Abstract
Background
A single NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) measurement is a strong prognostic factor in adult congenital heart disease. This study investigates NT‐proBNP profiles within patients with adult congenital heart disease and relates these to cardiovascular events.
Methods and Results
In this prospective cohort, 602 patients with adult congenital heart disease were enrolled at the outpatient clinic (years 2011–2013). NT‐proBNP was measured at study inclusion in 595 patients (median age 33 [IQR 25–41] years, 58% male, 90% NYHA I) and at subsequent annual visits. The primary end point was defined as death, heart failure, hospitalization, arrhythmia, thromboembolic event, or cardiac intervention; the secondary end point as death or heart failure. Repeated measurements were analyzed using linear mixed models and joint models. During a median follow‐up of 4.4 [IQR 3.8–4.8] years, a total of 2424 repeated measurements were collected. Average NT‐proBNP increase was 2.9 pmol/L the year before the primary end point (n=199, 34%) and 18.2 pmol/L before the secondary end point (n=58, 10%), compared with 0.3 pmol/L in patients who remained end point‐free (P‐value for difference in slope 0.006 and <0.001, respectively). In patients with elevated baseline NT‐proBNP (>14 pmol/L, n=315, 53%), repeated measurements were associated with the primary end point (HR per 2‐fold higher value 2.08; 95% CI 1.31–3.87; P<0.001) and secondary end point (HR 2.47; 95% CI 1.13–5.70; P=0.017), when adjusted for the baseline measurement.
Conclusions
NT‐proBNP increased before the occurrence of events, especially in patients who died or developed heart failure. Serial NT‐proBNP measurements could be of additional prognostic value in the annual follow‐up of patients with adult congenitive heart disease with an elevated NT‐proBNP.
Keywords: biomarker, congenital heart disease, natriuretic peptide, prognosis, serial measurements
Subject Categories: Biomarkers, Congenital Heart Disease
Clinical Perspective
What Is New?
NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) is associated with the risk of death or heart failure in patients with adult congenital heart disease (ACHD).
ACHD patients without cardiovascular events during 5‐year follow‐up had stable and low NT‐proBNP levels.
NT‐proBNP levels generally increased before the occurrence of an adverse event in patients with ACHD, especially before death or heart failure.
Repeated NT‐proBNP measurements were useful to improve prognostication in patients with elevated (>14 pmol/L) baseline values, since higher values, as well as (large) variations were associated with an increased risk of adverse cardiovascular events.
What Are the Clinical Implications?
ACHD patients with low NT‐proBNP concentrations (<14 pmol/L) have a favorable prognosis, and serial measurements will therefore be of limited additional value.
ACHD patients with elevated NT‐proBNP concentrations (>14 pmol/L) have an increased risk of death or heart failure.
ACHD patients with elevated NT‐proBNP concentrations that have additional increments over time represent a population that should receive close follow‐up.
A congenital heart defect is the most prevalent congenital anomaly, with an occurrence of 9 per 1000 live births.1 Because of the success of pediatric cardiology and cardiothoracic surgery, the population of patients with adult congenital heart disease (ACHD) is rapidly growing. Currently around 2.3 million patients with ACHD are estimated to be alive in Europe, already outnumbering children with congenital heart disease.2 Although survival has improved, residual lesions may cause progressive exercise intolerance and late complications such as arrhythmia, heart failure and sudden death.3, 4 This expanding demographic phenomenon is causing serious issues about the optimal management of patients with ACHD. Accurate biomarkers that enable risk stratification and monitoring of disease progression are therefore clearly needed.
Natriuretic peptides are firmly established diagnostic and prognostic tools in a variety of cardiovascular conditions, such as heart failure and coronary heart disease.5, 6 Previous studies have shown that NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) is also a strong independent prognostic factor in patients with ACHD.7, 8, 9 However, NT‐proBNP is known to have a considerable intra‐individual biological variability10 and progression of disease may lead to substantial changes in the NT‐proBNP level over time.11, 12, 13, 14 Repeated NT‐proBNP measurements could therefore provide additional prognostic information. The aim of this study was to investigate the temporal evolution of NT‐proBNP within individual patients and to relate this to cardiovascular events in a prospective cohort study of patients with ACHD.
Methods
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure upon reasonable request.
Study Design and Population
This prospective cohort study was conducted at the outpatient clinic of a tertiary care center between April 2011 and April 2013, that is responsible for the clinical care of all patients with ACHD in the South‐West region of The Netherlands. A total of 602 consecutive patients with a moderate or complex type of congenital heart defect15 who routinely visited the outpatient clinic were enrolled in the study cohort. Exclusion criteria were: aged <18 years, pregnancy, mild cardiac lesion (isolated atrial or ventricular septal defect), not capable of understanding and signing informed consent, or kidney failure (creatinine >200 μmol/L).
According to the study protocol, participants underwent physical examination by a cardiologist, 12‐lead electrocardiography, echocardiography and venous blood sampling at the day of study inclusion. During four subsequent years, all patients were structurally followed‐up by yearly visits to the ACHD outpatient clinic, including physical examination by a cardiologist, 12‐lead electrocardiography, and venous blood sampling. During follow‐up, patients were treated in accordance with ESC guidelines.16 Presence of pulmonary hypertension was defined as a right ventricular systolic pressure of >40 mm Hg, as measured by the tricuspid valve regurgitation maximal velocity (in patients with a systemic left ventricle) or mitral valve regurgitation maximal velocity (in patients with a systemic right ventricle) and the estimated right atrial pressure, in accordance with the guidelines.17 In patients with pulmonary stenosis we subtracted the gradient across the pulmonary valve from the right ventricular systolic pressure.18 The study protocol was approved by the Erasmus MC medical ethics committee and all participants provided written informed consent. Other details of the study protocol have been described previously.9, 19
N‐terminal pro‐B‐type natriuretic peptide
NT‐proBNP was measured at inclusion and at the scheduled yearly follow‐up visits. Therefore, a maximum of five subsequent measurements per patient were collected. NT‐proBNP measurements were not part of standard clinical care at the time this study was conducted. All measurements were performed for research purposes only, and decisions on patient management were made independently of any NT‐proBNP level. NT‐proBNP was directly measured in fresh serum samples at the clinical chemistry laboratory of the Erasmus MC, using a commercial electrochemiluminescence immunoassay (Roche Diagnostics, Rotkreuz, Switzerland). The limit of detection was 0.6 pmol/L, and all values below the limit of detection were reported as <0.6 pmol/L. Elevated NT‐proBNP was defined as >14 pmol/L (≈>125 pg/mL), based on the recommended low cut‐off for the diagnosis of heart failure in patients presenting with non‐acute symptoms.20
Definition and Assessment of Events
The primary end point was defined before the collection of data as a composite of the following adverse (cardiovascular) events: all‐cause mortality, heart failure (requiring initiation or change in heart failure medication, or requiring hospital admission), hospitalization for cardiac reasons (including heart failure, arrhythmia, thromboembolic event, cardiac intervention, endocarditis, or other cardiac reasons requiring admission 24 hours or longer), arrhythmia (symptomatic and recorded, or requiring treatment), thromboembolic event (ischemic cerebrovascular accident, pulmonary embolism or myocardial infarction), or any cardiac intervention (surgical or percutaneous). The secondary composite end point was defined as a composite of all‐cause mortality or heart failure.
