Abstract
Background
Elderly patients with acute coronary syndrome (ACS) are at high risk for ischemic and bleeding events. This study aimed to evaluate the clinical effectiveness and safety of dual loading antiplatelet therapy for patients 75 years and older undergoing percutaneous coronary intervention for ACS.
Methods and Results
The Improving Care for Cardiovascular Disease in China‐ACS project was a collaborative study of the American Heart Association and Chinese Society of Cardiology. A total of 5887 patients 75 years and older with ACS who had percutaneous coronary intervention and received dual antiplatelet therapy with aspirin and P2Y12 inhibitors (clopidogrel or ticagrelor) between November 2014 and June 2017 were enrolled. The primary effectiveness and safety outcomes were in‐hospital major adverse cardiovascular events and major bleeding. Hazard ratios (HRs) of in‐hospital outcomes with different loading statuses of antiplatelet therapy were estimated using Cox proportional hazard models with multivariate adjustment. A propensity score–matched analysis was also conducted. Compared with patients receiving a dual nonloading dose, patients taking a dual loading dose had increased risks of both major adverse cardiovascular events (HR, 1.66, 95% confidence interval, 1.13–2.44; [P=0.010]) and major bleeding (HR, 2.34, 95% confidence interval, 1.75–3.13; [P<0.001]). Among 3284 propensity score–matched patients, a dual loading dose was associated with a 1.36‐fold risk of major adverse cardiovascular events (HR, 1.36; 95% confidence interval, 0.88–2.11 [P=0.168]) and a 2.08‐fold risk of major bleeding (HR, 2.08; 95% confidence interval, 1.47–2.93 [P<0.001]).
Conclusions
A dual loading dose of antiplatelet therapy was associated with increased major bleeding risk but not with decreased major adverse cardiovascular events risk among patients 75 years and older undergoing percutaneous coronary intervention for ACS in China.
Clinical Trial Registration
URL: http://www.ClinicalTrials.gov. Unique identifier: NCT02306616.
Keywords: acute coronary syndrome, antiplatelet therapy, elderly, loading dose, percutaneous coronary intervention
Subject Categories: Clinical Studies, Platelets, Aging, Percutaneous Coronary Intervention, Myocardial Infarction
Clinical Perspective
What Is New?
This is the first registry study with a relatively large sample size focused on the effectiveness and safety of a dual loading versus nonloading dose of aspirin and P2Y12 receptor inhibitor in patients 75 years and older with acute coronary syndromes undergoing percutaneous coronary intervention.
Our study shows that a dual loading dose of antiplatelet therapy is not associated with reduced risk of in‐hospital major adverse cardiovascular events, but with significantly increased risk of major bleeding among patients 75 years and older with acute coronary syndromes undergoing percutaneous coronary intervention in China.
What Are the Clinical Implications?
There is a considerable proportion of patients older than 75 years with acute coronary syndromes seen in clinical practice, but there is still a relative lack of evidence on treatment strategies for these patients.
The potential risks and benefits regarding use of a dual loading dose of aspirin and P2Y12 receptor inhibitors should be carefully considered in patients 75 years and older with acute coronary syndromes undergoing percutaneous coronary intervention.
Introduction
Over the past 2 decades, oral dual antiplatelet therapy with aspirin and a P2Y12 receptor inhibitor has become the cornerstone for treating patients with acute coronary syndrome (ACS).1 For patients with ACS undergoing percutaneous coronary intervention (PCI), a loading dose of dual antiplatelet therapy is strongly recommended as early as possible or at the time of PCI by the latest guidelines.2, 3, 4, 5
However, the elderly, especially those 75 years and older, who account for a large proportion of patients with ACS in clinical practice, were underrepresented, or even excluded, in randomized trials that provided evidence for guidelines.6 These patients are generally more vulnerable to the adverse effects of a loading dose of antithrombotic drugs. One of the most noteworthy complications is bleeding, which is associated with prolonged hospitalization and increased mortality.7, 8, 9 However, recommendations in the guidelines are the same for all ages, except for those receiving thrombolytic therapy.2, 5
An increasing number of researches have been performed focusing on antiplatelet therapy for elderly patients, such as the the POPular AGE (Ticagrelor or Prasugrel Versus Clopidogrel in Elderly Patients With an Acute Coronary Syndrome and a High Bleeding Risk: Optimization of Antiplatelet Treatment in High‐Risk Elderly) study and the Elderly‐ACS 2 Study.10, 11 However, there are limited data regarding the effectiveness and safety of a dual loading dose of antiplatelet therapy in patients 75 years and older with ACS undergoing PCI.
In this study, we aimed to evaluate whether receiving a dual loading dose of antiplatelet agents is appropriate for patients 75 years and older with ACS who underwent PCI during hospitalization.
Methods
For the concern about intellectual property and patient privacy, the data, analytic methods, and study materials of this study will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Design
The CCC‐ACS (Improving Care for Cardiovascular Disease in China‐ACS) project is a nationwide registry and quality improvement study with an ongoing database focusing on quality of ACS care. This study was launched in 2014 as a collaborative initiative of the American Heart Association and Chinese Society of Cardiology. Details of the design and methodology of the CCC‐ACS project have been published.12 A standard web‐based data collection platform (Oracle Clinical Remote Data Capture, Oracle) was used. Trained data abstractors in the participating hospitals reported the required data, which they abstracted from the patients' medical records. Eligible patients were consecutively reported to the CCC‐ACS database for each month before the middle of the following month. Third‐party clinical research associates were hired to perform quality audits to ensure that cases were reported consecutively rather than selectively. Additionally, ≈5% of reported cases from every participating center were randomly selected every 3 months. Selected data were then compared with the original medical records to ensure accuracy and completeness. According to the quality audit reports, the data in this study were appropriately reported with a low incidence of missing data or error. The quality audit reports were also fed back to each center regularly to ensure data quality.
Study Population
On the basis of principal discharge diagnosis, 63 641 patients with ACS from 145 hospitals were registered from November 1, 2014, to June 30, 2017. Of these, 6384 patients 75 years and older who received a known dose of both aspirin and P2Y12 receptor inhibitor (clopidogrel or ticagrelor) without switching drugs within 24 hours of first medical contact and received PCI during hospitalization were included in this study. Of these 6384 patients enrolled in our study, 497 patients were excluded, including 31 patients with treatment of warfarin within 2 weeks before admission, 91 patients with fibrinolytic therapy, 59 patients who died within 24 hours of admission, and 316 patients lacking important clinical data (Figure 1, Data S1). Institutional review board approval was granted for this research by the ethics committee of Beijing Anzhen Hospital, Capital Medical University. No informed consent was required.
Figure 1.
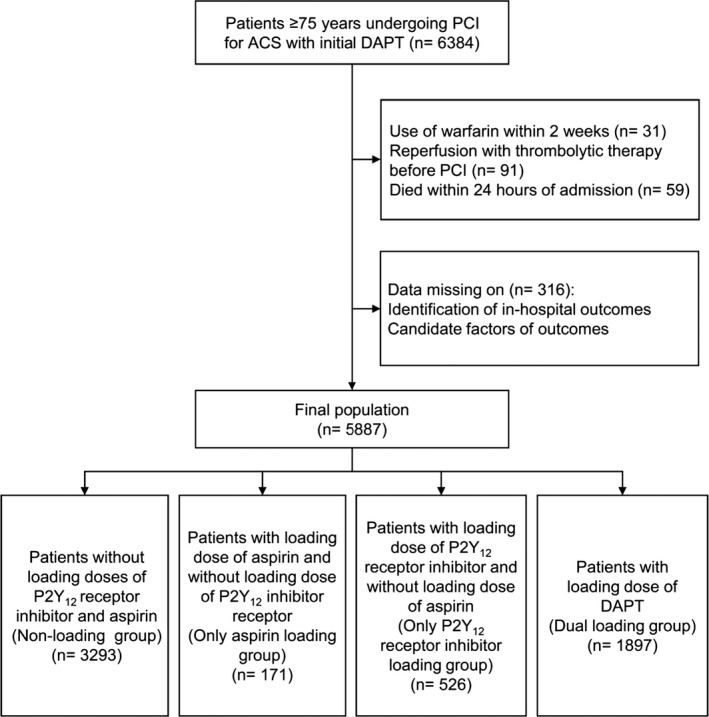
Flow diagram of selection of the study population. ACS indicates acute coronary syndrome; DAPT, dual antiplatelet therapy; PCI, percutaneous coronary intervention.
