Abstract
Preservatives used in topical glaucoma medications have a plethora of well-described toxic effects on the ocular surface. Such ocular toxicity is manifest clinically as ocular surface disease (OSD) and has been confirmed in epidemiologic, prospective clinical trials and studies in which patients are switched from preservative-added to preservative-free topical therapy. Such toxicity has implications not only for tolerability, but also for adherence and persistence with therapy that is known to be poor in glaucoma. Glaucoma medication is now widely available in preservative-free formulations, and the question arises as to which patients should receive preservative-free glaucoma therapy in preference to preservative-added medication. A case can be made for several subpopulations of patients who might particularly benefit from preservative-free medication: patients with existing OSD, older patients, younger adult patients, female patients, pediatric and juvenile patients, patients who work in air-conditioned environments or who use electronic screens frequently, patients with medical risk factors for OSD, patients in whom trabecular surgery may become indicated in the future, contact lens users, perhaps patients with Asian ethnicity and patients with severe or treatment-refractory glaucoma. Whilst arguments could be made for selecting patients for preservative-free medication on the basis of their existing risk of OSD, collectively, these patients form a significant proportion of the glaucoma patient population as a whole and, in the absence of any cost premium or positive indication for preservative-added medication, preservative-free glaucoma medication for all patients seems an appropriate strategy.
Keywords: glaucoma, topical therapy, preservative toxicity, preservative-free glaucoma medication, ocular surface disease
Introduction
Preservatives are added to topical ophthalmic preparation to prevent bacterial contamination. In many cases, they were required by regulatory authorities and pharmacopoeias, although alternative methods of avoiding contamination are now available. However, evidence has accumulated that while the acute toxicity of substances such as benzalkonium chloride (BAK), novel detergents (such as Polyquad) and peroxide derivatives (such as Oxyd) may have appeared acceptable in the past, long-term use causes serious deleterious effects on the ocular surface.1–5 Clinically, the effects of BAK generally manifest as ocular surface disease (OSD) that is common in glaucoma patients receiving long-term topical medication.6–8 Moreover, there is increasing evidence suggesting that the associated inflammation may jeopardize the outcome of glaucoma surgery.9–11
As a symptomless, but sight-threatening condition, glaucoma requires long-term, usually life-long treatment. In common with other insidious illnesses, gaining patient acceptance and adherence with treatment is essential if chronic deterioration and ultimate loss of vision is to be avoided. While a number of barriers to good adherence exist, the adverse effects of medication are among the most significant.12–14 Improvements in the tolerability of topical glaucoma medication are likely to have a beneficial effect on adherence as well as the quality of life of patients; indeed, preliminary evidence suggests that ameliorating OSD in glaucoma patients can improve their clinical outcome, and that adherence patterns established early in treatment improves long-term adherence.15–18 The objective of this review is to summarize current understanding of the toxicity of topical ocular therapy preservatives and to identify which patients would benefit from a switch to preservative-free glaucoma medication.
Preclinical studies
The ability of BAK to disrupt the tear film has been known for decades; in 1975, Wilson et al19 showed that instillation of BAK-containing drops caused disruption of the tear film in both rabbits and humans (Figure 1). Preservatives exert their toxic impact on the ocular surface by multiple mechanisms including cellular viability, apoptosis, neurotoxicity, effects on the trabecular network and damage to DNA.1,4,7,20–22
Figure 1.
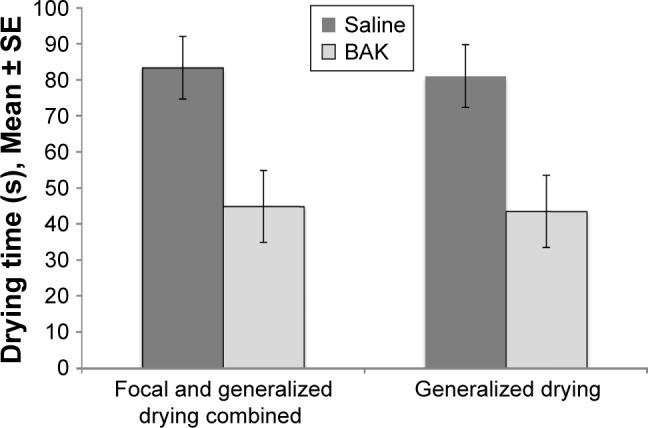
Tear film disruption caused by instillation of a single drop of BAK (0.01%).
Notes: Sixteen healthy volunteers, pretreated with a local anesthetic, received two drops of BAK or saline. The time until the appearance of focal or generalized drying was measured. Adapted by permission from BMJ Publishing Group Ltd. Effect of benzalkonium chloride on the stability of the precorneal tear film in rabbit and man, Wilson WS, Duncan AJ, Jay JL, British Journal of Ophthalmology. 59(11):667–669. © 1975.19
Abbreviation: BAK, benzalkonium chloride.
Although there have been criticisms of the evidence of BAK toxicity in preclinical studies (in particular, the concentrations of BAK employed),23 the weight of evidence appears to support a significant toxic effect of BAK on the ocular surface.1,3,4,21
Clinical studies of BAK toxicity in glaucoma treatment
Epidemiologic studies
BAK toxicity has been identified in a number of epidemiologic studies conducted in glaucoma patients receiving topical therapy.24–29 The first published of these studies was in 1999, which showed that a number of symptoms were more common among 725 patients receiving preservative-added eye drops, rather than among 125 patients using preservative-free eye drops for treatment of their glaucoma. Discomfort or pain on instillation, presence of symptoms of ocular irritation and clinical conjunctival signs on ocular examination were all more common in patients using preservative-added eye drops than in those using preservative-free drops.29
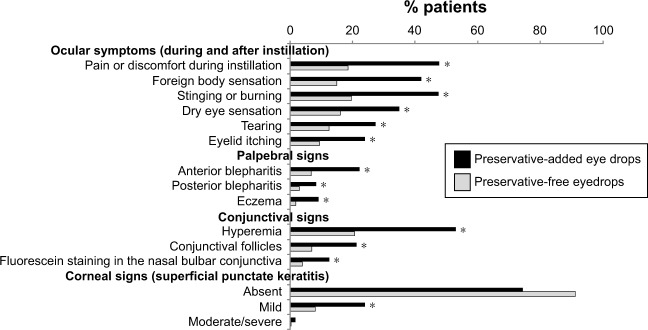
A multinational study examined patient-reported symptoms as well as palpebral, conjunctival and corneal signs in 9,658 patients using preservative-added or preservative-free beta-blocker eye drops. Preservative-added drops were used by 74% and preservative-free eye drops by 12% (combination therapy and unknown drop type accounted for the remainder).25 Reported symptoms, as well as all palpebral, conjunctival and corneal signs were significantly more frequent in patients using preservative-containing drops than in those using preservative-free drops (Figure 2). Patients who reduced their dosage or switched to preservative-free drops experienced a significant amelioration of their symptoms as well as clinical signs.
Figure 2.
Ocular symptoms and signs with preservative-added and preservative-free glaucoma medications.
Notes: Exactly 9,659 patients receiving glaucoma medication were entered into a multicenter cross-sectional epidemiologic survey. Patients were questioned regarding ocular symptoms and they underwent clinical examination. The chart shows the proportion of patients displaying each symptom or clinical sign (*p<0.001). Republished with permission of Wichtig Editore srl, from Ocular symptoms and signs with preserved and preservative-free glaucoma medications, Jaenen N, Baudouin C, Pouliquen P, Manni G, Figueiredo A, Zeyen T, Volume 17, edition 3, Copyright 2007; permission conveyed through Copyright Clearance Center, Inc.25
In addition to increased prevalence of symptoms and clinical signs in patients taking preservative-added compared with preservative-free eye drops, epidemiologic studies in subjects given BAK preservative-added eye drops have also demonstrated similar effects to those seen in animal experiments; increase in inflammatory markers, diminished goblet cells and impression cytology changes, suggestive of increased apoptosis, as well as corneal permeability changes were observed.30–33
Prospective studies
The epidemiologic studies described above suggest that BAK has toxicity properties that may be relevant in patients, but prospective studies provide yet more convincing evidence.
