumulative incidence curves; patients with shorter follow‐up were censored at the end of their observation period. Death was treated as a competing risk factor in analyzing components. Arm comparisons are based on proportional hazard models adjusting for the stratification variables EuroSCORE, diabetes mellitus status and the concomitant use of cholesterol or lipid lowering medications, and incorporated study centers as a random effect by the logistic regression adjusting, to evaluate the therapeutic effect.
Transesophageal echocardiography data were analyzed using a general linear model with repeated measures (pre‐ and post‐operative) and randomization group (RIPC and sham‐RIPC) as between subject factor.
Analysis of POCD data were in accordance to the analysis performed previously.5 The 1 standard deviation (SD) criterion and the summarized Z‐score were determined to analyze the individual change in performance. Except for MMSE, RAVLT 1 to 3, RAVLT LT, DSST and the VFT tests, all data underwent a logarithmic transformation. The 1‐SD criterion was used to analyze how many patients of each group were cognitively deteriorated or potentially improved. A cognitive change was assumed if the preoperative to postoperative difference in ≥2 tasks assessing different cognitive domains (memory, motor skills, attention and executive function) exceeded >1‐SD of the corresponding preoperative test (1‐SD criterion). The Z‐score criterion was performed test‐specifically and as the sum of each Z‐score (postoperative result subtracted from preoperative test result, divided by the preoperative SD of the group) to analyze the individual change in performance. If required the algebraic sign was changed so that a positive discrepancy indicated deterioration, a negative discrepancy improvement. The sum of each Z‐score for all tests was calculated and the patients in the treatment groups were compared using a 1‐way ANOVA. In case of Z‐scores >1.96 on 2 individual tests or the combined Z‐score, POCD was assumed.
Results
No Beneficial Effect of RIPC After 1 Year
No significant differences 1 year after surgery could be found between the groups (RIPC versus sham‐RIPC) on the composite primary end point (16.4% versus 16.9%) and its individual components (all‐cause mortality 3.4% versus 2.5%, myocardial infarction 7.0% versus 9.4%, stroke 2.2% versus 3.1%, and acute renal failure 7.0% versus 5.7%; Table 1). The Kaplan–Meier‐Plot shows the event‐free time to primary end point in the RIPC and the sham‐RIPC group up to 1 year after surgery (Figure). The between‐group differences were not significant.
Table 1.
Long‐Term Follow‐Up
| Variable | RIPC (n=692) | Sham‐RIPC (n=693) |
|---|---|---|
| Composite end point (365 d after surgery)—(%) | 16.4 (13.6–19.1) | 16.9 (14.0–19.6) |
| All‐cause mortality | 3.4 (2.0–4.8) | 2.5 (1.3–3.7) |
| Myocardial infarction | 7.0 (5.0–8.9) | 9.4 (7.2–11.7) |
| Stroke | 2.2 (1.0–3.3) | 3.1 (1.8–4.4) |
| Acute renal failure | 7.0 (5.0–8.9) | 5.7 (3.9–7.4) |
Rate estimates with 95% confidence interval were derived from respective time to event or cumulative incidence curves; patients with shorter follow‐up were censored at the end of their observation period. Death was treated as a competing risk factor in analyzing components. RIPC indicates remote ischemic preconditioning.
Figure 1.
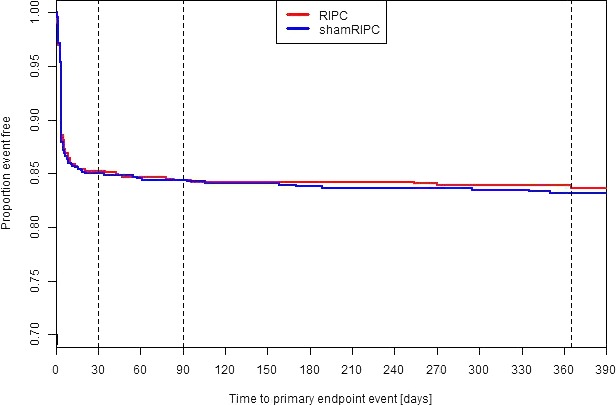
Kaplan–Meier‐plots of proportion event‐free time to primary end point. Kaplan–Meier‐Plots are shown for the two intervention groups. Event‐free survival did not differ significantly between the two intervention groups (Cox regression analysis, with adjustment for the stratification variables). RIPC indicates remote ischemic preconditioning.
No Significant Differences Between the Groups in Myocardial Dysfunction
In 808 of 1403 patients (58.2%) intraoperative transesophageal echocardiography was documented (RIPC: n=397, sham‐RIPC n=411). After cardiopulmonary bypass, earlymax/atrialmax ratio (systole) as well as left ventricular outflow tract Vmax, left ventricular ejection fraction, fractional shortening (Teichholz), left ventricular ejection fraction (Teichholz) and MPI (diastole) were increased, while deceleration time (DTearly) (systole) as well as left ventricular outflow tract VTI, left ventricular ejection time, mitral close‐to‐open time, left ventricular end‐diastolic volume and left ventricular end systolic volume (diastole) were decreased irrespective of group assignment (Table 2). Comparing the randomization group effect on before‐after differences no significant difference could be found, only for left ventricular end systolic volume (P=0.03).
