Abstract
Background
This study aimed to investigate the protective effect of eicosapentaenoic acid (EPA) on rats with polycystic ovary syndrome (PCOS).
Material/Methods
Rats with PCOS were intraperitoneally injected with different doses of EPA. Levels of follicle stimulating hormone (FSH), luteinizing hormone (LH), and testosterone (T) were measured using corresponding kits. HE staining was used to observe lesions in ovarian tissue. Levels of inflammatory factors in ovarian tissue of rats were detected by ELISA. RT-PCR was to detect the expression of SREBP1 mRNA and Western blot was used to detect the expression of SREBP1 and TLR4 protein.
Results
The levels of LH and T were significantly higher and FDH was significantly lower in the Model group compared with the Control group. EPA treatment increased the number of follicular cell layers and promoted maturation of oocytes. Levels of IL-1β, TNF-α, and IL-18 were significantly reduced after EPA treatment. Content of IL-10 was significantly increased after EPA treatment. Expression levels of SREBP1 and TLR4 were significantly deceased after EPA treatment.
Conclusions
EPA can improve PCOS through the SREBP1/TLR4 pathway.
MeSH Keywords: Adrenal Cortex Hormones, Eicosapentaenoic Acid, Polycystic Ovary Syndrome
Background
As one of the most common endocrine disorders in females, polycystic ovary syndrome (PCOS) affects about 7% of women of reproductive age [1]. PCOS in not only the main cause of anovulatory infertility but is also an important risk factor for type II diabetes mellitus [2]. Previous studies indicated that various populations have PCOS [3,4]. At present, the most accepted definition of PCOS is the chronic anovulation associated with hyperandrogenism in females who do not have any pituitary gland or adrenal diseases [5]. The pathology of PCOS is unclear. Abnormal gonadotropin secretion was found to be responsible for hyperandrogenism and anovulation [6]. At the hormonal level, the production of excessive luteinizing hormone during the development of PCOS can stimulate production of testosterone and inhibit the production of follicle stimulating hormone, leading to the arrest of ovarian folliculogenesis [7]. Although a variety of treatments have been developed to treat PCOS, many treatments have failed to provide satisfactory outcomes [8,9]. Therefore, it would be of great clinical value to identify new drugs that can effectively and safely treat PCOS.
Eicosapentaenoic acid (EPA), which is also called icosapentaenoic acid, is a type of omega-3 fatty acid. EPA is therapeutically effective in various human diseases, including cardiovascular disease [10], depressive disorder [11], and nonalcoholic fatty liver disease [12]. It has been shown that omega-3 fatty acids supplementation can significantly improve the conditions of patients with PCOS by interacting with different signaling pathways [13]. A recent study also showed that treatment with omega-3 fatty can regulate the levels of sex hormones in postmenopausal women [14]. Therefore, in the present study, we hypothesized that EPA may have a therapeutic effect on PCOS.
In this study, a rat PCOS model was established by subcutaneous injection of DHEA to induce the production of excessive androgen. Different doses of EPA were used to treat rats with PCOS, and the effects of EPA treatment on levels of various hormones, inflammatory factors, and expression levels of SREBP1 and TLR4 were investigated.
Material and Methods
Animals
Fifty SD female rats (21 days old) were purchased from Suzhou Aiermaite Technology Co., Ltd. (license number SCXK (Su) 2014-0007, Suzhou, China). All animal experiments were performed in accordance with the Institutional Animal Care Committee of People’s Hospital of Wuhan University.
Grouping and model establishment
Rats were randomly divided into 5 groups: Control group, PCOS group (Model group), EPA low-dose group (6 μg/kg, EPA-L), EPA middle-dose group (12 μg/kg, EPA-M), and EPA high-dose group (18 μg/kg, EPA-H), 10 rats in each group. Rats in the Model, EPA-L, EPA-M, and EPA-H groups were subcutaneously injected with DHEA (Sinopharm Chemical Reagent, Co., Ltd., 6 mg/100 g·d/0.2 ml sesame oil) to induce PCOS model with excessive androgen. Rats in the Control group were injected with the same volume of sesame oil. After model establishment, rats were injected with the corresponding concentrations of EPA (0.01 ml, Sigma, St. Louis, USA) diluted in serum-free DMEM medium (Sigma, St. Louis, USA) for 15 days. The same volume of DMEM medium and saline was used in the Model group and Control group.
