Abstract
Background
Fertilization as one of the measures in restoring degraded soil qualities has been introduced on arid steppes in recent decades. However, the fertilization use efficiency on arid steppes varies greatly between steppe types and years, enhancing uncertainties and risks in introducing fertilizations on such natural system to restore degraded steppes.
Methods
The experiment was a completely randomized design with five fertilization treatments, 0 (Control), 60 kg P ha−1 (P), 100 kg N ha−1 (N), 100 kg N ha−1 plus 60 kg P ha−1 (NP), and 4,000 kg sheep manure ha−1 (M, equaling 16.4 kg P ha−1 and 81.2 kg N ha−1). Soils were sampled from a degraded arid steppe which was consecutively applied with organic and inorganic fertilizers for three years. We analyzed the diversity and abundance of soil bacteria and fungi using high-throughput sequencing technique, measured the aboveground biomass, the soil chemical properties (organic carbon, available and total phosphorus, available and total nitrogen, and pH), and the microbial biomass nitrogen and microbial biomass carbon.
Results
In total 3,927 OTU (operational taxonomic units) for bacteria and 453 OTU for fungi were identified from the tested soils. The Ace and Chao of bacteria were all larger than 2,400, which were almost 10 times of those of fungi. Fertilizations had no significant influence on the richness and diversity of the bacteria and fungi. However, the abundance of individual bacterial or fungi phylum or species was sensitive to fertilizations. Fertilization, particularly the phosphorus fertilizer, influenced more on the abundance of the AMF species and colonization. Among the soil properties, soil pH was one of the most important soil properties influencing the abundance of soil bacteria and fungi.
Discussion
Positive relationships between the abundance of bacteria and fungi and the soil chemical properties suggested that soil bacteria and fungi communities in degraded steppes could be altered by improving the soil chemical properties through fertilizations. However, it is still not clear whether the alteration of the soil microbe community is detrimental or beneficial to the degraded arid steppes.
Keywords: Soil Degradation, Diversity, High-throughput sequencing, Arid steppe, Fertilization, Soil microbe
Introduction
Steppe is the largest land ecosystem in China, which plays important roles in providing life necessities, ecological services and recreations for human beings (Du et al., 2014; Ren, Lu & Fu, 2016). However, long-term drought, frequent human activities and overgrazing have caused severe steppe degradation such as decreases of soil qualities and productivities (Zhao et al., 2009; Bai et al., 2012). Fertilization as one of the measures in restoring degraded soil qualities has been introduced on steppes in recent decades, mainly concentrating on its efficiency in improving soil nutrient conditions (Gong et al., 2011), increasing aboveground biomass and vegetation coverage (Zhou et al., 2016; Yang, Ruijven & Du, 2011), and enriching ecological biodiversity (Symstad & Jonas, 2011). However, the fertilization use efficiencies in steppes varied greatly between steppe types and years (Su, Li & Yang, 2014; Zhou et al., 2016; Yang, Ruijven & Du, 2011). This increased the uncertainties in restoring the degraded steppes through fertilization and the risk in introducing more fertilization into such natural system.
Fertilizations influence both the aboveground biomass and the belowground microbe biomass. Soil microbial communities play critical roles in ecosystem function and regulate key processes such as carbon and nitrogen cycles (Balser & Firestone, 2005; Bragazza et al., 2015). In a Tibetan alpine meadow, nitrogen (N) and phosphorus (P) additions reduced the biomass of the whole microbial community, gram negative bacteria and fungi (Guo et al., 2017). In a subtropical forest in East China, soil microbial biomass was enhanced by mixed N fertilization (Guo et al., 2011). The different responses of the soil microbes to fertilizations, on one hand, are attributed to the differences in the contents of soil total carbon (C), N and P (Yu et al., 2013), and the differences of soil water conditions and plant species (Huang et al., 2018). On the other hand, the soil microbe communities in different ecosystems are different, and thus their responses to similar fertilizations might also be different.
During the long-term evolution process, the soil, plants and microbes have co-evolved, forming relatively stable relationships under certain ecosystem (terHorst, Lennon & Lau, 2014). In this relationship, genetic variation in plant phenotypes can change soil processes and biotic communities, whereas soil gradients and microbial communities can influence the expression and evolution of plant phenotypes (Van Nuland et al., 2016). When exterior N and P are input into local ecosystem, the given balance between soils, plants and microbes will be destroyed, and a new balance will be formed. For example, in a 50-year-old fertilization experiment, the composition of soil nitrate-reducing, denitrifying and total bacterial communities co-varied with primary production and both were strongly linked to soil properties (Hallin et al., 2009). In paddy soils, Bai et al. (2015) reported that anammox was more common and active in alkaline soils than in acidic soils, and the anammox activities were likely to be regulated by soil chemical properties such as pH, salinity and redox potential.
Soil microbes are mainly consisted of bacteria, fungi and Archaea. They are involved in catalyzing the transitions between compounds of N, P and C, benefiting the plant uptake and microbial propagation (Van Nuland et al., 2016; Zhang et al., 2017). In a paddy soil, the application of organic fertilizer increased the abundance of the copiotrophic bacteria such as Proteobacteria phylum, and the relative abundance of Agaricomycetes and Orbiliomycetes classes of fungi (Wang et al., 2017). In an arable soil, arbuscular mycorrhiza fungi (AMF) diversity and richness significantly decreased under long-term P fertilizations (Lin et al., 2012). In a temperate steppe in Inner Mongolia, N fertilization changed ammonia-oxidizing bacteria (AOB) community composition and increased AOB abundance in both May and August, but significantly decreased ammonia-oxidizing archaea (AOA) abundance in May (Chen et al., 2014). In a temperate desert, nitrogen fertilization increased the relative abundance of Ascomycota phylum and decreased Actinobacteria phylum in spring (Huang et al., 2018). Such changes in soil microbe communities induced by environmental changes might have detrimental effects on the relationship between soil microbes and the plants, and thus negatively influence the productivity. For example, the changes in the fungal to bacterial (F:B) ratio in the soil microbial biomass can lead to increases in total microbial community C:N and declines in biomass turnover rates, both of which can reduce microbial N uptake (Waring, Averill & Hawkes, 2013). The decrease of AMF diversity and abundance after fertilization will limit plant nutrient uptake under low soil phosphorus and drought conditions (Wu et al., 2011a).
The marker gene amplification metagenomics represent an important way to acquire information on the microbial communities present in complex environments like soil (Lombard et al., 2011), which allow to identify soil microbes that are unable to be cultured in medium. Marker gene amplification metagenomics, typically using the 16S and 18S ribosomal RNA, have been applied in studying the uncultured microbial populations in various environments (Handelsman, 2009; Oulas et al., 2015). Therefore, in the current study, using high-throughput sequencing technique, we analyzed the soil microbe communities including bacteria and fungi in soils of a degraded steppe which has been fertilized for consecutively three years. The responses of the microbial biomass nitrogen (MBN) and microbial biomass carbon (MBC) as well as the aboveground biomass and the soil chemical properties to fertilizations were also measured. The main objective of the current study was to clarify how the soil microbe communities respond to three years’ fertilization on a degraded arid steppe and their relationship with soil chemical properties and aboveground biomass.
Methods
Study site
The experimental site is located in Arigalangtu (43°50′23″N;116°09′57″E), 15 km away from Xilinhaote, Inner Mongolia, China. Average annual precipitation is 350 mm and average annual temperature is 1.7 °C during the last 50 years, whereas the precipitation in 2014, 2015 and 2016 were 255 mm, 412 mm and 309 mm, respectively. The vegetation is a typical steppe, located in an open high plain with altitude reaching approximately 1,290 m. Based on plant species investigation, Stipa krylovii is the dominant species, dry weight of which accounts for ca. 68%, followed by Allium bidentatum and Leymus chinensis, accounting for ca. 18%, with the remaining consisting of Convolvulus ammannii, Cleistogenes squarrosa, Salsola collina, Agropyron michnoi, and Carex korshinskyi, et al. The soil is mainly light Kastanozems (FAO soil classification). Soil texture averages 21% clay, 30% silt and 49% sand. This area has been continuously grazed by sheep and cattle for at least 20 years before 2014, showing severe deleterious symptoms, mainly low plant productivity and low plant coverage, typical in the most degraded steppes in Inner Mongolia (Pei, Fu & Wan, 2008; Schönbach et al., 2011).
In June 2014, about 50 km2 of this degraded steppe were fenced and excluded from grazing. Soils (0–20 cm) were sampled and analyzed for basic chemical properties. The concentrations of alkali dispersed N (AN), available phosphorus (AP, Olsen-P) and available potassium (AK) were 69.79, 3.32 and 133.07 mg kg−1, respectively. The concentrations of total nitrogen (TN), total phosphorus (TP), total potassium (TK), and organic carbon (OC) were 1.16, 0.34, 16.21, and 17.75 g kg−1. The soil pH was 7.75 (soil:water = 1:5).
Experimental design
The experiment was a completely randomized design with five fertilization treatments, 0 (Control), 60 kg P ha−1 (P), 100 kg N ha−1 (N), 100 kg N ha−1 plus 60 kg P ha−1 (NP), and 4,000 kg sheep manure ha−1 (M, equaling 16.4 kg P ha−1 and 81.2 kg N ha−1). The inorganic phosphorus fertilizer was superphosphate (14% P); the nitrogen fertilizer was urea (46% N); the sheep manure was collected from farmer’s feedlot and composted for 3 months (April to June), air dried, and sieved (<2 mm) before being applied to the field. Each treatment replicated three times with random complete block design. Each plot size reached 400 m2 (20 m ×20 m). Fertilizers were applied on steppe surface in rainy day for three times with once in each year, July 2014, June 2015 and June 2016. The steppes were cut for hay once a year at the end of growing season in late August and early September each year.
Aboveground biomass and root samples
Aboveground biomass was measured in each month during the growing season, June, July and August of 2016. In each of the sampling plot, plants were cut at the ground level from three quadrats (1 m × 1 m), dried at 75 °C for 48 h, and weighed. Meanwhile, fine roots from three plant species, Cleistogenes squarrosa, Stipa krylovii and Leymus chinensis, were sampled, cleaned, and stored in FAA (5 mL formalin (38% formaldehyde ) + 5 mL acetic acid + 90 mL alcohol (70%)) solution.
Collection of soil samples
After the aboveground biomass were harvested, soil samples were collected in June (before fertilization), July (one month after fertilization) and August (two month after fertilization) 2016. In total, nine soil cores (6 cm in diameter) from three quadrats in each plot were collected at 0–10 cm and 10–20 cm soil layers in each month, bulked into one composite soil sample for each plot from each soil layer. The soils were further divided into two parts, part one was air-dried, sieved to 2 mm for soil chemical property analysis; part two was stored in 4 °C for MBC and MBN analysis. For soil samples in July 2016, ca. 100 g of fresh soil per sample was stored at −80 °C for DNA extraction. July was the growing month that most plant species were in their vigorous growth stage.
Soil chemical analysis
Organic carbon (OC) was determined by oxidation with potassium dichromate in a concentrated sulfuric acid medium and the excess dichromate was measured using Mohr’s salt (K2Cr2O7–H2SO4) (Yeomans & Bremner, 1988). Dried soils (1.000 g) were digested in 5 mL H2SO4 and then determined for soil total nitrogen (TN) by Kjeldahl method (Bao, 2005). Total phosphorus (TP) was measured using NaOH fusion and Mo-Sb colorimetric procedure (Bao, 2005). Total potassium was measured using NaOH fusion and flame photometry (Bao, 2005). Dried soils (2.500 g) were mixed and shaken in 50 mL 0.5 mol L−1 NaHCO3 for 30 min, and then the supernatant was analyzed for available phosphorus (AP) using Mo-Sb colorimetric procedure (Bao, 2005). Dried soils (2.000 g) were digested in 10 mL 1 mol L−1 NaOH solution for 24 h and titrated with 0.01 mol L−1 1/2 H2SO4 for alkali dispelled nitrogen (AN) (Bao, 2005). Dried soils (5.000 g) were digested in 50 mL 1 mol L-1 NH4Ac solution for 15 min, and then the supernatant was analyzed for available potassium (AK) using flame photometer method (Bao, 2005). Soil pH value was determined in a soil:water solution (1:5) using a pH meter (Bao, 2005).
