Abstract
Colorectal cancer (CRC) is one of the most common cancers worldwide, usually with poor prognosis because many CRC patients are diagnosed at an advanced stage. Therefore, novel potential diagnostic and prognostic biomarkers are urgently needed. MicroRNAs have been reported to regulate a variety of biological processes, such as cell proliferation, differentiation and apoptosis. Accumulating studies have demonstrated that miR-490-3p could regulate the development and progression of multiple cancers, but its clinical significance and molecular mechanism in CRC are still elusive. Here, we try to further elucidate the regulatory mechanism of miR-490-3p in CRC. In the present study, miR-490-3p expression level observably down-regulated in CRC tissues and cell lines, and miR-490-3p expression in CRC tissues was significantly associated with TNM stage, histological grade, tumor size and overall survival (OS). In addition, we observed miR-490-3p expression was also decreased in CRC plasmas and could act as a promising diagnostic biomarker for screening CRC. Further studies in vitro demonstrated Voltage Dependent Anion Channel 1 (VDAC1) which highly expressed in CRC tissues and cell lines is a direct target of miR-490-3p, and miR-490-3p could markedly inhibit CRC cells proliferation, metastasis, invasion and anti-apoptosis through suppressing VDAC1/AMPK/mTOR pathway. These results indicated that miR-490-3p functions as a tumor suppressor in CRC, and may be a novel potential diagnostic and prognostic biomarker for CRC.
Keywords: colorectal cancer, miR-490-3p, VDAC1, biomarker.
Introduction
Colorectal cancer is the fourth most deadly cancer (after lung, liver and stomach cancer), killing almost 700,000 people every year 1. The International Agency for Research on Cancer (IARC) reported that CRC accounts for 6.3% of all malignancies mortality in China 2. It is such a pity that a lack of reliable biomarkers has been leading to late diagnosis, advanced TNM stage and consequent unfavorable 5-year survival rate of CRC. Thus, identifying novel diagnostic and prognostic biomarkers might be helpful to reduce the mortality of CRC patients 3. Although mounting studies have verified that genetic and epigenetic changes are related to the development and progression of CRC, the molecular mechanism of CRC pathogenesis is so complicated that the therapeutic method of patients still remains limited. Therefore, the identification of new sensitive and specific biomarkers and novel therapeutic strategies of CRC is in urgent demand.
It has been demonstrated that microRNAs (miRNAs), a type of endogenous small non-coding RNAs (18-25 nucleotides in length), can functionally carry out biological effects through direct binding to 3' untranslated regions (UTRs) of their target mRNAs by inducing mRNAs degradation and/or translational repression 4. Mounting studies have demonstrated that aberrant miRNAs play a vital role in the pathogenetic processes of cancers 5-7, and may act as potential biomarkers for cancer diagnosis, therapy and prognosis 8-10.
MiR-490-3p, a newly discovered miRNA, has been previously shown to be significantly associated with the carcinogenesis of several cancers. For instance, Zhang et al. found that miR-490-3p could modulate cell growth and epithelial to mesenchymal transition (EMT) of hepatocellular carcinoma by targeting endoplasmic reticulum-Golgi intermediate compartment protein 3 (ERGIC3) 11. Jia et al. observed miR-490-3p could inhibit the growth and invasiveness of triple-negative breast cancer by repressing the expression of tankyrase 2 (TNKS2) 12. In CRC, although Xu et al. have reported that miR-490-3p could inhibit CRC metastasis by targeting transforming growth factor beta receptor 1 (TGFβR1) 13, the molecular mechanism of CRC is so complicated that further researches are in urgent demand.
VDAC1, a 31 kDa pore-forming protein, was found in the outer mitochondrial membrane (OMM) of all eukaryotes acting as a gatekeeper for the entry and exit of mitochondrial metabolites, thus controlling cross talk between mitochondria and the rest of cell 14. Accumulating studies have demonstrated that VDAC1 is significantly associated with the regulation of mitochondria-mediated apoptosis and implicated in multiple cancers 15-17. For example, Arif et al. reported that silencing VDAC1 expression using siRNA could inhibit cancer cell proliferation and growth 18. Head et al. demonstrated that antifungal drug itraconazole targets VDAC1 to modulate the AMPK/mTOR signaling axis in endothelial cells 19, but whether miR-490-3p/VDAC1/AMPK/mTOR pathway exists in CRC has not been reported.
In the current study, we found that miR-490-3p was not only significantly reduced in CRC tissues, cell lines and plasmas, but also a novel potential prognostic and diagnostic biomarker for CRC. Studies in vitro demonstrated that miR-490-3p could inhibit cell proliferation, migration, invasion and apoptosis by targeting to VDAC1/AMPK/mTOR signaling pathway.
Materials and methods
Bioinformatics analysis
The potential target genes of miR-490-3p were predicted using TargetScan (http://www.targetscan.org/) and miRanda (http://www.microrna.org/). The relative expression of miR-490-3p and VDAC1 mRNA in CRC tissues was also searched in NCBI Gene Expression Omnibus (GEO) database and The Cancer Genome Atlas (TCGA) database respectively.
Clinical specimens
Forty-three matched CRC tissues and adjacent normal tissues (ANTs) were collected from CRC patients who underwent primary surgery resection at Nanjing First Hospital Affiliated to Nanjing Medical University. All specimens were snap-frozen after surgery and pathologically confirmed, followed by being stored at -80 °C until RNA extraction. 55 CRC plasma specimens were collected from patients diagnosed as colorectal cancer, and 35 healthy plasma specimens were collected from people who had physical examinations. None of patients recruited in this study received chemotherapy or radiation therapy before specimens collected. Moreover, This study was approved by the ethics committees of Nanjing First Hospital, and written informed consents were obtained from all participants.
Cell culture and transfection
The colonic mucosal epithelial cell (FHC) and CRC cell lines (HCT116, HCT8, SW480 and HT29) were cultured in Dulbecco's Modifed Eagle's Medium (DMEM) supplemented with 100 µl fetal bovine serum (FBS), 10 µl penicillin and 10 µl streptomycin per mL medium in humidified atmosphere containing 5% CO2 at 37°C. All cell lines were obtained from the Chinese Academy Medical Science (China). The miR-490-3p mimic (miR-mimic) and negative control (NC) were purchased from GenePharma (Shanghai, China). The corresponding sequences were as followings: miR-mimic: 5'-CAACCUGGAGGACUCCAUGCUGGCAUGGAGUCCUCCAGGUUGUU-3' and NC: 5'-UUCUCCGAACGUGUCACGUTTACGUGACACGUUCGGAGAATT-3'. The miRNA transfection was conducted at a concentration of 100 nM using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer's instructions.
Plasmid constructs and luciferase reporter assay
Both wild-type (Wt) and mutant (Mut) VDAC1 3'- untranslated regions (UTRs) were amplified by PCR and cloned into the pMIR-Report construct (Ambion, USA). For luciferase assay, HCT116 and SW480 cells plated in 24 well plates were co-transfected with Wt or Mut VDAC1 plasmid along with miR-mimic or NC respectively. Transfection was conducted using Lipofectamine 2000 in accordance with the procedure of manufacture (Invitrogen, USA). Cells were collected at 48h after transfection for luciferase reporter assay analyzed using a Dual-luciferase Reporter Assay system (Promega, USA), and renilla luciferase was used for normalization.
