Abstract
Aim:
Our aim was to evaluate physicians’ attitudes toward pharmacogenetic testing before and after pharmacogenetic education.
Methods:
In total, 12 physicians (˜40% response rate) completed a survey with eight questions on 10-point scales on their attitudes toward pharmacogenetic testing before and after a 1-h grand rounds presentation on pharmacogenetics. Differences in question scores overall, among training levels (resident/fellow/attending), and specific drugs (clopidogrel/simvastatin/warfarin) were assessed using Wilcoxon signed-rank and exact Kruskal–Wallis tests.
Results & conclusion:
The scores for all eight questions increased, with statistically significant (p < 0.05) increases for four out of eight questions. The scores were similar among training levels, but the postscores for clopidogrel were significantly higher than for simvastatin and warfarin. In conclusion, brief pharmacogenetic education can significantly affect physicians’ attitudes toward pharmacogenetic testing.
Keywords: : clopidogrel, education, pharmacogenetics, physician, simvastatin, survey, warfarin
Pharmacogenetics is the area of personalized medicine in which a patient's genetic information is used to guide their drug therapy, and it can potentially decrease patients’ risk for adverse drug effects [1] and drug ineffectiveness [2]. Despite the publication of numerous pharmacogenetic US FDA recommendations [3] and clinical guidelines [4], pharmacogenetic testing is not frequently used by physicians. In a 2008 nationwide survey of US physicians, only 12.9% had ordered a pharmacogenetic test within the past 6 months [5]. Positive physician attitudes toward pharmacogenetic testing are necessary if pharmacogenetic testing is to become a part of healthcare, but currently physicians’ attitudes toward pharmacogenetics vary [5–15]. In a 2012–2013 survey of 101 internal medicine physicians, 47% did not know or thought that it was mostly false that genotype data are useful for making prescribing decisions [8]. In interviews of 60 primary care physicians performed in 1998, the leading barrier to the clinical implementation of genetic testing was their ‘uncertainty of the clinical utility’ [15].
Varying physician attitudes toward pharmacogenetic testing could stem from multiple variables. One potential variable is differing levels of pharmacogenetic education. Indeed, only 29% of physicians in 2008 reported receiving any pharmacogenetic education [5]. Another potential variable is the limited availability of randomized controlled trials clearly demonstrating the clinical utility of pharmacogenetic testing. Warfarin is one of the few examples where pharmacogenetic testing was evaluated in randomized controlled trials [16,17], but these trials’ results are mixed. Another potential variable leading to differing physician attitudes toward pharmacogenetic testing could be inconsistencies between pharmacogenetic clinical guidelines and recommendations. For example, the FDA [18], the American Heart Association/American College of Cardiology (AHA/ACC) [19], and the Clinical Pharmacogenetics Implementation Consortium (CPIC) [2] each have different recommendations regarding the interpretation of pharmacogenetic testing for clopidogrel.
Many cross-sectional studies have described physicians’ attitudes toward pharmacogenetic testing [5–15], but to our knowledge, no studies have assessed whether pharmacogenetic education can change their attitudes. Even when physicians are educated on current pharmacogenetic data, they may still perceive pharmacogenetics as having uncertain clinical utility, due to the lack of randomized controlled trials and inconsistent clinical guidelines. Therefore, the goal of this study was to determine whether pharmacogenetic education can change physicians’ attitudes toward pharmacogenetic testing.
Methods
Subjects
A convenience sample of physicians, attending a Cardiology Grand Rounds continuing medical education session at the Ohio State University Wexner Medical Center in March 2015, was asked to participate in this survey study. The surveys were placed on a table next to the sign-in sheet for continuing education credit at the doorway to the seminar, and it was left to the physicians’ discretion to take a survey into the seminar and complete it. No incentives were given for survey participation. Survey participants gave verbal informed consent before the seminar was started. This study was determined to be exempted from institutional review board review by the institutional review board at the Ohio State University.