The soil microbial biomass carbon (MBC) and microbial biomass nitrogen (MBN) was measured by fumigation-extraction procedure (Brookes et al., 1985). Ten grams of fresh soil samples fumigated with chloroform and non-fumigated were extracted with 50 ml of 0.5 mol L−1 K2SO4 separately. Total carbon and nitrogen in the subsequent filtrates were measured by the methods used for soil samples as mentioned above.
A: extractable organic C in fumigated.
B: extractable organic C non-fumigated soil.
0.38: The conversion factor for microbial biomass carbon.
C: extractable organic N in fumigated.
D: extractable organic N non-fumigated soil.
0.45: The conversion factor for microbial biomass nitrogen.
Assessment of AMF colonization
Approximately 2 g of fine roots were cut into 1 cm pieces, cleared in 2% KOH (w/v) at 90 °C for 60 min and rinsed three times in water on a fine sieve. The root samples were acidified in 2% HCl (v/v) for 30 min and then stained in 0.05% (w/v) trypan blue in lactoglycerol for 30 min at 90 °C. Root segments of each species were selected randomly from the stained samples. Four replicates of 15 roots per slide were assessed for the presence or absence of AMF structures (arbuscules, vesicles and thick hyphae) using a stereomicroscope. AMF colonization was distinguished from non-mycorrhizal fungi as described by Callaway et al. (2003). Colonization was expressed as mycorrhizal frequency (F%), mycorrhizal intensity (M%) and arbuscular density (A%) according to the method of Trouvelot et al. (1986).
DNA extraction and PCR amplification
Microbial DNA was extracted from 30 samples using the E.Z.N.A.® Kit (Omega Bio-tek, Norcross, GA, US) according to manufacturer’s protocols. The V3-V4 region of the bacteria 16S ribosomal RNA gene were amplified by PCR (95 °C for 3 min , followed by 27/35 cycles at 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 45 s and a final extension at 72°C for 10 min, 10 °C until halted by user) using primers 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-ATGCAGGGACTA CHVGGGTWT CTAAT-3′). The fungal sequence of 18S rRNA genes was amplified using primers SSU0817F (5′-TTAGCATGGAATAATRRAATAGGA-3′) and SSU1196R (5′-TCTGGACCT GGTGAGTTTCC-3′). PCR reactions were performed in triplicate 20 µL mixture containing 4 µL of 5 × FastPfu Buffer, 2 µL of 2.5 mM dNTPs, 0.8 µL of each primer (5 µM), 0.4 µL of FastPfu Polymerase, and 10 ng of template DNA. Bacterial and fungal PCR products were pooled separately to be sequenced in their runs.
Illumina MiSeq sequencing
Amplicons were extracted from 2% agarose gels and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, US) according to the manufacturer’s instructions and quantified using QuantiFluor™-ST (Promega, Madison, WI, USA). Purified amplicons were pooled in equimolar and paired-end sequenced (2 × 300) on an Illumina MiSeq platform (Illumina, San Diego, CA, USA) according to the standard protocols. All the sequences of bacteria and fungi were deposited into the NCBI Sequence Read Archive database (SRP126759).
Statistical analysis
Raw fastq files were demultiplexed, quality-filtered by Trimmomatic and merged by FLASH with the following criteria: (i) the reads were truncated at any site receiving an average quality score <20 over a 50 bp sliding window. (ii) Primers were exactly matched allowing 2 nucleotide mismatching, and reads containing ambiguous bases were removed. (iii) Sequences whose overlap longer than 10 bp were merged according to their overlap sequence. Operational Taxonomic Units (OTUs) were clustered with 97% similarity cutoff using UPARSE (version 7.1 http://drive5.com/uparse/) and chimeric sequences were identified and removed using UCHIME. The OTU representative sequences were analyzed by RDP Classifier (http://rdp.cme.msu.edu/) against the silva (SSU123)16S rRNA database using confidence threshold of 70% (Amato et al., 2013), while the representative sequences of the fungi were analyzed using UNITE database (Abarenkov et al., 2010).
The alpha and beta diversity analyses were performed using the high quality sequences. To assess the diversity of the samples, we used the observed richness estimator Sobs, the coverage estimator Ace, the richness estimator Chao1, the Good’s coverage and the Shannon and Simpson diversity indices. To determine the effects of fertilization regimes on bacterial and fungal community compositions, permutational multivariate analysis was performed using the‘vegan’ package in R software (Team RC, 2014). The correlation analysis of the relative abundance of the bacterial and fungal taxa was held at a phylum level (abundance of the top 50 species) using the R package.
Statistical analyses were performed with SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). Results were expressed as mean ± standard error (SE). Nonparametric variables were mathematically transformed to improve symmetry. The correlation between relative abundance of certain OTU/genus/phylum and environmental factors was performed by Spearman’s correlation analyses. P < 0.05 was considered to be statistically significant according to least significant difference test. The Glomeromycota sequences were aligned and tested using the best-fit model with MEGA 4.0 (Tamura et al., 2007). For the phylogenetic analysis, the maximum likelihood method (Nei & Kumar, 2000) was used to construct the tree with MEGA 4.0 using DNA sequences selected according to their sequence similarity to the reference data in GenBank.
Results
Soil chemical properties and aboveground biomass
The contents of soil OC and TN showed no significant difference between fertilization treatments at 0–10 cm or 10–20 cm and in three sampling months (Table 1). The ratios of C/N ranged from 6.61 to 13.84 and their responses to fertilization differed between sampling months and soil layers. At 0–10 cm soil layer, C/N ratio in non-fertilized plots was higher than those in fertilized plot in June, lower than those in P and N fertilized plots in July, and unchanged in August. The contents of AN changed insignificantly after fertilization and varied slightly between months, with the AN contents at 0–10 cm soil layer higher than those at 10–20 cm soil layer. The contents of AP were lower than 5 mg kg−1 in non-fertilized plots in all sampling months, which increased after phosphorus or manure fertilization. The AP contents at 0–10 cm soil layer were higher than those at 10–20 cm soil layer. The soil pH level ranged from 7.37 to 7.85 at 0–10 cm soil layer and from 7.71 to 8.19 at 10–20 cm soil layer. Overall, though fertilizations had a trend in increasing the aboveground biomass in all sampling months, no significant difference could be observed between fertilized and non-fertilized plots (Table 2).
Table 1. Effects of fertilizations on the soil chemical properties.
| De | Tr | OC g kg−1 | TN g kg−1 | C/N | AN mg kg−1 | AP mg kg−1 | pH |
|---|---|---|---|---|---|---|---|
| June 2016 | |||||||
| 0–10 cm | CK | 10.09 ± 0.23a | 0.87 ± 0.02a | 11.57 ± 0.05a | 98.70 ± 1.21a | 3.58 ± 1.31c | 7.83 ± 0.11a |
| P | 9.69 ± 0.23a | 0.98 ± 0.03a | 9.92 ± 0.36b | 100.80 ± 5.28a | 14.43 ± 0.51a | 7.75 ± 0.09a | |
| N | 10.85 ± 0.68a | 1.07 ± 0.02a | 10.13 ± 0.57b | 103.60 ± 2.80a | 6.33 ± 2.35bc | 7.58 ± 0.16a | |
| NP | 9.96 ± 0.13a | 1.16 ± 0.01a | 8.59 ± 0.13c | 107.10 ± 3.21a | 13.91 ± 0.55a | 7.75 ± 0.09a | |
| M | 10.80 ± 0.29a | 1.12 ± 0.02a | 9.67 ± 0.17b | 101.50 ± 12.38a | 10.48 ± 3.27ab | 7.82 ± 0.06a | |
| 10–20 cm | CK | 8.34 ± 0.59a | 0.88 ± 0.06a | 9.56 ± 0.56ab | 79.80 ± 4.85a | 1.83 ± 0.80b | 7.99 ± 0.09a |
| P | 7.77 ± 0.29a | 0.91 ± 0.05a | 8.53 ± 0.17bc | 83.13 ± 3.20a | 6.25 ± 1.09a | 7.71 ± 0.12a | |
| N | 8.38 ± 0.09a | 0.94 ± 0.03a | 8.97 ± 0.36bc | 72.80 ± 1.40a | 4.17 ± 0.36ab | 7.82 ± 0.09a | |
| NP | 7.67 ± 0.01a | 0.92 ± 0.01a | 8.33 ± 0.11c | 86.63 ± 6.26a | 2.83 ± 0.36ab | 8.02 ± 0.12a | |
| M | 9.52 ± 0.12a | 0.91 ± 0.02a | 10.45 ± 0.38a | 72.80 ± 1.40a | 3.67 ± 2.10ab | 8.04 ± 0.08a | |
| July 2016 | |||||||
| 0–10 cm | CK | 10.51 ± 0.40a | 1.05 ± 0.02a | 10.00 ± 0.47c | 88.90 ± 9.42a | 2.92 ± 0.10c | 7.85 ± 0.19a |
| P | 12.13 ± 0.47a | 0.88 ± 0.001a | 13.84 ± 0.55a | 97.30 ± 3.90a | 19.44 ± 1.83a | 7.75 ± 0.14a | |
| N | 12.44 ± 0.18a | 1.03 ± 0.05a | 12.15 ± 0.64b | 98.70 ± 9.15a | 3.79 ± 0.83c | 7.37 ± 0.15a | |
| NP | 12.13 ± 0.83a | 1.18 ± 0.04a | 10.28 ± 0.61c | 109.90 ± 5.47a | 11.25 ± 2.66b | 7.59 ± 0.16a | |
| M | 10.83 ± 0.18a | 1.19 ± 0.05a | 9.12 ± 0.21c | 93.10 ± 8.94a | 4.23 ± 1.51c | 7.63 ± 0.15a | |
| 10–20 cm | CK | 9.46 ± 0.24a | 0.88 ± 0.02a | 10.71 ± 0.12a | 72.10 ± 2.80a | 2.52 ± 0.12b | 8.05 ± 0.12a |
| P | 9.60 ± 0.14a | 0.85 ± 0.03a | 11.32 ± 0.47a | 74.90 ± 9.10a | 4.16 ± 0.38a | 8.19 ± 0.16a | |
| N | 9.77 ± 0.65a | 0.91 ± 0.01a | 10.74 ± 0.78a | 77.70 ± 9.92a | 2.15 ± 0.34b | 7.86 ± 0.03a | |
| NP | 8.53 ± 0.42a | 1.00 ± 0.03a | 8.55 ± 0.29b | 89.60 ± 6.22a | 2.75 ± 0.66b | 8.04 ± 0.09a | |
| M | 10.38 ± 0.65a | 0.99 ± 0.06a | 10.51 ± 0.31a | 90.3 ± 10.57a | 1.98 ± 0.19b | 8.00 ± 0.07a | |
| August 2016 | |||||||
| 0–10 cm | CK | 9.25 ± 0.05a | 1.26 ± 0.03a | 7.37 ± 0.18a | 92.17 ± 6.27a | 3.38 ± 0.57c | 7.78 ± 0.12a |
| P | 11.32 ± 0.08a | 1.23 ± 0.01a | 9.23 ± 0.14a | 94.73 ± 3.06a | 15.17 ± 3.47a | 7.64 ± 0.10a | |
| N | 10.29 ± 0.87a | 1.42 ± 0.12a | 7.28 ± 0.41a | 106.6 ± 15.16a | 3.22 ± 0.50c | 7.45 ± 0.12b | |
| NP | 9.00 ± 0.44a | 1.36 ± 0.01a | 6.61 ± 0.35a | 107.10 ± 8.17a | 11.85 ± 2.49ab | 7.44 ± 0.06b | |
| M | 10.15 ± 0.00a | 1.23 ± 0.04a | 8.29 ± 0.24a | 92.26 ± 2.03a | 8.17 ± 2.23bc | 7.49 ± 0.03a | |
| 10–20 cm | CK | 9.50 ± 0.16a | 1.09 ± 0.003a | 8.75 ± 0.12b | 80.73 ± 3.93a | 2.31 ± 0.30c | 8.07 ± 0.15a |
| P | 10.66 ± 0.21a | 1.02 ± 0.01a | 10.43 ± 0.15b | 77.93 ± 2.63a | 5.32 ± 1.64ab | 8.00 ± 0.08a | |
| N | 8.78 ± 0.32a | 1.15 ± 0.07a | 6.88 ± 0.21b | 74.20 ± 10.01a | 2.28 ± 0.27c | 8.04 ± 0.28a | |
| NP | 7.91 ± 0.13a | 1.12 ± 0.03a | 7.06 ± 0.27b | 72.80 ± 1.85a | 7.35 ± 1.02a | 7.92 ± 0.03a | |
| M | 9.78 ± 0.02a | 1.00 ± 0.05a | 9.82 ± 0.50a | 78.26 ± 7.40a | 3.62 ± 0.18bc | 7.76 ± 0.01a | |
Notes.
