Abstract
We analyzed the impact of growth at either 350 (ambient) or 700 (elevated) μL L−1 CO2 on key elements of the C4 pathway (photosynthesis, carbon isotope discrimination, and leaf anatomy) using the C4 cereal sorghum (Sorghum bicolor L. Moench.). Gas-exchange analysis of the CO2 response of photosynthesis indicated that both carboxylation efficiency and the CO2 saturated rate of photosynthesis were lower in plants grown at elevated relative to ambient CO2. This was accompanied by a 49% reduction in the phosphoenolpyruvate carboxylase content of leaves (area basis) in the elevated CO2-grown plants, but no change in Rubisco content. Despite the lower phosphoenolpyruvate carboxylase content, there was a 3-fold increase in C isotope discrimination in leaves of plants grown at elevated CO2 and bundle sheath leakiness was estimated to be 24% and 33%, respectively, for the ambient and elevated CO2-grown plants. However, we could detect no difference in quantum yield. The ratio of quantum yield of CO2 fixation to PSII efficiency was lower in plants grown at elevated CO2, but only when leaf internal was below 50 μL L−1. This suggests a reduction in the efficiency of the C4 cycle when [CO2] is low, and also implies increased electron transport to acceptors other than CO2. Analysis of leaf sections using a transmission electron microscope indicated a 2-fold decrease in the thickness of the bundle sheath cell walls in plants grown at elevated relative to ambient CO2. These results suggest that significant acclimation to increased CO2 concentrations occurs in sorghum.
The C4 photosynthetic pathway differs from the C3 pathway in that it involves two carboxylation steps rather than one. In the first step, CO2 is fixed into C4 acids by phosphoenolpyruvate carboxylase (PEPC) in mesophyll cells. In the second step, these C4 acids are transported into bundle sheath cells, where they are decarboxylated and the CO2 is refixed by Rubisco. Efficient functioning of the C4 pathway is facilitated by the distinctive Kranz anatomy of C4 leaves that allows separation of the two carboxylation steps while at the same time maintaining short diffusion pathways for the transfer of metabolites (Leegood, 1997). Another important structural feature is the very low permeability of bundle sheath cell walls, which minimizes leakage of accumulated CO2 back to the mesophyll (Hatch et al., 1995). This distinctive combination of biochemistry and anatomy has been estimated to result in a 3- to 20-fold increase in the CO2 concentration in bundle sheath cells, relative to that in the surrounding air (Jenkins, 1997; Laisk and Edwards, 1998). The main advantages of possessing the C4 pathway arise both directly and indirectly, from the improved carboxylation efficiency (CE) with which Rubisco operates in bundle sheath cells relative to that in the mesophyll of C3 plants. This improved efficiency is the result of both the higher substrate concentration (CO2) around Rubisco and the suppression of photorespiration (oxygenation reaction of Rubisco).
The improved operating efficiency of Rubisco produces secondary advantages for C4 plants with respect to both water- and nitrogen-use efficiencies (Sage and Pearcy, 1987; Long, 1999). Based on an estimated bundle sheath CO2 concentration of 10 to 100 times that in air, it has been calculated that C4 photosynthesis needs only 13% to 20% of the Rubisco required by C3 plants to sustain the same carbon fixation rate (Long, 1999). However, others have suggested that the bundle sheath CO2 concentration may be lower than this (e.g. Laisk and Edwards, 1998) and thus the amount of Rubisco required may be as much as 42% of that found in C3 plants. C4 plants also allocate significant amounts of N to PEPC and the ratio of PEPC to Rubisco activity has been shown to decline as N becomes more limiting (Sage et al., 1987). The preferential allocation of N to Rubisco, rather than PEPC, probably helps to prevent a build up of CO2 in the bundle sheath above carboxylation capacity, thus reducing the potential for increased leakiness. When grown at very low N, the advantage of C4 photosynthesis over C3 tends to decline and photosynthetic nitrogen use efficieny of C3 plants may be higher (Sage and Pearcy, 1987). Furthermore, under limiting N, C4 plants become more responsive to elevated CO2 concentrations and there is some evidence, based on δ13-C values of plant tissue, of an impairment of the CO2-concentrating mechanism under these conditions (Wong and Osmond, 1991). Growth at elevated CO2 concentrations was also found to result in an increase in carbon isotope discrimination (Δ) for the C4 crop, maize (Vogel, 1980) and the C4 savannah grass, Eragrostis pilosa (Watling and Press, 1998). Measurements of Δ in C4 plants have also been shown to vary in response to other environmental variables such as water availability (Buchmann et al., 1996; Saliendra et al., 1996) and light (Buchmann et al., 1996). Models relating C4 photosynthesis to Δ suggest that changes in Δ are largely the result of increases in bundle sheath leakiness (Farquhar et al., 1989). However, measurements of on-line isotope discrimination during gas-exchange found little or no short-term response to environmental variables in C4 plants (Henderson et al., 1992), suggesting that the observed long-term variations in Δ may represent acclimatory responses.
It has been known for some time that environmental variables, such as water availability and salinity, can trigger switches between C3 and crassulacean acid metabolism photosynthesis in some plants (Winter, 1985). A small number of species have also been reported to exhibit shifts between C3 and C4 characteristics in response to environmental variables. These species include sedges from the genus Eleocharis (Ueno, 1996a, 1996b) and grasses from the tribe Orcuttieae (Keeley, 1998), both of which develop C3-like traits when they are in aquatic environments, but become more C4-like when in the terrestrial phase. Another example is the aquatic plant Hydrilla verticillata that switches from C3 to C4 photosynthesis when CO2 availability declines (Reiskind et al., 1997). Despite such examples, and the impacts of both N and CO2 reported above, the extent to which C4 photosynthesis may be regulated by environmental variables remains relatively unexplored, especially in comparison with the C3 pathway.
Under circumstances where CO2 concentrations are high, as may be the case, at least internally, for the aquatic sedges and grasses, there is no particular advantage in operating a CO2-concentrating mechanism such as the C4 pathway. This is because as [CO2] in the environment increases, the efficiency of C3 photosynthesis will improve, relative to C4 photosynthesis, because of the extra cost of operating a CO2-concentrating mechanism that is incurred by the C4 pathway (two extra ATP are required for regeneration of phosphoenolpyruvate [PEP]; Kanai and Edwards, 1999). Thus, under high [CO2], C3 photosynthesis becomes energetically more favorable than C4. Furthermore, when [CO2] is high, C4 efficiency may be further compromised because the supply of C4 acids may exceed Rubisco carboxylation capacity, resulting in increased leakiness of CO2 from the bundle sheath. In an analogous situation, increased leakiness has been demonstrated for transgenic Flaveria bidentis, in which levels of Rubisco in bundle sheath cells were reduced (von Caemmerer et al., 1997).
