Abstract
Objective: The aim of the present study was to determine the prognostic significances of markers of preoperative systemic inflammatory response (SIR) in patients with ovarian clear cell carcinoma (OCCC).
Methods: A total of 109 patients diagnosed with OCCC that underwent primary cytoreductive surgery and adjuvant platinum-based chemotherapy from 2009 to 2012 were enrolled in this retrospective study. SIR markers were calculated from complete blood cell counts determined before surgery. Receiver operating characteristic (ROC) curve analysis was used to determine optimal cut-off values for neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and platelet-to-lymphocyte ratio (PLR). Prognostic significances with respect to overall survival (OS) and progression-free survival (PFS) were determined by Kaplan-Meier curve and multivariate Cox regression analysis.
Results: The optimized NLR, LMR and PLR cut-off values as determined by ROC curve analysis for PFS and OS were 2.3, 4.2, and 123.6, respectively. When the cohort was divided using these optimized cut-offs, NLR and LMR were found to be significantly associated with clinicopathologic factors, NLR with FIGO stage, the presence of malignant ascites, and platinum response, and LMR with FIGO stage, lymph node metastasis, malignant ascites, and platinum response. Kaplan-Meier analysis revealed a high NLR (> 2.3) was significantly associated with low 5-year PFS and OS rates and that a high LMR was significantly associated with high 5-year PFS and OS rates. Multivariate analysis identified FIGO stage, residual mass, and platinum response as independent prognostic factors of PFS, and FIGO stage, residual mass, platinum response, and LMR as independent prognostic factors of OS.
Conclusions: Markers of systemic inflammatory response provide useful prognostic information and lymphocyte-to-monocyte ratio is the most reliable independent prognostic factor of overall survival in patients with ovarian clear cell carcinoma.
Keywords: Ovarian clear cell carcinoma, systemic inflammatory response, neutrophil-to-lymphocyte ratio, lymphocyte-monocyte ratio, platelet-to-lymphocyte ratio, survival.
Introduction
Epithelial ovarian cancer (EOC) remains as the most lethal gynecologic cancer and a leading cause of cancer-related death in women 1. Effective screening strategies are lacking, and the majority of women are still diagnosed with advanced stage disease. Ovarian clear cell carcinoma (OCCC) is a type of epithelial ovarian cancer and differs from high grade serous ovarian cancer (HGSC) in terms of its clinical, histopathological, and genetic features 2. OCCC is frequently diagnosed in Asian women and shows greater higher resistance to first-line platinum and taxane-based chemotherapy than HGSC. Furthermore, patients with stage Ic-IV OCCC have much poorer prognoses than patients with serous carcinoma, and thus, pointed efforts are required to develop more effective treatment strategies and to identify prognostic factors 3.
The factors known to influence treatment outcomes in OCCC are FIGO stage, LN status, and the presence of endometriosis and residual tumor after primary cytoreductive surgery 4,5. However, most of these prognostic biomarkers are limited to intraoperative surgical findings and postoperative pathological features, and thus, clinically useful preoperative prognostic factors that accurately predict chemotherapeutic response and prognosis are needed to improve survival rates in OCCC.
Accumulating evidence indicates inflammation is a hallmark of cancer, and that tumor-associated inflammatory microenvironments facilitate tumor growth and metastasis. Several inflammatory response-related biomarkers in peripheral blood, such as, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR), have been widely investigated as potentially useful prognostic markers in different cancers. Furthermore, it has been established that blood cells associated with host inflammation and immunity, such as, neutrophils, lymphocytes, monocytes and platelets, can be influenced by the cytokines secreted by tumors 6.
The prognostic and predictive values of several preoperative hematologic parameters have been investigated in epithelial ovarian cancer (EOC). Of these potentially useful parameter, a low LMR has been shown to be significantly associated with clinicopathological characteristics, indicative of poor prognosis and disease aggressiveness in patients with several solid tumor types 7. In addition, NLR and PLR values have assisted the identification of patients with a poor prognosis and have been shown to have potential clinical value for predicting platinum resistance in EOC 8. However, few studies have evaluated the prognostic significance of markers of systemic inflammatory response (SIR) in terms of predicting survival in OCCC. Therefore, we undertook the present study to investigate the prognostic values of preoperative SIR markers in OCCC.
Materials and Methods
1. Subjects
One hundred and nine OCCC patients that had underwent primary debulking and adjuvant paclitaxel and carboplatin chemotherapy at university hospitals between April 2007 and June 2012 were retrospectively enrolled in this study, which was approved by the Institutional Review Board (IRB) of Pusan National University Hospital. Patients with any inflammatory condition, except endometriosis, or another malignancy were excluded because of possible influences on laboratory test results. Clinical, pathological, and preoperative complete blood count (CBC) variables were subjected to analysis. No patient received neoadjuvant chemotherapy. Histological diagnoses were based on the WHO criteria, and all microscope slides were reviewed by an experienced gynecologic pathologist. Clinicopathologic characteristics, such as, age, FIGO stage, LN metastasis, malignant ascites, endometriosis, preoperative CA125 level, residual mass, and platinum response were documented and subjected to analysis (Table 1).
Table 1.