Different lowercase letters after the data within each soil layer represented significance at P < 0.05 (l.s.d).
De, depth; Tr, treatment; CK, Control; P, 60 kg P ha−1; N, 100 kg N ha−1; NP, 60 kg P ha−1 plus 100 kg N ha−1; M, 4,000 kg sheep manure ha−1. OC, organic C; TN, total N; AN, alkali dispelled N; AP, available P. Values were the means of three replicates ± SE.
Table 2. Effects of fertilizations on total aboveground biomass (kg DM ha−1).
| June | July | August | |
|---|---|---|---|
| CK | 702.9 ± 7.42a | 863.4 ± 106.9a | 897.4 ± 21.1a |
| P | 705.3 ± 53.69a | 1,087.7 ± 108.9a | 1,095.0 ± 23.8a |
| N | 692.0 ± 65.0a | 1,062.5 ± 94.4a | 937.0 ± 21.8a |
| NP | 755.1 ± 36.1a | 1,131.0 ± 46.1a | 1,089.2 ± 9.5a |
| M | 747.3 ± 79.5a | 1,013.9 ± 81.8a | 856.6 ± 122.2a |
Notes.
Different lowercase letter after the data in each month represented significance at P < 0.05 level (l.s.d).
CK, Control; P, 60 kg P ha−1; N, 100 kg N ha−1; NP, 60 kg P ha−1 plus 100 kg N ha−1; M, 4,000 kg sheep manure ha−1.
Soil microbial biomass carbon and nitrogen
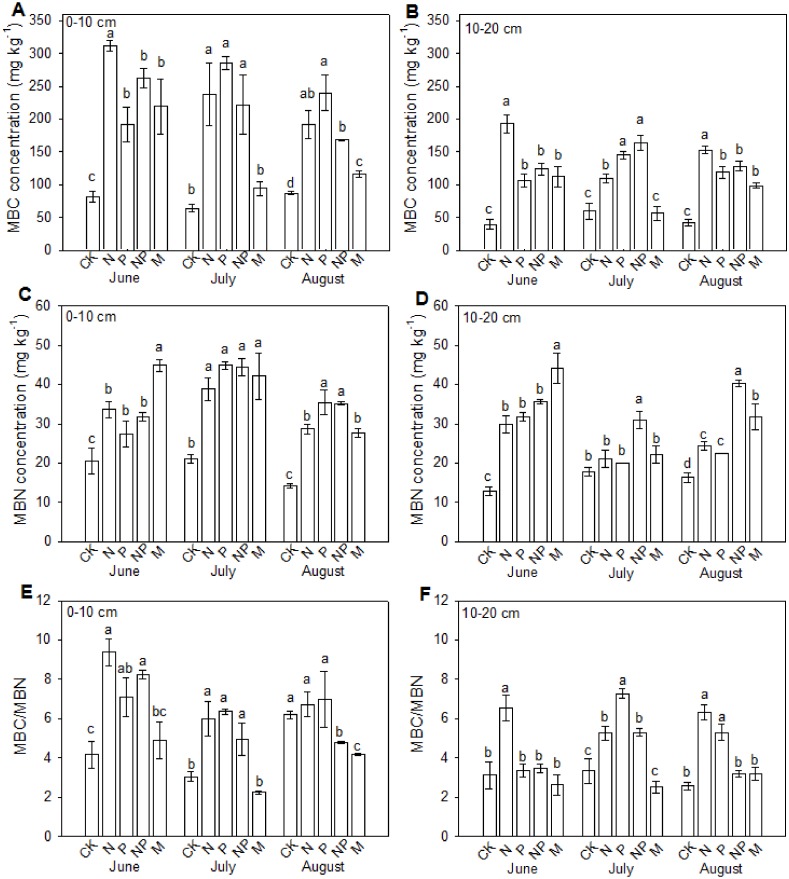
The influences of fertilizations on MBC and MBN varied between treatments and soil layers (Fig. 1). Overall, the MBC and MBN at 0–10 cm soil layer were higher than those at 10–20 cm soil layer. Phosphorus, nitrogen and manure fertilizations increased the MBC and MBN contents in all sampling months, excepting for insignificant changes of MBC in manure fertilized plot in July at both soil layers and insignificant changes of MBN in all fertilizations in July at 10–20 cm soil layer excepting NP treatment. In June, the MBC in N, NP and manure fertilized plots showed no significant differences, which were significantly lower than those in P fertilized plots at both soil layers. In July, the MBC in manure fertilized plots was significantly lower than those in P, N and NP fertilized plots at both soil layers. In August, the MBC in N and P fertilized plots was the highest and that in manure plots was the lowest at 0–10 cm soil layer, whereas the MBC in P fertilized plot was significantly higher than those in other treatments at 10–20 cm soil layer. The MBN was the highest in manure fertilized plots at both soil layers in June, showed no difference between treatments in July excepting for higher MBN in NP plot at 10–20 cm soil layer, and was the highest in NP plot at both soil layers in August. The ratio between MBC and MBN was all larger than 2. At 0–10 cm soil layer, the ratio increased in fertilized plots excepting for manure fertilized plots in all sampling months and NP fertilized plot in August. At 10–20 cm soil layer, the ratio increased only in P plots in June, in P, N and NP plots in July, and in P and N plots in August.
Figure 1. Effects of continuous fertilization on soil microbial biomass C and microbial biomass N at 0–10 cm (A, C, E) and 10–20 cm (B, D, F) soil layers.
CK, Control; P, 60 kg P ha−1; N, 100 kg N ha−1; NP, 60 kg P ha−1 plus 100 kg N ha−1; M, 4,000 kg sheep manure ha−1. Data were the average of three replicates (±SE). Different lowercase letters above the data bar represented significance at P < 0.05 level (l.s.d).
Biodiversity and abundance of bacterial and fungi
In this study, 16S rRNA genes were used as phylogenetic makers to determine changes in bacterial and fungi community structure. In total 1,120,431 bacterial sequences and 1,129,387 fungi sequences were obtained from 30 soil samples, which were further clustered into 3,927 OTU for bacteria and 453 OTU for fungi, according to 97% similarities. Based on these OTUs, the Richness estimator, Ace and Chao, and diversity index, Shannon and Simpson, were further analyzed (Table 3). Overall, the Ace and Chao of bacteria were all larger than 2,400, which were almost 10 times of those of fungi. No significant differences of richness estimators could be observed for bacteria and fungi at both soil layers. The Shannon index of bacteria ranged from 6.16 to 6.49 for bacteria, significantly higher than those for fungi which were all lower than 3.5. The Simpson index of fungi ranged from 5.93 to 9.05, significantly higher than those of bacteria which were all lower than 0.8. Bacteria had higher Shannon index and lower Simpson index at 0–10 cm soil layer than those at 10–20 cm soil layer, whereas no obvious difference could be observed for fungi.
Table 3. Diversity indices of soil bacterial and fungi communities in degraded arid steppes as influenced by fertilizations.
| Treatment | Richness estimator | Diversity index | |||||
|---|---|---|---|---|---|---|---|
| Ace | Chao | Shannon | Simpson (%) | Coverage (%) | |||
| 0–10 cm | Bacterial | CK | 2,752.09 ± 31.90a | 2,732.96 ± 44.32a | 6.49 ± 0.01a | 0.45 ± 0.01a | 96.97 |
| P | 2,695.87 ± 76.79a | 2,711.19 ± 82.01a | 6.42 ± 0.04a | 0.48 ± 0.06a | 96.96 | ||
| N | 2,654.69 ± 35.76a | 2,691.37 ± 33.05a | 6.36 ± 0.06a | 0.52 ± 0.09a | 97.02 | ||
| NP | 2,626.71 ± 81.73a | 2,635.74 ± 81.80a | 6.42 ± 0.11a | 0.44 ± 0.02a | 97.08 | ||
| M | 2,629.60 ± 29.36a | 2,612.58 ± 21.75a | 6.41 ± 0.05a | 0.46 ± 0.08a | 97.07 | ||
| Fungi | CK | 182.89 ± 3.20a | 183.40 ± 2.76a | 3.49 ± 0.06a | 5.93 ± 0.81a | 99.91 | |
| P | 164.53 ± 12.04a | 167.97 ± 15.55a | 3.36 ± 0.12a | 6.19 ± 0.98a | 99.91 | ||
| N | 185.92 ± 8.21a | 187.41 ± 8.85a | 3.32 ± 0.13a | 7.38 ± 1.92a | 99.9 | ||
| NP | 173.17 ± 8.04a | 171.69 ± 6.77a | 3.37 ± 0.05a | 6.34 ± 0.74a | 99.92 | ||
| M | 198.31 ± 6.25a | 196.54 ± 1.24a | 3.24 ± 0.31a | 9.05 ± 4.44a | 99.89 | ||
| 10–20 cm | Bacterial | CK | 2,547.23 ± 47.37a | 2,566.96 ± 65.75a | 6.21 ± 0.03a | 0.65 ± 0.06a | 97.09 |
| P | 2,495.59 ± 88.77a | 2,505.43 ± 104.85a | 6.22 ± 0.06a | 0.73 ± 0.10a | 97.22 | ||
| N | 2,508.50 ± 57.66a | 2,515.83 ± 35.21a | 6.16 ± 0.11a | 0.71 ± 0.15a | 97.08 | ||
| NP | 2,644.51 ± 2.29a | 2,649.42 ± 28.10a | 6.29 ± 0.05a | 0.57 ± 0.05a | 96.93 | ||
| M | 2,644.29 ± 64.92a | 2,640.09 ± 78.60a | 6.30 ± 0.09a | 0.56 ± 0.08a | 96.97 | ||
| Fungi | CK | 158.80 ± 10.50a | 161.38 ± 9.69a | 3.22 ± 0.04a | 7.13 ± 0.41a | 99.92 | |
| P | 149.36 ± 5.74a | 150.86 ± 7.12a | 3.38 ± 0.06a | 6.24 ± 0.54 | 99.95 | ||
| N | 167.98 ± 7.79a | 167.94 ± 7.30a | 3.33 ± 0.09a | 6.58 ± 1.05a | 99.93 | ||
| NP | 167.16 ± 9.63a | 165.80 ± 9.62a | 3.29 ± 0.04a | 7.14 ± 0.21a | 99.93 | ||
| M | 178.72 ± 11.88a | 176.12 ± 9.69a | 3.20 ± 0.12a | 8.16 ± 1.58a | 99.91 | ||
Notes.
Different lowercase letter after the data in each soil layer represented significance at P < 0.05 level (l.s.d).