Detection of hormone contents in serum of rats
Rats were fasted overnight and blood was extracted from the abdominal aorta. Through centrifugation (3000 rpm for 10 min), serum was separated from blood. Levels of follicle stimulating hormone (FSH), luteinizing hormone (LH), and testosterone (T) in serum were measured by use of the corresponding kit (Uscn Life Science, Inc., Wuhan, China).
HE staining to observe lesions in ovarian tissue of rats
Ovarian tissue was fixed and embedded with paraffin. The embedded tissue was cut into 5-μm sections, followed by dewaxing with xylene 2 times for 5 min each time. Hydration was performed by passing through a grades series of ethanol. After staining with hematoxylin (Solarbio, Beijing, China) for 5 min, slides were washed with tap water and treated with 1% hydrochloric acid-ethanol. After washing with water, eosin (Solarbio, Beijing, China) staining was performed for 3 min and excessive dye was washed out. The sections were treated with ethanol for 5 min and xylene for 10 min and sealed with neutral resin. Pathological changes were observed under an optical microscope (Olympus, Japan).
ELISA to detect the contents of inflammatory factors in ovarian tissue
Ovarian tissue was collected and coagulation and appendages were removed. Ovarian tissue was mixed with precooled saline to prepare 10% ovarian tissue homogenate, followed by centrifugation at 3000 rpm for 10 min to collect the supernatant. Levels of TNF-α, IL-1β, IL-10, and IL-18 in ovarian tissues were detected by use of ELISA kits (R&D Systems, USA).
RT-PCR to detect the expression of SREBP1 gene
Total RNA was extracted from ovarian tissue using Trizol (Takara, Japan). Purity and integrity of RNA samples were checked and reverse transcription was performed using a reverse transcription kit (Takara, Japan). The SYBR Green master kit (Takara, Japan) and cDNA were used to prepare the reaction system. The following primers were used: SREBP1 forward: 5′-GCCATCGACTACATCCGCTT-3′; SREBP1 reverse: 5′-TGGGCTTTTCACCTGGTTATCC-3′; β-actin forward: 5′-TAAAGACCTCTATGCCAACACAGT-3; β-actin reverse: 5′-CACGATGGAGGGGCCGGACTCATC-3. Reaction conditions were: 95°C for 2.5 min, followed by 40 cycles of 95°C for 15 s and 60°C for 45 s. Ct values were processed using 2−ΔΔCt method and relative expression level of SREBP1 was normalized to endogenous control.
Western blot analysis to detect the expression of SREBP1 and TLR4 protein
Total protein was extracted from ovarian tissue, and 10 μg of protein from each sample was subjected to electrophoresis using 10–20% Ready Gels under 100 V. After transfer to PVDF membranes (Millipore, USA), membranes were washed and blocked with 5% skim milk (Shanghai Bright Dairy Co., Ltd., Shanghai, China). After washing, membranes were incubated with rabbit anti-TLR4 polyclonal antibody (1: 2000, ab28481, Abcam, Hong Kong, China), rabbit anti-SREBP1 polyclonal antibody (1: 1000, ab62352, Abcam, Hong Kong, China), or rabbit anti-β-actin polyclonal antibody (1: 2000, ab8227, Abcam, Hong Kong, China) overnight at 4°C. After washing with TBST 3 times, goat anti-rabbit IgG secondary antibody (1: 3000, ab6721, Abcam, Hong Kong, China) was incubated with the membranes overnight at 4°C. After washing with TBST 3 times, chemiluminescence method was used to detect the signal. Relative expression of each protein was normalized to the grey-scale value of endogenous control β-actin.
Statistical analysis
SPSS19.0 statistical software was used. All data are expressed as mean ± standard deviation (SD). Comparison among multiple groups were performed by one-way ANOVA and p<0.05 was considered to be statistically significant.
