Abstract
Given the widespread availability of effective anti-retroviral therapy, engagement of HIV-infected persons in care is a global priority. We reviewed 51 studies, published in the past decade, assessing strategies for improving linkage to and retention in HIV care. The review included studies from highly resourced settings (HRS) and resource-poor settings (RPS), specifically the USA and sub-Saharan Africa. In HRS, strength-based case management was best supported for improving linkage and retention in care; peer navigation and clinic-based health promotion were supported for improving retention. In RPS, point of care CD4 testing was best supported for improving linkage to care; decentralization, and task-shifting for improving retention. Novel interventions continue to emerge in HRS and RPS, yet many strategies have not been adequately evaluated. Further consideration should be given to analyses that identify which interventions, or combinations of interventions, are most effective, cost-effective, scalable, and aligned with patient preferences for HIV care.
Keywords: HIV, Antiretroviral therapy, Treatment cascade, Engagement in care, Linkage to care, Retention, Healthcare utilization, Patient-centered care
Introduction
With the advent of effective antiretroviral therapy (ART) in 1996, the trajectory of the HIV pandemic has improved dramatically [1]. Benefits of access to ART and retention in HIV care include decreased risk of transmission, attenuation of chronic end-organ effects of untreated viremia, and an overall increase in life expectancy [2–6]. Given the unequivocal benefits of HIV care, major initiatives have focused on improving engagement in HIV care. In the USA, the 2010 National HIV/AIDS Strategy (NHAS) provided 5-year benchmarks for improved engagement in HIV care. By 2015, the strategy aims to “increase the proportion of newly diagnosed persons linked to care within 3 months of diagnosis from 65% to 85%” and increase the number of patients in Ryan White-funded clinics that meet criteria for retention in care from 73 to 80% [7]. On a global scale, the Treatment 2015 Initiative launched by the Joint United Nations Programme on HIV/AIDS forwarded the goal of having 15 million people on ART worldwide by 2015 [8]. Unfortunately, as of the end of 2011, 30% of all persons diagnosed with HIV in the USA were not engaged in care [9••]. In sub-Saharan Africa (SSA), which accounts for two thirds of all HIV-infected persons worldwide, only 32% of infected persons were on ART as of December 2012 [8]. In this article, we review recent published literature on interventions to improve linkage and retention in care with a focus on the USA and sub-Saharan Africa (SSA).
Definitions of Engagement in Care
The US Health Resources and Services Administration HIV/AIDS Bureau (HRSA-HAB) defines timely “linkage to care” as an initial encounter with an HIV care provider within 90 days of diagnosis [10]. At present, no universally accepted criterion for timely linkage to HIV care exists for resource-poor settings (RPS) [11•]. Definitions for “retention in care” are more variable. HRSA-HAB criteria for adequate retention in care is two kept visits at least 90 days apart in a 12 month period, the standard used for performance assessments of Ryan White-funded clinics and the preferred measure for NHAS benchmarks [7, 10]. However, the optimal measure of adequate engagement is still an area of active research. Mugavero and colleagues compared measures based on kept visits to measures based on enumeration of missed visits and concluded that no single measure significantly outperformed others in quantifying adequate engagement [12•]. Accordingly, heterogeneous measures of linkage and retention were used in the studies included in this review.
State of Engagement in Care
In 2011, Gardner and colleagues presented the HIV “treatment cascade” to depict the state of the HIV epidemic in the USA, stepwise from diagnosis to viral suppression [13]. Recent estimates indicate that only 66% of HIV-infected persons in the USA had an initial care encounter within 90 days of diagnosis. Furthermore, only 37% met HRSA-HAB criteria for retention in care [14]. Large cohort analyses have corroborated these findings. For example, the North American AIDS Cohort Collaboration on Research and Design (NAACCORD) followed over 61,000 HIV-infected persons in the USA and Canada since 2006. Within this population, only 75% met HRSA-HAB criteria for retention in care, highlighting the difficulty in keeping patients engaged in care even in highly scrutinized cohorts [15•]. Many studies have examined attrition of patients on ART in SSA. A systematic review of attrition rates among persons on ART in SSA, based on 33 studies covering over 226,000 patients, revealed that at 36 months, only 64.6% remained on ART [16••]. Few studies assessed losses in the continuum of care prior to ART initiation [17]. Kranzer and colleagues attempted to reconstruct the treatment cascade in SSA using aggregated data from previously published studies. In their analysis, 50% of persons diagnosed with HIV completed evaluation for ART eligibility. Of those started on ART, 65% were retained in care, although no standardized definition of retention was used [18•].
Current estimates of linkage and retention in care, and a list of key strategies for their improvement identified in this review, are summarized in Fig. 1.
Fig. 1.
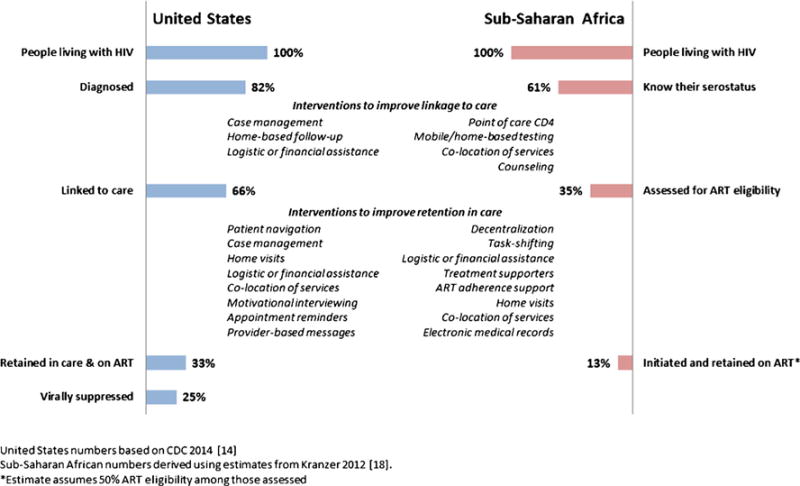
“Treatment cascade” in highly resourced and resource-poor settings and interventions to improve engagement in care
Strategies to Improve Linkage to HIV Care in Highly Resourced Settings
In 2012, an expert panel from the International Association of Physicians in AIDS Care (IAPAC) released guidelines for improving entry into and retention in HIV care. After reviewing available evidence, the panel recommended two strategies for improving linkage to care based on the published literature (see Table 1 for a description of the IAPAC Grading Scale): (1) strength-based case management sessions for newly diagnosed persons (level of evidence: high; strength of recommendation: moderate); (2) intensive outreach for individuals not engaged in care within 6 months of diagnosis (level of evidence: medium; strength of recommendation: optional) [19••]. The highest quality of evidence supporting strength-based case management as a strategy for improving linkage to care comes from the Antiretroviral Treatment and Access Study (ARTAS), a randomized controlled trial conducted at four academic HIV care centers in the USA totaling 273 participants (see Table 2). Based on the strength-based theory of cognitive behavioral therapy, providers assist patients in identifying and re-iterating their strongest crisis resolution skills and outline a plan to address the crisis based on identified strengths. Participants were assigned to either five sessions of strength-based counseling in 90 days or the standard of care (SOC). At the end of the study, 78% of patients in the intervention group visited an HIV clinician within 6 months of diagnosis compared to 60% in the control group (RR 1.36, p<0.001) [20]. A follow-up single-arm study was conducted in 10 health departments and community-based care centers around the USA that showed similar linkage to care rates (79%) [21]. Notably, a study of 104 inmates in North Carolina reported no significant improvement in linkage rates after discharge from incarceration [22].
Table 1.
IAPAC grading scale for quality of the body of evidence and strength of recommendations [19••]
| Quality or strength | Interpretation |
|---|---|
| Quality of the body of evidence | |
| Excellent | 1. RCT evidence without important limitations 2. Overwhelming evidence from observational studies |
| High | 1. RCT evidence with important limitations 2. Strong evidence from observational studies |
| Medium | 1. RCT evidence with critical limitations 2. Observational study evidence without important limitations |
| Low | 1. Observational study evidence with important or critical limitations |
| Strength of recommendation | |
| Strong | Almost all patients should receive the recommended course of action |
| Moderate | Most patients should receive the recommended course of action. However, other choices may be appropriate for some patients |
| Optional | There may be consideration for this recommendation on the basis of individual patient circumstances. Not recommended routinely |
RCT randomized, controlled trial
Table 2.
