Abstract
Background
Chronic obstructive pulmonary disease (COPD) patients are at increased risk of respiratory related complications after cardiac surgery. It is unclear whether transcatheter aortic valve replacement (TAVR) or surgical aortic valve replacement (SAVR) results in favorable outcomes among COPD patients.
Methods and Results
Patients were identified from the Nationwide Inpatient Sample database from 2011 to 2014. Patients with age ≥60, COPD, and either went transarterial TAVR or SAVR were included in the analysis. A 1:1 propensity‐matched cohort was created to examine the outcomes. A matched pair of 1210 TAVR and 1208 SAVR patients was identified. Respiratory‐related complications such as tracheostomy (0.8% versus 5.8%; odds ratio [OR], 0.14; P<0.001), acute respiratory failure (16.4% versus 23.7%; OR, 0.63; P=0.002), reintubation (6.5% versus 10.0%; OR, 0.49; P<0.001), and pneumonia (4.5% versus 10.1%; OR, 0.41; P<0.001) were significantly less frequent with TAVR versus SAVR. Use of noninvasive mechanical ventilation was similar between TAVR and SAVR (4.1% versus 4.8%; OR, 0.84; P=0.41). Non‐respiratory‐related complications, such as in‐hospital mortality (3.3% versus 4.2%; OR, 0.64; P=0.035), bleeding requiring transfusion (9.9% versus 21.7%; OR, 0.38; P<0.001), acute kidney injury (17.7% versus 25.3%; OR, 0.63; P<0.001), and acute myocardial infarction (2.4% versus 8.4%; OR, 0.19; P<0.001), were significantly less frequent with TAVR than SAVR. Cost ($56 099 versus $63 146; P<0.001) and hospital stay (mean, 7.7 versus 13.0 days; P<0.001) were also more favorable with TAVR than SAVR.
Conclusions
TAVR portended significantly fewer respiratory‐related complications compared with SAVR in COPD patients. TAVR may be a preferable mode of aortic valve replacement in COPD patients.
Keywords: chronic obstructive pulmonary disease, surgical aortic valve replacement, transcatheter aortic valve replacement, transcutaneous aortic valve implantation
Subject Categories: Aortic Valve Replacement/Transcather Aortic Valve Implantation, Cardiovascular Surgery
Clinical Perspective
What Is New?
Patients with chronic obstructive pulmonary disease may benefit significantly by experiencing lower in‐hospital mortality as well as fewer respiratory‐related outcomes, including tracheostomy, re‐intubation, pneumonia, and acute respiratory failure.
Cost and length of stay were significantly lower and shorter in the transcatheter aortic valve replacement group in chronic obstructive pulmonary disease patients compared with surgical aortic valve replacement.
What Are the Clinical Implications?
Symptomatic, severe aortic stenosis patients with chronic obstructive pulmonary obstructive disease should strongly be considered for transcatheter rather than surgical aortic valve replacement given the significantly lower in‐hospital mortality rates and respiratory‐related complications.
Further study is warranted to further investigate whether different stage of chronic obstructive pulmonary disease similarly benefit from transcatheter compared with surgical aortic valve replacement strategy.
Chronic obstructive pulmonary disease (COPD) is a common comorbidity that portends significant impact on decision making among candidates undergoing aortic valve replacement for severe, symptomatic aortic stenosis.1 Surgical aortic valve replacement (SAVR), compared with transcatheter aortic valve replacement (TAVR), may require longer duration of mechanical ventilation, thus adversely affecting patients’ outcomes especially among those with COPD. Therefore, TAVR may confer added advantage over SAVR in COPD patients requiring aortic valve replacement. The negative impact of COPD has been evaluated in both TAVR and SAVR for those with and without COPD.2, 3, 4, 5 However, comparative outcome data between TAVR versus SAVR have not been extensively investigated.
TAVR could potentially offer clinical benefit especially in COPD patients given that it could be performed under local or moderate sedation without mechanical ventilation. In addition, cost and length of stay may favor TAVR compared with SAVR.
The main purpose of this analysis was to better define the optimal mode of aortic valve replacement in COPD patients using the Nationwide Inpatient Sample (NIS) database.
Methods
The data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure because the NIS database is publicly available data. The requirement for institutional review board approval was waived for the same reason.
Data Source
The NIS was used to derive patient‐relevant information between January 2011 and December 2014. The NIS is the largest publicly available all‐payer administrative claims‐based database and contains encounter‐level information of hospital stays compiled in a uniform format with privacy protection of individual patients. Each year, over 7 million hospital stays are sampled nationwide, which, when weighted, estimates more than 35 million hospitalizations annually. These data are stratified to represent ≈20% of US inpatient hospitalizations across different hospital and geographical regions (random sample). National estimates of the entire US hospitalized population were calculated using the Agency for Healthcare Research and Quality sampling and weighting method (https://www.hcup-us.ahrq.gov/).