Pharmacogenetic education
The grand rounds presentation was taught by a licensed pharmacist with additional PhD and postdoctoral training in pharmacogenetics. Clopidogrel, simvastatin and warfarin were chosen as examples because they were deemed to be the most pertinent to the attendees’ scope of practice. Moreover, clopidogrel, simvastatin and warfarin are the only cardiovascular drugs with clinical recommendations by CPIC for the interpretation of test results [1,2,20]. The presentation was 45 min in length with an additional 15 min available for questions. The outline for the presentation is displayed in Box 1 and consisted of the following sections: an introduction to pharmacogenetics; a patient case; cardiovascular pharmacogenetic examples; practical considerations of testing; revisiting the patient case; future directions of cardiovascular pharmacogenetics and a summarization. The introduction contained five trivia questions based on direct-to-consumer pharmacogenetic testing, advertisement of pharmacogenetics in the media, and the FDA Table of Pharmacogenomic Biomarkers in Drug Labeling [3]. The introduction also defined pharmacogenetics and reviewed pharmacogenetic nomenclature (e.g., SNP, genotype, diplotype, carrier, among others). The patient case, based on a real-life patient (Box 2), included two questions for reflection: “How would you interpret this genetic data?” and “How would it affect your clinical decision making?” The pharmacogenetic examples included the genetic mechanism, clinical evidence, clinical context and current clinical recommendations for clopidogrel, simvastatin and warfarin. The clinical evidence was based on primary literature identified from PubMed and The Pharmacogenomics Knowledge Base [21]. The clinical context compared the effects of genetic polymorphisms on each of the three drugs to the effects of other factors that are already considered in drug therapy, such as drug–drug and food–drug interactions. The review of clinical recommendations came from pharmacogenetic recommendations and test interpretation from the FDA [3], CPIC [1,2,20] and AHA/ACC [19], where applicable. This review also described incongruities between these organizations’ recommendations for clopidogrel, simvastatin and warfarin. The practical considerations for pharmacogenetic testing included specific examples characterizing its accessibility, affordability and timeliness with respect to both institutional and national options. The audience again considered the patient case using the concepts presented during presentation. The future directions of cardiovascular pharmacogenetics introduced β-blocker efficacy in heart failure [22,23], and the seminar concluded with a summary of all of the information presented.
Box 1. . Outline of 1-h grand rounds presentation on pharmacogenetics.
-
Introduction to PGx
Trivia
Concept
Nomenclature
Patient case
-
Cardiovascular PGx examples: clopidogrel, simvastatin and warfarin:
Genetic mechanism
Clinical evidence
Clinical context
Clinical recommendations
-
Practical considerations of PGx testing:
Availability, affordability and timeliness
Benefits and risks
Patient case
Future direction of cardiovascular PGx: β-blocker efficacy in heart failure
Summary
PGx: Pharmacogenetics
Box 2. . Patient case, based on a real patient, presented during grand rounds presentation.
CC: CR is a 62 yo WM that presents to your cardiology clinic today for hospital discharge f/u
HPI: 3 weeks ago CR presented to the ED with STEMI. He was loaded with 600 mg clopidogrel and sent emergently to the cath lab, where in stent thrombosis in the LAD was discovered. Angioplasty restored flow
PMH: CAD (s/p DES to LAD in December 2014), afib, HTN and HL
Meds: Aspirin 81 mg q.d., clopidogrel 75 mg q.d., simvastatin 40 mg q.d., metoprolol XL 50 mg q.d. and warfarin 6 mg q.d.
CR bought his own genetic testing. He ‘Googled’ that these genes can affect his medications. He asks, “What does this mean Doc?”
| CYP2C19 | SLCO1B1 | CYP2C9 | VKORC1 |
| *1/*2 | rs4149056 | *1/*2 | -1639G>A |
| CC | GG | ||
Afib: Atrial fibrilation; CC: Chief complaint; CAD: Coronary artery disease; DES: Drug-eluting stent; ED: Emergency department; f/u: Follow-up; HPI: History of present illness; HL: Hyperlipidemia; HTN: Hypertension; LAD: Left anterior descending; Meds: Medications; PMH: Past medical history; q.d.: Every day; STEMI: ST-segment-elevated myocardial infarction; s/p: Status post; WM: White male; Yo: Year-old.