Although there have been a number of papers in which the impact of elevated CO2 concentration on growth of C4 plants has been examined (for review, see Wand et al., 1999), few have explored the possibility that the C4 pathway itself may be sensitive to changes in CO2 concentration. In this paper we report the results of an experiment designed to explore the extent to which key features of the C4 syndrome, specifically leaf anatomy, photosynthetic light and CO2 utilization, Δ, and enzyme contents may be affected by increased CO2 concentrations. We grew the C4 crop, sorghum (Sorghum bicolor L. Moench.), at both 350 and 700 μL L−1 CO2 and found evidence suggesting modification of the C4 pathway, at both anatomical and metabolic levels, in the plants grown at elevated CO2.
RESULTS
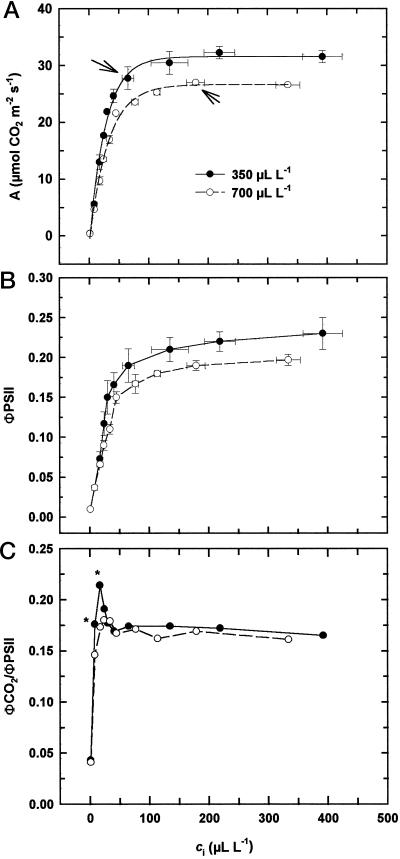
In interpreting the CO2 response of photosynthesis in sorghum, we have used the model of C4 photosynthesis developed by von Caemmerer and Furbank (1999) in which the initial slope of the A/ci response is an indicator of PEPC activity (CE), whereas the CO2 saturated rate (Asat), is determined by either Rubisco activity, the rate of PEP regeneration, the electron transport rate, or PEPC activity if it is very low. This model has been supported by data obtained both from mutants deficient in PEPC (Dever et al., 1997), and transgenic plants with reduced amounts of Rubisco (von Caemmerer et al., 1997). There was a significant [CO2] effect on the A/ci response of sorghum in our experiment(Fig. 1a). In the plants grown at the higher CO2 concentration CE was 28% lower and Asat was 16% lower, although this latter value was not statistically significant (Table I). These results suggest that growth at elevated CO2 had a significant impact on PEPC activity and possibly on some or all of the components that determine Asat. Despite these changes, rates of assimilation were similar when plants were measured at growth [CO2] (indicated by arrows in Fig. 1a). In addition, there was no difference in the CO2 compensation point 1.42 and 1.51 μL L−1, respectively, for plants grown at either ambient or elevated CO2, implying that rates of photorespiration were equally low in both.
Figure 1.
The relationship between ci and CO2 assimilation rate (a), quantum yield of PSII (ΦPSII; b), and ratio of the quantum yields of CO2 assimilation and PSII (ΦCO2/ΦPSII; c) for S. bicolor grown at ambient (350 μL L−1) or elevated (700 μL L−1) CO2. The arrows in a indicate the CO2 assimilation rate at growth CO2 concentration. For clarity, error bars have not been included in c; the asterisks indicate where there was a significant difference at α = 0.05.
Table I.
CE and the Asat (μmol CO2 m−2 s−1) for sorghum grown at either 350 or 700 μL L−1 CO2
| Growth [CO2] | CE | Asat |
|---|---|---|
| μL L−1 | ||
| 350 | 1.16 (0.04)a | 31.7 (1.3)a |
| 700 | 0.83 (0.02)b | 26.7 (0.3)a |
Parameters were determined using the data shown in Figure 1. Values are means ± se, n = 3. Means superscripted with the same letter are not significantly different at α = 0.05.
Chlorophyll (Chl) fluorescence measurements indicated that PSII efficiency (ΦPSII) varied with ci in a similar way to A in both the ambient- and elevated-CO2 grown plants (Fig. 1b). However, when ci was below 50 μL L−1, the ratio of CO2 fixation (ΦCO2) to ΦPSII, which is a measure of the energy efficiency of CO2 fixation, was lower in the elevated CO2-grown plants (Fig. 1c). Thus, at low values of ci, less CO2 was fixed per electron transported in the elevated CO2-grown plants than in their ambient CO2-grown counterparts. In conjunction with the gas-exchange data, this provides further evidence of a reduction in the efficiency of the C4 cycle in sorghum grown at elevated CO2. However, it also suggests an increase in electron transport to processes other than CO2 fixation, such as photorespiration, O2 reduction (Mehler reaction), or nitrogen assimilation.
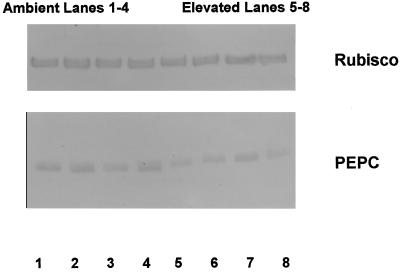
PEPC and Rubisco contents of the same leaves used for gas-exchange measurements were determined from western blots. The PEPC content (area basis) of sorghum grown at elevated CO2 was 51% of that found in the ambient CO2-grown plants, but there was no change in Rubisco content with growth CO2 (Table II and Fig. 2). The lower PEPC content of the elevated CO2-grown sorghum is consistent with the lower CE observed in these plants; however, the lower Asat does not appear to have been the result of any change in Rubisco content and instead, may have been due to the decline in PEPC and/or the changes in PEP regeneration and electron transport. Despite the difference in PEPC content, there was no significant difference in either leaf N or chl content (area basis) between the two CO2 treatments (Table II). Two previous studies with sorghum have also found that leaf N did not vary significantly with [CO2] (Reeves et al., 1994; Henning et al., 1996).
