Abstract
Background
The International Classification of Diseases (ICD) coding system does not recognize type 2 myocardial infarction (MI) as a separate entity; therefore, patients with type 2 MI continue to be categorized under the general umbrella of non–ST‐segment–elevation myocardial infarction (NSTEMI). We aim to evaluate the impact of type 2 MI on hospital‐level NSTEMI metrics and discuss the implications for quality and public reporting.
Methods and Results
We conducted a single‐center retrospective analysis of 1318 patients discharged with a diagnosis of NSTEMI between July 2013 and October 2014. The Third Universal Definition was used to define type 1 and type 2 MI. Weighted Kaplan–Meier curves were used to analyze risk of mortality and readmission. Overall, 1039 patients met NSTEMI criteria per the Third Universal Definition; of those, 264 (25.4%) had type 2 MI. Patients with type 2 MI were older, were more likely to have chronic kidney disease, and had lower peak troponin levels. Compared with type 1 MI patients, those with type 2 MI had higher inpatient mortality (17.4% versus 4.7%, P<0.0001) and were more likely to die from noncardiovascular causes (71.7% versus 25.0%, P<0.0001). Despite weighting for patient characteristics and discharge medications, patients with type 2 MI had higher mortality at both 30 days (risk ratio: 3.63; 95% confidence interval, 1.67–7.88) and 1 year (risk ratio: 1.98; 95% confidence interval, 1.44–2.73) after discharge. Type 2 MI was also associated with a lower 30‐day cardiovascular‐related readmission (risk ratio: 0.49; 95% confidence interval, 0.12–2.06).
Conclusions
NSTEMI metrics are significantly affected by type 2 MI patients. Type 2 MI patients have distinct etiologies, are managed differently, and have higher mortality compared with patients with type 1 MI. Moving forward, it may be appropriate to exclude type 2 MI data from NSTEMI quality metrics.
Keywords: coronary artery disease, mortality, myocardial infarction, readmission, troponin
Subject Categories: Quality and Outcomes, Mortality/Survival, Coronary Artery Disease, Acute Coronary Syndromes, Myocardial Infarction
Clinical Perspective
What Is New?
In current US clinical practice, type 2 myocardial infarction (MI) patients are grouped under the general umbrella of non–ST‐segment–elevation myocardial infarction (NSTEMI).
Understanding the impact of type 2 MI on overall NSTEMI outcomes is crucial.
Our results show that type 2 MI has significantly higher mortality than plaque‐rupture type 1 MI and has a significant impact on overall NSTEMI mortality.
Patients with type 2 MI are discharged less frequently on medications, part of acute coronary syndrome core measures, which may affect the overall rates at which patients are discharged on these medications in an NSTEMI population.
What Are the Clinical Implications?
Type 2 MI may skew MI metrics at different institutions, which could lead to inaccurate assessment of quality and performance measures by MI quality review programs; if assessments are based on patient populations that the metrics were not designed for, a result could be inappropriate penalties for healthcare providers.
Moving forward, it seems prudent to categorize type 2 MI patients separately, instead of under the general umbrella of NSTEMI, and to exclude these patients from acute MI quality metrics.
In 2007, the European Society of Cardiology, American College of Cardiology, American Heart Association, and World Heart Federation task force released an expert consensus document redefining myocardial infarction (MI) into 5 types.1 Given the increased sensitivity of biomarker assays, the Third Universal Definition of MI was published in 2012.2 Type 1 MI was referred to as acute coronary syndrome (ACS) caused by atherosclerotic plaque rupture, ulceration, fissure, or erosion leading to intraluminal thrombus formation and obstructed coronary blood flow. Type 2 MI was defined as MI not caused by plaque rupture but secondary to an imbalance between myocardial oxygen demand and supply related to an underlying cause. Type 3 MI is defined as cardiac death due to MI, and types 4 and 5 are MI associated with revascularization procedures.
Type 2 MI is common and has been associated with worse prognosis.3, 4, 5, 6 Although there has been widespread acceptance of the concept of type 2 MI, the current International Classification of Diseases (ICD) coding system does not recognize type 2 MI as a separate entity; therefore, its impact on hospital‐level MI outcomes has not been studied because these patients continue to be categorized under the general umbrella of non–ST‐segment–elevation myocardial infarction (NSTEMI). Compounding this is the fact that guidelines‐based post‐ACS treatment is expected to be followed in all patients with a diagnosis of MI. Although aspirin, statins, and beta blockers may have a role in the management of some patients with type 2 MI, using guideline‐based ACS therapy in every case of type 2 MI may be inappropriate. The purpose of this study, therefore, was to assess differences in patient characteristics, treatment, mortality, and hospital readmissions between type 2 and type 1 MI patients and to evaluate their potential impact on hospital‐level MI metrics and implications for quality and public reporting.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Patient Identification
Chart review was performed on consecutive patients discharged from a single healthcare center between July 1, 2013, and October 31, 2014, with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) code 410 (acute MI) at discharge. The requirement of patients to give informed consent was waived, and the study was approved by the institutional review board. All charts were reviewed, including admission notes, cardiology consultation notes, discharge summaries, and other notes relevant to abstract demographics and comorbidities, including age, sex, history of hypertension, diabetes mellitus, hyperlipidemia, tobacco abuse (current or remote), history of percutaneous coronary intervention, history of coronary artery bypass grafting, peripheral artery disease, chronic kidney disease, and body mass index (BMI). Clinical variables collected were chief complaint at presentation, primary diagnosis at admission, initial and peak cardiac troponin I (cTnI), ischemic ECG changes (ST‐segment depression, T‐wave inversion, and new left bundle‐branch block), echocardiographic data from studied hospitalization, coronary angiographic findings including details of intervention, and discharge medications restricted to aspirin, statin, beta blocker, and P2Y12 inhibitors.
Patients were excluded if they (1) were discharged with a diagnosis of ST‐segment–elevation MI; (2) did not meet the biomarker criteria of the universal definition; (3) were transferred to our facility from another facility, to prevent any discrepancy between different types of troponins used at different facilities; (4) did not have troponins measured within 24 hours of admission; or (5) presented with cardiac arrest in the emergency department. In patients who were readmitted, the earlier hospitalization was indexed to prevent duplication of patients. Each patient was classified as type 1 or type 2 MI using the Third Universal definition of MI2 criteria.