Survey
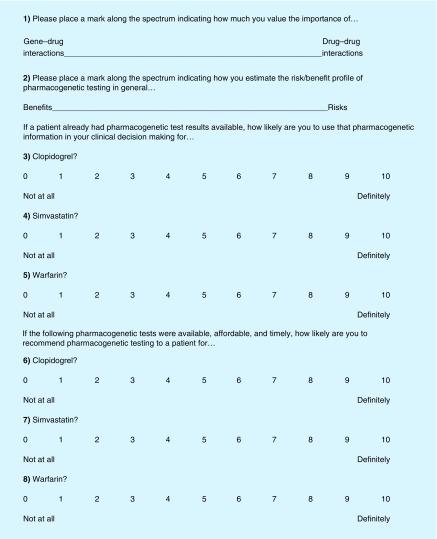
Physicians’ attitudes toward pharmacogenetic testing were assessed with eight questions in four domains (Figure 1). Domain 1 was the perceived importance of gene-drug interactions relative to drug–drug interactions (question #1). Domain 2 was the perceived risk/benefit profile of pharmacogenetic testing (question #2). Domain 3 was the likelihood of using pharmacogenetic test results to inform clinical decisions when patient genotypes are already available (questions #3–5). Domain 4 was the likelihood of recommending pharmacogenetic testing when patient genotypes were not already available (questions #6–8), barring commonly perceived barriers (e.g., availability, affordability, and timeliness [5–15]). The rationale for domain 1 is based on the current debate over the levels of evidence required for the clinical implementation of pharmacogenetics [24–27]. The rationale for domain 2 is based on the decision to weigh the risk/benefit profile prior to pharmacogenetic testing, which physicians must weigh prior to any type of clinical testing. The rationale for domain 3 is based on the recent popularity of direct-to-consumer genetic testing [28], and thus some patients may already have genetic test results available when they see their physician. The rationale for domain 4 was to determine whether the physicians would recommend pharmacogenetic testing in the absence of already available test results and potential barriers, such as availability, affordability and timeliness. Domains 3 and 4 were separated by the three drugs presented (clopidogrel, simvastatin and warfarin) to determine whether their attitudes differed by drug. We asked the physicians to complete the same survey immediately before and after the grand rounds presentation. On the first survey, we were interested to determine whether attitudes varied by training level because of the specialized nature of pharmacogenomics and asked for respondents’ clinical position (e.g., resident, fellow or attending). Questions #1 and 2 were structured as a spectrum with one of two variables on each end of the spectrum. The participants were asked to place a mark along the spectrum, indicating how they value the relative importance of the two factors. The spectrum response style was chosen for questions #1 and 2 because the participants were asked to weigh the relative importance of two factors (question #1: gene–drug vs drug–drug interactions and question #2: risks vs benefits of pharmacogenetic testing). The remaining questions (#3–8) were structured as 10-point scales, with 0 meaning ‘not at all’ and 10 meaning ‘definitely’.
Figure 1. . Pre- and post-presentation survey.
Statistical analysis