CK, Control; P, 60 kg P ha−1; N, 100 kg N ha−1; NP, 60 kg P ha−1 plus 100 kg N ha−1; M, 4,000 kg sheep manure ha−1.
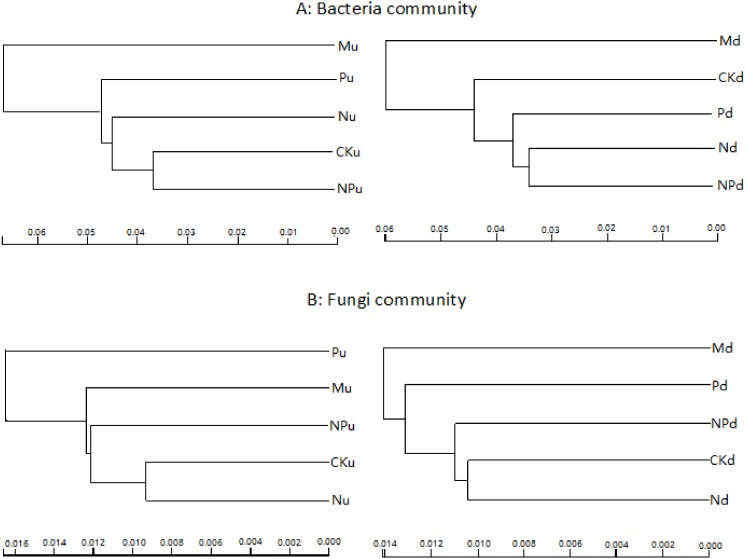
Cluster analysis based on sequencing data of bacteria and fungi indicated that manure fertilization had the greatest influence on soil microbe communities (Fig. 2). The soil bacterial sequences in manure fertilized plots were separated with those in the other treatments at both soil layers. For fungi, sequences in phosphorus fertilized plot were separated with other treatments at 0–10 cm soil layer, while those in manure fertilized plots were separated with other treatments at 10–20 cm soil layer.
Figure 2. Overall structural changes of bacterial communities (A) and fungi communities (B) from sequencing data as influenced by fertilizations.
The clustering was based on the weighted distance tree in Unifrac. CK, Control; P, 60 kg P ha−1; N, 100 kg N ha−1; NP, 60 kg P ha−1 plus 100 kg N ha−1; M, 4,000 kg sheep manure ha−1. Lowercase letter “u” after treatment represented samples from 0–10 cm soil layer; “d” represented samples from 10–20 cm soil layer.
Communities of soil bacteria and fungi
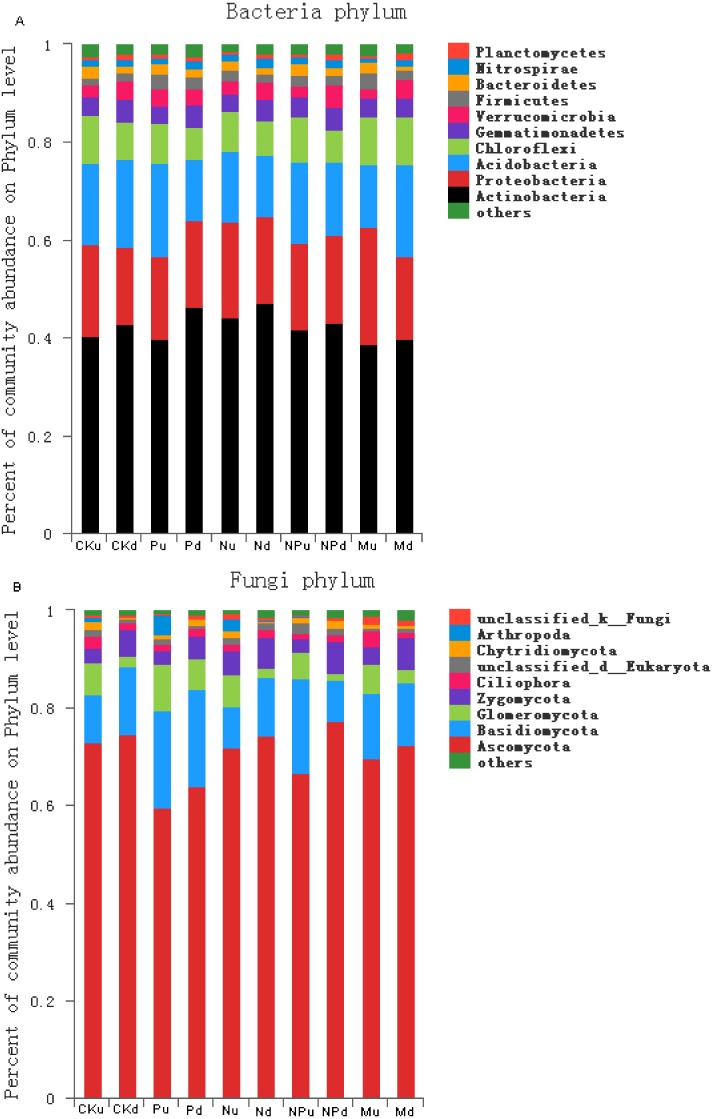
In the tested soils, the dominant bacteria species with abundance larger than 1% included Actinobacteria (42.21%), Proteobacteria (18.44%), Acidobacteria (15.71%), Chloroflexi (8.27%), Gemmatimonadetes (4.06%), Verrucomicrobia (3.18), Firmicutes (2.21%), Bacteroidetes (1.79%), and Nitrospirae (1.34%) (Fig. 3A, Fig. S1). At 0–10 cm soil layer, manure fertilization had a trend in reducing the abundance of Nitrospirae (P < 0.05) and increasing the abundance of Proteobacteria (P < 0.05) (Table S1). At 10–20 cm soil layer, the abundance of Chloroflexi in manure fertilized plot was significantly higher than those in non-fertilized, P, N and NP fertilized plots.
Figure 3. Proportions of the bacterial phyla (A) and fungal phyla (B) with the relative abundance higher than 1%.
CK, Control; P, 60 kg P ha−1; N, 100 kg N ha−1; NP, 60 kg P ha−1 plus 100 kg N ha−1; M, 4,000 kg sheep manure ha−1. Lowercase letter “u” after treatment represented samples from 0–10 cm soil layer; “d” represented samples from 10–20 cm soil layer.
The dominant fungi species with abundance larger than 1% included Ascomycota (71.26%), Basidiomycota (13.50%), Glomeromycota (4.67%), Zygomycota (4.56%), Ciliophora (1.60%), Chytridiomycota (1.25%) (Fig. 3B, Fig. S2). At 0–10 cm soil layer, the abundance of Ciliophora was the highest in manure fertilized plot (P < 0.05) (Table S2). At 10–20 cm soil layer, the abundance of Chytridiomycota increased in P and NP treatments when compared with the non-fertilized plot, whereas unchanged in N and M treatments.
AMF colonization and diversity
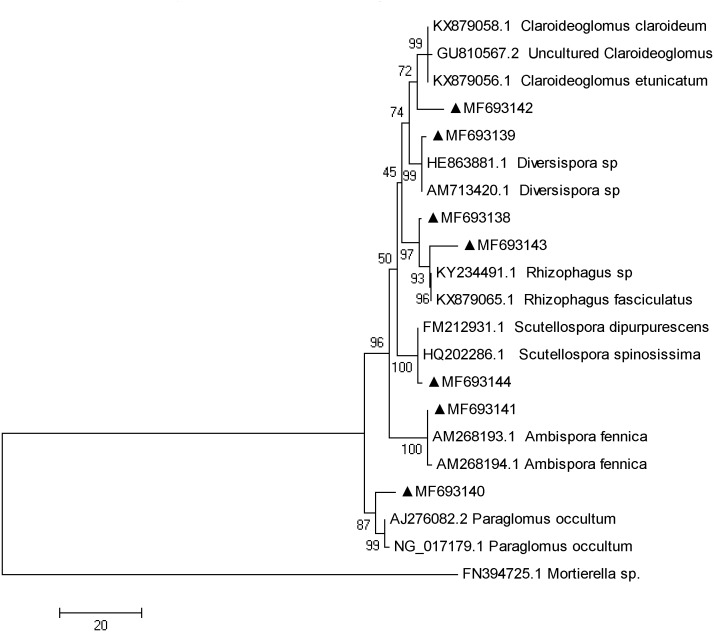
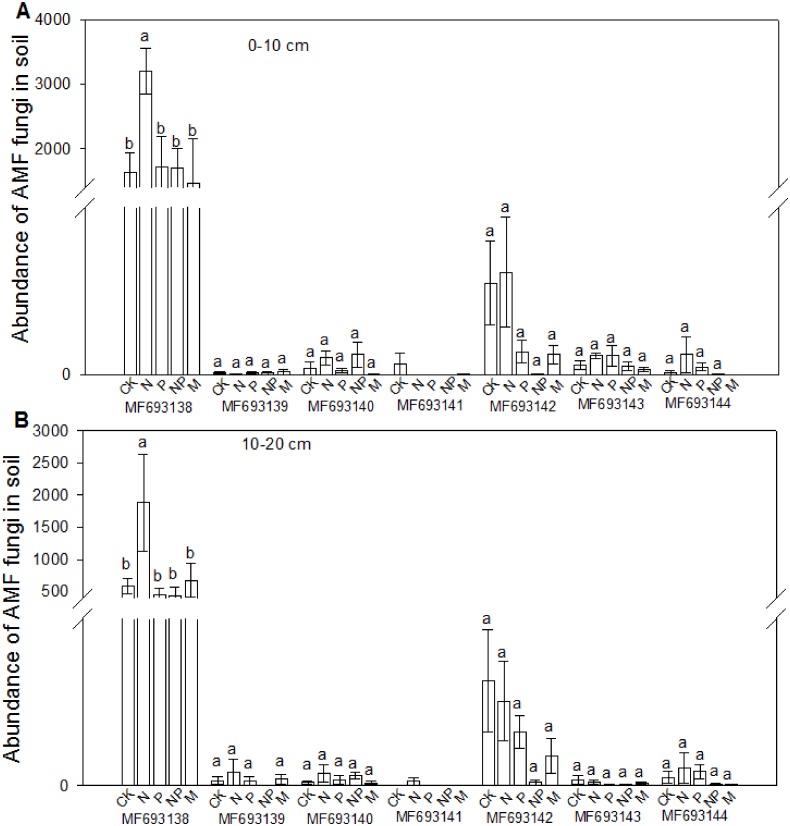
The fungi sequences were further blasted in NCBI and seven sequences matched with the published AMF sequences (Fig. 4). Cluster analysis separated these sequences into two groups, with MF693140 clustered with paraglomus occultum in one group, and the other six sequences in another group. MF693141 was clustered with Ambispora fennica; MF693144 was clustered with Scutellospora species; MF693143 and MF693138 were clustered with Rhizophagus species; MF693139 was clustered with Diversispora species; MF693142 was clustered with Claroideglomus species. The abundance of MF693138 was the highest (Fig. 5). P fertilization significantly increased the abundance of MF693138, whereas N, NP or manure fertilization showed no significant difference when compared with the non-fertilized plot at both soil layers. The abundance of other AMF sequences was low and showed no variation between treatments.
Figure 4. Phylogenetic tree inferred from the fungi sequences of AMF.
Numbers above branches indicate the bootstrap support (1,000 replicates). Cluster was conducted according to the maximum likelihood method. GenBank accession numbers followed by a black triangle (▴) represent the sequences obtained in the present study. Names followed by codes represent the sequences downloaded from GenBank.
Figure 5. Effects of fertilizations on the abundance of AMF in soils at 0–10 cm (A) and 10–20 cm (B).
CK, Control; P, 60 kg P ha−1; N, 100 kg N ha−1; NP, 60 kg P ha−1 plus 100 kg N ha−1; M, 4,000 kg sheep manure ha−1. Different lowercase letters above the data bar represented significance at P < 0.05 level (l.s.d).