Type 1 MI
Detection of fall or rise of troponin with at least 1 value >99th percentile marked by the manufacturer. A 20% increase or decrease in the value of cTnI was marked as the threshold to meet the biomarker criteria (when initial cTnI was ≥0.5 ng/mL). In case the initial cTnI was close to 99th percentile (0.045–0.5 ng/mL), at least ≥3‐SD change (≥30% change) was required.7 If the biomarker criteria were met, one of the following additional criteria had to be satisfied: (1) symptoms suggestive of ischemia, (2) new or presumed ST‐T wave changes or left bundle‐branch block, (3) development of pathological Q waves in the ECG, (4) intracoronary thrombus noted on coronary angiography or autopsy, (5) new wall motion abnormality noted on echocardiography.
Type 2 MI
Fulfillment of criteria for type 1 MI as described, along with identification of an alternative (noncoronary) explanation known to cause a supply/demand mismatch either by decreasing myocardial oxygen supply or by increasing myocardial oxygen demand. The “condition other than coronary artery disease contributing to an imbalance between myocardial oxygen supply and/or demand” was defined as an alternate factor. The presence of an underlying alternate factor known to induce a supply/demand mismatch was required for a diagnosis of type 2 MI to be considered along with fulfillment of criteria of MI, as per the Third Universal Definition of MI.
Biomarker Criteria
The 99th percentile of the reference range for cTnI (Siemens Healthcare Diagnostics) used at our institution was determined to be 0.045 ng/mL, and a coefficient of variance <10% was estimated to be <0.04 ng/mL. A value >0.045 ng/mL was an initial requisite, along with a rise or fall pattern, as defined by the universal definition. Samples of cTnI were drawn during clinical care. Peak troponin levels were defined as the highest troponin level obtained during the hospitalization course, regardless of the number of values obtained.
Statistical Analyses
Patient characteristics, comorbidities, and ACS core measure medications (aspirin, statins, and beta blockers) prescribed at discharge were compared using χ2 and Wilcoxon–Mann–Whitney tests, as appropriate. P<0.05 was considered statistically significant. A Wilcoxon–Mann–Whitney test was also used to assess differences in peak troponin levels between type 1 and type 2 MI.
Mortality
Among patients who died during their hospitalization, the death summary from medical records was used to evaluate the cause of death, which was categorized as cardiovascular 8 and noncardiovascular causes. Both overall inpatient mortality and noncardiovascular related inpatient mortality were compared for type 1 and type 2 MI patients, using multivariable logistic regression after adjusting for confounding factors.
Kaplan–Meier survival curves were used to estimate the 30‐day and 1‐year risk of mortality after discharge among patients with type 1 and type 2 MI. Both risk differences and risk ratios (RRs) were calculated. Follow‐up mortality data were obtained by searching for the date of birth for each patient in the North Carolina State Center for Health Statistics and confirmed using the name and sex of each patient. To prevent missing death records, mortality was cross‐checked by searching for the name and then confirming the date of birth for each patient. This strategy helped us detect a few patients (≈1–2%) who had either the wrong date of birth listed or the name incorrectly spelled in the medical record or the mortality database. Follow‐up time for each patient was calculated by counting the number of days between their discharge and date of death. If no death date was found, the patient was assumed to be alive and to have 365 days of follow‐up. Patients who died during their inpatient hospitalization were excluded from all survival analyses.
Weighted Kaplan–Meier curves were used to estimate the standardized cumulative 30‐day and 1‐year risk of mortality after discharge.9 Standardized estimates were weighted using inverse probability of treatment weights to account for potential confounding by weighting patients so that the exposure is effectively randomized across the included covariates. Briefly, the inverse probability of treatment weight for each patient was estimated using multivariable logistic regression that modeled the probability of having type 2 MI, compared with type 1, using patient age, sex, BMI, hypertension, diabetes mellitus, hyperlipidemia, peripheral artery disease, chronic kidney disease, smoking status (current or remote), previous percutaneous coronary intervention, and previous coronary artery bypass grafting and whether aspirin, statins, or beta‐blockers were prescribed at discharge. Both age and BMI were modeled as restricted cubic splines to allow for the most flexibility in the continuous variables. Weights were stabilized using the marginal (ie, overall) probability of having a type 2 MI in the cohort. Forty‐one patients (4.3%) were missing BMI and/or discharge medication data and thus were excluded from all standardized analyses.
Thirty‐Day Readmission
The 30‐day readmission data, including primary discharge diagnosis code, were obtained from the cardiac quality committee by linking the account numbers from the original MI hospitalization and subsequent readmissions within 30 days following discharge. Readmissions were further categorized into cardiovascular (defined as a readmission related to MI, congestive heart failure, cerebrovascular accident, cardiac arrhythmia, cardiac device complication, or structural heart abnormality) and noncardiovascular causes. Similar to mortality, Kaplan–Meier survival curves were used to estimate the standardized 30‐day risk of any readmission and cardiovascular‐related readmission among patients discharged alive. Death was treated as a competing risk using an Aalen–Johansen estimator in all readmission analyses. Weighted survival curves, using the same inverse probability of treatment weight models described earlier, were used to estimate the standardized risk of any readmission and cardiovascular readmission.
Confidence intervals (CIs) for both the crude and standardized cumulative incidence of mortality and readmission were calculated using the standard deviation estimated from nonparametric bootstrapping. Specifically, 500 resamples with replacement were conducted, and the risk difference and RR were calculated using the procedures outlined above, and the standard deviation from the resamples was calculated.
All analyses were performed using SAS 9.4 (SAS Institute).
Results
A total of 1727 consecutive hospitalizations were coded as acute MI, including 299 hospitalizations for ST‐segment–elevation MI. After application of inclusion criteria, 1039 patients were included in the final analysis (Figure 1. Patients with cardiac arrest were excluded. We did not identify any patients meeting the definition of type 4 or type 5 MI. Of 1039 patients, 775 (74.6%) were classified as having type 1 MI and 264 (25.4%) were classified as having type 2 MI. Patients with type 2 MI were older (73 versus 65 years, P<0.0001), less likely to have hyperlipidemia (50.0% versus 57.1%, P=0.05), more likely to have chronic kidney disease (25.2% versus 16.8%, P=0.003), less likely to smoke (30% versus 42%, P=0.0006), and had lower BMI (26 versus 29, P<0.0001) compared with patients with type 1 MI (Table 1. Patients with type 2 MI were also less likely to undergo coronary angiography (25.8% versus 78.6%, P<0.0001), percutaneous coronary intervention (12.1% versus 46.8%, P<0.0001), and coronary artery bypass grafting (1.5% versus 11.7%, P<0.0001). No other significant differences in clinical characteristics were observed.