Clinicopathologic characteristics of 109 patients with ovarian clear cell carcinoma
| Characteristics | No. of patients (%) |
|---|---|
| Age (yr) | 50 (24-77) |
| <50 | 55 (50.5) |
| ≥50 | 54 (49.5) |
| FIGO stage | |
| I/II | 64 (58.7) |
| III/IV | 45 (41.3) |
| LN metastasis | |
| No | 86 (78.9) |
| Yes | 23 (21.1) |
| Malignant ascites | |
| No | 71 (65.1) |
| Yes | 38 (34.9) |
| Endometriosis | |
| No | 85 (78.0) |
| Yes | 24 (22.0) |
| CA-125 (Unit/mL) | 88.2 (8.2-1086.0) |
| <88.2 | 55 (50.5) |
| ≥88.2 | 54 (49.5) |
| Residual mass | |
| <1 cm | 85 (78.0) |
| ≥1 cm | 24 (22.0) |
| Platinum response | |
| Sensitive | 85 (78.0) |
| Resistant | 24 (22.0) |
| WBC (per μL), median (range) | 6800.0 (3200.0-14080.0) |
| ANC (per μL), median (range) | 3981.2 (1219.5-17159.2) |
| ALC (per μL), median (range) | 2023.1 (318.5-39178.2) |
| AMC (per μL), median (range) | 416.4 (77.7-2631.8) |
| Platelet (× 103/μL), median (range) | 280.0 (66.0-799.0) |
FIGO, International Federation of Gynecology and Obstetrics; LN, lymph node; CA-125, cancer antigen 125; WBC, white blood cell; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; AMC, absolute monocyte count.
2. Data extraction
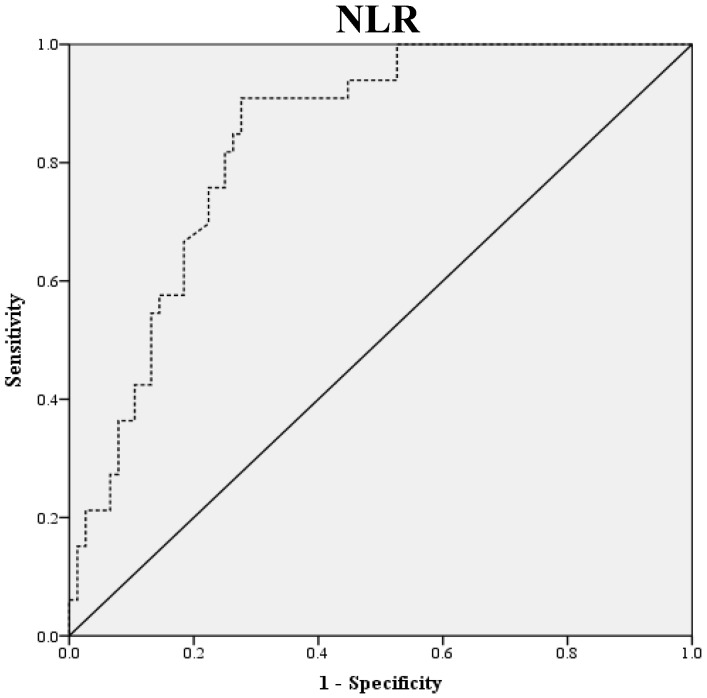
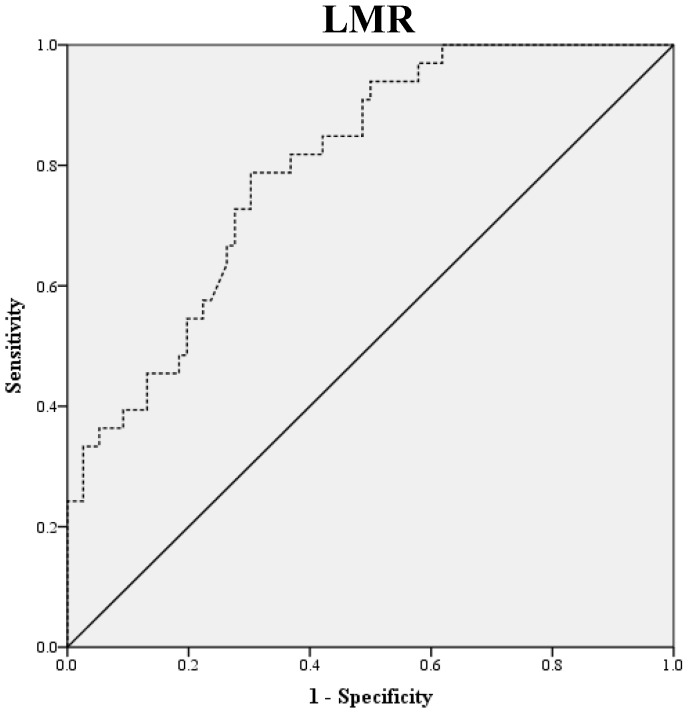
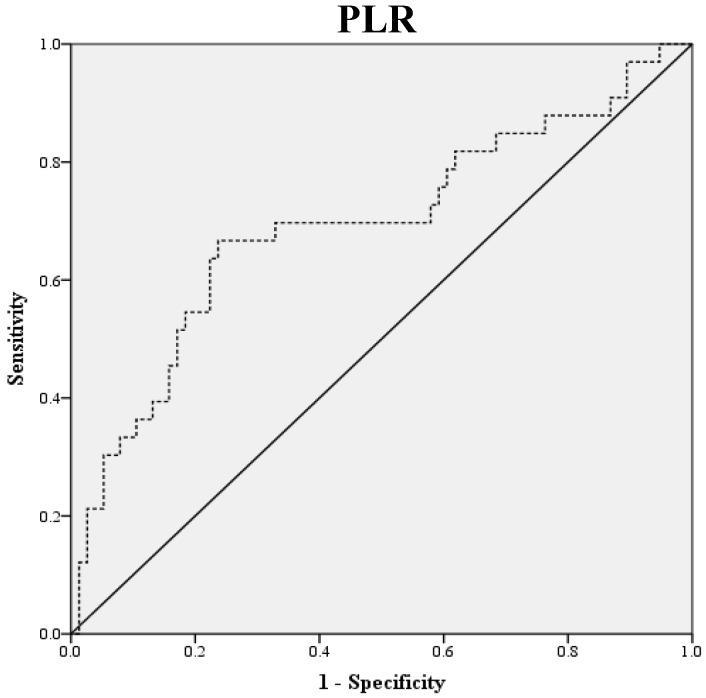
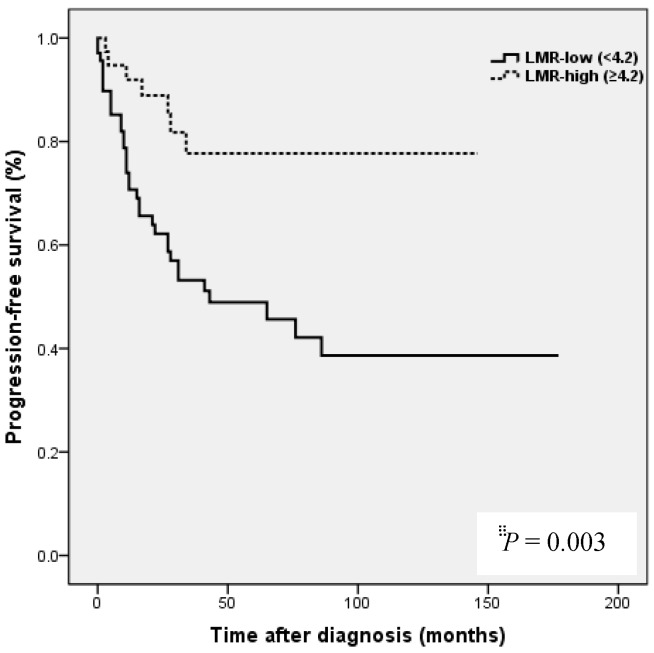
Preoperative blood samples were drawn 1 to 2 weeks prior to surgery. NLR was defined as absolute neutrophil count divided by absolute lymphocyte count, and PLR was defined as absolute platelet count divided by absolute lymphocyte count. LMR was defined as the ratio of absolute lymphocyte count and absolute monocyte count. ROC curve analysis was used to obtain optimal NLR, LMR and PLR cutoff values for predicting survival outcomes. The optimal NLR, LMR and PLR were 2.3, 4.2 and 123.6, respectively and this value was used as the cut-off for the allocations to low and high groups (Fig. 1). OS was accurately defined as time from surgery to death, whereas PFS was defined as time from surgery to tumor recurrence.
Figure 1.
