ABSTRACT
Background: Little is known about the normal range of metal levels in unstimulated saliva, nor whether these might impact Candida carriage in healthy individuals. Both are important in determining which populations are at risk for candidiasis, as the availability of metal ions can influence the growth and pathogenesis of Candida albicans.
Objective: We quantified salivary metals of healthy individuals to determine the correlation with C. albicans oral colonization.
Design: Unstimulated whole saliva was collected from healthy adults and plated to determine fungal carriage, and metal content was measured using ICP-mass spectrometry (ICP-MS).
Results: Zinc was most abundant, followed by iron, copper, manganese, and nickel. Cultivable oral Candida carriage was found in 48% of people. Total protein levels did not differ in salivas from donors with or without carriage. However, innate fungicidal activity was increased in donors with carriage; correlations between levels of several metals were stronger in salivas with fungal carriage, indicating a shift in the oral environment. Concentrations of copper and manganese, as well as age and gender, were significantly predictive of carriage levels in a multiple regression model.
Conclusions: Salivary copper and manganese content along with age and gender could be used as a predictive metric for individuals that are more susceptible to Candida overgrowth.
KEYWORDS: Salivary diagnostics, candidiasis, innate immunity, saliva, salivary metals
Introduction
Metal ions including iron, zinc, copper, nickel, and manganese are found in trace amounts in the saliva of healthy adults [1,2] although their exact physiological function is unknown. Salivary metal levels are quite variable and are believed to be affected by diet [3], salivary flow rates [2], occupational exposure [4], and quantity of metal-binding salivary proteins [5]. Analyses of salivary metal content has been related to exposure to toxic metals [2,4], metal-leaching dental devices [6], or the relationship to disease states [7,8]. Previous data describing normal metal levels in healthy adults are limited and scattered, and these studies were hampered by the fact that salivary copper, nickel, iron, and manganese are generally below the limit of detection by even the most sensitive spectro-analytical methods [1,2] including atomic absorption spectroscopy (AAS) or inductively coupled plasma atomic emission spectroscopy (ICP-AES). Therefore, we examined metal levels in the saliva of healthy adults using ICP-mass spectrometry (ICP-MS) that is capable of detecting part per trillion (ppt) concentrations of these elements [9].
It is likely that oral metal levels have a profound impact on the composition of the oral microbial community due to nutritional immunity [7,10] wherein the host sequesters physiologically important metals away from colonizing microbiota [11]. Saliva contains many proteins involved in nutritional immunity [5,10,12]. Calprotectin is the classic example of a metal-sequestering protein [10] that binds manganese, zinc, and iron with nanomolar to picomolar affinity, and inhibits microbial growth by disrupting superoxide dismutase function [13,14]. Salivary amylase [15], ferritin [16], Histatin 5 [17], gustin [18], and lactoferrin (functioning synergistically with calprotectin) [19] are all metal binding proteins expected to have a role in nutritional immunity.
Candida albicans is an opportunistic pathogen responsible for 50–90% of oral candidiasis [20]; however, it is a commensal organism in healthy individuals. Opportunistic infections by commensal organisms are unique from other types of microbial infection, because they are caused by microbiota that is already present in the host. The mechanistic switch between the commensal and pathogenic state is not well understood, but commensal and pathogenic proliferation appears to be controlled by the same genetic circuit [21]. C. albicans has extensive cellular machinery to respond to metal availability in the host environment; therefore, nutritional immunity is likely to be one mechanism that controls proliferation and pathogenesis of commensal Candida in the human oral environment.
Carriage of oral Candida varies widely in both colonized healthy people and those with oral candidiasis. Moreover, salivary colony forming units (CFU) are used for delineating overall levels of C. albicans colonization or infection [22]. There is a substantial overlap in the range of carriage in asymptomatic carriers (2–888 CFU/mL saliva) and those with disease (22–5,000 CFU/mL saliva) [22]. Moreover, average salivary carriage in healthy individuals is considered to be between 300 and 500 CFU/mL [23]. High C. albicans carriage is associated with higher levels of calprotectin in saliva [10], suggesting that nutritional immunity has an important role in maintaining commensal levels of C. albicans. C. albicans has nutritional requirements for physiologically important metals including iron, zinc, manganese, and copper [24]. In addition, several fungal virulence factors including the yeast to hyphae switch (induced by high concentrations of zinc and inhibited by high concentrations of copper [25]), superoxide dismutase (SOD) expression (toggled by copper availability [26]), and zinc induced aggregation of C. albicans [27] are regulated by extracellular metals.
We hypothesized that salivary metal levels in healthy adults influence C. albicans carriage levels, and measured salivary metal concentrations of five major metals by ICP-MS to determine their relationship with oral C. albicans.