Figure 1.
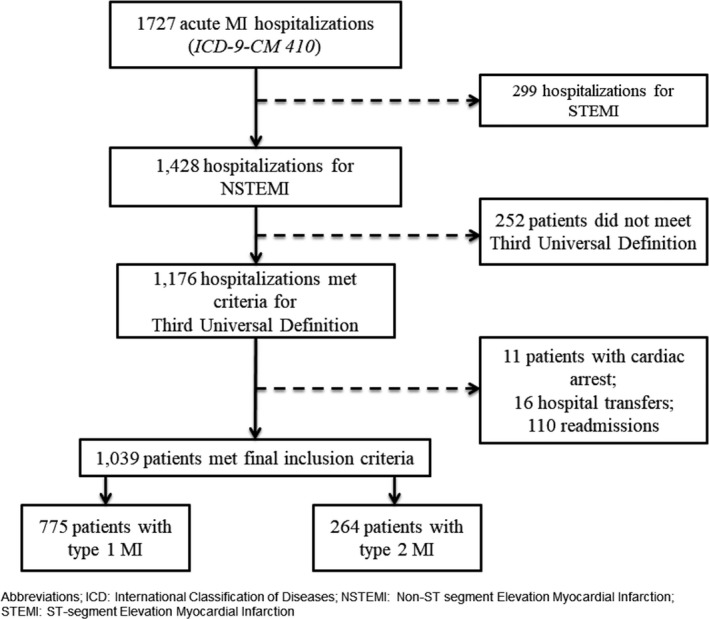
Consortium selection diagram for inclusion in the final analysis. ICD‐9‐CM indicates International Classification of Diseases, Ninth Revision, Clinical Modification; MI, myocardial infarction; NSTEMI, non–ST‐segment–elevation myocardial infarction; STEMI, ST‐segment–elevation myocardial infarction.
Table 1.
Patient Characteristics Stratified by Type of MI
| MI Type 1 (n=775) | MI Type 2 (n=264) | P Value | |
|---|---|---|---|
| Age, y, median (IQR) | 65 (55–76) | 73 (64–81) | <0.0001 |
| Male sex, n (%) | 434 (56) | 127 (48) | 0.03 |
| BMI, kg/m2, median (IQR) | 29 (25–35) | 26 (23–31) | <0.0001 |
| Hypertension, n (%) | 642 (83) | 225 (86) | 0.27 |
| Diabetes mellitus, n (%) | 371 (48) | 110 (42) | 0.09 |
| Hyperlipidemia, n (%) | 441 (57) | 131 (50) | 0.05 |
| PAD, n (%) | 111 (14) | 46 (18) | 0.21 |
| CKD, n (%) | 130 (17) | 66 (25) | 0.003 |
| Smoker, n (%) | 327 (42) | 80 (30) | 0.0006 |
| Previous PCI, n (%) | 209 (27) | 56 (21) | 0.07 |
| Previous CABG, n (%) | 112 (15) | 51 (19) | 0.06 |
| Ejection fraction, %, median (IQR) | 55 (40–55) | 53 (38–55) | 0.05 |
| Ischemic ECG, n (%)a | 369 (48) | 120 (45) | 0.48 |
| Initial troponin, ng/mL, median (IQR) | 0.33 (0.33–1.34) | 0.36 (0.10–1.16) | 0.57 |
| Initial troponin, n (%) | |||
| 1–2.9× URLb | 270 (35) | 79 (30) | 0.15 |
| 3–4.9× URL | 78 (10) | 33 (13) | 0.30 |
| ≥5× URL | 425 (55) | 152 (58) | 0.47 |
| Peak troponin, ng/mL, median (IQR) | 2.53 (0.66–10.00) | 1.57 (0.48–3.72) | <0.0001 |
| Peak troponin, n (%) | |||
| 1–2.9× URL | 48 (6) | 19 (7) | 0.56 |
| 3–4.9× URL | 34 (4) | 17 (6) | 0.19 |
| ≥5× URL | 693 (89) | 228 (86) | 0.18 |
| Procedures during index hospitalization | |||
| Coronary angiography, n (%) | 609 (79) | 68 (26) | <0.0001 |
| PCI, n (%) | 363 (47) | 32 (12) | <0.0001 |
| CABG, n (%) | 91 (12) | 4 (2) | <0.0001 |
| Medications on dischargec | |||
| Aspirin, n (%) | 637 (87) | 154 (73) | <0.0001 |
| Statin, n (%) | 646 (89) | 153 (72) | <0.0001 |
| Beta blocker, n (%) | 645 (88) | 165 (78) | 0.0001 |
| P2Y12 platelet inhibitor, n (%) | 430 (59) | 72 (34) | <0.0001 |
| Number of medications, median (IQR) | 3 (2–3) | 2 (2–3) | <0.0001 |
BMI indicates body mass index; CABG, coronary artery bypass grafting; CKD, chronic kidney disease; IQR, interquartile range; MI, myocardial infarction; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; URL, upper reference limit.
Ischemic ECG changes were defined as (1) new ST‐T wave changes, (2) new left bundle‐branch block, and (3) new Q waves. In cases of unknown baseline ECG, abnormality was assumed to be new.
Upper reference of our assay (cardiac troponin I) was 0.045 ng/mL.
Among patients alive at discharge, n=957; 10 type 1 MI patients and 7 type 2 MI patients were missing medication data; documented contraindication was not taken into consideration to assess medication use rates.
The median peak troponin levels for patients with type 1 MI (2.5 ng/mL; interquartile range: 0.7–10.0 ng/mL) was significantly higher than the median peak troponin levels for patients with type 2 MI (1.6 ng/mL; interquartile range: 0.5–3.7 ng/mL), P<0.0001 (Figure 2. The nonischemic triggers for type 2 MI (alternate factors) included infection or sepsis (28.0%), respiratory failure (17.8%), congestive heart failure (10.6%), hypertension (7.2%), and anemia (7.2%; Figure 3. The median length of stay was shorter for patients with type 1 compared with type 2 MI (4 days [interquartile range: 2–8] versus 7 days [interquartile range: 4–14], P<0.0001).
Figure 2.