Table 2.
Intraoperative Myocardial Performance
| Before CPB | After CPB | P Values | |||||
|---|---|---|---|---|---|---|---|
| RIPC Mean (CI) | Sham‐RIPC Mean (CI) | RIPC Mean (CI) | Sham‐RIPC Mean (CI) | Before and After CPB | Randomization Group Effect Before CPB | Randomization Group Effect on Before—After Difference | |
| LOVT Vmax, m/s |
1.53 (1.16–1.89) [n=372] |
1.47 (1.22–1.73) [n=385] |
1.69 (1.20–2.18) [n=346] |
1.74 (1.26–2.23) [n=348] |
0.07 | 0.85 | 0.33 |
| LOVT VTI, cm |
25.1 (23.0–27.2) [n=373] |
23.7 (21.7–25.7) [n=283] |
21.3 (20.4–22.1) [n=347] |
21.4 (20.4–22.5) [n=346] |
0.00 | 0.68 | 0.31 |
| LVET, ms |
347 (339–354) [n=361] |
344 (337–351) [n=377] |
285 (279–291) [n=337] |
288 (281–294) [n=346] |
0.00 | 0.79 | 0.15 |
| MCO, ms |
449 (440–458) [n=371] |
447 (438–456) [n=377] |
379 (370–388) [n=347] |
378 (368–388) [n=346] |
0.00 | 0.52 | 0.46 |
| DTEarly, ms |
233 (224–242) [n=369] |
224 (215–233) [n=383] |
190 (182–199) [n=348] |
195 (186–203) [n=350] |
0.00 | 0.69 | 0.08 |
| E/A ratio |
1.11 (1.05–1.17) [n=383] |
1.15 (1.09–1.21) [n=382] |
1.14 (1.07–1.20) [n=351] |
1.16 (1.10–1.22) [n=350] |
0.19 | 0.75 | 0.84 |
| LVEDV, mL |
78 (74–81) [n=356] |
79 (75–83) [n=376] |
69 (65–72) [n=332] |
69 (65–72) [n=347] |
0.00 | 0.69 | 0.21 |
| LVESV, mL |
33 (30–35) [n=354] |
34 (32–37) [n=372] |
29 (27–31) [n=331] |
29 (27–31) [n=343] |
0.00 | 0.37 | 0.03 |
| LVEF, % |
60 (59–62) [n=362] |
59 (58–60) [n=374] |
61 (60–62) [n=338] |
59 (58–61) [n=346] |
0.11 | 0.07 | 0.72 |
| FS (Teich), % |
39 (38–41) [n=362] |
39 (37–40) [n=380] |
41 (39–42) [n=339] |
39 (38–41) [n=351] |
0.02 | 0.15 | 0.63 |
| LVEF (Teich), % |
65 (64–67) [n=363] |
63 (61–64) [n=376] |
66 (65–68) [n=336] |
64 (62–65) [n=349] |
0.00 | 0.01 | 0.94 |
The data of transesophageal echocardiography are described before and after cardiopulmonary bypass. Data were analyzed using a general linear model with repeated measures (pre‐ and post‐operative) and randomization group (RIPC and sham‐RIPC) as between subject factor. CPB indicates cardiopulmonary bypass; CI, confidence interval; DTEarly, deceleration time; E/A Ratio, earlymax/atrialmax ratio; FS, fractional shortening by Teichholz; LVEDV, left ventricular end‐diastolic volume by Simpson; LVEF, left ventricular ejection fraction by Simpson and by Teichholz; LVESV, left ventricular end‐systolic volume by Simpson; LVET, left ventricular ejection time; LVOT Vmax, maximal speed in left ventricular outflow tract; LVOT VT, ventricular arrhythmia including left ventricular outflow tract; MCO, mitral close‐to‐open time.
No Relevant Between‐Group Differences in Postoperative Neurocognitive Dysfunction
Neurocognitive assessment was performed in 331 (23.6%) out of 1403 randomized patients of those 273 (19.5%) patients (RIPC n=141, sham‐RIPC n=132) underwent POCD testing before and 5 to 7 days after surgery, and 126 (9.0%) patients (RIPC n=61 patients, sham‐RIPC n=65) before and 3 months after surgery.
Concerning 1‐SD criterion, POCD was present in 67 (47.5%) versus 71 (53.8%) patients (RIPC versus sham‐RIPC) 5 to 7 days (P=0.60) and in 17 (27.9%) versus 18 (27.7%) patients 3 months after surgery (P=0.84; Table 3). An improvement of cognition was found in 22 (15.6%) versus 19 (14.4%) patients 5 to 7 days and in 14 (23.0%) versus 18 (27.7%) patients 3 months after surgery.