Table II.
PEPC and Rubisco content (area basis) and N and Chl concentrations for sorghum grown at either 350 or 700 μL L−1 CO2
| Growth [CO2] | PEPC | Rubisco | N | Total Chl |
|---|---|---|---|---|
| μL L−1 | % 350 CO2 | % 350 CO2 | g m−2 | μmol m−2 |
| 350 | 100 (15.0)a | 100 (7.6)a | 0.59 (0.01)a | 371.0 (18.0)a |
| 700 | 51.0 (8.1)b | 95.0 (6.8)a | 0.56 (0.02)a | 392.0 (10.0)a |
Values are means ± se. n = 5. Means superscripted with the same letter are not significantly different at α = 0.05.
Figure 2.
Western blots of Rubisco and PEPC for leaf samples taken from S. bicolor grown at ambient (350 μL L−1) or elevated (700 μL L−1) CO2.
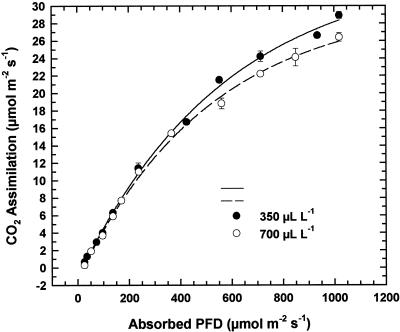
Measurements of Δ made on dried leaf material indicated a significant increase in discrimination against 13C when plants were grown at elevated relative to ambient CO2 (Table III). Bundle sheath leakiness (φ), calculated on the basis of the ratio of internal [CO2] to external [CO2] (ci/ca) observed during gas-exchange measurements, was also higher in the elevated CO2-grown plants than in those grown at ambient CO2 (Table III). The magnitude of φ is determined by both the physical conductance of bundle sheath cell walls and also the extent of PEPC over-cycling, which occurs if the delivery of CO2 to the bundle sheath is in excess of its utilization by the C3 cycle (Farquhar et al., 1989; von Caemmerer and Furbank, 1999). In the current experiment it is unlikely that PEPC over-cycling was significantly higher in the plants grown at elevated CO2 because of their lower PEPC to Rubisco ratio, relative to ambient CO2-grown plants. Thus the higher φ may have been due to changes in bundle sheath conductance and/or the higher ci in the plants grown at elevated CO2. Increased φ should also result in a decline in the light-use efficiency of C4 plants, because CO2 that leaks from the bundle sheath is either lost or refixed by PEPC in the mesophyll, thus increasing the energy expended per CO2 fixed. However, when we measured the photon flux density (PFD) response of photosynthesis in our experiment, there was no difference in quantum yield between the ambient and elevated CO2 grown sorghum (Fig. 3).
Table III.
Δ obtained from leaf dry matter and estimated bundle-sheath leakiness (φ) for sorghum grown at either 350 or 700 μL L−1 CO2
| Growth [CO2] | Δ | φ |
|---|---|---|
| μL L−1 | ‰ | % |
| 350 | 1.05 (0.18)a | 24.0 (0.6)a |
| 700 | 3.51 (0.09)b | 33.0 (0.3)b |
The ci/ca values used to estimate φ were obtained during gas-exchange measurements and were 0.19 and 0.26, respectively, for 350 or 700 μL L−1 CO2-grown plants, measured at growth CO2. Values are means ± se, n = 5. Means superscripted with the same letter are not significantly different at α = 0.05.
Figure 3.
The relationship between absorbed PFD and CO2 assimilation rate for S. bicolor grown at ambient (350 μL L−−1) or elevated (700 μL L−1) CO2.
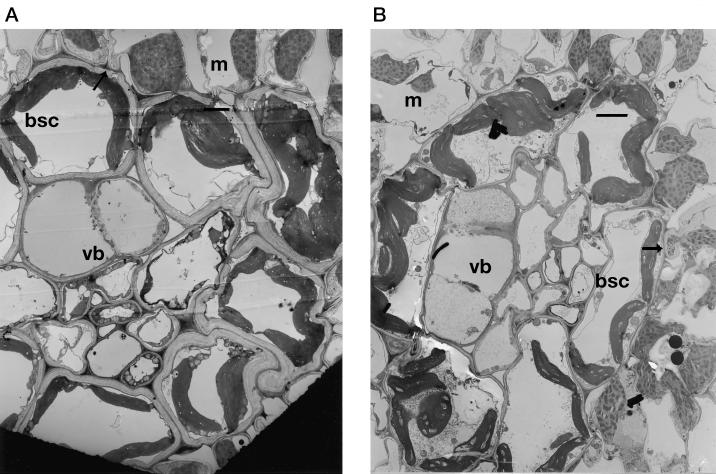
Leaf sections taken from the youngest fully expanded leaves of the sorghum plants were analyzed using a transmission electron microscope. Examination of the micrographs indicated that plants grown at ambient CO2 had significantly thicker bundle sheath cells walls than elevated CO2-grown plants (Fig. 4). Sections from three plants at each CO2 concentration were analyzed and on average, bundle sheath cell walls of the ambient CO2-grown plants were twice as thick as those of the elevated CO2-grown plants, (3.6 ± 0.3 and 1.6 ± 0.1 μm, respectively). This anatomical data provides further evidence that the decline in C4 pathway efficiency observed in the sorghum plants grown at elevated CO2 may be, at least partly, the result of changes in the conductance of bundle sheath cell walls to CO2.
Figure 4.
Transmission electron micrographs of leaf sections showing bundle sheaths from S. bicolor grown at either 350 (a) or 700 μL L−1 CO2 (b). bsc, Bundle sheath cell; m, mesophyll; vb, vascular bundle. Scale bar = 15 μm (both micrographs). Bundle sheath cell walls (indicated by arrows) were approximately twice as thick in ambient relative to elevated CO2 grown plants.