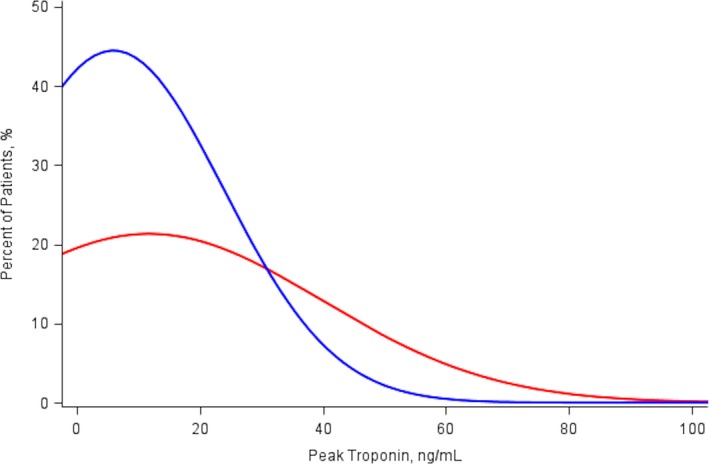
Distribution of peak troponin levels for patients with type 1 MI (red line) and type 2 MI (blue line). MI indicates myocardial infarction.
Figure 3.
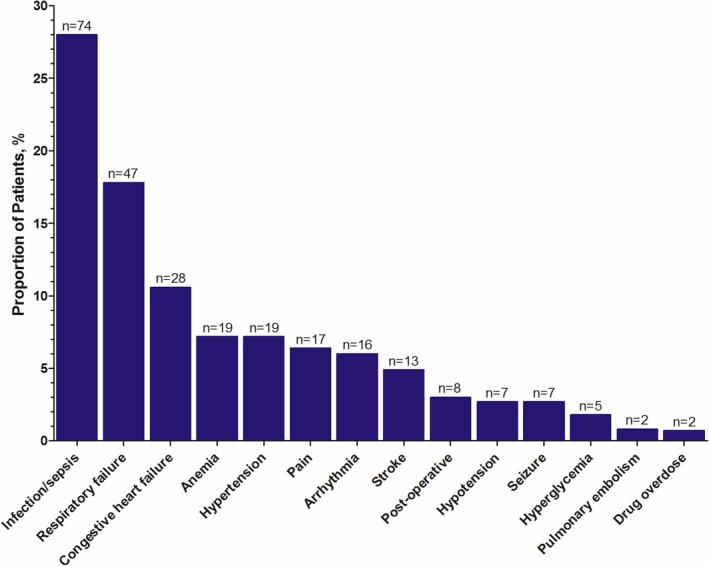
Prevalence of alternative factors in patients with type 2 myocardial infarction.
Overall. 82 patients (7.9%) died during their index hospitalization. Patients with type 2 MI were more likely to die than those with type 1 MI (17.4% versus 4.7%, P<0.0001). Patients with type 2 MI were also more likely to die from noncardiovascular causes (71.7% versus 25.0%, P<0.0001; Figure 4. Specifically, among type 1 MI patients, 23 (63.9%) died from MI, 8 (22.2%) died from infection or sepsis, 2 (5.6%) died from cardiac arrhythmias, 1 (2.8%) died from congestive heart failure, 1 (2.8%) died from stroke, and 1 (2.8%) died from respiratory failure. In comparison, among patients with type 2 MI, 23 (50.0%) died from infection or sepsis, 5 (10.9%) died from sudden cardiac death, 4 (8.7%) died from stroke, 4 (8.7%) died from cancer, 3 (6.5%) died from respiratory failure, 3 (6.5%) died from congestive heart failure, 2 (4.4%) died from gastrointestinal bleeds, 1 (2.2%) died from cardiac arrhythmia, and 1 (2.2%) died from cervical injury. Even after adjusting for patient demographics and comorbidities, type 2 MI was still significantly associated with increased odds of inpatient mortality (adjusted odds ratio: 3.76; 95% CI, 2.24–6.29) and noncardiovascular causes (adjusted odds ratio: 6.47; 95% CI, 1.74–23.99).
Figure 4.
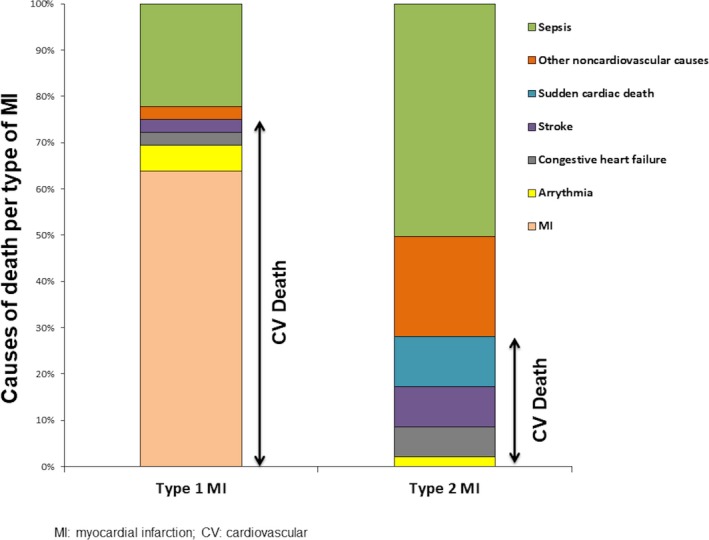
Comparison of causes of mortality between type 1 and type 2 MI. CV indicates cardiovascular; MI, myocardial infarction.
Medication data were available for 98.4% of patients discharged alive (n=942). Although the majority of patients were discharged on multiple medications, patients with type 1 MI were more likely to be discharged on aspirin (87.4% versus 72.6%, P<0.0001), statins (88.5% versus 72.2%, P<0.0001), and beta blockers (88.4% versus 77.8%, P=0.0001) compared with patients with type 2 MI (Table 1. Patients with type 1 MI were also more likely to be discharged on P2Y12 platelet inhibitors (59.0% versus 34.1%, P<0.0001).