AMF colonization was measured in three plant species, C. squarrosa, S. krylovii and L. chinensis (Table 4). The colonization (F%), colonization intensity (M%) and arbuscular (A%) varied among sampling months and plant species. Fertilization significantly influenced AMF colonization, however, no similar trend could be observed in different sampling months and plant species.
Table 4. Effects of fertilization on AMF colonization of Cleistogenes squarrosa, Stipa krylovii and Leymus chinensis in different growing months.
| Month | Treatment | F% | M% | A% | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cleistogenes squarrosa | Stipa krylovii | Leymus chinensis | Cleistogenes squarrosa | Stipa krylovii | Leymus chinensis | Cleistogenes squarrosa | Stipa krylovii | Leymus chinensis | ||
| June | CK | 82.67 ± 2.40b | 92.00 ± 3.06a | 90.00 ± 2.31b | 7.59 ± 1.55b | 13.86 ± 5.30a | 12.62 ± 5.20b | 0.52 ± 0.08c | 2.16 ± 0.16b | 0.66 ± 0.36c |
| P | 82.67 ± 2.40b | 91.33 ± 3.53a | 93.33 ± 3.53ab | 11.33 ± 2.26ab | 13.01 ± 2.05a | 13.37 ± 1.65b | 2.79 ± 0.37bc | 3.56 ± 1.15ab | 3.62 ± 0.58bc | |
| N | 88.67 ± 2.67ab | 95.33 ± 1.33a | 100.00 ± 0a | 17.03 ± 0.28a | 21.73 ± 4.42a | 20.00 ± 4.09ab | 5.41 ± 1.09a | 5.45 ± 1.62a | 4.70 ± 1.56b | |
| NP | 92.00 ± 1.15a | 90.00 ± 5.03a | 98.00 ± 1.15a | 16.34 ± 4.99ab | 8.15 ± 2.01a | 18.25 ± 2.21ab | 3.73 ± 1.14ab | 0.61 ± 0.06b | 5.25 ± 0.56b | |
| M | 83.33 ± 0.67b | 93.33 ± 1.33a | 94.67 ± 1.76ab | 10.93 ± 2.30ab | 13.21 ± 4.84a | 29.23 ± 4.82a | 0.78 ± 0.08c | 1.21 ± 0.32b | 11.33 ± 1.89a | |
| July | CK | 90.67 ± 0.67c | 87.33 ± 0.67c | 100.00 ± 0a | 18.65 ± 1.20b | 7.13 ± 0.50c | 13.09 ± 0.05d | 14.06 ± 0.56b | 0.87 ± 0.05c | 5.54 ± 0.16d |
| P | 96.67 ± 0.67b | 100.00 ± 0a | 100.00 ± 0a | 26.41 ± 0.54a | 43.25 ± 0.95b | 22.11 ± 1.26c | 18.14 ± 1.30a | 23.05 ± 0.73ab | 14.23 ± 0.18b | |
| N | 100.00 ± 0a | 98.67 ± 1.33a | 98.00 ± 1.15a | 17.16 ± 2.13b | 34.79 ± 8.91b | 34.75 ± 1.02a | 5.67 ± 0.10c | 17.36 ± 3.60b | 19.82 ± 0.14a | |
| NP | 82.67 ± 0.67e | 92.00 ± 1.15b | 90.67 ± 0.67b | 8.47 ± 0.30c | 13.11 ± 0.50c | 13.91 ± 0.14d | 3.06 ± 0.11d | 2.04 ± 0.25c | 7.00 ± 0.12c | |
| M | 88.67 ± 0.67d | 100.00 ± 0a | 98.67 ± 1.33a | 3.43 ± 0.65d | 58.17 ± 0.44a | 29.80 ± 1.04b | 0.88 ± 0.20e | 27.40 ± 3.25a | 14.53 ± 0.19b | |
| August | CK | 91.67 ± 0.88b | 87.33 ± 0.67d | 98.67 ± 1.33a | 22.63 ± 2.17a | 20.95 ± 3.37b | 26.73 ± 1.22b | 2.94 ± 0.58b | 3.68 ± 1.50c | 14.51 ± 0.25a |
| P | 96.67 ± 0.67a | 95.33 ± 0.67b | 98.00 ± 2.00a | 22.47 ± 0.94a | 36.41 ± 4.42a | 30.37 ± 0.33a | 16.27 ± 0.89a | 18.41 ± 1.63b | 12.00 ± 0.45b | |
| N | 88.67 ± 0.67c | 95.33 ± 0.67b | 98.67 ± 1.33a | 9.91 ± 0.54b | 25.68 ± 0.55b | 8.43 ± 1.26d | 2.91 ± 0.12b | 3.17 ± 0.55c | 6.41 ± 0.27d | |
| NP | 80.67 ± 0.67d | 91.33 ± 0.67c | 97.33 ± 1.33a | 7.67 ± 0.59b | 23.31 ± 0.34b | 19.29 ± 0.13c | 1.22 ± 0.09c | 4.38 ± 0.55c | 10.82 ± 0.07bc | |
| M | 91.33 ± 0.67b | 100.00 ± 0a | 96.00 ± 2.31a | 24.97 ± 0.52a | 40.53 ± 0.63a | 22.14 ± 0.95c | 3.73 ± 0.27b | 24.76 ± 0.93a | 9.97 ± 0.76c |
Notes.
F%, colonization; M%, colonization intensity; A%, arbuscular. Different lowercase letter after the data in each soil layer represented significance at P < 0.05 level (l.s.d). CK, Control; P, 60 kg P ha−1; N, 100 kg N ha−1; NP, 60 kg P ha−1 plus 100 kg N ha−1; M, 4,000 kg sheep manure ha−1.
Relationship between soil chemical properties and microbial community
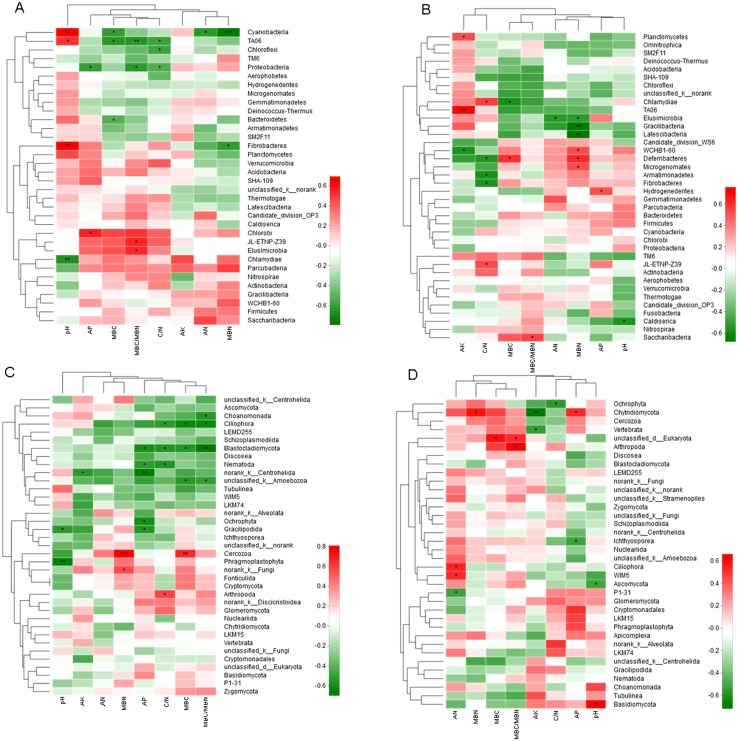
Correlation Heatmap indicated that soil chemical properties significantly influenced the abundance of bacteria and fungi (Fig. 6). At 0–10 cm soil layer, cluster analysis separated soil pH with other environmental factors affecting the microbe abundance. The soil pH was positively correlated with the abundance of three phyla and negatively correlated with one phylum. The ratio of MBC/MBN was positively correlated with the abundance of two phyla and negatively correlated with two phyla. Both MBC and soil C/N were negatively correlated with three phyla. AN and MBN were further separated with other environmental factors. At 10–20 cm soil layer, MBN influenced more on the bacterial abundance, which was positively correlated with three phyla and negatively correlated with three phyla. C/N was positively correlated with two phyla and negatively correlated with three phyla. MBC/MBN, AN, AP and pH, were all correlated with one phylum, positively or negatively.
Figure 6. Spearman Correlation Heatmap between bacterial and soil properties at 0–10 cm (A) and 10–20 cm (B) and fungi and soil properties at 0–10 cm (C) and 10–20 cm (D).
AN, alkaline hydrolyzed N; AP, available P; AK, available potassium; MBC, microbial biomass C; MBN, microbial biomass N. The R values are displayed in different colors and the right color card of the heat map is a color partition of different R values. 0.01 < P ≤ 0.05∗ 0.001 < P ≤ 0.01∗∗ P ≤ 0.001∗∗∗. CK, Control; P, 60 kg P ha−1; N, 100 kg N ha−1; NP, 60 kg P ha−1 plus 100 kg N ha−1; M, 4,000 kg sheep manure ha−1. Lowercase letter “u” after treatment represented samples from 0–10 cm soil layer; “d” represented samples from 10–20 cm soil layer.
At 0–10 cm soil layer, factors affecting the abundance of fungi were separated into two groups, pH and other factors. Most of the soil chemical properties negatively influenced the fungi abundance, with the positive correlation was only observed with MBN (two phyla), C/N (one phylum), and MBC (one phylum). There were five and four fungi phyla were negatively correlated with AP and MBC/MBN, respectively. AN had no significant influence on any fungi phylum. At 10–20 cm, factors affecting the abundance of fungi were separated into two groups, with AN, MBN, MBC and MBC/MBN in one group and the others in second group. Factors in the first group were all positively correlated with fungi abundance excepting for a negative correlation with AN. Factors in the second group were mainly negatively correlated with the abundance of fungi excepting for a positive correlation with AP and pH.
Discussion
Fertilizations have been applied in many ecosystems to improve crop production or soil qualities (Mitran et al., 2016; Paredes et al., 2011). In the tested arid steppes, though the contents of soil AP increased significantly after P, NP or manure fertilizations after three years consecutive fertilizations, the aboveground biomass of the tested steppes was not improved, suggesting that sole fertilization in such ecosystem is not efficient in improving steppe productivity. This, on one hand, might be attributed to the low rainfall during growing season (142 mm from May to July), which limited the fertilizer efficiency (Bai et al., 2007; Fang et al., 2005), and thus the plant growth (Austin, 2002; Jobbagy, Sala & Paruelo, 2002). In a degraded steppe in northern China, Chen, Hooper & Lin (2011) also reported that nitrogen fertilization might only be effective in increasing rangeland production in wet years. On the other hand, fertilization might alter other properties of this ecosystem, such as soil microbe activities, which thus offset the positive effect of the fertilizations (Kautz, Wirth & Ellmer, 2004; Plaza et al., 2004).
During the long-term evolution, the soil microbe and the plant species have adapted to their growing environments, and their adaptation is relatively stable under certain conditions (Lau & Lennon, 2011). In differently managed Belgian grasslands, Denef et al. (2009) reported that soil habitats significantly differed in microbial community structure as well as in gram-positive bacterial rhizodeposit-C uptake. In the current study, phosphorus, nitrogen and manure fertilizations increased MBC and MBN, suggesting that the propagation of soil microbes were improved when exterior nutrients were input into the degraded steppes. In a temperate desert, Huang et al. (2018) also reported that nitrogen fertilization increased microbial biomass by 14.0% and fungal biomass by 30.0% in winter. In central high land soils of Kenya, Kamaa et al. (2011) reported that organic inputs had a positive effect on both bacterial and fungal diversity with or without chemical fertilizers.
