Abstract
Background
Heart rate (HR) recovery has been investigated in specific patient cohorts, but there is less information about the role of HR recovery in general populations. We investigated whether HR recovery has long‐term prognostic significance in primary prevention.
Methods and Results
Exercise tests performed between 1993 and 2010 on patients aged 30 to 79 years without cardiovascular disease were included. Mortality was determined from Mayo Clinic records and Minnesota Death Index. Total, cardiovascular, and non‐cardiovascular mortality was reported according to HR recovery <13 bpm using Cox regression. 19 551 patients were included, 6756 women (35%), age 51±10 years. There were 1271 deaths over follow‐up of 12±5 years. HR recovery declined after age 60, and was also lower according to diabetes mellitus, hypertension, obesity, current smoking, and poor cardiorespiratory fitness but not sex or β‐blockers. Adjusting for these factors, abnormal HR recovery was a significant predictor of total (hazard ratio [95% confidence interval]=1.56 [1.384–1.77]), cardiovascular (1.95 [1.57–2.42]), and non‐cardiovascular death (1.41 [1.22–1.64]). Hazard ratios for cardiovascular death according to abnormal HR recovery were significant in all age groups (30–59, 60–69, 70–79), in both sexes, in patients with and without hypertension, obesity, and diabetes mellitus, but not in patients taking β‐blockers, current smokers, and patients with normal cardiorespiratory fitness.
Conclusions
HR recovery is a powerful prognostic factor predicting total, cardiovascular, and non‐cardiovascular death in a primary prevention cohort. It performs consistently well according to sex, age, obesity, hypertension, and diabetes mellitus but shows diminished utility in patients taking β‐blockers, current smokers, and patients with normal cardiorespiratory fitness.
Keywords: exercise testing, heart rate recovery, mortality, primary prevention
Subject Categories: Exercise, Risk Factors, Primary Prevention
Clinical Perspective
What Is New?
We give a detailed description about the role and prognostic performance of HR recovery in a large primary prevention population.
We show that heart rate (HR) recovery is associated with a number of cardiovascular risk factors including diabetes mellitus, hypertension, current smoking and poor cardiorespiratory fitness but is not affected by sex or use of HR‐lowering drug.
What Are the Clinical Implications?
The exercise test is an important prognostic tool with the ability to predict mortality.
Using nonelectrocardiographic parameters such as HR recovery, we can predict not only total mortality but show that abnormal HR recovery is an even stronger predictor of cardiovascular death and predicts also non‐cardiovascular death.
While HR recovery performs equally well in all age groups and in both men and women, it is less useful in patients with normal cardiorespiratory fitness or in those taking a β‐blocker.
It is important for both good patient care and healthcare economics to gain the most information possible from each test performed. This is true for the exercise test, which provides both diagnostic and prognostic information.1 This means not only looking at the ST‐segment response but also accurately reporting and identifying the significance of functional aerobic capacity (FAC) and exercise heart rate (HR) responses, including the HR recovery.
Ever since the concept of HR recovery on the exercise test was proposed by Cole and colleagues in 1999,2 investigations have consistently demonstrated its ability to identify higher‐risk patients in mixed cohorts (with and without documented coronary artery disease),3 in patients referred for nuclear stress testing and possible coronary angiography,4 and patients with symptomatic coronary artery disease.5 HR recovery is impaired in patients with obstructive sleep apnea,6 silent myocardial ischemia,7 heart failure with preserved ejection fraction,8 and type 2 diabetes mellitus,9 although these studies did not report long‐term outcomes. We have also recently published data showing that HR recovery is one of several exercise HR responses that are impaired in patients with diabetes mellitus and that it contributes significantly to the increased all‐cause mortality versus patients without diabetes mellitus.10 The prognostic performance of HR recovery in a primary prevention population without documented coronary artery disease has not been fully examined. In a primary prevention cohort, cardiovascular deaths may not constitute the majority of all deaths, so it is important to know if noncardiovascular deaths are also related to HR recovery.
Another issue is whether adjusting the HR recovery for different subgroups will improve its performance. Among exercise test prognostic factors, FAC is adjusted for age and sex, whereas peak exercise HR is adjusted for age and β‐blocker use: either ignored in β‐blocked patients or given a different age‐adjusted target.11 It is therefore essential to determine if HR recovery varies by age, sex, and β‐blocker use and whether adjustment for any of these factors improves its performance.12 These questions represent the focus of the current investigation while also validating the prognostic value of HR recovery in a primary prevention cohort.
Methods
Due to patient confidentiality issues, the data, analytic methods, and study materials will not be made available online to other researchers for purposes of reproducing the results or replicating the procedure. However, the corresponding author may be able to provide some materials upon request. This was a retrospective study approved by the Mayo Clinic Rochester Institutional Review Board. Subjects not consenting to have their data used in research under Minnesota Statute (§144.335) were excluded.13 The Mayo Integrated Stress Center database was queried for the period September 21, 1993, through December 20, 2010.
Study Population
Inclusion criteria were as follows: nonimaging stress test, Minnesota resident, symptom‐limited treadmill test performed on the Bruce protocol, aged 30 to 79 years. Tests were excluded if (1) the patient had a documented history of cardiovascular disease, including ischemic heart diseases, heart failure, cardiac surgery, structural or valvular heart diseases, major arrhythmias, defibrillator or pacemaker, congenital heart diseases, cerebrovascular diseases, and peripheral vascular diseases; (2) the test was not symptom limited but stopped because of ST changes, major arrhythmias, or abnormal blood pressure response; (3) active recovery for at least 1 minute was not completed; or (4) peak exercise or 1‐minute recovery HR were impaired by a paroxysmal arrhythmia. Where multiple qualifying tests were available for a given patient, the first test chronologically was chosen to maximize follow‐up.