Patients were prospectively and systematically followed for fatal and non‐fatal events by a yearly clinical evaluation at our institution according the study protocol until August 1, 2016. We retrieved information from electronic patient charts and also checked the survival status of patients in the Municipal Population Register. Suspect end point events were adjudicated by two experienced investigators (V.J.M.B. and J.W.R.‐H.) without knowledge of NT‐proBNP levels.
Statistical Analysis
Variables are presented as mean±standard deviation or median [interquartile range (IQR)], depending on the distribution of data. NT‐proBNP had a skewed distribution and was therefore log2 transformed for further analysis.
We adjudicated each component of the primary end point (death, heart failure, hospitalization for cardiac reasons, arrhythmia, thromboembolic event, and cardiac intervention) separately. In other words, patients were followed until the occurrence of the specific end point of interest and were not censored at another end point type. This was important because patients frequently experienced multiple event types simultaneously or subsequently. For patients with multiple events, event‐free survival was defined as the time from enrollment to the occurrence of the first event. Patients without any cardiovascular event were censored at the end of the follow‐up duration. Cox proportional hazards regression was performed to identify associations between baseline NT‐proBNP and the study end points. We conducted multivariable analyses with adjustment for baseline age (years), sex, congenital diagnosis (moderate versus complex), NYHA class (I versus II–III), cardiac medication use (angiotensin‐converting‐enzyme inhibitors, angiotensin II receptor blockers, ß‐blockers, diuretics, calcium blockers, or anti‐arrhythmic drugs), loss of sinus rhythm, systemic ventricular function (normal, mildly, moderately, or severely impaired), body mass index (kg/m2), saturation <90%, ≥1 re‐intervention after initial corrective surgery, and estimated glomerular filtration range (eGFR; mL/min, log2‐transformed). Data on covariates were 99.3% complete; we used imputation of the mean to account for missing values.
We developed linear mixed‐effects (LME) models to describe the temporal NT‐proBNP evolution, while accounting for the correlation between subsequent NT‐proBNP measurements within individuals. In patients with study end points, only NT‐proBNP measurements before the study end point were used. The model building and selection strategy for NT‐proBNP is further detailed in Data S1 and Table S1. We developed models with adjustment for the full set of covariates that were used in the Cox models. The proportion of within‐subject variation was calculated as the residual variance of the LME model divided by the total variance. To illustrate the average NT‐proBNP evolution in patients with and without events, an interaction term in the fixed part of the model was used to allow different slopes in patients with and without events. We additionally developed LME models with time point zero denoted as the moment at which patients experienced an event, or when they were censored because of reaching the end of the follow‐up duration without experiencing an event. Repeated NT‐proBNP measurements were denoted as years before this moment (ie, on a negative timescale). These models were used to calculate the increase in NT‐proBNP in the year before an event. To provide additional insight in the NT‐proBNP dynamics over time, we expressed the association between baseline and follow‐up NT‐proBNP values by the Spearman rank correlation coefficient. In addition, we calculated quartiles of the change in NT‐proBNP concentration in the first year (Δ year 1–year 0) and related this to baseline patient characteristics and study end points. Hence, a positive value indicates an increase in NT‐proBNP in the first year, and a negative value indicates a decrease.
We used joint modeling (JM) to investigate the association between the individual NT‐proBNP evolutions over time and the occurrence of study end points.21 JM combines the LME model estimating the NT‐proBNP level of an individual patient at time t, with a Cox model to relate that estimated value with the incidence of study end points after time t. Results are presented as hazard ratios (HR) with 95% confidence intervals (CI) for a 2‐fold higher value of the NT‐proBNP level at any time t between 0 and 4 years follow‐up. HRs were adjusted for the baseline NT‐proBNP level by including baseline NT‐proBNP as a separate variable in the Cox model. In addition, we constructed multivariate LME models including both serial NT‐proBNP and serial eGFR measurements, which were used in a multivariate joint model to investigate the additive value of serial NT‐proBNP measurements on top of serial eGFR measurements.
We repeated the JM analyses when stratified for normal versus elevated NT‐proBNP level at baseline, and stratified for the absence or presence of pulmonary hypertension. In addition, multiple explorative sub analyses were performed to check the robustness of specific assumptions with regard to end point definitions. Because a linear random slopes term did not significantly improve the fit of the model, we only used random intercepts and therefore did not calculate HRs for the slope. Finally, although the present study was not specifically designed to investigate NT‐proBNP profiles in patients with a cardiac intervention, we performed explorative post‐hoc analyses to evaluate whether we could detect a decrease in NT‐proBNP levels after a surgical or percutaneous valve intervention among individual patients.
We used IBM SPSS Statistics for Windows, version 21.0 (IBM Corp, Armonk, NY), and the nlme and JMBayes packages in R statistical software, version 3.3.1 (available at: https://www.r-project.org).22 Two‐sided P‐values <0.05 were considered statistically significant.