Cell proliferation assay
Cell proliferation was measured by CCK-8 kit (KeyGEN BioTECH, China) according to the manufacturer's instructions. Briefly, cells were plated into the 96-well plate at a density of 1.0 × 103 cells/well and incubated for 24 h, 48 h and 72 h. The absorbance was measured at 450 nm on a microplate reader (Infinite M200 PRO; TECAN, Switzerland).
Wound healing assay
HCT116 and SW480 cells digested and plated into 6-well culture plates were transfected with miR-mimic or NC and incubated for 24 h, respectively. Then 200 μl pipette tips were used to scrape three parallel lines and cells were washed with PBS twice, after which cells were cultured in 10% FBS DMEM. Photographs were taken at 0 and 24 h after wounding under a microscope (Olympus, Japan). Migration was assessed by measuring the change of scratch area.
Cell invasion assay
Matrigel (BD Biosciences, USA) was used to coat the top chamber of 6.5 mm Transwell chambers (8 µm pore size; BD Biosciences, USA). Then, 500 μl DMEM containing 10% FBS was added to the bottom chamber while 200 μl serum-free MEM was added to the top chamber, in which 1.5×105 cells per well had been seeded. After 24 h, cells that had not passed through the Matrigel were removed using cotton swabs, but cells that had passed through the Matrigel to the underside of the filter were fixed with 90% alcohol and stained by 0.05% crystal violet. All the cells were counted under an inverted microscope (Olympus, Japan) and the number of stained cells represented invasiveness.
Cell apoptosis assay
HCT116 and SW480 cells were transfected with miR-mimic or NC in six-well plates respectively. After transfection for 48 h, the cells were collected and washed with PBS twice. For apoptosis analysis, the collected cells (3×105) were mixed with 500 µl Binding Buffer and detected by Annexin V-FITC and PI (KeyGEN BioTECH, China) staining according to the manufacturer's instructions, followed by flow cytometry analysis.
RNA extraction and quantitative real-time PCR (qRT-PCR) analysis
Total RNA was isolated from tissues, cells and plasmas using TRIzol LS reagent (Invitrogen, USA) according to the manufacturer's instructions. Hairpin-itTM microRNA Normalization RT-PCR Quantitation Kit (GenePharma, China) was performed for the reverse transcription and quantitative PCR of miR-490-3p. Elegans miR-39-3p was used as external reference, and U6 snRNA was used as internal control. Real-Time PCR for detecting VDAC1 mRNA expression were performed in 20 μl reactions following the SYBR® Premix Ex TaqTM Kit (Takara, China) and β-actin was used as internal control. 2 μg RNA was reverse transcribed and cDNA was synthesized using PrimeScript RT-PCR Kit (Takara, China). The forward and reverse primers of VDAC1 were 5'-AATGACGGGACAGAGTTTG-3' and 5'-TCAGGCTGGAGTTGTTCAC-3'; the forward and reverse primers of β-actin were 5'-CGTCTTCCCCTCCATCG-3' and 5'-CTCGTTAATGTCACGCAC-3'. The relative expression of miR-490-3p and VDAC1 mRNA was calculated using 2-ΔΔCt.
Western blot analysis
Cultured cells were collected and lysed to harvest protein. Denatured protein was separated by 10% SDS-PAGE, transferred to PVDF membranes. The membranes were blocked with TBST that contains 5% skimmed milk, then blocked with primary antibodies (rabbit anti-AMPK, 1:5000, ab32047; rabbit anti-p-AMPK, 1:5000, ab133448; rabbit anti-mTOR, 1:2000, ab2732; anti-p-mTOR, 1:2000, ab109268; rabbit anti-VDAC1, 1:5000, ab15895; Abcam, UK. rabbit anti-β-actin 13E5, 1: 10000, CST, USA) overnight at 4℃. After being washed, the membranes were incubated with anti-IgG conjugated to horseradish peroxidase at room temperature for 1 h. Bands were visualized using the enhanced chemiluminescence system (ECL) reagent (KeyGEN BioTECH, China).
Immunohistochemistry
Paraffin sections were deparaffinised, rehydrated, and incubated with 3% hydrogen peroxide. Antigen retrieval was obtained after microwaving the slides in 0.01 M citrate buffer. After antigen retrieval, the slides were incubated with 10% normal goat serum for 15min at room temperature followed by incubating with anti-VDAC1 (1:500, ab15895, Abcam, UK) overnight at 4°C and then washed with TBST (30 min). Thereafter, samples were incubated with secondary antibody (Dako, Denmark) for 30 min at room temperature. For visualization, the tissue sections were treated with 3,3'-diaminobenzidine solution and then stained by hematoxylin. All slides were observed under a microscope and images were taken by a digital camera.
Statistical analysis
All the data were expressed as means ± SD (standard deviation) from three independent experiments and analyzed by the Student's t-test or one-way analysis of variance (ANOVA) using SPSS 19.0 (IBM, USA) or GraphPad Prism 5.0 (GraphPad Software, USA). The Kaplan-Meier method with the log-rank test was used to calculate overall survival (OS) rates for comparisons. The Cox proportional hazards model was used in the univariate and multivariate analysis. The area under the receiver operating characteristic (ROC) curve (AUC) was performed to evaluate the feasibility of plasma miR-490-3p as a biomarker for CRC diagnosis, and P < 0.05 was considered to be statistically significant.