Results
Levels of FSH, LH, and T in serum of rats in different groups
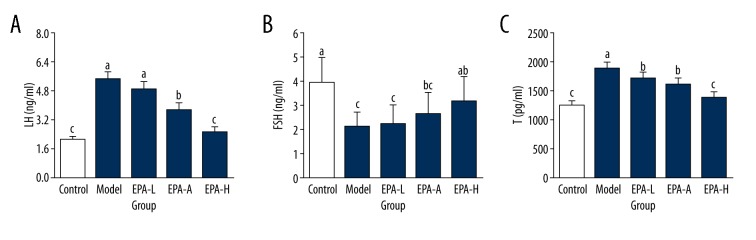
As shown in Figure 1, levels of LH and T in serum of rats in the Model group were 5.48±0.41 ng/ml and 1897.62±106.75 pg/ml, respectively, which were significantly higher than in the Control group (P<0.05). In contrast, content of FSH was 2.16±0.59 ng/ml, which was significantly lower than that of the Control group (P<0.05).Compared with the Model group, levels of LH and T were significantly reduced and level of FSH was significantly increased in the EPA-M and EPA-H groups (P<0.05), and no significant differences in levels of LH and T were found between the EPA-H group and Control group (P>0.05). Those data suggest that EPA can significantly reduce the levels of LH, FSH, and T in serum of rats with PCOS.
Figure 1.
Levels of LH (A), FSH (B), and T (C) in serum of rats in different groups. The different letters (a, b, c) mean significant differences (P<0.05).
Histopathological changes in ovarian tissue of each group
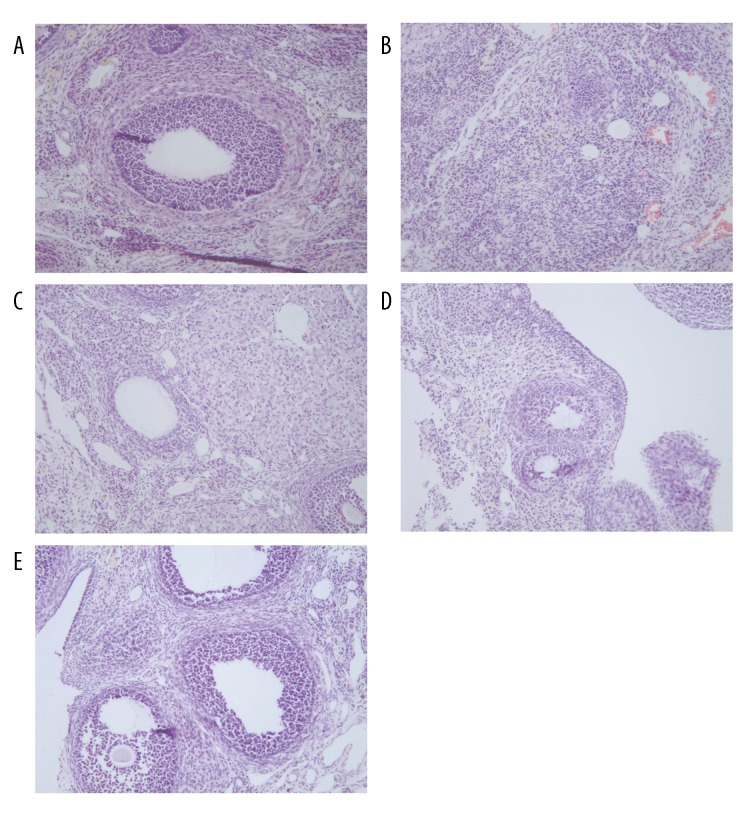
Multiple corpus luteum and follicles were observed in the Control group, oocytes were seen in nearly mature follicles, and there were fewer follicular cell layers (Figure 2A). Multiple primary follicles and few follicles were observed in the Model group, no oocytes were seen in nearly mature follicles, granulosa cells were arranged loosely, there were fewer layers, and the follicular cell layer was thicker (Figure 2B). Histopathological changes in ovarian tissue in EPA groups were improved compared with the Model group, multiple corpus luteum and follicles were observed, oocytes were seen in nearly mature follicles, granulosa cells were arranged tightly and the layers were increased, and the ovarian cell layer was thin (Figure 2C–2E). Pathological damages were markedly attenuated by EPA treatment.
Figure 2.
HE staining to show histopathological changes in ovarian tissue of each group (×40). (A) Control group; (B) Model group; (C) EPA-L group; (D) EPA-M group; (E) EPA-H group.