Dual Antiplatelet Therapy Within 24 Hours of First Medical Contact
According to the type and dose of antiplatelet therapy received within 24 hours of first medical contact, patients were divided into 4 groups as follows: loading with neither aspirin nor P2Y12 receptor inhibitor (nonloading group); only loading with aspirin, but not with P2Y12 receptor inhibitor (only aspirin loading group); only loading with P2Y12 receptor inhibitor, but not with aspirin (only P2Y12 receptor inhibitor loading group); and loading with both aspirin and P2Y12 receptor inhibitor (dual loading group). The loading dose of aspirin was defined as ≥150 mg and the loading dose of P2Y12 receptor inhibitor was defined as ≥300 mg of clopidogrel or ≥180 mg of ticagrelor. There were 1997 patients (96.6% of patients with aspirin loading) taking a 300‐mg dose of aspirin and 1407 patients (84.0% of patients with clopidogrel loading) taking a 300‐mg dose of clopidogrel and 744 patients (99.7% of patients with ticagrelor loading) taking a 180‐mg dose of ticagrelor. The nonloading dose of aspirin was <150 mg and the nonloading dose of P2Y12 receptor inhibitor was defined as 75 to 150 mg of clopidogrel or 90 to 135 mg of ticagrelor. There were 3796 patients (99.4% of patients with aspirin nonloading) taking a 100‐mg dose of aspirin. In addition, there were 3004 patients (94.4% of patients with clopidogrel nonloading) taking a 75‐mg dose of clopidogrel and 283 patients (97.6% of patients with ticagrelor nonloading) taking a 90‐mg dose of ticagrelor. The detailed doses of each oral antiplatelet drug are provided in supplemental files.
In‐Hospital Outcomes
In this study, the primary effectiveness outcome was major adverse cardiovascular event (MACE), including cardiac death, myocardial infarction (MI), stent thrombosis, and ischemic stroke during hospitalization. The secondary effectiveness outcome was in‐hospital all‐cause death. The primary safety outcome in our study was in‐hospital major bleeding, including intracranial bleeding, retroperitoneal bleeding, decline in hemoglobin levels ≥4 g/dL during hospitalization, transfusion with overt bleeding, or bleeding requiring surgical intervention.13 We also examined all bleeding events as our secondary safety outcome, including all documented bleeding (intracranial bleeding, retroperitoneal bleeding, access‐site bleeding, gastrointestinal bleeding, skin or mucosa bleeding, and other sites bleeding) or a decline in hemoglobin levels ≥3 g/dL during hospitalization. Minor bleeding was defined as bleeding events excluding major bleeding.
Statistical Analysis
Demographic information, medical history, clinical and procedural characteristics, and in‐hospital outcomes of the participants were described by the different loading statuses of antiplatelet therapy (Tables 1 and 2). Continuous variables were shown as mean±SD or median (interquartile range) according to different distributions. Categorical variables were presented as the number (percentage). Differences in various characteristics among the groups were compared using 1‐way ANOVA, Kruskal–Wallis test, and chi‐square test. The characteristics between the dual loading group and the nonloading group were further compared by t test, Wilcoxon test, and chi‐square test.
Table 1.
Baseline Characteristics
| Unmatched | Propensity Score–Matched | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nonloading (n=3293) | Only Aspirin Loading (n=171) | Only P2Y12 Receptor Inhibitor Loading (n=526) | Dual Loading (n=1897) | P Value of 4 Groupsa | P Value of Nonloading and Dual Loading Groupsb | Nonloading (n=1642) | Dual Loading (n=1642) | P Valueb | |
| Age, y | 79.91±3.66 | 80.20±3.44 | 79.96±4.05 | 80.20±4.02 | 0.059 | 0.008 | 80.06±3.74 | 80.06±3.92 | 0.998 |
| Male | 2072 (62.9) | 107 (62.6) | 343 (65.2) | 1162 (61.3) | 0.371 | 0.233 | 1010 (61.5) | 1024 (62.4) | 0.615 |
| Comorbidity | |||||||||
| Previous MI | 314 (9.5) | 16 (9.4) | 45 (8.6) | 96 (5.1) | <0.001 | <0.001 | 81 (4.9) | 91 (5.5) | 0.433 |
| Previous PCI | 395 (12.0) | 19 (11.1) | 45 (8.6) | 113 (6.0) | <0.001 | <0.001 | 105 (6.4) | 106 (6.5) | 0.943 |
| Previous CABG | 22 (0.7) | 3 (1.8) | 5 (1.0) | 3 (0.2) | 0.007 | 0.011 | 6 (0.4) | 3 (0.2) | 0.317 |
| Hypertension | 2413 (73.3) | 116 (67.8) | 375 (71.3) | 1346 (71.0) | 0.157 | 0.071 | 1184 (72.1) | 1161 (70.7) | 0.374 |
| Dyslipidemia | 287 (8.7) | 16 (9.4) | 33 (6.3) | 141 (7.4) | 0.137 | 0.106 | 110 (6.7) | 121 (7.4) | 0.453 |
| Diabetes mellitus | 962 (29.2) | 54 (31.6) | 141 (26.8) | 531 (28.0) | 0.475 | 0.349 | 436 (26.6) | 445 (27.1) | 0.723 |
| Renal failure history | 92 (2.8) | 2 (1.2) | 10 (1.9) | 35 (1.8) | 0.094 | 0.033 | 31 (1.9) | 33 (2.0) | 0.801 |
| Heart failure history | 103 (3.1) | 5 (2.9) | 15 (2.9) | 29 (1.5) | 0.006 | <0.001 | 31 (1.9) | 27 (1.6) | 0.596 |
| Hemorrhagic stroke | 32 (1.0) | 3 (1.8) | 4 (0.8) | 12 (0.6) | 0.352 | 0.199 | 12 (0.9) | 12 (0.7) | 0.562 |
| Ischemic stroke | 418 (12.7) | 21 (12.3) | 75 (14.3) | 210 (11.1) | 0.171 | 0.084 | 184 (11.2) | 186 (11.3) | 0.912 |
| Laboratory examinations | |||||||||
| Serum creatinine, mg/dL | 1.10 (0.78–1.20) | 1.03 (0.78–1.14) | 1.03 (0.76–1.15) | 1.04 (0.64–1.17) | 0.180 | 0.063 | 1.05 (0.77–1.17) | 1.05 (0.76–1.18) | 0.933 |
| Hemoglobin, g/dL | 12.60 (11.50–13.90) | 12.97 (11.70–14.40) | 12.88 (11.80–14.10) | 12.87 (11.70–14.10) | <0.001 | <0.001 | 12.81 (11.70–14.00) | 12.74 (11.60–14.00) | 0.284 |
| Clinical conditions | |||||||||
| GRACE score ≥140 | 2499 (75.9) | 133 (77.8) | 407 (77.4) | 1443 (76.1) | 0.846 | 0.884 | 1236 (75.3) | 1282 (78.1) | 0.058 |