DISCUSSION
Responses of C4 Photosynthesis to Elevated CO2
We observed significant [CO2] effects on photosynthetic characteristics of the C4 crop sorghum, with plants grown at elevated CO2 having lower CE than their ambient CO2-grown counterparts. According to the model of C4 photosynthesis developed by von Caemmerer and Furbank (1999), this is consistent with a decline in the PEPC content of leaves, as the initial slope of the A/ci response is proportional to PEPC activity and Asat may also decline if PEPC activity is very low, because CO2 levels in the bundle sheath will not be saturating for Rubisco. Similar changes in the A/ci response have been reported both for mutants of the C4 dicot Amaranthus edulis, with reduced amounts of PEPC (Dever et al., 1997), and also for Amaranthus retroflexus in which PEPC content varied with N availability (Sage et al., 1987). In agreement with the predictions of the model and with these earlier reports, we found that PEPC content of the plants grown at elevated CO2 was only 51% that of the plants grown at ambient CO2. In contrast, there was no difference in the Rubisco content of leaves from the two CO2 treatments. Maroco et al. (1998) also found no change in Rubisco content for heterozygous PEPC mutants of A. edulis with a similar reduction in PEPC content to that which we observed for the plants grown at elevated CO2. In an earlier paper (Watling and Press, 1997) we reported that [CO2] had no impact on photosynthesis of sorghum grown at elevated and ambient CO2. At present we are unable to account entirely for this difference. However, the level of N supplied to plants was higher in the former study than the present one, and nitrogen supply can affect PEPC:Rubisco ratios (Sage et al., 1987) and the response of C4 plants to [CO2] (Wong and Osmond, 1991; Ghannoum and Conroy, 1998).
Although the changes in the A/ci response that we observed for elevated CO2-grown sorghum are entirely consistent with the concurrent decline in PEPC content, they could also be explained by changes in bundle sheath conductance. As modeled by von Caemmerer and Furbank (1999), increases in the permeability of the bundle sheath to CO2 can cause a decline in both CE and Asat because of increased leakage of CO2 from the bundle sheath. These predictions are supported by work with transgenic F. bidentis, in which expression of carbonic anhydrase in the bundle sheath was increased, resulting in increased leakage of bicarbonate from the bundle sheath and a decline in both CE and Asat (Ludwig et al., 1998). Our data also suggest that there was an increase in φ in the plants grown at elevated CO2, and this was accompanied by significant changes in the physical characteristics of the bundle sheath cell walls as indicated by electron microscopy. Increases in φ can be the result of an increased PEPC to Rubisco ratio (over-cycling of PEPC), and/or changes in the physical conductance of the bundle sheath to CO2 (Farquhar et al., 1989). However, as we observed a decline in the PEPC to Rubisco ratio, it is most likely that the increased φ was due to changes in bundle sheath conductance, perhaps exacerbated by the increase in ci. If this is the case, it is possible that the decline in PEPC content was a response to the increase in leakiness, brought about by the change in bundle sheath conductance, rather than a direct response to increased [CO2]. If there had been no decline in the PEPC to Rubisco ratio, the magnitude of φ would have been even higher and C4 efficiency further compromised. Maroco et al. (1998) also observed a decline in PEPC content in transgenic F. bidentis with reduced amounts of Rubisco, although von Caemmerer et al. (1997) did not.
The high concentrations of CO2 in bundle sheath cells of C4 plants act to suppress the oxygenase reaction of Rubisco, but do not remove it altogether, as has been demonstrated through measurements of Gly metabolism in maize (Marek and Stewart, 1983), 18O2 labeling also in maize (de Veau and Burris, 1989), NH4+ production in A. edulis (Lacuesta et al., 1997), and increased O2-sensitivity, relative to wild-type plants, in PEPC-deficient mutants of A. edulis (Maroco et al., 1998). If bundle sheath conductance was greater in sorghum grown at elevated CO2, as is implied by our data, then it might be expected that the plants would show an increased sensitivity to O2. Although we did not make direct measurements of the O2 sensitivity of photosynthesis in our experiment, we did find a decrease in the ΦCO2 to ΦPSII ratio, at low ci, for the plants grown at elevated as compared with ambient CO2. This implies both a decline in the energy efficiency of CO2 fixation and also an increase in electron transport to acceptors other than CO2 and is consistent with increased rates of photorespiration in the elevated CO2-grown plants when exposed to low [CO2]. At higher CO2 concentrations, the ΦCO2 to ΦPSII ratio was similar in both ambient- and elevated CO2-grown plants. Presumably, this was because the ratio of CO2 to O2 in the bundle sheath cells increased as both PEPC activity and ci increased. Despite the decline in the ΦCO2 to ΦPSII ratio observed at low ci, we did not observe any significant increase in CO2 compensation point for elevated CO2-grown sorghum, as might be expected if photorespiration rates had increased. However, it is possible that the differences in photorespiration were too small to be detected by the gas-exchange system we used, whereas small changes in energy-use efficiency of CO2 fixation were detected by the Chl fluorescence measurements.
Theory predicts that increases in φ in C4 plants should be accompanied by a decline in the quantum yield of CO2 fixation, because CO2 diffusing from the bundle sheath is either lost or refixed by PEPC in the mesophyll, increasing the energy expended per CO2 fixed (Farquhar, 1983; Hatch et al., 1995). In this context, quantum yields have been reported to vary between both the different C4 subtypes and C4 monocots and dicots; this has been attributed to variation in φ postulated to be the result of differences in bundle sheath conductance associated with the presence or absence of a suberin lamella in cell walls (Hattersley, 1982; Ehleringer and Pearcy, 1983; Ohsugi et al., 1988). However, concurrent measurements of quantum yield and φ have rarely been made in the same plants. Furthermore, von Caemmerer et al. (1997) were able to demonstrate a significant increase in φ for transgenic F. bidentis with reduced Rubisco content, but found no difference in quantum yield between the transgenic and wild-type plants. In our experiment, although the isotope data indicated that there had been a significant increase in φ for sorghum grown at elevated CO2, we also could not detect any difference in quantum yield. Von Caemmerer et al. (1997) suggested that the inability to find a correlation between φ and quantum yield may be due to two factors. First, the extent to which the Q-cycle contributes to proton translocation is unknown, but may be significant in C4 plants (Furbank et al., 1990). And second, the relationship between φ and the quantum requirement of CO2 fixation is non-linear, so that a relatively large increase in φ actually has a rather small impact on quantum yield, which may be undetectable. However, if the latter is true, it is then difficult to argue that increases in φ are significantly disadvantageous to C4 plants.