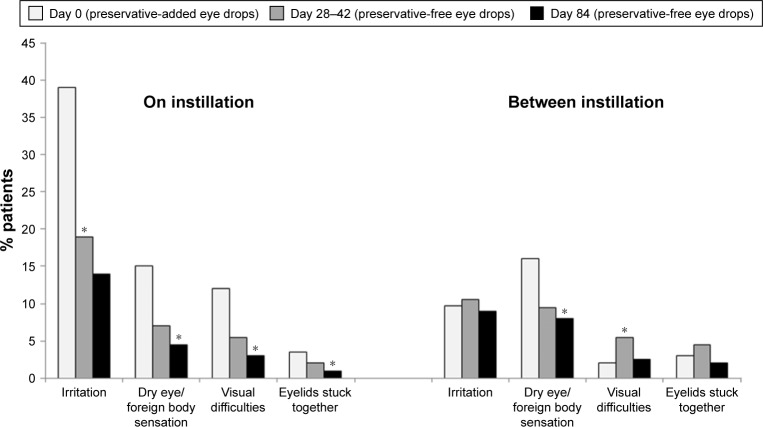
A multicenter, international, prospective, investigator-masked study compared preservative-added and preservative-free latanoprost eye drops in two parallel groups of glaucoma patients.34 Although there was no disadvantage for the preservative-free eye drops in terms of efficacy, there were statistically significant tolerability advantages. Conjunctival hyperemia, subjective symptoms upon instillation and subjective symptoms between instillations were all significantly diminished in patients using preservative-free latanoprost, compared with the preservative-added product. There was a tendency for symptoms to worsen during treatment with the preservative-added eye drops, but to improve when patients were switched to the preservative-free medication. For example, the proportion of patients with moderate to severe conjunctival hyperemia increased almost threefold during the 84 days of study in the patients receiving preservative-added latanoprost eye drops, but fell by 35% in those receiving preservative-free latanoprost eye drops (Figure 3). Similarly, although the differences were not large, total ocular symptom scores fell slightly in the preservative-free group and increased in the preservative-added eye drops group. Symptoms on instillation of eye drops (comprising pruritus, burning/stinging, blurred vision, sticky eye sensation, eye dryness sensation, foreign body sensation) expressed as a score were significantly reduced from day 42 onward (Figure 4). Such benefits in tolerability are likely to be reflected in improved adherence.15–17
Figure 3.
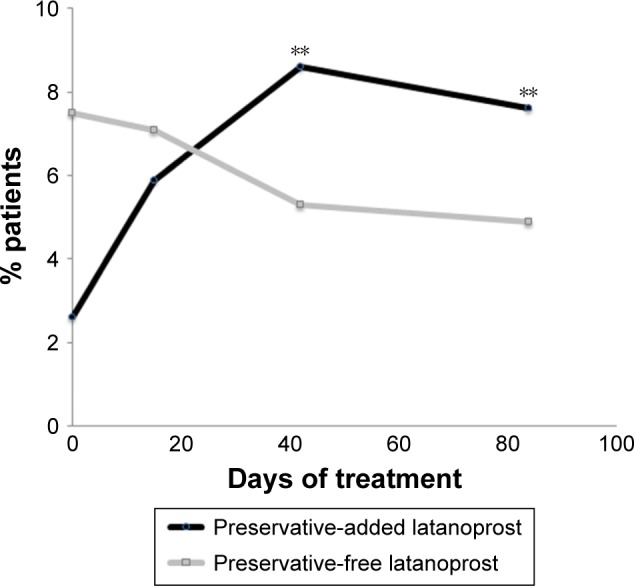
Hyperemia in patients receiving preservative-added or preservative-free glaucoma medication.
Notes: A multicenter, single-blind, parallel-group study compared the safety and efficacy of preservative-added and preservative-free latanoprost eye drops in 463 patients with glaucoma or ocular hypertension. The graph shows the proportion of patients with moderate or severe conjunctival hyperemia during 84 days of treatment (**p<0.01). Adapted by permission from BMJ Publishing Group Ltd. Efficacy and safety of preservative-free latanoprost eyedrops, compared with BAK-preserved latanoprost in patients with ocular hypertension or glaucoma, Rouland JF, Traverso CE, Stalmans I, et al; T2345 Study Group. 97(2):196–200. © 2013.34
Figure 4.
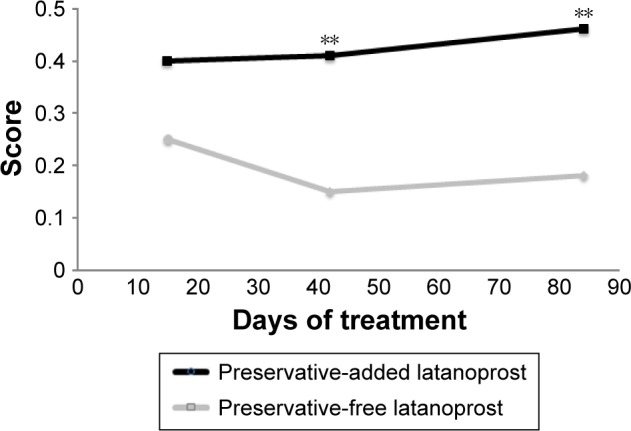
Ocular signs and symptoms in patients receiving preservative-added or preservative-free glaucoma medication.
Notes: A multicenter, single-blind, parallel-group study compared the safety and efficacy of preservative-added and preservative-free latanoprost eye drops in 463 patients with glaucoma or ocular hypertension. The graph shows total subjective ocular score (pruritus, burning/stinging, blurred vision, sticky eye sensation, eye dryness sensation, foreign body sensation) during 84 days of treatment (**p<0.01). Adapted by permission from BMJ Publishing Group Ltd. Efficacy and safety of preservative-free latanoprost eyedrops, compared with BAK-preserved latanoprost in patients with ocular hypertension or glaucoma, Rouland JF, Traverso CE, Stalmans I, et al; T2345 Study Group. 97(2):196–200. © 2013.34
A recent prospective study, in which 40 glaucoma patients were randomized to preservative-added or preservative-free timolol preparations, confirmed the toxicity of BAK preservative-added glaucoma medication.35 After 12 months, intraepithelial goblet cell density was significantly lower in patients who received preservative-added medication compared with those who received preservative-free medication (48.3 vs 86.8) or controls (88.9). Tear break-up time (BUT) was curtailed in patients who received preservative-added timolol compared with those who received preservative-free timolol (8.12 vs 11.27 s) or controls (12.10 s).
Switch studies
1999
If the toxicity differences between preservative-added and preservative-free medications apparent from laboratory studies and observed in epidemiologic and prospective studies are of genuine clinical significance, then it should be possible to observe improvements in patients’ symptoms and adherence when their medication is switched from a preservative-added to a preservative-free formulation. There have been a number of such “switch studies” that have shown this to be the case. The first of these, although not originally conceived as a switch study, was undertaken by Levrat et al.29 This epidemiologic survey of 919 patients of 125 French ophthalmologists was an early indication of the toxic effects of BAK in glaucoma medication. However, data were also collected on 164 patients who switched from preservative-added to preservative-free eye drops and at a mean follow-up of 3.3 months, the incidence of reported symptoms and objective signs had statistically significantly diminished by threefold to fourfold.