Clinical Data
Demographic and clinical information were collected prospectively at the time of the stress study. HR and other exercise data were uploaded into the database electronically from the GE CASE stress testing systems (Milwaukee, WI). Patient characteristics including age, sex, anthropometrics, and comorbidities were extracted from patient medical charts and patient interview at the time of the exercise test. We specifically looked for diabetes mellitus, hypertension, obesity (defined as body mass index ≥30 kg/m2), current smoking, and use of an HR‐lowering drug (β‐blocker or nondihydropyridine calcium channel blocker).
Exercise Test Protocol and Variables
Symptom‐limited treadmill exercise testing was performed on usual medications using the standard Bruce protocol according to American College of Cardiology/American Heart Association guidelines.14, 15 Resting HR and blood pressure measurements were obtained in the standing position. Symptoms, blood pressure, HR, rating of perceived exertion, and workload were electronically entered into the database during the final minute of each stage of exercise, peak exercise, 1 and 3 minutes of active recovery at 1.7 MPH/0% grade, and 6 minutes post peak exercise in seated recovery.
Exercise test interpretation data including reason for termination, symptoms, abnormal signs, and exercise electrocardiographic analysis were added to the database immediately after the test. FAC was expressed as 100%×actual performance time/predicted performance time based on previous publications from our laboratory.16 Peak HR was also expressed as percent predicted peak HR.17 HR recovery was calculated as peak exercise HR minus HR at 1 minute of active recovery at 1.7 MPH/0% grade. An abnormal exercise ECG was defined as any ST depression or elevation >1.0 mm irrespective of the resting ECG, while an abnormal exercise ECG was considered positive only if the resting ECG did not present with significant ST‐T abnormalities, the patient was not taking digitalis, and rate‐related left bundle branch block did not occur.
Mortality Outcomes
Outcomes were taken from Mayo Clinic patient records and the Minnesota Death Index, which was reviewed in March 2016. A death was considered to be cardiovascular‐related if a cardiovascular condition was included among the first 3 listed causes in the Minnesota Death Index; otherwise, the death was considered non‐cardiovascular. Mortality data were classified using the International Classification of Diseases, Ninth Revision (ICD‐9; 391, 391.9, 394–398, 402, 404, 410–414, 415–417, 420–429, 430–438, 440–448, 451–454, 456–459) and ICD‐9 (I101, I05–I09, I11, I13, I20–I25, I26–I28, I30–I52, I60–69, I70–I79, I80–89) codes.
Statistical Analysis
Patient characteristics, outcomes, and exercise data were analyzed by decade of age. Differences among continuous variables by age group were assessed by analysis of variance under the general linear model with multiple comparisons handled by Tukey's method, while Pearson's chi‐squared test of continuity was used to test age group differences in discrete variables. Similar to a previous paper on peak HR by age and sex from our laboratory,17 the first step in the analysis was to determine factors that significantly affect HR recovery using stepwise multivariate regression, then to create a “pure cohort” by eliminating patients with those factors. This allowed us to identify the true physiologic change in HR recovery with age and sex.
The next step in the analysis was to determine if the standard definition of abnormal HR recovery of <13 bpm would predict the outcomes of total death, cardiovascular death, and non‐cardiovascular death in this primary prevention data set using the whole cohort. Cox proportional hazards regression was employed for this analysis. Further analyses were stratified by age, sex, FAC, presence of hypertension, diabetes mellitus, current smoking, and use of an HR‐lowering drug. Differences in hazard ratios between different strata were determined by the Z‐score method. Statistics were performed using SAS 9.4 (Raleigh, NC). P<0.05 was considered significant for all analyses.
Results
Study Population
A total of 19 551 patients were available for analysis. Their demographic and clinical data, stratified by decade of age, are provided in Table 1, along with the long‐term outcome data, shown here in Table 1 for convenience. Diabetes mellitus and hypertension rates increased progressively with age, while obesity and current smoking showed an opposite trend. Not surprisingly, there were relatively more women in the older age groups. Poor cardiorespiratory fitness (CRF)—identified by an FAC <80%—was highest in the youngest age group, likely reflecting referral bias in younger patients.
Table 1.