Results
Patient Characteristics
A total of 595 patients underwent NT‐proBNP measurement at study inclusion. The median age was 33 [IQR 25–41] years and 346 (58%) were male. Surgical repair of the congenital defect before study entry was performed in 540 patients (91%) at a median age of 3.7 [IQR 0.8–11.9] years), and 534 patients (90%) were in NYHA functional class I. The underlying congenital diagnoses were congenital aortic stenosis (n=137), aortic coarctation (n=109), repaired tetralogy of Fallot (including pulmonary atresia with ventricular septal defect, n=177), transposition of the great arteries (TGA) corrected by atrial switch operation (Mustard, n=65), TGA corrected by arterial switch operation (n=24), congenitally corrected TGA (n=20), complex TGA with ventricular septal defect or double‐outlet right ventricle corrected with a Rastelli type repair (n=11), functionally univentricular heart (n=43), and pulmonary arterial hypertension after a (corrected) atrial or ventricular septal defect (n=9). The majority of patients were in sinus rhythm (n=514, 87%). Systemic ventricular function was graded as normal (n=298, 50%), mildly impaired (n=210, 35%), moderately impaired (n=69, 12%), or severely impaired (n=18, 3%). Right ventricular systolic pressure was available in 420 patients (71%), of whom 33 patients (7.9%) had pulmonary hypertension. A flowchart of the patient selection and detailed clinical, electrocardiographic and echocardiographic characteristics of the study cohort, also expressed per baseline NT‐proBNP quartile, have been previously reported.9
Cardiovascular Events
Patients were followed‐up during a median of 4.4 [IQR 3.8–4.8] years. Survival status according to the Municipal Population Register and detailed follow‐up data about non‐fatal events were available in 590 patients (99.2%). The primary end point occurred in 199 patients (34%) and the secondary end point in 58 patients (10%). In total, 16 patients died. Causes of death were: end‐stage heart failure (n=8); cardiac arrest (n=4); sudden death, presumed cardiac (n=3); other (n=1). Other components of the primary end point were heart failure (n=52), hospitalization for cardiac reasons (n=151), arrhythmia (n=110), thromboembolic event (n=25), and cardiac intervention (n=113).
Prognostic Value of Baseline NT‐proBNP
Baseline NT‐proBNP was significantly associated with the primary end point (crude HR per 2‐fold higher value 1.55 [95% CI 1.43–1.68], P<0.001) and with the secondary end point (crude HR per 2‐fold higher value 2.30 [95% CI 1.97–2.68], P<0.001). The Kaplan‐Meier estimate of primary end point‐free survival at 4 years of follow‐up was 84.1% in patients with normal baseline NT‐proBNP (<14 pmol/L) and 55.1% in patients with elevated baseline NT‐proBNP. Secondary end point‐free survival at 4 years of follow‐up was 99.3% in patients with normal baseline NT‐proBNP (<14 pmol/L) and 83.2% in patients with elevated baseline NT‐proBNP. After complete adjustment for all covariates, the strength of the association was slightly attenuated but remained significant for both the primary end point (adjusted HR per 2‐fold higher value 1.39 [95% CI 1.23–1.57], P<0.001) and the secondary end point (adjusted HR per 2‐fold higher value 1.80 [95% CI 1.42–2.28], P<0.001). The prognostic value of baseline NT‐proBNP in this cohort has been previously published (using standardized HRs).9 Of note, baseline eGFR measurements were not predictive of the primary and secondary end point in these multivariable models (adjusted HR per 2‐fold higher value 1.00 [95% CI 0.58–1.72], P=0.993 and 2.49 [95% CI 0.78–7.89], P=0.123, respectively).
Temporal NT‐proBNP Evolution
During the follow‐up, a median of 4 yearly repeated NT‐proBNP measurements were collected per patient, resulting in a total of 2424 measurements. For the analysis of the primary end point 2009 measurements were available, and for the analysis of the secondary end point 2319 measurements were available. Figures 1 and 2 describe the average NT‐proBNP profiles in patients with and without the primary and secondary end point, respectively. Individual NT‐proBNP profiles are depicted in Figures S1 and S2. Of the total variation in the NT‐proBNP level, 89% was attributable to variation between subjects and 11% to variation within subjects.
Figure 1.
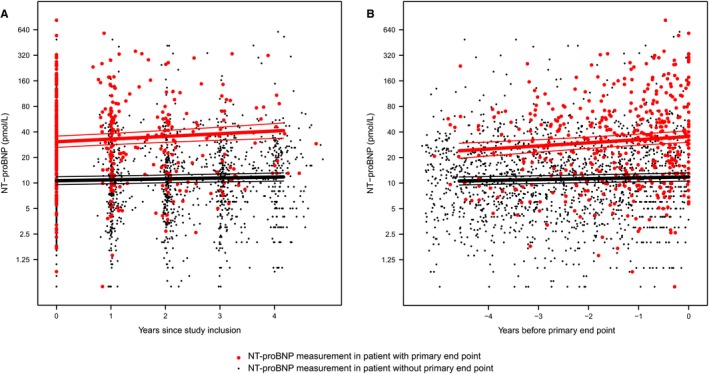
Average NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) evolution in patients with and without the primary end point. NT‐proBNP measurements after the event were deleted, therefore a total of 2009 measurements that occurred before the primary end point were used in this analysis. A, time point zero is denoted as the date of study inclusion (and first NT‐proBNP measurement). B, time point zero is denoted as the moment at which patients either experienced an event, or when they were censored because of reaching the end of the follow‐up duration without experiencing an event. We displayed all repeated NT‐proBNP measurements as years before this moment (ie, on a negative timescale).
Figure 2.
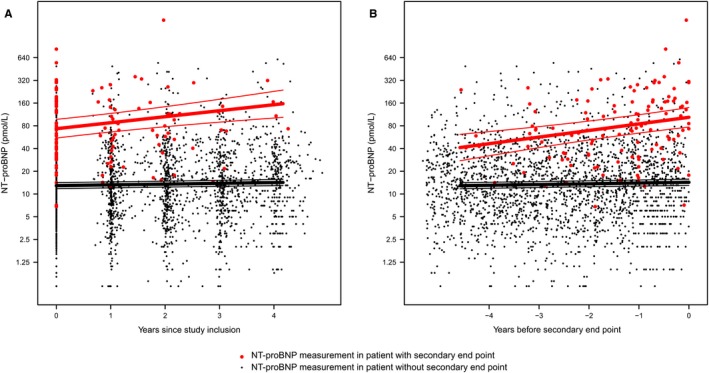
Average NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) evolution in patients with and without the secondary end point. NT‐proBNP measurements after the event were deleted, therefore a total of 2319 measurements that occurred before the secondary end point were used in this analysis. A, time point zero is denoted as the date of study inclusion (and first NT‐proBNP measurement). B, time point zero is denoted as the moment at which patients either experienced an event, or when they were censored because of reaching the end of the follow‐up duration without experiencing an event. We displayed all repeated NT‐proBNP measurements as years before this moment (ie, on a negative timescale).
The average baseline NT‐proBNP (intercept) in patients who reached the primary end point was 30.6 (95% CI 25.8–36.2) pmol/L, compared with 10.6 (95% CI 9.6–11.8) pmol/L in patients who remained event‐free during the follow‐up (P‐value <0.001). The average NT‐proBNP increase in the last year before the primary end point was 2.9 pmol/L, compared with 0.3 pmol/L in patients without the end point (P‐value for difference in slope 0.006).