In total 3,927 OTU for bacteria and 453 OTU for fungi were identified from the tested soils. Fertilizations had no significant influence on the diversity and richness of soil bacteria or fungi at both soil layers. However, Wang et al. (2017) reported that inorganic fertilizer significantly decreased the Chao and ACE richness indexes of bacterial community, but increased that of the fungal community. Farmer et al. (2017) reported that long-term fertilizer addition greatly reduced the population of bacteria as well as its richness in a brown soil. The inconsistency between our results with these studies might be as a result of the differences in precipitation, soil type or fertilizer types or rates applied to the soil. In the Gurbantunggut Desert, Northwestern China, water addition increased bacterial and fungal diversity and abundance in summer but not in winter and spring (Huang et al., 2018). Francioli et al. (2016) reported that organic fertilization increased bacterial diversity, and stimulated microbial groups that were known to prefer nutrient-rich environments. These results suggested that the responses of the abundance of bacteria and fungi to fertilizations might be differed among microbe species, causing insignificant changes of the total richness and diversity in arid steppes.
Cluster analysis based on the sequence data indicated that bacterial and fungi communities in manure fertilized plots were separated with other fertilizations at both soil layers, excepting fungi communities in P fertilized plots which were separated with other fertilizations at 0–10 cm soil layer. This suggested that the sensitivities of the steppe soil microbes differed in their responses to soil nutrients and fertilizer types. Wang, Ji & Gao (2016) reported that the prokaryotic community structure in manure fertilized soil was different from that in N and P and NP fertilized soils. Long-term application of inorganic fertilizer resulted in a decrease of bacterial diversity, whereas manure application increased microbe diversity (Sapp et al., 2015; Sun et al., 2015).
In the present study, high-throughput sequencing revealed that the predominant bacteria phyla were Acidobacteria, Proteobacteria, Actinobacteria and Chloroflexi, accounting for an average 84.63% of the total bacterial sequences; the predominant fungal phylum was Ascomycota, which accounted for an average 71.26% of the total fungal sequences. These results were consistent with the previous studies based on agricultural soils (Feng et al., 2015; Su et al., 2017; Wu et al., 2011b). Further analysis confirmed that the abundance of soil bacteria and fungi phylum differed in their responses to fertilizers. For example, nitrogen fertilization increased the abundance of Nitrospira and gram negative bacteria, among which Nitrifier oxidizes the nitrite into nitrate, benefiting the plant nitrogen use efficiency (Kant, 2017). Manure relatively increased the abundance of Proteobacteria, which might be attributed to the higher organic carbon input form manures. Fierer, Bradford & Jackson (2007) reported that Proteobacteria, especially the Betaproteobacteria were attributed and favored by nutrient-rich conditions with high C content. At 10–20 cm soil layer, manure fertilizations relatively increased the abundance of Chloroflexi, facultative anaerobic bacteria, among which Chlorobi has also been shown to respond differently to the N and P inputs in a steppe ecosystem (Pan et al., 2014). For fungi, manure increased the abundance of Ciliophora, P and NP increased the abundance of Chytridiomycota, and P increased the abundance of AMF MF693138. These fungi might differ in their nutrient requirements during propagation (Dong & Yao, 2005; Zhu et al., 2008), and the variations of soil chemical properties caused after fertilization resulted in such response differences.
AMF are widely distributed in natural soils, which improve plant nutrient uptake and drought resistances (Valyi, Rillig & Hempel, 2015; Tilman, Wedin & Knops, 1996). In the tested soils, in total seven AMF sequences were identified, being clustered with Paraglomus occultum, Ambispora fennica, Scutellospora species, Rhizophagus species, Diversispora, or Claroideglomus species. Though most of these AMF species were also observed in other steppes (Wang et al., 2014; Hiiesalu et al., 2014), no Glomus species was identified in the tested soils, G. mosseae of which has been reported from all continents except the Antarctic (Guo et al., 2016; Rosendahl, McGee & Morton, 2009), reflecting its wide adaptation and positive roles in different ecosystems (Smith & Read, 1997). The possible reason might be related to the soil properties of the current degraded steppe. A study with non-degraded, moderately degraded and severely degraded steppes in Inner Mongolia has shown that different AMF species showed different distributions among steppes, suggesting that both biotic and abiotic factors were important in determining the AMF communities (Tian et al., 2009). Fertilization, particularly the P fertilizer, influenced the abundance of the AMF species in the tested steppes, confirming that the AMF diversity was driven by soil properties. Variations of AMF colonization were also observed between sampling months and fertilizations. However, no similar trend could be observed in different months and plant species. This suggested that the AMF species colonizing each plant might be different between plant species (Guo et al., 2016), which attributed to their response variations to fertilizations.
In many ecological systems, soil pH is a very important ecological factor for soil microbes (Fierer & Jackson, 2006; Rousk, Brookes & Baath, 2010). Heatmap analysis further indicated that soil pH in the tested steppe was also one of the most important soil properties influencing the abundance of soil bacteria and fungi. Though fertilization had no significant influence on soil pH, soil pH differed between two soil layers, causing the difference of microbial communities. Besides soil pH, other soil chemical properties, such as the concentrations of AP, AN, AK, C/N, MBN, MBC, and MBC/MBN all had positive or negative influences on bacterial and fungi communities. Farmer et al. (2017) reported that soil phosphorus and pH positively influenced the bacterial richness. In a semi-arid steppe, N inputs significantly increased the relative abundance of Proteobacteria and Firmicutes but reduced the abundance of Acidobacteria, Nitrospirae and Chloraflexi, whereas P additions significantly affected Armatimonadetes and Chlorobi (Ling et al., 2017). These results suggested that soil bacteria and fungi communities in degraded steppes could be altered by improving the soil chemical properties through fertilizations. During nature restoration the efficiency of nutrient cycling can increase by shift in microorganism composition (Morrien et al., 2017). Three years’ fertilization improved soil qualities and altered soil bacteria and fungi communities; however, it is still a long term procedure to restore such degraded steppe into original status, including soil properties (McLauchlan, Hobbie & Post, 2006), plant species composition (Zhan, Li & Cheng, 2007), and the network between soil microbe and plants (Hamonts et al., 2017).
Conclusions
Fertilizations had no significant influence on the richness and diversity of the bacteria and fungi. However, the abundance of individual bacterial or fungi species was sensitive to fertilizations, particularly to manure application, mainly attributing to the variations of soil chemical properties caused by fertilizations. Fertilization, particularly the P fertilization, influenced the abundance of the AMF species and the AMF colonization in the tested steppes, suggesting that the AMF diversity was driven by soil properties. Among the soil properties, soil pH in the tested steppe was one of the most important soil properties influencing the abundance of soil bacteria and fungi. Soil bacteria and fungi communities in degraded steppes could be altered by improving the soil chemical properties through fertilizations. However, it is still not clear whether the alteration of the microbe community is detrimental or beneficial to the degraded arid steppes.
Supplemental Information
Different lowercase letters after the data within each soil layer represented significance at P < 0.05 (l.s.d). De, depth; Tr, treatment; CK, Control; P, 60 kg P ha−1; N, 100 kg N ha−1; NP, 60 kg P ha−1 plus 100 kg N ha−1; M, 4,000 kg sheep manure ha−1. Values were the means of three replicates ±SE.
Different lowercase letters after the data within each soil layer represented significance at P < 0.05 (l.s.d). De, depth; Tr, treatment; CK, Control; P, 60 kg P ha−1; N, 100 kg N ha−1; NP, 60 kg P ha−1 plus 100 kg N ha−1; M, 4,000 kg sheep manure ha−1. Values were the means of three replicates ±SE. “-”
Averaged across all treatments.
Averaged across all treatments.
Acknowledgments
We appreciate the help from Mr. Jianjun Chen for field sampling and fertilization.
Abbreviations
- AMF
arbuscular mycorrhizal fungi
- AN
alkali dispelled nitrogen
- AOA
ammonia-oxidizing archaea
- AOB
ammonia-oxidizing bacteria
- AP
available phosphorus
- MBC
microbial biomass carbon
- MBN
microbial biomass nitrogen
- OTUs
operational taxonomic units
- TN
total nitrogen
- OC
organic carbon
- TP
total phosphorus
Funding Statement
The work was supported by the National Key Basic Research Program of China (No. 2014CB138806) and National Natural Science Foundation of China (No. 31670407). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Luhua Yao performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Dangjun Wang and Lin Kang performed the experiments, analyzed the data, authored or reviewed drafts of the paper, approved the final draft.
Dengke Wang performed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Yong Zhang conceived and designed the experiments, performed the experiments, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Xiangyang Hou conceived and designed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Yanjun Guo conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
NCBI Sequence Read Archive database, SRP126759.