Methods
Saliva collection
Written informed consent was obtained from medically healthy donors over the age of 18 before demographic information was collected (UB Federalwide Assurance ID#: FWA00008824). Individuals were excluded if they self-reported acute illness, dry mouth or use of antibiotics, or smoking cigarettes within 1 year of donation. Additionally, salivary flow was noted, and no donors exhibited a defect in saliva production. Donors were asked to fast from food or drink, besides water, for 2 h prior to saliva collection. Saliva was collected between 8 and 11 AM. Candida colonization can be described as a stable population in the oral cavity that must adhere to host surfaces to be maintained [23]. Therefore, we determined CFU per millilitre whole saliva as an aggregate indication of oral colonization. Donors were asked to vigorously rinse with 200 mL distilled water prior to saliva collection to ensure that Candida came from host colonization and not environmental contamination. Then, 10 mL of unstimulated whole saliva (WS) was collected into tubes on ice containing 20 μL of 5 X protease inhibitor (Complete Protease Inhibitor Cocktail, 04693116001, Roche). WS was plated (500 μL) onto YPD (1% Yeast, 2% Peptone, 2% Dextrose) plates containing 50 units/mL penicillin and 50 µg/mL streptomycin (10,000 units/mL penicillin, 10 mg/mL streptomycin, P4333, Sigma), incubated for 48 h at 30°C to quantitate Candida spp. CFU per mL of saliva. In one case, where detected Candida exceeded 9,000 CFU/mL, the donor was examined for visible plaques and saliva was collected again 3 months later to confirm stable commensal colonization. For samples positive for Candida spp., salivas were plated on CHROMagar™ Candida (CHROMagar, Paris, France) to identify Candida species. All donor salivas contained only C. albicans, except for one donor in which only Candida krusei was recovered.
Saliva processing
For all other assays, WS was centrifuged at 900 × g for 5 min at 4°C to remove debris and cells. Then, clarified WS was collected from the supernatant and stored at −80°C. Protein concentration of clarified WS was determined by bicinchoninic acid (BCA) assay (Pierce BCA Protein Assay Kit). Clarified WS was used for ICP-MS to determine salivary metal concentrations, and innate candidacidal assays.
Fungicidal activity of salivas
Innate candidacidal activity of clarified WS was determined by incubating C. albicans wild-type (WT) CAI-4 cells with WS, or 10 mM Sodium Phosphate Buffer (NaPB) as a control, for 1 h followed by microdilution plate assay. Per cent killing activity of WS was calculated as percentage reduction in CFU compared to control.
Detection of metals by ICP-MS
Clarified WS and control saliva spiked at 2 ng/mL with internal standards were digested and analysed by the bioanalytical services at RTI International (Research Triangle Park, NC). Analysis was conducted using a Thermo Element 2 SF-ICP-MS equipped with an ESI SC2-DX autosampler. The limit of quantification (LOQ) was reported as the lowest concentration calibration standard that exhibited acceptable recovery. The limit of detection (LOD) was calculated as the standard deviation of the digested method blank samples, multiplied by the Student’s t-factor at a 99% confidence interval (4,541 at 3 degrees of freedom). For elements where the reported LOD was greater than LOQ, the LOQ was reported to be equal to the LOD.
Statistical methods
To address detection limits of metals, after log transformation of data, observations that were less than the LOD were replaced by the conditional expected value using the Richardson and Ciampi algorithm [28] that is used when data are normally or approximately normally distributed [29]. Descriptive statistics including mean, standard deviation, and selected percentiles were calculated for all variables. Spearman correlations were computed to examine the bivariate dependences among the five metal variables in both groups of detected carriage or no detected carriage.
Owing to a greater proportion of zero counts in carriage, analyses of this dependent variable were based on a zero inflated negative binomial model (ZINB) [30]. Iron, nickel, copper, manganese, and zinc were examined in separate models and together in a single model. The donor covariates age and gender were added to examine the robustness of results. Likelihood ratio tests were utilized to determine the significance of predictors. Statistical analyses were performed using GraphPad Prism or SAS 9.4 software [31]. Principle component analysis was applied using the ClustVis data visualization web tool [32], and correlation matrix plots were generated with the software, R [33].