Details of reviewed studies assessing strategies to improve linkage to HIV care
| First author (year) |
Study dates | Location | Sample size (population) |
Design | Intervention | Primary outcome | Results |
|---|---|---|---|---|---|---|---|
| Highly resourced settings | |||||||
| Bocour [23] (2013) |
2007–2011 | New York City | 10,095 (newly diagnosed) | 2 groups, retrospective | Home-based assessment of patient’s knowledge of treatment plan by Field Services Unit (FSU) | CD4 reported within 90 days of diagnosis | 79% (FSU) vs. 66% (non-FSU) p<0.0001 |
| Craw [21] (2008) |
Apr 2005–Oct 2006 | Anniston, AL; Atlanta; Baltimore; Baton Rouge, LA; Chicago; Columbia, SC; Jacksonville, FL; Kansas City; Miami; Richmond, VA | 626 | Single-arm, prospective | 5 strength-based case management sessions over 90 days | Initial visit with HIV care provider within 6 months of enrollment | 79% |
| Gardner [20] (2005) |
Mar 2001–May 2002 | Los Angeles, Atlanta, Miami, Baltimore | 273 (newly diagnosed) | 2-arm, randomized | Strength-based case management sessions | Initial visit with HIV care provider within 6 months of discharge | 78 vs. 64% (intervention vs. control) Adjusted RR 1.36 p<0.001 |
| Naar-King [24] (2007) |
Not reported | Detroit Los Angeles; Portland, OR; Washington, DC | 119 | Single-arm prospective, observational | Case management, financial assistance | Initial visit with HIV care provider within 6 months of enrollment | 92% |
| Wohl [22] (2011) |
Not reported | North Carolina | 104 (inmates) | 2-arm, randomized | Series of strength-based counseling sessions between 3 months pre-discharge and 6 months post-discharge vs. SOC | Initial visit with HIV care provider within 6 months of discharge | 90.7% (intervention) vs. 89.1% (control) p> 0.5 |
| Resource-poor settings | |||||||
| Faal [25] (2011) |
Aug–Dec 2009 | South Africa | 344 | 3-arm, randomized | Point of care (POC) CD4: POC CD4 vs. receipt of results after 1 week (SOQ+ written material on HIV care pathway vs. SOC only | Enrollment in HIV care within 1 month of pre-ART care or within 3 months of ART initiation | POC vs. 2 standard arms combined RR 2.1 (95% CI 1.39–3.17) |
| Govindasamy [26] (2013) |
Mar 2010–Sep 2011 | South Africa | 9806 screened | Single-arm, prospective, observational | Mobile testing unit and referral service | Initial visit within 1, 3, or 6 months depending on CD4 at diagnosis | 5.5% tested positive 51.3% were linked to care in recommended time frame |
| Jani [27] (2011) |
Sep 2009–Mar 2010 | Mozambique | 929 | Single population, retrospective, pre-post | Point of care CD4 vs. standard of care | CD4 staging appointment within 90 days of enrollment | 21% pre vs. 57% post Adjusted OR 0.20 (95% CI 0.15–0.27) |
| Killiam [28] (2010) |
Jul 2007–Jul 2008 | Zambia | 716 (pre) 846 (post) |
Single population, pre-post | Integration of ART care into antenatal care | Enrollment in ART care within 60 days of diagnosis | 25.6% (pre) vs. 44.4% (post) Adjusted OR 2.06 (95% CI 1.27–3.34) |
| Larson [29] (2012) |
May–Oct 2010 | South Africa | 508 | 2-arm, prospective, observational | Point of care CD4 vs. SOC | Initial visit with provider within 8 weeks of diagnosis | 59.4% in POC group 46.7% in SOC group Adjusted RR 1.25 (95% CI 1.00–1.57) |
| Muhamedi [30] (2011) |
Jul 2009–Jun 2010 | Uganda | 400 | 2-arm, randomized | Counseling after new diagnosis of HIV vs. SOC | Initial appointment within 3 months of diagnosis | 61.5% (intervention) 38.5% (control) Adjusted RR 1.80 (95% CI 1.40–2.10) |
| Patten [31] (2013) |
May 2010–April 2011, Aug 2011–July 2012 | South Africa | 272 (pre) 304 (post) |
Single population, observational, pre-post | Point of care CD4 testing vs. SOC | Initiation of ART within 90 days of diagnosis for ART eligibility | 38% (pre) 43% (pre) v. 50% (post), p = 0.5 |
| van Rooyen [32] (2013) |
Mar 2011–Mar 2012 | South Africa | 671 | Single-arm, observational | Home-based point of care test, home-based counseling | Attendance of visit at 1-month post-diagnosis | 99% |
| Waxrnan [33] (2007) |
Jan–Apr 2006 | Kenya | 1339 screened | Retrospective, single-arm | Testing and referral in emergency department | Attendance of initial encounter at 1-month post-diagnosis | 22.7% tested positive; 65% compliant with 1-month visit |
CI confidence interval, HR hazard ratio, LTFU lost to follow up, OR odds ratio, RR risk ratio, SOC standard of care
Evidence supporting the efficacy of outreach efforts to improve linkage in care is mostly observational (Table 2). Naar-King and colleagues published a study of programmatic data from 10 sites under the HRSA Special Projects of National Significance (HRSA-SPNS) Outreach Initiative. The Outreach Initiative provided a combination of “community outreach programs, case management, motivational interventions and ancillary services” to study participants. Among 119 newly diagnosed persons enrolled during the study period, 92% visited an HIV clinician within 6 months of diagnosis [24]. A similar study with 334 minority men who have sex with men (MSM) enrolled at eight SPNS-funded clinics showed that 87% of newly diagnosed patients were linked to an HIV provider within 90 days of diagnosis [34]. Notably, our review did not identify studies assessing the effectiveness of appointment reminders post-diagnosis, transportation services, financial incentives, or peer navigators in improving linkage to HIV care.
Strategies to Improve Retention in HIV Care in Highly Resourced Settings
The 2012 IAPAC panel synthesized data available to recommend one strategy for improving retention in care—peer/paraprofessional navigation (level of evidence: medium; strength of recommendation: optional) [19••]. The most notable study supporting use of peer navigators was conducted at four HRSA-SPNS Outreach Initiative sites involving 437 HIV-positive patients with a history of suboptimal engagement in care (Table 3). As described by the authors, peer navigators assist patients in optimizing utilization of available clinical resources, developing effective communication with providers and maneuvering through the complexity of multidisciplinary treatment [35]. In the 12 months following the implementation of the peer navigator program, the proportion of patients who attended two clinic visits in a 6-month period increased from 64 to 79%. Another study of 51 disengaged HIV-infected women reported an increase in the proportion of women who attended all clinic visits over a 6-month period from 10 to 58% after the implementation of a nurse-patient navigator program. This program was part of an intervention that included transportation assistance [36].
Table 3.