ROC Curve Analysis for NLR, LMR, and PLR in prediction of 109 patients with ovarian clear cell carcinoma. The area under the curve for the NLR, LMR, and PLR were 0.838 (95% confidence interval [CI], 0.764 to 0.911; P< 0.0001), 0.798 (95% confidence interval [CI], 0.714 to 0.882; P< 0.0001) and 0.693 (95% confidence interval [CI], 0.576 to 0.810; P=0.011), respectively.
3. Statistical analysis
The Chi-square test was used to analyze differences between proportions, and the OS and PFS curves were obtained by Kaplan-Meier analysis using the log-rank test. Cox regression analysis was used to determine hazard ratios (HR) and multivariate analysis. The analysis was conducted using SPSS, version 18.0 (SPSS Inc., Chicago, IL, USA). All presented P-values are two-sided, and statistical significance was considered at P<0.05.
Results
1. Patient characteristics
Patient' baseline characteristics are shown in Table 1. Median age of the 109 study subjects was 50 years (range 24-77 years). As previously reported, early stage disease was more common than advanced disease in our patients; 64 (58.7 %) had disease stage I to II, and 45 (41.3%) had disease stage III to IV. Twenty-four patients (22.0 %) were endometriosis- associated. Eighty-five patients (78.0%) were optimally debulked at primary surgery with less than 1 cm of residual disease and all had platinum-sensitive disease.
2. Relations between SIR markers and clinicopathologic characteristics
When patients were stratified using the optimal cut-off values for NLR, LMR and PLR, 33 (30.3%) patients were allocated to the low NLR group and 76 (69.7%) to the high NLR group, 69 (63.3%) to the low LMR group and 40 (36.7%) to the high LMR group, and 17 (15.6%) to the low PLR group and 92 (84.4%) to the high PLR group (Table 2). The low and high NLR groups differed significantly in terms of FIGO stage (P = 0.005), malignant ascites (P = 0.047) and platinum response (P = 0.008). The low and high LMR groups differed significantly in terms of FIGO stage (P=0.026), LN metastasis (P=0.031), malignant ascites (P=0.013) and platinum response (P=0.021). However, no significant differences were found between the low and high PLR groups in terms of age, FIGO stage, LN metastasis, malignant ascites, the presence of endometriosis, CA-125 level, residual mass, or platinum response (all P values > 0.05).
Table 2.