Results
Descriptive statistics for saliva donors
Descriptive statistics for the entire group of subjects (n = 42), and individuals with detected carriage (n = 20) are reported in Table 1. The age of all subjects ranged from 21 to 80 years, and the mean age was 35 ± 14 years. Twenty out of the 42 subjects (48%) in our study had oral carriage of Candida. Of those with detected carriage (2–9,776 CFU/mL), ages ranged from 22 to 62 years with a mean age of 36 ± 12 years. Total salivary protein ranged from 0.45 to 4.44 mg/mL with a mean of 2.05 ± 0.9 mg/mL. In general, zinc was the most abundant metal in saliva, at 94.18 ± 152.54 ng/mL, followed by iron (22.3 ± 30.2 ng/mL), copper (13.43 ± 18.81 ng/mL), manganese (5,626 ± 9,724 ng/mL), and nickel (0.545 ± 0.728 ng/mL). Innate fungicidal activity of saliva ranged from 0 to 32.0% with an average of 9.2 ± 8.6%. Among individuals with detected carriage, the hierarchy of metal abundances was conserved: zinc (95.04 ± 120.90 ng/mL), iron (22.9 ± 30.9 ng/mL), copper (17.66 ± 22.29 ng/mL), manganese (5,541 ± 7,019 ng/mL), and nickel (0.741 ± 0.965 ng/mL). For these individuals, innate fungicidal activity of saliva ranged from 0 to 22.2% with an average of 11.9 ± 8.4%. Total protein in individuals with carriage had an identical range to all donors and a mean of 2.08 ± 1.11 mg/mL.
Table 1.
Demographic variables of saliva donors.
| Variable | N | Mean | St. Dev. | Minimum | Median | Maximum | 90th Pctl. | 95th Pctl. | 99th Pctl. | |
|---|---|---|---|---|---|---|---|---|---|---|
| All donors | Age (Years) | 42 | 35 | 14 | 21 | 30 | 80 | 61 | 63 | 80 |
| Carriage (CFU/mL) | 42 | 356 | 1535 | 0 | 0 | 9776 | 440 | 728 | 9776 | |
| Fungicidal activity (%) | 42 | 9.2 | 8.6 | 0.0 | 4.6 | 32.0 | 20.3 | 21.6 | 32.0 | |
| Total protein (mg/mL) | 42 | 2.05 | 0.90 | 0.45 | 1.99 | 4.44 | 2.99 | 3.98 | 4.44 | |
| Iron (ng/mL) | 42 | 22.3 | 30.2 | 1.4 | 13.9 | 144.0 | 50.4 | 80.7 | 144.0 | |
| Zinc (ng/mL) | 42 | 94.18 | 152.54 | 2.43 | 37.65 | 831.00 | 189.00 | 389.00 | 831.00 | |
| Copper (ng/mL) | 42 | 13.43 | 18.81 | 0.10 | 6.37 | 79.40 | 25.40 | 60.30 | 79.40 | |
| Manganese (ng/mL) | 42 | 5.626 | 9.724 | 0.044 | 2.370 | 53.100 | 15.600 | 20.200 | 53.100 | |
| Nickel (ng/mL) | 42 | 0.545 | 0.728 | 0.157 | 0.157 | 3.150 | 1.270 | 1.990 | 3.150 | |
| Detected carriage | Age (Years) | 20 | 36 | 12 | 22 | 33 | 62 | 52 | 57 | 62 |
| Carriage (CFU/mL) | 20 | 747 | 2186 | 2 | 80 | 9776 | 728 | 2240 | 9776 | |
| Fungicidal activity (%) | 20 | 11.9 | 8.4 | 0.0 | 14.8 | 22.2 | 20.3 | 21.0 | 22.2 | |
| Total protein (mg/mL) | 20 | 2.08 | 1.11 | 0.45 | 1.92 | 4.44 | 3.98 | 4.06 | 4.44 | |
| Iron (ng/mL) | 20 | 22.9 | 30.9 | 1.4 | 7.7 | 108.0 | 71.5 | 80.7 | 108.0 | |
| Zinc (ng/mL) | 20 | 95.04 | 120.90 | 2.43 | 56.80 | 428.00 | 173.00 | 389.00 | 428.00 | |
| Copper (ng/mL) | 20 | 17.66 | 22.29 | 0.10 | 8.73 | 79.40 | 58.50 | 60.30 | 79.40 | |
| Manganese (ng/mL) | 20 | 5.541 | 7.019 | 0.044 | 2.150 | 20.200 | 15.600 | 18.700 | 20.200 | |
| Nickel (ng/mL) | 20 | 0.741 | 0.965 | 0.157 | 0.157 | 3.150 | 1.990 | 3.040 | 3.150 |
Histograms of the frequency of C. albicans carriage and metal concentrations illustrate that the data in all cases were heavily skewed towards zero (Figure 1). Interestingly, in all cases the median value of the dataset was considerably lower than the mean value, illustrating that a relatively small number of high values for carriage and metal concentration contributed to the higher means. The small number of large values also contributed to a large standard deviation of the mean. Box-and-whisker plots denote minimum, median, and maximum values, and inter-quartile ranges. Coefficients of variation (CV) were calculated to compare the variability between metals. The greatest variability was found for manganese (CV = 172.8%) and zinc (CV = 162.0%), while copper (CV = 140.1%), iron (CV = 135.4%), and nickel (CV = 133.6%) each had less variability.
Figure 1.