Table 3.
Neurocognitive Assessment
| 5 to 7 d After Surgery | 3 mo After Surgery | |||||
|---|---|---|---|---|---|---|
| RIPC | sham‐RIPC | P Value | RIPC | sham‐RIPC | P Value | |
| Memory | ||||||
| RAVLT 1‐3 | −0.13 (−1.03) | −0.08 (1.02) | 0.70 | 0.00 (1.06) | 0.17 (1.19) | 0.41 |
| RAVLT LT | −0.01 (0.98) | 0.06 (0.93) | 0.55 | 0.12 (1.13) | 0.11 (1.04) | 0.95 |
| Motor skills | ||||||
| PBT dominant | 0.52 (0.96) | 0.42 (1.15) | 0.48 | 0.07 (0.79) | −0.11 (0.71) | 0.19 |
| PBT non‐dominant | 0.51 (0.86) | 0.53 (1.69) | 0.93 | −0.15 (0.82) | −0.13 (0.71) | 0.91 |
| Attention | ||||||
| STROOP I | 0.29 (1.04) | 0.57 (0.93) | 0.02 | 0.32 (1.06) | 0.35 (1.11) | 0.86 |
| STROOP II | 0.41 (0.94) | 0.61 (1.21) | 0.13 | −0.22 (1.02) | −0.02 (0.88) | 0.25 |
| STROOP III | 0.08 (1.00) | 0.02 (0.90) | 0.63 | −0.24 (0.80) | −0.51 (0.78) | 0.06 |
| TMT A | 0.19 (0.95) | −0.02 (0.79) | 0.05 | −0.04 (0.68) | −0.11 (0.63) | 0.58 |
| TMT B | 0.14 (0.93) | 0.17 (0.88) | 0.83 | −0.19 (0.79) | −0.30 (0.69) | 0.44 |
| Digit span forwards | 0.14 (0.68) | 0.26 (0.79) | 0.16 | 0.10 (0.83) | 0.21 (0.81) | 0.45 |
| Digit span backwards | 0.31 (0.96) | 0.26 (0.68) | 0.65 | 0.04 (0.94) | 0.10 (0.94) | 0.70 |
| DSST | 0.23 (0.64) | 0.26 (0.62) | 0.71 | −0.14 (0.52) | −0.21 (0.54) | 0.49 |
| Executive function | ||||||
| VFT semantic | 0.80 (0.92) | 0.94 (0.93) | 0.24 | 0.06 (1.03) | 0.23 (1.02) | 0.38 |
| VFT phonetic | −0.20 (1.03) | −0.37 (0.97) | 0.15 | 0.13 (1.06) | −0.26 (1.08) | 0.04 |
| Summarized Z‐score | 3.38 (5.68) | 3.82 (5.73) | 0.56 | 0.05 (4.64) | −0.50 (4.76) | 0.53 |
Data are presented as mean (SD). There are no between‐group differences. Z‐score was calculated by subtracting the postoperative test result from the preoperative test result, divided by the preoperative SD of the group. Positive signs indicate deterioration, negative signs indicate improvement. Further details of the single tests are provided in the pilot study.5 DSST indicates Digit Symbol Substitution Test; PBT dominant, Purdue Pegboard Test with preferred hand; PBT non‐dominant, Purdue Pegboard Test with nonpreferred/other hand; RAVLT LT, Rey's Auditory Verbal Learning Test long‐term memory; RAVLT, Rey's Auditory Verbal Learning Test first to third presentation of words (I‐III, short‐term memory); RIPC, remote ischemic preconditioning; STROOP, Stroop Color Word Interference Test, first to third run (I–III); TMT, Trail Making Test part A and B; Digit Span Test (forward and backwards); VFT, Verbal Fluency Test including semantic and phonetic categories.
Comparing the Z‐scores and the summarized Z‐scores, no significant between‐group differences were found, except for STROOP1 after 5 to 7 days (P=0.02), which may be because of multiple testing (Table 3). Comparing the time points 5 to 7 days and 3 months after surgery, we observed an improvement of neurocognitive function in both groups (Table 3).
Discussion
Cardiac surgery is associated with predictable risks of myocardial, neurologic, and renal ischemia/reperfusion injury. RIPC could be an easy, low‐risk, and cost‐effective therapeutic strategy to counteract these risks. Various clinical studies demonstrated protection of the heart, kidney, and brain by using surrogate end points in patients with cardiovascular surgery,6, 7, 8, 13, 14, 15 coronary intervention16, 17 or stroke,18 and some of them even reported improved clinical outcome,6, 7 while others reported neutral effects.5, 19, 20 In our multicenter RIPHeart Study3 including 1403 cardio surgery patients we found no short‐term benefits of RIPC referring to a composite end point with all‐cause mortality, myocardial infarction, stroke and acute renal failure. Here, we present that no significant differences were found between the treatment groups 1 year after surgery. Similar findings were observed in the ERICCA Study9 including 1612 surgery cardiac patients with no significant benefits 1 year after surgery. Very recently, we performed a Cochrane meta‐analysis21 and found no evidence that RIPC has a treatment effect on clinical outcomes (measured as a composite end point including all‐cause mortality, non‐fatal myocardial infarction or any new stroke, or both, assessed at 30 days after surgery), while we found moderate‐quality evidence that RIPC reduces the cardiac troponin T and I release measured at 72 hours after surgery.