Study Population
COPD patients aged ≥60 years with aortic stenosis who underwent SAVR (35.21, 35.22) or transarterial TAVR (35.05) during the study period were identified using International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM) codes. We excluded patients with a diagnosis of aortic insufficiency (395.1, 746.4, and 396.3) without a diagnosis of aortic stenosis (395.0, 395.2, 396.0, 396.2, 424.1, and 746.3) and those who had concomitant coronary artery bypass graft (36.1), mitral valve surgery (35.23, 35.24), pulmonary valve surgery (35.25, 35.26), tricuspid valve surgery (35.27, 35.28), and atrium or ventricular septum defect closure (35.5, 35.6, and 35.7) because combined surgery may affect the outcomes.
Covariates
Data on patient‐ and hospital‐level characteristics were provided for each patient in the NIS. However, identity variables were not included to preserve both patient and hospital privacies. Other potentially confounding exposures were included presence or absence of the Elixhauser comorbidities.
We examined both respiratory‐ and non‐respiratory‐related outcomes. Respiratory‐related outcomes included tracheostomy, noninvasive mechanical ventilation, reintubation, acute respiratory failure, and pneumonia. Non‐respiratory‐related outcomes were inpatient mortality, bleeding requiring transfusion acute kidney Injury, venous thromboembolism, stroke, postoperative sepsis, need for permanent pacemaker, and acute myocardial infarction. All of the clinical outcomes were estimated in‐hospital because only in‐hospital outcomes are available in the NIS database. Assessment of healthcare resource was performed by comparing nonroutine home discharge rate, cost, and length of stay. Hospital cost data were queried from the hospital accounting reports from the Centers for Medicare and Medicaid Services.
To account for demographic factors, we included race (whites, non‐Hispanic blacks, Hispanics, and others), sex (female and male), health insurance type (Medicare, Medicaid, private, self‐pay, and others), and income level based on the ZIP code (lowest quartile, second quartile, third quartile, and highest quartile). We also included for hospital‐level factors: region (Northeast, Midwest, South, and West), teaching status (rural, urban nonteaching, and urban teaching), and hospital size (small, medium, and large sizes).
Outcome and exposures were all identified with ICD‐9‐CM codes (Data S1, Table S1). To estimate the cost of hospitalization, the NIS data were merged with cost‐to‐charge ratios available from the Healthcare Cost and Utilization Project. We estimated the cost of each inpatient stay by multiplying the total hospital charge with cost‐to‐charge ratios.
Propensity Score
We performed a comparative analysis between outcomes of TAVR and SAVR in COPD patients. To account for potential confounding factors and reduce the effect of selection bias, a propensity‐score–matching model was developed to derive 2 matched groups for comparative outcomes analysis. Propensity score was calculated using multivariable logistic regression models derived from hospital level, clinical, and demographic covariates, including the Elixhauser comorbidities (Table S2). For calculation of the propensity score, the dependent variable was the TAVR versus SAVR procedure use. We performed matching on the propensity score implementing a greedy algorithm (gmatch macro) to construct a balanced match of TAVR cases to SAVR cases in a 1:1 ratio using a caliper of 0.1. Finally, 1210 TAVR cases were selected for the propensity‐matched population. We assessed the success of the match by performing McNemar's test for categorical variables and paired t test for normally distributed continuous variables.
Statistical Analysis
National estimates were calculated using the hospital discharge/trend weight provided for the NIS. Descriptive statistics are presented as percentages for categorical variables and as means with SDs for continuous variables. Baseline characteristics were compared using a chi‐square test for categorical variables and an independent‐samples t test for continuous variables. Univariate logistic regression was performed to estimate odds ratios (ORs) with 95% confidence intervals to compare tracheostomy, pneumonia, noninvasive mechanical ventilation, inpatient mortality, bleeding requiring transfusion, reintubation, acute respiratory failure, acute kidney injury, venous thromboembolism, postoperative sepsis, acute myocardial infarction, new pacemaker implantation, stroke, and nonroutine home discharge. Discrete numerical variables with an overdispersed count distributions—length of stay and continuous variables with a right‐skewed spread—cost of hospitalization were modeled using a generalized linear regression (GENMOD) with a negative binomial function and gamma function, respectively. The GENMOD procedures were performed using the CLASS, WEIGHT, and REPEATED statements to account for the clustered sampling and in‐hospital correlations. Matched categorical variables were presented as percentages and compared using McNemar's test. Matched continuous variables were presented as means with SDs and compared using a paired‐samples t test with the STRATA, CLUSTER, and WEIGHT and the SURVEYMEANS procedure to account for the complex clustered sampling methodology as recommended by the Healthcare Cost and Utilization Project. All variables were 100% present besides race (6.56%), health insurance type (0.17%), and average household income by ZIP code (2.13%). Because race, health insurance type and health insurance type were all missing at random, all missing observations for the 3 variables were excluded, and a complete case analysis was performed.
All the statistical tests were 2‐tailed, P<0.05 was chosen a priori, and the P values, ORs, and 95% confidence intervals were reported. All the statistical models were performed in SAS software (version 9.4; SAS Institute Inc, Cary, NC).