Baseline Characteristics and Outcomes in the 5 Age Groups for the Full Clinical Cohort
| Age: 30 to 39 (N=2664) | Age: 40 to 49 (N=6703) | Age: 50 to 59 (N=6047) | Age: 60 to 69 (N=3112) | Age 70 to 79 (N=1025) | P Value | |
|---|---|---|---|---|---|---|
| Age, y | 35.7±2.81 | 44.7±2.82 | 54.0±2.93 | 63.9±2.84 | 73.0±2.65 | <0.0001 |
| Female | 810 (30.4)1 | 2170 (32.4)1 | 2097 (34.7)2 | 2171 (40.8)3 | 408 (39.8)3 | <0.0001 |
| BMI, kg/m2 | 29.2±6.33 | 28.8±5.12 | 28.8±5.12 | 28.4±4.72 | 27.2±4.01 | <0.0001 |
| Hypertension | 285 (10.7)1 | 1000 (14.9)2 | 1492 (24.7)3 | 1059 (34.0)4 | 444 (43.3)5 | <0.0001 |
| Diabetes mellitus | 83 (3.1)1 | 273 (4.1)1 | 382 (6.3)2 | 248 (8.0)3 | 95 (9.3)3 | <0.0001 |
| Current smoker | 416 (16.2)4 | 892 (13.7)3 | 633 (10.8)2 | 196 (6.6)1 | 53 (5.3)1 | <0.0001 |
| Obesity | 1046 (39.3)4 | 2410 (36.0)2,3 | 2259 (37.4)3,4 | 1072 (34.4)2 | 255 (24.9)1 | <0.0001 |
| Poor CRF | 866 (32.5)4 | 1532 (21.4)1,2 | 1245 (20.6)1 | 724 (23.3)2,3 | 278 (27.1)3 | <0.0001 |
| Deaths | 40 (1.5)1 | 158 (2.4)1 | 287 (4.8)2 | 437 (14.0)3 | 350 (34.2)4 | <0.0001 |
| Cardiovascular death | 11 (0.4)1 | 50 (0.8)1 | 69 (1.1)1 | 133 (4.3)2 | 142 (13.8)3 | <0.0001 |
| Non‐cardiovascular death | 29 (1.1)1 | 108 (1.6)1 | 218 (3.6)2 | 304 (9.8)3 | 208 (20.3)4 | <0.0001 |
Continuous data are presented as mean±SD; categorical data as N (percentage of sample). Poor cardiorespiratory fitness (CRF) defined as functional aerobic capacity (FAC) <80% predicted for age and sex on exercise test. Different superscripts indicate a statistically significant difference between age groups. Superscript 1 is arbitrarily set at the lowest value for each variable. BMI indicates body mass index.
Exercise Test Results
Table 2 provides the exercise test data for the full clinical cohort of 19 551 patients. Because of the large sample size, even minor differences, such as in resting HR or highest rating of perceived exertion, reached statistical significance, although some age trends were pronounced, such as the well‐documented trend toward decreasing peak exercise HR with age.17 HR recovery also declined significantly with age, while the proportion of patients with HR recovery <13 bpm increased significantly. Not surprisingly, the number of patients taking HR‐lowering drugs increased significantly with age. The frequency of both positive and all abnormal exercise ECGs also increased steadily with age.
Table 2.
Exercise Test Results for the Full Clinical Cohort
| Age: 30 to 39 (N=2664) | Age: 40 to 49 (N=6703) | Age: 50 to 59 (N=6047) | Age: 60 to 69 (N=3112) | Age 70 to 79 (N=1025) | P Value | |
|---|---|---|---|---|---|---|
| FAC, % | 88.4±19.31 | 94.9±20.22 | 97.1±21.13 | 96.9±23.53 | 93.8±24.72 | <0.0001 |
| Resting HR, bpm | 78.7±13.25 | 77.1±12.84 | 76.4±12.73 | 75.5±12.92 | 74.0±12.41 | <0.0001 |
| Peak exercise HR, bpm | 176.6±15.35 | 170.8±15.74 | 162.9±16.03 | 152.7±17.32 | 142.7±17.31 | <0.0001 |
| Percent pred. peak HR | 96.3±8.31 | 97.6±8.82,3 | 98.0±9.53 | 97.1±10.92 | 95.9±11.51 | <0.0001 |
| HR‐lowering drug use | 142 (5.3)1 | 436 (6.5)1 | 563 (9.3)2 | 424 (13.6)3 | 196 (19.2)4 | <0.0001 |
| Peak HR <85% pred. | 241 (9.1)1 | 568 (8.5)1 | 583 (9.6)1 | 415 (13.3)2 | 169 (16.5)3 | <0.0001 |
| HR recovery | 21.2±8.15 | 20.5±8.04 | 18.9±7.93 | 16.0±7.52 | 12.9±7.41 | <0.0001 |
| HR recovery <13 bpm | 340 (12.8)1 | 993 (14.8)1 | 1279 (21.2)2 | 1022 (32.8)3 | 512 (50.0)4 | <0.0001 |
| Resting SBP, mm Hg | 118.8±14.91 | 120.0±15.62 | 124.0±17.33 | 129.5±18.74 | 133.2±20.35 | <0.0001 |
| Resting DBP, mm Hg | 79.7±11.02 | 79.7±11.12 | 80.5±10.83 | 79.9±10.92,3 | 77.9±11.31 | <0.0001 |
| Peak SBP, mm Hg | 171.0±23.61 | 174.1±23.62 | 179.4±24.63 | 182.3±25.24 | 177.4±20.23 | <0.0001 |
| Peak DBP, mm Hg | 72.9±16.81 | 74.9±15.82 | 77.4±15.53 | 78.7±15.14 | 77.2±14.83,4 | <0.0001 |
| Highest RPE | 18.3±0.84 | 18.3±0.83,4 | 18.2±0.83 | 18.0±0.92 | 17.9±1.01 | <0.0001 |
| Positive exercise ECG | 17 (0.6)1 | 150 (2.2)2 | 266 (4.4)3 | 217 (7.0)4 | 95 (9.3)5 | <0.0001 |
| Abnormal exercise ECG | 62 (2.3)1 | 300 (4.5)2 | 474 (7.8)3 | 356 (11.4)4 | 146 (14.2)5 | <0.0001 |
Continuous data are presented as mean±SD; categorical data as number (percentage of sample). Functional aerobic capacity (FAC) defined as 100%×actual time/predicted time on Bruce protocol for age and sex. Rating of perceived exertion measured on standard Borg Scale (6–20). Positive exercise ECG defined by standard methods. Abnormal exercise ECG defined as positive or abnormal but not diagnostic due to resting ST‐T abnormalities. Different superscripts indicate a statistically significant difference between age groups. Superscript 1 is arbitrarily set at the lowest value for each variable. DBP indicates diastolic blood pressure; HR, heart rate; RPE, rating of perceived exertion; and SBP, systolic blood pressure.