In a non‐selected subgroup of patients, we evaluated whether RIPC may have beneficial effects on acute post‐cardiopulmonary bypass myocardial dysfunction. Myocardial ischemia/reperfusion injury after cardiac surgery is well known to have an impact on clinical recovery, and experimental studies found specifically less myocardial ischemia/reperfusion injury and improved cardiac function following RIPC.22, 23, 24, 25, 26 Based on the echocardiography methods used, here, we could not find any difference between groups. Possibly, the negative impact of the surgery itself could mask any slight effect of RIPC. Furthermore, biased results could be caused by the selected parameters, eg, in the case of ventricle asymmetry the M Mode is highly prone to error, but ejection fraction determined by Simpson method also did not show any benefits.
The use of the intravenous anesthetic propofol has also been repeatedly discussed as a potential confounding factor that may interfere and inhibit RIPC`s protective effects.27, 28, 29 A small study in patients undergoing CABG surgery revealed a beneficial effect of RIPC if general anesthesia was maintained with isoflurane that was not observed upon using propofol.30 In line with this, a recent multicenter trial using volatile anesthetics for maintaining anesthesia showed that RIPC significantly reduced acute kidney injury in cardiac surgical patients with a high risk for this complication that was maintained up to 1 year after surgery.31 Thus, despite basic science data clearly point into a different direction,32 it cannot be ruled out completely that the use of propofol may have blunted or even abolished the organ protective effects of RIPC.
Alternatively, and more likely, the risk of the population studied in our trial may have been too low to show a meaningful effect of an intervention aiming to reduce the sequelae of ischemia/reperfusion injury; in other words: in most instances the perioperative course may have been too smooth and without hemodynamic fluctuations that may have induced an ischemia and reperfusion injury modifiable by RIPC.
In line with this assumption, recent experimental findings indeed suggested that RIPC may offer advantages if ischemia/reperfusion injury is severe.18, 33, 34, 35, 36, 37 England et al recently performed a pilot blinded placebo‐controlled trial in 26 patients with acute ischemic stroke with a National Institutes of Health Stroke Scale (NIHSS) score of 5. Compared with sham‐RIPC, there was a significant decrease in day 90 NIHSS score in the RIPC group, median NIHSS score 1 versus 3 suggesting improved neurological outcome.38 Zhao et al assigned 189 subjects with severe carotid artery stenosis undergoing carotid artery stenting to RIPC, sham‐RIPC, and no intervention (control) groups performed twice daily for 2 weeks before intervention. The incidence of new diffusion‐weighted imaging lesions in the RIPC group (16%) was significantly lower than in the sham group (37%) and the control group (41%), again suggesting improved neurological outcome.39 To evaluate the impact of RIPC on the incidence of POCD, a comprehensive neurocognitive test battery was used in a subgroup of unselected 331 patients. POCD was found 5 to 7 days in 67 versus 71 patients and 3 months after surgery in 17 versus 18 patients, with no relevant differences between the groups. The number of missing data was reasonably high, so that the lower number of patients completing the 3 months follow‐up might present a limitation. Similar results were already shown in our pilot study,5 although the sample size was considerably smaller. Joung et al evaluated the effect of RIPC on POCD in 70 patients who underwent off‐pump coronary artery bypass graft surgery. RIPC did not reduce the incidence of POCD in the immediate postoperative period (28.6% [n=10] in the control versus 31.4% [n=11] in the RIPC group).40
In conclusion, RIPC had no beneficial effect on 1‐year follow‐up, intraoperative myocardial dysfunction and postoperative neurocognitive dysfunction in cardiac surgery patients undergoing propofol‐based anesthesia.