2003
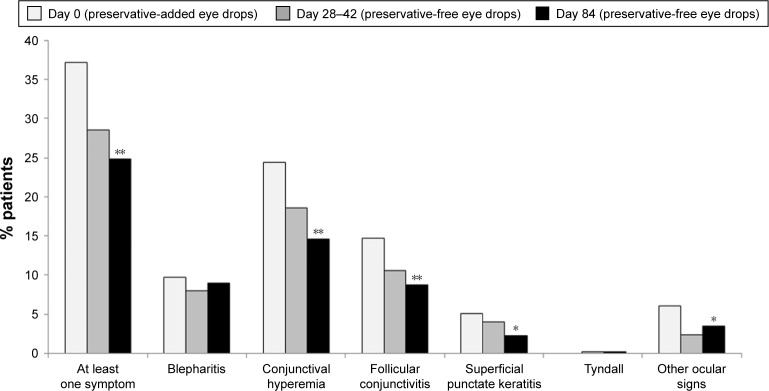
A prospective open clinical trial in which 435 patients with glaucoma or ocular hypertension were switched from BAK preservative-added timolol to the same regimen of preservative-free timolol demonstrated statistically significant reductions in symptoms and clinical signs on or between instillation (Figures 5 and 6).26
Figure 5.
Reduction in ocular symptoms following switch from preservative-added eye drops to preservative-free eye drops.
Notes: In an open, prospective clinical trial, 435 glaucoma patients were switched from their previous preservative-added timolol medication to a preservative-free timolol preparation. Ocular symptoms at and between instillation were recorded during 84 days of treatment (*p<0.01). Reproduced from Bron A, Chiambaretta F, Pouliquen P, Rigal D, Rouland JF. Efficacy and safety of substituting a twice-daily regimen of timolol with a single daily instillation of nonpreserved beta-blocker in patients with chronic glaucoma or ocular hypertension. Français D’Ophtalmologie 2003; volume 26, issue 7:pages 668–674. Copyright © 2003, Elsevier Masson SAS. All rights reserved.26
Figure 6.
Reduction in clinical signs following switch from preservative-added to preservative-free eye drops.
Notes: In an open, prospective clinical trial, 435 glaucoma patients were switched from their previous preservative-added timolol medication to a preservative-free timolol preparation. Clinical signs were recorded during 84 days of treatment (*p<0.01, **p<0.05). Reproduced from Bron A, Chiambaretta F, Pouliquen P, Rigal D, Rouland JF. Efficacy and safety of substituting a twice-daily regimen of timolol with a single daily instillation of nonpreserved beta-blocker in patients with chronic glaucoma or ocular hypertension. Français D’Ophtalmologie 2003; volume 26, issue 7:pages 668–674. Copyright © 2003, Elsevier Masson SAS. All rights reserved.26
2010
OSD improved significantly among 678 glaucoma or ocular hypertension patients switched from preservative-added to preservative-free glaucoma medication in a prospective, double-masked, randomized controlled, 12-week trial.36 Intraocular pressure (IOP) was not significantly different between preservative-added and preservative-free groups.
2014
Switching from a preservative-added to a preservative-free preparation had no deleterious effects on IOP. An interesting recent study15 describes a series of four patients with open-angle glaucoma that was poorly controlled by their current preservative-added medication. All patients reported ocular discomfort and all had previously unrecognized OSD. Modification of their treatment to control OSD, including switching to preservative-free glaucoma medication and intensive treatment of the OSD, not only markedly improved symptomatic and clinical signs of OSD, but also resulted in a clinically significant improvement in IOP control.
In a recent open prospective study, a switch from preservative-added to preservative-free glaucoma medication resulted in improvements in corneal and conjunctival staining, erythema, conjunctival hyperemia and follicular hyperplasia, as well as in Schirmer’s test and tear BUT, which were associated with reduction in reports of dry eye and foreign body sensation. Improvements in IOP were not compromised by the switch to preservative-free medication.37
2016
A combined analysis of two Phase III clinical trials in which glaucoma patients were switched from a preservative-added prostaglandin to a preservative-free prostaglandin medication showed reductions of two thirds in the incidence of ocular surface symptoms (irritation/burning/stinging, foreign body sensation, tearing, itching and dry eye sensation) and a halving of the incidence of blepharitis, abnormal staining and conjunctival hyperemia. A large majority of patients preferred the preservative-free preparation.38
Studies in which patients are switched from preservative-added to preservative-free topical medication provide compelling evidence not only for the clinical toxicity of BAK preservative-added eye drops, but also that such toxicity can be ameliorated or avoided by adopting preservative-free medications in favor of BAK preparations. Importantly, all of the studies show that switching to a preservative-free formulation did not compromise IOP control. While improving tolerability is a laudable objective in its own right, in the context of glaucoma treatment, it has even more important implications in improving adherence and long-term visual outcome.
Adherence, persistence and quality of life
Adherence and persistence with glaucoma treatment is notoriously poor, particularly among patients new to therapy and, as any ophthalmologist will recognize, is a major barrier to the effective management of IOP.12,39–41 In clinical trials, topical adverse effects are the most common reason for drop-out and are a significant source of poor adherence in clinical situations as well.42,43 A recent study completed in Singapore links adherence with quality of life in glaucoma patients.44 Topical adverse events are among the most frequently reported reasons for treatment switching therapy and, as the foregoing discussion shows, it is now recognized that preservatives are responsible for at least some, and possibly most, of these local adverse events and that removing the preservative from the patient’s medication can improve both quality of life and adherence to treatment.1,45–47
Direct evidence for the improvement in quality of life that can be achieved by a switch from a preservative-added to preservative-free therapy in glaucoma comes from a recent study in which patients were switched to a preservative-free medication and their quality of life was determined using the Glaucoma Symptom Scale. Scores for symptoms and functioning improved significantly after 8 weeks following the switch.48
Ameliorating patients’ symptoms and improving quality of life are worthwhile goals for the clinician; however, they are yet more important if they can improve adherence and persistence with therapy and ultimately preserve the patient’s vision.
Patient satisfaction with treatment can be considered an overarching parameter that measures patients’ attitude to their medication. A recent study of patient satisfaction with glaucoma treatment showed that, while most patients were satisfied with their treatment, factors significantly associated with dissatisfaction included the presence of OSD, hyperemia, ocular signs, symptoms during and between instillation and the use of tear substitutes. The study also illustrated the frequency of ocular symptoms (Figure 7) and their onset with the commencement of glaucoma therapy (Figure 8).49
Figure 7.
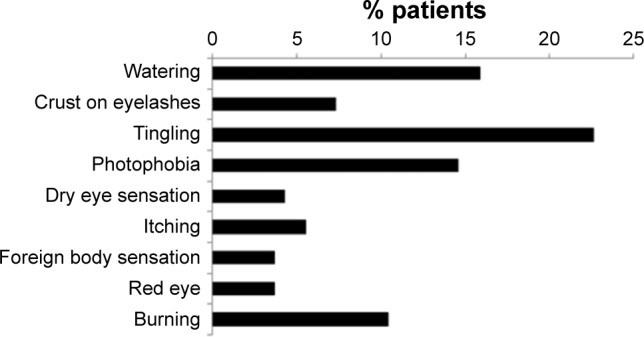
Proportion of patients experiencing symptoms between eye drop instillations.
Notes: In a cross-sectional epidemiologic survey, 164 patients suffering from glaucoma treated with topical prostaglandins were queried regarding their experience of ocular symptoms between eye drop instillations. Chart shows the proportion of patients reporting each symptom. Copyright ©2015. Dove Medical Press. Reproduced from Lemij HG, Hoevenaars JG, van der Windt C, Baudouin C. Patient satisfaction with glaucoma therapy: reality or myth? Clin Ophthalmol. 2015;9:785–793.49
Figure 8.