Patients with type 2 MI were also significantly more likely to die within 30 days after discharge (11.9% versus 2.2%, P<0.0001) and 1 year after discharge (34.9% versus 12.4%, P<0.0001; Figure 5. Patients with type 2 MI were 5 times more likely to die within 30 days of hospital discharge (RR: 5.50; 95% CI, 2.73–11.08) and almost 3 times more likely to die within 1 year (RR: 2.80; 95% CI, 2.13–3.67; Table 2. After weighting for patient demographics, comorbidities, and medications prescribed at discharge, the patients with type 2 MI were still almost 4 times more likely to die within 30 days after discharge (RR: 3.63; 95% CI, 1.67–7.88) and 2 times more likely to die within 1 year (RR: 1.98; 95% CI, 1.44–2.73) compared with patients with type 1 MI (Table 2. Although patients with type 2 MI made up only 25.4% of our patient population, they constituted 56.1% of all NSTEMI patients who died during hospitalization, 61.9% of those who died within 30 days after discharge, and 45.2% of those who died within 1 year after discharge.
Figure 5.
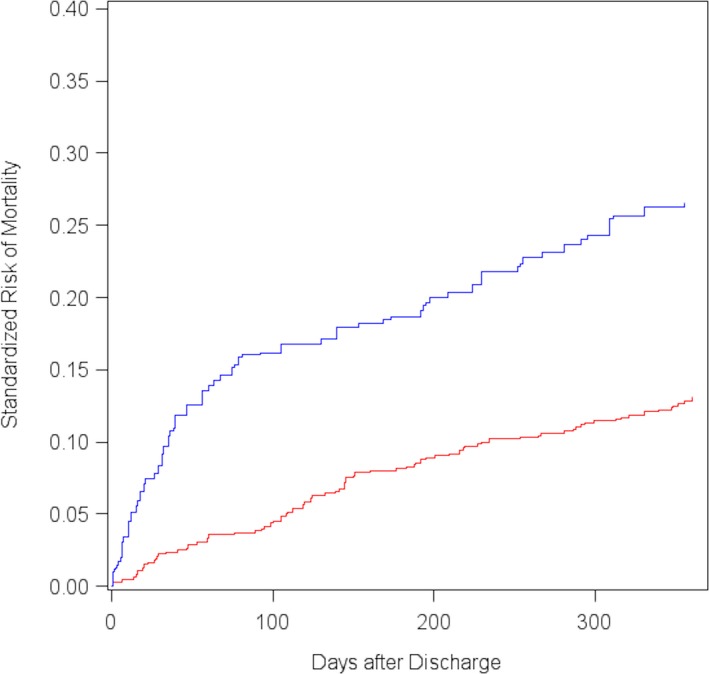
Standardized 1‐year cumulative risk of mortality after hospital discharge among patients with type 1 MI (red line) and type 2 MI (blue line). MI indicates myocardial infarction.
Table 2.
Crude and Standardized Risk of Mortality After Hospital Discharge Stratified by MI Type Among Patients Admitted With NSTEMI
| Mortality, % | RD | 95% CIa | RR | 95% CIa | ||
|---|---|---|---|---|---|---|
| Type 2 MI | Type 1 MI | |||||
| Crude | ||||||
| 30‐day | 11.9 | 2.2 | 0.10 | 0.05–0.14 | 5.50 | 2.73–11.08 |
| 1‐year | 34.8 | 12.4 | 0.22 | 0.15–0.29 | 2.80 | 2.13–3.67 |
| Standardizedb | ||||||
| 30‐day | 8.1 | 2.2 | 0.06 | 0.02–0.10 | 3.63 | 1.67–7.88 |
| 1‐year | 25.9 | 13.1 | 0.13 | 0.06–0.20 | 1.98 | 1.44–2.73 |
CI indicates confidence interval; MI, myocardial infarction; NSTEMI, non–ST‐segment–elevation myocardial infarction; RD, risk difference; RR, risk ratio.
CIs were determined using the standard deviation estimated from 500 nonparametric bootstrap resamples.
Standardized for patient age, sex, body mass index, hypertension, diabetes mellitus, hyperlipidemia, peripheral vascular disease, chronic kidney disease, smoking status, previous percutaneous coronary intervention, previous coronary artery bypass grafting, and whether aspirin, statins, or beta blockers were prescribed at discharge.
Overall, 90 patients (9.4%) were readmitted within 30 days of discharge. Among patients with type 1 MI, there were 79 readmissions (10.7%), with 46 readmissions resulting from a cardiovascular cause. Specifically, 28 patients (35.3%) were admitted with MI, 11 (13.9%) for congestive heart failure, 3 (3.8%) for device complications, 2 (2.5%) for stroke, 1 (1.3%) for cardiac arrhythmia, and 1 (1.3%) for a structural heart abnormality. Only 11 patients (5.1%) with type 2 MI were readmitted; of those, 3 (27.3%) were readmitted for congestive heart failure, 1 (9.1%) for MI, and 7 (63.6%) for noncardiovascular reasons. Both before and after weighting, readmission rates were lower for type 2 MI compared with patients with type 1 MI (RR: 0.48; 95% CI, 0.26–0.88) and (RR: 0.57; 95% CI, 0.28–1.16; Table 3, Figure 6. Similarly, patients with type 2 MI were less likely to be readmitted for cardiovascular reasons (Table 3.
Table 3.
Crude and Standardized Risk of 30‐Day Readmission After Hospital Discharge, Stratified By MI Type Among Patients Admitted With NSTEMI
| Readmission, % | RD | 95% CIa | RR | 95% CIa | ||
|---|---|---|---|---|---|---|
| Type 2 MI | Type 1 MI | |||||
| Any readmission | ||||||
| Crude | 5.0 | 10.6 | −0.06 | −0.09 to −0.02 | 0.48 | 0.26–0.88 |
| Standardizedb | 5.8 | 10.2 | −0.04 | −0.09 to 0.00 | 0.57 | 0.28–1.16 |
| Cardiovascularc readmission | ||||||
| Crude | 1.8 | 6.1 | −0.04 | −0.06 to −0.02 | 0.30 | 0.11–0.86 |
| Standardizedb | 2.9 | 5.9 | −0.03 | −0.07 to 0.01 | 0.49 | 0.12–2.06 |
CI indicates confidence interval; MI, myocardial infarction; NSTEMI, non–ST‐segment–elevation myocardial infarction; RD, risk difference; RR, risk ratio.
CIs determined using the standard deviation estimated from 500 nonparametric bootstrap resamples.
Standardized for patient age, sex, body mass index, hypertension, diabetes mellitus, hyperlipidemia, peripheral vascular disease, chronic kidney disease, smoking status, previous percutaneous coronary intervention, previous coronary artery bypass grafting, and whether aspirin, statins, or beta blockers were prescribed at discharge.