Appendix
RIPHeart (Remote Ischemic Preconditioning for Heart Surgery) Study Collaborators
Aachen (Department of Anesthesiology, Medical Faculty RWTH Aachen University, Aachen, Germany): Ana Stevanovic, Rolf Rossaint, Marc Felzen, (Department of Thoracic and Cardiovascular Surgery): Andreas Goetzenich; 195 patients; Berlin (Department of Anesthesiology and Intensive Care Medicine, Charité‐Universitätsmedizin Berlin, Campus Charité Mitte, Berlin, Germany): Tobias Moormann, Katharina Chalk; 37 patients; Bonn (Department of Anesthesiology and Intensive Care Medicine, University Hospital Bonn, Bonn, Germany): Pascal Knuefermann, Thomas Recht, Andreas Hoeft; 73 patients; Duesseldorf (Department of Anesthesiology and Intensive Care Medicine, University Hospital Duesseldorf, Germany): Michael Winterhalter; 65 patients; Frankfurt am Main (Department of Anesthesiology, Intensive Care Medicine and Pain Therapy, University Hospital Frankfurt, Frankfurt am Main, Germany): Sonja Iken, Carolin Wiedenbeck, Gerhard Schwarzmann, Simone Lindau, (Department of Thoracic and Cardiovascular Surgery): Andreas Zierer, (Internal Medicine III: Cardiology, Angiology, Nephrology): Stephan Fichtlscherer; 117 patients; Giessen (Department of Cardiovascular Surgery, University of Giessen, Germany): Gerold Goerlach, Matthias Wollbrueck, Ursula Boening; (Department of Anesthesiology): Markus Weigand; 148 patients; Goettingen (Department of Anesthesiology and Intensive Care Medicine, University Hospital Goettingen, Germany): Julia Strauchmann, Konrad August; 91 patients; Jena (Department of Anesthesiology and Intensive Care Medicine, Jena University Hospital, Jena, Germany): Kai U. Morsbach, Markus Paxian, Konrad Reinhard; 76 patients; Kiel (Department of Anesthesiology and Intensive Care Medicine, University Hospital Schleswig‐Holstein, Campus Kiel, Germany): Jens Scholz, Jochen Renner, Ole Broch, Helga Francksen, Bernd Kuhr; 237 patients; Luebeck (Department of Anesthesiology, University Hospital Luebeck, Luebeck, Germany): Hermann Heinze, Hauke Paarmann; (Department of Cardiac and Thoracic Vascular Surgery): Hans‐Hinrich Sievers, Stefan Klotz; 56 patients; Magdeburg (Department of Anesthesiology, University Hospital Magdeburg, Germany); Thomas Hachenberg; 14 patients; Mainz (Department of Anesthesiology, Medical Center of Johannes Gutenberg‐University, Mainz, Germany): Christian Werner, Susanne Mauff; 116 patients; Rostock (Clinic of Anesthesiology and Intensive Care Medicine, University Hospital Rostock, Rostock, Germany): Angela Alms, Stefan Bergt; 146 patients; Wuerzburg (Department of Anesthesiology, University Hospital Wuerzburg, Wuerzburg, Germany): Norbert Roewer; 32 patients.
Sources of Funding
This work was funded by the German Research Foundation (ME 3559/1‐1).
Disclosures
Dr Bein reports receiving fees for serving on advisory boards from Pulsion Medical Systems, 3M, and Merck Sharp & Dohme; acting as medical advisor to the Medicines Company; receiving consulting and lecture fees from Edwards Lifesciences, CSL Behring, Orion Pharma, AbbVie, and GE Healthcare; receiving devices for research purposes from 3M; and receiving grant support from AbbVie and GE Healthcare. Dr Böning reports receiving honoraria for presentations from Maquet, Bayer, AstraZeneca, and Orion Pharma. Dr Stehr reports receiving honoraria and travel support from Teva/Ratiopharm. Dr Heringlake reports receiving consulting and lecture fees from Orion Pharma. Dr Sander reports receiving grant support from Ratiopharm, Pulsion Medical Systems, Edwards Lifesciences, the Medicines Company, and Fresenius Medical Care. The remaining authors have no disclosures to report.
Acknowledgments
We would like to thank all perfusionists, physicians and, nurses of all participating study centers who have been involved in the care of our study patients.
(J Am Heart Assoc. 2018;7:e008077 DOI: 10.1161/JAHA.117.008077.)
Contributor Information
Patrick Meybohm, Email: patrick.meybohm@kgu.de.