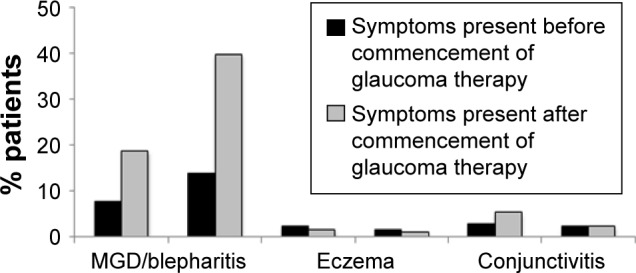
Emergence of ocular symptoms on commencement of glaucoma treatment.
Notes: In a cross-sectional epidemiologic survey, 164 patients suffering from glaucoma treated with topical prostaglandins were queried regarding their experience of ocular symptoms between eye drop instillations. Chart shows the proportion of patients reporting each symptom before and after the commencement of glaucoma treatment. Copyright ©2015. Dove Medical Press. Reproduced from Lemij HG, Hoevenaars JG, van der Windt C, Baudouin C. Patient satisfaction with glaucoma therapy: reality or myth? Clin Ophthalmol. 2015;9:785–793.49
Abbreviation: MGD, meibomian gland dysfunction.
Effects of preservatives on other aspects of glaucoma treatment
It seems clear that preservatives in eye drops can be the cause of both hyperemia and OSD in glaucoma patients. Dry eye can interfere with glaucoma diagnostic tests and deleteriously affect the long-term outcome of trabeculectomy.10,50,51
Patients undergoing topical glaucoma therapy have reduced corneal (and conjunctival) sensitivity.52 A number of studies have suggested that preservatives are responsible for neurotoxic effects in the cornea; for example, reduction in corneal sensitivity in glaucoma patients correlates with the frequency of instillation of preservative-added treatments.53 Supporting this contention is the finding that stromal nerve fibers are significantly reduced in mice treated with BAK-containing eye drops. Moreover, in human studies, sub-basal nerve density is reduced in patients treated with preservative-added eye drops compared with that in patients treated with preservative-free eye drops and in untreated control subjects.54,55 Such a diminution of corneal sensitivity may explain why glaucoma patients frequently have clinical signs of OSD on examination, yet remain satisfied with their treatment and do not volunteer symptoms.49
The study of Martone et al described above additionally investigated a suite of parameters among control subjects, glaucoma patients treated with preservative-free eye drops and those given preservative-added eye drops.55 The study showed significantly better clinical scores for Schirmer test, esthesiometry and tear BUT in preservative-free-treated patients than in those given preservative-added medication. IOP was controlled equally well by preservative-free and preservative-added medication. Confocal microscopy examination showed advantages for preservative-free vs preservative-added medication in a number of parameters, though not all achieved statistical significance.
Indeed, the apoptotic effects of BAK may even promote glaucomatous changes in the eye or antagonize the effects of glaucoma medication by its effects on apoptosis in trabecular cells.56–61
Preservative-free glaucoma therapy for which patients?
Because of the more widespread availability of preservative-free formulations of topical glaucoma medications, consideration needs to be given as to which patients would most benefit from being switched to a preservative-free formulation. A number of patient subgroups can be identified who are at higher risk of OSD, or in whom OSD would be particularly unwelcome.
Newly diagnosed patients with pre-existing OSD
Glaucoma and OSD are commonly comorbid conditions; in some cases, a glaucoma patient’s OSD may be related to their glaucoma medication, but OSD can be present in treatment-naive patients. In addition, OSD, dry eye and glaucoma are all diseases typically more prevalent in older patients.62–65 Exacerbating OSD in such patients can be avoided by starting them on topical therapy that excludes the preservatives that can result in ocular surface toxicity during the long-term treatment the patients will likely require.
Elderly
Glaucoma is a progressive disease and is not only more common in the elderly, but in its later stages, it also often requires the use of more frequent application of eye drops and/or the use of multiple medications, thus increasing the risk of precipitating OSD.
A number of epidemiologic and other studies have demonstrated the increased risk of OSD in older patients.62,66–69 The aging eye is susceptible to OSD for a number of pathophysiologic reasons including functional changes in the lacrimal glands, meibomian glands and goblet cells. Perhaps most importantly, aging is one of the most important risk factors for meibomian gland dysfunction (MGD).70 Consequent changes in the composition of the tear film give rise to a propensity to inflammation.71,72 Anatomic changes such as conjunctivochalasis and eyelid laxity, more common in older populations, also predispose to OSD.71,72 Elderly patients may also be more sensitive to the effects of OSD than the younger patient populations.
Younger patients
Although predominantly a disease of older age, glaucoma can occur in individuals below the age of 40 years. Early-onset glaucoma is generally more difficult to treat and progresses more rapidly than glaucoma arising in older patients.73 Consequently, younger patients tend to be treated more aggressively. Because glaucoma requires long-term, normally life-long therapy, younger patients with extended life expectancy will be exposed for a longer period of time to the cumulative toxicity of preservatives in their medication. Avoiding extended exposure may prevent the development of ocular symptoms and aid adherence and persistence in younger patients.
Pediatric and juvenile patients
Glaucoma is relatively uncommon in pediatric and juvenile patients and is generally treated by surgical methods.74 However, topical medications are used prior to surgery and in those patients whose IOP is not adequately controlled following surgery. Clearly, such patients face many years of topical therapy, during which ocular surface toxicity is unwelcome and likely to impact adherence and persistence. Moreover, as discussed previously, OSD precipitated by the preservatives in topical glaucoma medication may impair the outcome of trabecular surgery.9,50
Work/leisure environments
Many offices and other workplaces are air-conditioned and, while the low humidity air and draft provided in these environments make the individuals physically comfortable, it can increase evaporative losses from their ocular surface and predispose them to dry eye. Other individuals (aircraft cabin staff, frequent air travelers, workers in arid environments, professional drivers) can be at risk from overexposure to draft and desiccated atmospheres.75–78
The use of electronic displays for both work and leisure is close to universal in the developed world and increasing in the developing world. Dry eye is a significant component of an established computer vision syndrome that also includes ocular discomfort, headache, eyestrain and temporary difficulties in accommodation.79,80 Both blink rate and blink amplitude are reduced during the use of display screens, increasing the susceptibility to evaporation of the ocular film, especially where, as is frequently the case, such screens are used in desiccated air-conditioned work environments.80 Moreover, there may be a relationship between blink rate and MGD that further exacerbates OSD in habitual computer screen users.81 Frequent computer use is itself associated with MGD.82
Patients with medical risk factors
A number of other medical conditions are associated with dry eye; collagen vascular disease, refractive surgery, hematopoietic stem cell transplantation, vitamin A deficiency and androgen insufficiency have high levels of evidence for an association with dry eye, while diabetes mellitus, human immunodeficiency virus infection, systemic chemotherapy, some types of cataract surgery, keratoplasty, sarcoidosis and ovarian dysfunction are also candidate risk factors with at least moderate evidence.62,83 Dry eye can also be iatrogenic; there is at least moderately strong evidence showing antihistamines, some antidepressants, diuretics and beta-blockers as risk factors for dry eye. While cancer itself is not a risk factor for dry eye, radiation therapy and some types of systemic chemotherapy are.62,83
Similarly, MGD has been associated with a number of medical treatments including antihistamines, antidepressants, antiandrogens and isotretinoin drugs used for treating benign prostatic hyperplasia.64 The degree of overlap between those drugs identified as potential risk factors for dry eye and those associated with MGD is notable. The list of medical conditions that may be associated with MGD is long, but includes androgen deficiency, atopy (and perhaps associated antihistamine use), benign prostatic hyperplasia, hypertension, menopause, Parkinson’s disease, rosacea, Sjögren’s syndrome and others.64
Clearly, there is a wide range of medical conditions and medical treatments that put patients at increased risk of OSD. Many of these conditions are more prevalent among older patients and, therefore, frequently comorbid with glaucoma. Avoiding preservatives in the glaucoma medication of patients already at risk of OSD because of pre-existing medical conditions or those that develop during the course of their glaucoma treatment would seem to be the responsible course of action.