In patients who reached the secondary end point, the average baseline NT‐proBNP was 73.7 (95% CI 56.1–96.8) pmol/L, compared with 12.9 (11.7–14.1) pmol/L in patients without the end point (P‐value <0.001). The average NT‐proBNP increase in the last year before the secondary end point was 18.2 pmol/L, compared with 0.3 pmol/L in patients without the secondary end point (P‐value for difference in slope <0.001).
The Spearman correlation coefficient of the baseline NT‐proBNP measurement with years 1, 2, and 3, respectively, was 0.84, 0.85, and 0.86 in subjects without any event; 0.85, 0.82, and 0.87 in subjects with the primary end point; and 0.88, 0.75, and 0.79 in subjects with the secondary end point (all P<0.001). In Table 1, quartiles of the change in NT‐proBNP concentration in the first year are related to baseline patient characteristics. Patients in the highest quartile (ie, with the largest increase in NT‐proBNP level within the first year) were significantly older, were more likely to be female, had a higher age at initial surgical repair, more frequently used cardiac medication, were in a higher NYHA class and had a worse systemic ventricular function. Of note, in this type of statistical analysis only a subset of the data is used and regression to the mean partly explains the differences in NT‐proBNP levels between patient subgroups. The exact results should therefore be carefully interpreted.
Table 1.
Association Between Baseline Patient Characteristics and Quartiles of the Difference in NT‐proBNP (pmol/L) Between Year 1 and Year 0
| No Measurement At Year 1 (n=33) | Change in NT‐proBNP (pmol/L) Calculated by Year 1 to Year 0 | P Value (n=562) | ||||
|---|---|---|---|---|---|---|
| Q1 (∆ <−4.1 pmol/L, n=140) | Q2 (∆ −4.1 to 0.0 pmol/L, n=144) | Q3 (∆ 0.0–5.1 pmol/L, n=138) | Q4 (∆ >5.1 pmol/L, n=140) | |||
| Clinical characteristics | ||||||
| Age, y | 32.8 [25.7–43.4] | 32.5 [25.5–41.4] | 31.2 [24.1–39.5] | 28.8 [22.2–37.6] | 37.0 [28.7–45.2] | <0.001 |
| Sex, male n (%) | 20 (61) | 69 (49) | 92 (64) | 93 (67) | 72 (51) | 0.003 |
| Surgical repair, n (%) | 30 (91) | 132 (94) | 124 (86) | 123 (89) | 131 (94) | 0.057 |
| Age at surgical repair, y | 1.7 [0.3–6.6] | 3.6 [0.9–13.8] | 3.4 [0.4–13.2] | 2.9 [0.8–9.4] | 6.5 [1.1–15.5] | 0.017 |
| Congenital diagnosis, n (%)a | 20 (61) | 83 (59) | 66 (46) | 72 (52) | 84 (60) | 0.054 |
| Cardiac medication use, n (%) | 9 (27) | 59 (42) | 38 (26) | 36 (26) | 70 (50) | <0.001 |
| Body mass index, kg/m2 | 24.2±5.4 | 24.9±4.5 | 24.9±4.6 | 24.2±4.2 | 24.9±4.0 | 0.423 |
| Heart rate, beats/minute | 72±17 | 73±13 | 73±13 | 73±13 | 76±13 | 0.261 |
| Systolic blood pressure, mm Hg | 122±12 | 127±18 | 128±16 | 126±16 | 125±16 | 0.531 |
| O2 saturation <90%, n (%) | 1 (3) | 4 (3) | 1 (1) | 6 (4) | 5 (4) | 0.309 |
| NYHA class, II to III n (%) | 4 (12) | 16 (11) | 7 (5) | 9 (7) | 25 (18) | 0.001 |
| ECG | ||||||
| Sinus rhythm, n (%) | 28 (85) | 117 (84) | 132 (92) | 121 (88) | 116 (83) | 0.108 |
| QRS duration, ms | 111 [97–132] | 115 [101–142] | 109 [99–126] | 112 [99–140] | 118 [100–152] | 0.069 |
| Echocardiogram | ||||||
| LA volume, mL/m2 b | 20 [14–27] | 22 [17–35] | 20 [15–25] | 20 [15–27] | 23 [16–34] | 0.009 |
| LV end‐diastolic volume, mL/m2 b | 57±13 | 67±20 | 61±17 | 65±19 | 62±20 | 0.125 |
| LV end‐systolic volume, mL/m2 b | 24±7 | 30±12 | 27±10 | 29±11 | 30±18 | 0.421 |
| LV ejection fraction, %b | 58±6 | 56±7 | 56±7 | 57±7 | 54±11 | 0.237 |
| RV fractional area change, % | 35±12 | 38±11 | 39±12 | 41±10 | 36±11 | 0.019 |
| Systemic ventricular function, n (%) | 0.001 | |||||
| Normal | 20 (61) | 67 (48) | 74 (51) | 86 (62) | 51 (36) | |
| Mildly impaired | 10 (30) | 45 (32) | 53 (37) | 44 (32) | 58 (42) | |
| Moderately impaired | 3 (9) | 21 (15) | 13 (9) | 7 (5) | 25 (18) | |
| Severely impaired | 0 (0) | 7 (5) | 4 (3) | 1 (1) | 6 (4) | |
| Laboratory results | ||||||
| eGFR, mL/min | 90 [89–90] | 90 [81–90] | 90 [83–90] | 90 [84–90] | 90 [79–90] | 0.048 |
| NT‐proBNP, pmol/L (year 0) | 14.4 [5.9–26.0] | 32.5 [18.7–59.0] | 8.9 [5.5–6.1] | 7.3 [3.7–15.3] | 24.2 [9.7–56.9] | <0.001 |
Values are reported as n (%), mean±SD or median [IQ1–IQ3]. Differences across quartiles of change are analyzed using the chi‐square test for categorical data, One‐Way ANOVA or Kruskal‐Wallis for continuous data (depending on the distribution). No trend tests were used, because not necessarily a linear relationship is assumed. eGFR indicates estimated glomerular filtration rate; LA, left atrial; LV, left ventricular; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; RV, right ventricular.
Congenital diagnosis of aortic stenosis, aortic coarctation or arterial switch operation (0) vs tetralogy of Fallot, Rastelli, systemic RV, univentricular heart or pulmonary arterial hypertension (1).