| Systolic blood pressure, mm Hg | 131.75±23.57 | 128.64±24.83 | 133.12±24.24 | 130.76±25.23 | 0.077 | 0.157 | 130.22±23.11 | 129.76±25.37 | 0.591 |
| Heart rate, beats per min | 76.40±16.00 | 74.37±15.07 | 77.70±16.78 | 76.96±17.64 | 0.083 | 0.249 | 76.83±16.44 | 76.85±17.94 | 0.980 |
| Killip class | <0.001 | <0.001 | 0.001 | ||||||
| Class I | 1855 (56.3) | 101 (59.1) | 295 (56.1) | 1254 (66.1) | 1004 (61.1) | 1024 (62.4) | |||
| Class II to III | 1237 (37.6) | 58 (33.9) | 205 (39.0) | 499 (26.3) | 546 (33.3) | 477 (29.0) | |||
| Class IV | 201 (6.1) | 12 (7.0) | 26 (4.9) | 144 (7.6) | 92 (5.6) | 141 (8.6) | |||
| Type of ACS | <0.001 | <0.001 | 0.350 | ||||||
| STEMI | 1681 (51.0) | 120 (70.2) | 318 (60.5) | 1442 (76.0) | 1172 (71.4) | 1196 (72.8) | |||
| NSTE‐ACS | 1612 (49.0) | 51 (29.8) | 208 (39.5) | 455 (24.0) | 470 (28.6) | 446 (27.2) | |||
| Hospital stays, d | 11.58 (7.00–13.00) | 10.26 (6.00–12.00) | 11.31 (7.00–13.00) | 11.82 (7.00–13.00) | 0.879 | 0.749 | 11.11 (7.00–13.00) | 12.17 (7.00–13.00) | 0.232 |
| Preadmission of oral antiplatelet therapy (within 2 wk until incidence of ACS) | |||||||||
| Aspirin | 951 (28.9) | 30 (17.5) | 170 (32.3) | 190 (10.0) | <0.001 | <0.001 | 167 (10.2) | 184 (11.2) | 0.337 |
| P2Y12 receptor inhibitor | 740 (22.5) | 27 (15.8) | 62 (11.8) | 113 (6.0) | <0.001 | <0.001 | 99 (6.0) | 109 (6.6) | 0.474 |
| In‐hospital medication | |||||||||
| Type of P2Y12 receptor inhibitor used within 24 h of first medical contact | <0.001 | <0.001 | <0.001 | ||||||
| Clopidogrel | 3021 (91.7) | 153 (89.5) | 306 (58.2) | 1371 (72.3) | 1484 (90.4) | 1207 (73.5) | |||
| Ticagrelor | 272 (8.3) | 18 (10.5) | 220 (41.8) | 526 (27.7) | 158 (9.6) | 435 (26.5) | |||
| ACEI or ARB | 1703 (51.7) | 80 (46.8) | 245 (46.6) | 866 (45.7) | <0.001 | <0.001 | 845 (51.5) | 744 (45.3) | <0.001 |
| β‐Blockers | 1838 (57.7) | 92 (55.4) | 262 (52.0) | 826 (46.0) | <0.001 | <0.001 | 883 (55.4) | 744 (45.3) | <0.001 |
| Statins | 3157 (96.0) | 163 (95.3) | 509 (97.0) | 1814 (95.6) | 0.562 | 0.529 | 1572 (95.8) | 1569 (95.6) | 0.734 |
| Glycoprotein IIb/IIIa inhibitors | 795 (24.1) | 66 (38.6) | 188 (35.7) | 742 (39.1) | <0.001 | <0.001 | 471 (28.7) | 636 (38.7) | <0.001 |
| Anticoagulant therapy | <0.001 | <0.001 | <0.001 | ||||||
| None | 925 (28.1) | 35 (20.5) | 107 (20.3) | 1419 (24.1) | 428 (26.1) | 301 (18.3) | |||
| UFH | 57 (1.7) | 1 (0.6) | 8 (1.5) | 100 (5.3) | 39 (2.4) | 91 (5.5) | |||
| LMWH | 2142 (65.0) | 115 (65.5) | 364 (69.2) | 1364 (71.9) | 1098 (66.9) | 1186 (72.2) | |||
| Fondaparinux | 98 (3.0) | 11 (6.4) | 25 (4.8) | 42 (2.2) | 46 (2.8) | 32 (1.9) | |||
| Others | 71 (2.2) | 12 (7.0) | 22 (4.2) | 39 (2.1) | 31 (1.9) | 32 (1.9) | |||
| Revascularization procedure | |||||||||
| Transradial access | 3094 (94.0) | 148 (86.5) | 490 (93.2) | 1731 (91.2) | <0.001 | <0.001 | 1523 (92.8) | 1502 (91.5) | 0.174 |
| Stents implantation | 2623 (79.7) | 148 (86.5) | 409 (77.8) | 1596 (84.1) | <0.001 | <0.001 | 1372 (83.6) | 1367 (83.3) | 0.815 |
| DES | 2508 (76.2) | 145 (84.8) | 394 (74.9) | 1586 (83.6) | <0.001 | <0.001 | 1341 (81.7) | 1355 (82.5) | 0.524 |
| In‐admission CABG after PCI | 24 (0.7) | 2 (1.2) | 1 (0.2) | 11 (0.6) | 0.405 | 0.528 | 9 (0.5) | 10 (0.6) | 0.819 |
Data are expressed as mean±SD, medians (25th–75th percentiles), or number (percentage). ACEI indicates angiotensin‐converting enzyme inhibitor; ACS, acute coronary syndrome; ARB, angiotensin receptor blocker; CABG, coronary artery bypass grafting; DES, drug‐eluting stent; GRACE, Global Registry of Acute Coronary Events; LMWH, low‐molecular‐weight heparin; MI, myocardial infarction; NSTE‐ACS, non–ST‐segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; STEMI, ST‐segment elevation myocardial infarction; UFH, unfractionated heparin.
Chi‐square tests were used for categorical variables. One‐way ANOVA and Kruskal–Wallis tests were performed for continuous variables with normal and skewed distribution, respectively.
Chi‐square tests were used for categorical variables. t and Wilcoxon tests were used for continuous variables with normal and skewed distribution, respectively.
Table 2.
In‐Hospital Outcomes Within 15 d After Hospitalizationa
| Unmatched | Propensity Score–Matched | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nonloading (n=3293) | Only Aspirin Loading (n=171) | Only P2Y12 Receptor Inhibitor Loading (n=526) | Dual Loading (n=1897) | P Value of 4 Groupsb | P Value of Nonloading and Dual Loading Groupsb | Nonloading (n=1642) | Dual Loading (n=1642) | P Valueb | |
| MACE | 57 (1.7) | 1 (0.6) | 9 (1.7) | 57 (3.0) | 0.009 | 0.003 | 34 (2.1) | 49 (3.0) | 0.153 |
| Death | 42 (1.3) | 1 (0.6) | 8 (1.5) | 50 (2.6) | 0.003 | <0.001 | 27 (1.6) | 44 (2.7) | 0.044 |
| Cardiac death | 41 (1.2) | 1 (0.6) | 7 (1.3) | 46 (2.4) | 0.009 | 0.001 | 27 (1.6) | 42 (2.6) | 0.068 |
| MI | 6 (0.2) | 1 (0.6) | 0 | 7 (0.4) | 0.205 | 0.195 | 0 | 0 | |
| Stroke | 15 (0.5) | 1 (0.6) | 4 (0.8) | 21 (1.1) | 0.049 | 0.006 | 8 (0.5) | 18 (1.1) | 0.049 |
| Ischemic stroke | 10 (0.3) | 0 | 2 (0.4) | 5 (0.3) | 0.920 | 0.796 | 6 (0.4) | 5 (0.3) | 0.763 |
| Stent thrombosis | 5 (0.2) | 0 | 0 | 4 (0.2) | 0.831 | 0.623 | 1 (0.1) | 3 (0.2) | 0.317 |
| All bleeding | 157 (4.8) | 9 (5.3) | 30 (5.7) | 203 (10.7) | <0.001 | <0.001 | 88 (5.4) | 181 (11.0) | <0.001 |
| Major Bleeding | 82 (2.5) | 4 (2.3) | 17 (3.2) | 117 (6.2) | <0.001 | <0.001 | 48 (2.9) | 106 (6.5) | <0.001 |
| Non–CABG‐related major bleeding | 81 (2.5) | 4 (2.3) | 17 (3.2) | 116 (6.1) | <0.001 | <0.001 | 47 (2.9) | 105 (6.4) | <0.001 |
Data are expressed as number (percentage). CABG indicates coronary artery bypass grafting; MACE, major adverse cardiovascular event; MI, myocardial infarction.