Clinical and pathologic characteristics according to NLR, LMR or PLR in 109 patients with ovarian clear cell carcinoma
| Characteristics | NLR-low (<2.3) |
NLR-high (≥2.3) |
P | LMR-low (<4.2) |
LMR-high (≥4.2) |
P |
PLR-low (<123.6) |
PLR-high (≥123.6) |
P |
|---|---|---|---|---|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | ||||
| Age (yr) | 0.786 | 0.638 | 0.453 | ||||||
| <50 | 16 (48.5) | 39 (51.3) | 36 (52.2) | 19 (47.5) | 10 (58.8) | 45 (48.9) | |||
| ≥50 | 17 (51.5) | 37 (48.7) | 33 (47.8) | 21 (52.5) | 7 (41.2) | 47 (51.1) | |||
| FIGO stage | 0.005 | 0.026 | 0.585 | ||||||
| I/II | 26 (78.8) | 38 (50.0) | 35 (50.7) | 29 (72.5) | 11 (64.7) | 53 (57.6) | |||
| III/IV | 7 (21.2) | 38 (50.0) | 34 (49.3) | 11 (27.5) | 6 (35.3) | 39 (42.4) | |||
| LN metastasis | 0.316 | 0.031 | 0.094 | ||||||
| No | 28 (84.8) | 58 (76.3) | 50 (72.5) | 36 (90.0) | 16 (94.1) | 70 (76.1) | |||
| Yes | 5 (15.2) | 18 (23.7) | 19 (27.5) | 4 (10.0) | 1 (5.9) | 22 (23.9) | |||
| Malignant ascites | 0.047 | 0.013 | 0.608 | ||||||
| No | 26 (78.8) | 45 (59.2) | 39 (56.5) | 32 (80.0) | 12 (70.6) | 59 (64.1) | |||
| Yes | 7 (21.2) | 31 (40.8) | 30 (43.5) | 8 (20.0) | 5 (29.4) | 33 (35.9) | |||
| Endometriosis | 0.712 | 0.634 | 0.267 | ||||||
| No | 25 (13.8) | 60 (23.1) | 55 (79.7) | 30 (75.0) | 15 (88.2) | 70 (76.1) | |||
| Yes | 8 (86.2) | 16 (76.9) | 14 (20.3) | 10 (25.0) | 2 (11.8) | 22 (23.9) | |||
| CA-125 (Unit/mL) | 0.269 | 0.113 | 0.173 | ||||||
| <88.2 | 19 (57.6) | 35 (46.1) | 29 (42.0) | 25 (62.5) | 11 (64.7) | 43 (46.7) | |||
| ≥88.2 | 14 (42.4) | 41 (53.9) | 40 (58.0) | 15 (37.5) | 6 (35.3) | 49 (53.3) | |||
| Residual mass | 0.254 | 0.178 | 0.150 | ||||||
| <1 cm | 28 (84.8) | 57 (75.0) | 51 (73.9) | 34 (85.0) | 11 (64.7) | 74 (80.4) | |||
| ≥1 cm | 5 (15.2) | 19 (25.0) | 18 (26.1) | 6 (15.0) | 6 (35.3) | 18 (19.6) | |||
| Platinum response | 0.008 | 0.021 | 0.870 | ||||||
| Sensitive | 31 (93.9) | 54 (71.1) | 49 (71.0) | 36 (90.0) | 13 (76.5) | 72 (78.3) | |||
| Resistant | 2 (6.1) | 22 (28.9) | 20 (29.0) | 4 (10.0) | 4 (23.5) | 20 (21.7) |
NLR, neutrophil-lymphocyte ratio; LMR, lymphocyte-monocyte ratio; PLR, platelet-lymphocyte ratio; FIGO, The International Federation of Gynecology and Obstetrics; LN, lymph node; CA-125, cancer antigen 125
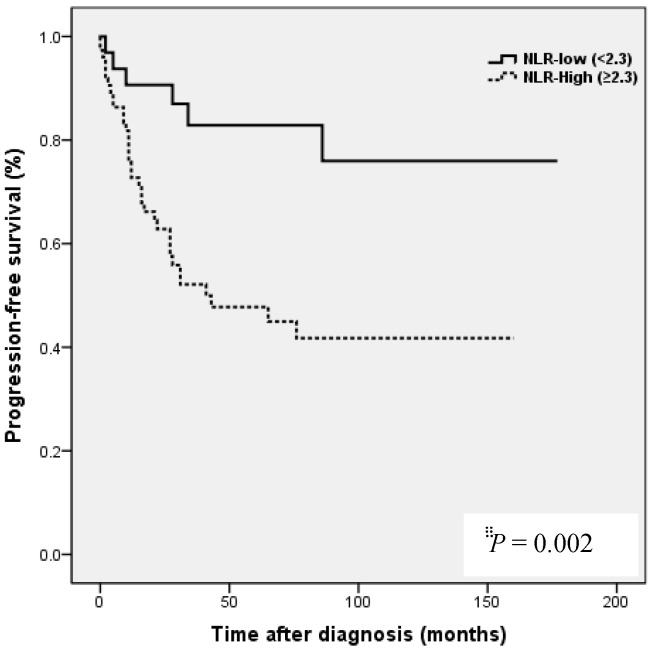
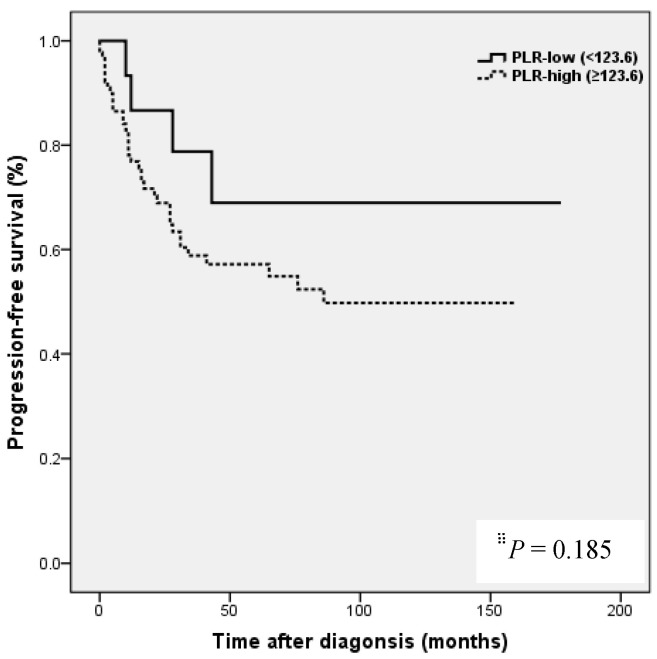
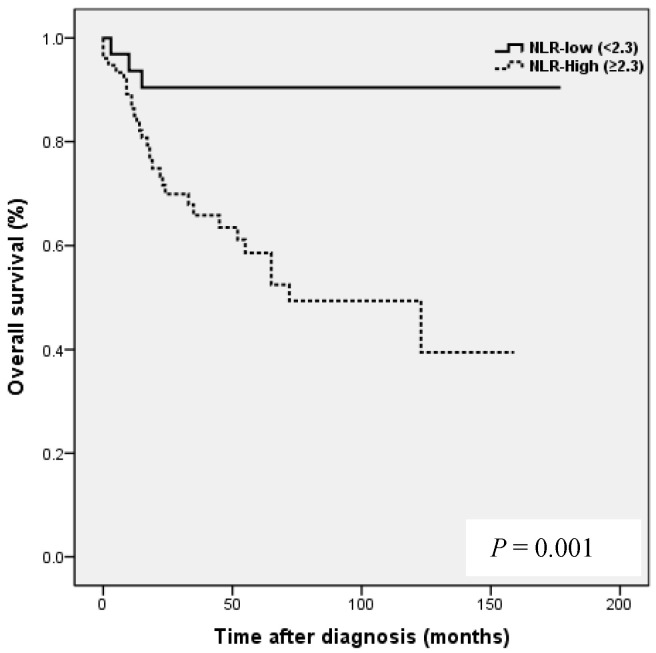
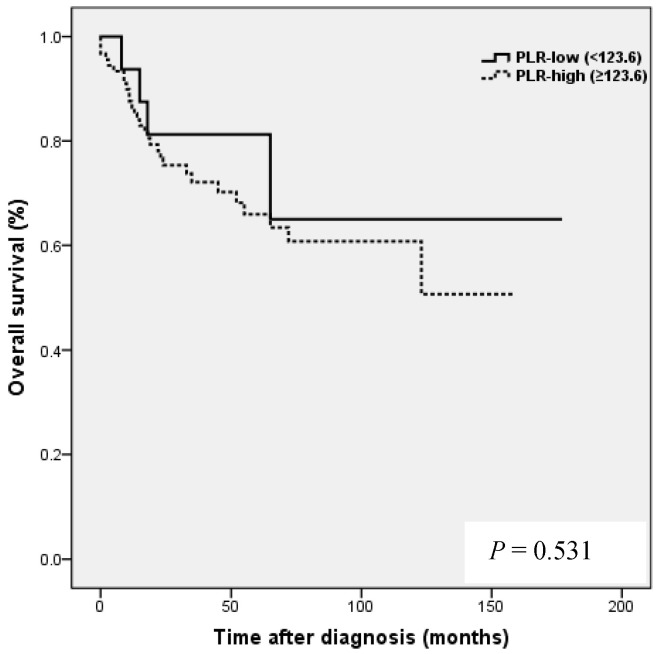