Presurgical patients
Trabeculotomy or filtration surgery is a common procedure used in patients in whom topical glaucoma therapy does not adequately control IOP or in those who fail to tolerate topical medication sufficiently well. A link between impaired outcome of trabecular surgery and long-term topical therapy has long been suspected.9,50 The role of toxic effects of preservatives in precipitating inflammatory and fibrotic changes in the ocular surface is now well recognized and some of the mechanisms have been elucidated.28,84–88 It would thus seem reasonable that patients in whom filtration surgery might become indicated in the future be spared the additional risk of a poor surgical outcome by using preservative-free, rather than preservative-added medication in the initial stages of their illness.
Women
Dry eye is more common in women, particularly after menopause when there is a specific increase in MGD.68,89–91 A dichotomy appears to exist regarding the benefit of hormone replacement therapy in improving the condition of the ocular surface in postmenopausal women; some studies have identified an improvement with hormone replacement therapy.92–96 On the other hand, The International Workshop on Meibomian Gland Dysfunction identified both menopause and hormone replacement therapy as risk factors for MGD.64 The Dry Eye Workshop identified hormone replacement as a risk factor, but although older age and female sex were risk factors with consistent evidence, the evidence for menopause per se was deemed as “unclear”.62 A possible explanation is that, estrogen may be a risk factor for OSD in premenopausal women, but that the loss of circulating androgens (androgen deficiency being a well-established risk factor) at menopause may be responsible for the postmenopausal increase in OSD.
Regardless of the mechanism, it is clear that women, older women in particular, are at higher risk of OSD than men, and that avoidance of preservative-induced toxicity would be of benefit in this population.
Contact lens use
The use of contact lenses is very common and is a frequent cause of dry eye, with around half of all contact lens wearers reporting symptoms of dry eye.97 Similarly, MGD is more common among contact lens wearers than the population at large, although whether this association is causal or not remains unclear.64 Nevertheless, contact lens use is a risk factor for OSD and the precautionary principle suggests avoiding additional risk in these patients by excluding preservatives from their topical glaucoma medication.
Ethnicity
Although methodological inconsistences exist between studies, a number of studies suggest that the prevalence of MGD is considerably higher among Asian populations than Caucasians.64 A higher rate of dry eye in Asian populations is also supported by suggestive evidence.62
Glaucoma
Although the severity or type of glaucoma is not known to be a risk factor for the development of OSD, patients with higher IOP are more likely to receive more frequent eye drops or treatment with more than one agent, both of which are known to be associated with an increased risk of dry eye.8,16 Patients receiving frequent instillations or multidrug therapy for severe glaucoma could usefully be considered for a switch to preservative-free medication.
Conclusion
There are a number of risk factors that can predispose to OSD among glaucoma patients: long treatment duration, age, sex, working and leisure environments, multiple medical comorbidities and their associated medication, and ethnicity. In such patients, it seems reasonable to avoid an additional risk of OSD caused by exposing them to the preservatives in their glaucoma therapy.
At first sight, there seems little medical rationale to choose a preservative-added medication over a preservative-free alternative. There have been suggestions that BAK enhances the penetration of active molecules into deeper ocular structures.98 However, this phenomenon is better considered as histologic toxicity, rather than a means of improving penetration; in any case, acute pharmacodynamic effects are not necessarily indicative of conditions during chronic sustained treatment.1 Until recently, the cost associated with single-dose or preservative-free multidose containers was a significant barrier to the use of preservative-free eye drops. However, preservative-free formulations are now widely available and often at little or no cost premium over preservative-added alternatives. Health economic studies comparing preservative-added and preservative-free glaucoma medications are lacking; but given an equivalent cost of acquisition, it seems likely that preservative-free formulations would have an advantage in cost-effectiveness (better persistence and adherence) and fewer consultations for OSD.
There are a number of reasons to choose a preservative-free topical therapy for glaucoma over a preservative-added treatment: improvements in patients’ quality of life, better adherence and persistence with therapy, and improving the outcome of trabecular surgery. The question arises as to which patients to choose for preservative-free treatment. However, perhaps the better question is: for which patients would an ophthalmologist choose to prescribe a preservative-added medication?
Footnotes
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.Baudouin C, Labbé A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29(4):312–334. doi: 10.1016/j.preteyeres.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Meloni M, Pauly A, Servi BD, Varlet BL, Baudouin C. Occludin gene expression as an early in vitro sign for mild eye irritation assessment. Toxicol In Vitro. 2010;24(1):276–285. doi: 10.1016/j.tiv.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Actis AG, Rolle T. Ocular surface alterations and topical antiglaucomatous therapy: a review. Open Ophthalmol J. 2014;8:67–72. doi: 10.2174/1874364101408010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aguayo Bonniard A, Yeung JY, Chan CC, Birt CM. Ocular surface toxicity from glaucoma topical medications and associated preservatives such as benzalkonium chloride (BAK) Expert Opin Drug Metab Toxicol. 2016;12(11):1279–1289. doi: 10.1080/17425255.2016.1209481. [DOI] [PubMed] [Google Scholar]
- 5.Freeman DP, Kahook MY. Preservatives in topical ophthalmic medications: historical and clinical perspectives. Expert Rev Ophthalmol. 2009;4(1):59–64. [Google Scholar]
- 6.Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17(5):350–355. doi: 10.1097/IJG.0b013e31815c5f4f. [DOI] [PubMed] [Google Scholar]
- 7.Anwar Z, Wellik SR, Galor A. Glaucoma therapy and ocular surface disease: current literature and recommendations. Curr Opin Ophthalmol. 2013;24(2):136–143. doi: 10.1097/ICU.0b013e32835c8aba. [DOI] [PubMed] [Google Scholar]
- 8.Fechtner RD, Godfrey DG, Budenz D, Stewart JA, Stewart WC, Jasek MC. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010;29(6):618–621. doi: 10.1097/ICO.0b013e3181c325b2. [DOI] [PubMed] [Google Scholar]
- 9.Broadway DC, Grierson I, O’Brien C, Hitchings RA. Adverse effects of topical antiglaucoma medication. II. The outcome of filtration surgery. Arch Ophthalmol. 1994;112(11):1446–1454. doi: 10.1001/archopht.1994.01090230060021. [DOI] [PubMed] [Google Scholar]
- 10.Baudouin C. Ocular surface and external filtration surgery: mutual relationships. Dev Ophthalmol. 2012;50:64–78. doi: 10.1159/000334791. [DOI] [PubMed] [Google Scholar]
- 11.Movahedan A, Djalilian AR. Cataract surgery in the face of ocular surface disease. Curr Opin Ophthalmol. 2012;23(1):68–72. doi: 10.1097/ICU.0b013e32834d90b7. [DOI] [PubMed] [Google Scholar]