Patients may have had >1 outcome in each category but were counted only once for overall events.
Chi‐square tests were used for categorical variables. Fisher exact test was used as appropriate.
Survival curves of MACE, all‐cause death, major bleeding, and all bleeding events were displayed using Kaplan–Meier curves and compared using log‐rank tests. Multivariable Cox proportional hazard model was performed to examine the association between different loading statuses and in‐hospital outcomes by controlling potentially confounding factors. Candidate adjustment variables included age, sex, previous MI, previous PCI, heart failure history, renal failure history, ischemic stroke history, hemorrhagic stroke history, diabetes mellitus, hypertension, preadmission use of P2Y12 receptor inhibitors, preadmission use of aspirin, preadmission use of β‐blockers, type of ACS, Killip classes, stent(s) implantation, type of stent(s), access site of PCI, baseline hemoglobin, elevated serum creatinine level, systolic blood pressure, heart rates, type of P2Y12 receptor inhibitor used within 24 hours of first medical contact, and use of angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, β‐blockers, statins, glycoprotein IIb/IIIa inhibitors, and anticoagulant therapy during hospitalization. After forward stepwise selection with entry and exit criteria setting at the P=0.05 and 0.1 level, respectively, the variables listed in Tables 3 and 4 were eventually included in the multivariable Cox proportional hazard model of MACE and major bleeding, respectively. Because the majority of patients were hospitalized for ≈2 weeks, this study only took incidents that occurred within 15 days of admission into account. Therefore, all Kaplan–Meier curves and performed Cox regression were constructed based on a 15‐day observation. Hazard ratios (HRs) for different variables and corresponding 95% confidence intervals (CIs) were reported.
Table 3.
Independent Predictors of Primary Effectiveness Outcomes (MACE)
| HR (95% CI) | P Value | |
|---|---|---|
| Whole study population | ||
| DAPT loading statuses | ||
| Only aspirin loading | 0.34 (0.05–2.49) | 0.291 |
| Only P2Y12 inhibitor loading | 0.98 (0.48–2.00) | 0.961 |
| Dual loading | 1.66 (1.13–2.44) | 0.010 |
| Age | 1.09 (1.04–1.13) | <0.001 |
| Female | 1.45 (1.01–2.07) | 0.043 |
| Renal failure history | 2.46 (1.21–5.03) | 0.013 |
| Heart rate | 1.01 (1.01–1.02) | 0.035 |
| STEMI | 1.80 (1.13–2.86) | 0.013 |
| Elevated serum creatinine level | 2.08 (1.32–3.30) | 0.002 |
| Killip class | ||
| Class II or III | 2.13 (1.38–3.28) | 0.001 |
| Class IV | 6.37 (3.94–10.29) | <0.001 |
| Glycoprotein IIb/IIIa inhibitors | 1.53 (1.05–2.22) | 0.025 |
| Transradial access | 0.61 (0.38–1.00) | 0.049 |
| Stent implantation | 0.48 (0.32–0.71) | <0.001 |
| Propensity score–matched population | ||
| Dual loading of antiplatelet therapy | 1.36 (0.88–2.11) | 0.168 |
| Age | 1.06 (1.01–1.11) | 0.018 |
| Female | 1.67 (1.09–2.57) | 0.020 |
| Renal failure history | 1.85 (0.70–4.87) | 0.212 |
| Heart rate | 1.01 (1.00–1.02) | 0.135 |
| STEMI | 1.41 (0.78–2.55) | 0.253 |
| Elevated serum creatinine level | 2.77 (1.65–4.65) | <0.001 |
| Killip class | ||
| Class II or III | 2.88 (1.68–4.95) | <0.001 |
| Class IV | 8.07 (4.43–14.69) | <0.001 |
| Glycoprotein IIb/IIIa inhibitors | 1.49 (0.95–2.35) | 0.083 |
| Transradial access | 0.76 (0.41–1.41) | 0.386 |
| Stent implantation | 0.42 (0.26–0.67) | <0.001 |
CI indicates confidence interval; DAPT, dual antiplatelet therapy; HR, hazard ratio; MACE, major adverse cardiovascular event; STEMI, ST‐segment elevation myocardial infarction.
Table 4.
Independent Predictors of Primary Safety Outcomes (Major Bleeding)
| HR (95% CI) | P Value | |
|---|---|---|
| The whole study population | ||
| DAPT loading statuses | ||
| Only aspirin loading | 0.86 (0.32–2.36) | 0.775 |
| Only P2Y12 inhibitor loading | 1.22 (0.72–2.06) | 0.462 |
| Dual loading | 2.34 (1.75–3.13) | <0.001 |
| Age | 1.04 (1.00–1.07) | 0.030 |
| Female | 1.10 (0.84–1.45) | 0.486 |
| Renal failure history | 2.00 (1.09–3.67) | 0.025 |
| Elevated serum creatinine level | 1.97 (1.36–2.86) | <0.001 |
| Killip class | ||
| Class II or III | 1.39 (1.04–1.86) | 0.028 |
| Class IV | 2.27 (1.52–3.38) | <0.001 |
| Glycoprotein IIb/IIIa inhibitors | 1.85 (1.41–2.42) | <0.001 |
| Transradial access | 0.48 (0.33–0.68) | <0.001 |
| Propensity score–matched population | ||
| Dual loading of antiplatelet therapy | 2.08 (1.47–2.93) | <0.001 |
| Age | 1.06 (1.02–1.10) | 0.002 |
| Female | 1.19 (0.86–1.64) | 0.291 |
| Renal failure history | 2.64 (1.28–5.47) | 0.009 |
| Elevated serum creatinine level | 1.51 (0.95–2.42) | 0.082 |
| Killip class | ||
| Class II or III | 1.64 (1.15–2.33) | 0.006 |
| Class IV | 2.73 (1.73–4.32) | <0.001 |
| Glycoprotein IIb/IIIa inhibitors | 1.68 (1.26–2.33) | 0.002 |
| Transradial access | 0.57 (0.37–0.89) | 0.013 |
CI indicates confidence interval; DAPT, dual antiplatelet therapy; HR, hazard ratio.
As most of the patients received either a dual loading dose of antiplatelet therapy or a dual nonloading dose in clinical practice, we compared the differences of in‐hospital outcomes between these 2 groups in a propensity score–matched population to minimize selection bias from the real world. Patients with a dual loading dose were matched 1:1 with patients randomly selected from the dual nonloading group with no replacement, on the basis of the nearest neighbor in terms of Mahalanobis distance with a caliper of 0.02. The propensity score of exposure to a dual loading dose was estimated with a logistic regression model with the variables of age, sex, first medical contact site, participating hospitals, diabetes mellitus, systolic blood pressure, heart rates, Killip classes, type of ACS, preadmission use of P2Y12 receptor inhibitors, preadmission use of aspirin, preadmission use of β‐blockers, previous MI, previous PCI, heart failure history, renal failure history, ischemic stroke history, hemorrhagic stroke history, and elevated serum creatinine level. The incidence of in‐hospital outcomes between the 2 propensity score–matched subsets were compared. As some characteristics were not well comparable between the 2 groups even after the propensity score matching, multivariable Cox proportional hazard model was further performed to compare the risk by adjusting factors, which were eventually included in the whole population by forward stepwise selection.
Subgroup analyses of primary outcomes were then performed based on important characteristics, including age (younger than 80 years or 80 years and older), sex (male or female), type of ACS (ST‐segment elevation MI or non–ST‐segment elevation ACS), preadmission antiplatelet therapy (no or yes), type of P2Y12 receptor inhibitor used within 24 hours of first medical contact (clopidogrel or ticagrelor), hypertension (no or yes), diabetes mellitus (no or yes), Global Registry of Acute Coronary Events score (≥140 or <140), Killip class I (no or yes), and glycoprotein IIb/IIIa inhibitors (no or yes).