Levels of inflammatory factors in ovarian tissue of each group
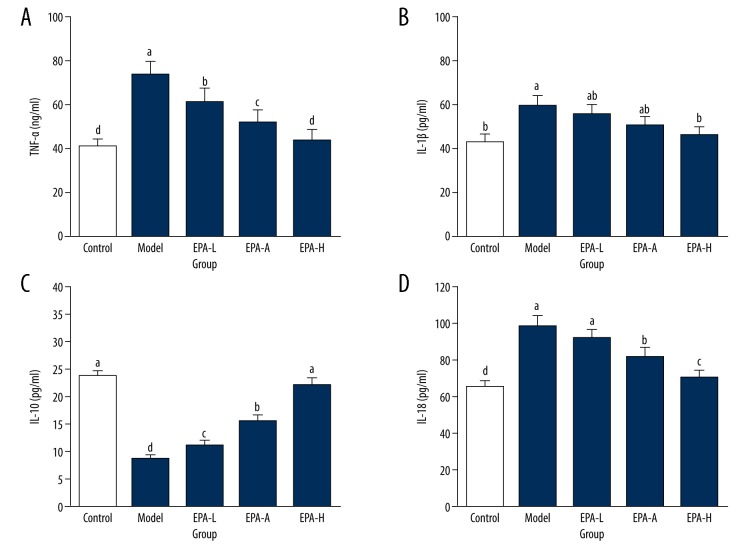
As shown in Figure 3, compared with the Control group, in the Model group the levels of IL-1β, IL-18, and TNF-α were significantly increased (P<0.05). Levels of IL-18, TNF-α, and IL-1β were significantly decreased in EPA groups compared with the Model group (P<0.05) except for the level of TNF-α in the EPA-L group, which showed no significant difference from the Model group (P>0.05). Content of IL-10 in the Model group was significantly lower than that in the Control group (23.69±1.08 pg/ml, P<0.05), while content of IL-10 was significantly increase in EPA groups compared with the Model group. In addition, no significant difference in content of IL-10 was found between the EPA-H group and Control group (P>0.05). These results suggest that EPA treatment can significantly reduce inflammatory response of ovarian tissue in PCOS rats.
Figure 3.
Levels of inflammatory factors in ovarian tissue of each group. (A) TNF-α; (B) IL-1 β; (C) IL-10; (D) IL-18. The different letters (a, b, c, d) mean significant differences (P<0.05).
Expression of SREBP1 in ovarian tissue of rats in each group
As shown in Figure 4, the expression level of SREBP1 mRNA was significantly increased (by 42%) in the Model group (P<0.05) compared with the Control group. Compared with the Model group, expression level of SREBP1 mRNA was significantly decreased by 6% (P<0.05), 14% (p<0.05), and 28% (P<0.05) in the EPA-L group, EPA-M group, and EPA-H group, respectively. These data suggest that EPA treatment can decrease the expression level of SREBP1 mRNA in ovarian tissue of PCOS rats.
Figure 4.
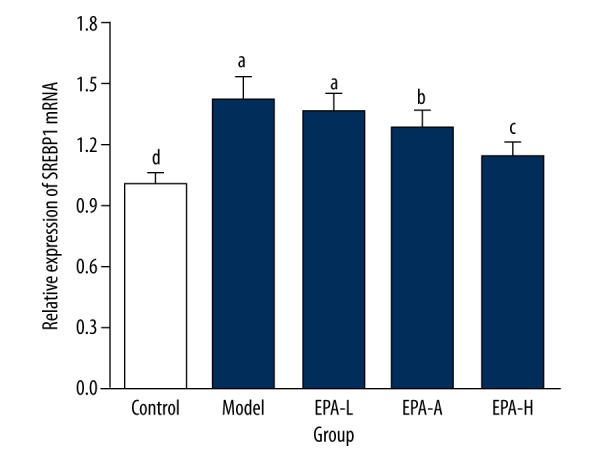
Expression of SREBP1 mRNA in ovarian tissue of rats in each group. The different letters (a, b, c, d) mean significant differences (P<0.05).