Results
Patient Characteristics
A total of unweighted 7548 (1595 TAVR and 5953 SAVR) patients were identified. In unmatched cohorts, as expected, TAVR patients were significantly older (80.6±7.1 versus 73.6±7.3 years; P<0.001), more often female (40.8% versus 34.1%; P<0.001), and had higher proportion of Elixhauser score ≥4 (71.6% versus 66.4%; P<0.001). After 1:1 propensity‐score matching, a matched pair of 1210 TAVR and 1208 SAVR patients was identified. Patient characteristics were well matched between the 2 groups (Tables 1 and 2).
Table 1.
Baseline Characteristics of COPD Participants Undergoing TAVR or SAVR Between 2011 and 2014 in the United States—Unmatched
| Total | TAVR | SAVR | P Value | |
|---|---|---|---|---|
| No. of observation, unweighted | 7548 | 1595 | 5953 | |
| No. of observation, weighted | 37 102 | 7945 | 29 157 | |
| Age, y, mean (SD) | 75.09 (7.81) | 80.63 (7.07) | 73.61 (7.32) | <0.0001 |
| Female, % | 35.59 | 40.84 | 34.16 | <0.0001 |
| Race/ethnicity, % | 0.006 | |||
| White | 88.35 | 89.30 | 88.10 | |
| Black | 3.90 | 3.20 | 4.09 | |
| Hispanic | 3.94 | 2.78 | 4.25 | |
| Asia | 0.81 | 0.88 | 0.79 | |
| Others | 3.00 | 3.84 | 2.78 | |
| COPD with oxygen dependence, % | 7.13 | 16.86 | 4.47 | <0.0001 |
| Peripheral vascular disease, % | 27.90 | 33.41 | 26.39 | <0.0001 |
| Hypertension, % | 77.30 | 78.93 | 76.86 | 0.081 |
| Rheumatological disorder, % | 4.15 | 6.03 | 3.64 | <0.0001 |
| Hypothyroidism, % | 15.26 | 19.93 | 13.98 | <0.0001 |
| Diabetes mellitus, % | 35.33 | 37.06 | 34.86 | 0.104 |
| Obesity, % | 21.41 | 18.37 | 21.73 | 0.004 |
| Chronic blood loss, % | 1.38 | 1.07 | 1.46 | 0.231 |
| Anemia deficiency, % | 22.98 | 26.64 | 21.98 | <0.0001 |
| Congestive heart failure, % | 4.47 | 13.03 | 2.14 | <0.0001 |
| Depression, % | 8.75 | 8.46 | 8.83 | 0.647 |
| Renal failure, % | 24.79 | 36.45 | 21.61 | <0.0001 |
| Liver disease, % | 2.12 | 2.18 | 2.11 | 0.849 |
| Lymphoma, % | 0.81 | 1.25 | 0.68 | 0.023 |
| Alcohol abuse, % | 3.29 | 1.56 | 3.76 | <0.0001 |
| Metastasis, % | 0.23 | 0.31 | 0.20 | 0.397 |
| Neurological disorders, % | 5.64 | 6.03 | 5.55 | 0.457 |
| Pulmonary circulatory disease, % | 1.68 | 4.59 | 0.89 | <0.0001 |
| Electrolyte, % | 36.01 | 25.60 | 38.85 | <0.0001 |
| Smoking, % | 49.69 | 38.85 | 52.64 | <0.0001 |
| Elixhauser score, % | 0.0004 | |||
| 0 to 1 | 2.25 | 1.88 | 2.35 | |
| 2 to 3 | 30.28 | 26.53 | 31.30 | |
| ≥4 | 67.47 | 71.59 | 66.35 | |
| Hospital bed size, % | <0.0001 | |||
| Small | 5.62 | 3.52 | 6.19 | |
| Medium | 19.62 | 16.13 | 20.57 | |
| Large | 74.77 | 80.35 | 73.24 | |
| Hospital location, % | <0.0001 | |||
| Rural | 2.26 | 0.63 | 2.70 | |
| Urban nonteaching | 24.19 | 9.53 | 28.18 | |
| Urban teaching | 73.55 | 89.84 | 69.11 | |
| Expected primary payer, % | <0.0001 | |||
| Medicare | 84.29 | 91.64 | 82.28 | |
| Medicaid | 1.68 | 0.56 | 1.99 | |
| Private | 11.74 | 5.85 | 13.35 | |
| Others | 2.28 | 1.94 | 2.38 | |
| Median household income in quartile, % | <0.0001 | |||
| 1st | 25.98 | 23.66 | 26.61 | |
| 2nd | 26.02 | 23.91 | 26.60 | |
| 3rd | 25.03 | 23.87 | 25.35 | |
| 4th | 22.96 | 28.56 | 21.44 | |
| Hospital region, % | 0.003 | |||
| Northeast | 22.78 | 24.81 | 22.22 | |
| Midwest | 20.31 | 18.36 | 20.85 | |
| South | 38.70 | 40.66 | 38.17 | |
| West | 18.21 | 16.17 | 18.76 |
COPD indicates chronic obstructive pulmonary disease; TAVR, transcatheter aortic valve replacement; SAVR, surgical aortic valve replacement.