Readmission diagnosis of myocardial infarction, congestive heart failure, cerebrovascular accident, cardiac arrhythmia, cardiac device complication, or structural heart abnormality.
Figure 6.
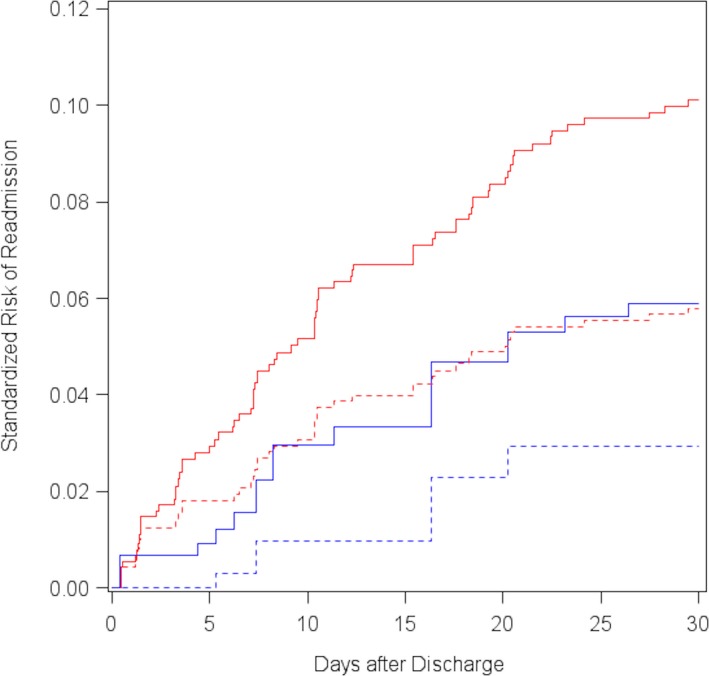
Standardized 30‐day cumulative risk of any hospital readmission (solid) and cardiovascular readmission (dashed) after discharge, among patients with type 1 MI (red line) and type 2 MI (blue line). MI indicates myocardial infarction.
Discussion
We analyzed data from 1039 consecutive patients with a discharge diagnosis of NSTEMI and found type 2 MI to be present in about a quarter of the patients discharged with NSTEMI. Patients with type 2 MI had significantly higher mortality than those with type 1 MI during hospitalization and at 30 days and 1 year after discharge. During hospitalization, patients with type 2 MI had lower median peak troponin levels, and most deaths were noncardiovascular. Patients with type 2 MI were older and more often female but less likely to have traditional coronary risk factors such as diabetes mellitus and hypertension compared with patients with type 1 MI. Patients with type 2 MI were less likely to undergo cardiac catheterization and revascularization and less likely to be discharged on medications that are part of post‐ACS care. Despite that, patients with type 2 MI were less likely to be readmitted, both overall and for cardiovascular causes within 30 days of discharge.
Although deaths secondary to type 1 MI have plateaued in the past decade, clinical outcomes with type 2 MI vary widely. In our study, patients with type 2 MI composed 25% of all NSTEMI patients but represented roughly half of all deaths. Frequently used risk scores such as the TIMI (Thrombolysis in Myocardial Infarction) and GRACE (Global Registration of Acute Coronary Events) models were developed from a cohort of patients with type 1 MI, and their applicability to type 2 MI is not known.7 Moreover, studies showing that the degree of biomarker elevation correlates with adverse cardiovascular outcomes were also performed in patients with type 1 MI.10, 11, 12 Despite higher all‐cause mortality, we found significantly lower peak troponin levels and lower prevalence of traditional coronary risk factors in the type 2 MI group. The fact that more than two‐thirds of all inpatient deaths in patients with type 2 MI were due to noncardiovascular causes suggests that mortality in these patients may not be estimated using traditional approaches.
The most likely reason for the higher mortality observed with type 2 MI is the presence of an alternate factor responsible for demand/supply mismatch. In our study, >70% of the deaths in the type 2 MI cohort were from noncardiovascular causes, compared with only a quarter of the deaths in the type 1 MI cohort. This suggests that although these alternate factors have potential to cause demand/supply mismatch leading to type 2 MI, the mortality among these patients is not secondary to a cardiac process but rather driven by their primary disease process, such as sepsis and other noncardiovascular causes. Troponin elevation in heart failure has been attributed to small vessel obstruction and endothelial dysfunction.13 Troponin elevation in stroke patients may occur related to myocardial stretch, endothelial dysfunction, microvascular spasm, cytokine‐mediated myocardial injury, or catecholamine‐mediated myocardial toxicity.14, 15 Compared with stroke, demand/supply mismatch in seizures has been largely attributed to massive catecholamine release related to muscular activity, leading to an elevation in heart rate, blood pressure, and contractility.16 A similar mechanism has been attributed to causing neurocardiogenic injury in intracranial hemorrhage.17, 18 The underlying mechanism of cardiac injury in sepsis continues to be investigated. Although sepsis is known to cause increased coronary blood flow,19 it also has been linked to improper oxygen utilization.20 Other mechanisms such as upregulation of endothelin 1 have also been demonstrated to play a role in these patients and could be the trigger for endothelial dysfunction.21 Furthermore, an increase in cardiac myocyte permeability in septic patients may also play a role in troponin leakage.22 Elevated levels of troponins in critical illness or after surgery can be caused by increased levels of both endogenous or exogenous catecholamines.23
A second potential explanation for the higher mortality in patients with type 2 MI are the differences in demographics and comorbidities. Patients with type 2 MI are older, are more often female, and have a higher prevalence of chronic kidney disease. However, even after accounting for patient demographics and comorbidities, type 2 MI patients still had significantly increased mortality in our analysis. A third potential explanation is the less frequent use of coronary angiography and revascularization in type 2 MI. Although we were not able to categorize postdischarge mortality into cardiovascular and noncardiovascular causes, almost all of the type 2 MI patients were readmitted at our institution for noncardiovascular reasons.