Expression of SREBP1 and TLR4 proteins in ovarian tissue
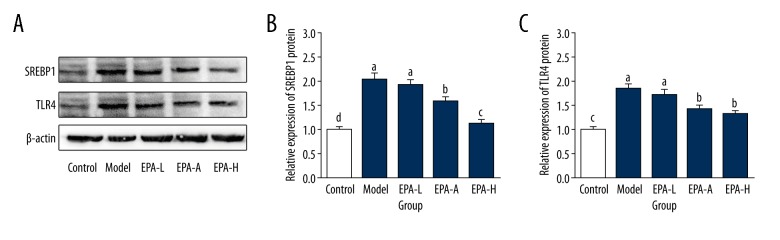
As shown in Figure 5, compared with the Control group, expression levels of SREBP1 and TLR4 proteins were significantly increased by 104% and 83% (P<0.05), respectively, in the Model group. No significant differences in expression levels of SREBP1 and TLR4 proteins were found between the EPA-L group and Model group (P>0.05). Expression levels of SREBP1 and TLR4 proteins were significantly reduced in the EPA-M group and EPA-H group compared with the Model group but were still significantly higher than those in the Control group (P<0.05). These results suggest that EPA can improve PCOS by reducing the expression levels of SREBP1 and TLR4 in ovarian tissue.
Figure 5.
Expression of SREBP1 and TLR4 proteins in ovarian tissue of different groups. (A) Representative Western blot result; (B) Relative expression levels of SREBP1 protein in ovarian tissue of different groups; (C) Relative expression levels of TLR4 protein in ovarian tissue of different groups. The different letters (a, b, c, d) mean significant differences (P<0.05).
Discussion
Although the pathogenesis of PCOS still has not been fully elucidated, abnormal production of sex hormones is closely related to the development of this disease [15]. The significantly increased level of testosterone is usually considered as a marker of hyperandrogenism in women with PCOS [14]. Abnormal gonadotropin secretion patterns, including reduced production of FSH, increased production of LH, and increased LH/FSH ratio, are the common hormonal features of PCOS [16]. Various vulnerable groups have PCOS [17,18]. During the development of PCOS, abnormally increased secretion of LH significantly reduces the production of testosterone and increases the secretion of FSH, which in turn leads to an increased LH/FSH ratio, resulting in arrest of ovarian folliculogenesis [7]. Thus, the regulation of level of LH has become a target in the treatment of PCOS. A recent study reported that treatment with myo-inositol can significantly improve the hormonal parameters by decreasing the level of LH, which in turn improved the conditions of PCOS [19]. In another study, orally administrated FSH was found to significantly reduce the severity of PCOS in a mouse model by reducing the number of cystic follicles and restoring estradiol level [20]. In our study, levels of LH and testosterone were significantly increased in rats with PCOS compared with the Control rats. However, treatment with medium and high doses of EPA significantly reduced the levels of LH and testosterone, indicating that EPA can improve the conditions of PCOS at the hormonal level.
Patients with PCOS are usually also have low-grade chronic inflammation [21]. More and more studies have shown that the metabolic effects of PCOS, including impaired glucose homeostasis, dyslipidemia, visceral obesity, and (potentially) cardiovascular disease are closely related to the low-grade chronic inflammatory state, and low-grade chronic inflammation together with hyperandrogenism and hyperinsulinemia contribute to the development of PCOS [22,23]. The roles of inflammatory cytokines such as TNF-α and IL-18 in the progression of PCOS have been widely studied, and the results suggest the utility of anti-inflammation-centered therapy for the treatment of PCOS [24]. TNF-α, IL-1β, and IL-18 are 3 proinflammatory factors that can drive inflammatory responses to various internal and external stimulations [25,26]. In contrast, IL-10 is an anti-inflammatory factor that achieves it biological functions by inhibiting inflammatory responses [27]. In our study, levels of TNF-α, IL-1β, and IL-18 in ovarian tissue of rats were significantly increased, while level of IL-10 was significantly decreased compared with the Control group, indicating the increased inflammatory response in rats with PCOS. EPA treatment significantly reduced levels of TNF-α, IL-1β, and IL-18 and increased the level of IL-10. These data suggest that EPA can improve the conditions of PCOS by inhibiting the inflammatory response.