- 12.Nordstrom BL, Friedman DS, Mozaffari E, Quigley HA, Walker AM. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005;140(4):598–606. doi: 10.1016/j.ajo.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 13.Kastelan S, Tomic M, Metez Soldo K, Salopek-Rabatic J. How ocular surface disease impacts the glaucoma treatment outcome. Biomed Res Int. 2013;2013:696328. doi: 10.1155/2013/696328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen Castel O, Keinan-Boker L, Geyer O, Milman U, Karkabi K. Factors associated with adherence to glaucoma pharmacotherapy in the primary care setting. Fam Pract. 2014;31(4):453–461. doi: 10.1093/fampra/cmu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baudouin C, Bechetoille A, Bron A, et al. Intérêt de la mesure de la qualité de vie (QDV) et de l’observance thérapeutique chez les patients atteints de glaucome chronique à angle ouvert. [Relevance of quality of life and treatment compliance measurement in patients with chronic open-angle glaucoma] J Fr Ophtalmol. 2000;23(10):1057–1064. French. [PubMed] [Google Scholar]
- 16.Rossi GC, Tinelli C, Pasinetti GM, Milano G, Bianchi PE. Dry eye syndrome-related quality of life in glaucoma patients. Eur J Ophthalmol. 2009;19(4):572–579. doi: 10.1177/112067210901900409. [DOI] [PubMed] [Google Scholar]
- 17.Batra R, Tailor R, Mohamed S. Ocular surface disease exacerbated glaucoma: optimizing the ocular surface improves intraocular pressure control. J Glaucoma. 2014;23(1):56–60. doi: 10.1097/IJG.0b013e318264cd68. [DOI] [PubMed] [Google Scholar]
- 18.Newman-Casey PA, Blachley T, Lee PP, Heisler M, Farris KB, Stein JD. Patterns of glaucoma medication adherence over four years of follow-up. Ophthalmology. 2015;122(10):2010–2021. doi: 10.1016/j.ophtha.2015.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson WS, Duncan AJ, Jay JL. Effect of benzalkonium chloride on the stability of the precorneal tear film in rabbit and man. Br J Ophthalmol. 1975;59(11):667–669. doi: 10.1136/bjo.59.11.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chibret H. Toxicité oculaire du benzalkonium. [Ocular toxicity of benzalkonium] Ann Pharm Fr. 2011;69(2):108–115. doi: 10.1016/j.pharma.2010.12.002. French. [DOI] [PubMed] [Google Scholar]
- 21.Huang C, Chen W, Chen Y, Liu Z. Toxicity research status of benzalkonium chloride on ocular surface. Zhonghua Yan Ke Za Zhi. 2014;50(4):303–306. Chinese. [PubMed] [Google Scholar]
- 22.Baudouin C. Detrimental effect of preservatives in eyedrops: implications for the treatment of glaucoma. Acta Ophthalmol. 2008;86(7):716–726. doi: 10.1111/j.1755-3768.2008.01250.x. [DOI] [PubMed] [Google Scholar]
- 23.Tressler CS, Beatty R, Lemp MA. Preservative use in topical glaucoma medications. Ocul Surf. 2011;9(3):140–158. doi: 10.1016/s1542-0124(11)70024-6. [DOI] [PubMed] [Google Scholar]
- 24.Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. 2002;86(4):418–423. doi: 10.1136/bjo.86.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaenen N, Baudouin C, Pouliquen P, Manni G, Figueiredo A, Zeyen T. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Ophthalmol. 2007;17(3):341–349. doi: 10.1177/112067210701700311. [DOI] [PubMed] [Google Scholar]
- 26.Bron A, Chiambaretta F, Pouliquen P, Rigal D, Rouland JF. Efficacy and safety of substituting a twice-daily regimen of timolol with a single daily instillation of nonpreserved beta-blocker in patients with chronic glaucoma or ocular hypertension. J Fr Ophtalmol. 2003;26(7):668–674. [PubMed] [Google Scholar]
- 27.Rouland JF. Etude CARAT: acceptabilité d’un collyre bêtabloquant sans conservateur dans le traitement du glaucome. [Acceptability of preservative-free beta-blocker eye drops in the treatment of glaucoma] Réflexions Ophtalmologiques. 2011;150:43–46. French. [Google Scholar]
- 28.Van Went C, Brasnu E, Hamard P, Baudouin C, Labbé A. Influence des pathologies de la surface oculaire sur le traitement du glaucome. [The influence of ocular surface diseases in the management of glaucoma] J Fr Ophtalmol. 2011;34(4):230–237. doi: 10.1016/j.jfo.2010.11.010. French. [DOI] [PubMed] [Google Scholar]
- 29.Levrat F, Pisella PJ, Baudouin C. Tolérance clinique des collyres antiglaucomateux conservés et non conservés. Résultats d’une enquête inédite en Europe. [Clinical tolerance of antiglaucoma eyedrops with and without a preservative. Results of an unpublished survey in Europe] J Fr Ophtalmol. 1999;22(2):186–191. French. [PubMed] [Google Scholar]
- 30.Campagna P, Macri A, Rolando M, et al. Chronic topical eye preservative-free beta-blocker therapy effect on the ocular surface in glaucomatous patients. Acta Ophthalmol Scand (Suppl) 1997;75(S224):53. doi: 10.1111/j.1600-0420.1997.tb00480.x. [DOI] [PubMed] [Google Scholar]
- 31.Pisella PJ, Lala E, Parier V, et al. Retentissement conjonctival des conservateurs: étude comparative de collyres bêta-bloquants conservés et non conservés chez des patients glaucomateux. [Effect of preservatives on the conjunctiva: a comparative study of beta-blocker eye drops with and without preservatives in glaucoma patients] J Fr Ophtalmol. 2003;26(7):675–679. French. [PubMed] [Google Scholar]
- 32.Pisella PJ, Debbasch C, Hamard P, et al. Conjunctival proinflammatory and proapoptotic effects of latanoprost and preserved and unpreserved timolol: an ex vivo and in vitro study. Invest Ophthalmol Vis Sci. 2004;45(5):1360–1368. doi: 10.1167/iovs.03-1067. [DOI] [PubMed] [Google Scholar]
- 33.Burstein NL. Preservative alteration of corneal permeability in humans and rabbits. Invest Ophthalmol Vis Sci. 1984;25(12):1453–1457. [PubMed] [Google Scholar]
- 34.Rouland JF, Traverso CE, Stalmans I, et al. T2345 Study Group Efficacy and safety of preservative-free latanoprost eyedrops, compared with BAK-preserved latanoprost in patients with ocular hypertension or glaucoma. Br J Ophthalmol. 2013;97(2):196–200. doi: 10.1136/bjophthalmol-2012-302121. [DOI] [PubMed] [Google Scholar]
- 35.Frezzotti P, Fogagnolo P, Haka G, et al. In vivo confocal microscopy of conjunctiva in preservative-free timolol 0.1% gel formulation therapy for glaucoma. Acta Ophthalmol. 2014;92(2):e133–e140. doi: 10.1111/aos.12261. [DOI] [PubMed] [Google Scholar]
- 36.Katz G, Springs CL, Craven ER, Montecchi-Palmer M. Ocular surface disease in patients with glaucoma or ocular hypertension treated with either BAK-preserved latanoprost or BAK-free travoprost. Clin Ophthalmol. 2010;4:1253–1261. doi: 10.2147/OPTH.S14113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iester M, Telani S, Frezzotti P, et al. Ocular surface changes in glaucomatous patients treated with and without preservatives beta-blockers. J Ocul Pharmacol Ther. 2014;30(6):476–481. doi: 10.1089/jop.2013.0216. [DOI] [PubMed] [Google Scholar]
- 38.Uusitalo H, Egorov E, Kaarniranta K, Astakhov Y, Ropo A. Benefits of switching from latanoprost to preservative-free tafluprost eye drops: a meta-analysis of two Phase IIIb clinical trials. Clin Ophthalmol. 2016;10:445–454. doi: 10.2147/OPTH.S91402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reardon G, Kotak S, Schwartz GF. Objective assessment of compliance and persistence among patients treated for glaucoma and ocular hypertension: a systematic review. Patient Prefer Adherence. 2011;5:441–463. doi: 10.2147/PPA.S23780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gurwitz JH, Glynn RJ, Monane M, Everitt DE, Gilden D, Smith N, Avorn J. Treatment for glaucoma: adherence by the elderly. Am J Public Health. 1993;83(5):711–716. doi: 10.2105/ajph.83.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeaw J, Benner JS, Walt JG, Sian S, Smith DB. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm. 2009;15(9):728–740. doi: 10.18553/jmcp.2009.15.9.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stewart WC, Demos CM, Turner MK, Stewart JA. Risk factors for subject withdrawals in clinical trials evaluating glaucoma medications. Graefes Arch Clin Exp Ophthalmol. 2010;248(7):1007–1012. doi: 10.1007/s00417-010-1339-4. [DOI] [PubMed] [Google Scholar]