Factors Affecting Heart Rate Recovery
For the next step in the analysis, stepwise multivariate regression to determine factors significantly affecting HR recovery was performed. The results are shown in Table 3. The N for Table 3 was 18 887, as 664 patients (3.4%) had 1 missing covariate, principally smoking status. Intercept and age were highly significant in the regression, and 5 other factors—poor CRF, obesity, current smoking, hypertension, and diabetes mellitus—all showed significant (negative) effects on HR recovery, whereas sex, taking an HR‐lowering drug, and an abnormal exercise ECG did not affect HR recovery significantly. Only age and poor CRF contributed more than 1% to the model R 2. Based on these results, we formed our pure cohort by eliminating patients with the 6 HR recovery‐lowering factors, leaving us 7852 patients.
Table 3.
Multivariate Regression Analysis to Determine Factors Affecting HR Recovery
| Variable | Parameter estimate | Standard error | Partial R 2 | Model R 2 | F | P Value |
|---|---|---|---|---|---|---|
| Intercept | 31.79 | 0.29 | 12 107.3 | <0.0001 | ||
| Age | −0.21 | 0.0055 | 0.071 | 0.071 | 1445.6 | <0.0001 |
| Poor CRF | −3.92 | 0.14 | 0.063 | 0.134 | 1368.1 | <0.0001 |
| Obesity | −1.32 | 0.12 | 0.0060 | 0.140 | 132.5 | <0.0001 |
| Current smoking | −1.90 | 0.18 | 0.0051 | 0.145 | 113.2 | <0.0001 |
| Hypertension | −1.08 | 0.15 | 0.0027 | 0.148 | 60.9 | <0.0001 |
| Diabetes mellitus | −1.46 | 0.25 | 0.0016 | 0.149 | 35.8 | <0.0001 |
CRF indicates cardiorespiratory fitness; HR, heart rate.
HR Recovery in Age Groups
Table 4 shows means, medians, and interquartile range for HR recovery and percentage of patients with HR recovery less than the traditional cut point of 13 bpm in the first minute of active recovery according to decade of age in the pure cohort. We propose that these data represent the true effect of age on HR recovery. At each age group, HR recovery in the pure cohort was higher than in the full cohort, as expected. Average HR recovery is relatively constant from ages 30 to 39 through ages 50 to 59, then begins to decrease more rapidly as age increases. The percentage of patients with an HR recovery below the traditional cut point of 13 bpm is only 8.4% at age 30 to 39 but rises to 41.5% by age 70 to 79.
Table 4.
Heart Rate Recovery Distribution in the Pure Cohort
| Age: 30 to 39 (N=1056) | Age: 40 to 49 (N=2967) | Age: 50 to 59 (N=2390) | Age: 60 to 69 (N=1116) | Age 70 to 79 (N=323) | P Value | |
|---|---|---|---|---|---|---|
| Resting HR, bpm | 76.1±12.72 | 74.8±12.41 | 74.6±12.11 | 75.1±12.11,2 | 73.8±11.11 | <0.001 |
| Peak exercise HR, bpm | 182.4±11.45 | 176.4±11.94 | 168.6±12.93 | 159.1±13.72 | 149.4±14.61 | <0.001 |
| Female (%) | 327 (31.0)1 | 990 (33.4)1,2 | 880 (36.8)2 | 509 (45.6)3 | 147 (45.5)3 | <0.001 |
| HR‐lowering drug use (%) | 23 (2.2)1,2 | 54 (1.8)1 | 60 (2.5)1,2 | 43 (3.8)2 | 23 (7.1)3 | <0.001 |
| Mean HR recovery, bpm | 22.9±8.34 | 22.3±8.24 | 21.1±8.03 | 18.4±7.42 | 14.9±7.31 | <0.001 |
| Median HR recovery, bpm | 22 | 22 | 21 | 18 | 14 | |
| Interquartile range, bpm | 17–28 | 17–27 | 16–26 | 13–23 | 10–20 | |
| HR recovery <13 bpm | 87 (8.4)1 | 298 (10.0)1 | 311 (13.0)2 | 240 (21.5)3 | 134 (41.5)4 | <0.001 |
Continuous data are presented as mean±SD; categorical data as number (percentage of sample). Pure cohort created by excluding patients with hypertension, diabetes mellitus, current smoking, obesity (BMI ≥30 kg/m2), and poor cardiorespiratory fitness (functional aerobic capacity <80% age‐sex predicted). Different superscripts indicate a statistically significant difference between groups. Superscript 1 is arbitrarily set at the lowest value for each variable. HR indicates heart rate.
Outcomes
There were a total of 1271 deaths (6.5%) in the full cohort over an average follow‐up of 12.4±5.0 years. Consistent with exclusion of baseline cardiovascular disease and residence in a state (Minnesota) with overall low cardiovascular death rates, there were actually more non‐cardiovascular deaths (867, 4.4%) than cardiovascular deaths (405, 2.1%). Not surprisingly, women were at a lower age‐adjusted risk of death (0.70 with 95% confidence interval [0.62–0.90]), compared with men.
Using the full clinical cohort, an abnormal HR recovery by the traditional value of <13 bpm in the first minute post peak exercise during active recovery was a significant risk factor for death from any cause, cardiovascular death, and even non‐cardiovascular death in this primary prevention cohort. Hazard ratios for an abnormal HR recovery predicting death, cardiovascular death, and also non‐cardiovascular death are shown in Figure 1. Three models are shown for each outcome: unadjusted; adjusted for age and sex; and fully adjusted for age, sex, diabetes mellitus, hypertension, obesity, current smoking, and poor CRF. The hazard ratios in all models for all outcomes are statistically significant. The hazard ratio for abnormal HR recovery for cardiovascular versus non‐cardiovascular death was significantly higher in the unadjusted (P<0.0001), age‐sex–adjusted models (P<0.001), and fully adjusted (P<0.02) models. We have therefore demonstrated that an abnormal HR recovery is a significant predictor of death, cardiovascular death, and even non‐cardiovascular death in our primary prevention cohort.