TLR4 also plays pivotal roles in proinflammatory signaling, and SREBP1 was proved to be able to promote the TLR4-induced proinflammatory responses by reprogramming fatty acid metabolism [28]. In our study, expression levels of SREBP 1 and TLR4 were found to be significantly increased in rats with PCOS compared with rats in the Control group, indicating that the proinflammatory responses mediated by the SREBP 1/TLR4 pathway are involved in the development of PCOS. After treatment with EPA, expression levels of SREBP 1 and TLR4 were significantly reduced, indicating that EPA treatment can improve the conditions of PCOS by inhibiting the SREBP 1/TLR4 pathway.
Changes in polycystic ovarian morphology caused by PCOS can significantly affect the normal functions of the ovaries [29]. Therefore, chemicals used to improve the histopathological changes in polycystic ovary syndrome are usually promising drugs. A recent study showed that melatonin can reduce oxidative damage in rats with PCOS induced by DHEA, indicating the therapeutic effects of this chemical on PCOS. In this study, histopathological changes in ovaries caused by PCOS were found to be significantly improved by EPA treatment, indicating that EPA treatment can improve the conditions of PCOS by reducing histopathological damages.
Conclusions
In conclusion, EPA treatment can improve the hormonal parameters, reduce inflammatory responses, and inhibit the SREBP1/TLR4 pathway in rats with PCOS. EPA treatment can also improve the histopathological damages caused by PCOS. However, our study is limited by its small sample size. Further studies with larger sample sizes are needed to confirm the conclusions of this study.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Flannery CA, Rackow B, Cong X, et al. Polycystic ovary syndrome in adolescence: Impaired glucose tolerance occurs across the spectrum of BMI. Pediatr Diabetes. 2013;14:42–49. doi: 10.1111/j.1399-5448.2012.00902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franks S. Polycystic ovary syndrome. Medicine. 2013;41:553–56. [Google Scholar]
- 3.Liang Y, Yi Y, Sun Q. The impact of migration on fertility under China’s underlying restrictions: a comparative study between permanent and temporary migrants. Soc Indic Res. 2014;116:307–26. [Google Scholar]
- 4.Liang Y, Chu P, Wang X. Health-related quality of life of Chinese earthquake survivors: A case study of five hard-hit disaster counties in Sichuan. Soc Indic Res. 2014;119:943–66. [Google Scholar]
- 5.Lujan ME, Jarrett BY, Brooks ED, et al. Updated ultrasound criteria for polycystic ovary syndrome: Reliable thresholds for elevated follicle population and ovarian volume. Hum Reprod. 2013;28:1361–68. doi: 10.1093/humrep/det062. [DOI] [PubMed] [Google Scholar]
- 6.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: An update on mechanisms and implications. Endocr Rev. 2012;33:981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update. 2008;14:367–78. doi: 10.1093/humupd/dmn015. [DOI] [PubMed] [Google Scholar]
- 8.Vu LC, Joe E, Kirk JK. Role of statin drugs for polycystic ovary syndrome. J Family Reprod Health. 2017;10:165–75. [PMC free article] [PubMed] [Google Scholar]
- 9.Palioura E, Diamanti-Kandarakis E. Polycystic ovary syndrome (PCOS) and endocrine disrupting chemicals (EDCs) Rev Endocr Metab Disord. 2015;16:365–71. doi: 10.1007/s11154-016-9326-7. [DOI] [PubMed] [Google Scholar]
- 10.Asztalos IB, Gleason JA, Sever S, et al. Effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular disease risk factors: A randomized clinical trial. Metabolism. 2016;65:1636–45. doi: 10.1016/j.metabol.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Beier AM, Lauritzen L, Galfalvy HC, et al. Low plasma eicosapentaenoic acid levels are associated with elevated trait aggression and impulsivity in major depressive disorder with a history of comorbid substance use disorder. J Psychiatr Res. 2014;57:133–40. doi: 10.1016/j.jpsychires.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scorletti E, Bhatia L, McCormick KG, et al. Effects of purified eicosapentaenoic and docosahexaenoic acids in nonalcoholic fatty liver disease: Results from the Welcome* study. Hepatology. 2014;60:1211–21. doi: 10.1002/hep.27289. [DOI] [PubMed] [Google Scholar]