Details of reviewed studies assessing strategies to improve retention in HIV care
| First author (year) | Study dates | Location | Sample size (population) |
Design | Intervention | Primary outcome | Results |
|---|---|---|---|---|---|---|---|
| Highly resourced settings | |||||||
| Andersen [36] (2007) |
Not reported | Detroit, MI | 61 (women) | 2-arm, prospective, pre-post | Nurse-based counseling and transportation assistance vs. transport alone | No missed appointments in 6 months | Transport plus: 10% pre 58% post Transport only: 21% pre vs. 61%post (at a 12-month follow-up) |
| Bocour [23] (2013) |
2007–2011 | New York City | 10,095 (newly diagnosed) | 2 groups, retrospective | Home-based assessment of patient’s knowledge of treatment plan by Field Services Unit (FSU) | Two CD4 counts separated by at least 90 days in a 12-month period | 84 vs. 87% (non-FSU vs. FSU) p<0.001 |
| Bradford [35] (2007) |
Oct 2003–Jun 2006 | Boston, Portland, Seattle, Washington, DC | 437 | Single-arm, prospective, pre-post | Patient navigation by trained staff | 2 or more clinic appointments in a 6-month period | 64% pre-intervention 79% 12 months post intervention |
| Cabral [40] (2007) |
2004–2006 | 10 US cities | 773 | Single-arm, retrospective, pre-post | Appointment reminders, transportation and housing assistance, and case management | Gap of 4 months or more for scheduled clinic visits | HR 0.45 (95% CI 0.26–0.78) |
| Davila [41] (2012) |
Jan 2002–Aug 2008 | Houston | 174 (Latino and Afiican-American youth) | 3 groups, retrospective | Co-location of youth-based services vs. youth-based services and educational support vs. no services | 3 or more quarters with at least 1 visit in a 12-month period | Referent: Youth services No youth services: OR 0.42 (95% CI 0.17–1.43) Youth services plus: OR 1.18 (95%CI 0.55–2.53) |
| Enriquez [42] (2007) |
Mar 2005–Mar 2007 | Kansas City | 43 (Latinos) | 1 group, retrospective, pre-post | Bilingual peer educators and case managers | Number of visits with HIV provider per year | Mean: 2.81 (pre) vs. 5.30 (post) |
| Gardner [20] (2005) |
Mar 2001–May 2002 | Atlanta, Baltimore, Miami, Los Angeles | 273 (newly diagnosed) | 2-arm, randomized | Strength-based case management sessions | 2 or more clinic visits in a 12-month period | 64 vs. 49% (intervention vs. control) RR 1.41 p<0.001 |
| Gardner [43•] (2012) |
May 2008–May 2010 | Baltimore; Boston; Birmingham, AL; Brooklyn, NY; Bronx, NY; Houston; Miami | 8535 | Single population, cross-sectional, pre-post | Provider-based messages, clinic posters, and brochures promoting care engagement | 2 consecutive HIV care visits separated by 90 days in a 12-month period | 48.6 vs. 52.2% (pre vs. post) |
| Gardner [44•] (2014) |
2010–2011 | Boston, Miami; Baltimore; Birmingham, AL; Houston; New York City | 1838 | 3-arm, randomized | Periodic face-to-face contact and periodic phone calls from interventionist (EC) vs. EC and strength-based skill building sessions vs. standard of care (SOC) | 2 or more visits separated by >90 days in a 12-month period | SOC 45.6% EC only 55.6% EC + skills 55.8% |
| Hightow-Weidman [34] (2011) |
Jun 2006–Aug 2009 | Chapel Hill, NC | 89 (Latino and African-American MSM) | 2 groups, retrospective | Strength-based case management, appointment coordination, and co-location of services | 3 or more HIV care visits in the first 12 months after enrollment | 80 vs. 67% (intervention vs. control) |
| Naar-King [38] (2009) |
Mar 2006 | Detroit | 87 (adolescents and young adults) | 2-arm, randomized | Motivational interviewing by case managers | Gaps in scheduled appointments over a 12-month period | Mean: 2.76 vs. 1.33 (pre vs. post) |
| Purcell [45] (2007) |
Aug 2001–March 2005 | Miami, New York City, San Francisco | 795 (injection drug users) | 2-arm, randomized | Peer 1-on-l vs. video-based mentoring sessions | 2 or more clinic appointments in a 6-month period | 69 vs. 64% (peer vs. video) at 12 months OR 1.14 (95% CI 0.82–1.58) |
| Willis [37] (2013) |
Oct 2009–Sep 2010 | Washington, DC | 5631 | Observational | On-site case management vs. standard clinic | 2 or more clinic visits separated by 90 days in a 12-month period | OR 4.13 (95% CI 1.93–8.85) |
| Wohl [39] (2011) |
Apr 2006–Apr 2009 | Los Angeles | 61 (Latino and African-American MSM) | 1 group, retrospective, post | Case management with strength-based counseling | 2 or more clinic appointments in a 6-month period | 70% at 6 months |
| Resource-poor settings | |||||||
| Alamo [46] (2012) | Oct 2008–June 2009 | Uganda | 6500 encounters | 1 group, prospective pre-post | Implementation of electronic medical record | LTFU: Absent 3 or more months after last scheduled appointment |
LTFU: 10.9 to 4.8% (pre vs. post) |
| Balcha [47] (2010) | Feb 2007–Feb 2009 | Ethiopia | 1709 | 2 groups, retrospective | Decentralization: Health center-based vs. hospital-based care |
LTFU: Absent 3 or more months after last scheduled appointment |
10% (health center-based) vs. 23% (hospital-based) |
| Bedelu [48] (2007) | Jan 2004–Jul 2006 | South Africa | 1025 | Prospective, observational | Decentralization: Health center-based vs. hospital-based care | LTFU: Not defined |
2% (clinic-based) vs. 19% (hospital-based) at 1 year |
| Braitstein [49] (2012) |
Mar 2007–Mar 2009 | Kenya | 4958 | 2 groups, retrospective | Express care for high-risk patients vs. SOC | LTFU: Absent 3 or more months after last scheduled appointment |
Adjusted HR 0.59 (95% CI 0.45–0.77) |
| Brennan [50•] (2011) |
Feb 2008–Jan 2009 | South Afiica | 2848 | 2-arm, prospective, matched | Decentralization/task-shifting: Down referral to health center (nurse) vs. hospital-based (physician) care |
LTFU: Absent 3 or more months after last scheduled appointment |
1.7% in down referral vs. 5.1% in hospital-based Combined attrition: RR 0.27 (95% CI 0.15–0.49) at 1 year |
| Chan [51] (2010) | October 2004–December 2008 | Malawi | 8093 | 2 groups, retrospective | Decentralization: Health center-based (rural) vs. hospital-based (urban) care |
LTFU: Absent 3 or more months after last scheduled appointment |
Rural vs. urban: Adjusted OR 0.48 (95% CI 0.40–0.58) |
| Decroo [52] (2010) | Feb 2008–May 2010 | Mozambique | 1384 | 1 group, retrospective | Self-forming groups for peer ART adherence support | Retention in care: No death or LTFU (absent for 3 or more months from care) |
97.5% retention rate at 13 months |
| Fatti [53] (2010) | 2004–2007 | South Afiica | 29,203 | 3 groups, retrospective | Decentralization: Regional hospital vs. district hospital vs. health center-based care (PHC) |
LTFU: Absent 3 or more months after last scheduled appointment |
LTFU (PHC referent) Adjusted HR: Regional hospital 2.19 (95% CI 1.94–2.47) District hospital 1.60 (95% CI 1.30–1.99), |
| Fatti [54] (2012) | 2004–2010 | South Afiica | 66,953 | 2 groups, prospective, observational | Protocolized home visits by patient advocates (PA) vs. standard of care | Attrition: Dead or absent from clinic for 180 days or more |
Adjusted HR 0.65 (95% CI 0.59–0.72) at a median of 14.8 months |
| Franke [55•] (2013) | June 2007–Aug 2008 | Rwanda | 610 | 2 groups, prospective, observational | Daily visits by community health workers (CHW), monthly food rations, accompanied to clinic by CHWs, and financial support vs. SOC | LTFU: Absent 2 or more months after last scheduled appointment |
Adjusted HR 0.17 (95% CI 0.09–0.35) at 1 year |
| Greig [56] (2012) | 2003–2007 | 9 SSA countries | 15,403 | Retrospective, observational | Decentralization: Integrated-based (local primary care) vs. vertical (HIV-only tertiary center) care |
LTFU: Absent 2 or more months after last scheduled appointment |
Integrated vs. vertical: HR 0.71 (95% CI 0.61–0.83) |
| Humphreys [57] (2010) |
Jan–Nov 2007 | Swaziland | 582 | 2-arm, prospective, non-randomized | Decentralization/task-shifting | LTFU: Absent 3 or more months after last scheduled appointment |
2.4 (nurse care) vs. 1.3 (standard care), RR 1.85 (95% CI 0.41–8.34) at 6 months |
| Jaffar/Amuron [58, 59•] (2009) | Feb 2005–Jan 2009 | Uganda | 1459 | 2-arm, cluster-randomized equivalence | Monthly home-based vs. standard clinic-based care | LTFU: Not defined |
1 vs. 2% (home vs. clinic) at median of 28 months of follow-up |
| Kohler [60] (2011) | 2005–2007 | Kenya | 1024 (ART-ineligible) | Prospective, observational | Received cotrimoxazole prophylaxis at diagnosis vs. SOC | LTFU: Absent 30 days or more months after last scheduled appointment |
Adjusted HR 2.64 (95% CI 1.95–3.57) at 1 year |
| Kimutsor [61] (2011) |
Mar–Sep 2010 | Uganda | 174 | 2-arm, randomized | Patient-selected treatment supporters (DOT, appointment accompaniment, group education) vs. SOC | Missed visits | 1 (TS group) vs. 7 (SOC) at 28 weeks |
| Lambdin [62] (2013) |