- 43.Nordmann JP, Auzanneau N, Ricard S, Berdeaux G. Vision related quality of life and topical glaucoma treatment side effects. Health Qual Life Outcomes. 2003;1(1):75. doi: 10.1186/1477-7525-1-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loon SC, Jin J, Jin Goh M. The relationship between quality of life and adherence to medication in glaucoma patients in Singapore. J Glaucoma. 2015;24(5):e36–e42. doi: 10.1097/IJG.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 45.Odberg T, Jakobsen JE, Hultgren SJ, Halseide R. The impact of glaucoma on the quality of life of patients in Norway. I. Results from a self-administered questionnaire. Acta Ophthalmol Scand. 2001;79(2):116–120. doi: 10.1034/j.1600-0420.2001.079002116.x. [DOI] [PubMed] [Google Scholar]
- 46.Bayer A, Weiler W, Oeverhaus U, Skrotzki FE, Stewart WC, Xplore Observation Group Two-year follow-up of latanoprost 0.005% monotherapy after changing from previous glaucoma therapies. J Ocul Pharmacol Ther. 2004;20(6):470–478. doi: 10.1089/jop.2004.20.470. [DOI] [PubMed] [Google Scholar]
- 47.Nordmann JP, Akesbi J. Améliorer l’adhérence au traitement des patients glaucomateux : le rôle du médecin. [Improve adherence in glaucoma patients: a doctor’s duty] J Fr Ophtalmol. 2011;34(6):403–408. doi: 10.1016/j.jfo.2011.04.003. French. [DOI] [PubMed] [Google Scholar]
- 48.Abegão Pinto L, Vandewalle E, Gerlier L, Stalmans I. Improvement in glaucoma patient quality of life by therapy switch to preservative-free timolol/dorzolamide fixed combination. Ophthalmologica. 2014;231(3):166–171. doi: 10.1159/000356468. [DOI] [PubMed] [Google Scholar]
- 49.Lemij HG, Hoevenaars JG, van der Windt C, Baudouin C. Patient satisfaction with glaucoma therapy: reality or myth? Clin Ophthalmol. 2015;9:785–793. doi: 10.2147/OPTH.S78918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rüfer F, Erb C. Einflüsse des trockenen Auges auf die Glaukomdiagnostik. [Influence of dry eye syndrome on glaucoma diagnostic procedures] Ophthalmologe. 2012;109(11):1082–1086. doi: 10.1007/s00347-012-2640-x. German. [DOI] [PubMed] [Google Scholar]
- 51.Broadway DC, Chang LP. Trabeculectomy, risk factors for failure and the preoperative state of the conjunctiva. J Glaucoma. 2001;10(3):237–249. doi: 10.1097/00061198-200106000-00017. [DOI] [PubMed] [Google Scholar]
- 52.Romero-Díaz de León L, Morales-León JE, Ledesma-Gil J, Navas A. Conjunctival and corneal sensitivity in patients under topical antiglaucoma treatment. Int Ophthalmol. 2016;36(3):299–303. doi: 10.1007/s10792-015-0115-1. [DOI] [PubMed] [Google Scholar]
- 53.Van Went C, Alalwani H, Brasnu E, et al. Évaluation de la sensibilité cornéenne chez les patients traités médicalement pour un glaucome ou une hypertonie oculaire. [Corneal sensitivity in patients treated medically for glaucoma or ocular hypertension] J Fr Ophtalmol. 2011;34(10):684–690. doi: 10.1016/j.jfo.2011.07.011. French. [DOI] [PubMed] [Google Scholar]
- 54.Sarkar J, Chaudhary S, Namavari A, et al. Corneal neurotoxicity due to topical benzalkonium chloride. Invest Ophthalmol Vis Sci. 2012;53(4):1792–1802. doi: 10.1167/iovs.11-8775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martone G, Frezzotti P, Tosi GM, et al. An in vivo confocal microscopy analysis of effects of topical antiglaucoma therapy with preservative on corneal innervation and morphology. Am J Ophthalmol. 2009;147(4):725–735.e1. doi: 10.1016/j.ajo.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 56.Lin Z, Liu X, Zhou T, et al. A mouse dry eye model induced by topical administration of benzalkonium chloride. Mol Vis. 2011;17:257–264. [PMC free article] [PubMed] [Google Scholar]
- 57.De Saint Jean M, Brignole F, Bringuier AF, Bauchet A, Feldmann G, Baudouin C. Effects of benzalkonium chloride on growth and survival of Chang conjunctival cells. Invest Ophthalmol Vis Sci. 1999;40(3):619–630. [PubMed] [Google Scholar]
- 58.Buron N, Micheau O, Cathelin S, Lafontaine PO, Creuzot-Garcher C, Solary E. Differential mechanisms of conjunctival cell death induction by ultraviolet irradiation and benzalkonium chloride. Invest Ophthalmol Vis Sci. 2006;47(10):4221–4230. doi: 10.1167/iovs.05-1460. [DOI] [PubMed] [Google Scholar]
- 59.Hamard P, Blondin C, Debbasch C, Warnet JM, Baudouin C, Brignole F. In vitro effects of preserved and unpreserved antiglaucoma drugs on apoptotic marker expression by human trabecular cells. Graefes Arch Clin Exp Ophthalmol. 2003;241(12):1037–1043. doi: 10.1007/s00417-003-0777-7. [DOI] [PubMed] [Google Scholar]
- 60.Yu AL, Fuchshofer R, Kampik A, Welge-Lüssen U. Effects of oxidative stress in trabecular meshwork cells are reduced by prostaglandin analogues. Invest Ophthalmol Vis Sci. 2008;49(11):4872–4880. doi: 10.1167/iovs.07-0984. [DOI] [PubMed] [Google Scholar]
- 61.Samples JR, Binder PS, Nayak S. The effect of epinephrine and benzalkonium chloride on cultured corneal endothelial and trabecular meshwork cells. Exp Eye Res. 1989;49(1):1–12. doi: 10.1016/0014-4835(89)90071-7. [DOI] [PubMed] [Google Scholar]
- 62.Sullivan DA, Rocha EM, Aragona P, et al. TFOS DEWS II Sex, Gender and Hormones Report. Ocul Surf. 2017;15(3):284–333. doi: 10.1016/j.jtos.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 63.Sharma A, Hindman HB. Aging: a predisposition to dry eyes. J Ophthalmol. 2014;2014:781683. doi: 10.1155/2014/781683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci. 2011;52(4):1994–2005. doi: 10.1167/iovs.10-6997e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80(5):389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rossi GC, Pasinetti GM, Scudeller L, Raimondi M, Lanteri S, Bianchi PE. Risk factors to develop ocular surface disease in treated glaucoma or ocular hypertension patients. Eur J Ophthalmol. 2013;23(3):296–302. doi: 10.5301/ejo.5000220. [DOI] [PubMed] [Google Scholar]
- 67.Ghosh S, O’Hare F, Lamoureux E, Vajpayee RB, Crowston JG. Prevalence of signs and symptoms of ocular surface disease in individuals treated and not treated with glaucoma medication. Clin Experiment Ophthalmol. 2012;40(7):675–681. doi: 10.1111/j.1442-9071.2012.02781.x. [DOI] [PubMed] [Google Scholar]
- 68.Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000;118(9):1264–1268. doi: 10.1001/archopht.118.9.1264. [DOI] [PubMed] [Google Scholar]
- 69.Moss SE, Klein R, Klein BE. Incidence of dry eye in an older population. Arch Ophthalmol. 2004;122(3):369–373. doi: 10.1001/archopht.122.3.369. [DOI] [PubMed] [Google Scholar]
- 70.Ezuddin NS, Alawa KA, Galor A. Therapeutic strategies to treat dry eye in an aging population. Drugs Aging. 2015;32(7):505–513. doi: 10.1007/s40266-015-0277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Foulks GN. Pharmacological management of dry eye in the elderly patient. Drugs Aging. 2008;25(2):105–118. doi: 10.2165/00002512-200825020-00003. [DOI] [PubMed] [Google Scholar]
- 72.Ding J, Sullivan DA. Aging and dry eye disease. Exp Gerontol. 2012;47(7):483–490. doi: 10.1016/j.exger.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnson AT, Drack AV, Kwitek AE, Cannon RL, Stone EM, Alward WL. Clinical features and linkage analysis of a family with autosomal dominant juvenile glaucoma. Ophthalmology. 1993;100(4):524–529. doi: 10.1016/s0161-6420(13)31615-7. [DOI] [PubMed] [Google Scholar]
- 74.Huang W. Pediatric glaucoma: a review of the basics. 2014. [Accessed August 16, 2016]. Available from: https://www.reviewofophthalmology.com/article/pediatric-glaucoma-a-review-of-the-basics.