The model developed by Farquhar (1983), describing the relationship between C4 photosynthesis and 13C discrimination, indicates that the magnitude of Δ in C4 plants is largely determined by the extent of φ. As described above, φ itself is a function of the PEPC to Rubisco ratio and the physical conductance of the bundle sheath to CO2. When C4 plants are grown at elevated CO2 concentrations, however, a third factor may influence the magnitude Δ. This is the proportion of CO2 fixed directly by Rubisco in the bundle sheath that has diffused in from the mesophyll, rather than being delivered via PEPC. If this proportion increases, as may occur when bundle sheath conductance increases in combination with an increase in ci and a decline in PEPC activity, as appears to occur in the elevated CO2 grown sorghum, then the opportunity for Rubisco to discriminate against 13CO2 increases and Δ will also increase. That is, under elevated CO2, there may be an increased exchange of CO2 between the atmosphere and the bundle sheath and this is reflected in the increase in Δ. This type of change in Δ may result either from an increase in the rate of diffusion of CO2 into the bundle sheath (indicating an increase in direct fixation of CO2 by Rubisco) or an increase in the rate of CO2 leakage from the bundle sheath into the atmosphere (i.e. CO2 that is lost from the bundle sheath, but not recycled by PEPC; Hatch et al., 1995). The former may be analogous to similar changes in Δ observed during transitions between the various phases of crassulacean acid metabolism photosynthesis (Roberts et al., 1997).
Environmental Regulation of C4
The benefits of operating the C4 pathway, relative to the C3 pathway, are greatest under conditions of high light and temperature and a low CO2 to O2 ratio. Thus, if the C4 syndrome is subject to environmental regulation, it might be expected to occur under those conditions that least favor C4 photosynthesis. In the current experiment sorghum was exposed to elevated CO2 concentrations under conditions of limiting N, and PFDs that were approximately one-half of those generally experienced in the regions where sorghum, and C4 grasses in general, predominate (Doggett, 1988). We observed changes in both photosynthetic and anatomical characteristics that suggested modifications of the C4 syndrome had occurred in response to the increased CO2 concentration. Similar modifications have been reported for grasses from the tribe Orcuttieae, which contains a number of species that have both aquatic and terrestrial phases in their life cycle (Keeley, 1998). One genus, Neostapfia, exhibits C4 characteristics in the terrestrial form, but in aquatic leaves there is a reduction in the thickness of bundle sheath cell walls, an increase in Δ, and a decline in the PEPC to Rubisco ratio, characteristics that are identical to those we observed for the elevated CO2 grown sorghum. In a second genus, Orcuttia, C4 activity is maintained in the aquatic plants, but in the absence of Kranz anatomy (Keeley, 1998). Similar changes have also been reported for the sedge Eleocharis vivipara on switching from a terrestrial to an aquatic habitat (Ueno, 1996a, 1996b). A further example of environmental regulation is given by the aquatic plant Hydrilla verticillata, which switches from C3 to C4 metabolism when CO2 concentrations decline (Reiskind et al., 1997). Expression of the C4 syndrome can also be affected by light availability. Maize seedlings that developed in low light or darkness were shown to have Rubisco mRNA present in both bundle sheath and mesophyll cells, whereas high-light grown seedlings showed localization of Rubisco to the bundle sheath cells only (Langdale et al., 1988). These observations indicate that expression of the C4 phenotype is flexible with respect to environmental factors, in at least some species.
There are a number of consequences that arise from the knowledge that the C4 phenotype may be subject to some level of environmental regulation. First, it may mean that C4 plants are more flexible in the face of environmental change than has previously been thought. In particular, this could have consequences for the persistence of C4-dominated communities in response to climate change and rising atmospheric CO2 concentrations, both in the future and the past (Cerling et al., 1997; Collatz et al., 1998). Second, the fact that C4 can be expressed in a variety of forms, including without the presence of the distinctive (and often diagnostic) Kranz anatomy (Keeley, 1998), means that it may be invisible in the fossil record. Of particular interest is the fact that the carbon isotope signatures of C4 plants can vary with environmental factors such as light, water availability and, as shown in our paper, [CO2]. This has obvious consequences for interpretation of paleoecological data that is based on carbon isotope signatures of fossil material.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Sorghum (Sorghum bicolor L. Moench. cv CSH-1) plants were grown in controlled environment cabinets (model SGC097, Fitotron, Sanyo-Gallenkamp, UK) at either 350 μL L−1 CO2 (ambient) or 700 μL L−1 CO2 (elevated). Although there was only one cabinet at each CO2 concentration, we have previously assessed chamber effects by conducting two identical experiments with sorghum in which CO2 treatment was alternated between the cabinets (i.e. the ambient CO2 cabinet in the first experiment became the elevated CO2 cabinet in the second experiment) and no significant differences were found with respect to growth and photosynthesis of the plants in the two experiments (J.R. Watling and M.C. Press, unpublished data). The CO2 concentration in each cabinet was monitored by infrared gas analyzers (IRGA; ADC2000, ADC, Hoddesdon, UK). In the elevated CO2 cabinet, the IRGA was also used to control introduction of pure CO2, via a solenoid valve, from an external cylinder. Because of a 2 to 3 min lag in the response time of the IRGA, actual [CO2] in the elevated cabinet ranged between 650 and 760 μL L−1 (mean of 706 μL L−1 CO2). Concentration of CO2 in the ambient cabinet remained stable throughout the experiment, only declining slightly to 330 μL L−1 in the later stages of the experiment as plants matured and canopy photosynthesis increased. Lighting in the cabinets was provided by a combination of fluorescent tubes (58 W, PLL-type, Philips, The Netherlands) and incandescent (tungsten) lamps. PFD in the cabinets, measured with a quantum sensor (Skye, calibrated by Skye Instruments, Wales, UK), was 800 μmol photons m−2 s−1 at plant height. A 12-h photoperiod was maintained throughout the experiment with day and night temperatures of 30°C and 23°C, respectively, and vapor-pressure differences of 1.7 and 1.1 kPa, respectively.
Plants were grown in washed sand and irrigated with 40% full strength Long Ashton solution modified such that N was at 20% (0.5 mol m−3 NH4NO3) via an automatic drip-irrigation system. Initially plants received 48 cm3 of nutrient solution each per day, this was increased to 96, 132, and 240 cm3 at 4, 6, and 8 weeks after sowing, respectively.