Results
The expression of miR-490-3p is downregulated in CRC tissue and cell lines
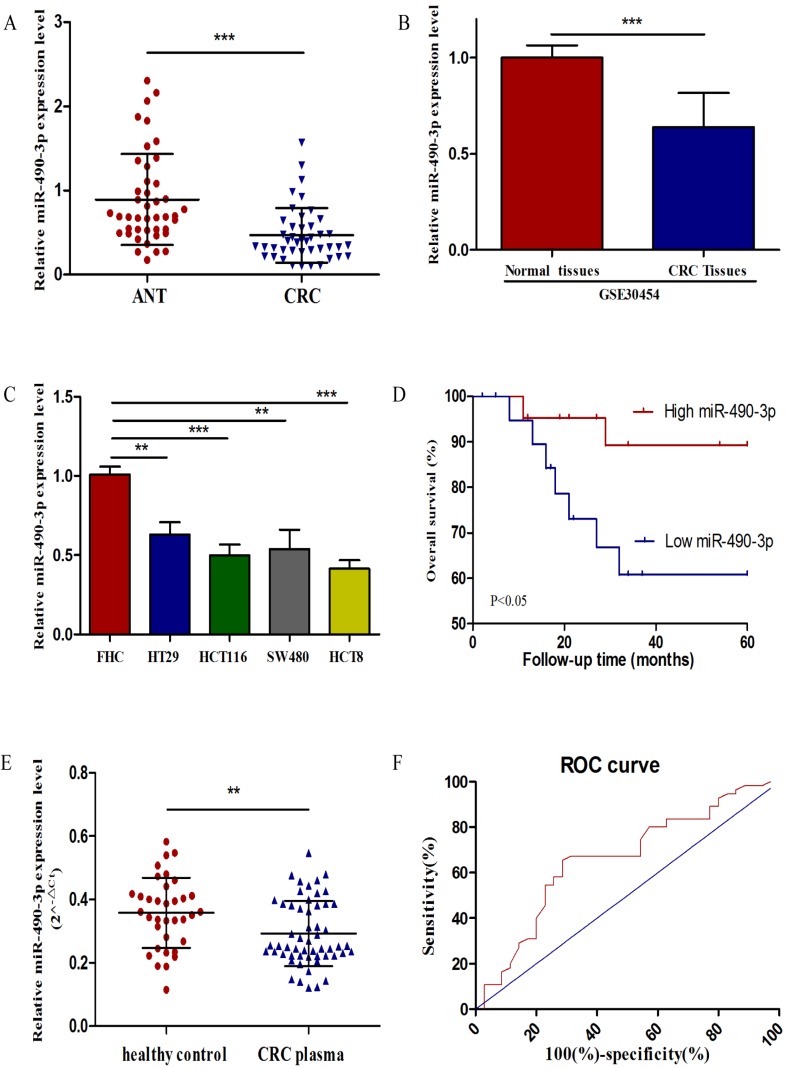
To estimate the expression level of miR-490-3p in 43 matched CRC tissues and ANTs, qRT-PCR was performed, and miR-490-3p was demonstrated to be significantly downregulated in CRC tissues when compared with ANTs (Figure 1A), which was supported by data from GSE30454 exhibiting a consistent result, shown in Figure 1B. In addition, the expression of miR-490-3p in CRC cell lines exhibited differently lower levels when compared to that in FHC (Figure 1C).
Figure 1.
The expression of miR-490-3p in CRC. A, analysis of miR-490-3p expression in 43 pairs of colorectal cancer and adjacent normal tissues. B, relative expression of miR-490-3p in 54 CRC tissues compared to 20 normal tissues (GSE30454). C, comparison of miR-490-3p expression in FHC and CRC cell lines. D, Kaplan-Meier curves for overall survival of patients with CRC categorized according to miR-490-3p expression. E, measurement of plasma miR-490-3p expression in 35 healthy controls and 55 CRC patients. F, ROC curve for miR-490-3p in CRC patients and healthy controls. **P < 0.01, ***P < 0.001.
Decreased miR-490-3p in CRC tissues are correlated with poor clinicopathological characteristics and prognosis of patients
To assess the correlation between miR-490-3p and clinicopathologic characteristics of CRC patients, the expression levels of miR-490-3p in CRC patients' tissues were categorized as high (n = 22) or low (n = 21) according to the median value of miR-490-3p expression. As shown in Table 1, miR-490-3p expression was significantly correlated with the TNM stage (P = 0.009), histological grade (P = 0.039) and tumor size (P = 0.044). However, no significant association was found between the expression of miR-490-3p and other parameters, such as age and gender. Moreover, we analyzed whether there was an association between the expression of miR-490-3p and clinical outcomes of CRC patients using Kaplan-Meier survival analysis. The result indicated that CRC patients with low miR-490-3p expression may have shorter overall survival time (P < 0.05), shown in Figure 1D. Univariate analysis identified that miR-490-3p expression and the TNM stage are associated with the OS of CRC patients (P < 0.05), but multivariate analysis indicated that the only TNM stage was an independent prognostic factor for the OS of CRC patients (Table 2).
Table 1.
Clinicopathological characteristics of patients with CRC
| Variables | Number | Expression of miR-490-3p | P value | |
|---|---|---|---|---|
| low | high | |||
| [1]Gender | ||||
| Male | 26 | 12 | 14 | |
| Female | 17 | 9 | 8 | 0.663 |
| [2]Age (year) | ||||
| >60 | 22 | 10 | 12 | |
| ≤60 | 21 | 11 | 10 | 0.65 |
| [3]TNM | ||||
| Ⅰ+Ⅱ | 21 | 6 | 15 | |
| Ⅲ+Ⅳ | 22 | 15 | 7 | 0.009 |
| [4]Histology grade | ||||
| well | 17 | 5 | 12 | |
| Moderate + poor | 26 | 16 | 10 | 0.039 |
| [5]Tumor size (cm) | ||||
| ≤4.5 | 27 | 10 | 17 | |
| >4.5 | 16 | 11 | 5 | 0.044 |
Table 2.
Univariate and multivariate analyses for OS of CRC patients
| Variables | univariate analysis | multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| miR-490-3p expression (low, high) | 2.248 | 1.291-3.914 | 0.007 | 1.355 | 0.608-3.021 | 0.458 |
| TNM (Ⅲ+Ⅳ,Ⅰ+Ⅱ) | 3.949 | 2.358-6.612 | <0.001 | 3.485 | 1.997-6.083 | 0.021 |
| Histology grade (moderate+poor, well) | 0.781 | 0.442-1.377 | 0.393 | 1.039 | 0.492-2.192 | 0.921 |
| Tumor size (>4.5, ≤4.5) | 1.262 | 0.742-2.146 | 0.39 | 1.088 | 0.638-1.856 | 0.756 |
| Gender (male, female) | 0.784 | 0.492-1.251 | 0.308 | 1.101 | 0.623-1.946 | 0.74 |
| Age (>60, ≤60) | 0.849 | 0.529-1.363 | 0.498 | 0.971 | 0.545-1.728 | 0.919 |
Plasma miR-490-3p might be a potential biomarker for CRC diagnosis
To determine whether plasma miR-490-3p could be used for diagnosing CRC, we compared plasma miR-490-3p level between healthy controls and patients with CRC. The relative expression of plasma miR-490-3p was calculated by 2-△Ct. The results showed that plasma miR-490-3p expression in CRC patients was significantly lower than that in the healthy controls (Figure 1E). A ROC curve was performed to distinguish CRC patients from healthy controls, and the AUC was up to 0.66 (95% CI: 0.54-0.78; P < 0.05) (Figure 1F). The cutoff value of plasma miR-490-3p from ROC curve was 0.3132 with 65.45% sensitivity and 71.43% specificity.