References
- Abarenkov et al. (2010).Abarenkov K, Nilsson RH, Larsson K-H, Alexander IJ, Eberhardt U, Erland S, Hoiland K, Kjoller R, Larsson E, Pennanen T, Sen R, Taylor AFS, Tedersoo L, Ursing BM, Vralstad T, Liimatainen K, Peintner U, Koljalg U. The UNITE database for molecular identification of fungi—recent updates and future perspectives. New Phytologist. 2010;186(2):281–285. doi: 10.1111/j.1469-8137.2009.03160.x. [DOI] [PubMed] [Google Scholar]
- Amato et al. (2013).Amato KR, Yeoman CJ, Kent A, Righini N, Carbonero F, Estrada A, Gaskins HR, Stumpf RM, Yildirim S, Torralba M, Gillis M, Wilson BA, Nelson KE, White BA, Leigh SR. Habitat degradation impacts black howler monkey (Alouatta pigra) gastrointestinal microbiomes. ISME Journal. 2013;7(7):1344–1353. doi: 10.1038/ismej.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin (2002).Austin AT. Differential effects of precipitation on production and decomposition along a rainfall gradient in Hawaii. Ecology. 2002;83(2):328–338. doi: 10.1890/0012-9658(2002)083[0328:deopop]2.0.co;2. [DOI] [Google Scholar]
- Bai et al. (2015).Bai R, Xi D, He JZ, Hu HW, Fang YT, Zhang LM. Activity, abundance and community structure of anammox bacteria along depth profiles in three different paddy soils. Soil Biology & Biochemistry. 2015;91:212–221. doi: 10.1016/j.soilbio.2015.08.040. [DOI] [Google Scholar]
- Bai et al. (2012).Bai Y, Wu J, Clark CM, Pan Q, Zhang L, Chen S, Wang Q, Han X, Wisley B. Grazing alters ecosystem functioning and C:N:P stoichiometry of grasslands along a regional precipitation gradient. Journal of Applied Ecology. 2012;49(6):1204–1215. doi: 10.1111/j.1365-2664.2012.02205.x. [DOI] [Google Scholar]
- Bai et al. (2007).Bai Y, Wu J, Pan Q, Huang J, Wang Q, Li F, Buyantuyev A, Han X. Positive linear relationship between productivity and diversity: evidence from the Eurasian Steppe. Journal of Applied Ecology. 2007;44(5):1023–1034. doi: 10.1111/j.1365-2664.2007.01351.x. [DOI] [Google Scholar]
- Balser & Firestone (2005).Balser TC, Firestone MK. Linking microbial community composition and soil processes in a California annual grassland and mixed-conifer forest. Biogeochemistry. 2005;73(2):395–415. doi: 10.1007/s10533-004-0372-y. [DOI] [Google Scholar]
- Bao (2005).Bao SD. Agricultural chemical analysis of soil. China Agriculture Press; Beijing: 2005. [Google Scholar]
- Bragazza et al. (2015).Bragazza L, Bardgett RD, Mitchell EAD, Buttler A. Linking soil microbial communities to vascular plant abundance along a climate gradient. New Phytologist. 2015;205(3):1175–1182. doi: 10.1111/nph.13116. [DOI] [PubMed] [Google Scholar]
- Brookes et al. (1985).Brookes PC, Landman A, Pruden G, Jenkinson DS. Chloroform fumigation and the release of soil-nitrogen—a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biology & Biochemistry. 1985;17(6):837–842. doi: 10.1016/0038-0717(85)90144-0. [DOI] [Google Scholar]
- Callaway et al. (2003).Callaway RM, Mahall BE, Wicks C, Pankey J, Zabinski C. Soil fungi and the effects of an invasive forb on grasses: neighbor identity matters. Ecology. 2003;84(1):129–135. doi: 10.1890/0012-9658(2003)084[0129:sfateo]2.0.co;2. [DOI] [Google Scholar]
- Chen, Hooper & Lin (2011).Chen Q, Hooper DU, Lin S. Shifts in species composition constrain restoration of overgrazed grassland using nitrogen fertilization in inner Mongolian steppe, China. PLOS ONE. 2011;6(3):e16909. doi: 10.1371/journal.pone.0016909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2014).Chen Y-L, Hu H-W, Han H-Y, Du Y, Wan S-Q, Xu Z-W, Chen B-D. Abundance and community structure of ammonia-oxidizing Archaea and Bacteria in response to fertilization and mowing in a temperate steppe in Inner Mongolia. FEMS Microbiology Ecology. 2014;89(1):67–79. doi: 10.1111/1574-6941.12336. [DOI] [PubMed] [Google Scholar]
- Denef et al. (2009).Denef K, Roobroeck D, Wadu M, Lootens P, Boeckx P. Microbial community composition and rhizodeposit-carbon assimilation in differently managed temperate grassland soils. Soil Biology & Biochemistry. 2009;41(1):144–153. doi: 10.1016/j.soilbio.2008.10.008. [DOI] [Google Scholar]
- Dong & Yao (2005).Dong CH, Yao YJ. Nutritional requirements of mycelial growth of Cordyceps sinensis in submerged culture. Journal of Applied Microbiology. 2005;99(3):483–492. doi: 10.1111/j.1365-2672.2005.02640.x. [DOI] [PubMed] [Google Scholar]
- Du et al. (2014).Du B, Zhen L, De Groot R, Goulden CE, Long X, Cao X, Wu R, Sun C. Changing patterns of basic household consumption in the Inner Mongolian grasslands: a case study of policy-oriented adoptive changes in the use of grasslands. Rangeland Journal. 2014;36(5):505–517. doi: 10.1071/rj14021. [DOI] [Google Scholar]
- Fang et al. (2005).Fang JY, Piao SL, Zhou LM, He JS, Wei FY, Myneni RB, Tucker CJ, Tan K. Precipitation patterns alter growth of temperate vegetation. Geophysical Research Letters. 2005;32 doi: 10.1029/2005gl024231. Article L21411. [DOI] [Google Scholar]
- Farmer et al. (2017).Farmer J, Zhang B, Jin X, Zhang P, Wang J. Long-term effect of plastic film mulching and fertilization on bacterial communities in a brown soil revealed by high through-put sequencing. Archives of Agronomy and Soil Science. 2017;63(2):230–241. doi: 10.1080/03650340.2016.1193667. [DOI] [Google Scholar]
- Feng et al. (2015).Feng Y, Yu Y, Tang H, Zu Q, Zhu J, Lin X. The contrasting responses of soil microorganisms in two rice cultivars to elevated ground-level ozone. Environmental Pollution. 2015;197:195–202. doi: 10.1016/j.envpol.2014.11.032. [DOI] [PubMed] [Google Scholar]
- Fierer, Bradford & Jackson (2007).Fierer N, Bradford MA, Jackson RB. Toward an ecological classification of soil bacteria. Ecology. 2007;88(6):1354–1364. doi: 10.1890/05-1839. [DOI] [PubMed] [Google Scholar]
- Fierer & Jackson (2006).Fierer N, Jackson RB. The diversity and biogeography of soil bacterial communities. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(3):626–631. doi: 10.1073/pnas.0507535103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francioli et al. (2016).Francioli D, Schulz E, Lentendu G, Wubet T, Buscot F, Reitz T. Mineral vs. organic amendments: microbial community structure, activity and abundance of agriculturally relevant microbes are driven by long-term fertilization strategies. Frontiers in Microbiology. 2016;7:16. doi: 10.3389/fmicb.2016.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong et al. (2011).Gong XY, Chen Q, Dittert K, Taube F, Lin S. Nitrogen, phosphorus and potassium nutritional status of semiarid steppe grassland in Inner Mongolia. Plant and Soil. 2011;340(1/2):265–278. doi: 10.1007/s11104-010-0577-x. [DOI] [Google Scholar]
- Guo et al. (2016).Guo Y, Du Q, Li G, Ni Y, Zhang Z, Ren W, Hou X. Soil phosphorus fractions and arbuscular mycorrhizal fungi diversity following long-term grazing exclusion on semi-arid steppes in Inner Mongolia. Geoderma. 2016;269:79–90. doi: 10.1016/j.geoderma.2016.01.039. [DOI] [Google Scholar]
- Guo et al. (2017).Guo H, Ye CL, Zhang H, Pan S, Ji YG, Li Z, Liu MQ, Zhou X, Du GZ, Hu F, Hu SJ. Long-term nitrogen & phosphorus additions reduce soil microbial respiration but increase its temperature sensitivity in a Tibetan alpine meadow. Soil Biology & Biochemistry. 2017;113:26–34. doi: 10.1016/j.soilbio.2017.05.024. [DOI] [Google Scholar]
- Guo et al. (2011).Guo P, Wang CY, Jia Y, Wang QA, Han GM, Tian XJ. Responses of soil microbial biomass and enzymatic activities to fertilizations of mixed inorganic and organic nitrogen at a subtropical forest in East China. Plant and Soil. 2011;338(1–2):355–366. doi: 10.1007/s11104-010-0550-8. [DOI] [Google Scholar]
- Hallin et al. (2009).Hallin S, Jones CM, Schloter M, Philippot L. Relationship between N-cycling communities and ecosystem functioning in a 50-year-old fertilization experiment. ISME Journal. 2009;3(5):597–605. doi: 10.1038/ismej.2008.128. [DOI] [PubMed] [Google Scholar]
- Hamonts et al. (2017).Hamonts K, Bissett A, Macdonald BCT, Barton PS, Manning AD, Young A. Effects of ecological restoration on soil microbial diversity in a temperate grassy woodland. Applied Soil Ecology. 2017;117:117–128. doi: 10.1016/j.apsoil.2017.04.005. [DOI] [Google Scholar]
- Handelsman (2009).Handelsman J. Metagenetics: spending our inheritance on the future. Microbial Biotechnology. 2009;2(2):138–139. doi: 10.1111/j.1751-7915.2009.00090_8.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiiesalu et al. (2014).Hiiesalu I, Partel M, Davison J, Gerhold P, Metsis M, Moora M, Oumlpik M, Vasar M, Zobel M, Wilson SD. Species richness of arbuscular mycorrhizal fungi: associations with grassland plant richness and biomass. New Phytologist. 2014;203(1):233–244. doi: 10.1111/nph.12765. [DOI] [PubMed] [Google Scholar]
- Huang et al. (2018).Huang G, Li L, Su YG, Li Y. Differential seasonal effects of water addition and nitrogen fertilization on microbial biomass and diversity in a temperate desert. Catena. 2018;161:27–36. doi: 10.1016/j.catena.2017.09.030. [DOI] [Google Scholar]
- Jobbagy, Sala & Paruelo (2002).Jobbagy EG, Sala OE, Paruelo JM. Patterns and controls of primary production in the Patagonian steppe: a remote sensing approach. Ecology. 2002;83(2):307–319. doi: 10.2307/2680015. [DOI] [Google Scholar]
- Kamaa et al. (2011).Kamaa M, Mburu H, Blanchart E, Chibole L, Chotte JL, Kibunja C, Lesueur D. Effects of organic and inorganic fertilization on soil bacterial and fungal microbial diversity in the Kabete long-term trial, Kenya. Biology and Fertility of Soils. 2011;47(3):315–321. doi: 10.1007/s00374-011-0539-3. [DOI] [Google Scholar]
- Kant (2017).Kant S. Understanding nitrate uptake, signaling and remobilisation for improving plant nitrogen use efficiency. Seminars in Cell & Developmental Biology. 2017;74:89–96. doi: 10.1016/j.semcdb.2017.08.034. [DOI] [PubMed] [Google Scholar]
- Kautz, Wirth & Ellmer (2004).Kautz T, Wirth S, Ellmer F. Microbial activity in a sandy arable soil is governed by the fertilization reuirne. European Journal of Soil Biology. 2004;40(2):87–94. doi: 10.1016/j.ejsobi.2004.10.001. [DOI] [Google Scholar]
- Lau & Lennon (2011).Lau JA, Lennon JT. Evolutionary ecology of plant-microbe interactions: soil microbial structure alters selection on plant traits. New Phytologist. 2011;192(1):215–224. doi: 10.1111/j.1469-8137.2011.03790.x. [DOI] [PubMed] [Google Scholar]
- Lin et al. (2012).Lin XG, Feng YZ, Zhang HY, Chen RR, Wang JH, Zhang JB, Chu HY. Long-term balanced fertilization decreases arbuscular mycorrhizal fungal diversity in an arable soil in North China revealed by 454 pyrosequencing. Environmental Science and Technology. 2012;46(11):5764–5771. doi: 10.1021/es3001695. [DOI] [PubMed] [Google Scholar]
- Ling et al. (2017).Ling N, Chen DM, Guo H, Wei JX, Bai YF, Shen QR, Hu SJ. Differential responses of soil bacterial communities to long-term N and P inputs in a semi-arid steppe. Geoderma. 2017;292:25–33. doi: 10.1016/j.geoderma.2017.01.013. [DOI] [Google Scholar]
- Lombard et al. (2011).Lombard N, Prestat E, Van Elsas JD, Simonet P. Soil-specific limitations for access and analysis of soil microbial communities by metagenomics. FEMS Microbiology Ecology. 2011;78(1):31–49. doi: 10.1111/j.1574-6941.2011.01140.x. [DOI] [PubMed] [Google Scholar]
- McLauchlan, Hobbie & Post (2006).McLauchlan KK, Hobbie SE, Post WM. Conversion from agriculture to grassland builds soil organic matter on decadal timescales. Ecological Applications. 2006;16(1):143–153. doi: 10.1890/04-1650. [DOI] [PubMed] [Google Scholar]