Kaplan-Meier analysis showed that patients in the high NLR group had poorer clinical outcomes in terms of five-year PFS (47.2 vs. 83.0%, P = 0.002) and OS rate (51.4 vs. 90.7%, P = 0.001) than patients in the low NLR group. Patients in the high LMR group had better clinical outcomes in terms of five-year PFS (76.2 vs. 39.8 %, P = 0.003) and OS rate (90.1 vs. 50.6 %, P <0.001) than patients in the low LMR group. However, no such significant differences were observed between the high and low PLR groups (PFS 72.0 vs. 56.2%, P=0.185), and OS (Fig. 2).
Figure 2.
Results of progression-free survival (PFS) and overall survival (OS) analysis with respect to NLR, LMR, and PLR in 109 patients with ovarian clear cell carcinoma.
3. Relationships between cancer- and host-related characteristics and prognosis
Age, FIGO stage, LN metastasis, malignant ascites, the presence of endometriosis, CA-125 level, residual mass, platinum response, and NLR, LMR, and PLR were included in the univariate analyses. Results obtained for PFS showed significant relations with FIGO stage (P < 0.001), LN metastasis (P = 0.005), malignant ascites (P = 0.003), the presence of endometriosis (P = 0.018), CA-125 level (P = 0.005), residual mass (P < 0.001), platinum response (P < 0.001), NLR (P = 0.005), and LMR (P = 0.004). However, COX multivariate analysis showed only the following were independent poor prognostic factors of PFS: advanced FIGO stage (III to IV) (hazard ratio [HR] = 4.027, 95% confidence interval [CI] = 1.756-9.233, P = 0.001), residual mass ≥1 cm (HR = 2.540, 95% CI = 1.084-5.948, P = 0.032) and resistant platinum response (HR = 3.595, 95% CI = 1.458-8.861, P = 0.005) (Table 3). Similarly, univariate analysis showed significant relations between the following variables and OS: FIGO stage (P < 0.001), LN metastasis (P = 0.004), malignant ascites (P < 0.001), the presence of endometriosis (P = 0.039), CA-125 level (P = 0.001), residual mass (P < 0.001), platinum response (P < 0.001), NLR (P = 0.007), and LMR (P = 0.001). However, COX multivariate analysis showed only the following were independent poor prognostic factor of OS, advanced FIGO stage (III to IV) (HR = 5.587, 95% CI = 1.951-15.999, P = 0.001), residual mass ≥1 cm (HR = 3.408, 95% CI = 1.434-8.099, P = 0.011, resistant platinum response (HR = 3.167, 95% CI = 1.023-7.086, P = 0.031), and a low LMR (HR = 2.655, 95% CI = 0.889-8.926, P = 0.038) (Table 4).
Table 3.