MiR-490-3p induces apoptosis and suppresses proliferation, migratory and invasion activities of CRC cells
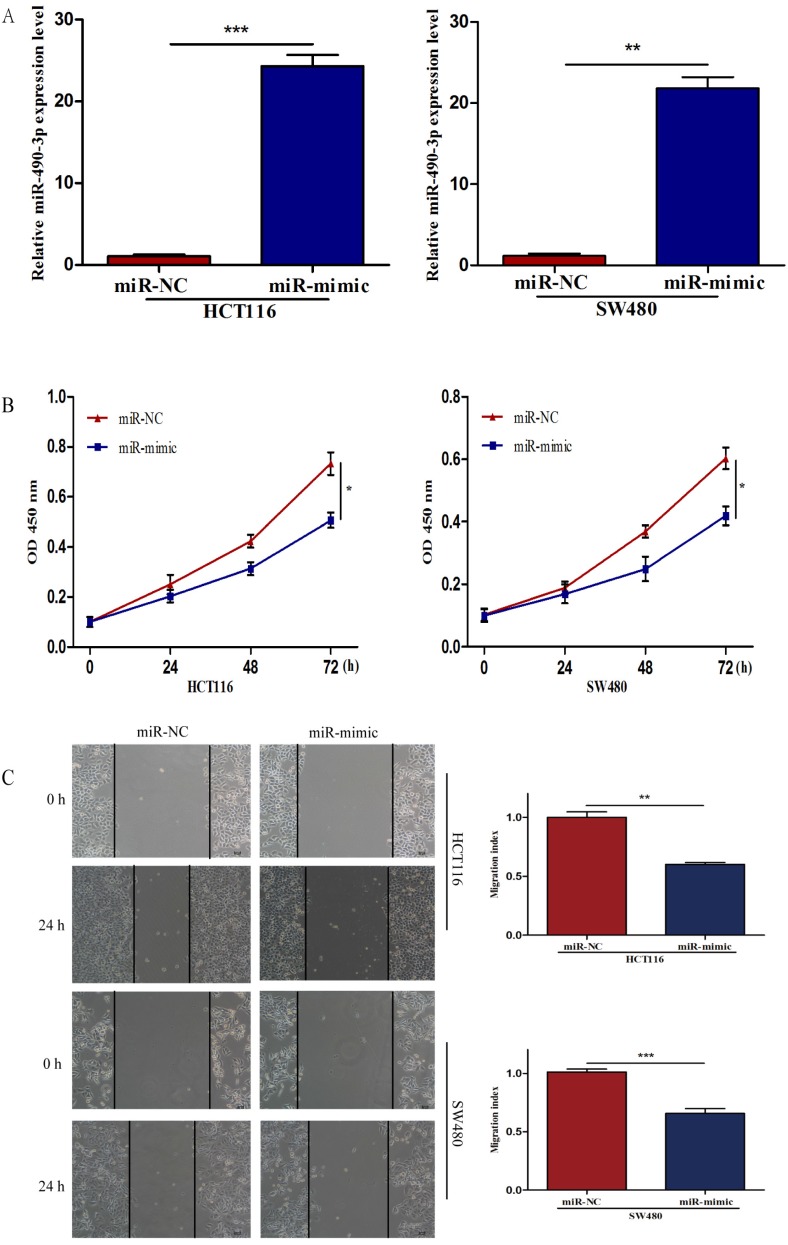
To further investigate the biological impact of miR-490-3p on CRC, both HCT116 and SW480 cells were transfected with miR-mimic and NC respectively, followed by qRT-PCR which was used to confirm the efficiency of transfection (Figure 2A). When compared with that of negative control cells, the viability of miR-490-3p mimic transfected cells was obviously lower (Figure 2B). Wound-healing assay exhibited a markedly decrease of cell migration in miR-490-3p mimic-transfected cells when compared to negative control cells (Figure 2C). Besides, Transwell assay showed that the invasive ability of cells in the miR-mimic group was significantly reduced compared with that in the NC group, shown in Figure 2D. To elucidate the role of miR-490-3p in cell apoptosis, we performed flow cytometric analysis and the apoptotic rate was significantly increased in miR-490-3p mimic transfected cells when compared to negative control cells (Figure 2E).
Figure 2.
Over-regulated miR-490-3p significantly inhibited the proliferation, migratory, invasion activities and promoted apoptotic rate of CRC cells (HCT116 and SW480). A, miR-490-3p highly expressed in HCT116 and SW480 cells after transfection with miR-mimic. B, CCK-8 assay was used to assess the proliferation ability of CRC cells transfected with miR-mimic or NC respectively. C, wound healing assay was used to elucidate the migratory ability of CRC cells transfected with miR-mimic or NC respectively. D, transwell invasion assay was used to assess the invasion ability of CRC cells transfected with miR-mimic or NC respectively. E, flow cytometry analysis was used to assess the apoptotic rate of CRC cells transfected with miR-mimic or NC respectively. * P < 0.05, **P < 0.01, ***P < 0.001.
VDAC1 is a direct target of miR-490-3p
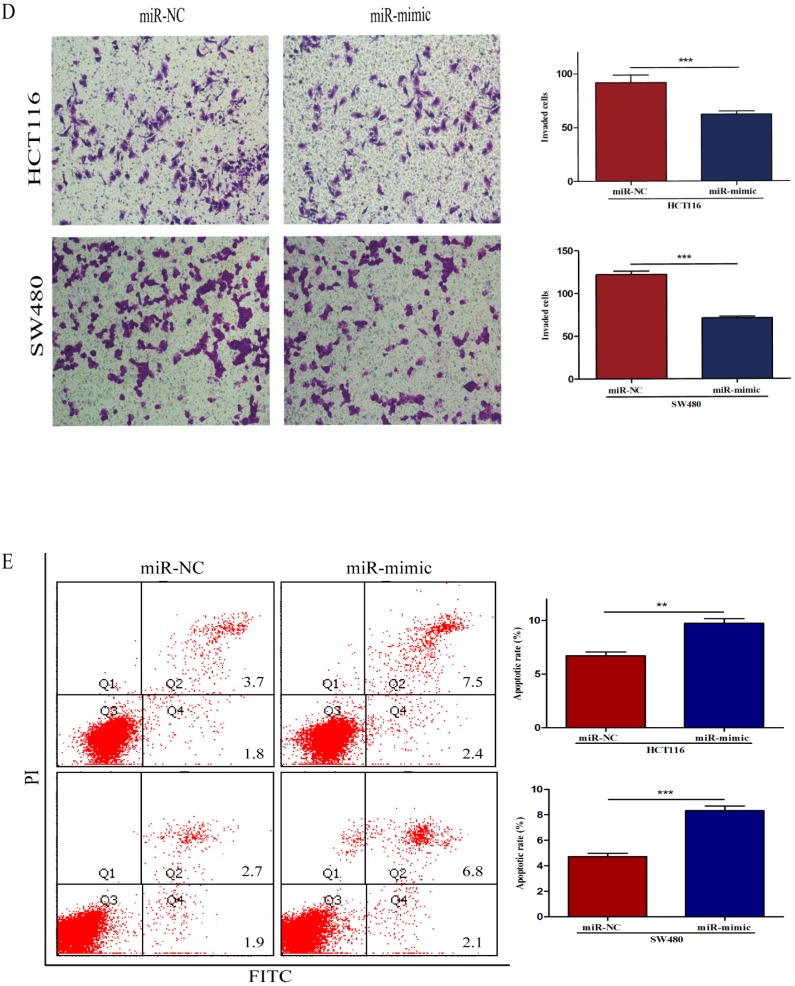
To further identify the potential molecular mechanisms underlying miR-490-3p-induced regulation of CRC biology, miRanda and TargetScan algorithms were used to analyze the potential targets of miR-490-3p together. We found that VDAC1 might be a potential target gene of miR-490-3p which was verified by luciferase reporter assay. The Wt and Mut 3'-UTR of VDAC1 was created (Figure 3A). As shown in Figure 3B, the luciferase activity of VDAC1 3'-UTR-Wt rather than VDAC1 3'-UTR-Mut was reduced by miR-490-3p mimic groups when compared to the negative control groups conforming that miR-490-3p could directly bind to the 3'-UTR of VDAC1. Subsequent qRT-PCR demonstrated that miR-490-3p overexpression markedly reduced the VDAC1 mRNA expression levels both in HCT116 and SW480 cells, shown in Figure 3C. Western blot assay demonstrated that tansfection with miR-490-3p mimic observably inhibited the protein expression levels of VDAC1 and phosphorylated mTOR, but increased the protein expression level of phosphorylated AMPK. Taken together, these data indicate miR-490-3p could directly target VDAC1 to modulate the AMPK/mTOR signaling axis in CRC. A model of VDAC1 inhibition mediated by miR-490-3p causing activation of AMPK and inhibition of mTOR was shown in Figure 3D.