Left‐sided volumes were not measured in patients with a systemic right ventricle, functionally univentricular heart, pulmonary hypertension (n=137) or a poor acoustic window.
Prognostic Value of Repeated Measurements
The HRs of the repeatedly measured NT‐proBNP value indicate the risk of the primary and secondary end point for a 2‐fold higher value of NT‐proBNP, measured at any time t between 0 and 4 years of follow‐up (Table 2). These HRs were consequently higher than the HRs of the single baseline NT‐proBNP value, calculated using Cox regression (as reported in the text above). This indicates that the repeated NT‐proBNP measurements that were taken during the follow‐up were more predictive of the primary and secondary end point than the single baseline value.
Table 2.
Hazard Ratios Per 2‐Fold Higher Value of the Biomarker, Calculated Using a Joint Model
| HR (95% CI) | P Value | |
|---|---|---|
| Primary end point | ||
| Repeated NT‐proBNP measurementsa | 1.63 (1.49–1.76) | <0.001 |
| Adjusted for baseline characteristicsa , b | 1.55 (1.35–1.79) | <0.001 |
| Additionally adjusted for baseline NT‐proBNPb , c | 2.05 (1.23–3.66) | <0.001 |
| Repeated NT‐proBNP and eGFR measurementsd | ||
| Repeated NT‐proBNP measurements | 1.60 (1.50–1.71) | <0.001 |
| Repeated eGFR measurements | 0.88 (0.67–1.16) | 0.266 |
| Secondary end point | ||
| Repeated NT‐proBNP measurementsa | 2.46 (2.04–2.87) | <0.001 |
| Adjusted for baseline characteristicsa , b | 2.10 (1.64–2.75) | <0.001 |
| Additionally adjusted for baseline NT‐proBNPb , c | 4.44 (1.50–13.7) | <0.001 |
| Repeated NT‐proBNP and eGFR measurementsd | ||
| Repeated NT‐proBNP measurements | 2.51 (2.20–2.92) | <0.001 |
| Repeated eGFR measurements | 1.30 (0.87–2.11) | 0.166 |
CI indicates confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
HR for a patient with a 2‐fold higher NT‐proBNP level than another patient at any point in time during the follow‐up.
Adjusted for age (years), sex (0–1), congenital diagnosis (moderate vs complex), NYHA class (I vs II–III), cardiac medication use (0–1), loss of sinus rhythm (0–1), systemic ventricular function (0–3), body mass index (kg/m2), saturation <90%, ≥1 re‐intervention after initial corrective surgery, and eGFR (mL/min, log2‐transformed).
HR for a patient with a 2‐fold higher NT‐proBNP level than another patient at any point in time during the follow‐up, when two patients with the same baseline NT‐proBNP level are compared.
Analyzed using a multivariate joint model (including both repeated NT‐proBNP and repeated eGFR measurements).
The NT‐proBNP level at any point in time during the follow‐up was even stronger associated with the study end points when adjustment was performed for the baseline NT‐proBNP level. That is when two patients with the same NT‐proBNP level at baseline were compared, higher values during follow‐up were strongly predictive of study end points (Table 2).
The stratified analysis reported in Table 3 shows that the repeated measurements seem to carry additional prognostic value on top of the baseline value in both patients with a normal NT‐proBNP level and patients with an elevated NT‐proBNP level at baseline. However, this association is probably underpowered in the group of patients with a normal baseline NT‐proBNP, since the event rates of the primary end point and especially the secondary end point (which may be considered as most clinically relevant in this matter) were very low in these patients. A stratified analysis based on the absence (n=387) or presence (n=33) of pulmonary hypertension showed repeated NT‐proBNP measurements were associated with the primary and secondary end point in both groups (P<0.001 for all analyses). Additional explorative sub analyses excluding cardiac interventions from the primary end point, and separately analyzing heart failure requiring hospitalization versus heart failure requiring medication change or initiation did not yield different conclusions.
Table 3.
Hazard Ratios Per 2‐Fold Higher Value of NT‐proBNP, Calculated Using a Joint Model: Stratified Analysis in Patients With Baseline BNP <14 pmol/L (Normal) and >14 pmol/L (Elevated)
| Baseline NT‐proBNP <14 pmol/L (Normal, n=280) | Baseline NT‐proBNP >14 pmol/L (Elevated, n=315) | |||||
|---|---|---|---|---|---|---|
| No. Cases | Crude HR (95% CI) | P Value | No. Cases | Crude HR (95% CI) | P Value | |
| Primary end point | 50 | 149 | ||||
| Repeated NT‐proBNP measurementsa | 1.61 (1.10–2.52) | 0.011 | 1.65 (1.46–1.86) | <0.001 | ||
| Adjusted for baseline NT‐proBNPb | 2.03 (0.88–4.71) | 0.098 | 2.08 (1.31–3.87) | <0.001 | ||
| Secondary end point | 2 | 56 | ||||
| Repeated NT‐proBNP measurementsa | 3.67 (0.41–63.2) | 0.284 | 2.30 (1.89–2.86) | <0.001 | ||
| Adjusted for baseline NT‐proBNPb | 6.62 (0.10–1026) | 0.435 | 2.47 (1.13–5.70) | 0.017 | ||
CI indicates confidence interval; HR, hazard ratio; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
HR for a patient with a 2‐fold higher NT‐proBNP level than another patient at any point in time during the follow‐up.
HR for a patient with a 2‐fold higher NT‐proBNP level than another patient at any point in time during the follow‐up, when two patients with the same baseline NT‐proBNP level are compared.
Finally, in Figure 3, the association between the change in NT‐proBNP level in the first year (Δ year 1–year 0) and the study end points is depicted.
Figure 3.
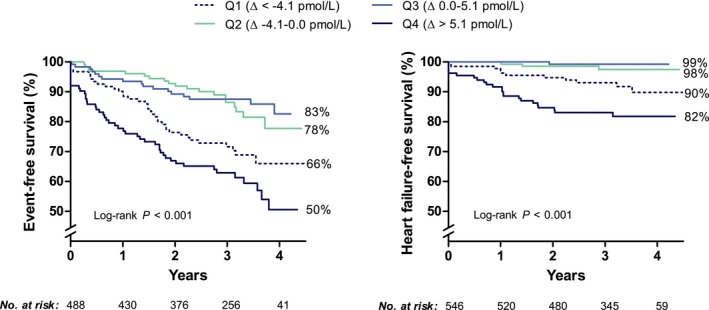
Association of the change in NT‐proBNP concentration in the first year (Δ year 1–year 0) with the primary and secondary study end point. Only a subset of the data could be used for this conventional type of analysis, because all measurements after year 1 were discarded, the time‐to‐event was recalculated from year 1 onwards and patients with a study end point in the first year were excluded. Hence, for the analysis of the primary and secondary end points, 488 and 546 patients were available, respectively.