Figure 1.
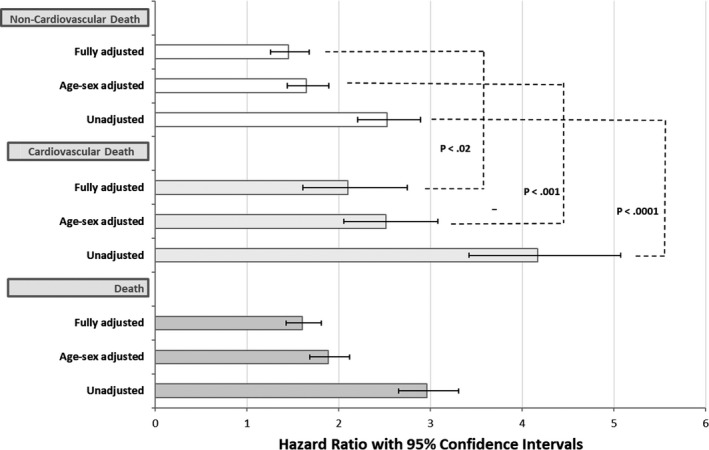
Hazard ratios with 95% confidence intervals for an abnormal heart rate recovery predicting death, cardiovascular death, and non‐cardiovascular death. Three models are shown for each outcome: unadjusted; adjusted for age and sex; fully adjusted for age, sex, diabetes mellitus, hypertension, obesity, current smoking, and poor cardiorespiratory fitness. Hazard ratios for cardiovascular and non‐cardiovascular death are compared by the Z‐score method.
For further analyses, we focused on cardiovascular mortality because its association with abnormal HR recovery was strongest.
We divided the full cohort according to 3 age groups, 30 to 59 (based on the minimal change in HR recovery across this age range, as mentioned above), then 60 to 69, and 70 to 79, as HR recovery declined significantly over these older age groups. Hazard ratios for cardiovascular death according to HR recovery in the 3 age groups are shown in Figure 2. Hazard ratios were statistically significant in all age groups and not significantly different among the 3 age groups.
Figure 2.
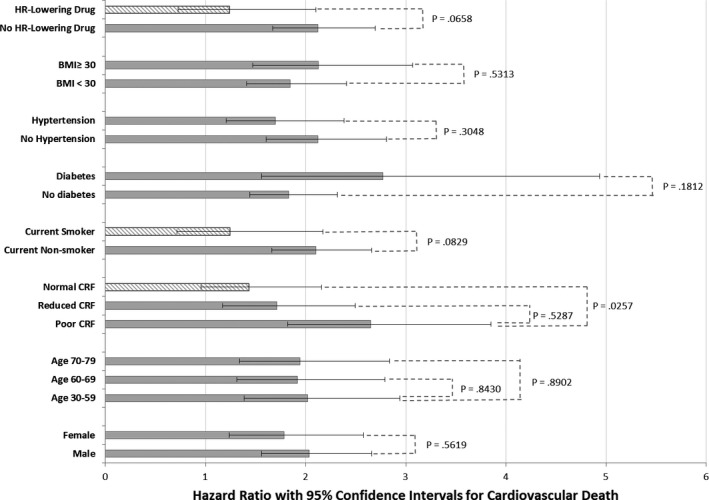
Hazard ratios with 95% confidence intervals for an abnormal heart rate (HR) recovery predicting cardiovascular death stratified by age, sex, presence of obesity, hypertension, diabetes mellitus, current smoking, use of HR‐lowering drug, and level of cardiorespiratory fitness (CRF). Poor CRF refers to functional aerobic capacity (FAC) <80%, reduced CRF to FAC 80% to 99%, and normal CRF to FAC ≥100% predicted. All models are fully adjusted for age, sex, diabetes mellitus, hypertension, obesity, current smoking, and poor CRF. Hazard ratios for cardiovascular death are compared by the Z‐score method. Hazard ratio bars filled with striped pattern indicate nonsignificant findings.
Figure 2 also shows the predictive value of abnormal HR recovery according to sex, 3 levels of CRF, smoking status, diabetes mellitus, hypertension, obesity, and use of an HR‐lowering drug. Abnormal HR recovery significantly predicted outcomes in all subgroups except current smokers, patients with normal CRF (≥100% FAC), and patients taking an HR‐lowering drug; in these subgroups, the confidence intervals in the hazard ratios included 1.0. As noted in the displayed P values, there was a significant difference in the hazard ratios in poor versus normal CRF, whereas the differences in hazard ratios according to HR‐lowering drug and current smoking were of borderline significance. On the other hand, abnormal HR recovery did not perform differently in males versus females; in reduced versus normal CRF; among the 3 age groups; or in patients with or without diabetes mellitus, hypertension, or obesity,
Discussion
This retrospective study confirmed the hypothesis that HR recovery has prognostic significance in a primary prevention cohort of almost 20 000 patients without a documented history of known cardiovascular disease. We show that HR recovery is a significant predictor of not only of total and cardiovascular death but non‐cardiovascular death as well. HR recovery is impaired by a number of cardiovascular risk factors including diabetes mellitus, hypertension, obesity, smoking, and poor CRF. HR recovery decreases significantly with age, especially above 50 years, but it performs equally well in all adult age groups. We also show that HR recovery predicts cardiovascular death equally in men versus women and patients with and without hypertension, diabetes mellitus, or obesity, but its prognostic performance is limited in patients with normal CRF, current smokers, and those taking HR‐lowering drugs.