the RIPHeart (Remote Ischemic Preconditioning for Heart Surgery) Study Collaborators:
Ana Stevanovic, Rolf Rossaint, Marc Felzen, Andreas Goetzenich, Tobias Moormann, Katharina Chalk, Pascal Knuefermann, Thomas Recht, Andreas Hoeft, Michael Winterhalter, Sonja Iken, Carolin Wiedenbeck, Gerhard Schwarzmann, Simone Lindau, Andreas Zierer, Stephan Fichtlscherer, Gerold Goerlach, Matthias Wollbrueck, Ursula Boening, Markus Weigand, Julia Strauchmann, Kai U. Morsbach, Markus Paxian, Konrad Reinhard, Jens Scholz, Jochen Renner, Ole Broch, Helga Francksen, Bernd Kuhr, Hermann Heinze, Hauke Paarmann, Hans‐Hinrich Sievers, Stefan Klotz, Thomas Hachenberg, Christian Werner, Susanne Mauff, Angela Alms, Stefan Bergt, and Norbert Roewer
References
- 1. Stoppe C, Meybohm P, Benstoem C, Goetzenich A. Remote ischemic preconditioning in cardiac anesthesia: a review focusing on translation. Minerva Anestesiol. 2017;83:610–623. [DOI] [PubMed] [Google Scholar]
- 2. Stoppe C, Meybohm P, Goetzenich A. [Remote ischaemic conditioning—an overview]. Anasthesiol Intensivmed Notfallmed Schmerzther. 2016;51:596–603. [DOI] [PubMed] [Google Scholar]
- 3. Meybohm P, Bein B, Brosteanu O, Cremer J, Gruenewald M, Stoppe C, Coburn M, Schaelte G, Boning A, Niemann B, Roesner J, Kletzin F, Strouhal U, Reyher C, Laufenberg‐Feldmann R, Ferner M, Brandes IF, Bauer M, Stehr SN, Kortgen A, Wittmann M, Baumgarten G, Meyer‐Treschan T, Kienbaum P, Heringlake M, Schon J, Sander M, Treskatsch S, Smul T, Wolwender E, Schilling T, Fuernau G, Hasenclever D, Zacharowski K; Collaborators RIS . A multicenter trial of remote ischemic preconditioning for heart surgery. N Engl J Med. 2015;373:1397–1407. [DOI] [PubMed] [Google Scholar]
- 4. Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, Ashley E, Vichare S, Di Salvo C, Kolvekar S, Hayward M, Keogh B, MacAllister RJ, Yellon DM. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet. 2007;370:575–579. [DOI] [PubMed] [Google Scholar]
- 5. Meybohm P, Renner J, Broch O, Caliebe D, Albrecht M, Cremer J, Haake N, Scholz J, Zacharowski K, Bein B. Postoperative neurocognitive dysfunction in patients undergoing cardiac surgery after remote ischemic preconditioning: a double‐blind randomized controlled pilot study. PLoS One. 2013;8:e64743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thielmann M, Kottenberg E, Kleinbongard P, Wendt D, Gedik N, Pasa S, Price V, Tsagakis K, Neuhauser M, Peters J, Jakob H, Heusch G. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single‐centre randomised, double‐blind, controlled trial. Lancet. 2013;382:597–604. [DOI] [PubMed] [Google Scholar]
- 7. Zarbock A, Schmidt C, Van Aken H, Wempe C, Martens S, Zahn PK, Wolf B, Goebel U, Schwer CI, Rosenberger P, Haeberle H, Gorlich D, Kellum JA, Meersch M; Renal RI . Effect of remote ischemic preconditioning on kidney injury among high‐risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA. 2015;313:2133–2141. [DOI] [PubMed] [Google Scholar]
- 8. Zimmerman RF, Ezeanuna PU, Kane JC, Cleland CD, Kempananjappa TJ, Lucas FL, Kramer RS. Ischemic preconditioning at a remote site prevents acute kidney injury in patients following cardiac surgery. Kidney Int. 2011;80:861–867. [DOI] [PubMed] [Google Scholar]
- 9. Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, Knight R, Kunst G, Laing C, Nicholas J, Pepper J, Robertson S, Xenou M, Clayton T, Yellon DM; Investigators ET . Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med. 2015;373:1408–1417. [DOI] [PubMed] [Google Scholar]
- 10. Meybohm P, Zacharowski K, Cremer J, Roesner J, Kletzin F, Schaelte G, Felzen M, Strouhal U, Reyher C, Heringlake M, Schon J, Brandes I, Bauer M, Knuefermann P, Wittmann M, Hachenberg T, Schilling T, Smul T, Maisch S, Sander M, Moormann T, Boening A, Weigand MA, Laufenberg R, Werner C, Winterhalter M, Treschan T, Stehr SN, Reinhart K, Hasenclever D, Brosteanu O, Bein B; Group RIPH‐SI . Remote ischaemic preconditioning for heart surgery. The study design for a multi‐center randomized double‐blinded controlled clinical trial—the ripheart‐study. Eur Heart J. 2012;33:1423–1426. [PubMed] [Google Scholar]
- 11. Murkin JM, Newman SP, Stump DA, Blumenthal JA. Statement of consensus on assessment of neurobehavioral outcomes after cardiac surgery. Ann Thorac Surg. 1995;59:1289–1295. [DOI] [PubMed] [Google Scholar]