Relationship of cancer- and host-related characteristics with progression-free survival (PFS) in 109 patients with ovarian clear cell carcinoma
| Characteristics | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (yr) (≥50 vs. <50) | 1.561 (0842-20896) | 0.158 | ||
| FIGO stage (III/IV vs. I/II) | 8.239 (3.994-16.995) | < 0.001 | 4.027 (1.756-9.233) | 0.001 |
| LN metastasis (Yes vs. No) | 2.640 (1.333-5.229) | 0.005 | 1.367 (0.575-3.254) | 0.479 |
| Malignant ascites (Yes vs. No) | 3.604 (1.929-6.733) | 0.003 | 0.992 (0.283-3.477) | 0.990 |
| Endometriosis (No vs. Yes) | 4.232 (1.307-13.697) | 0.018 | 2.685 (0.968-5.667) | 0.155 |
| CA-125 (Unit/mL) (≥88.2 vs. <88.2) | 3.567 (1.838-8.924) | 0.005 | 1.769 (0.659-4.746) | 0.257 |
| Residual mass (≥1 cm vs. <1 cm) | 4.577 (2.392-8.757) | < 0.001 | 2.540 (1.084-5.948) | 0.032 |
| Platinum response (Resistant vs. Sensitive) | 10.105 (5.227-19.535) | < 0.001 | 3.595 (1.458-8.861) | 0.005 |
| NLR (≥2.3 vs. <2.3) | 3.495 (1.466-8.331) | 0.005 | 1.810 (0.714-4.587) | 0.211 |
| LMR (<4.2 vs. ≥4.2) | 3.065 (1.416-6.634) | 0.004 | 1.251 (0.689-3.716) | 0.427 |
| PLR (≥123.6 vs. <123.6) | 1.974 (0.703-5.539) | 0.197 | ||
HRs was obtained from Cox's proportional hazard model. HR, hazard ratio; CI, confidence interval; NLR, neutrophil-lymphocyte ratio; LMR, lymphocyte-monocyte ratio; PLR, platelet-lymphocyte ratio; FIGO, The International Federation of Gynecology and Obstetrics; LN, lymph node; CA-125, cancer antigen 125
Table 4.
Relationship of cancer- and host-related characteristics with overall survival (OS) in 109 patients with ovarian clear cell carcinoma
| Characteristics | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (yr) (≥50 vs. <50) | 1.451 (0.730-2.885) | 0.287 | ||
| FIGO stage (III/IV vs. I/II) | 9.330 (3.823-22.766) | < 0.001 | 5.587 (1.951-15.999) | 0.001 |
| LN metastasis (Yes vs. No) | 2.928 (1.416-6.056) | 0.004 | 1.073 (0.439-2.619) | 0.878 |
| Malignant ascites (Yes vs. No) | 4.095 (2.006-8.358) | < 0.001 | 1.223 (0.479-3.126) | 0.674 |
| Endometriosis (No vs. Yes) | 4.827 (1.155-20.178) | 0.039 | 2.115 (0.669-6.935) | 0.263 |
| CA-125 (Unit/mL) (≥88.2 vs. <88.2) | 3.732 (1.674-8.319) | 0.001 | 1.720 (0.587-4.8866) | 0.578 |
| Residual mass (≥1 cm vs. <1 cm) | 4.847 (2.361-9.952) | < 0.001 | 3.408 (1.434-8.099) | 0.011 |
| Platinum response (Resistant vs. Sensitive) | 8.403 (4.044-17.459) | < 0.001 | 3.167 (1.023-7.086) | 0.031 |
| NLR (≥2.3 vs. <2.3) | 5.071 (1.546-16.633) | 0.007 | 2.145 (0.529-8.700) | 0.285 |
| LMR (<4.2 vs. ≥4.2) | 6.603 (2.015-21.646) | 0.001 | 2.655 (0.889-8.926) | 0.038 |
| PLR (≥123.6 vs. <123.6) | 1.393 (0.490-3.962) | 0.535 | ||
HRs was obtained from Cox's proportional hazard model. HR, hazard ratio; CI, confidence interval; NLR, neutrophil-lymphocyte ratio; LMR, lymphocyte-monocyte ratio; PLR, platelet-lymphocyte ratio; FIGO, The International Federation of Gynecology and Obstetrics; LN, lymph node; CA-125, cancer antigen 125
Discussion
EOC is the most lethal gynecologic cancer and a major cause of cancer-related death in women 1. This high mortality is mainly due to difficulties associated with early diagnosis, the development of resistance to chemotherapeutic agents, and recurrence. OCCC often has a poorer prognosis than high grade serous carcinoma mainly because of its resistance to standard chemotherapy regimens. Known prognostic factors in OCCC include age, FIGO stage, LN status, the presence of endometriosis, and residual tumor after primary cytoreductive surgery 4,5. However, the abilities of these conventional intraoperative and postoperative factors to predict survival are inadequate. To date, few reliable preoperative biomarkers have been identified that can predict the prognosis in OCCC.
The association between inflammation and tumorigenesis was first reported by Virchow in 1863 9 and available evidence indicates chronic inflammation plays a major role in cancer development and progression, and in therapeutic response 9-11. Moreover, accumulating evidence suggests systemic inflammatory response (SIR) is a key determinant of outcome in patients with cancer, and several authors have suggested reported hematological markers of SIR, such as, NLR, PLR, and LMR, might serve as independent prognostic markers of survival.
Of these suggested markers of SIR, LMR (absolute lymphocyte count (ALC) / absolute monocyte count (AMC)), has attracted increasing attention recently, and has been suggested to be related to survival in malignant lymphoma 12 and in numerous solid tumors 13-16. Lymphocytes represent host anti-tumor immune response, and cause cytotoxic cell death and inhibit tumor cell proliferation and migration 10, 17. Furthermore, lymphocytes that migrate into tumor microenvironments evolve into tumor-infiltrating lymphocytes (TILs), and in a recent meta-analysis, it was suggested TIL might be a prognostic biomarker in ovarian cancer 18. On the other hand, monocytes can differentiate into tumor-associated macrophages, which promote tumor proliferation 19, tumor progression and metastasis by producing various cytokines in different cancer types. In a previous study, in which we assessed the prognostic value of LMR in a cohort of 234 EOC patients that underwent primary debulking surgery and adjuvant chemotherapy, we found a high LMR was strongly correlated with age, serum CA-125 level, FIGO stage and malignant ascites, and that 5-year PFS and OS rates were better for those with a high LMR (PFS, 62.5% vs. 40.0%, P<0.0001; OS 67.2% vs. 42.2% P<0.0001, respectively) 20. In addition, LMR was found to predict the outcome of primary surgical cytoreduction in EOC patients, and LMR, age, CA125 level, and WBC count were found to be reliable predictors of suboptimal cytoreduction 20. Although the prognostic value of SIR has been established in EOC, its value in OCCC has not been well studied 21-23, and few studies have compared the prognostic values of SIR, NLR, and PLR in OCCC, but the prognostic value of LMR has yet to be evaluated in OCCC.