The first comprehensive study of HR recovery was from Cole et al in 1999 and included patients who were referred for a first symptom‐limited exercise test and single‐photon‐emission computed tomography with thallium scintigraphy. These patients were candidates for initial angiography.2 Lauer postulated that HR recovery is a reflection of decreased vagal activity and established the formula calculating HR recovery as peak HR (HR at 1 minute in the active recovery) and defined it as abnormal if <13 bpm. Extended analyses on 9454 patients undergoing myocardial perfusion imaging confirmed that HR recovery is a powerful predictor of overall mortality independent of workload, presence of myocardial perfusion defects, and Duke treadmill score.3 Other studies have investigated the predictive value of HR recovery in various high‐risk populations such as heart failure, diabetes mellitus, and coronary artery disease.5, 18, 19, 20, 21 They all confirmed that HR recovery is an independent predictor of both cardiovascular and total mortality in their specific patient populations. In an American Heart Association statement published in 2005 in Circulation, exercise testing was identified as an important tool of assessing and refining prognosis, particularly when emphasis is placed on non‐ECG measures including HR recovery.22
Data on HR recovery in lower‐risk cohorts are more limited. The prognostic importance (all‐cause mortality and nonfatal myocardial infarction) of HR recovery has been confirmed in patients with chest pain with low Duke treadmill scores.23 Our group also previously identified the prognostic role of HR recovery in 2014 in a smaller population with 6546 patients, focusing on the association of HR recovery with exercise test parameters and all‐cause mortality.24 The ability of HR recovery to predict non‐cardiovascular mortality has not been previously addressed.
Two prior studies investigated the effect of sex on HR recovery and mortality.25, 26 Both suggested that abnormal HR recovery was independent of sex and had similar prognostic value in both men and women, as our study confirmed.
Two prior studies reported HR recovery in older patients,27, 28 but they did not compare the impact of age on its prognostic value, as we have done here.
Although obesity, hypertension, and diabetes mellitus are all affected the HR recovery, abnormal HR recovery had similar prognostic significance in patients with or without obesity, hypertension, and diabetes mellitus. Smokers also had lower HR recovery, but abnormal HR recovery was not a significant predictor of cardiovascular death in current smokers. We speculate that the profound effect of smoking on cardiovascular mortality may follow a different mechanism (acute plaque rupture) than other risk factors, including age, sex, obesity, hypertension, and poor CRF, but the limited utility of HR recovery in current smokers may also reflect low statistical power attributable to the relatively small number of current smokers in the cohort (11%).
Another important clinical question about HR recovery interpretation is the use of HR‐lowering drugs, mainly β‐blockers. Of the 1761 (8.9%) patients taking any HR‐lowering drug, almost all (1667, 94.7%) were taking a β‐blocker. Although there was no difference in HR recovery in patients taking or not taking HR‐lowering drug, an abnormal HR recovery did not significantly predict cardiovascular death in patients taking an HR‐lowering drug. We explain this paradoxical finding by pointing out that an abnormal HR recovery indicates reduced parasympathetic tone with a resultant imbalance between sympathetic and parasympathetic tone. Blocking sympathetic tone may restore that balance and eliminate the hazard associated with the abnormal HR recovery. However, as may also have been the case with current smoking, the small number of patients in this primary prevention cohort taking a β‐blocker may have also limited our ability to show significance in this group. Prior studies have tested the role of β‐blockers on HR recovery. One study found significant impact of β‐blocker use on HR recovery, but they included patients with cardiovascular diseases.29 Karnik et al stated that β‐blocker use improves HR recovery in patients with positive exercise stress echocardiography, does not affect HR recovery in patients with negative exercise stress echocardiography, and can be used for mortality prediction,30 the latter finding in contrast to what we have observed in a low‐risk primary prevention cohort. Also in apparent contrast to our findings, the study of Myers et al, which included 1910 male veterans, suggested that β‐blockade had minimal impact on the prognostic power of HR recovery.31 The interaction of β‐blockers and HR recovery may require further clarification.
Our study is also the first to stratify HR recovery according to CRF. Better CRF is clearly associated with better HR recovery, while the prognostic significance of an abnormal HR recovery is inversely related to CRF. The limited utility of HR recovery in patients with normal CRF may be a consequence of the low rate of cardiovascular mortality (109 deaths, 1.4%) in this subgroup. The principle that “fitness trumps other risk factors” has also been observed with respect to obesity and diabetes mellitus,32, 33 and also applies to ST‐segment changes during exercise.1, 34 What is more important, perhaps, is the observation that abnormal HR recovery is a strong predictor of cardiovascular death in patients with poor CRF, indicating that abnormal HR recovery is more than just “deconditioning.”