There remains some debate regarding the management of patients with type 2 MI. The current ICD coding system does not recognize type 2 MI as a separate entity and continues to group these patients under the general umbrella of NSTEMI. This leads to enforcement of guidelines that are part of post‐ACS care for all patients with type 2 MI. Although aspirin, statin, and beta blockers may have a role in the management of some patients with type 2 MI,7 using guideline‐based ACS therapy in every patient with type 2 MI may be inappropriate and could result in adverse events. In our analysis, type 2 MI patients were discharged with guideline‐based ACS therapy at significantly lower levels than type 1 MI patients. In our study, the rates of guideline‐based ACS therapies at discharge for the type 1 MI group were slightly lower compared with the real‐world utilization reports from the ACTION Registry largely because we did not exclude patients with a documented contraindication to these medications from the total number of patients.24 Despite a lower rate of guideline‐based therapies at discharge, the 30‐day readmissions for MI were lower in the type 2 MI group. Although we treated mortality after discharge as a competing risk, we were unable to incorporate inpatient mortality into these models (because discharge medication usage was missing for these patients). It is possible that the higher inpatient mortality in type 2 MI patients contributed to us finding a lower 30‐day readmission risk in this analysis.
Little is known about the optimal management of type 2 MI, emphasizing the importance of better delineating type 2 MI from type 1 MI, as the optimal management of the 2 groups is likely different. In addition, if they are not appropriately differentiated, MI metrics at different institutions may be skewed and could lead to inaccurate assessment of quality and performance measures by MI quality review programs, based on patient populations for which the metrics were not designed, and inappropriate penalties for healthcare providers. Moreover, in most research on NSTEMI, including randomized trials and observational studies, NSTEMI patients are grouped as 1 cohort and type 2 MI is not isolated from the population. This approach is likely to have a major impact on the way the end points are assessed because outcomes can be skewed against the group with a higher type 2 MI percentage in the NSTEMI cohort. This issue needs to be further investigated.
Data from the SWEDEHEART (Swedish Web System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies) registry found higher crude mortality in patients with type 2 MI, compared with type 1 MI, that attenuated after adjustment for demographics, comorbidities, and treatment.3 In contrast, we used the Third Universal Definition to distinguish between type 1 and type 2 MI and to provide additional morbidity data by comparing their influence on rehospitalizations. Gaggin et al used the Third Universal Definition to distinguish between type 1 and type 2 MI; however, they limited their study to those undergoing coronary or peripheral angiography.4 We used ICD‐9 codes to define NSTEMI patients, regardless of whether they underwent angiography, and thus were able to evaluate the effect of type 2 MI on hospital‐level MI metrics. Smilowitz et al attempted to distinguish type 2 MI using the Third Universal Definition; however, their study was limited to a comparison between type 2 MI and myocardial injury.5 In a recently published study, Neumann et al enrolled 1548 patients (188 with type 1 MI and 99 with type 2 MI) who presented with suspected MI and used the Third Universal Definition to distinguish type 1 from type 2 MI.6 In contrast to our study, the investigators found no mortality difference between type 1 and type 2 MI, likely because their analysis was limited to patients with suspected MI. In addition, fewer type 1 and type 2 MI patients were included in the study.6
Limitations
The single‐center nature of our study may limit the generalizability of our findings; however, this allowed us to use the Third Universal Definition of MI as a stringent inclusion criterion. Reliance on ICD coding for NSTEMI at discharge may have led to some selection bias. We were able to observe only prescribed medications at discharge; therefore, we assumed medication compliance. It is likely that some patients may have been misclassified as being on more guideline‐directed cardiovascular medications than they were, but this misclassification is expected to be the same across types of MI.
In addition, mortality data were obtained from the North Carolina State Center for Health Statistics. The Center for Health Statistics was not allowed to release data on deaths that occurred out of state, which constituted about 1.5% of all deaths for the calendar years of the study. This is comparable to the data obtained from the Social Security Death Index, which has overall accuracy of 95% and has been found to be even more accurate in older individuals.25 We were also unable to categorize postdischarge mortality into cardiovascular or noncardiovascular causes, and readmission data were restricted to the data available from our institution; therefore, we may have missed any rehospitalizations that occurred at other institutions.
We were also limited in the analysis we could conduct on 30‐day readmission because of a low number of readmissions, despite having causes for readmission. Moreover, although we observed a lower incidence of readmission both before and after weighing, we were underpowered to detect a significant difference in our analyses, which controlled for confounding. The adjudication of type 1 versus type 2 MI was performed by a single reviewer (S.A.) who was blinded to outcomes using a prespecified criterion. In the setting of doubt, the cases were discussed with a second reviewer (P.K.) to reach a consensus on type 1 versus type 2 MI. The adjudication by a single reviewer only was due to limitation in resources and funding, and we understand that this approach may lead to observational biases in which the ascertainment of type 1 versus type 2 MI could be influenced by a reviewer's clinical beliefs and biases. Nevertheless, because the reviewer used a prespecified criterion, we expect that bias would have been minimized.
Finally, as with all analyses, there is the potential for unmeasured confounding.
Conclusions
The NSTEMI metrics at our institution are significantly affected by type 2 MI patients. Despite representing only 25% of all NSTEMI patients, type 2 MI patients represent >50% of all inpatient NSTEMI deaths, with the majority due to noncardiovascular causes. Furthermore, these patients are discharged on medications that are part of ACS quality measures at a lower rate than type 1 MI patients. Interestingly, patients with type 2 MI had lower readmission rates. Consequently, it is important to recognize that type 2 MI patients have distinct etiologies, are often managed differently, and have significantly different prognoses compared with patients with type 1 MI. Moving forward, it seems prudent to categorize type 2 MI patients separately, instead of under the general umbrella of NSTEMI, and to exclude these patients from acute MI quality metrics. A multicenter study with a larger sample size is needed to confirm our findings and to better understand how we can improve outcomes in this high‐risk population.
Disclosures
Dr Qamar is supported by a postdoctoral training grant from the National Heart, Lung, and Blood Institute of the National Institute of Health (T32 HL 007604). The remaining authors have no relevant disclosures to report.
(J Am Heart Assoc. 2018;7:e008661 DOI: 10.1161/JAHA.118.008661.)