Jan 2006–Jan 2008 | Mozambique | 11,775 | 2 groups, retrospective, observational | Decentralization: Integrated-based (local primary care) care vs. vertical-based (HIV-only tertiary center) care |
LTFU: Absent 2 or more months after last scheduled appointment |
Integrated vs. vertical: HR 1.75 (95% CI 1.04–2.94) |
| Massaquoi [63] (2009) |
Jun 2006–June 2007 | Malawi | 4074 | 2 groups, retrospective | Decentralization: Hospital-based vs. health center-based care |
In care and alive on ART | 85% in both hospital and health center-based groups |
| McGuire [64] (2012) |
Aug 2001–Dec 2008 | Malawi | 15,412 | 2 groups, retrospective | Decentralization: Health center-based (decentralized) vs. hospital-based care (centralized) |
Attrition: Death or LTFU (absent for 2 or more months after last scheduled appointment) |
Attrition rates at 2 years: 9.9 per 100 person years (decentralized) vs. 20.8 per 100 person years (centralized) |
| Odale [65] (2012) | 2007–2010 | Nigeria | 6408 | 2 groups, retrospective | Decentralization: Secondary vs. tertiary hospital-based care |
Attrition: Death or LTFU (absent for 3 or more months after last scheduled appointment) |
10.7% at secondary hospitals and 19.6% tertiary hospitals (p<0.001) at 24 months |
| Same [66•] (2010) | Feb 2005–Jan 2009 | South Africa | 812 | 2-arm, randomized, non-inferiority | Task-shifting: Nurse vs. physician-based HIV care |
Missed 3 consecutive study visits (LTFU) | 4% in nurse group and 15.4% in physician group (HR 1.42, 95% CI 0.63–3.20) at 120 weeks |
| Selke [67] (2010) | Mar 2006–Apr 2008 | Kenya | 208 | 2-arm, randomized | Monthly home visits + clinic visits every 3 months vs. monthly clinic visits (SOC) | LTFU (definition not reported) | 5.2 vs. 4.5% (intervention vs. control), p>0.5 at 24 months |
| Shumbusho [68] (2009) |
Sep 2005–Mar 2008 | Rwanda | 1076 | 1 group, retrospective | Task-shifting: Nurse-based care |
LTFU: Absent 3 or more months after last scheduled appointment |
91% at 24 months |
| Torpey [69] (2008) | Mar–Apr 2007 | Zambia | 3903 (pre), 4972 (post) | 1 group, pre-post | Adherence support by community-based volunteers | LTFU: Missing 3 consecutive monthly pharmacy appointments |
15% (pre) vs. 0% (post) at 12 months |
CI confidence interval, HR hazard ratio, LTFU lost to follow up, OR odds ratio, RR risk ratio, SOC standard of care
Strength-based case management has also been validated as a technique to retain patients in HIV care. As part of the ARTAS, patients were followed for at least 12 months to evaluate longitudinal engagement in care outcomes. Patients who were randomized to the five-session case management protocol were more likely to meet the HRSA definition for retention in care (64 vs. 49%) [20]. More recently, a large retrospective study of 14 HIV care facilities in Washington, DC investigated retention in care in facilities with on-site case management compared to those without it using the enhanced HIV/AIDS reporting system (eHARS). Among 5631 prevalent cases captured in the analysis, persons who attended a clinic with on-site case management were more likely to be retained in care (adjusted OR 4.13, 95% CI 1.93–8.85) [37]. Smaller, single-arm studies have also reported improvements in retention in care due to case management [38, 39].
Recent efforts have been aimed at identifying less labor-intensive interventions to improve retention. The largest of these was a pre-post intervention analysis conducted at six university-based HIV care centers as part of the Retention in Care Study. The intervention consisted solely of brochures, examination room posters, and brief standardized verbal messages from clinic staff. Of 11,039 patients reviewed during the year-long intervention period, 52.7% kept their next two visits compared to 49.3% of 10,018 patients reviewed in the pre-intervention year—a relative increase of 7% over the study period [43•]. The second part of the Retention in Care Study assessed the added value of individual components of a prototypical “package” intervention to identify which component had the greatest impact on retention. This 3-arm study randomized 1838 patients at six US academic clinics to enhanced contact with a trained interventionist (face-to face meetings at each clinic visit, interim contact phone calls, clinic reminder phone calls 1 and 7 days prior to scheduled visit), enhanced contact + skills building (one-on-one training session on personal organization, problem solving, identification of unmet needs, and strength-based interactions), or SOC. No difference was identified in the proportion of persons who attended one clinic visit in three consecutive 4-month intervals between the enhanced contact-only and enhanced contact + skills groups (55.8 vs. 55.6%). However, participants in both groups were more likely to meet the retention in care outcome than participants randomized to SOC (RR 1.22, 95% CI 1.09–1.36) [44•].
The published literature on strategies to improve retention in groups with known high rates of disengagement remains sparse. A single-site study of 41 Spanish-speaking patients in Kansas City reported a doubling in average clinic appointments per year after the implementation of a bilingual patient support team (case manager, peer educator, nurse practitioner) [42]. A sub-study of the HRSA-SPNS Outreach Initiative focused on 773 high-risk persons (minorities, drug abusers) reported that persons with nine or more contacts were less likely to have a gap in scheduled visits of more than 4 months (HR 0.45, 95% CI 0.26–0.78) [40]. Another study of 966 HIV-positive intravenous drug users in four US cities randomized participants to a 10-session strength-based interactive peer mentoring session or an 8-session video mentoring protocol. There was no significant difference between the two groups in improvement in retention in care [45].
Strategies to Improve Linkage to Care in Resource-Poor Settings
A major barrier to adequate HIV care in SSA is the number of clinical encounters required prior the initiation of antiretroviral therapy (ART) (Fig. 1). An important point of disengagement from HIV care is the delay between initial diagnosis and laboratory staging with CD4 testing, with attrition rates as high as 56% by some estimates [17]. Accordingly, the most robust evidence for improving linkage to care in SSA is centered around immediate/point of care (POC) CD4 testing at the time of diagnosis. The largest randomized trial was a study of 508 patients in Johannesburg diagnosed as part of a mobile HIV counseling and testing campaign, where participants offered POC CD4 testing were more likely to attend their initial clinic assessment (RR 1.25, 95% CI 1.00–1.57) [29]. Another study of 344 newly diagnosed South African patients randomized to either same-day CD4 testing/results, informational leaflet with HIV care pathway and standard CD4 testing (with results in 7 days), or standard CD4 testing alone. Persons who received same-day CD4 testing/results were twice as likely to report to their initial encounter with an HIV provider as patients in the other two study arms [25]. The importance of POC CD4 testing has also been demonstrated in less-resourced African countries. An observational cohort study of 929 patients from four HIV care clinics in Mozambique showed a declines in attrition prior to CD4 staging (57 to 21%) and loss to follow up prior to ARV (64 to 33%) and an increase in patients initiating ART (12 to 22%) following POC CD4 [27].
Other innovative approaches for improving linkage to care in SSA have been examined. A pre-post observational cohort study in Zambia (n=13,917) evaluated co-location of antenatal care and HIV care for newly diagnosed pregnant women. ARV eligible women who were diagnosed at co-located clinics were significantly more likely to start therapy within 60 days of diagnosis than women in standard clinics (OR 2.06, 95% CI 1.27–3.34) [28]. Several studies examined the role of home-based counseling and testing (HBCT) in improving linkage to care [31]. A randomized trial of 400 newly diagnosed persons in eastern Uganda looked at the added benefit of post-test counseling and monthly home visits after diagnosis in enhancing linkage to care. Persons who received home visits were more likely to present for their initial appointment within 90 days of diagnosis than persons who did not (RR 1.80, 95% CI 1.40–2.10) [30]. A pilot study of HBCT coupled with POC CD4 testing in South Africa showed that 99% of 201 persons who tested positive reported for their initial clinic visit within 90 days of diagnosis [32]. Incorporation of HIV testing into urgent care encounters may also be associated with improved linkage rates. A study in a large referral hospital in Kenya reported an 87% linkage to care rate among the 312 patients who tested positive for HIV during a 12-month emergency department HIV testing campaign [33].