NT‐proBNP Response after Cardiac Intervention
Of the 113 patients with a cardiac intervention, 41 patients underwent a surgical intervention (valvular replacement, n=35; valvular repair, n=4; aortic surgery, n=1; and defect closure, n=1) and 72 patients underwent a percutaneous intervention (ablation, n=21; pacemaker or ICD implantation, n=20; valve replacement, n=16; coronary intervention, n=5; defect closure, n=4; valvular balloon dilatation, n=4; aortic stenting, n=2). To explore whether interventions with a volume or pressure load reduction on the heart were followed by a decrease in NT‐proBNP level among individual patients, we plotted the NT‐proBNP profiles of all patients with a surgical valve intervention (valve replacement or valve repair) and with a percutaneous valve intervention (valve replacement or balloon dilatation) in Figure S3. Within individual patients, large reductions in the NT‐proBNP level were observed after the valvular intervention, especially in the surgical valve intervention group. Other exploratory analyses are shown in Figure S4 (NT‐proBNP profiles in patients with elective versus non‐elective cardiac interventions) and in Figure S5 (NT‐proBNP profiles in patients with sudden cardiac death).
Discussion
In this study, we describe yearly repeated NT‐proBNP measurements in adult patients with congenital heart disease for the first time. Baseline NT‐proBNP levels in patients with an event were markedly higher, especially in patients who developed heart failure or died. In addition, NT‐proBNP levels generally increased before the occurrence of an event, whereas patients without any cardiovascular event exhibited stable and low NT‐proBNP levels. Consequently, when 2 patients with the same NT‐proBNP level at baseline were compared, higher values measured during the follow‐up were strongly indicative of events. Repeated measurements were especially of value in patients with an elevated NT‐proBNP at baseline.
Repeated Biomarker Measurements
Repeated NT‐proBNP sampling allows the quantification of a change in levels over time. Although, in general, NT‐proBNP concentrations were relatively stable over time and the average increase in patients with the primary end point was modest, the occurrence of death or heart failure was preceded by a substantial change in NT‐proBNP level. The annual increase before the occurrence of death or heart failure was ≈25% of the baseline value, and may therefore be considered clinically meaningful. This is in line with previous studies in patients with chronic heart failure, showing that changes in NT‐proBNP level over time are associated with an individual's prognosis.11, 12, 13, 14
Using all repeated NT‐proBNP measurements in the JM yielded a higher HR compared with using only the baseline value in the Cox model. This may be explained by the fact that the JM incorporates all NT‐proBNP values that were subsequently taken during the follow‐up, and were thus also measured closer to the occurrence of adverse events. A measurement close to the occurrence of an adverse event could better reflect an individual's disease status at that time. Accordingly, in a study on chronic heart failure patients by Masson et al, a single NT‐proBNP value at 4 months of follow‐up was found to have a greater prognostic accuracy than baseline NT‐proBNP.11
Although the exact results must be interpreted with great caution because only a subset of the data is used and regression to the mean can play a role, the conventional statistical analyses (Figure 3) confirm our main analysis, and show that the 25% of patients with the largest increase in NT‐proBNP during the first year (>5.1 pmol/L) were at highest risk of the primary and secondary study end point. Interestingly, the 25% of patients with the largest decrease in NT‐proBNP (<−4.1 pmol/L) also had a high risk of the study end points. These data suggest that variations in NT‐proBNP level are associated with an increased risk of adverse events, and that the observed direction of change is dependent on the coincidental moment on which the biomarker is measured (note that our patients were clinically stable). Furthermore, patients with more variation in their NT‐proBNP level also appear to have higher baseline NT‐proBNP levels (Table 1). Comparable findings have been reported by Masson et al in patients with chronic heart failure, showing that patients with the largest absolute change in NT‐proBNP level within the first 4 months had a higher all‐cause mortality risk than patients with a relative stable level.11 Of note, the strength of evaluating >2 measurements per patient with LME models (as applied in this study), is that it becomes possible to adjust for this variation and to quantify gradual increases in the average NT‐proBNP level over time.
Regression to the Mean
An extreme measurement has the tendency to become less extreme when it is measured again, because of the intra‐patient fluctuations around a true mean. This statistical phenomenon is called regression to the mean, and especially occurs when an analyzed variable has a substantial within‐subject variability over time.23, 24, 25 NT‐proBNP is known to have a substantial intra‐individual biological variation.10 Regression to the mean is therefore an important concept to consider when serial measurements are analyzed, and differences between two subsequent measurements cannot be directly translated into a true increase or decrease. Multiple (>2) measurements provide more accurate estimations of an individual's true average NT‐proBNP level, and better allow analysis of biomarker evolution over time.
Most of the studies that have evaluated repeated NT‐proBNP measurements to date, have assessed the difference between two subsequent NT‐proBNP measurements and related relative, absolute and/or categorical changes to adverse events.11, 12, 13, 14 This is the first time that multiple repeated NT‐proBNP values are investigated, and that JM techniques are applied. An important advantage of JM is that the LME model part takes into account the random within‐subject variation (11% of the total variation in our study). Because this allows adjustment for the inherent biological variation and regression to the mean, this sophisticated statistical technique produces more reliable estimates of the true NT‐proBNP profile of individual patients.
Clinical Implications
Patients with an elevated NT‐proBNP level at baseline are clearly at higher risk of adverse cardiovascular events.9 In these patients, it seems reasonable to regularly check the NT‐proBNP level by an annual measurement, because especially the increase in the year before the occurrence of death or heart failure was substantial (≈25%). Moreover, in this group the repeated measurements were independently associated with study end points when adjustment was performed for the baseline NT‐proBNP measurement. Patients with high NT‐proBNP levels seem to have more biological variation; therefore, it could be important to perform multiple (>2) measurements, to detect a gradual increase in the NT‐proBNP over time. Patients with a high NT‐proBNP level and patients with a substantial increase over time may require more frequent follow‐up visits or timely initiation or expansion of heart failure medication. The optimal frequency of repeated measurements cannot be determined from these data and warrants future research.