The scores for the spectrum-response questions #1 and 2 were scaled to 10 so that the values were directly comparable to the rest of the questions on 10-point scales. The scores for questions #1 and 2 were collected by measuring the distance of their mark on the spectrum to the right side of the spectrum. The total length of the spectrum was 11.6 cm, and thus the score was scaled to 10 with the following scaling factor: (measured distance in centimeters)*(10/11.6). The scores for all survey questions were summarized by the median ± quartiles. Changes in scores (post-presentation minus pre-presentation) for each question were assessed using Wilcoxon signed-rank tests. Differences in pre-presentation, post-presentation and changes in scores among the different training levels (resident/fellow/attending) and different drugs (clopidogrel/simvastatin/warfarin) were assessed using exact Kruskal–Wallis tests. All statistical analyses were performed using SAS version 9.3 (NC, USA), and p < 0.05 was considered statistically significant.
Results
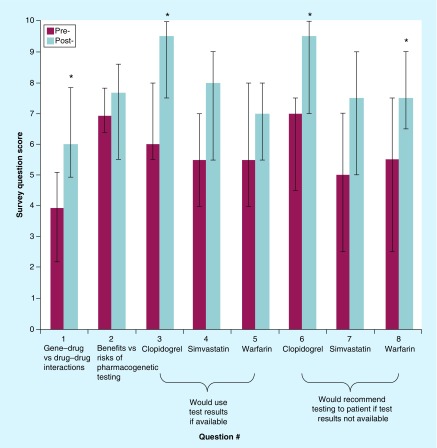
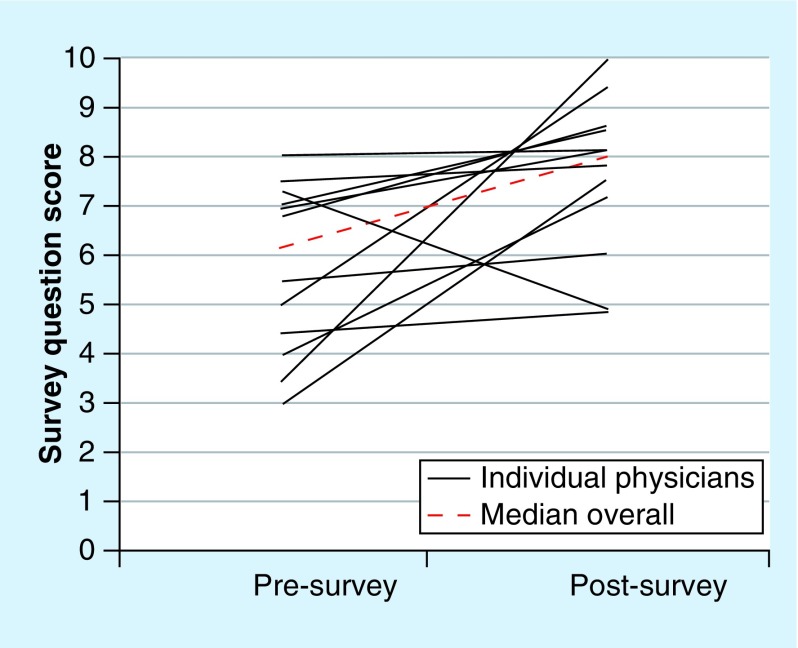
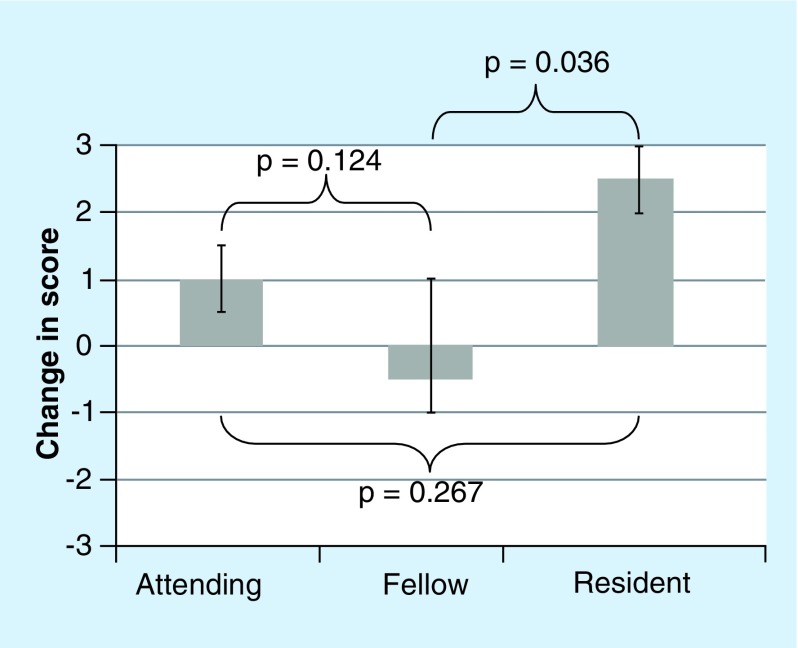
A total of 12 physicians (two residents, six fellows and four attendings) completed the eight-question survey on their attitudes toward pharmacogenetic testing before and after the grand rounds presentation on pharmacogenetics. The total number of physicians attending the grand rounds presentation was not officially counted, but we estimated that approximately 30 physicians attended, yielding an approximate 40% response rate. Despite the small sample size (n = 12), the change in score (post-presentation minus pre-presentation) was statistically significant for four out of eight questions (Figure 2). The overall median pre-presentation and post-presentation scores ± quartiles for all eight questions was 6 ± 3 and 8 ± 2, respectively, yielding an overall change in scores of +2 (Figure 3; p = 0.012). The changes in scores were similar for all training levels (resident vs fellow vs attending) for seven out of eight questions (p > 0.05). The change in score for question #5 (“If a patient already had pharmacogenetic test results available, how likely are you to use that pharmacogenetic information in your clinical decision making for warfarin?”) significantly differed by training level (p = 0.022 for resident vs fellow vs attending; Figure 4). The change in score for question #5 was similar for the attendings and fellows (p > 0.05), but the change in score was significantly larger for the residents (+2.5) than for the fellows (-0.5; p = 0.036; Figure 4). For the two sets of drug-specific questions (questions #3–5 and #6–8), only clopidogrel had a statistically significant change in score for both questions (question #3: p = 0.001; question #6: p = 0.006). Warfarin only had a statistically significant change in one out of two drug-specific questions (question #8: p = 0.043), and neither drug-specific question score for simvastatin significantly changed (questions #4 and 7 p > 0.05).
Figure 2. . Median (± quartiles) survey question scores before and after the grand rounds presentation on pharmacogenetics (n = 12 physicians).
*Statistically significant difference in pre- and post-presentation scores via Wilcoxon signed-rank test (p < 0.05).
Figure 3. . Individual physician (n = 12) pre- and post-survey question scores (solid lines) with the overall median pre- and post-survey question score (dashed line; p = 0.012).