All P values were 2‐tailed and a P<0.05 was considered statistically significant. All statistical analyses were conducted with SPSS 23.0 (IBM) and STATA 12.0 (StataCorp).
Results
Patients' Characteristics
A total of 5887 patients were enrolled in this study, with a mean age of 80.02 (SD 3.81) years and 37.4% women. The patients included 3293 in the nonloading group, 171 in the only aspirin loading group, 526 in the only P2Y12 receptor inhibitors loading group, and 1897 in the dual loading group.
Characteristics of the study population were shown in Table 1. Among the 4 groups, there were fewer patients in the dual loading group with a history of MI (5.1%), PCI (6.0%), coronary artery bypass grafting (0.2%), heart failure (1.5%), and preadmission use of aspirin (10.0%) and P2Y12 receptor inhibitors (6.0%). When comparing the clinical conditions at admission, patients in the dual loading group had a lower proportion of Killip class II to III (26.3%) but a higher proportion of class IV (7.6%). The dual loading group had more patients with ST‐segment elevation MI (76.0%). Additionally, when comparing the treatment during hospitalization, patients in the dual loading group tended to use ticagrelor (27.7%), glycoprotein IIb/IIIa inhibitors (39.1%), and low‐molecular‐weight heparin (71.9%) and have stent implantation (84.1%). Patients in the nonloading group had the highest proportion of clopidogrel use (91.7%) and transradial access (94.0%) among the 4 groups. When we only compared the baseline characteristics between the nonloading group and the dual loading group, the above significant difference still existed. In addition, there were statistically significant differences in age (79.91±3.66 versus 80.20±4.02) and history of renal failure (2.8% versus 1.8%) between the nonloading and dual loading groups.
After propensity score matching, postmatching absolute standardized differences were <10% for all covariates (Figure S1). A total of 3284 cases, 1642 in each of the dual loading and nonloading groups, were matched. The characteristics of the dual loading and nonloading groups were recompared. In propensity score–matched population, there were no significant differences of baseline characteristics between the 2 groups except for Killip class and some in‐hospital medications (Table 1).
Effectiveness Outcomes
In‐hospital effectiveness outcomes within 15 days of admission were examined according to the different loading statuses. Among the whole study population, the incidence of in‐hospital effectiveness outcome was much higher in the dual loading group (MACE: 3.0%; all‐cause death: 2.6%) compared with the other groups (Table 2, Figure 2), mainly resulting from a high incidence of cardiac death. Cumulative hazards of MACE and all‐cause death were also much higher in the dual loading group compared with other groups (Figure 3).
Figure 2.
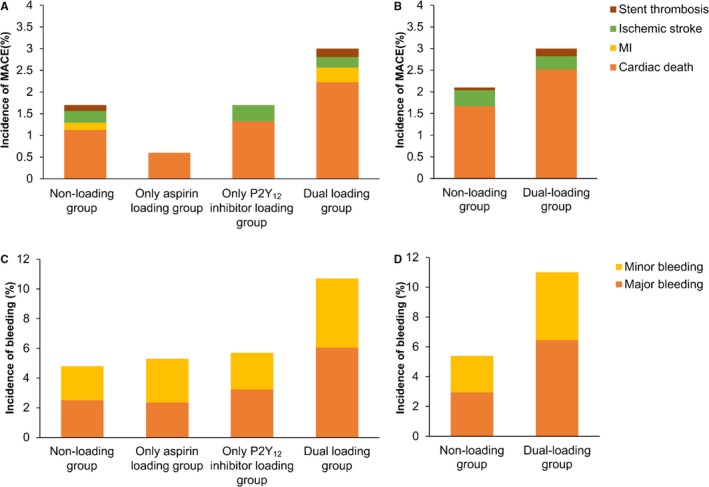
In‐hospital outcomes within 15 days after hospitalization. The incidence of in‐hospital primary effectiveness outcomes (major adverse cardiovascular event [MACE]) was higher in the dual loading group compared with other groups, mainly resulting from a higher proportion of cardiac death, in both the whole study population (A) and the propensity score–matched population (B). The incidence of both major bleeding and all bleeding was much higher in the dual loading group, compared with the nonloading group in both the whole study population (C) and the propensity score–matched population (D). The proportion of component outcomes is also shown by dosage categories. MI indicates myocardial infarction.
Figure 3.
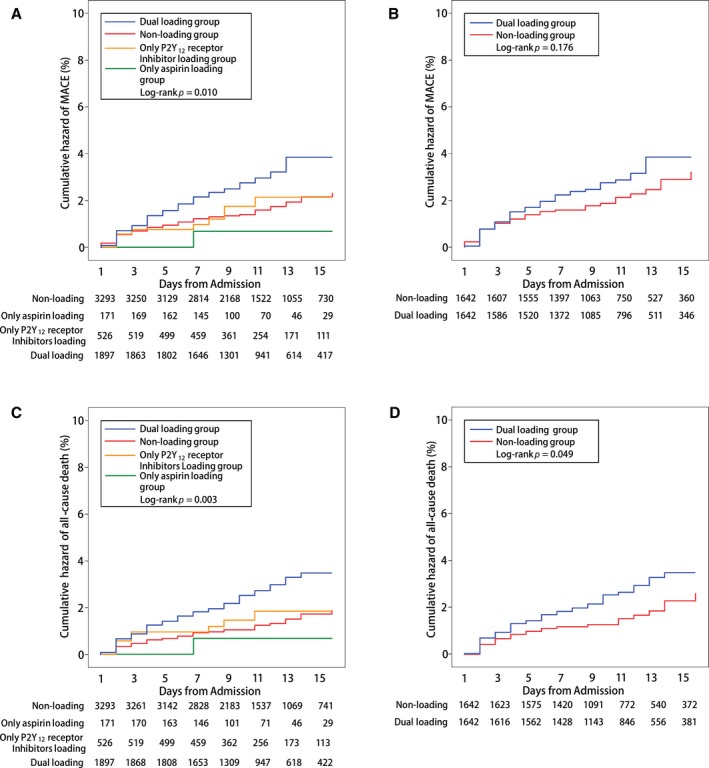
Cumulative Kaplan–Meier curve estimates of effectiveness outcomes during the 15‐day in‐hospital period. A and B, Data for the primary effectiveness outcomes of a major adverse cardiovascular event (MACE) in the whole study population and the propensity score–matched population, respectively. C and D, Data for the secondary effectiveness outcomes of all‐cause death in the whole study population and the propensity score–matched population, respectively.
In the multivariate‐adjusted analysis, a dual loading dose of aspirin and a P2Y12 receptor inhibitor was associated with higher risk of in‐hospital MACE (HR, 1.66; 95% CI, 1.13–2.44 [P=0.010]) (Table 3) and all‐cause death (HR, 1.78; 95% CI, 1.15–2.76 [P=0.010]) (Table S1) compared with dual nonloading of antiplatelet therapy.
After propensity score matching, the incidence of in‐hospital MACE (3.0% versus 2.1%; P=0.153) and all‐cause death (2.7% versus 1.6%; P=0.044) were still higher in the dual loading group but without statistical significance in MACE (Table 2, Figure 2). After multivariate‐adjusted analyses, compared with the nonloading dose of antiplatelet agents, the dual loading dose of antiplatelet agents was associated with increased risk of MACE (HR, 1.36; 95% CI, 0.88–2.11 [P=0.168]) and all‐cause death (HR, 1.57; 95% CI, 0.95–2.59 [P=0.079]), but without statistical significance (Table 3, Table S2).
Additionally, considering the impact of severe clinical conditions, we conducted further analysis by excluding patients with cardiac shock and cardiac arrest at admission, who were at the highest risk of death. We still did not observe a lower risk of in‐hospital MACE in the dual loading group (whole population: HR, 1.85; 95% CI, 1.16–2.96 [P=0.010] and propensity score–matched population: HR, 1.80, 95% CI, 1.04–3.13 [P=0.035]).