In contrast, in patients with a normal NT‐proBNP level at baseline (<14 pmol/L, 47% of our study population) the NT‐proBNP concentration was relatively stable and the event rates were much lower. Especially the risk of death and heart failure was extremely low, which emphasizes the high negative predictive value of NT‐proBNP.9 Because of these low event rates, no significant additional value of repeated measurements could be shown in this group. Our pragmatic interpretation of these results is as follows: because the absolute risk of events is low in this group, even an increased relative risk for higher values during follow‐up (which did not reach statistical significance in our study) would add limited information. It therefore seems unnecessary to repeat NT‐proBNP measurements annually in these patients to obtain additional prognostic information. The clinical implication of not needing annual repeated measurements in patients with normal NT‐proBNP also includes a possible positive cost‐effect, because these measurements are relatively expensive. It may be advisable to update information on NT‐proBNP levels at a lower frequency, for instance every 4 or 5 years. To provide evidence for this, studies with a much longer follow‐up duration are needed.
Finally, NT‐proBNP might be useful as a biomarker to detect a response to successful interventions, because we observed large NT‐proBNP reductions among individual patients, especially after surgical valve interventions. Whether this could actually improve the patient selection and timing for re‐intervention warrants future studies including more frequent NT‐proBNP measurements that are scheduled at pre‐defined time points both before and after valvular interventions.
Study Limitations
Although we performed multivariable adjustment for multiple covariates such as age, sex, congenital diagnosis, NYHA class, cardiac medication use, rhythm, systemic ventricular function, body mass index, saturation, re‐intervention, and eGFR, this is an observational study subject to residual confounding. For instance, our cohort consisted of a relatively heterogeneous group of multiple congenital diagnoses, and we could only adjust for a dichotomized variable indicating a moderate (0) or complex (1) type of congenital diagnosis. Other variables that were not taken into account in this analysis may also play a role in the prognostication of patients with ACHD. Of note, patients with mild cardiac lesions such as isolated atrial or ventricular septal defect were excluded in this study, because the expected event rate in these patients is very low. This should be considered when these results are compared with other cohorts of patients with ACHD.
In this study, only repeated NT‐proBNP and repeated eGFR measurements were analyzed. However, also changes in other biomarkers with less day‐to‐day variations or other patient characteristics could play a role in the prognostication of patients with ACHD. We did not take repeated 2‐dimensional echocardiographic measurements into account, because these are known to have a relatively large measurement error and we therefore did not consider these suitable for accurate quantification of serial changes.26 Other repeatedly assessed clinical variables were not available. Ideally, updating multiple variables during each follow‐up visit could lead to more precise and individualized dynamic predictions.
Finally, we used composite end points in this study, and it should be taken into account that the individual components are not all of similar clinical importance to the patient.
Conclusions
A single measurement of NT‐proBNP at any time during the follow‐up is of important prognostic value in patients with ACHD who routinely visit the outpatient clinic. In patients with a normal NT‐proBNP level, it is not needed to annually repeat NT‐proBNP measurements to obtain additional prognostic information. In patients with an elevated NT‐proBNP level, yearly repeated measurements were strongly predictive of study end points and provided significant prognostic information beyond the baseline NT‐proBNP level. In these patients, serial measurements may therefore aid in the further optimization of individual follow‐up strategies, medical therapies, and timing of re‐interventions.
Sources of Funding
This study was supported by a grant from the Dutch Heart Foundation, The Hague, The Netherlands (grant number 2015T029) to Baggen.
Disclosures
None.
Supporting information
Data S1. Supplemental Methods.
Table S1. Linear Mixed‐Effects Model Building and Selection
Figure S1. Individual NT‐proBNP profiles in patients without and with the primary end point.
Figure S2. Individual NT‐proBNP profiles in patients without and with the secondary end point.
Figure S3. NT‐proBNP profiles in patients with a surgical or percutaneous valve intervention (at time point 0).
Figure S4. NT‐proBNP profiles in patients with elective versus non‐elective cardiac interventions. Of the 113 cardiac interventions, 17 were non‐elective (pacemaker or ICD implantation, n=10; surgical aortic valve replacement, n=4; percutaneous pulmonary valve dilatation, n=1; coronary intervention, n=1; ablation, n=1). No clear differences in the NT‐proBNP profiles were observed, probably because the group of patients with a non‐elective intervention was relatively small and heterogeneous.
Figure S5. NT‐proBNP profiles in patients with sudden cardiac death. The 7 patients with sudden cardiac death had higher NT‐proBNP levels at baseline that increased over time in all patients. Because of the low (expected) number of patients with sudden cardiac death, we did not aim to make predictions for this specific end point.
(J Am Heart Assoc. 2018;7:e008349 DOI: 10.1161/JAHA.117.008349.)
References
- 1. van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, Roos‐Hesselink JW. Birth prevalence of congenital heart disease worldwide: a systematic review and meta‐analysis. J Am Coll Cardiol. 2011;58:2241–2247. [DOI] [PubMed] [Google Scholar]
- 2. Webb G, Mulder BJ, Aboulhosn J, Daniels CJ, Elizari MA, Hong G, Horlick E, Landzberg MJ, Marelli AJ, O'Donnell CP, Oechslin EN, Pearson DD, Pieper EP, Saxena A, Schwerzmann M, Stout KK, Warnes CA, Khairy P. The care of adults with congenital heart disease across the globe: current assessment and future perspective: a position statement from the international society for adult congenital heart disease (ISACHD). Int J Cardiol. 2015;195:326–333. [DOI] [PubMed] [Google Scholar]
- 3. Bolger AP, Coats AJ, Gatzoulis MA. Congenital heart disease: the original heart failure syndrome. Eur Heart J. 2003;24:970–976. [DOI] [PubMed] [Google Scholar]