Gas Exchange and Chl a Fluorescence
Net CO2 assimilation rates and Chl a fluorescence characteristics were determined simultaneously, using the youngest, fully expanded leaf of 45- to 50-d-old plants. An open gas-exchange system was used with a Parkinson-type leaf chamber (PLC-3, ADC, Hoddesdon, UK). Actinic light was supplied, via a fiber optic bundle, from a KL 1500 light source (Schott, Mainz, Germany), and the same fiber optic bundle was connected to two other KL 1500 light sources to provide the saturating pulses for determination of the Chl a fluorescence parameters Fm and Fm′. Input gases (N2, O2, and CO2) were mixed using mass flow controllers (AFC 260, ASM, Bithoven, The Netherlands). Prior to the addition of CO2, N2 and O2 were bubbled through water and then dried to a set humidity using a condenser coil immersed in a temperature controlled water bath. Differences in the concentrations of CO2 and H2O entering and leaving the leaf chamber were measured with an IRGA (LCA-3, ADC, Hoddesdon, UK) and gas-exchange parameters were calculated using the equations of von Caemmerer and Farquhar (1981). Measurements were made at a leaf temperature of 30°C and a leaf to air vapor-pressure difference of 1.7 kPa.
Chl a fluorescence was determined using a pulse amplitude modulated fluorometer (PAM 103, Walz, Effeltrich, Germany). The quantum yield of PSII in the light (ΦPSII) was calculated as ΦPSII = (Fm′ − Fs)/Fm′ (Genty et al., 1989). The quantum yield of CO2 fixation (ΦCO2) was calculated as ΦCO2 = A/absorbed PFD, assuming a leaf absorptivity of 85% (Oberhuber and Edwards, 1993).
The response of A to ci was assessed by varying the concentration of CO2 entering the leaf chamber (O2 was maintained at 210 mL L−1). Measurements for the A/ci response were made at a PFD of 1,200 μmol m−2 s−1. Light response curves of photosynthesis were measured at a ca of 350 μL L−1 and a range of PFDs. Curve fitting software (Sigma Plot for Windows 4.0) was used to analyze both the A/ci and PFD responses using a three component exponential function of the form:
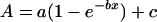 |
1 |
where A = steady-state assimilation rate and x = ci or PFD. Using this equation, the Asat was calculated as a + c and the CE as the slope at A = 0 (calculated as b[a + c]). The quantum yield of photosynthesis was calculated in a similar fashion to CE.
SDS-PAGE and Western Blotting
Proteins were extracted from the same leaves that had been used for gas-exchange measurements. Leaf discs (0.56 cm2) were collected, immediately frozen in liquid N2, and then ground in 300 μL of extraction buffer (180 mol m−3 Bicine [N,N′-bis(2-hydroxyethylglycine)]-KOH, pH 9.0, 5.0 mol m−3 DTT (dithiothreitol), and 1.0% [w/v] SDS). The extracts were centrifuged at 14,000g for 2 min then solubilization buffer (62.5 mol m−3 Tris [Tris(hydroxymethyl)-aminomethane]-HCl, pH 6.8, 20% [v/v] glycerol, 2.5% [w/v] SDS, and 5% [v/v] 2-mercaptoethanol) was combined with an aliquot of the supernatant in a ratio of 1:1 (v/v) and boiled in a water bath for 90 s. Proteins were separated using SDS-PAGE. The separated proteins were transferred from gels to polyvinylidene difluoride membranes (Immobilon-P, Millipore, Bedford, MA). Following transfer, membranes were blocked in 4% milk/Tris-buffered saline (TBS; 20 mol m−3 Tris and 140 mol m−3 NaCl, pH 7.4) for 1 h and then probed with antiserum to either Rubisco (1:1,000 in 4% milk/TBS) or PEPC (1:10,000 in 4% milk/TBS) for 45 min. Membranes were washed several times with TBS and then probed with the secondary antibody, antirabbit IgG peroxidase complex (Sigma, Poole, Dorset, UK). Immunoreactive bands were visualized by enhanced chemiluminescence (ECL Kit, Amersham Life Sciences, Buckinghamshire, UK) and recorded on x-ray film (X-Omat, Kodak Eastman, Rochester, NY). Band densities on the exposed film were quantified by computerized video imaging. Previous determinations indicated that band densities were within the linear range.
Chl and N Determination
Dried sorghum leaf tissue was analyzed for nitrogen using a modified Kjeldahl technique. Samples of dried tissue (50 mg) were digested in concentrated H2SO4-salycilic acid in the presence of a catalyst (CuSO4-Li2SO4) for 5 h at 365°C. The resulting digest was diluted to a known volume with distilled H2O and analyzed with a colorimetric assay using a flow injection analysis system (Tecator 5042 Detector and 5012 Analyzer, Tecator, UK). Leaf discs collected from the same leaves used for gas-exchange were analyzed for their Chl content using the method of Porra et al. (1989).
Stable Carbon Isotope Discrimination
Samples of dried and ground sorghum leaf tissue were analyzed for their stable carbon isotope composition. In each case about 1 mg of plant material was combusted and the relative abundance of 13C and 12C was determined using the mass spectrometer facilities at the University of Newcastle upon Tyne (UK; Europa Scientific 20/20 MS, interfaced with an ANCA SL prep unit, Europa Scientific, Crewe, UK). Gas samples from the growth cabinets were analyzed with a trace gas prep unit interfaced to the same mass spectrometer. Carbon isotope compositions of the plant material and source gas in the growth cabinets were determined relative to the Pee Dee Belemnite standard and discrimination against 13C (Δ) was calculated using Equation 2.
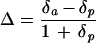 |
2 |
where δa is the δ13-C value of the source air in the growth cabinets and δp is the δ13-C value of the plant material. The δa values (means ± se) for the ambient and elevated CO2 cabinets were −11.45‰ (±0.22) and −18.62‰ (±0.24), respectively. Sampling of gas in both cabinets was carried out over a single day, with 3 samples collected every 2 h between 9 am and 5 pm. The same cylinder of CO2 was used to enrich the elevated CO2 cabinet throughout the experiment.
φ to CO2 was estimated using the equations derived by Farquhar et al. (1989) for C4 photosynthesis. Ideally, when using these equations, values of Δ and ci/ca should be obtained from concurrent gas-exchange measurements. However, in this case we used the Δ obtained from the dried leaf material and the ci/ca values measured during gas-exchange of the same plants (corresponding to growth-CO2 concentrations) to provide an approximation of φ for the plants in our experiment. Using this approach, φ was estimated using Equation 3.
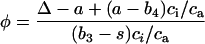 |
3 |
where a (4.4‰) is the fractionation occurring during diffusion of CO2 in air, b4 (−5.7‰) is the combined fractionation due to PEPC (2.2‰) and the activity of carbonic anhydrase in the mesophyll, b3 (30‰) is the fractionation by Rubsico and s (1.8‰) is the fractionation associated with leakage of CO2 from the bundle sheath to the mesophyll (von Caemmerer et al., 1997).