Figure 4. . Median changes in score ± quartiles for question #5 by physician training level.
(“If a patient already had pharmacogenetic test results available, how likely are you to use that pharmacogenetic information in your clinical decision making for warfarin?”). An exact Kruskal–Wallis test indicated a statistically significant difference overall (attending vs fellow vs resident; p = 0.022).
With respect to the absolute values of the pre- and post-presentation scores, and not the changes in scores, the pre- and postpresentation scores for all of the questions were similar for all training levels (resident vs fellow vs attending; p > 0.05) and all three drugs (clopidogrel vs simvastatin vs warfarin; p > 0.05), except for one question. The post-presentation scores significantly differed by drug for the following question: “If a patient already had pharmacogenetic test results available, how likely are you to use that pharmacogenetic information in your clinical decision making for…” (clopidogrel vs simvastatin vs warfarin; p = 0.014). The post-presentation score for this question for clopidogrel was 9.5 out of 10, which was significantly higher than for simvastatin (8 out of 10; p = 0.036 vs clopidogrel) and warfarin (7 out of 10; p = 0.006 vs clopidogrel).
Discussion
Physicians’ perception of the clinical utility of pharmacogenetics is necessary for pharmacogenetics to become a part of healthcare. Even when physicians are educated on current pharmacogenetic data, they may still perceive pharmacogenetics as having uncertain clinical utility due to the lack of randomized controlled trials and inconsistent clinical guidelines. However, in this small survey study, we demonstrated that as little as 1 h of pharmacogenetic education can significantly change physicians’ attitudes toward pharmacogenetic testing for clopidogrel and partially for warfarin. Therefore, this study suggests that physicians’ attitudes toward pharmacogenetics can significantly change with a brief amount of pharmacogenetic education and despite the lack of randomized controlled trial data and consistent clinical guidelines. Moreover, attitude changes were drug specific. They were partially responsive for warfarin and not responsive for simvastatin. This deserves further inquiry, but we speculate a multivariate causality due to declining prevalence of simvastatin in current clinical practice, inconsistent clinical practice guidelines, and/or variable quality of the current evidence.
Despite the small sample size, the scores for all eight survey questions increased with statistically significant increases for four out of eight survey questions. Regardless of training level (resident vs fellow vs attending), physicians’ attitudes toward pharmacogenetics were mostly similar. The only instance where their attitudes significantly differed by training level was in regard to clinical decision-making for warfarin when the patient's pharmacogenetic test results were already available; the residents’ attitudes significantly changed more with education than the fellows. Physicians’ attitudes also differed for the three drugs presented, with significantly higher (near maximum) scores for clopidogrel, compared with simvastatin and warfarin. We believe that these differing responses by drug are not due to differences in the pharmacogenetic education (the same topics were covered for each drug), but it could be due to the quality of available evidence and prescribing preferences. For example, although the survey did not have a free-form response option, one physician wrote on their survey, “Do not use simvastatin much anymore.” Moreover, at the study site, warfarin therapy is largely managed by pharmacists in an anticoagulation specialty clinic.
The attitudes toward pharmacogenetics of this small group of physicians significantly changed despite the lack of randomized controlled trial data and consistent clinical guidelines (which were presented in the grand rounds), suggesting that pharmacogenetic education can still play a role in the absence of those factors. These results do not suggest that randomized controlled trials and consistent clinical guidelines are not important. Perhaps physicians are willing to accept levels of evidence for pharmacogenetics similar to other clinical factors that lack randomized controlled trials or consistent clinical guidelines but yet influence therapy decisions. For example, drug–drug and drug–food interactions also lack randomized controlled trials and consistent clinical guidelines, but physicians may perceive them as important nonetheless. As with every clinical intervention, appropriate use is an important goal in an efficient and effective health system, and for topics with varying amounts of evidence like pharmacogenetics, an informed discussion between patients and providers is paramount. However, informed discussions on pharmacogenetic testing may be limited by the pervasive gaps in education on pharmacogenetics in our current clinical workforce [5,29]. More comprehensive pharmacogenetic education than could feasibly be achieved in this study will be necessary to fill the current gaps in clinical pharmacogenetic education. Due to the time constraint (only 1 h) and the audience (cardiovascular), we had to be selective in the number (only three) and types (only cardiovascular) of pharmacogenetic examples that could be presented.