Figure 3.
MiR-490-3p directly targets VDAC1 in CRC cells (HCT116 and SW480). A, VDAC1 was predicted to be a theoretical target of miR-490-3p. B, dual-luciferase reporter assay indicated that miR-490-3p binds to VDAC1 mRNA 3'UTR directly. C, quantitative real-time PCR and western blot analysis indicated that overexpression of miR-490-3p could inhibit the expression of VDAC1 mRNA and the protein expression levels of VDAC1 and phosphorylated mTOR, but increase the protein expression level of phosphorylated AMPK. * P < 0.05, **P < 0.01.
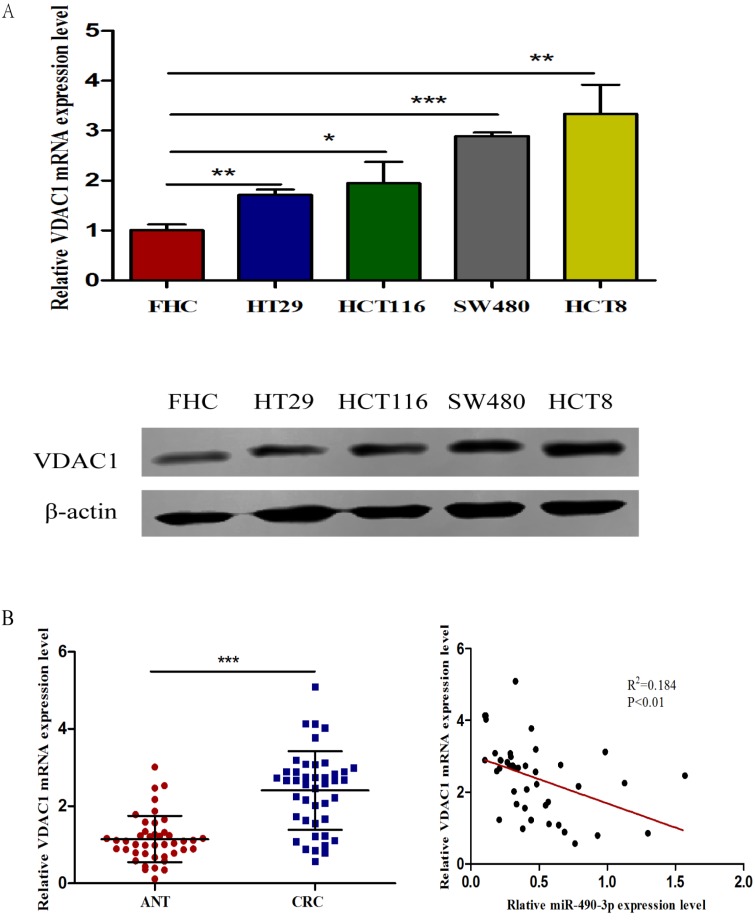
The expression of VDAC1 is upregulated in CRC tissue and cell lines
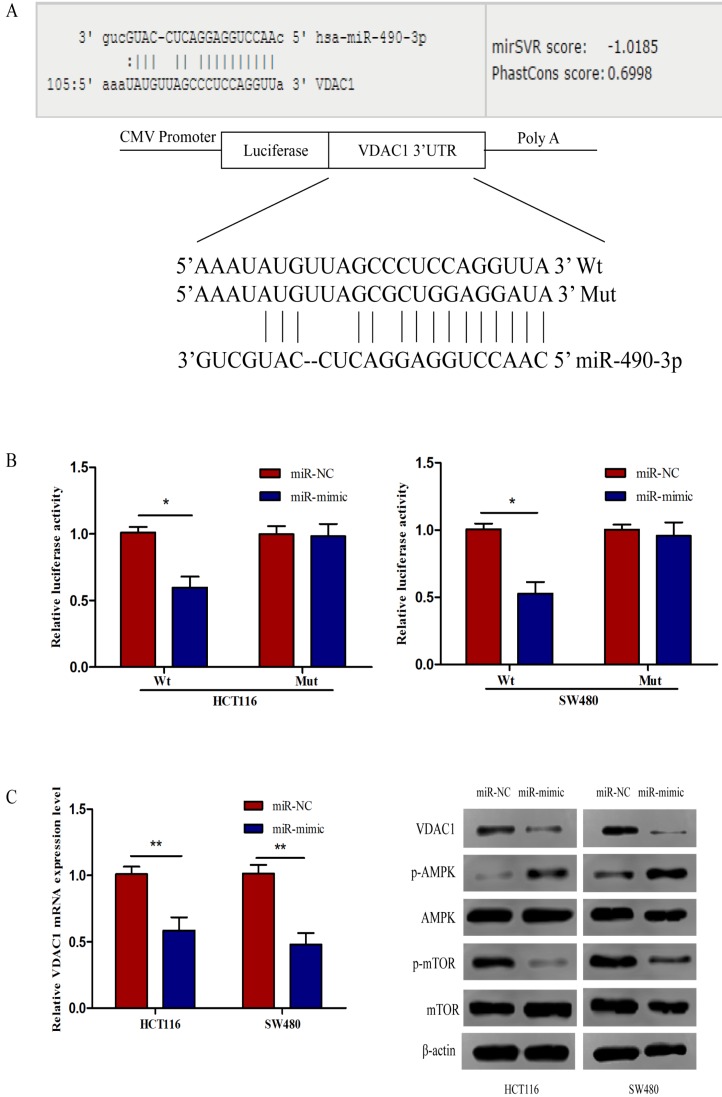
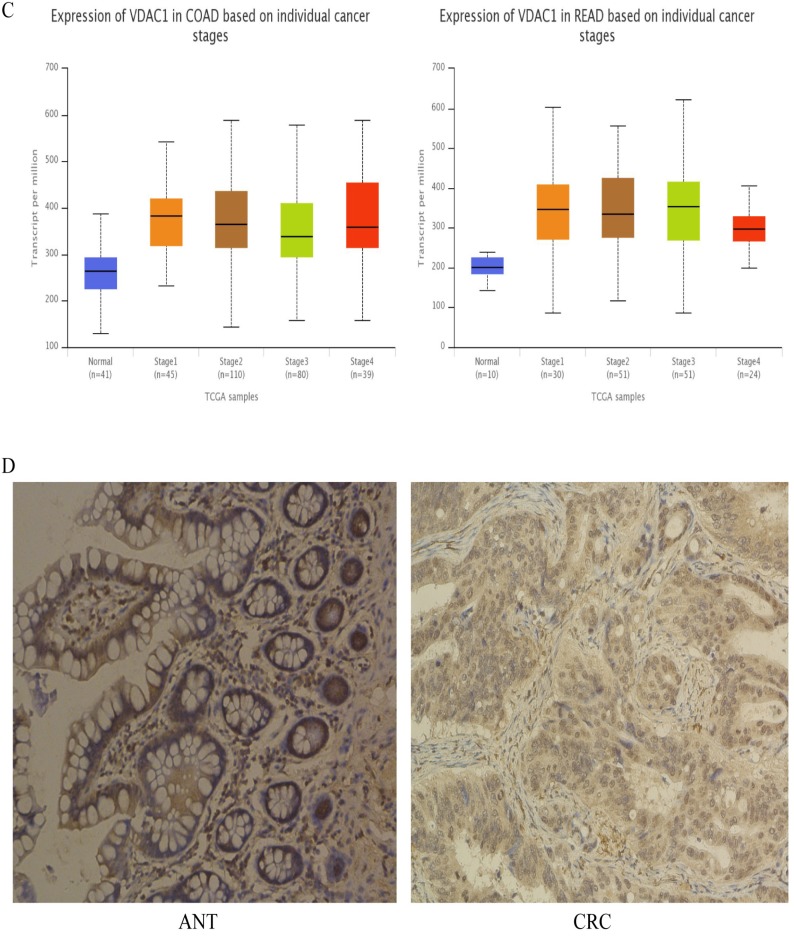
To assess the expression of VDAC1 mRNA and protein in cells, qRT-PCR and western blot analysis was conducted using RNA and protein samples extracted from FHC, HCT8, HT29, HCT116 and SW480 cells respectively. The Results showed that the expression of both VDAC1 mRNA and protein was significantly higher in CRC cell lines when compared with FHC (Figure 4A). Besides, qRT-PCR and IHC analysis were done in 43 matched CRC tissues and adjacent normal tissues to further evaluate the expression of VDAC1 mRNA and protein in tissues. Results from qRT-PCR revealed a significant upregulation of VDAC1 mRNA which is consistent with the result of TCGA database 20 (Figure 4C), and a negative correlation was found between VDAC1 mRNA and miR-490-3p expression in CRC tissues (Figure 4B). IHC analysis revealed that protein levels of VDAC1 were highly expressed in CRC tissues when compared with ANTs (Figure 4D).
Figure 4.
The expression of VDAC1 is upregulated in CRC tissue and cell lines. A, VDAC1 mRNA and protein is upregulated in CRC cell lines. B, VDAC1 mRNA highly expressed in CRC tissues and negatively associated with corresponding miR-490-3p expression levels. C, high expression of VDAC1 mRNA in CRC based on individual cancer stages were obtained from TCGA samples. D, representative immunohistochemical staining for VDAC1 from CRC tissues and matched ANTs. * P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Colorectal cancer is one of the most common cancers in the world and the prognosis for CRC patients is usually poor. At present, due to a lack of early disease-specific symptoms, lots of CRC patients are diagnosed at an advanced stage when treatment is not effective 21. Therefore, novel diagnostic and prognostic biomarkers and therapeutic targets are urgently needed to improve the outcomes of CRC patients.