Strategies to Improve Retention in Care in Resource-Poor Settings
Numerous approaches for improving retention in HIV care in RPS have been investigated. Two strategies have been described repeatedly in the literature: (1) decentralization of HIV care from tertiary care centers to community-based clinics [48] and (2) shifting responsibility of routine HIV care from over-committed consultant physicians to protocol-based care using clinical officers and nurses (“task-shifting”) [70]. These approaches highlight the importance of easy access to skilled providers and medical infrastructure above all else in the retention of HIV-infected persons in care in RPS. In accordance with WHO/UNAIDS recommended strategies for the scale up of ARV/HIV care in Africa, many studies have validated the significance of accessible primary treatment centers for sustainable provision of care, especially for HIV-infected persons who live outside of urban centers [8]. Brennan and colleagues conducted a prospective analysis of 2772 HIV-infected persons on ART in South Africa. Of these, 693 were “down-referred” to primary treatment centers and managed by nurses for routine ART care. After adjusting for confounders, persons who were down-referred were less likely to die (HR 0.2, 95% CI 0.2–0.6) and less likely to be lost to follow up (LTFU; HR 0.3, 95% CI 0.4–0.9) than persons who remained at the tertiary referral center [50•]. A randomized controlled trial of 582 HIV-infected persons on ART in Swaziland showed that persons assigned to decentralized, nurse-run health centers were significantly less likely to miss an appointment than persons who continued care at the referral hospital (RR 0.37, 95% CI 0.29–0.60), although LTFU rates were similar in both groups [57]. Retrospective studies from various countries in SSA have corroborated the effectiveness of decentralization in retaining HIV-infected persons in care. The largest of these studies was a retrospective cohort study of 29,203 persons in four provinces in South Africa assessing three tiers of care: community-level primary health centers (PHCs), district hospitals, and tertiary regional hospitals. At 24 months of follow-up, 80.1% of persons cared for at PHCs were retained in care compared to 71.5% in district hospitals and 68.7% in regional hospitals [53]. Retrospective analyses from Nigeria, Ethiopia, Malawi, Mozambique, Uganda, and Democratic Republic of Congo also support decentralization as a strategy for improving retention (Table 3) [47, 51, 56, 62, 63, 65].
Task-shifting has also been assessed repeatedly in the literature. The largest trial to examine the quality of care provided by mid-level providers was the CIPRA-SA study conducted in South Africa. Eight hundred and twelve patients were randomized to either physician-monitored or nurse-monitored ART care. After 120 weeks of follow-up, treatment outcomes were similar in the two groups. Importantly, there were no significant differences in the rate of LTFU (17.2% in nurse group vs. 15.4% in physician group) [66•]. Prospective studies in Swaziland and Rwanda, in contrast, reported findings in favor of task-shifting [57, 68]. To enhance accessibility and improve care engagement, home-based services provided by community-based adherence support (CBAS) workers have also been evaluated. The most significant study in favor of this approach was a cluster-randomized equivalence trial of 1453 HIV-infected persons in Uganda. Participants were randomized to home-based care (monthly home visits by CBAS workers with ART delivery coupled with facility visits every 6 months) or SOC (facility visits every 3 months). Findings from the study revealed similar rates of death, withdrawal, and virological failure. The LTFU rates were also similar between the two groups (1% home-based, 2% facility-based) at 12 months [58, 59•]. A large observational study of approximately 67,000 South Africans on ART reported that at the end of the 5-year study period, 79.1% (95% CI 77.7–80.4) of persons who received any CBAS support were retained in care compared to 73.6% (95% CI 72.6–74.5%) of persons who received no support. The adjusted hazard ratio for LTFU was 0.63 (95% CI 0.59–0.68) [54]. A retrospective pre-post study from Zambia (n=8875) showed that after the implementation of adherence support workers who also engaged in home ART delivery, LTFU rates in the study area dropped from 15 to 0% [69]. Not all studies have reported results in favor of community-based services. A randomized study (n=239) from Kenya reported no significant difference in LTFU among persons who received home-based adherence support compared to those that did not (5.2% in intervention group, 4.5% in control group, p>0.5 at 12 months) [67].
Other approaches have been investigated to improve retention in care in RPS, including methods as innovative as electronic medical record implementation [46]. Studies describing co-location of HIV services with primary care services have yielded mixed results [71]. A retrospective cohort study of 11,775 patients who received care in 17 clinics in Mozambique showed a higher risk of attrition for persons who received care at integrated clinics than at HIV-only clinics (HR 1.75, 95% CI 1.04–2.94) [62]. However, a larger retrospective study of Médecins Sans Frontières (MSF) clinics in nine SSA countries showed significantly lower LTFU in patients cared for at integrated clinics than in HIV-only clinics (aHR 0.71, 95% CI 0.61–0.83) [56]. Providing free opportunistic infection prophylaxis for infected persons not yet eligible for ART may also be an effective strategy for improving retention. A pre-post observational study in Nairobi showed that ART-ineligible patients were significantly more likely to be LTFU prior to the implementation of free cotrimoxazole prophylaxis than after the program’s implementation (adjusted HR 2.64, 95% CI 1.95–3.97, p<0.001) [60]. A few studies have looked at self-selected treatment supporters to improve retention. A randomized trial of 174 HIV-infected persons in Uganda showed that patients who were assigned to have a self-selected treatment supporter were not more likely to keep all their clinic appointments than patients not assigned a treatment supporter (OR 1.19 95% CI, 0.74–1.91), although fewer missed appointments were missed in the treatment support group [61]. A study in Mozambique (n=1384) examined the effectiveness of self-forming ART counseling and home-delivery groups for patients clinically stable on ART, with a 97.5% retention rate at 13 months [52]. Simple approaches like triaging patients by disease severity on presentation appears to impact retention in care as well. An observational study of an express care triage program for patients with late-stage AIDS on presentation showed that patients enrolled in the program with CD4 count <100 cells/mm3 on presentation were less likely to be LTFU than persons who remained in routine care (adjusted HR 0.62, 95% CI 0.55–0.70) [49].
Ultimately, programs that combine multiple approaches appear to be the most effective [72]. A recent observational study of 610 patients in Rwanda examined the impact of a multipronged community support program that combined standard ARV care with daily CBAS worker visits, monthly food rations, attendance to routine clinic appointments with assigned CBAS workers, and transportation stipends. Persons enrolled in the program were more likely to achieve the retention in care outcome (attendance of all clinic visits in the 12-month study period) than persons who received SOC (RR 1.08, 95% CI 1.01–1.15) [55•]. On a larger scale, a review of 349 clinics in 10 African countries showed that patients who attended clinics with two or more adherence support services were less likely to be LTFU (RR 0.84, 95% CI 0.73–0.96) [73]. Further efforts to elucidate which components of these combination support programs have the greatest impact on improving retention are warranted.
Discussion
In this review, we present a concise overview of the recent evidence on strategies to improve engagement in care as they relate to the treatment continuum in HRS and RPS (Fig. 1). Overall, there is a relative paucity of high-quality prospective studies assessing the effectiveness of individual strategies to improve engagement. The dearth of evidence highlights the importance of published guidelines based on expert opinion like the 2012 IAPAC guidelines [19••]. As part of this update, we reviewed a number of abstracts from recent major HIV/AIDS conferences held in the last 2 years. Promising data were presented on the use of city-wide surveillance systems to re-link patients to care as well as the use of phone appointment reminders to improve retention [74–79]. Future publications regarding these novel strategies would be a welcome addition to the evolving literature in this field.
In highly resourced settings, the benefits of on-site case managers to address unmet needs not directly related to ART appear to be strongly supported by the published literature. The ability of case managers to provide strength-based counseling and other forms of motivational interviewing, to serve as patient navigators, and to coordinate transportation/financial assistance when needed proves them as an invaluable resource for improving care engagement. Recent studies also showed that low-cost interventions like the use of brochures and posters in clinic may be effective and grossly underutilized [43•]. In resource-limited settings, a number of high-quality studies have supported the use of POC CD4 testing to improve initial linkage to care. While highly effective in cutting down the time it takes to establish HIV care, the financial feasibility of employing this intervention on a larger scale is unclear. HIV testing in settings outside of the conventional clinic interface (home, emergency departments, primary care clinics, etc.) may be an effective way to move the interface of care and thus facilitate linkage to HIV services. Along the lines of moving the “interface of care” closer to the patient, increasing the number of HIV care centers to facilitate local access (“decentralization”) and growing the number of available HIV providers for routine care (task-shifting) seem to be logical approaches supported by some evidence. Patient-centered models like self-selected peer navigators and self-forming ART counseling/home-delivery groups have also proven to be effective in severely resource-limited settings.