Others have researched pharmacogenetic education [30,31] and set pharmacogenetic educational standards [32,33], but to our knowledge, only a single previous study has assessed the effect of pharmacogenetic education on the clinical utility of pharmacogenetic testing [34]. Specifically, as part of the investigation by Owusu-Obeng et al. at the University of Florida Health Personalized Medicine Program, the CYP2C19 testing rate for clopidogrel in 2012 was 63% in the initial months of implementation and then 98% by the end of 1 year [34]. Owusu-Obeng et al. perceived that the effective implementation of CYP2C19 testing was largely due to the educational efforts led by a pharmacist, who ‘led clinician group discussions, participated in patient care activities in targeted clinical services, conducted professional seminars and in-services, and created written patient and provider educational materials’ [34]. Although highly effective, such intensive educational efforts are currently not scalable to the thousands of currently practicing physicians with limited pharmacogenetic education. Our approach demonstrated that a single 1-h pharmacogenetic seminar, conducted by a pharmacist during an existing education time (grand rounds continuing medical education), can still effectively change physicians’ attitudes. However, the difference in educational intensity between our study and the study by Owusu-Obeng et al. probably reflects the educational intensity required to change action, and not just attitudes.
As with any survey study, selection bias, response bias and internal reliability are potential limitations. Our study used a small sample size from a single, academic site, which may limit its generalizability, and it may be underpowered to detect differences in attitudes between training levels. Additionally, demographic data, such as the age and gender of the survey participants, was not collected. Cardiology Grand Rounds was the venue for this educational presentation, and thus it may not be applicable to other practice specialties. The grand rounds presentation was taught by a licensed pharmacist with additional PhD and postdoctoral training in pharmacogenetics; therefore, it is unknown whether pharmacogenetic education would be as effective if taught by instructors with other training backgrounds. Our survey only assessed physicians’ attitudes toward pharmacogenetics and not actual clinical implementation of testing or long-term effects on their attitudes. Without this information, we are unable to infer the clinical significance from the magnitude of attitude changes we observed.
Conclusion
A 1-h Grand Rounds presentation on pharmacogenetics significantly changed the attitudes of a small group of physicians toward pharmacogenetic testing. Physicians’ attitudes were mostly similar regardless of training level (resident, fellow or attending), but their attitudes significantly changed more for clopidogrel than for simvastatin or warfarin. After this proof-of-concept intervention, we intend to expand it to a larger sample size in order to better characterize physicians’ attitudes toward pharmacogenetic testing within cardiology.
Executive summary.
Background
Despite the publication of numerous pharmacogenetic clinical guidelines and recommendations, physicians’ attitudes toward pharmacogenetic testing vary.
Our goal was to evaluate physicians’ attitudes toward pharmacogenetic testing before and after pharmacogenetic education.
Methods
Physicians were surveyed with eight questions on their attitudes toward pharmacogenetic testing before and after a 1-h grand rounds presentation on pharmacogenetics.
Differences in survey scores overall, among training levels (resident/fellow/attending), and specific drugs (clopidogrel/simvastatin/warfarin) were assessed using Wilcoxon signed-rank and exact Kruskal–Wallis tests.
Results
In total, 12 physicians completed the survey (approximate 40% response rate).
Scores for all eight questions increased, with four out of eight significantly increasing (p < 0.05).
Score changes were mostly similar regardless of training level (resident/fellow/attending), but the scores changed significantly more for clopidogrel than for simvastatin or warfarin.
Conclusion
A 1-h grand rounds presentation on pharmacogenetics significantly changed the attitudes of a small group of physicians toward pharmacogenetic testing.
Acknowledgements
The authors would like to acknowledge the physicians that completed the survey.
Footnotes
Financial & competing interests disclosure
JA Luzum is funded by a postdoctoral fellowship from the American Heart Association (14POST20100054) and the NIH student loan repayment program (L30 HL110279-02). JA Luzum is a consultant for Gnome Diagnostics, LLC, a pharmacogenetic testing company. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Ramsey LB, Johnson SG, Caudle KE, et al. The clinical pharmacogenetics implementation consortium guideline for SLCO1B1 and simvastatin-induced myopathy: 2014 update. Clin. Pharmacol. Ther. 2014;96(4):423–428. doi: 10.1038/clpt.2014.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott SA, Sangkuhl K, Stein CM, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin. Pharmacol. Ther. 2013;94(3):317–323. doi: 10.1038/clpt.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics/ucm083378.htm United States Food and Drug Administration: Table of Pharmacogenomic Biomarkers in Drug Labels 2015.