Safety Outcomes
The incidence of major bleeding and all bleeding events within 15 days of admission was significantly higher in the dual loading group than in the nonloading group in the whole study population (major bleeding: 6.2% versus 2.5%, P<0.001; all bleeding: 10.7% versus 4.8%, P<0.001) and the propensity score–matched population (major bleeding: 6.4% versus 2.9%, P<0.001; all bleeding: 11.0% versus 5.4%, P<0.001) (Table 2, Figure 2). The higher cumulative hazard of bleeding could be identified in the Kaplan–Meier curves (Figure 4).
Figure 4.
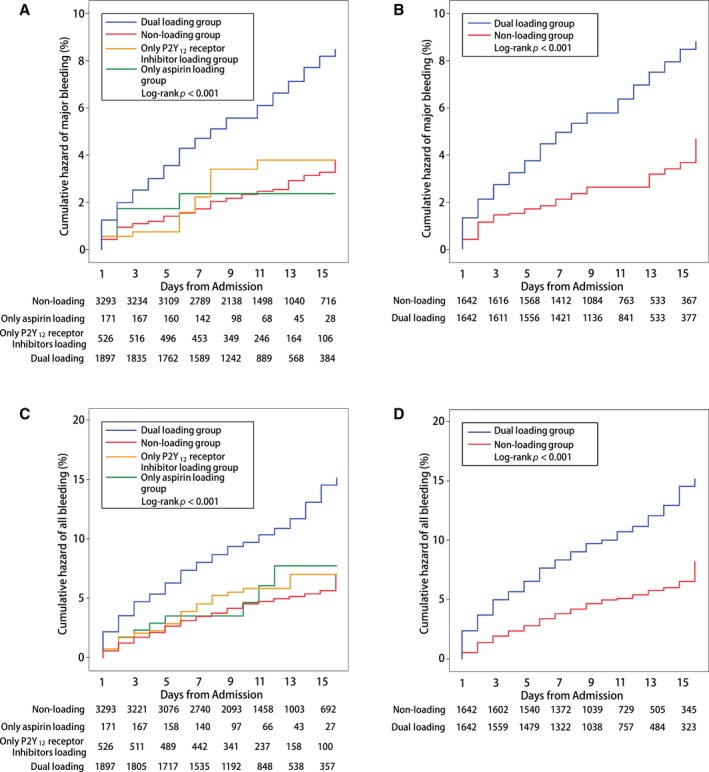
Cumulative Kaplan–Meier curve estimates of safety outcomes during the 15‐day in‐hospital period. A and B, Data for the primary safety outcomes of major bleeding in the whole study population and the propensity score–matched population, respectively. C and D, Data for the secondary safety outcomes of all‐cause death in the whole study population and the propensity score–matched population, respectively.
In the multivariate analysis, the dual loading dose of aspirin and a P2Y12 receptor inhibitor was associated with a 2‐fold risk of in‐hospital major bleeding (whole population: HR, 2.34; 95% CI, 1.75–3.13 [P<0.001] and propensity score–matched population: HR, 2.08; 95% CI, 1.47–2.93 [P<0.001]) (Table 4) and all bleeding events (whole population: HR, 1.98; 95% CI, 1.59–2.47 [P<0.001] and propensity score–matched population: HR, 1.97; 95% CI, 1.52–2.55 [P<0.001]) (Table S2).
Considering that patients in our study who received ticagrelor were more likely to take the loading dose, we used cross analyses between dosage and type of P2Y12 inhibitor in the multivariate analysis in the propensity score–matched population to evaluate whether ticagrelor added to the risk of bleeding. However, compared with clopidogrel, ticagrelor was not associated with increased risk of major bleeding and all bleeding in this study. In addition, adding the type of P2Y12 inhibitor did not change the association between loading dose and bleeding in the model (Tables S3 and S4).
Subgroup Analyses
Subgroup analyses were performed based on important baseline information in the whole study population and propensity score–matched population (Figures S2 through S5). Dual loading of aspirin and P2Y12 receptor inhibitors was associated with an increased risk of MACE in most of the subgroups. No interactions were found in different subgroups in both the whole study population and the propensity score–matched population. Dual loading of antiplatelet therapy was also associated with increased risk of major bleeding in all subgroups. Hypertension and diabetes mellitus modified the association between a dual loading dose of antiplatelet therapy and major bleeding in the whole study population. A dual loading dose was associated with a 1.9‐fold increased risk of major bleeding in patients without hypertension (HR, 1.87; 95% CI, 1.33–2.63) but a 3.8‐fold risk in patients with hypertension (HR, 3.82; 95% CI, 2.05–7.13 [P value for interaction=0.026]). A dual loading dose was associated with a 1.4‐fold risk of major bleeding in patients without diabetes mellitus (HR, 1.38; 95% CI, 0.80–2.37) and a 2.7‐fold risk in patients with diabetes mellitus (HR, 2.68; 95% CI, 1.88–3.82 [P value for interaction=0.045]). The effect of hypertension was still observed in the propensity score–matched population (P value for interaction=0.011). No other interactions were found.
Discussion
Our study is the first registry study to examine the effect of dual loading versus nonloading doses of antiplatelet therapy on 15‐day in‐hospital outcomes of patients 75 years and older with ACS undergoing PCI. The present study showed that using dual loading antiplatelet therapy with aspirin and a P2Y12 receptor inhibitor within 24 hours of first medical contact significantly increased the risk of major bleeding but was not associated with reduced risk of MACE.
Aging results in a series of physiological changes and comorbidities, which narrow the therapeutic ranges of several drugs and increase the risk of adverse drug‐drug interactions.8 This could potentially make older patients more prone to side effects and less to predictable effectiveness.14 In current clinical practice, all patients with ACS undergoing PCI are recommended to receive standard therapy of antiplatelet agents, irrespective of age.15 However, patients who are 75 years or older are often underrepresented or even excluded in randomized trials. Previous studies have determined that older patients have an independent risk of bleeding.9 Independent of the underlying disorders, all antiplatelet drugs variously amplify age‐related major risks of bleeding.14 Therefore, when older patients receive a dual loading dose of antiplatelet therapy after having ACS, they might be at the highest risk of bleeding. In addition, few studies evaluated the effectiveness and safety of a dual loading dose of antiplatelet therapy.1, 16, 17 Most of the recommendations in the guidelines for loading doses of aspirin and a P2Y12 receptor inhibitor as early as possible or at the time of PCI were mostly based on observational data and experts' opinions, as no randomized controlled trials are available to inform this strategy.2, 3, 4, 5 Recommendations on a loading dose of aspirin in ACS were originally from the period when only aspirin could be applied for oral antiplatelet therapy, and few studies evaluated the effect of loading dose, compared with nonloading dose in the acute phase.17, 18 The majority of studies on P2Y12 receptor inhibitors evaluated the effectiveness of dual antiplatelet therapy (clopidogrel plus aspirin) versus aspirin alone or compared the effect of different kinds of P2Y12 receptor inhibitors19, 20, 21 or the effect of a high loading dose of P2Y12 receptor inhibitors with a low loading dose of P2Y12 receptor inhibitors.16, 22, 23, 24 Only 1 registry study compared the effect of a loading dose of clopidogrel with a standard dose in patients older than 75 years.25 However, the use of aspirin was not mentioned in this study. The relatively small sample size of 791 patients in this study might not have been able to detect statistical significance in early complications. Therefore, whether older patients need to use a dual loading dose of antiplatelet therapy was based on relatively limited evidence.