- 13.Nasri K, Hantoushzadeh S, Aghadavod E, et al. The effects of Omega-3 fatty acids supplementation on gene expression involved in the insulin and lipid signaling pathway in patients with polycystic ovary syndrome. Horm Metab Res. 2017;49:446–51. doi: 10.1055/s-0042-122782. [DOI] [PubMed] [Google Scholar]
- 14.Young LR, Raatz SK, Thomas W, et al. Total dietary fat and omega-3 fatty acids have modest effects on urinary sex hormones in postmenopausal women. Nutr Metab (Lond) 2013;10:36. doi: 10.1186/1743-7075-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murri M, Insenser M, Fernández-Durán E, et al. Non-targeted profiling of circulating microRNAs in women with polycystic ovary syndrome (PCOS): Effects of obesity and sex hormones. Metabolism. :2018. doi: 10.1016/j.metabol.2018.01.011. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Chen MJ, Ho HN. Hepatic manifestations of women with polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2016;37:119–28. doi: 10.1016/j.bpobgyn.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Liang Y, Wang X. Developing a new perspective to study the health of survivors of Sichuan earthquakes in China: A study on the effect of post-earthquake rescue policies on survivors’ health-related quality of life. Health Res Policy Syst. 2013;11:1–12. doi: 10.1186/1478-4505-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang Y, Wang P. Influence of prudential value on the subjective well-being of chinese urban-rural residents. Social Indicators Research. 2014;118:1–19. [Google Scholar]
- 19.Tessaro I, Modina SC, Franciosi F, et al. Effect of oral administration of low-dose follicle stimulating hormone on hyperandrogenized mice as a model of polycystic ovary syndrome. J Ovarian Res. 2015;8:64. doi: 10.1186/s13048-015-0192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spritzer PM, Lecke SB, Satler F, Morsch DM. Adipose tissue dysfunction, adipokines, and low-grade chronic inflammation in polycystic ovary syndrome. Reproduction. 2015;149:R219–27. doi: 10.1530/REP-14-0435. [DOI] [PubMed] [Google Scholar]
- 21.Shorakae S, Teede H, de Courten B, et al. The emerging role of chronic low-grade inflammation in the pathophysiology of polycystic ovary syndrome. Semin Reprod Med. 2015;33:257–69. doi: 10.1055/s-0035-1556568. [DOI] [PubMed] [Google Scholar]
- 22.Ebejer K, Calleja-Agius J. The role of cytokines in polycystic ovarian syndrome. Gynecol Endocrinol. 2013;29:536–40. doi: 10.3109/09513590.2012.760195. [DOI] [PubMed] [Google Scholar]
- 23.Tao T, Wu P, Wang Y, Liu W. Comparison of glycemic control and β-cell function in new onset T2DM patients with PCOS of metformin and saxagliptin monotherapy or combination treatment. BMC Endocr Disord. 2018;18:14. doi: 10.1186/s12902-018-0243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kistowska M, Gehrke S, Jankovic D, et al. IL-1β drives inflammatory responses to propionibacterium acnes in vitro and in vivo. J Invest Dermatol. 2014;134:677–85. doi: 10.1038/jid.2013.438. [DOI] [PubMed] [Google Scholar]
- 25.Gracie JA, Forsey RJ, Chan WL, et al. A proinflammatory role for IL-18 in rheumatoid arthritis. J Clin Invest. 1999;104:1393–401. doi: 10.1172/JCI7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ip WKE, Hoshi N, Shouval DS, et al. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science. 2017;356:513–19. doi: 10.1126/science.aal3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oishi Y, Spann NJ, Link VM, et al. SREBP1 contributes to resolution of pro-inflammatory TLR4 signaling by reprogramming fatty acid metabolism. Cell Metab. 2017;25:412–27. doi: 10.1016/j.cmet.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dewailly D, Lujan ME, Carmina E, et al. Definition and significance of polycystic ovarian morphology: A task force report from the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update. 2013;20:334–52. doi: 10.1093/humupd/dmt061. [DOI] [PubMed] [Google Scholar]
- 29.Pai SA, Majumdar AS. Protective effects of melatonin against metabolic and reproductive disturbances in polycystic ovary syndrome in rats. J Pharm Pharmacol. 2014;66:1710–21. doi: 10.1111/jphp.12297. [DOI] [PubMed] [Google Scholar]