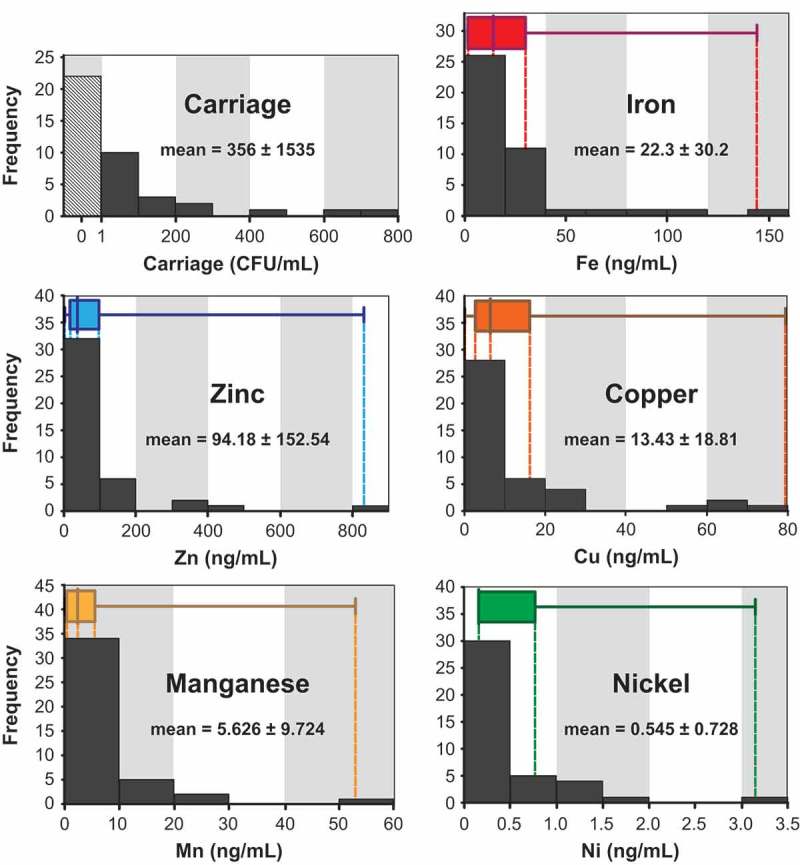
C. albicans carriage and salivary metal concentrations among all donors. Histograms of carriage (CFU/mL), iron (ng/mL), zinc (ng/mL), copper (ng/mL), manganese (ng/mL), and nickel (ng/mL) are combined with box and whisker plots showing minimum value, lower quartile, median, upper quartile, and maximum value for each variable (n = 42). Dotted lines indicate where box and whisker plots intersect with the x-axis. Sample means are reported in the same units as corresponding histograms.
Donors with Candida carriage have higher salivary metal pair covariance than donors without carriage
Total protein and innate fungicidal activity of donors with and without detected carriage groups were compared using t-tests (Figure 2(a)). We found that average total protein levels were not significantly different between groups; however, there was a significant (P = 0.047) increase in the innate fungicidal activity of donor salivas with detected carriage compared with donors without carriage. We expected that if C. albicans carriage induced nutritional immunity, one indication of this would be that different metals would co-vary in donor salivas with carriage and without, since many proteins implicated in nutritional immunity bind multiple metals. Therefore, we asked whether salivary metals had greater covariance with other metals when salivary samples were divided into carriage and no carriage groups (Figure 2(b)). Indeed, the strongest correlations between metal pairs differed between these two groups. Correlations were classified as significant (P ≤ 0.05) or not significant (P > 0.05), and the strength of the correlation, as indicated by the coefficient r, was defined as strong (r ≥ 0.7 shown in red), moderate (0.5 < r < 0.7 shown in yellow), weak (r ≤ 0.5 shown in blue), or non-significant (P > 0.05 shown in grey). We observed a strong correlation between nickel and copper that was similar in both groups; however, we observed many more differences in metals between groups. There was a strong correlation between iron and manganese in donor saliva with carriage, which was only moderate in donors without carriage. We also observed a strong correlation between manganese and copper in donors with carriage, which was again only moderate in donors without carriage. Correlations between zinc and nickel, as well as zinc and copper, were also higher in individuals with carriage than without. Thus, metal correlations become stronger for multiple metal pairs in groups with the presence of Candida, suggesting differences in the oral environment with respect to metals between individuals with and without carriage.
Figure 2.

Inherent fungicidal activity and correlations between multiple metals are increased in donor salivas with C. albicans carriage compared to salivas without C. albicans. (A) Box and whisker plots compare groups of no carriage and carriage by total protein level as determined by BCA and fungicidal activity. (* indicates P < 0.05) (B) Correlation matrices were generated of donors with (n = 20) and without (n = 22) detected Candida carriage. For each correlation matrix, Spearman correlations for the indicated metals are listed in the top right with P values in parenthesis. Bivariate scatter plots of log-transformed metal pairs are organized on the bottom left. Distributions of log(metal) for each of the indicated metals are organized on the diagonal. Units are log(ng/mL). Red indicates strong correlation (r ≥ 0.7), yellow indicates moderate correlation (0.5 < r < 0.7), and blue indicates weak correlation(r ≤ 0.5). Grey indicates that the correlation was not significant (P > 0.05).