Table 2.
Baseline Characteristics of COPD Participants Undergoing TAVR or SAVR Between 2011 and 2014 in the United States—Matched 1: 1
| Total | TAVR | SAVR | P Value | |
|---|---|---|---|---|
| No. of observation, unweighted | 2418 | 1210 | 1208 | |
| No. of observation, weighted | 11 910 | 6030 | 5879 | |
| Age, y, mean (SD) | 79.39 (6.86) | 79.52 (7.19) | 79.25 (6.50) | 0.335 |
| Female, % | 40.06 | 41.55 | 40.55 | 0.616 |
| Race/Ethnicity, % | 0.996 | |||
| White | 89.18 | 89.37 | 88.97 | |
| Black | 3.19 | 3.15 | 3.23 | |
| Hispanic | 3.15 | 3.15 | 3.14 | |
| Asia | 1.00 | 0.99 | 1.01 | |
| Others | 3.49 | 3.33 | 3.64 | |
| COPD with oxygen dependence, % | 12.71 | 12.55 | 12.87 | 0.814 |
| Coagulation disorder, % | 22.80 | 24.04 | 21.52 | 0.140 |
| Peripheral vascular disease, % | 30.29 | 29.76 | 30.83 | 0.568 |
| Hypertension, % | 79.49 | 79.06 | 79.92 | 0.604 |
| Rheumatological disorder, % | 5.66 | 5.63 | 5.68 | 0.967 |
| Hypothyroidism, % | 18.25 | 19.17 | 17.31 | 0.238 |
| Diabetes mellitus, % | 36.72 | 37.11 | 36.33 | 0.692 |
| Obesity, % | 20.17 | 19.57 | 20.79 | 0.459 |
| Chronic blood loss, % | 0.986 | 0.912 | 1.06 | 0.709 |
| Anemia deficiency, % | 24.85 | 24.64 | 25.07 | 0.810 |
| Congestive heart failure, % | 6.35 | 6.71 | 5.97 | 0.452 |
| Depression, % | 8.79 | 8.67 | 8.92 | 0.831 |
| Renal failure, % | 33.64 | 32.37 | 33.93 | 0.769 |
| Liver disease, % | 2.44 | 2.38 | 2.50 | 0.844 |
| Lymphoma, % | 1.45 | 1.40 | 1.49 | 0.866 |
| Alcohol abuse, % | 1.54 | 1.64 | 1.43 | 0.670 |
| Metastasis, % | 0.46 | 0.41 | 0.50 | 0.758 |
| Neurological disorder, % | 5.97 | 5.63 | 6.31 | 0.485 |
| Pulmonary circulatory disease, % | 2.48 | 2.74 | 2.21 | 0.414 |
| Electrolyte, % | 28.11 | 28.43 | 27.77 | 0.718 |
| Smoking, % | 40.52 | 39.62 | 41.43 | 0.364 |
| Elixhauser score, % | 0.727 | |||
| 0 to 1 | 2.00 | 2.23 | 1.78 | |
| 2 to 3 | 29.07 | 29.93 | 29.21 | |
| ≥4 | 68.92 | 68.83 | 69.01 | |
| Hospital bed size, % | 0.781 | |||
| Small | 3.98 | 4.22 | 3.73 | |
| Medium | 17.87 | 17.59 | 18.16 | |
| Large | 78.15 | 78.18 | 78.11 | |
| Hospital location, % | 0.953 | |||
| Rural | 0.72 | 0.74 | 0.69 | |
| Urban nonteaching | 11.68 | 11.85 | 11.51 | |
| Urban teaching | 87.60 | 87.40 | 87.80 | |
| Expected primary payer, % | 0.739 | |||
| Medicare | 91.66 | 91.48 | 91.86 | |
| Medicaid | 0.91 | 0.74 | 1.09 | |
| Private | 5.60 | 5.89 | 5.31 | |
| Others | 1.82 | 1.90 | 1.75 | |
| Median household income in quartile, % | 0.934 | |||
| 1st | 23.78 | 23.41 | 24.16 | |
| 2nd | 23.79 | 23.83 | 23.76 | |
| 3rd | 24.51 | 25.02 | 24.00 | |
| 4th | 27.91 | 27.74 | 28.08 | |
| Hospital region, % | 0.917 | |||
| Northeast | 26.05 | 25.77 | 26.34 | |
| Midwest | 18.25 | 17.85 | 18.66 | |
| South | 39.47 | 39.87 | 39.05 | |
| West | 16.23 | 16.50 | 15.95 |
COPD indicates chronic obstructive pulmonary disease; TAVR, transcatheter aortic valve replacement; SAVR, surgical aortic valve replacement.