- 12. Rubens FD, Boodhwani M, Mesana T, Wozny D, Wells G, Nathan HJ, Cardiotomy I. The cardiotomy trial: a randomized, double‐blind study to assess the effect of processing of shed blood during cardiopulmonary bypass on transfusion and neurocognitive function. Circulation. 2007;116:I89–I97. [DOI] [PubMed] [Google Scholar]
- 13. Heusch G, Musiolik J, Kottenberg E, Peters J, Jakob H, Thielmann M. STAT5 activation and cardioprotection by remote ischemic preconditioning in humans: short communication. Circ Res. 2012;110:111–115. [DOI] [PubMed] [Google Scholar]
- 14. Remote Preconditioning Trialists G , Healy DA, Khan WA, Wong CS, Moloney MC, Grace PA, Coffey JC, Dunne C, Walsh SR, Sadat U, Gaunt ME, Chen S, Tehrani S, Hausenloy DJ, Yellon DM, Kramer RS, Zimmerman RF, Lomivorotov VV, Shmyrev VA, Ponomarev DN, Rahman IA, Mascaro JG, Bonser RS, Jeon Y, Hong DM, Wagner R, Thielmann M, Heusch G, Zacharowski K, Meybohm P, Bein B, Tang TY. Remote preconditioning and major clinical complications following adult cardiovascular surgery: systematic review and meta‐analysis. Int J Cardiol. 2014;176:20–31. [DOI] [PubMed] [Google Scholar]
- 15. Eitel I, Stiermaier T, Rommel KP, Fuernau G, Sandri M, Mangner N, Linke A, Erbs S, Lurz P, Boudriot E, Mende M, Desch S, Schuler G, Thiele H. Cardioprotection by combined intrahospital remote ischaemic perconditioning and postconditioning in ST‐elevation myocardial infarction: the randomized lipsia conditioning trial. Eur Heart J. 2015;36:3049–3057. [DOI] [PubMed] [Google Scholar]
- 16. Botker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S, Lassen JF, Christiansen EH, Krusell LR, Kristensen SD, Thuesen L, Nielsen SS, Rehling M, Sorensen HT, Redington AN, Nielsen TT. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727–734. [DOI] [PubMed] [Google Scholar]
- 17. Hoole SP, Heck PM, Sharples L, Khan SN, Duehmke R, Densem CG, Clarke SC, Shapiro LM, Schofield PM, O'Sullivan M, Dutka DP. Cardiac remote ischemic preconditioning in coronary stenting (CRISP Stent) study: a prospective, randomized control trial. Circulation. 2009;119:820–827. [DOI] [PubMed] [Google Scholar]
- 18. Hougaard KD, Hjort N, Zeidler D, Sorensen L, Norgaard A, Hansen TM, von Weitzel‐Mudersbach P, Simonsen CZ, Damgaard D, Gottrup H, Svendsen K, Rasmussen PV, Ribe LR, Mikkelsen IK, Nagenthiraja K, Cho TH, Redington AN, Botker HE, Ostergaard L, Mouridsen K, Andersen G. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: a randomized trial. Stroke. 2014;45:159–167. [DOI] [PubMed] [Google Scholar]
- 19. Hong DM, Lee EH, Kim HJ, Min JJ, Chin JH, Choi DK, Bahk JH, Sim JY, Choi IC, Jeon Y. Does remote ischaemic preconditioning with postconditioning improve clinical outcomes of patients undergoing cardiac surgery? Remote ischaemic preconditioning with postconditioning outcome trial. Eur Heart J. 2014;35:176–183. [DOI] [PubMed] [Google Scholar]
- 20. Rahman IA, Mascaro JG, Steeds RP, Frenneaux MP, Nightingale P, Gosling P, Townsend P, Townend JN, Green D, Bonser RS. Remote ischemic preconditioning in human coronary artery bypass surgery: FROM promise to disappointment? Circulation. 2010;122:S53–S59. [DOI] [PubMed] [Google Scholar]
- 21. Benstoem C, Stoppe C, Liakopoulos OJ, Ney J, Hasenclever D, Meybohm P, Goetzenich A. Remote ischaemic preconditioning for coronary artery bypass grafting (with or without valve surgery). Cochrane Database Syst Rev. 2017;5:CD011719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang L, Wang G, Du Y, Ji B, Zheng Z. Remote ischemic preconditioning reduces cardiac troponin I release in cardiac surgery: a meta‐analysis. J Cardiothorac Vasc Anesth. 2014;28:682–689. [DOI] [PubMed] [Google Scholar]
- 23. Venugopal V, Hausenloy DJ, Ludman A, Di Salvo C, Kolvekar S, Yap J, Lawrence D, Bognolo J, Yellon DM. Remote ischaemic preconditioning reduces myocardial injury in patients undergoing cardiac surgery with cold‐blood cardioplegia: a randomised controlled trial. Heart. 2009;95:1567–1571. [DOI] [PubMed] [Google Scholar]
- 24. Cheung MM, Kharbanda RK, Konstantinov IE, Shimizu M, Frndova H, Li J, Holtby HM, Cox PN, Smallhorn JF, Van Arsdell GS, Redington AN. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol. 2006;47:2277–2282. [DOI] [PubMed] [Google Scholar]