Salivary metal levels predict increased carriage but not acquisition of Candida
To validate the predictive value of salivary metals for the amount of carriage measured in an individual, donors with detected carriage were included in a predictive model in which the natural log of metal concentration was used as the predictor value (Supplemental Table 1). Interestingly, we found that for iron (P = 0.022), manganese (P < 0.001), and copper (P = 0.001), a unit increase was significant in predicting an increase in carriage count. Specifically, for a unit increase in iron, there was a 134% increase in the expected carriage count. For a unit increase in manganese there was an 89% increase in expected carriage count, and for copper a 220% increase. Zinc and nickel were not significantly predictive of the amount of carriage.
Next multiple regression analysis was used to remove the influence of variability in age and gender on the predictive model. When demographic variables age and gender were considered (Supplemental Table 2), only copper was still significantly predictive of carriage, with a 174% increase in expected carriage count per unit increase of copper (showing that the relationship between copper and carriage count was not connected to age and gender). We also used multiple regression analysis to consider the variability of all five metals, age, and gender at once (Supplemental Table 3). Copper (P = 0.050) remained predictive of carriage in a model where the variability of the other four metals, age, and gender was removed, so that a unit increase in copper resulted in a 323% increase in expected carriage. In this model, manganese (P = 0.035) also became predictive of carriage, with a unit increase in manganese resulting in a 148% increase in expected carriage count. All other metals were not significantly predictive of carriage. Interestingly, age was a highly significant (P = 0.002) predictor in this model, so that there was a 20% expected increase in carriage count per increased year of age. Gender was also a significant (P = 0.028) predictor of carriage levels, so that females had a 97% decrease in expected carriage count compared to males. These results indicate that there was an interaction amongst age, gender, and the five metals in this study, and of the five metals, copper and manganese are the best predictors for increased carriage count in our donor group.
Next, we questioned whether any single salivary metal could predict if an individual was a Candida carrier using a ZINB model (Supplemental Table 1). For this analysis, donors with zero carriage as well as carriers were incorporated. Although we found that salivary manganese and copper in donors with carriage were predictive of an increase in the expected carriage count, none of the five metals singly could predict with significance the presence or absence of Candida in saliva of all individuals. Thus, salivary metals do not influence the acquisition of Candida, but do modulate subsequent levels of oral Candida.
The relationship between salivary metal levels and Candida count is non-monotonic
Spearman correlations identify monotonic relationships between variables; however, we suspected that the interaction between metal levels and carriage was more complex, because oral metal levels are known to be influenced by multiple factors. As metals were found to be predictive of increased carriage count, and not Candida acquisition, we focused on the donor group with detected carriage. To better visualize the relationship between salivary metal levels and carriage count, we examined metal levels of individuals with carriage levels (Figure 3). As expected from our predictive model (Supplemental Table 1), we found that low carriage is generally accompanied by comparatively low salivary metal content. However, we were surprised to note that individuals with the highest carriage generally did not have the highest metal levels. We normalized and averaged together all five metals to visualize the relationship between Candida carriage and the trend of metal levels. When donors were organized by increasing carriage, we found a distribution of higher metal levels centred around the middle of the donor group; whereas, another lower metal distribution was centred around those with higher carriage.
Figure 3.

Metal content and C. albicans carriage levels in donor salivas have a non-monotonic relationship. Graphs of individual donor measurements are organized by increasing carriage (CFU/mL) overlaid with the corresponding concentration of iron, zinc, copper, manganese, nickel (ng/mL), or the average of all five metals after feature scaling. The carriage axes are cut off at 1,000 CFU/mL for clarity, with CFU labelled at the bottom of the graph for carriage greater than 1,000.
To better understand this relationship, we used principle component analysis (PCA) to model the effects of multiple metals, age, and gender on the grouping of individual Candida carriers (Figure 4). Age and gender were included because they were significantly predictive of the carriage level in the predictive model where all five metals were considered. A model of the first two principle components (Figure 4 left panel) shows clear clustering of the lowest carriage and highest, demonstrating that the metals measured in this study have an interaction with carriage level. Copper was the only metal to retain significant prediction of carriage when age and gender were considered. Therefore, PCA was also performed with only copper, age, and gender (Figure 4 right panel). Of note, individuals with the highest carriage appeared to cluster more closely with individuals with the lowest carriage, compared to individuals in the middle of the donor group; indicating that individuals with low and high carriage, though still distinctly clustering, were more closely related to each other than to individuals in the middle of the group. Thus, PCA suggests that an individual’s salivary metal profile, in addition to their age and gender, is an indication of their likelihood to have different levels of Candida carriage; and these parameters could be used as a predictive metric for carriers that are more susceptible to Candida overgrowth.