- 75.Paschides CA, Stefaniotou M, Papageorgiou J, Skourtis P, Psilas K. Ocular surface and environmental changes. Acta Ophthalmol Scand. 1998;76(1):74–77. doi: 10.1034/j.1600-0420.1998.760113.x. [DOI] [PubMed] [Google Scholar]
- 76.Wolkoff P. “Healthy” eye in office-like environments. Environ Int. 2008;34(8):1204–1214. doi: 10.1016/j.envint.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 77.Wolkoff P. Ocular discomfort by environmental and personal risk factors altering the precorneal tear film. Toxicol Lett. 2010;199(3):203–212. doi: 10.1016/j.toxlet.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 78.Wolkoff P, Nojgaard JK, Franck C, Skov P. The modern office environment desiccates the eyes? Indoor Air. 2006;16(4):258–265. doi: 10.1111/j.1600-0668.2006.00429.x. [DOI] [PubMed] [Google Scholar]
- 79.Blehm C, Vishnu S, Khattak A, Mitra S, Yee RW. Computer vision syndrome: a review. Surv Ophthalmol. 2005;50(3):253–262. doi: 10.1016/j.survophthal.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 80.Rosenfield M. Computer vision syndrome: a review of ocular causes and potential treatments. Ophthalmic Physiol Opt. 2011;31(5):502–515. doi: 10.1111/j.1475-1313.2011.00834.x. [DOI] [PubMed] [Google Scholar]
- 81.Pimenidi MK, Polunin GS, Safonova TN. Meibomian gland disfunction in computer vision syndrome. Vestn Oftalmol. 2010;126(6):49–52. Russian. [PubMed] [Google Scholar]
- 82.Fenga C, Aragona P, Cacciola A, et al. Meibomian gland dysfunction and ocular discomfort in video display terminal workers. Eye (Lond) 2008;22(1):91–95. doi: 10.1038/sj.eye.6703025. [DOI] [PubMed] [Google Scholar]
- 83.Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015;112(5):71–81. doi: 10.3238/arztebl.2015.0071. quiz 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boimer C, Birt CM. Preservative exposure and surgical outcomes in glaucoma patients: the PESO study. J Glaucoma. 2013;22(9):730–735. doi: 10.1097/IJG.0b013e31825af67d. [DOI] [PubMed] [Google Scholar]
- 85.Thieme H, van der Velden KK. Konservierungsmittel aus Sicht der Glaukomchirurgie. [Preservatives from the perspective of glaucoma surgery] Ophthalmologe. 2012;109(11):1073–1076. doi: 10.1007/s00347-012-2642-8. German. [DOI] [PubMed] [Google Scholar]
- 86.Baudouin C, Pisella PJ, Fillacier K, et al. Ocular surface inflammatory changes induced by topical antiglaucoma drugs: human and animal studies. Ophthalmology. 1999;106(3):556–563. doi: 10.1016/S0161-6420(99)90116-1. [DOI] [PubMed] [Google Scholar]
- 87.Baudouin C. Mechanisms of failure in glaucoma filtering surgery: a consequence of antiglaucomatous drugs? Int J Clin Pharmacol Res. 1996;16(1):29–41. [PubMed] [Google Scholar]
- 88.Baudouin C. Side effects of antiglaucomatous drugs on the ocular surface. Curr Opin Ophthalmol. 1996;7(2):80–86. doi: 10.1097/00055735-199604000-00014. [DOI] [PubMed] [Google Scholar]
- 89.Tan LL, Morgan P, Cai ZQ, Straughan RA. Prevalence of and risk factors for symptomatic dry eye disease in Singapore. Clin Exp Optom. 2015;98(1):45–53. doi: 10.1111/cxo.12210. [DOI] [PubMed] [Google Scholar]
- 90.Paulsen AJ, Cruickshanks KJ, Fischer ME, et al. Dry eye in the beaver dam offspring study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014;157(4):799–806. doi: 10.1016/j.ajo.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Versura P, Campos EC. Menopause and dry eye. A possible relationship. Gynecol Endocrinol. 2005;20(5):289–298. doi: 10.1080/09513590400027257. [DOI] [PubMed] [Google Scholar]
- 92.Jin X, Lin Z, Liu Y, Lin L, Zhu B. Hormone replacement therapy benefits meibomian gland dysfunction in perimenopausal women. Medicine (Baltimore) 2016;95(31):e4268. doi: 10.1097/MD.0000000000004268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Affinito P, Di Spiezio Sardo A, Di Carlo C, et al. Effects of hormone replacement therapy on ocular function in postmenopause. Menopause. 2003;10(5):482–487. doi: 10.1097/01.GME.0000063568.84134.35. [DOI] [PubMed] [Google Scholar]
- 94.Feng Y, Feng G, Peng S, Li H. The effect of hormone replacement therapy on dry eye syndrome evaluated with Schirmer test and break-up time. J Ophthalmol. 2015;2015:420302. doi: 10.1155/2015/420302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guaschino S, Grimaldi E, Sartore A, et al. Visual function in menopause: the role of hormone replacement therapy. Menopause. 2003;10(1):53–57. doi: 10.1097/00042192-200310010-00009. [DOI] [PubMed] [Google Scholar]
- 96.Pelit A, Bagis T, Kayaselcuk F, Dursun D, Akova Y, Aydin P. Tear function tests and conjunctival impression cytology before and after hormone replacement therapy in postmenopausal women. Eur J Ophthalmol. 2003;13(4):337–342. doi: 10.1177/112067210301300402. [DOI] [PubMed] [Google Scholar]
- 97.Gomes JAP, Azar DT, Baudouin C, et al. TFOS DEWS II Iatrogenic Report. Ocul Surf. 2017;15(3):511–538. doi: 10.1016/j.jtos.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 98.Majumdar S, Hippalgaonkar K, Repka MA. Effect of chitosan, benzalkonium chloride and ethylenediaminetetraacetic acid on permeation of acyclovir across isolated rabbit cornea. Int J Pharm. 2008;348(1–2):175–178. doi: 10.1016/j.ijpharm.2007.08.017. [DOI] [PubMed] [Google Scholar]