Clinical Outcomes in Propensity‐Matched Cohorts
Tracheostomy (0.8% versus 5.8%; OR, 0.14; P<0.001), acute respiratory failure (16.4% versus 23.7%; OR, 0.63; P=0.002), reintubation (6.5% versus 10.0%; OR, 0.49; P<0.001), and pneumonia (4.5% versus 10.1%; OR, 0.41; P<0.001) were significantly lower in TAVR than SAVR. Use of noninvasive mechanical ventilation was similar in TAVR and SAVR groups (4.1% versus 4.8%; OR, 0.84; P=0.41).
Regarding non‐respiratory‐related clinical events, in‐hospital mortality (3.3% versus 4.2%; OR, 0.64; P=0.035), bleeding requiring transfusion (9.9% versus 21.7%; OR, 0.38; P<0.001), acute kidney injury (17.7% versus 25.3%; OR, 0.63; P<0.001), postoperative sepsis (0.83% versus 4.3%; OR, 0.19; P<0.001), and acute myocardial infarction (2.4% versus 8.4%; OR, 0.19; P<0.001) were significantly less frequent in TAVR than SAVR. New pacemaker placement was more frequently observed in TAVR (9.8% versus 6.2%; OR, 1.6; P=0.001). Other secondary end points not related to the respiratory system, including venous thromboembolism (1.3% versus 1.3%; OR, 1.01; P=0.98), and stroke (1.2% versus 1.2%; OR, 0.99; P=0.97) were comparable between groups (Figures 1 and 2).
Figure 1.
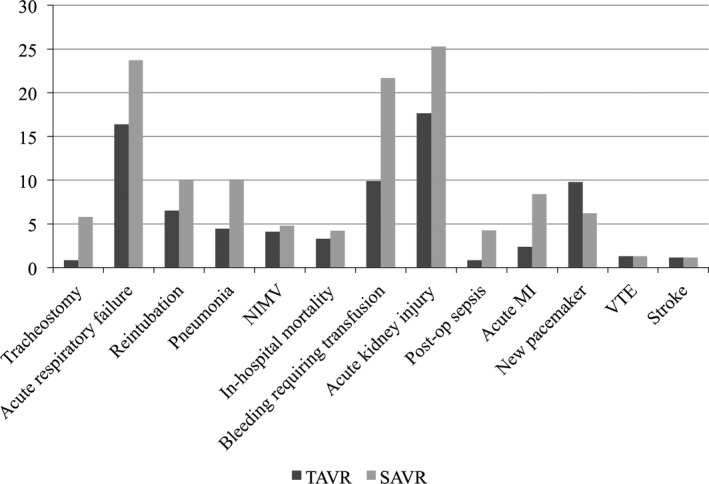
Incidence of perioperative complications. MI indicates myocardial infarction; NIMV, noninvasive mechanical ventilation; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement; VTE, venous thromboembolism.
Figure 2.
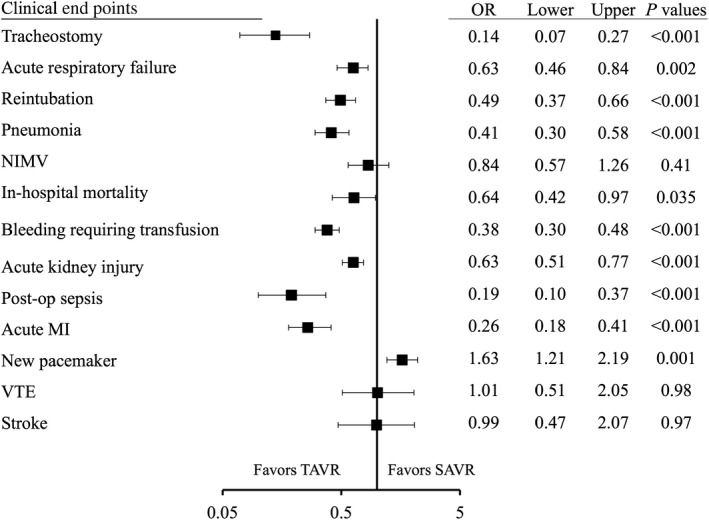
Forest plot for clinical end points between TAVR and SAVR in COPD patients. COPD indicates chronic pulmonary obstructive disease; MI, myocardial infarction; NIMV, noninvasive mechanical ventilation; OR, odds ratio; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement; VTE, venous thromboembolism.
Healthcare resources were utilized less in TAVR as represented by significantly lower cost ($56 099 versus $63 146; P<0.001), shorter hospital stay (mean, 7.7 versus 13.0 days; P<0.001), and less nonroutine home discharge (64.4% versus 80.9%; OR, 0.43; P<0.001) compared with SAVR.
Discussion
In this study from a large national database, we compared in‐hospital clinical outcomes for both respiratory‐related and non‐respiratory‐related end points between TAVR and SAVR in COPD patients. Most of the respiratory‐related outcomes significantly favored TAVR compared with SAVR. In addition, several non‐respiratory‐related outcomes were significantly lower in TAVR than SAVR.