Figure 4.

Salivary metals contribute to clustering by C. albicans carriage levels. Bivariate scatter plots of the first two principle components of a PC analysis of the variables age, gender, iron, zinc, copper, manganese and nickel, or the variables copper, age, and gender. Axis percentages indicate the amount of variation in the dataset that is explained by that principle component. Donors are labelled on a colour spectrum by increasing carriage.
Discussion
Although a handful of previous studies have examined salivary metals using less sensitive quantification methods [1,34], this is the first study to report metal concentrations in whole saliva for healthy adults along with their fungal carriage rates. Candida carriage in healthy adults has been reported after cultivation to occur in up to 75% of people [35]; however, our group of healthy donors had only 48% carriage, of which all but one were C. albicans. Other studies have assessed carriage in unstimulated WS of healthy adults; one study (n = 97) reported a slightly higher rate of carriage (56%), with a reported median of 40 CFU/mL for carriers and counts ranging from 4 to 2,732 CFU/mL [36]. Another study (n = 39) with a 46% rate of carriage reported a mean of 244 CFU/mL and a range from 2 to 888 CFU/mL saliva [22]. In this study, we found a median of 80 CFU/mL for carriers, a mean of 747 CFU/mL, and a range of 2–9,776 CFU/mL. Because the range of detected CFU/mL saliva in these studies was so great, the large differences in mean and median are not unexpected; in comparison, one study reported that the mean CFU/mL in patients with candidiasis was over 1,500 [22].
Studies that have looked into salivary metal content of healthy adults report a wide array of concentrations in unstimulated WS. One study reported concentrations of select metals to be lower than our findings; Zn at an average of 13.5 ± 12.2 ng/mL in unstimulated WS, Mn at 2.94 ± 2.84 ng/mL, and Cu at 1.53 ± 1.33 ng/mL [2]. Other studies found higher concentrations. Chicharro et al. reported 10 times the average concentration of Cu, 100 times the concentration of Zn, and over 200 times the concentration of Fe and Mn as our findings [37]. Wang et al. reported Zn at 1,180 ± 214 ng/mL, Cu at 822 ± 159 ng/mL, but also Mn at 2.7 ± 1.5 ng/mL [4]. Environmental contamination of trace metals is a concern in salivary metal studies and may account for the extreme variation in results.
Interestingly, the innate fungicidal activity of donor salivas with carriage was significantly higher, suggesting that antifungal proteins were induced without a change in total protein. As both calprotectin and Histatin 5 bind multiple different metals [14,17] related to their antimicrobial activity, this may reflect early host innate immune responses to higher Candida carriage. Of particular interest was our finding of the robust interaction between carriage with manganese and copper. Copper is a required nutrient for C. albicans (serving as a cofactor for C. albicans SODs which protect cells from oxidative stress [26]) and also has inherent antifungal and antimicrobial activity by participating in Fenton reactions to generate harmful reactive oxygen species [38,39]. Moreover, Histatin 5 binds copper with high affinity, and this increases the killing activity of the peptide [40]. Although manganese, different from many transition metals, is not prone to Fenton reactions [41], it is important for the function of antioxidant SOD proteins in C. albicans, particularly when copper is unavailable [26]. Reciprocally, in the oral environment, calprotectin sequesters manganese as the mechanism of its antimicrobial activity [39]. Thus, there are several possible mechanisms by which copper and manganese modulate Candida in the oral environment.
Although there is a perception that women tend towards higher levels of Candida carriage [42,43], we found that healthy adult women had 97% less expected carriage than men when age and the five metals were held constant. We also noted that when gender and the five metals were held constant, increasing age was predictive of higher carriage count, suggesting that the age-related changes in saliva predispose individuals to higher carriage independently from the five major salivary metals.
It is probable that individuals with high carriage count are at greater risk for progression to oral candidiasis, although there is still very little known about the cues that govern the switch between fungal commensalism and pathogenesis. As metals are so important to the virulence of C. albicans and to the antimicrobial activity of saliva, our data suggest better parameters for what constitutes low, middle, or high oral carriage in respect to salivary metal levels. We identified alterations in salivary metals at around 20 CFU/mL and another change around 260 CFU/mL. These parameters are relevant to actual changes in metals in the oral environment, rather than arbitrary cutoffs for low and high carriage. This is an important distinction to make, because the tri-modal pattern of metal levels over increasing carriage increases the possibility that individuals with high carriage versus mid-level carriage will respond differently to changes in external metal levels. These findings may reflect two separate facets of Candida commensalism: first, that increased salivary metal levels promote fungal proliferation at lower carriage counts; second, that high levels of Candida in the oral cavity deplete salivary metals, either due to nutritional requirements of the yeast or induced mechanisms of nutritional immunity by the host.