A nuanced understanding of social, cultural, and economic barriers is critical for customizing the most effective approach for a population of interest. Some studies have shown that patient behaviors in response to well-intentioned interventions may be far different then anticipated, due to factors overlooked by investigators or policy makers. For example, a prospective cohort study of 754 HIV-infected patients in Tanzania showed that despite rapid decentralization of care centers, 98% of patients never changed their primary treatment center and 75% continued to receive care at established tertiary care centers after 3.5 years of follow-up [80]. Although the authors posited stigma of receiving HIV care close to home and patient mistrust of the quality of care at newer treatment centers as possible reasons for their observations, the only way to ascertain the rationale for such behavioral patterns is direct patient input. Studies like these highlight the importance of including the input of patients at the conception of strategies to improve engagement. Rigorous methods to assess patient preferences, such as discrete choice experiments, hold promise for informing the development of new interventions [81].
There were a number of significant gaps in the scope of the published literature. Data supporting approaches based on assistance with barriers to care (e.g., transportation assistance, substance abuse counseling, housing assistance), appointment reminders via telephone or e-mail, community-wide health promotion-related media, and partner/family counseling are surprisingly sparse. Studies investigating the role of each of these approaches in improving care engagement are warranted. Finally, although many interventions are delivered simultaneously, identifying what component of the interventions is the most significant is difficult. The aforementioned Retention in Care Study is the only high-quality study we identified that attempts to deconstruct components of a “package” intervention [44•]. More studies like this are needed.
Conclusion
This review presents a number of intervention strategies implementable on the clinic/provider level in HRS and RPS. However, the dichotomization of approaches by resource availability should not take away from the potential applicability of most of the presented concepts in any locale. This survey of the published literature has identifies numerous gaps in our understanding of what works best in engaging and maintaining patients in the unique model of life-saving but life-long HIV care. More research in the areas of counseling services in RPS, the efficacy of clinic-based health promotion and partner/family services and the role of technology for linkage, retention, and re-linkage in care are needed. Equally important is the determination of which combinations of interventions are most impactful, scalable, and cost-effective, and most aligned with patients’ preferences for HIV care. Lastly, as the body of evidence on successful interventions grows, approaches supported by the evidence should be promptly incorporated into state- and national-level strategies to “close the leaks” in the ever-important cascade of HIV care.
Acknowledgments
Nathan M. Thielman reports receiving other support from Cubist Pharmaceuticals as a consultant and other support from The France Foundation for consultant work in developing CME activity. In addition, Dr. Thielman is a coinventor for a patent, Stable Glutamine Derivatives for Oral and Intravenous Rehydration and Nutrition Therapy issued to the University of Virginia Patents Foundation.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest N. Lance Okeke and Jan Ostermann declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
N. Lance Okeke, Division of Infectious Diseases, Department of Medicine, Duke University Medical Center, Durham, NC, USA; 315 Trent Drive, Room 181, Durham, NC 27710, USA.
Jan Ostermann, Duke Global Health Institute, Duke University, Durham, NC, USA.
Nathan M. Thielman, Division of Infectious Diseases, Department of Medicine, Duke University Medical Center, Durham, NC, USA Duke Global Health Institute, Duke University, Durham, NC, USA; 310 Trent Drive, Room 201, Durham, NC 27708, USA.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS) Global Report: UNAIDS Report on the Global AIDS Epidemic 2012. UNAIDS; Geneva, Switzerland: 2012. Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/20121120_UNAIDS_Global_Report_2012_with_annexes_en.pdf. Accessed 9 Jul 2014. [Google Scholar]
- 2.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giordano TP, Gifford AL, White AC, Jr, Suarez-Almazor ME, Rabeneck L, Hartman C, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007;44(11):1493–9. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- 4.Horstmann E, Brown J, Islam F, Buck J, Agins BD. Retaining HIV-infected patients in care: where are we? Where do we go from here? Clin Infect Dis. 2010;50(5):752–61. doi: 10.1086/649933. [DOI] [PubMed] [Google Scholar]
- 5.Robbins GK, Daniels B, Zheng H, Chueh H, Meigs JB, Freedberg KA. Predictors of antiretroviral treatment failure in an urban HIV clinic. J Acquir Immune Defic Syndr. 2007;44(1):30–7. doi: 10.1097/01.qai.0000248351.10383.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park WB, Choe PG, Kim SH, Jo JH, Bang JH, Kim HB, et al. One-year adherence to clinic visits after highly active antiretroviral therapy: a predictor of clinical progress in HIV patients. J Intern Med. 2007;261(3):268–75. doi: 10.1111/j.1365-2796.2006.01762.x. [DOI] [PubMed] [Google Scholar]
- 7.White House Office of National AIDS Policy. National HIV/AIDS Strategy for the United States Washington, DC 2010. Available at: http://www.whitehouse.gov/sites/default/files/uploads/NHAS.pdf2. Accessed July 9 2014.
- 8.Joint United Nations Programme on HIV/AIDS (UNAIDS) Treatment 2015. Geneva, Switzerland: 2012. Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2013/JC2484_treatment-2015_en.pdf. Accessed 9 Jul 2014. [Google Scholar]
- 9••.Mugavero MJ, Amico KR, Horn T, Thompson MA. The state of engagement in HIV care in the United States: from cascade to continuum to control. Clin Infect Dis. 2013;57(8):1164–71. doi: 10.1093/cid/cit420. A useful update on the status of engagement in the USA. [DOI] [PubMed] [Google Scholar]
- 10.United States Department of Health and Human Services. HIV core indicators. Washington, DC: 2012. Available at: http://blog.aids.gov/2012/08/secretary-sebelius-approves-indicators-for-monitoring-hhs-funded-hiv-services.html. Accessed July 9 2014. [Google Scholar]
- 11•.Fox MP, Larson B, Rosen S. Defining retention and attrition in pre-antiretroviral HIV care: proposals based on experience in Africa. Trop Med Int Health. 2012;17(10):1235–44. doi: 10.1111/j.1365-3156.2012.03055.x. A review of measures of engagement in HIV care in resource-poor settings. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12•.Mugavero MJ, Westfall AO, Zinski A, Davila J, Drainoni ML, Gardner LI, et al. Measuring retention in HIV care: the elusive gold standard. J Acquir Immune Defic Syndr. 2012;61(5):574–80. doi: 10.1097/QAI.0b013e318273762f. A study attempting to assess the most comprehensive measures for engagement in HIV care. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. HIV in the United States: the stages of care. Available at: http://www.cdc.gov/nchhstp/newsroom/docs/2012/Stages-of_Care-FactSheet-508.pdf. Accessed July 9 2014.