- 4.Relling MV, Klein TE. CPIC: Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin. Pharmacol. Ther. 2011;89(3):464–467. doi: 10.1038/clpt.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanek EJ, Sanders CL, Taber KA, et al. Adoption of pharmacogenomic testing by US physicians: results of a nationwide survey. Clin. Pharmacol. Ther. 2012;91(3):450–458. doi: 10.1038/clpt.2011.306. [DOI] [PubMed] [Google Scholar]; •• A survey of 10,303 physicians across the USA demonstrating that early and future adopters of pharmacogenetic testing were more likely to have received training in pharmacogenomics, but only 29% of physicians had pharmacogenetic education.
- 6.Haga SB, Burke W, Ginsburg GS, Mills R, Agans R. Primary care physicians’ knowledge of and experience with pharmacogenetic testing. Clin. Genet. 2012;82(4):388–394. doi: 10.1111/j.1399-0004.2012.01908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A national survey of primary care physicians demonstrating that the majority anticipate pharmacogenetic testing would soon become a valuable tool to inform drug response, but only a minority felt comfortable ordering pharmacogenetic tests.
- 7.Hoop JG, Lapid MI, Paulson RM, Roberts LW. Clinical and ethical considerations in pharmacogenetic testing: views of physicians in 3 “early adopting” departments of psychiatry. J. Clin. Psychiatry. 2010;71(6):745–753. doi: 10.4088/JCP.08m04695whi. [DOI] [PubMed] [Google Scholar]
- 8.Overby CL, Erwin AL, Abul-Husn NS, et al. Physician attitudes toward adopting genome-guided prescribing through clinical decision support. J. Pers. Med. 2014;4(1):35–49. doi: 10.3390/jpm4010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raghavan S, Vassy JL. Do physicians think genomic medicine will be useful for patient care? Per. Med. 2014;11(4):424–433. doi: 10.2217/pme.14.25. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Review of the literature on physician attitudes regarding the usefulness and limitations of genomic testing.
- 10.Bernhardt BA, Zayac C, Gordon ES, Wawak L, Pyeritz RE, Gollust SE. Incorporating direct-to-consumer genomic information into patient care: attitudes and experiences of primary care physicians. Per. Med. 2012;9(7):683–692. doi: 10.2217/pme.12.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant RW, Hivert M, Pandiscio JC, Florez JC, Nathan DM, Meigs JB. The clinical application of genetic testing in Type 2 diabetes: a patient and physician survey. Diabetologia. 2009;52(11):2299–2305. doi: 10.1007/s00125-009-1512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fargher EA, Eddy C, Newman W, et al. Patients’ and healthcare professionals’ views on pharmacogenetic testing and its future delivery in the NHS. Pharmacogenomics. 2007;8(11):1511–1519. doi: 10.2217/14622416.8.11.1511. [DOI] [PubMed] [Google Scholar]
- 13.Rogausch A, Prause D, Schallenberg A, Brockmoller J, Himmel W. Patients’ and physicians’ perspectives on pharmacogenetic testing. Pharmacogenomics. 2006;7(1):49–59. doi: 10.2217/14622416.7.1.49. [DOI] [PubMed] [Google Scholar]
- 14.Zachry WM, 3rd, Armstrong EP. Health care professionals’ perceptions of the role of pharmacogenomic data. J. Manag. Care Pharm. 2002;8(4):278–284. doi: 10.18553/jmcp.2002.8.4.278. [DOI] [PubMed] [Google Scholar]
- 15.Mountcastle-Shah E, Holtzman NA. Primary care physicians’ perceptions of barriers to genetic testing and their willingness to participate in research. Am. J. Med. Genet. 2000;94(5):409–416. doi: 10.1002/1096-8628(20001023)94:5<409::aid-ajmg13>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 16.Pirmohamed M, Burnside G, Eriksson N, et al. A randomized trial of genotype-guided dosing of warfarin. N. Engl. J. Med. 2013;369(24):2294–2303. doi: 10.1056/NEJMoa1311386. [DOI] [PubMed] [Google Scholar]; • A randomized clinical trial of genotype-guided versus standard dosing of warfarin therapy in Europe that found that genotype-guided warfarin therapy significantly increased the time within therapeutic range.
- 17.Kimmel SE, French B, Kasner SE, et al. A pharmacogenetic versus a clinical algorithm for warfarin dosing. N. Engl. J. Med. 2013;369(24):2283–2293. doi: 10.1056/NEJMoa1310669. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A randomized clinical trial of genotype-guided versus clinical variable guided warfarin therapy in the USA that did not find that genotype information improved time within the therapeutic range.
- 18.www.accessdata.fda.gov/drugsatfda_docs/label/2015/020839s061lbl.pdf Plavix®, prescribing information. US Food and Drug Administration: Clopidogrel prescribing information 2015.