This study establishes the concentration ranges of five metals in the saliva of healthy adults that are valuable in defining metrics of the oral environment. We found that salivary copper and manganese are predictive of increased levels of Candida carriage, and we suggest new definitions of cutoffs for low, middle, and high oral C. albicans carriage based upon salivary metals to identify individuals that are at risk for Candida overgrowth.
Funding Statement
This work was supported by the National Center for Advancing Translational Sciences [UL1TR001412], National Institute of Dental and Craniofacial Research [R01DE010641], National Institute of Dental and Craniofacial Research [R01DE022720].
Acknowledgments
This work was supported by R01DE010641 and R01DE022720 funded by the National Institute of Dental and Craniofacial Research, National Institutes of Health; as well as UL1TR001412 funded by the National Center for Advancing Translational Sciences, National Institutes of Health.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
Supplemental data for this article can be accessed here.
References
- [1]. Garhammer P, Hiller KA, Reitinger T, et al. Metal content of saliva of patients with and without metal restorations. Clin Oral Investig. 2004;8(4):238–10. [DOI] [PubMed] [Google Scholar]
- [2]. Kim YJ, Kim YK, Kho HS.. Effects of smoking on trace metal levels in saliva. Oral Dis. 2010;16(8):823–830. [DOI] [PubMed] [Google Scholar]
- [3]. Watanabe M, Asatsuma M, Ikui A, et al. Measurements of several metallic elements and matrix metalloproteinases (MMPs) in saliva from patients with taste disorder. Chem Senses. 2005;30(2):121–125. [DOI] [PubMed] [Google Scholar]
- [4]. Wang D, Du X, Zheng W. Alteration of saliva and serum concentrations of manganese, copper, zinc, cadmium and lead among career welders. Toxicol Lett. 2008;176(1):40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Arnold J, Sangwaiya A, Manglam V, et al. Presence of hepcidin-25 in biological fluids: bile, ascitic and pleural fluids. World J Gastroenterol. 2010;16(17):2129–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Mikulewicz M, Chojnacka K. Trace metal release from orthodontic appliances by in vivo studies: a systematic literature review. Biol Trace Elem Res. 2010;137(2):127–138. [DOI] [PubMed] [Google Scholar]
- [7]. Borella P, Fantuzzi G, Aggazzotti G. Trace elements in saliva and dental caries in young adults. Sci Total Environ. 1994;153(3):219–224. [DOI] [PubMed] [Google Scholar]
- [8]. Canatan D, Akdeniz SK. Iron and ferritin levels in saliva of patients with thalassemia and iron deficiency anemia. Mediterr J Hematol Infect Dis. 2012;4(1):e2012051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Ammann AA. Inductively coupled plasma mass spectrometry (ICP MS): a versatile tool. J Mass Spectrom. 2007;42(4):419–427. [DOI] [PubMed] [Google Scholar]
- [10]. Sweet SP, Denbury AN, Challacombe SJ. Salivary calprotectin levels are raised in patients with oral candidiasis or Sjögren’s syndrome but decreased by HIV infection. Oral Microbiol Immunol. 2001;16(2):119–123. [DOI] [PubMed] [Google Scholar]
- [11]. Potrykus J, Ballou ER, Childers DS, et al. Conflicting interests in the pathogen-host tug of war: fungal micronutrient scavenging versus mammalian nutritional immunity. PLoS Pathog. 2014;10(3):e1003910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Hibino K, Samaranayake LP, Hagg U, et al. The role of salivary factors in persistent oral carriage of Candida in humans. Arch Oral Biol. 2009;54(7):678–683. [DOI] [PubMed] [Google Scholar]
- [13]. Kehl-Fie TE, Chitayat S, Hood MI, et al. Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus . Cell Host Microbe. 2011;10(2):158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Nakashige TG, Zhang B, Krebs C, et al. Human calprotectin is an iron-sequestering host-defense protein. Nat Chem Biol. 2015;11(10):765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Hong JH, Duncan SE, Dietrich AM, et al. Interaction of copper and human salivary proteins. J Agric Food Chem. 2009;57(15):6967–6975. [DOI] [PubMed] [Google Scholar]
- [16]. Almeida RS, Brunke S, Albrecht A, et al. The hyphal-associated adhesin and invasin Als3 of Candida albicans mediates iron acquisition from host ferritin. PLoS Pathog. 2008;4(11):e1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Melino S, Santone C, Di Nardo P, et al. Histatins: salivary peptides with copper(II)- and zinc(II)-binding motifs: perspectives for biomedical applications. Febs J. 2014;281(3):657–672. [DOI] [PubMed] [Google Scholar]
- [18]. Shatzman AR, Henkin RI. Metal-binding characteristics of the parotid salivary protein gustin. Biochim Biophys Acta. 1980;623(1):107–118. [DOI] [PubMed] [Google Scholar]