- 4. Webb GD. Challenges in the care of adult patients with congenital heart defects. Heart. 2003;89:465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, Clopton P, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC, McCullough PA; Breathing Not Properly Multinational Study I . Rapid measurement of B‐type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–167. [DOI] [PubMed] [Google Scholar]
- 6. Kragelund C, Gronning B, Kober L, Hildebrandt P, Steffensen R. N‐terminal pro‐B‐type natriuretic peptide and long‐term mortality in stable coronary heart disease. N Engl J Med. 2005;352:666–675. [DOI] [PubMed] [Google Scholar]
- 7. Eindhoven JA, van den Bosch AE, Jansen PR, Boersma E, Roos‐Hesselink JW. The usefulness of brain natriuretic peptide in complex congenital heart disease: a systematic review. J Am Coll Cardiol. 2012;60:2140–2149. [DOI] [PubMed] [Google Scholar]
- 8. Popelova JR, Kotaska K, Tomkova M, Tomek J. Usefulness of N‐terminal pro‐brain natriuretic peptide to predict mortality in adults with congenital heart disease. Am J Cardiol. 2015;116:1425–1430. [DOI] [PubMed] [Google Scholar]
- 9. Baggen VJ, van den Bosch AE, Eindhoven JA, Schut AW, Cuypers JA, Witsenburg M, de Waart M, van Schaik RH, Zijlstra F, Boersma E, Roos‐Hesselink JW. Prognostic value of N‐terminal pro‐B‐type natriuretic peptide, troponin‐T, and growth‐differentiation factor 15 in adult congenital heart disease. Circulation. 2017;135:264–279. [DOI] [PubMed] [Google Scholar]
- 10. Meijers WC, van der Velde AR, Muller Kobold AC, Dijck‐Brouwer J, Wu AH, Jaffe A, de Boer RA. Variability of biomarkers in patients with chronic heart failure and healthy controls. Eur J Heart Fail. 2017;19:357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Masson S, Latini R, Anand IS, Barlera S, Angelici L, Vago T, Tognoni G, Cohn JN, Val‐He FTI. Prognostic value of changes in N‐terminal pro‐brain natriuretic peptide in val‐heft (Valsartan Heart Failure Trial). J Am Coll Cardiol. 2008;52:997–1003. [DOI] [PubMed] [Google Scholar]
- 12. Savarese G, Musella F, D'Amore C, Vassallo E, Losco T, Gambardella F, Cecere M, Petraglia L, Pagano G, Fimiani L, Rengo G, Leosco D, Trimarco B, Perrone‐Filardi P. Changes of natriuretic peptides predict hospital admissions in patients with chronic heart failure: a meta‐analysis. JACC Heart Fail. 2014;2:148–158. [DOI] [PubMed] [Google Scholar]
- 13. Savarese G, Hage C, Orsini N, Dahlstrom U, Perrone‐Filardi P, Rosano GM, Lund LH. Reductions in N‐terminal pro‐brain natriuretic peptide levels are associated with lower mortality and heart failure hospitalization rates in patients with heart failure with mid‐range and preserved ejection fraction. Circ Heart Fail. 2016;9:e003105. [DOI] [PubMed] [Google Scholar]
- 14. Zile MR, Claggett BL, Prescott MF, McMurray JJ, Packer M, Rouleau JL, Swedberg K, Desai AS, Gong J, Shi VC, Solomon SD. Prognostic implications of changes in n‐terminal pro‐B‐type natriuretic peptide in patients with heart failure. J Am Coll Cardiol. 2016;68:2425–2436. [DOI] [PubMed] [Google Scholar]
- 15. Warnes CA, Liberthson R, Danielson GK, Dore A, Harris L, Hoffman JI, Somerville J, Williams RG, Webb GD. Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol. 2001;37:1170–1175. [DOI] [PubMed] [Google Scholar]
- 16. Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N, Gatzoulis MA, Gohlke‐Baerwolf C, Kaemmerer H, Kilner P, Meijboom F, Mulder BJ, Oechslin E, Oliver JM, Serraf A, Szatmari A, Thaulow E, Vouhe PR, Walma E; Task Force on the Management of Grown‐up Congenital Heart Disease of the European Society of C, Association for European Paediatric C, Guidelines ESCCfP . ESC Guidelines for the management of grown‐up congenital heart disease (new version 2010). Eur Heart J. 2010;31:2915–2957. [DOI] [PubMed] [Google Scholar]
- 17. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck‐Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H, Luis Zamorano J. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67–119.26320113 [Google Scholar]
- 18. Roberts JD, Forfia PR. Diagnosis and assessment of pulmonary vascular disease by Doppler echocardiography. Pulm Circ. 2011;1:160–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eindhoven JA, van den Bosch AE, Ruys TP, Opic P, Cuypers JA, McGhie JS, Witsenburg M, Boersma E, Roos‐Hesselink JW. N‐terminal pro‐B‐type natriuretic peptide and its relationship with cardiac function in adults with congenital heart disease. J Am Coll Cardiol. 2013;62:1203–1212. [DOI] [PubMed] [Google Scholar]
- 20. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A; ESC Committee for Practice Guidelines . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 21. Rizopoulos D. Dynamic predictions and prospective accuracy in joint models for longitudinal and time‐to‐event data. Biometrics. 2011;67:819–829. [DOI] [PubMed] [Google Scholar]
- 22. Rizopoulos D, Ghosh P. impliciian semiparametric multivariate joint model for multiple longitudinal outcomes and a time‐to‐event. Stat Med. 2011;30:1366–1380. [DOI] [PubMed] [Google Scholar]
- 23. Bland JM, Altman DG. Some examples of regression towards the mean. BMJ. 1994;309:780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol. 2005;34:215–220. [DOI] [PubMed] [Google Scholar]
- 25. Pocock SJ, Bakris G, Bhatt DL, Brar S, Fahy M, Gersh BJ. Regression to the mean in SYMPLICITY HTN‐3: implications for design and reporting of future trials. J Am Coll Cardiol. 2016;68:2016–2025. [DOI] [PubMed] [Google Scholar]
- 26. Gopal AS, Shen Z, Sapin PM, Keller AM, Schnellbaecher MJ, Leibowitz DW, Akinboboye OO, Rodney RA, Blood DK, King DL. Assessment of cardiac function by three‐dimensional echocardiography compared with conventional noninvasive methods. Circulation. 1995;92:842–853. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental Methods.
Table S1. Linear Mixed‐Effects Model Building and Selection
Figure S1. Individual NT‐proBNP profiles in patients without and with the primary end point.
Figure S2. Individual NT‐proBNP profiles in patients without and with the secondary end point.
Figure S3. NT‐proBNP profiles in patients with a surgical or percutaneous valve intervention (at time point 0).
Figure S4. NT‐proBNP profiles in patients with elective versus non‐elective cardiac interventions. Of the 113 cardiac interventions, 17 were non‐elective (pacemaker or ICD implantation, n=10; surgical aortic valve replacement, n=4; percutaneous pulmonary valve dilatation, n=1; coronary intervention, n=1; ablation, n=1). No clear differences in the NT‐proBNP profiles were observed, probably because the group of patients with a non‐elective intervention was relatively small and heterogeneous.
Figure S5. NT‐proBNP profiles in patients with sudden cardiac death. The 7 patients with sudden cardiac death had higher NT‐proBNP levels at baseline that increased over time in all patients. Because of the low (expected) number of patients with sudden cardiac death, we did not aim to make predictions for this specific end point.