NLR and PLR have been shown to be independent prognostic markers of adverse clinical outcomes in EOC 8,24,25 and specifically in OCCC 21-23. In EOC, NLR known to be strongly associated with poor clinicopathological features, a high tumor burden, stage, preoperative CA125 level, and ascites at surgery in EOC 24. In patients with OCCC, NLR has been reported to be associated with advanced FIGO stage, intraperitoneal metastasis, more ascites, elevated CA-125, and platinum resistance 21, and in the same study, high NLR and PLR were associated with poor prognosis and failure to response to treatment in OCCC 21. In the present study, a high NLR was significantly associated with advanced FIGO stage (III to IV), presence of malignant ascites, and platinum resistance (Table 2). In addition, survival analysis showed that high NLR was associated with poorer PFS and OS than low NLR (Fig. 2). However, although univariate analysis showed NLR was significantly associated with PFS and OS, these relations were not supported by multivariate analysis. A high preoperative PLR has also been shown to be associated with advanced disease stage and reduced survival in EOC 23,25. Supoken et al. showed a high PLR was associated with adverse outcomes, advanced stage, resistance to primary treatment, and decreased survival in OCCC 23, and Kim et al. reported that high PLR and NLR were associated with advanced-stage disease, non-complete response, and platinum-resistance in OCCC 22. However, in the present study, no significant correlation was found between PLR level and age, FIGO stage, LN metastasis, malignant ascites, the presence of endometriosis, CA-125 level, residual mass, or platinum response (all, P > 0.05) (Table 2), and survival analysis showed that a high PLR was not significantly associated with poorer PFS or OS (Fig. 2). This discrepancy may have been caused by sample size differences and the different PLR cut-off values used. In previous studies, PLR cut-offs varied between 140 and 300 23. As a result, the prognostic meaning of PLR remains unclear. In the present study, a high LMR was significantly associated with early FIGO stage (I to II), no LN metastasis, no malignant ascites, and higher platinum response in OCCC (Table 2). Kaplan-Meier analysis supported these positive relations as it show patients in the high LMR group had significantly higher PFS (P=0.003) and OS (P <0.001) than patients in low LMR group (Fig. 2). These findings suggest that a high LMR caused by increased levels of peripheral lymphocytes, increases immune surveillance and response to adjuvant chemotherapy, and thus improves PFS and OS. Furthermore, univariate analysis showed LMR was significantly and positively associated with PFS and OS, and multivariate analysis showed that a high LMR independently predicted better OS, but not better PFS (Table 4). We further analyzed the absolute count of neutrophil, lymphocyte or monocyte in patients with ovarian clear cell carcinoma with or without endometriosis. The absolute count of neutrophil, lymphocyte or monocyte was not upregulated in patients with clear cell ovarian cancer (Table S1).
The strength of our study is that it represents a first attempt to evaluate the prognostic value of LMR in OCCC. Recent studies on SIR markers in OCCC did not include LMR as a potential prognostic marker 21-23. OCCC has been well demonstrated to be associated with chronic inflammation. Kato et al. reported that plasma cell rich inflammatory stroma and cancer cells were responsible for inducing inflammation and stimulating plasma cell differentiation in a paracrine manner in OCCC 26. Moreover, a large number of patients with OCCC despite its rarity were included.
Some limitations of the present study warrant consideration. First, due to the retrospective nature of the study, we were unable to fully confirm the prognostic significance of LMR. Second, LMR is a non-specific marker of inflammation, and though we excluded patients with any inflammatory condition, laboratory results may have been affected by the presence of other unrecognized systemic inflammatory diseases.
In conclusion, this is the first study to assess the prognostic value of LMR in patients with OCCC, and it shows a high LMR is associated with better survival, and suggests LMR might be an independent prognostic factor of survival. Thus, our findings indicate preoperative SIR measurements might provide a straightforward, convenient means of identifying OCCC patients with a poor prognosis.