- [19]. Okutomi T, Tanaka T, Yui S, et al. Anti-Candida activity of calprotectin in combination with neutrophils or lactoferrin. Microbiol Immunol. 1998;42(11):789–793. [DOI] [PubMed] [Google Scholar]
- [20]. Martins N, Ferreira IC, Barros L, et al. Candidiasis: predisposing factors, prevention, diagnosis and alternative treatment. Mycopathologia. 2014;177(5–6):223–240. [DOI] [PubMed] [Google Scholar]
- [21]. Perez JC, Kumamoto CA, Johnson AD. Candida albicans commensalism and pathogenicity are intertwined traits directed by a tightly knit transcriptional regulatory circuit. PLoS Biol. 2013;11(3):e1001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Epstein JB, Pearsall NN, Truelove EL. Quantitative relationships between Candida albicans in saliva and the clinical status of human subjects. J Clin Microbiol. 1980;12(3):475–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Cannon RD, Chaffin WL. Oral colonization by Candida albicans . Crit Rev Oral Biol Med. 1999;10(3):359–383. [DOI] [PubMed] [Google Scholar]
- [24]. Mayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence. 2013;4(2):119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Harrison JJ, Ceri H, Yerly J, et al. Metal ions may suppress or enhance cellular differentiation in Candida albicans and Candida tropicalis biofilms. Appl Environ Microbiol. 2007;73(15):4940–4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Li CX, Gleason JE, Zhang SX, et al. Candida albicans adapts to host copper during infection by swapping metal cofactors for superoxide dismutase. Proc Natl Acad Sci U S A. 2015;112(38):E5336–E5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Kumar R, Breindel C, Saraswat D, et al. Candida albicans Sap6 amyloid regions function in cellular aggregation and zinc binding, and contribute to zinc acquisition. Sci Rep. 2017;7(1):2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Richardson DB, Ciampi A. Effects of exposure measurement error when an exposure variable is constrained by a lower limit. Am J Epidemiol. 2003;157(4):355–363. [DOI] [PubMed] [Google Scholar]
- [29]. Nie L, Chu H, Liu C, et al. Linear regression with an independent variable subject to a detection limit. Epidemiology. 2010;21(Suppl 4):S17–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Ridout M, Cgb D, Hinde J. Models for count data with many zeros. Proceedings of the XIXth international biometric conference; Cape Town 1998. p. 179–192. [Google Scholar]
- [31]. SAS Institute Inc SAS® 9.4 language reference: concepts. 6th ed Cary, NC: SAS Institute Inc; 2016. [Google Scholar]
- [32]. Metsalu T, Vilo J. ClustVis: a web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acids Res. 2015;43(W1):W566–W570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. R Core Team R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- [34]. López-Jornet P, Juan H, Alvaro PF. Mineral and trace element analysis of saliva from patients with BMS: a cross-sectional prospective controlled clinical study. J Oral Pathol Med. 2014;43(2):111–116. [DOI] [PubMed] [Google Scholar]
- [35]. Cannon RD, Holmes AR, Mason AB, et al. Oral Candida: clearance, colonization, or candidiasis? J Dent Res. 1995;74(5):1152–1161. [DOI] [PubMed] [Google Scholar]
- [36]. Zhou PR, Hua H, Liu XS. Quantity of Candida colonies in saliva: a diagnostic evaluation for oral candidiasis. Chin J Dent Res. 2017;20(1):27–32. [DOI] [PubMed] [Google Scholar]
- [37]. Chicharro JL, Serrano V, Ureña R, et al. Trace elements and electrolytes in human resting mixed saliva after exercise. Br J Sports Med. 1999;33(3):204–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol. 2012;10(8):525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Crawford A, Wilson D. Essential metals at the host-pathogen interface: nutritional immunity and micronutrient assimilation by human fungal pathogens. FEMS Yeast Res. 2015;15(7):fov071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Conklin SE, Bridgman EC, Su Q, et al. Specific histidine residues confer histatin peptides with copper-dependent activity against Candida albicans . Biochemistry. 2017;56(32):4244–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Aguirre JD, Culotta VC. Battles with iron: manganese in oxidative stress protection. J Biol Chem. 2012;287(17):13541–13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Torres SR, Peixoto CB, Caldas DM, et al. Relationship between salivary flow rates and Candida counts in subjects with xerostomia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93(2):149–154. [DOI] [PubMed] [Google Scholar]
- [43]. Loster JE, Wieczorek A, Loster BW. Correlation between age and gender in Candida species infections of complete denture wearers: a retrospective analysis. Clin Interv Aging. 2016;11:1707–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