- 25. Hausenloy DJ, Erik Botker H, Condorelli G, Ferdinandy P, Garcia‐Dorado D, Heusch G, Lecour S, van Laake LW, Madonna R, Ruiz‐Meana M, Schulz R, Sluijter JP, Yellon DM, Ovize M. Translating cardioprotection for patient benefit: position paper from the working group of cellular biology of the heart of the european society of cardiology. Cardiovasc Res. 2013;98:7–27. [DOI] [PubMed] [Google Scholar]
- 26. Iliodromitis EK, Farmakis D, Kremastinos DT. Biomarkers of myocardial injury as surrogate end‐points for remote ischemic conditioning trials. Int J Cardiol. 2011;149:379. [DOI] [PubMed] [Google Scholar]
- 27. Heusch G. Cardioprotection: chances and challenges of its translation to the clinic. Lancet. 2013;381:166–175. [DOI] [PubMed] [Google Scholar]
- 28. Heusch G, Gersh BJ. ERICCA and RIPheart: two nails in the coffin for cardioprotection by remote ischemic conditioning? Probably not!. Eur Heart J. 2016;37:200–202. [DOI] [PubMed] [Google Scholar]
- 29. Meybohm P, Stoppe C, Zacharowski K. Dubious effects by the choice of anesthetics in remote ischemic preconditioning. J Thorac Dis. 2016;8:E1549–E1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kottenberg E, Thielmann M, Bergmann L, Heine T, Jakob H, Heusch G, Peters J. Protection by remote ischemic preconditioning during coronary artery bypass graft surgery with isoflurane but not propofol—a clinical trial. Acta Anaesthesiol Scand. 2012;56:30–38. [DOI] [PubMed] [Google Scholar]
- 31. Zarbock A, Kellum JA, Van Aken H, Schmidt C, Kullmar M, Rosenberger P, Martens S, Gorlich D, Meersch M. Long‐term effects of remote ischemic preconditioning on kidney function in high‐risk cardiac surgery patients: follow‐up results from the renalrip trial. Anesthesiology. 2017;126:787–798. [DOI] [PubMed] [Google Scholar]
- 32. Hausenloy DJ, Iliodromitis EK, Andreadou I, Papalois A, Gritsopoulos G, Anastasiou‐Nana M, Kremastinos DT, Yellon DM. Investigating the signal transduction pathways underlying remote ischemic conditioning in the porcine heart. Cardiovasc Drugs Ther. 2012;26:87–93. [DOI] [PubMed] [Google Scholar]
- 33. Hahn CD, Manlhiot C, Schmidt MR, Nielsen TT, Redington AN. Remote ischemic per‐conditioning: a novel therapy for acute stroke? Stroke. 2011;42:2960–2962. [DOI] [PubMed] [Google Scholar]
- 34. Wegener S, Gottschalk B, Jovanovic V, Knab R, Fiebach JB, Schellinger PD, Kucinski T, Jungehulsing GJ, Brunecker P, Muller B, Banasik A, Amberger N, Wernecke KD, Siebler M, Rother J, Villringer A, Weih M; Stroke MRIiASSGotGCN . Transient ischemic attacks before ischemic stroke: preconditioning the human brain? A multicenter magnetic resonance imaging study. Stroke. 2004;35:616–621. [DOI] [PubMed] [Google Scholar]
- 35. Zhao H. The protective effects of ischemic postconditioning against stroke: from rapid to delayed and remote postconditioning. Open Drug Discov J. 2011;5:138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tropak MB, Shi H, Li J, Dai X, Redington AN, Askalan R. Potent neuroprotection induced by remote preconditioning in a rat model of neonatal cerebral hypoxic‐ischemic injury. J Thorac Cardiovasc Surg. 2011;142:233–235. [DOI] [PubMed] [Google Scholar]
- 37. Xu T, Gong Z, Zhu WZ, Wang JF, Li B, Chen F, Deng XM. Remote ischemic preconditioning protects neurocognitive function of rats following cerebral hypoperfusion. Med Sci Monit. 2011;17:BR299–BR304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. England TJ, Hedstrom A, O'Sullivan S, Donnelly R, Barrett DA, Sarmad S, Sprigg N, Bath PM. Recast (remote ischemic conditioning after stroke trial): a pilot randomized placebo controlled phase ii trial in acute ischemic stroke. Stroke. 2017;48:1412–1415. [DOI] [PubMed] [Google Scholar]
- 39. Zhao W, Meng R, Ma C, Hou B, Jiao L, Zhu F, Wu W, Shi J, Duan Y, Zhang R, Zhang J, Sun Y, Zhang H, Ling F, Wang Y, Feng W, Ding Y, Ovbiagele B, Ji X. Safety and efficacy of remote ischemic preconditioning in patients with severe carotid artery stenosis before carotid artery stenting: a proof‐of‐concept, randomized controlled trial. Circulation. 2017;135:1325–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Joung KW, Rhim JH, Chin JH, Kim WJ, Choi DK, Lee EH, Hahm KD, Sim JY, Choi IC. Effect of remote ischemic preconditioning on cognitive function after off‐pump coronary artery bypass graft: a pilot study. Korean J Anesthesiol. 2013;65:418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]