Supplementary Material
Supplementary Table S1.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Okamoto A, Glasspool RM, Mabuchi S, Matsumura N, Nomura H, Itamochi H, Takano M, Takano T, Susumu N, Aoki D, Konishi I, Covens A, Ledermann J, Mezzanzanica D, Steer C, Millan D, McNeish IA, Pfisterer J, Kang S, Gladieff L, Bryce J, Oza A. Gynecologic Cancer InterGroup (GCIG) consensus review for clear cell carcinoma of the ovary. Int J Gynecol Cancer. 2014;24:S20–25. doi: 10.1097/IGC.0000000000000289. [DOI] [PubMed] [Google Scholar]
- 3.Köbel M, Kalloger SE, Santos JL, Huntsman DG, Gilks CB, Swenerton K. Tumor type and substage predict survival in stage I and II ovarian carcinoma: insights and implications. Gynecol Oncol. 2010;116:50–56. doi: 10.1016/j.ygyno.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 4.Bennett JA, Dong F, Young RH, Oliva E. Clear cell carcinoma of the ovary: evaluation of prognostic parameters based on a clinicopathological analysis of 100 cases. Histopathology. 2015;66:808–815. doi: 10.1111/his.12514. [DOI] [PubMed] [Google Scholar]
- 5.Miyamoto M, Takano M, Goto T, Kato M, Sasaki N, Tsuda H, Furuya K. Clear cell histology as a poor prognostic factor for advanced epithelial ovarian cancer: a single institutional case series through central pathologic review. J Gynecol Oncol. 2013;24:37–43. doi: 10.3802/jgo.2013.24.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lippi G, Meschi T, Nouvenne A, Mattiuzzi C, Borghi L. Neutrophil gelatinase-associated lipocalin in cancer. Adv Clin Chem. 2014;64:179–219. doi: 10.1016/b978-0-12-800263-6.00004-5. [DOI] [PubMed] [Google Scholar]
- 7.Gu L, Li H, Chen L, Ma X, Li X, Gao Y, Zhang Y, Xie Y, Zhang X. Prognostic role of lymphocyte to monocyte ratio for patients with cancer: evidence from a systematic review and meta-analysis. Oncotarget. 2016;7:31926–1942. doi: 10.18632/oncotarget.7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miao Y, Yan Q, Li S, Li B, Feng Y. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are predictive of chemotherapeutic response and prognosis in epithelial ovarian cancer patients treated with platinum-based chemotherapy. Cancer Biomark. 2016;17:33–40. doi: 10.3233/CBM-160614. [DOI] [PubMed] [Google Scholar]
- 9.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 11.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li YL, Gu KS, Pan YY, Jiao Y, Zhai ZM. Peripheral blood lymphocyte/monocyte ratio at the time of first relapse predicts outcome for patients with relapsed or primary refractory diffuse large B-cell lymphoma. BMC Cancer. 2014;14:341. doi: 10.1186/1471-2407-14-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang R, Cai XY, Yang ZH, Yan Y, Zou X, Guo L, Sun R, Luo DH, Chen QY, Huang PY, Xiang YQ, Lu X, Wang L, Xia WX, Mai HQ, Chen MY. Elevated peripheral blood lymphocyte-to-monocyte ratio predicts a favorable prognosis in the patients with metastatic nasopharyngeal carcinoma. Chin J Cancer. 2015;34:237–246. doi: 10.1186/s40880-015-0025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ni XJ, Zhang XL, Ou-Yang QW, Qian GW, Wang L, Chen S, Jiang YZ, Zuo WJ, Wu J, Hu X, Shao ZM. An elevated peripheral blood lymphocyte-to-monocyte ratio predicts favorable response and prognosis in locally advanced breast cancer following neoadjuvant chemotherapy. PLoS One. 2014;9:e111886. doi: 10.1371/journal.pone.0111886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu P, Shen H, Wang G, Zhang P, Liu Q, Du J. Prognostic significance of systemic inflammation-based lymphocyte- monocyte ratio in patients with lung cancer: based on a large cohort study. PLoS One. 2014;9:e108062. doi: 10.1371/journal.pone.0108062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Zhang F, Sheng XG, Zhang SQ. Decreased pretreatment lymphocyte/monocyte ratio is associated with poor prognosis in stage Ib1-IIa cervical cancer patients who undergo radical surgery. Onco Targets Ther. 2015;8:1355–1362. doi: 10.2147/OTT.S82174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin EY, Pollard JW. Role of infiltrated leucocytes in tumour growth and spread. Br J Cancer. 2004;90:2053–2058. doi: 10.1038/sj.bjc.6601705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Wang J, Chen R, Bai Y, Lu X. The prognostic value of tumor-infiltrating T lymphocytes in ovarian cancer. Oncotarget. 2017;8:15621–15631. doi: 10.18632/oncotarget.14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Eo W, Kim HB, Lee YJ, Suh DS, Kim KH, Kim H. Preoperative Lymphocyte-Monocyte Ratio Is a Predictor of Suboptimal Cytoreduction in Stage III-IV Epithelial Ovarian Cancer. J Cancer. 2016;7:1772–1779. doi: 10.7150/jca.15724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, Lu J, Lu Y, Zhou J, Wang Z, Liu H, Xu C. Prognostic significance and predictors of the system inflammation score in ovarian clear cell carcinoma. PLoS One. 2017;12:0177520. doi: 10.1371/journal.pone.0177520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HS, Choi HY, Lee M, Suh DH, Kim K, No JH, Chung HH, Kim YB, Song YS. Systemic Inflammatory Response Markers and CA-125 Levels in Ovarian Clear Cell Carcinoma: A Two Center Cohort Study. Cancer Res Treat. 2016;48:250–258. doi: 10.4143/crt.2014.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Supoken A, Kleebkaow P, Chumworathayi B, Luanratanakorn S, Kietpeerakool C. Elevated preoperative platelet to lymphocyte ratio associated with decreased survival of women with ovarian clear cell carcinoma. Asian Pac J Cancer Prev. 2014;15:10831–10836. doi: 10.7314/apjcp.2014.15.24.10831. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Hong N, Robertson M, Wang C, Jiang G. Preoperative red cell distribution width and neutrophil-to-lymphocyte ratio predict survival in patients with epithelial ovarian cancer. Sci Rep. 2017;7:43001. doi: 10.1038/srep43001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asher V, Lee J, Innamaa A, Bali A. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clin Transl Oncol. 2011;13:499–503. doi: 10.1007/s12094-011-0687-9. [DOI] [PubMed] [Google Scholar]
- 26.Kato N, Kurotaki H, Uchigasaki S, Fukase M, Kurose A. Ovarian clear cell carcinoma with plasma cell-rich inflammatory stroma: a clear cell carcinoma subgroup with distinct clinicopathological features. Histopathology. 2016;68:588–595. doi: 10.1111/his.12783. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1.