Our study observed that a dual loading dose of antiplatelet therapy was associated with increased risk of major bleeding but not with decreased risk of MACE compared with dual nonloading antiplatelet therapy among patients 75 years or older with ACS undergoing PCI. These findings were consistent in the propensity score–matched population and subgroup analyses. The increased risk of major bleeding observed in the dual loading group is of concern. In our study, we found that the incidence of major bleeding was as high as 6.2% in the dual nonloading group, compared with 2.5% in the dual nonloading group. Bleeding is especially dangerous for older patients because it cannot only extend the length of hospital stay but may also result in death.26 Additionally, bleeding can lead to the occurrence of ischemic events because antithrombotic therapy might be stopped when major bleeding occurs.27 Therefore, further research is urgently required for studying the effectiveness and safety of antiplatelet therapy in patients 75 years and older.
Study Limitations
This was a real‐word study. Therefore, the dose application of antiplatelet drugs in this study was not randomized but based on the doctor's judgment considering the patients' condition. However, after propensity score matching, we still observed a higher risk of bleeding in the dual loading group, but not with a lower risk of MACE. In addition, this study only analyzed in‐hospital outcomes but without long‐term evaluation. However, a previous study has shown that the different effects of initial antiplatelet agents occurred within 10 days according to different drug types or dosages.28 Therefore, the effect of antiplatelet agents administrated within 24 hours of first medical contact would be expected to mainly be observed during hospitalization. Another limitation of our study is that we did not collect treatment information on proton pump inhibitors during hospitalization, which could lower the risk of bleeding, especially gastrointestinal bleeding. Further studies should take this issue into account. In addition, major bleeding was mainly defined based on the magnitude of decrease in hemoglobin, and the detailed information about bleeding site was unavailable in this study. Finally, all patients in this study were Chinese. Whether this result can be extrapolated to patients in non–East Asia needs further study.
Conclusions
Our study suggests that a dual loading dose of antiplatelet drugs within 24 hours of first medical contact were associated with increased risk of major bleeding but not with decreased risk of MACE among patients 75 years and older with ACS undergoing PCI. Therefore, clinicians should be cautious about administering a dual loading dose of antiplatelet therapy to patients 75 years and older with ACS undergoing PCI. However, more research is still needed to evaluate the effectiveness and safety of dual loading doses of antiplatelet therapy in this population.
Sources of Funding
The CCC‐ACS project is a collaborative study of the American Heart Association (AHA) and Chinese Society of Cardiology. The AHA has been funded by Pfizer for quality improvement initiatives through an independent grant for learning and change.
Disclosures
None.
Supporting information
Data S1. Supplemental methods.
Table S1. Independent Predictors of Secondary Effectiveness Outcomes (All‐Cause Death)
Table S2. Independent Predictors of Secondary Safety Outcomes (All Bleeding)
Table S3. Independent Predictors of Major bleeding (Cross Analyses Between Dosage and Type of P2Y12 Inhibitors)
Table S4. Independent Predictors of All Bleeding (Cross Analyses Between Dosage and Type of P2Y12 Inhibitors)
Figure S1. Absolute standardized differences before and after propensity score matching. ACS indicates acute coronary syndrome; MI, myocardial infarction; PCI, percutaneous coronary intervention. *Postmatching standardized difference <10% indicates excellent covariate balance.
Figure S2. Subgroup analyses for primary effectiveness outcomes of the whole study population. ACS indicates acute coronary syndrome; CI, confidence interval; GP, glycoprotein; GRACE, Global Registry of Acute Coronary Events; HR, hazard ratio.
Figure S3. Subgroup analyses for primary safety outcomes of the whole study population. ACS indicates acute coronary syndrome; CI, confidence interval; GP, glycoprotein; GRACE, Global Registry of Acute Coronary Events; HR, hazard ratio.
Figure S4. Subgroup analyses for primary effectiveness outcomes of the propensity score–matched population. ACS indicates acute coronary syndrome; CI, confidence interval; GP, glycoprotein; GRACE, Global Registry of Acute Coronary Events; HR, hazard ratio.
Figure S5. Subgroup analyses for primary safety outcomes of the propensity score–matched population. ACS indicates acute coronary syndrome; CI, confidence interval; GP, glycoprotein; GRACE, Global Registry of Acute Coronary Events; HR, hazard ratio.
Appendix S1. CCC‐ACS Investigators.
Acknowledgments
We acknowledge the contribution of all investigators in the participating hospitals of the project.
(J Am Heart Assoc. 2018;7:e008100 DOI: 10.1161/JAHA.117.008100.)
References
- 1. Cuisset T, Verheugt FW, Mauri L. Update on antithrombotic therapy after percutaneous coronary revascularisation. Lancet. 2017;390:810–820. [DOI] [PubMed] [Google Scholar]
- 2. O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis‐Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–e425. [DOI] [PubMed] [Google Scholar]
- 3. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ. 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:e344–e426. [DOI] [PubMed] [Google Scholar]
- 4. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S, Baumgartner H, Gaemperli O, Achenbach S, Agewall S, Badimon L, Baigent C, Bueno H, Bugiardini R, Carerj S, Casselman F, Cuisset T, Erol C, Fitzsimons D, Halle M, Hamm C, Hildick‐Smith D, Huber K, Iliodromitis E, James S, Lewis BS, Lip GY, Piepoli MF, Richter D, Rosemann T, Sechtem U, Steg PG, Vrints C, Luis Zamorano J; Management of Acute Coronary Syndromes in Patients Presenting without Persistent, S. T. Segment Elevation of the European Society of Cardiology . 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: Task Force for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 5. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimsky P. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 6. Dodd KS, Saczynski JS, Zhao Y, Goldberg RJ, Gurwitz JH. Exclusion of older adults and women from recent trials of acute coronary syndromes. J Am Geriatr Soc. 2011;59:506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alonso Salinas GL, Sanmartin Fernandez M, Pascual Izco M, Marco Del Castillo A, Rincon Diaz LM, Lozano Granero C, Valverde Gomez M, Pastor Pueyo P, Del Val Martin D, Pardo Sanz A, Monteagudo Ruiz JM, Recio‐Mayoral A, Salvador Ramos L, Marzal Martin D, Camino Lopez A, Jimenez Mena M, Zamorano Gomez JL. Frailty predicts major bleeding within 30 days in elderly patients with acute coronary syndrome. Int J Cardiol. 2016;222:590–593. [DOI] [PubMed] [Google Scholar]
- 8. Andreotti F, Rocca B, Husted S, Ajjan RA, ten Berg J, Cattaneo M, Collet JP, De Caterina R, Fox KA, Halvorsen S, Huber K, Hylek EM, Lip GY, Montalescot G, Morais J, Patrono C, Verheugt FW, Wallentin L, Weiss TW, Storey RF; E. S. C. Thrombosis Working Group . Antithrombotic therapy in the elderly: expert position paper of the European Society of Cardiology Working Group on Thrombosis. Eur Heart J. 2015;36:3238–3249. [DOI] [PubMed] [Google Scholar]
- 9. Li L, Geraghty OC, Mehta Z, Rothwell PM. Age‐specific risks, severity, time course, and outcome of bleeding on long‐term antiplatelet treatment after vascular events: a population‐based cohort study. Lancet. 2017;390:490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qaderdan K, Ishak M, Heestermans AA, de Vrey E, Jukema JW, Voskuil M, de Boer MJ, van't Hof AW, Groenemeijer BE, Vos GJ, Janssen PW, Bergmeijer TO, Kelder JC, Deneer VH, ten Berg JM. Ticagrelor or prasugrel versus clopidogrel in elderly patients with an acute coronary syndrome: optimization of antiplatelet treatment in patients 70 years and older—rationale and design of the POPular AGE study. Am Heart J. 2015;170:981–985.e981. [DOI] [PubMed] [Google Scholar]