- 15•.Althoff KN, Rebeiro P, Brooks JT, Buchacz K, Gebo K, Martin J, et al. Disparities in the quality of HIV care when using US Department of Health and Human Services indicators. Clin Infect Dis. 2014;58(8):1185–9. doi: 10.1093/cid/ciu044. A study measuring linkage and retention in care in a well monitored large cohort in the USA and Canada. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011;8(7):e1001056. doi: 10.1371/journal.pmed.1001056. A comprehensive review of the state of retention in care in sub-Saharan Africa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilmarx PH, Mutasa-Apollo T. Patching a leaky pipe: the cascade of HIV care. Curr Opin HIVAIDS. 2013;8(1):59–64. doi: 10.1097/COH.0b013e32835b806e. [DOI] [PubMed] [Google Scholar]
- 18•.Kranzer K, Govindasamy D, Ford N, Johnston V, Lawn SD. Quantifying and addressing losses along the continuum of care for people living with HIV infection in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2012;15(2):17383. doi: 10.7448/IAS.15.2.17383. A large review attempting to re-construct the “treatment cascade” in sub-Saharan Africa from derived estimates of previously published studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Thompson MA, Mugavero MJ, Amico KR, Cargill VA, Chang LW, Gross R, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med. 2012;156(11):817–33. doi: 10.7326/0003-4819-156-11-201206050-00419. Comprehensive expert-based guidelines on strategies for improving engagement in HIV care. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gardner LI, Metsch LR, Anderson-Mahoney P, Loughlin AM, del Rio C, Strathdee S, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS. 2005;19(4):423–31. doi: 10.1097/01.aids.0000161772.51900.eb. [DOI] [PubMed] [Google Scholar]
- 21.Craw JA, Gardner LI, Marks G, Rapp RC, Bosshart J, Duffus WA, et al. Brief strengths-based case management promotes entry into HIV medical care: results of the antiretroviral treatment access study-II. J Acquir Immune Defic. 2008;47(5):597–606. doi: 10.1097/QAI.0b013e3181684c51. [DOI] [PubMed] [Google Scholar]
- 22.Wohl DA, Scheyett A, Golin CE, White B, Matuszewski J, Bowling M, et al. Intensive case management before and after prison release is no more effective than comprehensive pre-release discharge planning in linking HIV-infected prisoners to care: a randomized trial. AIDS Behav. 2011;15(2):356–64. doi: 10.1007/s10461-010-9843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boccour A, Renaud TC, Udeagu CC, Shepard CW. HIV partner services are associated with timely linkage to medical care. AIDS. 2013;27(18):2961–3. doi: 10.1097/QAD.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 24.Naar-King S, Bradford J, Coleman S, Green-Jones M, Cabral H, Tobias C. Retention in care of persons newly diagnosed with HIV: outcomes of the Outreach Initiative. AIDS Patient Care STDS. 2007;21(Suppl 1):S40–8. doi: 10.1089/apc.2007.9988. [DOI] [PubMed] [Google Scholar]
- 25.Faal M, Naidoo N, Glencross DK, Venter WD, Osih R. Providing immediate CD4 count results at HIV testing improves ART initiation. J Acquir Immune Defic. 2011;58(3):e54–9. doi: 10.1097/QAI.0b013e3182303921. [DOI] [PubMed] [Google Scholar]
- 26.Govindasamy D, Kranzer K, van Schaik N, et al. Linkage to HIV, TB and non-communicable disease care from a mobile testing unit in Cape Town, South Africa. PLoS One. 2013;8(11):e80017. doi: 10.1371/journal.pone.0080017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jani IV, Sitoe NE, Alfai ER, Chongo PL, Quevedo JI, Rocha BM, et al. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: an observational cohort study. Lancet. 2011;378(9802):1572–9. doi: 10.1016/S0140-6736(11)61052-0. [DOI] [PubMed] [Google Scholar]
- 28.Killam WP, Tambatamba BC, Chintu N, Rouse D, Stringer E, Bweupe M, et al. Antiretroviral therapy in antenatal care to increase treatment initiation in HIV-infected pregnant women: a stepped-wedge evaluation. AIDS. 2010;24(1):85–91. doi: 10.1097/QAD.0b013e32833298be. [DOI] [PubMed] [Google Scholar]
- 29.Larson BA, Schnippel K, Ndibongo B, Xulu T, Brennan A, Long L, et al. Rapid point-of-care CD4 testing at mobile HIV testing sites to increase linkage to care: an evaluation of a pilot program in South Africa. J Acquir Immune Defic. 2012;61(2):e13–7. doi: 10.1097/QAI.0b013e31825eec60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muhamadi L, Tumwesigye NM, Kadobera D, Marrone G, Wabwire-Mangen F, Pariyo G, et al. A single-blind randomized controlled trial to evaluate the effect of extended counseling on uptake of pre-antiretroviral care in Eastern Uganda. Trials. 2011;12:184. doi: 10.1186/1745-6215-12-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patten GE, Wilkinson L, Conradie K, Isaakidis P, Harries AD, Edginton ME, et al. Impact on ART initiation of point-of-care CD4 testing at HIV diagnosis among HIV-positive youth in Khayelitsha, South Africa. J Int AIDS Soc. 2013;16:18518. doi: 10.7448/IAS.16.1.18518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Rooyen H, Barnabas RV, Baeten JM, Phakathi Z, Joseph P, Krows M, et al. High HIV testing uptake and linkage to care in a novel program of home-based HIV counseling and testing with facilitated referral in KwaZulu-Natal, South Africa. J Acquir Immune Defic. 2013;64(1):e1–8. doi: 10.1097/QAI.0b013e31829b567d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waxman MJ, Kimaiyo S, Ongaro N, Wools-Kaloustian KK, Flanigan TP, Carter EJ. Initial outcomes of an emergency department rapid HIV testing program in western Kenya. AIDS Patient Care STDS. 2007;21(12):981–6. doi: 10.1089/apc.2007.0075. [DOI] [PubMed] [Google Scholar]
- 34.Hightow-Weidman LB, Jones K, Wohl AR, Futterman D, Outlaw A, Phillips G, 2nd, et al. Early linkage and retention in care: findings from the outreach, linkage, and retention in care initiative among young men of color who have sex with men. AIDS Patient Care STDS. 2011;25(Suppl 1):S31–8. doi: 10.1089/apc.2011.9878. [DOI] [PubMed] [Google Scholar]
- 35.Bradford JB, Coleman S, Cunningham W. HIV system navigation: an emerging model to improve HIV care access. AIDS Patient Care STDS. 2007;21(Suppl 1):S49–58. doi: 10.1089/apc.2007.9987. [DOI] [PubMed] [Google Scholar]
- 36.Andersen M, Hockman E, Smereck G, Tinsley J, Milfort D, Wilcox R, et al. Retaining women in HIV medical care. J Assoc Nurses AIDS Care. 2007;18(3):33–41. doi: 10.1016/j.jana.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Willis S, Castel AD, Ahmed T, Olejemeh C, Frison L, Kharfen M. Linkage, engagement, and viral suppression rates among HIV-infected persons receiving care at medical case management programs in Washington, DC. J Acquir Immune Defic. 2013;64(Suppl 1):S33–41. doi: 10.1097/QAI.0b013e3182a99b67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naar-King S, Outlaw A, Green-Jones M, Wright K, Parsons JT. Motivational interviewing by peer outreach workers: a pilot randomized clinical trial to retain adolescents and young adults in HIV care. AIDS Care. 2009;21(7):868–73. doi: 10.1080/09540120802612824. [DOI] [PubMed] [Google Scholar]
- 39.Wohl AR, Garland WH, Wu J, Au CW, Boger A, Dierst-Davies R, et al. Ayouth-focused case management intervention to engage and retain young gay men of color in HIV care. AIDS Care. 2011;23(8):988–97. doi: 10.1080/09540121.2010.542125. [DOI] [PubMed] [Google Scholar]
- 40.Cabral HJ, Tobias C, Rajabiun S, Sohler N, Cunningham C, Wong M, et al. Outreach program contacts: do they increase the likelihood of engagement and retention in HIV primary care for hard-to-reach patients? AIDS Patient Care STDS. 2007;21(Suppl 1):S59–67. doi: 10.1089/apc.2007.9986. [DOI] [PubMed] [Google Scholar]
- 41.Davila JA, Miertschin N, Sansgiry S, et al. Centralization of HIV services in HIV-positive African-American and Hispanic youth improves retention in care. AIDS Care. 2013;25(2):202–6. doi: 10.1080/09540121.2012.689811. [DOI] [PubMed] [Google Scholar]
- 42.Enriquez M, Farnan R, Cheng AL, Almeida A, Del Valle D, PulidoParra M, et al. Impact of a bilingual/bicultural care team on HIV-related health outcomes. J Assoc Nurses AIDS Care. 2008;19(4):295–301. doi: 10.1016/j.jana.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 43•.Gardner LI, Marks G, Craw JA, Wilson TE, Drainoni ML, Moore RD, et al. A low-effort, clinic-wide intervention improves attendance for HIV primary care. Clin Infect Dis. 2012;55(8):1124–34. doi: 10.1093/cid/cis623. First study to explore the role of clinic-based health promotion media for improving retention in care. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Gardner LI, Giordano TP, Marks G, Wilson TE, Craw JA, Drainoni ML, et al. Enhanced personal contact with HIV patients improves retention in primary care: a randomized trial in 6 US HIV clinics. Clin Infect Dis. 2014;59(5):725–34. doi: 10.1093/cid/ciu357. High-quality randomized trial assessing individual components of a “package” intervention to improve retention in care. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Purcell DW, Latka MH, Metsch LR, Latkin CA, Gomez CA, Mizuno Y, et al. Results from a randomized controlled trial of a peer-mentoring intervention to reduce HIV transmission and increase access to care and adherence to HIV medications among HIV-seropositive injection drug users. J Acquir Immune Defic. 2007;46(Suppl 2):S35–47. doi: 10.1097/QAI.0b013e31815767c4. [DOI] [PubMed] [Google Scholar]