Conclusion
This study confirmed the hypothesis that HR recovery has prognostic significance in a primary prevention cohort. A unique finding is that an abnormal HR recovery predicts not only total mortality, as previously demonstrated, but is an even stronger predictor of cardiovascular death and, surprisingly, also predicts non‐cardiovascular death. We demonstrate that impaired HR recovery is associated with a number of well‐established cardiovascular risk factors, including diabetes mellitus, hypertension, current smoking, and poor CRF, but is not affected by patient sex or taking an HR‐lowering drug. We further show that HR recovery performs equally well in all adult age groups and in both men and women and patients stratified by obesity, hypertension, or diabetes mellitus. On the other hand, HR recovery is less useful in patients with normal CRF, in current smokers, or in those taking a β‐blocker. We strongly endorse previous recommendations that HR recovery should be measured and reported on every exercise test.14
Strength and Limitations
The strengths of our study include a large consecutive cohort with complete mortality follow‐up over a long time period. We have also divided mortality according to cardiovascular and non‐cardiovascular death. Exercise test data were robust and complete, and important data on comorbidities and pharmacotherapies were available. Our study reflected the limited racial diversity seen in Minnesota, so our results may not be applicable to more diverse racial or ethnic groups. Overall mortality was low, reflecting the status of Minnesota as a state with low total and cardiovascular mortality. However, we might speculate that exercise HR responses might be even more important in a higher‐risk population.
Exercise tests were conducted in a clinical environment, and patients were instructed to exercise to subjective fatigue. Gas exchange was not measured to confirm the level of metabolic effort by respiratory exchange ratio. We are using CRF at a single time point. The exercise test may thus reflect recent, rather than lifetime, physical activity patterns for some patients.
We identified HR recovery as the change during the first minute of the active recovery period; we thus cannot comment on the value of HR recovery as measured over different time points, such as at 2, 3 or 5 minutes post exercise or where an active recovery was not employed (as in stress echocardiography).
For our nonimaging noncardiopulmonary stress tests, we generally used the Bruce protocol, so we did not have a sufficient number of patients of cycle ergometer or treadmill tests on other protocols to perform similar analyses of HR recovery.
Sources of Funding
This work was supported by internal funding from the Department of Cardiovascular Medicine, Mayo Clinic.
Disclosures
None.
Acknowledgments
The authors would like to acknowledge the critical assistance of Laurie Barr for her help in extracting these data from the Mayo Integrated Stress Center database.
(J Am Heart Assoc. 2018;7:e008143 DOI: 10.1161/JAHA.117.008143.)
The abstract of this work was presented at the American Heart Association Scientific Sessions, November 11 to 15, 2017, in Anaheim, CA.
References
- 1. Kligfield P, Lauer MS. Exercise electrocardiogram testing: beyond the ST segment. Circulation. 2006;114:2070–2082. [DOI] [PubMed] [Google Scholar]
- 2. Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart‐rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–1357. [DOI] [PubMed] [Google Scholar]
- 3. Nishime EO, Cole CR, Blackstone EH, Pashkow FJ, Lauer MS. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA. 2000;284:1392–1398. [DOI] [PubMed] [Google Scholar]
- 4. Cole CR, Foody JM, Blackstone EH, Lauer MS. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med. 2000;132:552–555. [DOI] [PubMed] [Google Scholar]
- 5. Aijaz B, Squires RW, Thomas RJ, Johnson BD, Allison TG. Predictive value of heart rate recovery and peak oxygen consumption for long‐term mortality in patients with coronary heart disease. Am J Cardiol. 2009;103:1641–1646. [DOI] [PubMed] [Google Scholar]
- 6. Maeder MT, Munzer T, Rickli H, Schoch OD, Korte W, Hurny C, Ammann P. Association between heart rate recovery and severity of obstructive sleep apnea syndrome. Sleep Med. 2008;9:753–761. [DOI] [PubMed] [Google Scholar]
- 7. Yamada T, Yoshitama T, Makino K, Lee T, Saeki F. Heart rate recovery after exercise is a predictor of silent myocardial ischemia in patients with type 2 diabetes. Diabetes Care. 2011;34:724–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Phan TT, Shivu GN, Abozguia K, Davies C, Nassimizadeh M, Jimenez D, Weaver R, Ahmed I, Frenneaux M. Impaired heart rate recovery and chronotropic incompetence in patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:29–34. [DOI] [PubMed] [Google Scholar]
- 9. Curtis JM, Horton ES, Bahnson J, Gregg EW, Jakicic JM, Regensteiner JG, Ribisl PM, Soberman JE, Stewart KJ, Espeland MA. Prevalence and predictors of abnormal cardiovascular responses to exercise testing among individuals with type 2 diabetes: the Look AHEAD (Action for Health in Diabetes) study. Diabetes Care. 2010;33:901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sydo N, Sydo T, Merkely B, Carta KG, Murphy JG, Lopez‐Jimenez F, Allison TG. Impaired heart rate response to exercise in diabetes and its long‐term significance. Mayo Clin Proc. 2016;91:157–165. [DOI] [PubMed] [Google Scholar]
- 11. Khan MN, Pothier CE, Lauer MS. Chronotropic incompetence as a predictor of death among patients with normal electrograms taking beta blockers (metoprolol or atenolol). Am J Cardiol. 2005;96:1328–1333. [DOI] [PubMed] [Google Scholar]