- 11. Ferri LA, Morici N, Grosseto D, Tortorella G, Bossi I, Sganzerla P, Cacucci M, Sibilio G, Tondi S, Toso A, Ferrario M, Gandolfo N, Ravera A, Mariani M, Corrada E, Di Ascenzo L, Petronio AS, Cavallini C, Moffa N, De Servi S, Savonitto S. A comparison of reduced‐dose prasugrel and standard‐dose clopidogrel in elderly patients with acute coronary syndromes undergoing early percutaneous revascularization: design and rationale of the randomized elderly‐ACS 2 study. Am Heart J. 2016;181:101–106. [DOI] [PubMed] [Google Scholar]
- 12. Hao Y, Liu J, Liu J, Smith SC Jr, Huo Y, Fonarow GC, Ma C, Ge J, Taubert KA, Morgan L, Guo Y, Zhang Q, Wang W, Zhao D; Investigators C‐A . Rationale and design of the improving care for cardiovascular disease in china (CCC) project: a national effort to prompt quality enhancement for acute coronary syndrome. Am Heart J. 2016;179:107–115. [DOI] [PubMed] [Google Scholar]
- 13. Mathews R, Peterson ED, Chen AY, Wang TY, Chin CT, Fonarow GC, Cannon CP, Rumsfeld JS, Roe MT, Alexander KP. In‐hospital major bleeding during ST‐elevation and non‐ST‐elevation myocardial infarction care: derivation and validation of a model from the ACTION Registry(R)‐GWTG. Am J Cardiol. 2011;107:1136–1143. [DOI] [PubMed] [Google Scholar]
- 14. Rocca B, Husted S. Safety of antithrombotic agents in elderly patients with acute coronary syndromes. Drugs Aging. 2016;33:233–248. [DOI] [PubMed] [Google Scholar]
- 15. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Juni P, Kastrati A, Kolh P, Mauri L, Montalescot G, Neumann FJ, Petricevic M, Roffi M, Steg PG, Windecker S, Zamorano JL, Levine GN. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2018;39:213–260. [DOI] [PubMed] [Google Scholar]
- 16. Siller‐Matula JM, Huber K, Christ G, Schror K, Kubica J, Herkner H, Jilma B. Impact of clopidogrel loading dose on clinical outcome in patients undergoing percutaneous coronary intervention: a systematic review and meta‐analysis. Heart. 2011;97:98–105. [DOI] [PubMed] [Google Scholar]
- 17. Antithrombotic Trialists C . Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Antithrombotic Trialists C , Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, Patrono C, Roncaglioni MC, Zanchetti A. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta‐analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial I . Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation. N Engl J Med. 2001;345:494–502. [DOI] [PubMed] [Google Scholar]
- 20. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA; Investigators P , Freij A, Thorsen M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. [DOI] [PubMed] [Google Scholar]
- 21. Fanaroff AC, Hasselblad V, Roe MT, Bhatt DL, James SK, Steg PG, Gibson CM, Ohman EM. Antithrombotic agents for secondary prevention after acute coronary syndromes: a systematic review and network meta‐analysis. Int J Cardiol. 2017;241:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Patti G, Barczi G, Orlic D, Mangiacapra F, Colonna G, Pasceri V, Barbato E, Merkely B, Edes I, Ostojic M, Wijns W, Di Sciascio G. Outcome comparison of 600‐ and 300‐mg loading doses of clopidogrel in patients undergoing primary percutaneous coronary intervention for ST‐segment elevation myocardial infarction: results from the ARMYDA‐6 MI (antiplatelet therapy for reduction of myocardial damage during angioplasty‐myocardial infarction) randomized study. J Am Coll Cardiol. 2011;58:1592–1599. [DOI] [PubMed] [Google Scholar]
- 23. Dangas G, Mehran R, Guagliumi G, Caixeta A, Witzenbichler B, Aoki J, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Rabbani LE, Parise H, Stone GW. Role of clopidogrel loading dose in patients with ST‐segment elevation myocardial infarction undergoing primary angioplasty: results from the HORIZONS‐AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trial. J Am Coll Cardiol. 2009;54:1438–1446. [DOI] [PubMed] [Google Scholar]
- 24. Mehta SR, Tanguay JF, Eikelboom JW, Jolly SS, Joyner CD, Granger CB, Faxon DP, Rupprecht HJ, Budaj A, Avezum A, Widimsky P, Steg PG, Bassand JP, Montalescot G, Macaya C, Di Pasquale G, Niemela K, Ajani AE, White HD, Chrolavicius S, Gao P, Fox KA, Yusuf S; Investigators C‐Ot . Double‐dose versus standard‐dose clopidogrel and high‐dose versus low‐dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT‐OASIS 7): a randomised factorial trial. Lancet. 2010;376:1233–1243. [DOI] [PubMed] [Google Scholar]
- 25. Puymirat E, Aissaoui N, Coste P, Dentan G, Bataille V, Drouet E, Mulak G, Carrie D, Blanchard D, Simon T, Danchin N. Comparison of efficacy and safety of a standard versus a loading dose of clopidogrel for acute myocardial infarction in patients >/= 75 years of age (from the FAST‐MI registry). Am J Cardiol. 2011;108:755–759. [DOI] [PubMed] [Google Scholar]
- 26. Steg PG, Huber K, Andreotti F, Arnesen H, Atar D, Badimon L, Bassand JP, De Caterina R, Eikelboom JA, Gulba D, Hamon M, Helft G, Fox KA, Kristensen SD, Rao SV, Verheugt FW, Widimsky P, Zeymer U, Collet JP. Bleeding in acute coronary syndromes and percutaneous coronary interventions: position paper by the Working Group on Thrombosis of the European Society of Cardiology. Eur Heart J. 2011;32:1854–1864. [DOI] [PubMed] [Google Scholar]
- 27. Halvorsen S, Storey RF, Rocca B, Sibbing D, Ten Berg J, Grove EL, Weiss TW, Collet JP, Andreotti F, Gulba DC, Lip GY, Husted S, Vilahur G, Morais J, Verheugt FW, Lanas A, Al‐Shahi Salman R, Steg PG, Huber K; E. S. C. Working Group on Thrombosis . Management of antithrombotic therapy after bleeding in patients with coronary artery disease and/or atrial fibrillation: expert consensus paper of the European Society of Cardiology Working Group on Thrombosis. Eur Heart J. 2017;38:1455–1462. [DOI] [PubMed] [Google Scholar]
- 28. Cadroy Y, Bossavy JP, Thalamas C, Sagnard L, Sakariassen K, Boneu B. Early potent antithrombotic effect with combined aspirin and a loading dose of clopidogrel on experimental arterial thrombogenesis in humans. Circulation. 2000;101:2823–2828. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental methods.
Table S1. Independent Predictors of Secondary Effectiveness Outcomes (All‐Cause Death)
Table S2. Independent Predictors of Secondary Safety Outcomes (All Bleeding)
Table S3. Independent Predictors of Major bleeding (Cross Analyses Between Dosage and Type of P2Y12 Inhibitors)
Table S4. Independent Predictors of All Bleeding (Cross Analyses Between Dosage and Type of P2Y12 Inhibitors)
Figure S1. Absolute standardized differences before and after propensity score matching. ACS indicates acute coronary syndrome; MI, myocardial infarction; PCI, percutaneous coronary intervention. *Postmatching standardized difference <10% indicates excellent covariate balance.
Figure S2. Subgroup analyses for primary effectiveness outcomes of the whole study population. ACS indicates acute coronary syndrome; CI, confidence interval; GP, glycoprotein; GRACE, Global Registry of Acute Coronary Events; HR, hazard ratio.
Figure S3. Subgroup analyses for primary safety outcomes of the whole study population. ACS indicates acute coronary syndrome; CI, confidence interval; GP, glycoprotein; GRACE, Global Registry of Acute Coronary Events; HR, hazard ratio.
Figure S4. Subgroup analyses for primary effectiveness outcomes of the propensity score–matched population. ACS indicates acute coronary syndrome; CI, confidence interval; GP, glycoprotein; GRACE, Global Registry of Acute Coronary Events; HR, hazard ratio.
Figure S5. Subgroup analyses for primary safety outcomes of the propensity score–matched population. ACS indicates acute coronary syndrome; CI, confidence interval; GP, glycoprotein; GRACE, Global Registry of Acute Coronary Events; HR, hazard ratio.
Appendix S1. CCC‐ACS Investigators.