Electron Microscopy
Leaf tissue was collected from 3 plants at each CO2 concentration at 54 d after sowing. In each case tissue samples were taken from a location one-half-way along the leaf and mid-way between the mid-vein and the leaf edge. Throughout the experiment leaf production rates were the same for plants in both CO2 treatments, therefore, we believe samples were collected from leaves that were at the same developmental stage. Samples were fixed in Karnovsky's solution (2% [w/v] paraformaldehyde and 2% [w/v] glutaraldehyde in 100 mol m−3 phosphate buffer) for 3 h at 4°C followed by three washes (30 min each) in 10% (w/v) Suc in 100 mol m−3 phosphate buffer. Secondary fixation was conducted at room temperature for 1 h in 2% (w/v) aqueous OsO4. Following secondary fixation, tissue samples were passed through an ethanol dehydration series (75%, 95%, and 100% [v/v] ethanol) with 15 min at each step and culminating in a final step at 100% ethanol dried over anhydrous CuSO4. The samples were then incubated twice (15 min each) in propylene oxide. Infiltration was achieved by incubation overnight in 1:1 propylene oxide:Araldite resin (Araldite resin; 1:1 CY212 resin:DDSA hardener, with accelerator 0.1 mL mL−1 araldite resin). Specimens were left in full-strength Araldite resin for 6 to 8 h at room temperature and then embedded in fresh Araldite resin for 48 h at 60°C. Ultrathin sections (70–90 nm) were cut on an ultramicrotome (Ultracut E, Reichert, Austria) and stained for 15 min with 3% (w/v) uranyl acetate in 50% (v/v) aqueous ethanol followed by 2 min with Reynold's lead citrate. The mounted sections were examined using a transmission electron microscope (CM10, Philips, Holland) at an accelerating voltage of 80 kV. Five separate sections were examined for each plant. As vein size varies across a sorghum leaf, comparisons were always made between veins of the same diameter.
Data Analysis
Where appropriate, data were analyzed using two sample t tests (Minitab 11.0). The response of ΦCO2/ΦPSII to ci was analyzed using ANOVA and a Tukey Test (Zar, 1984).
ACKNOWLEDGMENTS
We would like to thank Profs. R.C. Leegood and F.I. Woodward for helpful discussions during the drafting of this paper.
LITERATURE CITED
- Buchmann N, Brooks JR, Rapp KD, Ehleringer JR. Carbon isotope composition of C4 grasses is influenced by light and water supply. Plant Cell Environ. 1996;19:392–402. [Google Scholar]
- Cerling TE, Harris JM, MacFadden BJ, Leakey MG, Quade J, Eisenmann V, Ehleringer JR. Global vegetation change through the Miocene and Pliocene. Nature. 1997;389:153–158. [Google Scholar]
- Collatz GJ, Berry JA, Clark JS. Effects of climate and atmospheric CO2 partial pressure on the global distribution of C4 grasses: present, past and future. Oecologia. 1998;114:441–454. doi: 10.1007/s004420050468. [DOI] [PubMed] [Google Scholar]
- de Veau EJ, Burris JE. Photorespiratory rates in wheat and maize as determined by 18O-labeling. Plant Physiol. 1989;90:500–511. doi: 10.1104/pp.90.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever LV, Bailey KJ, Leegood RC, Lea PJ. Control of photosynthesis in Amaranthus edulis mutants with reduced amounts of PEP carboxylase. Aust J Plant Physiol. 1997;24:469–476. [Google Scholar]
- Doggett H. Sorghum. London: Longman Group; 1988. [Google Scholar]
- Ehleringer J, Pearcy RW. Variation in quantum yield for CO2 uptake among C3 and C4 plants. Plant Physiol. 1983;73:555–559. doi: 10.1104/pp.73.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD. On the nature of carbon isotope discrimination in C4 species. Aust J Plant Physiol. 1983;10:205–226. [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT. Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:503–537. [Google Scholar]
- Furbank RT, Jenkins CLD, Hatch MD. C4 photosynthesis: quantum requirements, C4 acid overhauling and Q-cycle involvement. Aust J Plant Physiol. 1990;17:1–7. [Google Scholar]
- Genty B, Briantais J-M, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta. 1989;990:87–92. [Google Scholar]
- Ghannoum O, Conroy JP. Nitrogen deficiency precludes a growth response to CO2 enrichment in C3 and C4 Panicum grasses. Aust J Plant Physiol. 1998;25:627–636. [Google Scholar]
- Hatch MD, Agostino A, Jenkins CLD. Measurement of the leakage of CO2 from bundle-sheath cells of leaves during C4 photosynthesis. Plant Physiol. 1995;108:173–181. doi: 10.1104/pp.108.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattersley PW. δ13C values of C4 types in grasses. Aust J Plant Physiol. 1982;9:139–154. [Google Scholar]
- Henderson SA, von Caemmerer S, Farquhar GD. Short-term measurements of carbon isotope discrimination in several C4 species. Aust J Plant Physiol. 1992;19:263–285. [Google Scholar]
- Henning FP, Wood CW, Rogers HH, Runion GB, Prior SA. Composition and decomposition of soybean and sorghum tissues grown under elevated atmospheric carbon dioxide. J Environ Qual. 1996;25:822–827. [Google Scholar]
- Jenkins CLD. The CO2 concentrating mechanism of C4 photosynthesis: bundle sheath cell CO2 concentration and leakage. Aust J Plant Physiol. 1997;24:543–547. [Google Scholar]
- Kanai R, Edwards GE. The biochemistry of C4 photosynthesis. In: Sage RF, Monson RK, editors. C4 Plant Biology. San Diego: Academic Press; 1999. pp. 49–87. [Google Scholar]
- Keeley JE. C4 photosynthetic modifications in the evolutionary transition from land to water in aquatic grasses. Oecologia. 1998;116:85–97. doi: 10.1007/s004420050566. [DOI] [PubMed] [Google Scholar]
- Lacuesta M, Dever LV, Muñoz-Rueda A, Lea PJ. A study of photorespiratory ammonia in the C4 plant Amaranthus edulis, using mutants with altered photosynthetic capacities. Physiol Plant. 1997;99:447–455. [Google Scholar]
- Laisk A, Edwards GE. Oxygen and electron flow in C4 photosynthesis: mehler reaction, photorespiration and CO2 concentration in the bundle sheath. Planta. 1998;205:632–645. [Google Scholar]