Respiratory‐Related Outcomes
There is a paucity of data regarding respiratory‐specific outcomes between TAVR versus SAVR in COPD patients. Dvir et al reported comparative outcomes between TAVR and SAVR in chronic lung disease, from a subgroup analysis of the PARTNER (Placement of Aortic Transcatheter valve) trial. However, there were no respiratory‐specific outcomes reported, which would be of great clinical interest in this specific population.6 Another study by Auffret et al reported outcomes between TAVR versus SAVR in lower‐surgical‐risk (Society of Thoracic Surgical Predicted Risk of Mortality score <4% was 60.1%, 4–8% was 39.9%) patients with COPD. The primary end point (composite of 30‐day respiratory mortality, prolonged ventilation, reintubation, tracheostomy, acute respiratory distress syndrome, pneumonia, or pneumothorax) was similar between the 2 groups (10.6% versus 7.4%; P=0.32). In addition, most of these clinical events were also similar between TAVR and SAVR.7
Our study differed in several aspects from the study by Auffret et al. Most important, we included much larger cohorts in both arms, which enabled more‐detailed assessment of each outcome by providing more‐robust statistical power. Secondary, the surgical risk of our cohort was higher because the indication for TAVR was mainly for high or prohibitive surgical risk during 2011–2014. The high‐surgical‐risk cohort could have experienced incremental benefit from transarterial TAVR, which is much less invasive compared with SAVR or transapical TAVR. Last, we only included patients who underwent transarterial TAVR. The aforementioned studies included 20% to 30% of transapical TAVR, which may lead to increased postrespiratory complication.7 The vast majority (>95%) of TAVR was performed under general anesthesia between 2011 and 2014 according to the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. This suggests that both transarterial and transapical TAVR were performed under general anesthesia.8 Therefore, the shorter intubation period may be 1 of the reasons for favorable respiratory‐related outcomes in TAVR.
Several explanations may account for the lower respiratory complication rates in transarterial TAVR compared with SAVR. First, intubation duration could have been shorter in transarterial TAVR and therefore resulted in fewer respiratory complications. Although the NIS database does not capture the duration of intubation postprocedure, the significantly higher rate of tracheostomy and reintubation strongly suggests a longer duration of ventilation in SAVR. Previous studies have suggested that longer invasive ventilation time has been associated with longer intensive unit and total hospital stay, ventilator‐associated pneumonia, and reintubation.9, 10 Second, SAVR requires open sternotomy whereas transarterial TAVR does not. Open cardiac surgery would likely cause more pain and hence increased use of analgesia, including opiates. These medications could suppress the respiratory drive and may result in respiratory failure, atelectasis, or aspiration pneumonia, therefore causing adverse respiratory complications.
Our study fills in the gap from previous studies by investigating (1) comparative outcome between transarterial TAVR versus SAVR in predominantly high‐risk patients, (2) respiratory‐specific outcomes, and (3) including larger cohorts with solid statistical adjustments.
Non‐Respiratory‐Related Outcomes and Healthcare Resource
Outcomes of non‐respiratory‐related outcomes generally mirrored outcomes of TAVR versus SAVR in unselected patients. Increased risk of bleeding, acute kidney injury, and myocardial infarction may have had been further enhanced by exclusion by of those who had transapical TAVR given that this approach has been reported to be associated with increased risk of these perioperative outcomes.11, 12, 13 Significantly lower in‐hospital mortality is likely the result of lower respiratory‐ as well as non‐respiratory‐related clinical events. In‐hospital mortality from the NIS for COPD patients was numerically lower for TAVR when compared with 30‐day mortality of the PARTNER trial and the French registry (3.3% versus 7.4% versus 10.8%, NIS, PARTNER, and French registry, respectively).3, 6 Mortality was also numerically lower in the NIS for COPD patients compared with the PARTNER trial in the SAVR cohort (4.2% versus 10.9%).6 These comparisons, however, should be cautiously interpreted given that the nature of the data differs significantly (randomized control study versus all‐inclusive) and our study only included transarterial TAVR.
The lower cost and nonroutine discharge and shorter hospital stay in TAVR patients was similar to previous studies in TAVR versus SAVR in unselected patients.14 Mean cost of TAVR was $55 916, and it was significantly lower than that of SAVR. Medicare cost analysis showed that TAVR hospital costs were higher compared with SAVR (median, $50 200 versus $45 500; P<0.01), mainly attributed to medical supply costs despite shorter hospital and intensive care unit stay.15 However, a previous study revealed that minimalist TAVR (use of local anesthesia, fully percutaneous access/closure, monitor with transthoracic echocardiography, and conscious sedation) was related with significantly lower cost ($45 485 versus $55 377) compared with conventional TAVR (use of endotracheal intubation, general anesthesia, transesophageal echocardiography, and pulmonary artery catheter hemodynamic monitoring), which was the predominant strategy during this study period.16 The minimalist TAVR approach could further reduce respiratory‐related complications and result in additional decrease in cost and length of stay in transarterial TAVR in COPD patients. Analysis of NIS data after 2015, which was not available when these analyses were performed, is required to prove this hypothesis. In the more‐recent data from a large commercial TAVR database, conscious sedation was used in 27.4% with the new‐generation, self‐expanding valves.17 Use of new‐generation valves and minimalist TAVR technique may portend further benefit, especially in COPD patients.