MicroRNA is a type of highly conserved non-coding RNA consisting of 18-25 nucleotides 5. Previous studies have reported that miRNAs were closely related to the development and progression of cancers, including pancreatic cancer 22, esophageal squamous cell carcinoma 23, lung cancer 24, gastric cancer 25, colorectal cancer 26 and osteosarcoma 27. MiR-490-3p is a newly identified microRNA and its biological functions in cancers is attracting more and more attentions 28. Chen et al. reported that microRNA-490-3p targets CDK1 and inhibits ovarian epithelial carcinoma tumorigenesis and progression 29; Li et al. showed that miR-490-3p regulates lung cancer metastasis by targeting poly r(C)-binding protein 1 30; Jia et al. declared that miR-490-3p could inhibit the growth and invasiveness in triple-negative breast cancer by repressing the expression of TNKS2 12; besides, Zhang et al. declared that miR-490-3p modulates cell growth and epithelial to mesenchymal transition of hepatocellular carcinoma cells by targeting endoplasmic reticulum-golgi intermediate compartment protein 3 (ERGIC3) 11.
In the present study, we found that the expression levels of miR-490-3p in CRC tissues and cells were significantly lower, and down-regulated expression of miR-490-3p in CRC tissues were significantly associated with TNM stage, histological grade, tumor size and poor prognosis of CRC patients. In order to investigate the biological functions of miR-490-3p in CRC, we evaluated the proliferation, apoptosis, metastasis and invasion activities of HCT116 and SW480 cells after overexpression of miR-490-3p. After being transfected with miR-490-3p mimic, both cell lines exhibited a marked increase of apoptosis rate and decrease of proliferation, metastasis and invasion activities when compared to negative control.
The prognosis of patients is significantly associated with cancer stages when diagnosed, with almost ninety percent of deaths could be prevented if patients diagnosed at early stage 31. The traditional Faecal Occult Blood Test (FOBT) and colonoscopy are still widely used for early detection of CRC patients, but the lack of high sensitivity and particular specificity of FOBT and the invasiveness and expensive cost of colonoscopy make both of them not suitable for screening the general population. In addition, the currently available serum tumor biomarkers are neither very sensitive nor specific such as CEA, CA19‐9 and CA72‐4 32. Therefore, novel potential biomarkers for early detection of CRC are urgently needed. Here, we are the first to report that the expression of plasma miR-490-3p in CRC patients was significantly lower compared to that in healthy subjects, and decreased plasma miR-490-3p may serve as a novel potential biomarker for early diagnosis of CRC patients with 65.45% sensitivity and 71.43% specificity. The cutoff value of plasma miR-490-3p from ROC curve was 0.3132.