- Langdale JA, Zelitch I, Miller E, Nelson T. Cell position and light influence C4 versus C3 patterns of photosynthetic gene expression in maize. EMBO J. 1988;7:3643–3651. doi: 10.1002/j.1460-2075.1988.tb03245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leegood RC. The regulation of C4 photosynthesis. Adv Biochem Res. 1997;26:251–316. [Google Scholar]
- Long SP. Ecology of C4 photosynthesis: environmental responses. In: Sage RF, Monson RK, editors. C4 Plant Biology. San Diego: Academic Press; 1999. pp. 215–249. [Google Scholar]
- Ludwig M, von Caemmerer S, Price GD, Badger MR, Furbank RT. Expression of tobacco carbonic anhydrase in the C4 dicot Flaveria bidentis. Plant Physiol. 1998;117:1071–1082. doi: 10.1104/pp.117.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek LF, Stewart GR. Photorespiratory glycine metabolism in corn leaf discs. Plant Physiol. 1983;73:118–120. doi: 10.1104/pp.73.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroco JP, Ku MSB, Lea PJ, Dever LV, Leegood RC, Furbank RT, Edwards GE. Oxygen requirementand inhibition of C4 photosynthesis: an analysis of C4 plants deficient in the C3 and C4 cycles. Plant Physiol. 1998;116:823–832. doi: 10.1104/pp.116.2.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhuber W, Edwards GE. Temperature dependence of the linkage of quantum yield of photosystem II to CO2 fixation in C4 and C3 plants. Plant Physiol. 1993;101:507–512. doi: 10.1104/pp.101.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsugi R, Samejima M, Chonan N, Murata T. δ13C values and the occurrence of suberized lamellae in some Panicum species. Ann Bot. 1988;62:53–59. [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE. Determination of accurate extinction coefficients and simultaneous equations for assaying chl a and b extracted with four different solvents: verification of the concentration of chl standards by atomic absorption spectroscopy. Biochim Biophys Acta. 1989;975:384–394. [Google Scholar]
- Reeves DW, Rogers HH, Prior SA, Wood CW, Runion GB. Elevated atmospheric carbon-dioxide effects on sorghum and soybean nutrient status. J Plant Nutr. 1994;17:1939–1954. [Google Scholar]
- Reiskind JB, Madsen TV, van Ginkel LC, Bowes G. Evidence that inducible C4-type photosynthesis is a chloroplastic CO2-concentrating mechanism in Hydrilla, a submersed monocot. Plant Cell Environ. 1997;20:211–220. [Google Scholar]
- Roberts A, Borland AM, Griffiths H. Discrimination processes and shifts in carboxylation during the phases of crassulacean acid metabolism. Plant Physiol. 1997;113:1283–1292. doi: 10.1104/pp.113.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Pearcy RW. The nitrogen use efficiency of C3 and C4 plants: II. Leaf nitrogen effects on the gas exchange characteristics of Chenopodium album (L.) and Amaranthus retroflexus (L.) Plant Physiol. 1987;84:959–963. doi: 10.1104/pp.84.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Pearcy RW, Seemann JR. The nitrogen use efficiency of C3 and C4 plants: III. Leaf nitrogen effects on the activity of carboxylating enzymes in Chenopodium album (L.) and Amaranthus retroflexus (L.) Plant Physiol. 1987;85:335–359. doi: 10.1104/pp.85.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliendra NZ, Meinzer FC, Perry M, Thom M. Associations between partitioning of carboxylase activity and bundle sheath leakiness to CO2, carbon isotope discrimination, photosynthesis and growth in sugarcane. J Exp Bot. 1996;47:907–914. [Google Scholar]
- Ueno O. Structural characterization of photosynthetic cells in an amphibious sedge, Eleocharis vivipara, in relation to C3 and C4 metabolism. Planta. 1996a;199:382–393. [Google Scholar]
- Ueno O. Immunocytochemical localization of enzymes involved in the C3 and C4 pathways in the photosynthetic cells of an amphibious sedge Eleocharis vivipara. Planta. 1996b;199:394–403. [Google Scholar]
- Vogel JC. Sitzungsberichte der Heidelberger Akademie der Wissenschaften: Mathematische-naturwissenschaftliche Klasse. Berlin: Springer-Verlag; 1980. Fractionation of the carbon isotopes during photosynthesis; pp. 111–135. [Google Scholar]
- von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Furbank RT. Modeling C4 photosynthesis. In: Sage RF, Monson RK, editors. C4 Plant Biology. San Diego: Academic Press; 1999. pp. 173–211. [Google Scholar]
- von Caemmerer S, Millgate A, Farquhar GD, Furbank RT. Reduction of ribulose-1,5-bisphosphate carboxylase/oxygenase by antisense RNA in the C4 plant Flaveria bidentis leads to reduced assimilation rates and increased carbon isotope discrimination. Plant Physiol. 1997;113:469–477. doi: 10.1104/pp.113.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand SJE, Midgley GF, Jones MH, Curtis PS. Responses of wild C4 and C3 grass (Poaceae) species to elevated atmospheric CO2 concentration: a meta-analytic test of current theories and perceptions. Global Change Biol. 1999;5:723–741. [Google Scholar]
- Watling JR, Press MC. How is the relationship between the C4 cereal Sorghum bicolor and the C3 root hemi-parasites Striga hermonthica and Striga asiatica affected by elevated CO2? Plant Cell Environ. 1997;20:1292–1300. [Google Scholar]
- Watling JR, Press MC. How does the C4 grass Eragrostis pilosa respond to elevated carbon dioxide and infection with the parasitic angiosperm Striga hermonthica? New Phytol. 1998;140:667–675. doi: 10.1046/j.1469-8137.1998.00303.x. [DOI] [PubMed] [Google Scholar]
- Winter K. Crassulacean acid metabolism. In: Barber J, Baker NR, editors. Topics in Photosynthesis. 6: Photosynthetic Mechanisms and the Environment. Amsterdam: Elsevier Science Publishers; 1985. pp. 329–387. [Google Scholar]
- Wong SC, Osmond CB. Elevated atmospheric partial pressure of CO2 and plant growth: III. Interactions between Triticum aestivum (C3) and Echinochloa frumantacea (C4) during growth in mixed culture under different CO2, N nutrition and irradiance treatments, with emphasis on below-ground responses estimated using the δ13C value of root biomass. Aust J Plant Physiol. 1991;18:137–152. [Google Scholar]
- Zar JH. Biostatistical Analysis. NJ: Prentice Hall; 1984. [Google Scholar]