Several limitations of our analysis should be acknowledged. First, we were unable to further assess the outcomes stratified to the severity of COPD. Those patients with only mild COPD patients may not have had benefit of transarterial TAVR and thus could have resulted in similar outcomes compared with TAVR. The subgroup analysis of TAVR versus SAVR in unselected patients suggested that 1‐year mortality and stroke did not differ when COPD was stratified by severity.14 However, because this was an exploratory analysis, further study is necessarily to determine whether or not the benefit of transarterial TAVR is applicable to all severity of COPD.
Second, this was a retrospective study using a large, national representative database, and baseline characteristics for TAVR and SAVR often differ owing to its different indication. We applied propensity‐matched analysis, but not all confounders were reported and could have resulted in biased results. However, we have included an extensive list for variable adjustments and we consider that results are robust. Third, the NIS does not capture the commonly used EuroSCORE or the Society of Thoracic Surgeons Score, and therefore we were unable to use these as propensity‐matched variables. Last, the NIS database is an administrative database that may be susceptible to coding errors. However, it has been applied to a wide variety of medical research and is considered a highly reliable database when analyzed appropriately.
In conclusion, TAVR portended significantly fewer respiratory‐related complications as well as certain non‐respiratory‐related complications compared with SAVR in COPD patients. In addition, TAVR resulted in less utilization of healthcare resources. TAVR may be a preferable mode of aortic valve replacement in COPD patients.
Disclosures
None.
Supporting information
Data S1.
Table S1. ICD‐9‐CM Codes
Table S2. Variables Used for Propensity Score Matching
(J Am Heart Assoc. 2018;7:e008408 DOI: 10.1161/JAHA.117.008408.)
References
- 1. Faggiano P, Frattini S, Zilioli V, Rossi A, Nistri S, Dini FL, Lorusso R, Tomasi C, Cas LD. Prevalence of comorbidities and associated cardiac diseases in patients with valve aortic stenosis. Potential implications for the decision‐making process. Int J Cardiol. 2012;159:94–99. [DOI] [PubMed] [Google Scholar]
- 2. Mok M, Nombela‐Franco L, Dumont E, Urena M, DeLarochellière R, Doyle D, Villeneuve J, Côté M, Ribeiro HB, Allende R, Laflamme J, DeLarochellière H, Laflamme L, Amat‐Santos I, Pibarot P, Maltais F, Rodés‐Cabau J. Chronic obstructive pulmonary disease in patients undergoing transcatheter aortic valve implantation: insights on clinical outcomes, prognostic markers, and functional status changes. JACC Cardiovasc Interv. 2013;6:1072–1084. [DOI] [PubMed] [Google Scholar]
- 3. Chopard R, Meneveau N, Chocron S, Gilard M, Laskar M, Eltchaninoff H, Iung B, Leprince P, Teiger E, Chevreul K, Prat A, Lievre M, Leguerrier A, Donzeau‐Gouge P, Fajadet J, Schiele F. Impact of chronic obstructive pulmonary disease on Valve Academic Research Consortium‐defined outcomes after transcatheter aortic valve implantation (from the FRANCE 2 Registry). Am J Cardiol. 2014;113:1543–1549. [DOI] [PubMed] [Google Scholar]
- 4. Spoon DB, Orszulak TA, Edell ES, Li Z, Nishimura RA. Risk of aortic valve replacement in patients with aortic stenosis and chronic obstructive pulmonary disease. J Heart Valve Dis. 2012;21:314–319. [PubMed] [Google Scholar]
- 5. Thourani VH, Chowdhury R, Gunter RL, Kilgo PD, Chen EP, Puskas JD, Halkos ME, Lattouf OM, Cooper WA, Guyton RA. The impact of specific preoperative organ dysfunction in patients undergoing aortic valve replacement. Ann Thorac Surg. 2013;95:838–845. [DOI] [PubMed] [Google Scholar]
- 6. Dvir D, Waksman R, Barbash IM, Kodali SK, Svensson LG, Tuzcu EM, Xu K, Minha S, Alu MC, Szeto WY, Thourani VH, Makkar R, Kapadia S, Satler LF, Webb JG, Leon MB, Pichard AD. Outcomes of patients with chronic lung disease and severe aortic stenosis treated with transcatheter versus surgical aortic valve replacement or standard therapy: insights from the PARTNER trial (placement of AoRTic TraNscathetER Valve). J Am Coll Cardiol. 2014;63:269–279. [DOI] [PubMed] [Google Scholar]