- 12. Morise AP. Heart rate recovery: predictor of risk today and target of therapy tomorrow? Circulation. 2004;110:2778–2780. [DOI] [PubMed] [Google Scholar]
- 13. Corrado D, Basso C, Pavei A, Michieli P, Schiavon M, Thiene G. Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program. JAMA. 2006;296:1593–1601. [DOI] [PubMed] [Google Scholar]
- 14. Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, Mark DB, McCallister BD, Mooss AN, O'Reilly MG, Winters WL Jr, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Russell RO, Smith SC Jr. ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (committee to update the 1997 exercise testing guidelines). Circulation. 2002;106:1883–1892. [DOI] [PubMed] [Google Scholar]
- 15. Gibbons RJ, Balady GJ, Beasley JW, Bricker JT, Duvernoy WF, Froelicher VF, Mark DB, Marwick TH, McCallister BD, Thompson PD, Winters WL Jr, Yanowitz FG, Ritchie JL, Cheitlin MD, Eagle KA, Gardner TJ, Garson A Jr, Lewis RP, O'Rourke RA, Ryan TJ. ACC/AHA guidelines for exercise testing: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (committee on exercise testing). Circulation. 1997;96:345–354. [DOI] [PubMed] [Google Scholar]
- 16. Daida H, Allison TG, Squires RW, Miller TD, Gau GT. Peak exercise blood pressure stratified by age and gender in apparently healthy subjects. Mayo Clin Proc. 1996;71:445–452. [DOI] [PubMed] [Google Scholar]
- 17. Sydo N, Abdelmoneim SS, Mulvagh SL, Merkely B, Gulati M, Allison TG. Relationship between exercise heart rate and age in men vs women. Mayo Clin Proc. 2014;89:1664–1672. [DOI] [PubMed] [Google Scholar]
- 18. Gayda M, Bourassa MG, Tardif JC, Fortier A, Juneau M, Nigam A. Heart rate recovery after exercise and long‐term prognosis in patients with coronary artery disease. Can J Cardiol. 2012;28:201–207. [DOI] [PubMed] [Google Scholar]
- 19. Arena R, Myers J, Abella J, Peberdy MA, Bensimhon D, Chase P, Guazzi M. The prognostic value of the heart rate response during exercise and recovery in patients with heart failure: influence of beta‐blockade. Int J Cardiol. 2010;138:166–173. [DOI] [PubMed] [Google Scholar]
- 20. Kubrychtova V, Olson TP, Bailey KR, Thapa P, Allison TG, Johnson BD. Heart rate recovery and prognosis in heart failure patients. Eur J Appl Physiol. 2009;105:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheng YJ, Lauer MS, Earnest CP, Church TS, Kampert JB, Gibbons LW, Blair SN. Heart rate recovery following maximal exercise testing as a predictor of cardiovascular disease and all‐cause mortality in men with diabetes. Diabetes Care. 2003;26:2052–2057. [DOI] [PubMed] [Google Scholar]
- 22. Lauer M, Froelicher ES, Williams M, Kligfield P. Exercise testing in asymptomatic adults: a statement for professionals from the American Heart Association Council on Clinical Cardiology, Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention. Circulation. 2005;112:771–776. [DOI] [PubMed] [Google Scholar]
- 23. Maddox TM, Ross C, Ho PM, Masoudi FA, Magid D, Daugherty SL, Peterson P, Rumsfeld JS. The prognostic importance of abnormal heart rate recovery and chronotropic response among exercise treadmill test patients. Am Heart J. 2008;156:736–744. [DOI] [PubMed] [Google Scholar]
- 24. Dhoble A, Lahr BD, Allison TG, Kopecky SL. Cardiopulmonary fitness and heart rate recovery as predictors of mortality in a referral population. J Am Heart Assoc. 2014;3:e000559. doi: 10.1161/JAHA.113.000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wandell PE, Carlsson AC, Theobald H. Effect of heart‐rate recovery on long‐term mortality among men and women. Int J Cardiol. 2010;144:276–279. [DOI] [PubMed] [Google Scholar]
- 26. Daugherty SL, Magid DJ, Kikla JR, Hokanson JE, Baxter J, Ross CA, Masoudi FA. Gender differences in the prognostic value of exercise treadmill test characteristics. Am Heart J. 2011;161:908–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kokkinos P, Myers J, Doumas M, Faselis C, Pittaras A, Manolis A, Kokkinos JP, Narayan P, Papademetriou V, Fletcher R. Heart rate recovery, exercise capacity, and mortality risk in male veterans. Eur J Prev Cardiol. 2012;19:177–184. [DOI] [PubMed] [Google Scholar]
- 28. Carnethon MR, Sternfeld B, Liu K, Jacobs DR Jr, Schreiner PJ, Williams OD, Lewis CE, Sidney S. Correlates of heart rate recovery over 20 years in a healthy population sample. Med Sci Sports Exerc. 2012;44:273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Desai MY, De la Pena‐Almaguer E, Mannting F. Abnormal heart rate recovery after exercise as a reflection of an abnormal chronotropic response. Am J Cardiol. 2001;87:1164–1169. [DOI] [PubMed] [Google Scholar]
- 30. Karnik RS, Lewis W, Miles P, Baker L. The effect of beta‐blockade on heart rate recovery following exercise stress echocardiography. Prev Cardiol. 2008;11:26–28. [DOI] [PubMed] [Google Scholar]
- 31. Myers J, Tan SY, Abella J, Aleti V, Froelicher VF. Comparison of the chronotropic response to exercise and heart rate recovery in predicting cardiovascular mortality. Eur J Cardiovasc Prev Rehabil. 2007;14:215–221. [DOI] [PubMed] [Google Scholar]
- 32. Oktay AA, Lavie CJ, Kokkinos PF, Parto P, Pandey A, Ventura HO. The interaction of cardiorespiratory fitness with obesity and the obesity paradox in cardiovascular disease. Prog Cardiovasc Dis. 2017;60:30–44. [DOI] [PubMed] [Google Scholar]
- 33. Myers J, McAuley P, Lavie CJ, Despres JP, Arena R, Kokkinos P. Physical activity and cardiorespiratory fitness as major markers of cardiovascular risk: their independent and interwoven importance to health status. Prog Cardiovasc Dis. 2015;57:306–314. [DOI] [PubMed] [Google Scholar]
- 34. Higgins JP, Higgins JA. Electrocardiographic exercise stress testing: an update beyond the ST segment. Int J Cardiol. 2007;116:285–299. [DOI] [PubMed] [Google Scholar]