As we know, one miRNA could own hundreds of target genes, forming a complex regulating network 33. Xu et al. reported that miR-490-3p represses CRC cell migration and invasion abilities partially by targeting to the TGF-β signaling pathway 13; Zheng et al. declared that epigenetic silencing of miR-490-3p promotes development of an aggressive colorectal cancer phenotype through activation of the Wnt/β-catenin signaling pathway 34; In addition, Dou et al. demonstrated that the colon cancer-associated transcript 1 (CCAT1)/miR-490-3p/cyclin-dependent kinase 1 (CDK1) regulatory pathway promotes the progression of hepatocellular 35. Here, we identified VDAC1 as a novel target gene of miR-490-3p. VDAC1 could serve as a hub protein and provide structural and functional anchoring sites for anti-apoptosis proteins, including HK, Bcl2 and Bcl-xL 14, 36, 37, inhibiting the release of apoptosis-inducing proteins from mitochondria to the cytosol, such as Cyto c, AIF and HTRA2/OMI 38-40. The oncogenic role of VDAC1 has been found in many cancers 41, 42. Zhang et al. reported that suppression of VDAC1 expression may inhibit cell proliferation and invasion of NSCLC by decreasing cell energy and metabolism 43; Brahimi et al. demonstrated that the expression of a truncated active form of VDAC1 in lung cancer associates with hypoxic cell survival and correlates with progression to chemotherapy resistance 44. In the present study, the expression of VDAC1 mRNA and protein were significantly higher in CRC tissues and cell lines when compared with adjacent normal tissues and FHC respectively. However, the expression of VDAC1 mRNA and protein were downregulated after tansfection with miR-490-3p mimic. Head et al. declared that antifungal drug itraconazole targets VDAC1 to modulate the AMPK/mTOR signaling axis in endothelial cells, and simultaneous targeting of NPC1 and VDAC1 by itraconazole leads to synergistic inhibition of mTOR signaling and angiogenesis 19, 45. Our study demonstrated that downregulated expression of VDAC1 modulated by miR-490-3p mimic could also increase phosphorylated AMPK, subsequently inhibiting the activation of mTOR. Indeed, the closure of VDAC1 has been recently declared to reduce mitochondrial energy conversion and decrease cytosolic ATP:ADP ratios 46. Therefore, it is logical that decreased expression of VDAC1 by small molecules such as miR-490-3p would lead to a drop in cellular ATP levels, causing an increase in the AMP:ATP ratio and the ensuing activation of AMPK. AMPK regulates mTOR through two alternative pathways, mediated by the tumor-suppressor protein TSC2 and the mTOR-binding partner raptor 47, 48. Taken together, these results indicate that miR-490-3p could modulate CRC cells proliferation, anti-apoptosis, metastasis and invasion by targeting VDAC1/AMPK/mTOR signaling pathway.
In conclusion, we identified a novel regulatory mechanism of miR-490-3p in CRC, and miR-490-3p was a promising potential diagnostic and prognostic biomarker for CRC. Targeting miR-490-3p may be a novel effective therapeutic strategy in CRC.
Acknowledgments
This project was supported by grants from the National Nature Science Foundation of China (No. 81472027, 81501820) to SKW and YQP; Key Project of Science and Technology Development of Nanjing Medicine (ZDX16005); Jiangsu Provincial Medical Innovation Team of the Project of Invigorating Health Care through Science, Technology and Education (Department of Laboratory Medicine of Nanjing First Hospital, Shukui Wang). Jiangsu Youth Medical Talents Training Project to B.H (QNRC2016066) and Y.P (QNRC2016074); Nanjing Medical Science and Technique Development Foundation to BSH (No. JQX13003).
References
- 1.Brody H. Colorectal cancer. Nature. 2015;521:S1. doi: 10.1038/521S1a. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Kronborg O, Jorgensen OD, Fenger C, Rasmussen M. Randomized study of biennial screening with a faecal occult blood test: results after nine screening rounds. Scandinavian journal of gastroenterology. 2004;39:846–51. doi: 10.1080/00365520410003182. [DOI] [PubMed] [Google Scholar]
- 4.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annual review of biochemistry. 2010;79:351–79. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 5.Zeng CY, Zhan YS, Huang J, Chen YX. MicroRNA7 suppresses human colon cancer invasion and proliferation by targeting the expression of focal adhesion kinase. Molecular medicine reports. 2016;13:1297–303. doi: 10.3892/mmr.2015.4643. [DOI] [PubMed] [Google Scholar]
- 6.Wu F, Mo Q, Wan X, Dan J, Hu H. NEAT1/has-mir-98-5p/MAPK6 axis is involved in non-small-cell lung cancer (NSCLC) development. Journal of cellular biochemistry; 2017. [DOI] [PubMed] [Google Scholar]
- 7.Sun Y, Zhang T, Wang C, Jin X, Jia C, Yu S. et al. MiRNA-615-5p functions as a tumor suppressor in pancreatic ductal adenocarcinoma by targeting AKT2. PloS one. 2015;10:e0119783. doi: 10.1371/journal.pone.0119783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber DG, Gawrych K, Casjens S, Brik A, Lehnert M, Taeger D. et al. Circulating miR-132-3p as a Candidate Diagnostic Biomarker for Malignant Mesothelioma. Disease markers. 2017;2017:9280170. doi: 10.1155/2017/9280170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Visone R, Croce CM. MiRNAs and cancer. The American journal of pathology. 2009;174:1131–8. doi: 10.2353/ajpath.2009.080794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Leva G, Croce CM. miRNA profiling of cancer. Current opinion in genetics & development. 2013;23:3–11. doi: 10.1016/j.gde.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang LY, Liu M, Li X, Tang H. miR-490-3p modulates cell growth and epithelial to mesenchymal transition of hepatocellular carcinoma cells by targeting endoplasmic reticulum-Golgi intermediate compartment protein 3 (ERGIC3) The Journal of biological chemistry. 2013;288:4035–47. doi: 10.1074/jbc.M112.410506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia Z, Liu Y, Gao Q, Han Y, Zhang G, Xu S. et al. miR-490-3p inhibits the growth and invasiveness in triple-negative breast cancer by repressing the expression of TNKS2. Gene. 2016;593:41–7. doi: 10.1016/j.gene.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Xu X, Chen R, Li Z, Huang N, Wu X, Li S. et al. MicroRNA-490-3p inhibits colorectal cancer metastasis by targeting TGFbetaR1. BMC cancer. 2015;15:1023. doi: 10.1186/s12885-015-2032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shoshan-Barmatz V, Ben-Hail D, Admoni L, Krelin Y, Tripathi SS. The mitochondrial voltage-dependent anion channel 1 in tumor cells. Biochimica et biophysica acta. 2015;1848:2547–75. doi: 10.1016/j.bbamem.2014.10.040. [DOI] [PubMed] [Google Scholar]
- 15.Benz R. Permeation of hydrophilic solutes through mitochondrial outer membranes: review on mitochondrial porins. Biochimica et biophysica acta. 1994;1197:167–96. doi: 10.1016/0304-4157(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 16.Shoshan-Barmatz V, De Pinto V, Zweckstetter M, Raviv Z, Keinan N, Arbel N. VDAC, a multi-functional mitochondrial protein regulating cell life and death. Molecular aspects of medicine. 2010;31:227–85. doi: 10.1016/j.mam.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Shoshan-Barmatz V, Mizrachi D, Keinan N. Oligomerization of the mitochondrial protein VDAC1: from structure to function and cancer therapy. Progress in molecular biology and translational science. 2013;117:303–34. doi: 10.1016/B978-0-12-386931-9.00011-8. [DOI] [PubMed] [Google Scholar]