- 7. Auffret V, Becerra Munoz V, Loirat A, Dumont E, Le Breton H, Paradis JM, Doyle D, De Larochellière R, Mohammadi S, Verhoye JP, Dagenais F, Bedossa M, Boulmier D, Leurent G, Asmarats L, Regueiro A, Chamandi C, Rodriguez‐Gabella T, Voisine E, Moisan AS, Thoenes M, Côté M, Puri R, Voisine P, Rodés‐Cabau J. Transcatheter aortic valve implantation versus surgical aortic valve replacement in lower‐surgical‐risk patients with chronic obstructive pulmonary disease. Am J Cardiol. 2017;120:1863–1868. [DOI] [PubMed] [Google Scholar]
- 8. Holmes DR Jr, Nishimura RA, Grover FL, Brindis RG, Carroll JD, Edwards FH, Peterson ED, Rumsfeld JS, Shahian DM, Thourani VH, Tuzcu EM, Vemulapalli S, Hewitt K, Michaels J, Fitzgerald S, Mack MJ; STS/ACC TVT Registry . Annual outcomes with transcatheter valve therapy: from the STS/ACC TVT Registry. J Am Coll Cardiol. 2015;66:2813–2823. [DOI] [PubMed] [Google Scholar]
- 9. Safdar N, Dezfulian C, Collard HR, Saint S. Clinical and economic consequences of ventilator‐associated pneumonia: a systematic review. Crit Care Med. 2005;33:2184–2193. [DOI] [PubMed] [Google Scholar]
- 10. Camp SL, Stamou SC, Stiegel RM, Reames MK, Skipper ER, Madjarov J, Velardo B, Geller H, Nussbaum M, Geller R, Robicsek F, Lobdell KW. Can timing of tracheal extubation predict improved outcomes after cardiac surgery? HSR Proc Intensive Care Cardiovasc Anesth. 2009;1:39–47. [PMC free article] [PubMed] [Google Scholar]
- 11. Elhmidi Y, Bleiziffer S, Deutsch MA, Krane M, Mazzitelli D, Lange R, Piazza N. Acute kidney injury after transcatheter aortic valve implantation: incidence, predictors and impact on mortality. Arch Cardiovasc Dis. 2014;107:133–139. [DOI] [PubMed] [Google Scholar]
- 12. Wang J, Yu W, Jin Q, Li Y, Liu N, Hou X, Yu Y. Risk factors for post‐TAVI bleeding according to the VARC‐2 bleeding definition and effect of the bleeding on short‐term mortality: a meta‐analysis. Can J Cardiol. 2017;33:525–534. [DOI] [PubMed] [Google Scholar]
- 13. Liebetrau C, Kim WK, Meyer A, Arsalan M, Gaede L, Blumenstein JM, Fischer‐Rasokat U, Wolter JS, Dörr O, Schillinger S, Troidl C, Nef HM, Hamm CW, Walther T, Möllmann H. Identification of periprocedural myocardial infarction using a high‐sensitivity troponin I assay in patients who underwent transcatheter aortic valve implantation. Am J Cardiol. 2017;120:1180–1186. [DOI] [PubMed] [Google Scholar]
- 14. Brennan JM, Thomas L, Cohen DJ, Shahian D, Wang A, Mack MJ, Holmes DR, Edwards FH, Frankel NZ, Baron SJ, Carroll J, Thourani V, Tuzcu EM, Arnold SV, Cohn R, Maser T, Schawe B, Strong S, Stickfort A, Patrick‐Lake E, Graham FL, Dai D, Li F, Matsouaka RA, O'Brien S, Li F, Pencina MJ, Peterson ED. Transcatheter versus surgical aortic valve replacement: propensity‐matched comparison. J Am Coll Cardiol. 2017;70:439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCarthy FH, Savino DC, Brown CR, Bavaria JE, Kini V, Spragan DD, Dibble TR, Herrmann HC, Anwaruddin S, Giri J, Szeto WY, Groeneveld PW, Desai ND. Cost and contribution margin of transcatheter versus surgical aortic valve replacement. J Thorac Cardiovasc Surg. 2017;154:1872–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Babaliaros V, Devireddy C, Lerakis S, Leonardi R, Iturra SA, Mavromatis K, Leshnower BG, Guyton RA, Kanitkar M, Keegan P, Simone A, Stewart JP, Ghasemzadeh N, Block P, Thourani VH. Comparison of transfemoral transcatheter aortic valve replacement performed in the catheterization laboratory (minimalist approach) versus hybrid operating room (standard approach): outcomes and cost analysis. JACC Cardiovasc Interv. 2014;7:898–904. [DOI] [PubMed] [Google Scholar]
- 17. Sorajja P, Kodali S, Reardon MJ, Szeto WY, Chetcuti SJ, Hermiller J Jr, Chenoweth S, Adams DH, Popma JJ. Outcomes for the commercial use of self‐expanding prostheses in transcatheter aortic valve replacement: a report from the STS/ACC TVT Registry. JACC Cardiovasc Interv. 2017;10:2090–2098. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Table S1. ICD‐9‐CM Codes
Table S2. Variables Used for Propensity Score Matching