References
- 1. Thygesen K, Alpert JS, White HD; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction , Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole‐Wilson PA, Gurfinkel EP, Lopez‐Sendon JL, Pais P, Mendis S, Zhu JR, Wallentin LC, Fernandez‐Aviles F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio‐Pulkki LM, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck‐Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez‐Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D, Al‐Attar N. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. [DOI] [PubMed] [Google Scholar]
- 2. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD; Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction , Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez‐Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035.22923432 [Google Scholar]
- 3. Baron T, Hambraeus K, Sundstrom J, Erlinge D, Jernberg T, Lindahl B; Group T‐As . Impact on long‐term mortality of presence of obstructive coronary artery disease and classification of myocardial infarction. Am J Med. 2016;129:398–406. [DOI] [PubMed] [Google Scholar]
- 4. Gaggin HK, Liu Y, Lyass A, van Kimmenade RR, Motiwala SR, Kelly NP, Mallick A, Gandhi PU, Ibrahim NE, Simon ML, Bhardwaj A, Belcher AM, Harisiades JE, Massaro JM, D'Agostino RB Sr, Januzzi JL Jr. Incident type 2 myocardial infarction in a cohort of patients undergoing coronary or peripheral arterial angiography. Circulation. 2017;135:116–127. [DOI] [PubMed] [Google Scholar]
- 5. Smilowitz NR, Weiss MC, Mauricio R, Mahajan AM, Dugan KE, Devanabanda A, Pulgarin C, Gianos E, Shah B, Sedlis SP, Radford M, Reynolds HR. Provoking conditions, management and outcomes of type 2 myocardial infarction and myocardial necrosis. Int J Cardiol. 2016;218:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neumann JT, Sorensen NA, Rubsamen N, Ojeda F, Renne T, Qaderi V, Teltrop E, Kramer S, Quantius L, Zeller T, Karakas M, Blankenberg S, Westermann D. Discrimination of patients with type 2 myocardial infarction. Eur Heart J. 2017;38:3514–3520. [DOI] [PubMed] [Google Scholar]
- 7. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ; ACC/AHA Task Force Members; Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons . 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2354–2394. [DOI] [PubMed] [Google Scholar]
- 8. Roth GA, Forouzanfar MH, Moran AE, Barber R, Nguyen G, Feigin VL, Naghavi M, Mensah GA, Murray CJ. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med. 2015;372:1333–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cole SR, Hernan MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75:45–49. [DOI] [PubMed] [Google Scholar]
- 10. Chia S, Senatore F, Raffel OC, Lee H, Wackers FJ, Jang IK. Utility of cardiac biomarkers in predicting infarct size, left ventricular function, and clinical outcome after primary percutaneous coronary intervention for ST‐segment elevation myocardial infarction. JACC Cardiovasc Interv. 2008;1:415–423. [DOI] [PubMed] [Google Scholar]
- 11. Hassan AK, Bergheanu SC, Hasan‐Ali H, Liem SS, van der Laarse A, Wolterbeek R, Atsma DE, Schalij MJ, Jukema JW. Usefulness of peak troponin‐T to predict infarct size and long‐term outcome in patients with first acute myocardial infarction after primary percutaneous coronary intervention. Am J Cardiol. 2009;103:779–784. [DOI] [PubMed] [Google Scholar]
- 12. Matetzky S, Sharir T, Domingo M, Noc M, Chyu KY, Kaul S, Eigler N, Shah PK, Cercek B. Elevated troponin I level on admission is associated with adverse outcome of primary angioplasty in acute myocardial infarction. Circulation. 2000;102:1611–1616. [DOI] [PubMed] [Google Scholar]
- 13. Januzzi JL Jr, Filippatos G, Nieminen M, Gheorghiade M. Troponin elevation in patients with heart failure: on behalf of the third Universal Definition of Myocardial Infarction Global Task Force: Heart Failure Section. Eur Heart J. 2012;33:2265–2271. [DOI] [PubMed] [Google Scholar]
- 14. Jespersen CM, Fischer Hansen J. Myocardial stress in patients with acute cerebrovascular events. Cardiology. 2008;110:123–128. [DOI] [PubMed] [Google Scholar]
- 15. Providencia R, Barra S, Paiva L. Atrial fibrillation, elevated troponin, ischemic stroke and adverse outcomes: understanding the connection. Clin Res Cardiol. 2013;102:701–711. [DOI] [PubMed] [Google Scholar]
- 16. Montepietra S, Cattaneo L, Granella F, Maurizio A, Sasso E, Pavesi G, Bortone E. Myocardial infarction following convulsive and nonconvulsive seizures. Seizure. 2009;18:379–381. [DOI] [PubMed] [Google Scholar]
- 17. Wybraniec MT, Mizia‐Stec K, Krzych L. Neurocardiogenic injury in subarachnoid hemorrhage: a wide spectrum of catecholamin‐mediated brain‐heart interactions. Cardiol J. 2014;21:220–228. [DOI] [PubMed] [Google Scholar]
- 18. Chen S, Li Q, Wu H, Krafft PR, Wang Z, Zhang JH. The harmful effects of subarachnoid hemorrhage on extracerebral organs. Biomed Res Int. 2014;2014:858496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cunnion RE, Schaer GL, Parker MM, Natanson C, Parrillo JE. The coronary circulation in human septic shock. Circulation. 1986;73:637–644. [DOI] [PubMed] [Google Scholar]
- 20. Powell RJ, Machiedo GW, Rush BF Jr, Dikdan G. Oxygen free radicals: effect on red cell deformability in sepsis. Crit Care Med. 1991;19:732–735. [PubMed] [Google Scholar]
- 21. Gupta A, Brahmbhatt S, Kapoor R, Loken L, Sharma AC. Chronic peritoneal sepsis: myocardial dysfunction, endothelin and signaling mechanisms. Front Biosci. 2005;10:3183–3205. [DOI] [PubMed] [Google Scholar]
- 22. Favory R, Neviere R. Significance and interpretation of elevated troponin in septic patients. Crit Care. 2006;10:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lim W, Qushmaq I, Devereaux PJ, Heels‐Ansdell D, Lauzier F, Ismaila AS, Crowther MA, Cook DJ. Elevated cardiac troponin measurements in critically ill patients. Arch Intern Med. 2006;166:2446–2454. [DOI] [PubMed] [Google Scholar]
- 24. Faridi KF, Peterson ED, McCoy LA, Thomas L, Enriquez J, Wang TY. Timing of first postdischarge follow‐up and medication adherence after acute myocardial infarction. JAMA Cardiol. 2016;1:147–155. [DOI] [PubMed] [Google Scholar]
- 25. Huntington JT, Butterfield M, Fisher J, Torrent D, Bloomston M. The Social Security Death Index (SSDI) most accurately reflects true survival for older oncology patients. Am J Cancer Res. 2013;3:518–522. eCollection 2013. [PMC free article] [PubMed] [Google Scholar]