- 19.Musunuru K, Hickey KT, Al-Khatib SM, et al. Basic concepts and potential applications of genetics and genomics for cardiovascular and stroke clinicians: a scientific statement from the American Heart Association. Circ. Cardiovasc. Genet. 2015;8(1):216–242. doi: 10.1161/HCG.0000000000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson JA, Gong L, Whirl-Carrillo M, et al. Clinical Pharmacogenetics Implementation Consortium Guidelines for CYP2C9 and VKORC1 genotypes and warfarin dosing. Clin. Pharmacol. Ther. 2011;90(4):625–629. doi: 10.1038/clpt.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hewett M, Oliver DE, Rubin DL, et al. PharmGKB: the pharmacogenetics knowledge base. Nucleic Acids Res. 2002;30(1):163–165. doi: 10.1093/nar/30.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talameh J, Garrand A, Ghali J, et al. Beta-1 adrenergic receptor genotype Ser49Gly is associated with beta-blocker survival benefit in patients with heart failure. J. Am. Coll. Cardiol. 2012;59(13 Suppl.):E861. [Google Scholar]
- 23.Talameh JA, McLeod HL, Adams KF, Jr, Patterson JH. Genetic tailoring of pharmacotherapy in heart failure: optimize the old, while we wait for something new. J. Card. Fail. 2012;18(4):338–349. doi: 10.1016/j.cardfail.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Altman RB. Pharmacogenomics: “noninferiority” is sufficient for initial implementation. Clin. Pharmacol. Ther. 2011;89(3):348–350. doi: 10.1038/clpt.2010.310. [DOI] [PubMed] [Google Scholar]
- 25.Ratain MJ, Johnson JA. Meaningful use of pharmacogenetics. Clin. Pharmacol. Ther. 2014;96(6):650–652. doi: 10.1038/clpt.2014.188. [DOI] [PubMed] [Google Scholar]
- 26.Gillis NK, Innocenti F. Evidence required to demonstrate clinical utility of pharmacogenetic testing: the debate continues. Clin. Pharmacol. Ther. 2014;96(6):655–657. doi: 10.1038/clpt.2014.185. [DOI] [PubMed] [Google Scholar]
- 27.Janssens AC, Deverka PA. Useless until proven effective: the clinical utility of preemptive pharmacogenetic testing. Clin. Pharmacol. Ther. 2014;96(6):652–654. doi: 10.1038/clpt.2014.186. [DOI] [PubMed] [Google Scholar]
- 28.Burton A. Are we ready for direct-to-consumer genetic testing? Lancet Neurol. 2015;14(2):138–139. doi: 10.1016/S1474-4422(15)70003-7. [DOI] [PubMed] [Google Scholar]
- 29.McCullough KB, Formea CM, Berg KD, et al. Assessment of the pharmacogenomics educational needs of pharmacists. Am. J. Pharm. Educ. 2011;75(3):51. doi: 10.5688/ajpe75351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salari K, Karczewski KJ, Hudgins L, Ormond KE. Evidence that personal genome testing enhances student learning in a course on genomics and personalized medicine. PLoS ONE. 2013;8(7):e68853. doi: 10.1371/journal.pone.0068853. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrates that the incorporation of personal genetic testing into a medical school course improved students’ self-assessed knowledge on genomics.
- 31.Formea CM, Nicholson WT, McCullough KB, et al. Development and evaluation of a pharmacogenomics educational program for pharmacists. Am. J. Pharm. Educ. 2013;77(1):10. doi: 10.5688/ajpe77110. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Demonstrates statistically significant improvement in pharmacists’ knowledge on pharmacogenomics 2 months after a 1-h case-based pharmacogenomics educational program.
- 32.National Coalition for Health Professionals Education in Genetics: Core competencies for all health professionals 2007. 2015. www.nchpeg.org/index.php?option=com_content&view=article&id=237&Itemid=84
- 33.US Department of Health & Human Services: Genetics education and training: report of the Secretary's Advisory Committee on genetics, health, and society 2011. 2015. www.genome.gov; •• A comprehensive review and report on the genetics education and training needs of health professionals, the public health workforce, patients and consumers.
- 34.Owusu-Obeng A, Weitzel KW, Hatton RC, et al. Emerging roles for pharmacists in clinical implementation of pharmacogenomics. Pharmacotherapy. 2014;34(10):1102–1112. doi: 10.1002/phar.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A comprehensive description of the role of pharmacists in the clinical implementation of pharmacogenetics, including education of other healthcare providers.