- 18.Arif T, Vasilkovsky L, Refaely Y, Konson A, Shoshan-Barmatz V. Silencing VDAC1 Expression by siRNA Inhibits Cancer Cell Proliferation and Tumor Growth In Vivo. Molecular therapy Nucleic acids. 2017;8:493. doi: 10.1016/j.omtn.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Head SA, Shi W, Zhao L, Gorshkov K, Pasunooti K, Chen Y. et al. Antifungal drug itraconazole targets VDAC1 to modulate the AMPK/mTOR signaling axis in endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E7276–85. doi: 10.1073/pnas.1512867112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi B. et al. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia (New York, NY) 2017;19:649–58. doi: 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coghlin C, Murray GI. Biomarkers of colorectal cancer: recent advances and future challenges. Proteomics Clinical applications. 2015;9:64–71. doi: 10.1002/prca.201400082. [DOI] [PubMed] [Google Scholar]
- 22.Ouyang H, Gore J, Deitz S, Korc M. microRNA-10b enhances pancreatic cancer cell invasion by suppressing TIP30 expression and promoting EGF and TGF-beta actions. Oncogene. 2014;33:4664–74. doi: 10.1038/onc.2013.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang L, Zhao Z, Zheng L, Xue L, Zhan Q, Song Y. Downregulation of miR-503 Promotes ESCC Cell Proliferation, Migration, and Invasion by Targeting Cyclin D1. Genomics, proteomics & bioinformatics. 2017;15:208–17. doi: 10.1016/j.gpb.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu CH, Lv DS, Li M, Sun G, Zhang XF, Bai Y. MicroRNA-4458 suppresses the proliferation of human lung cancer cells in vitro by directly targeting Lin28B. Acta pharmacologica Sinica; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng J, Wang X, Zhu W, Chen S, Feng C. MicroRNA-630 Suppresses Epithelial-to-Mesenchymal Transition by Regulating FoxM1 in Gastric Cancer Cells. Biochemistry Biokhimiia. 2017;82:707–14. doi: 10.1134/S0006297917060074. [DOI] [PubMed] [Google Scholar]
- 26.Zhou C, Cui F, Li J, Wang D, Wei Y, Wu Y, MiR-650 represses high-risk non-metastatic colorectal cancer progression via inhibition of AKT2/GSK3beta/E-cadherin pathway. Oncotarget; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu W, Xu G, Liu H, Li T. MicroRNA-490-3p regulates cell proliferation and apoptosis by targeting HMGA2 in osteosarcoma. FEBS letters. 2015;589:3148–53. doi: 10.1016/j.febslet.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 28.Hamfjord J, Stangeland AM, Hughes T, Skrede ML, Tveit KM, Ikdahl T. et al. Differential expression of miRNAs in colorectal cancer: comparison of paired tumor tissue and adjacent normal mucosa using high-throughput sequencing. PloS one. 2012;7:e34150. doi: 10.1371/journal.pone.0034150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen S, Chen X, Xiu YL, Sun KX, Zhao Y. MicroRNA-490-3P targets CDK1 and inhibits ovarian epithelial carcinoma tumorigenesis and progression. Cancer letters. 2015;362:122–30. doi: 10.1016/j.canlet.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Feng Q, Wei X, Yu Y. MicroRNA-490 regulates lung cancer metastasis by targeting poly r(C)-binding protein 1. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2016;37:15221–8. doi: 10.1007/s13277-016-5347-9. [DOI] [PubMed] [Google Scholar]
- 31.Smith RA, von Eschenbach AC, Wender R, Levin B, Byers T, Rothenberger D. et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. Also: update 2001-testing for early lung cancer detection. CA: a cancer journal for clinicians. 2001;51:38–75. doi: 10.3322/canjclin.51.1.38. quiz 7-80. [DOI] [PubMed] [Google Scholar]
- 32.Carpelan-Holmstrom M, Louhimo J, Stenman UH, Alfthan H, Haglund C. CEA, CA 19-9 and CA 72-4 improve the diagnostic accuracy in gastrointestinal cancers. Anticancer research. 2002;22:2311–6. [PubMed] [Google Scholar]
- 33.Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–8. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng K, Zhou X, Yu J, Li Q, Wang H, Li M. et al. Epigenetic silencing of miR-490-3p promotes development of an aggressive colorectal cancer phenotype through activation of the Wnt/beta-catenin signaling pathway. Cancer letters. 2016;376:178–87. doi: 10.1016/j.canlet.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 35.Dou C, Sun L, Jin X, Han M, Zhang B, Li T. Long non-coding RNA colon cancer-associated transcript 1 functions as a competing endogenous RNA to regulate cyclin-dependent kinase 1 expression by sponging miR-490-3p in hepatocellular carcinoma progression. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2017;39:1010428317697572. doi: 10.1177/1010428317697572. [DOI] [PubMed] [Google Scholar]
- 36.Azoulay-Zohar H, Israelson A, Abu-Hamad S, Shoshan-Barmatz V. In self-defence: hexokinase promotes voltage-dependent anion channel closure and prevents mitochondria-mediated apoptotic cell death. The Biochemical journal. 2004;377:347–55. doi: 10.1042/BJ20031465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arbel N, Shoshan-Barmatz V. Voltage-dependent anion channel 1-based peptides interact with Bcl-2 to prevent antiapoptotic activity. The Journal of biological chemistry. 2010;285:6053–62. doi: 10.1074/jbc.M109.082990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES. et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–89. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 39.Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE. et al. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 40.Li W, Srinivasula SM, Chai J, Li P, Wu JW, Zhang Z. et al. Structural insights into the pro-apoptotic function of mitochondrial serine protease HtrA2/Omi. Nature structural biology. 2002;9:436–41. doi: 10.1038/nsb795. [DOI] [PubMed] [Google Scholar]
- 41.Arif T, Vasilkovsky L, Refaely Y, Konson A, Shoshan-Barmatz V. Silencing VDAC1 Expression by siRNA Inhibits Cancer Cell Proliferation and Tumor Growth In Vivo. Molecular therapy Nucleic acids. 2014;3:e159. doi: 10.1038/mtna.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu CH, Lin YW, Wu TF, Ko JL, Wang PH. Clinical implication of voltage-dependent anion channel 1 in uterine cervical cancer and its action on cervical cancer cells. Oncotarget. 2016;7:4210–25. doi: 10.18632/oncotarget.6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang G, Jiang G, Wang C, Zhong K, Zhang J, Xue Q. et al. Decreased expression of microRNA-320a promotes proliferation and invasion of non-small cell lung cancer cells by increasing VDAC1 expression. Oncotarget. 2016;7:49470–80. doi: 10.18632/oncotarget.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brahimi-Horn MC, Ben-Hail D, Ilie M, Gounon P, Rouleau M, Hofman V. et al. Expression of a truncated active form of VDAC1 in lung cancer associates with hypoxic cell survival and correlates with progression to chemotherapy resistance. Cancer research. 2012;72:2140–50. doi: 10.1158/0008-5472.CAN-11-3940. [DOI] [PubMed] [Google Scholar]
- 45.Head SA, Shi WQ. Simultaneous Targeting of NPC1 and VDAC1 by Itraconazole Leads to Synergistic Inhibition of mTOR Signaling and Angiogenesis. 2017; 12: 174-82. [DOI] [PMC free article] [PubMed]
- 46.Maldonado EN, Lemasters JJ. ATP/ADP ratio, the missed connection between mitochondria and the Warburg effect. Mitochondrion. 2014;19(Pt A):78–84. doi: 10.1016/j.mito.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laplante M, Sabatini DM. mTOR signaling at a glance. Journal of cell science. 2009;122:3589–94. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS. et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Molecular cell. 2008;30:214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]