- Mitran et al. (2016).Mitran T, Mani PK, Basak N, Mazumder D, Roy M. Long-term manuring and fertilization influence soil inorganic phosphorus transformation vis-a-vis rice yield in a rice-wheat cropping system. Archives of Agronomy and Soil Science. 2016;62(1):1–18. doi: 10.1080/03650340.2015.1036747. [DOI] [Google Scholar]
- Morrien et al. (2017).Morrien E, Hannula SE, Snoek LB, Helmsing NR, Zweers H, De Hollander M, Soto RL, Bouffaud ML, Buee M, Dimmers W, Duyts H, Geisen S, Girlanda M, Griffiths RI, Jorgensen HB, Jensen J, Plassart P, Redecker D, Schmelz RM, Schmidt O, Thomson BC, Tisserant E, Uroz S, Winding A, Bailey MJ, Bonkowski M, Faber JH, Martin F, Lemanceau P, De Boer W, Van Veen JA, Van der Putten WH. Soil networks become more connected and take up more carbon as nature restoration progresses. Nature Communications. 2017;8:10. doi: 10.1038/ncomms14349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei & Kumar (2000).Nei M, Kumar S. Molecular evolution and phylogenetics. Oxford University Press; New York: 2000. [Google Scholar]
- Oulas et al. (2015).Oulas A, Pavloudi C, Polymenakou P, Pavlopoulos GA, Papanikolaou N, Kotoulas G, Arvanitidis C, Iliopoulos I. Metagenomics: tools and insights for analyzing next-generation sequencing data derived from biodiversity studies. Bioinformatics and Biology Insights. 2015;9:75–88. doi: 10.4137/bbi.s12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan et al. (2014).Pan Y, Cassman N, De Hollander M, Mendes LW, Korevaar H, Geerts Rob HEM, Van Veen JA, Kuramae EE. Impact of long-term N, P, K, and NPK fertilization on the composition and potential functions of the bacterial community in grassland soil. FEMS Microbiology Ecology. 2014;90(1):195–205. doi: 10.1111/1574-6941.12384. [DOI] [PubMed] [Google Scholar]
- Paredes et al. (2011).Paredes C, Menezes-Blackburn D, Cartes P, Gianfreda L, Luz Mora M. Phosphorus and nitrogen fertilization effect on phosphorus uptake and phosphatase activity in ryegrass and tall fescue grown in a Chilean andisol. Soil Science. 2011;176(5):1. doi: 10.1097/SS.0b013e3182147fd3. [DOI] [Google Scholar]
- Pei, Fu & Wan (2008).Pei SF, Fu H, Wan CG. Changes in soil properties and vegetation following exclosure and grazing in degraded Alxa desert steppe of Inner Mongolia, China. Agriculture Ecosystems & Environment. 2008;124(1–2):33–39. doi: 10.1016/j.agee.2007.08.008. [DOI] [Google Scholar]
- Plaza et al. (2004).Plaza C, Hernandez D, Garcia-Gil JC, Polo A. Microbial activity in pig slurry-amended soils under semiarid conditions. Soil Biology & Biochemistry. 2004;36(10):1577–1585. doi: 10.1016/j.soilbio.2004.07.017. [DOI] [Google Scholar]
- Ren, Lu & Fu (2016).Ren Y, Lu Y, Fu B. Quantifying the impacts of grassland restoration on biodiversity and ecosystem services in China: a meta-analysis. Ecological Engineering. 2016;95:542–550. doi: 10.1016/j.ecoleng.2016.06.082. [DOI] [Google Scholar]
- R Core Team (2014).R Core Team . R Foundation for Statistical Computing; Vienna: 2014. [Google Scholar]
- Rosendahl, McGee & Morton (2009).Rosendahl S, McGee P, Morton JB. Lack of global population genetic differentiation in the arbuscular mycorrhizal fungus Glomus mosseae suggests a recent range expansion which may have coincided with the spread of agriculture. Molecular Ecology. 2009;18(20):4316–4329. doi: 10.1111/j.1365-294X.2009.04359.x. [DOI] [PubMed] [Google Scholar]
- Rousk, Brookes & Baath (2010).Rousk J, Brookes PC, Baath E. Investigating the mechanisms for the opposing pH relationships of fungal and bacterial growth in soil. Soil Biology & Biochemistry. 2010;42(6):926–934. doi: 10.1016/j.soilbio.2010.02.009. [DOI] [Google Scholar]
- Sapp et al. (2015).Sapp M, Harrison M, Hany U, Charlton A, Thwaites R. Comparing the effect of digestate and chemical fertiliser on soil bacteria. Applied Soil Ecology. 2015;86:1–9. doi: 10.1016/j.apsoil.2014.10.004. [DOI] [Google Scholar]
- Schönbach et al. (2011).Schönbach P, Wan H, Gierus M, Bai Y, Müller K, Lin L, Susenbeth A, Taube F. Grassland responses to grazing: effects of grazing intensity and management system in an Inner Mongolian steppe ecosystem. Plant and Soil. 2011;340(1/2):103–115. doi: 10.1007/s11104-010-0366-6. [DOI] [Google Scholar]
- Smith & Read (1997).Smith S, Read D. Mycorrhizal symbiosis. Second Edition Academic Press; London: 1997. [Google Scholar]
- Su, Li & Yang (2014).Su J, Li X, Yang H. Effects of fertilization on population density and productivity of herbaceous plants in desert steppe. Sciences in Cold and Arid Regions. 2014;6(3):219–225. [Google Scholar]
- Su et al. (2017).Su P, Lou J, Brookes PC, Luo Y, He Y, Xu J. Taxon-specific responses of soil microbial communities to different soil priming effects induced by addition of plant residues and their biochars. Journal of Soils and Sediments. 2017;17(3):674–684. doi: 10.1007/s11368-015-1238-8. [DOI] [Google Scholar]
- Sun et al. (2015).Sun R, Zhang X-X, Guo X, Wang D, Chu H. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biology & Biochemistry. 2015;88:9–18. doi: 10.1016/j.soilbio.2015.05.007. [DOI] [Google Scholar]
- Symstad & Jonas (2011).Symstad AJ, Jonas JL. Incorporating biodiversity into rangeland health: plant species richness and diversity in great plains grasslands. Rangeland Ecology & Management. 2011;64(6):555–572. doi: 10.2111/rem-d-10-00136.1. [DOI] [Google Scholar]
- Tamura et al. (2007).Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- TerHorst, Lennon & Lau (2014).TerHorst CP, Lennon JT, Lau JA. The relative importance of rapid evolution for plant-microbe interactions depends on ecological context. Proceedings of the Royal Society B: Biological Sciences. 2014;281(1785):8. doi: 10.1098/rspb.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian et al. (2009).Tian H, Gai JP, Zhang JL, Christie P, Li XL. Arbuscular mycorrhizal fungi in degraded typical steppe of Inner Mongolia. Land Degradation & Development. 2009;20(1):41–54. doi: 10.1002/ldr.876. [DOI] [Google Scholar]
- Tilman, Wedin & Knops (1996).Tilman D, Wedin D, Knops J. Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature. 1996;379(6567):718–720. doi: 10.1038/379718a0. [DOI] [Google Scholar]
- Trouvelot et al. (1986).Trouvelot A, Fardeau JC, Plenchette C, Gianinazzi S, Gianinazzapearson V. Nutritional balance and symbiotic expression in mycorrhizal wheat. Physiologie Vegetale. 1986;24(2):300–300. [Google Scholar]
- Valyi, Rillig & Hempel (2015).Valyi K, Rillig MC, Hempel S. Land-use intensity and host plant identity interactively shape communities of arbuscular mycorrhizal fungi in roots of grassland plants. New Phytologist. 2015;205(4):1577–1586. doi: 10.1111/nph.13236. [DOI] [PubMed] [Google Scholar]
- Van Nuland et al. (2016).Van Nuland ME, Wooliver RC, Pfennigwerth AA, Read QD, Ware IM, Mueller L, Fordyce JA, Schweitzer JA, Bailey JK. Plant-soil feedbacks: connecting ecosystem ecology and evolution. Functional Ecology. 2016;30(7):1032–1042. doi: 10.1111/1365-2435.12690. [DOI] [Google Scholar]
- Wang et al. (2017).Wang JC, Song Y, Ma TF, Raza W, Li J, Howland JG, Huang QW, Shen QR. Impacts of inorganic and organic fertilization treatments on bacterial and fungal communities in a paddy soil. Applied Soil Ecology. 2017;112:42–50. doi: 10.1016/j.apsoi1.2017.01.005. [DOI] [Google Scholar]
- Wang et al. (2014).Wang Q, Bao YY, Liu XW, Du GX. Spatio-temporal dynamics of arbuscular mycorrhizal fungi associated with glomalin-related soil protein and soil enzymes in different managed semiarid steppes. Mycorrhiza. 2014;24(7):525–538. doi: 10.1007/s00572-014-0572-9. [DOI] [PubMed] [Google Scholar]
- Wang, Ji & Gao (2016).Wang Y, Ji HF, Gao CQ. Differential responses of soil bacterial taxa to long-term P, N, and organic manure application. Journal of Soils and Sediments. 2016;16(3):1046–1058. doi: 10.1007/s11368-015-1320-2. [DOI] [Google Scholar]
- Waring, Averill & Hawkes (2013).Waring BG, Averill C, Hawkes CV. Differences in fungal and bacterial physiology alter soil carbon and nitrogen cycling: insights from meta-analysis and theoretical models. Ecology Letters. 2013;16(7):887–894. doi: 10.1111/ele.12125. [DOI] [PubMed] [Google Scholar]
- Wu et al. (2011a).Wu FS, Dong MX, Liu YJ, Ma XJ, An LZ, Young JPW, Feng HY. Effects of long-term fertilization on AM fungal community structure and Glomalin-related soil protein in the Loess Plateau of China. Plant and Soil. 2011a;342(1–2):233–247. doi: 10.1007/s11104-010-0688-4. [DOI] [Google Scholar]
- Wu et al. (2011b).Wu M, Qin H, Chen Z, Wu J, Wei W. Effect of long-term fertilization on bacterial composition in rice paddy soil. Biology and Fertility of Soils. 2011b;47(4):397–405. doi: 10.1007/s00374-010-0535-z. [DOI] [Google Scholar]
- Yang, Ruijven & Du (2011).Yang Z, Van Ruijven J, Du G. The effects of long-term fertilization on the temporal stability of alpine meadow communities. Plant and Soil. 2011;345(1–2):315–324. doi: 10.1007/s11104-011-0784-0. [DOI] [Google Scholar]
- Yeomans & Bremner (1988).Yeomans JC, Bremner JM. A rapid and precise method for routine determination of organic carbon in soil. Communications in Soil Science and Plant Analysis. 1988;13:1467–1476. doi: 10.1080/00103628809368027. [DOI] [Google Scholar]
- Yu et al. (2013).Yu WT, Bi ML, Xu YG, Zhou H, Ma Q, Jiang CM. Microbial biomass and community composition in a Luvisol soil as influenced by long-term land use and fertilization. Catena. 2013;107:89–95. doi: 10.1016/j.catena.2013.02.010. [DOI] [Google Scholar]
- Zhan, Li & Cheng (2007).Zhan X, Li L, Cheng W. Restoration of Stipa kryloviisteppes in Inner Mongolia of China: assesment of seed banks and vegetation composition. Journal of Arid Environments. 2007;68(2):298–307. doi: 10.1016/j.jaridenv.2006.05.012. [DOI] [Google Scholar]
- Zhang et al. (2017).Zhang Y, Dong SK, Gao QZ, Liu SL, Ganjurjav H, Wang XX, Su XK, Wu XY. Soil bacterial and fungal diversity differently correlated with soil biochemistry in alpine grassland ecosystems in response to environmental changes. Scientific Reports. 2017;7:43077. doi: 10.1038/srep43077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao et al. (2009).Zhao W, Chen S, Han X, Lin G. Effects of long-term grazing on the morphological and functional traits of Leymus chinensis in the semiarid grassland of Inner Mongolia, China. Ecological Research. 2009;24(1):99–108. doi: 10.1007/s11284-008-0486-0. [DOI] [Google Scholar]
- Zhou et al. (2016).Zhou X, Wang Y, Zhang P, Guo Z, Chu C, Du G. The effects of fertilization on the trait-abundance relationships in a Tibetan alpine meadow community. Journal of Plant Ecology. 2016;9(2):144–152. doi: 10.1093/jpe/rtv043. [DOI] [Google Scholar]
- Zhu et al. (2008).Zhu Y, Pan J, Qiu J, Guan X. Optimization of nutritional requirements for mycelial growth and sporulation of entomogenous fungus Aschersonia aleyrodis webber. Brazilian Journal of Microbiology. 2008;39(4):770–775. doi: 10.1590/S1517-83822008000400032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Different lowercase letters after the data within each soil layer represented significance at P < 0.05 (l.s.d). De, depth; Tr, treatment; CK, Control; P, 60 kg P ha−1; N, 100 kg N ha−1; NP, 60 kg P ha−1 plus 100 kg N ha−1; M, 4,000 kg sheep manure ha−1. Values were the means of three replicates ±SE.
Different lowercase letters after the data within each soil layer represented significance at P < 0.05 (l.s.d). De, depth; Tr, treatment; CK, Control; P, 60 kg P ha−1; N, 100 kg N ha−1; NP, 60 kg P ha−1 plus 100 kg N ha−1; M, 4,000 kg sheep manure ha−1. Values were the means of three replicates ±SE. “-”
Averaged across all treatments.
Averaged across all treatments.
Data Availability Statement
The following information was supplied regarding data availability:
NCBI Sequence Read Archive database, SRP126759.