- 46.Alamo ST, Wagner GJ, Sunday P, Wanyenze RK, Ouma J, Kamya M, et al. Electronic medical records and same day patient tracing improves clinic efficiency and adherence to appointments in a community based HIV/AIDS care program, in Uganda. AIDS Behav. 2012;16(2):368–74. doi: 10.1007/s10461-011-9996-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balcha TT, Jeppsson A. Outcomes of antiretroviral treatment: a comparison between hospitals and health centers in Ethiopia. J Int Assoc AIDS Care. 2010;9(5):318–24. doi: 10.1177/1545109710367518. [DOI] [PubMed] [Google Scholar]
- 48.Bedelu M, Ford N, Hilderbrand K, Reuter H. Implementing antiretroviral therapy in rural communities: the Lusikisiki model of decentralized HIV/AIDS care. J Infect Dis. 2007;196(Suppl 3):S464–8. doi: 10.1086/521114. [DOI] [PubMed] [Google Scholar]
- 49.Braitstein P, Siika A, Hogan J, Kosgei R, Sang E, Sidle J, et al. A clinician-nurse model to reduce early mortality and increase clinic retention among high-risk HIV-infected patients initiating combination antiretroviral treatment. J Int AIDS Soc. 2012;15(1):7. doi: 10.1186/1758-2652-15-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50•.Brennan AT, Long L, Maskew M, Sanne I, Jaffray I, MacPhail P, et al. Outcomes of stable HIV-positive patients down-referred from a doctor-managed antiretroviral therapy clinic to a nurse-managed primary health clinic for monitoring and treatment. AIDS. 2011;25(16):2027–36. doi: 10.1097/QAD.0b013e32834b6480. High-quality prospective study assessing the role of de-centralization/task-shifting in HIV care in sub-Saharan Africa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chan AK, Mateyu G, Jahn A, Schouten E, Arora P, Mlotha W, et al. Outcome assessment of decentralization of antiretroviral therapy provision in a rural district of Malawi using an integrated primary care model. Trop Med Int Health. 2010;15(Suppl 1):90–7. doi: 10.1111/j.1365-3156.2010.02503.x. [DOI] [PubMed] [Google Scholar]
- 52.Decroo T, Telfer B, Biot M, Maikere J, Dezembro S, Cumba LI, et al. Distribution of antiretroviral treatment through self-forming groups of patients in Tete Province, Mozambique. J Acquir Immune Defic. 2011;56(2):e39–44. doi: 10.1097/QAI.0b013e3182055138. [DOI] [PubMed] [Google Scholar]
- 53.Fatti G, Grimwood A, Bock P. Better antiretroviral therapy outcomes at primary healthcare facilities: an evaluation of three tiers of ART services in four South African provinces. PLoS One. 2010;5(9):e12888. doi: 10.1371/journal.pone.0012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fatti G, Meintjes G, Shea J, Eley B, Grimwood A. Improved survival and antiretroviral treatment outcomes in adults receiving community-based adherence support: 5-year results from a multicentre cohort study in South Africa. J Acquir Immune Defic. 2012;61(4):e50–8. doi: 10.1097/QAI.0b013e31826a6aee. [DOI] [PubMed] [Google Scholar]
- 55•.Franke MF, Kaigamba F, Socci AR, Hakizamungu M, Patel A, Bagiruwigize E, et al. Improved retention associated with community-based accompaniment for antiretroviral therapy delivery in rural Rwanda. Clin Infect Dis. 2013;56(9):1319–26. doi: 10.1093/cid/cis1193. Recent study assessing the role of a multi-level intervention in improving adherence and care retention in a severely resource poor setting. [DOI] [PubMed] [Google Scholar]
- 56.Greig J, O’Brien DP, Ford N, Spelman T, Sabapathy K, Shanks L. Similar mortality and reduced loss to follow-up in integrated compared with vertical programs providing antiretroviral treatment in sub-Saharan Africa. J Acquir Immune Defic. 2012;59(5):e92–8. doi: 10.1097/QAI.0b013e31824206c7. [DOI] [PubMed] [Google Scholar]
- 57.Humphreys CP, Wright J, Walley J, Mamvura CT, Bailey KA, Ntshalintshali SN, et al. Nurse led, primary care based antiretroviral treatment versus hospital care: a controlled prospective study in Swaziland. BMC Health Serv Res. 2010;10:229. doi: 10.1186/1472-6963-10-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jaffar S, Amuron B, Foster S, Birungi J, Levin J, Namara G, et al. Rates of virological failure in patients treated in a home-based versus a facility-based HIV-care model in Jinja, southeast Uganda: a cluster-randomised equivalence trial. Lancet. 2009;374(9707):2080–9. doi: 10.1016/S0140-6736(09)61674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59•.Amuron B, Levin J, Birunghi J, Namara G, Coutinho A, Grosskurth H, et al. Mortality in an antiretroviral therapy programme in Jinja, south-east Uganda: a prospective cohort study. AIDS Res Ther. 2011;8:39. doi: 10.1186/1742-6405-8-39. Large randomized study examining the role of home-based care in improving retention in care in sub-Saharan Africa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kohler PK, Chung MH, McGrath CJ, Benki-Nugent SF, Thiga JW, John-Stewart GC. Implementation of free cotrimoxazole prophylaxis improves clinic retention among antiretroviral therapy-ineligible clients in Kenya. AIDS. 2011;25(13):1657–61. doi: 10.1097/QAD.0b013e32834957fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kunutsor S, Walley J, Muchuro S, Katabira E, Balidawa H, Namagala E, et al. Improving adherence to antiretroviral therapy in sub-Saharan African HIV-positive populations: an enhanced adherence package. AIDS Care. 2012;24(10):1308–15. doi: 10.1080/09540121.2012.661833. [DOI] [PubMed] [Google Scholar]
- 62.Lambdin BH, Micek MA, Sherr K, Gimbel S, Karagianis M, Lara J, et al. Integration of HIV care and treatment in primary health care centers and patient retention in central Mozambique: a retrospective cohort study. J Acquir Immune Defic. 2013;62(5):e146–52. doi: 10.1097/QAI.0b013e3182840d4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Massaquoi M, Zachariah R, Manzi M, Pasulani O, Misindi D, Mwagomba B, et al. Patient retention and attrition on antiretroviral treatment at district level in rural Malawi. Trans R Soc Trop Med Hyg. 2009;103(6):594–600. doi: 10.1016/j.trstmh.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 64.McGuire M, Pinoges L, Kanapathipillai R, et al. Treatment initiation, program attrition and patient treatment outcomes associated with scale-up and decentralization of HIV care in rural Malawi. PLoS One. 2012;7(10):e38044. doi: 10.1371/journal.pone.0038044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Odafe S, Torpey K, Khamofu H, Ogbanufe O, Oladele EA, Kuti O, et al. The pattern of attrition from an antiretroviral treatment program in Nigeria. PLoS One. 2012;7(12):e51254. doi: 10.1371/journal.pone.0051254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66•.Sanne I, Orrell C, Fox MP, Conradie F, Ive P, Zeinecker J, et al. Nurse versus doctor management of HIV-infected patients receiving antiretroviral therapy (CIPRA-SA): a randomised non-inferiority trial. Lancet. 2010;376(9734):33–40. doi: 10.1016/S0140-6736(10)60894-X. High-quality randomized trial assessing task-shifting as a strategy for the management of stable HIV-infected patients in sub-Saharan Africa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Selke HM, Kimaiyo S, Sidle JE, Vedanthan R, Tierney WM, Shen C, et al. Task-shifting of antiretroviral delivery from health care workers to persons living with HIV/AIDS: clinical outcomes of a community-based program in Kenya. J Acquir Immune Defic. 2010;55(4):483–90. doi: 10.1097/QAI.0b013e3181eb5edb. [DOI] [PubMed] [Google Scholar]
- 68.Shumbusho F, van Griensven J, Lowrance D, Turate I, Weaver MA, Price J, et al. Task shifting for scale-up of HIV care: evaluation of nurse-centered antiretroviral treatment at rural health centers in Rwanda. PLoS Med. 2009;6(10):e1000163. doi: 10.1371/journal.pmed.1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Torpey KE, Kabaso ME, Mutale LN, Kamanga MK, Mwango AJ, Simpungwe J, et al. Adherence support workers: a way to address human resource constraints in antiretroviral treatment programs in the public health setting in Zambia. PLoS One. 2008;3(5):e2204. doi: 10.1371/journal.pone.0002204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Geng EH, Nash D, Kambugu A, Zhang Y, Braitstein P, Christopoulos KA, et al. Retention in care among HIV-infected patients in resource-limited settings: emerging insights and new directions. Curr HIV/AIDS Rep. 2010;7(4):234–44. doi: 10.1007/s11904-010-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pfeiffer J, Montoya P, Baptista AJ, Karagianis M, Pugas Mde M, Micek M, et al. Integration of HIV/AIDS services into African primary health care: lessons learned for health system strengthening in Mozambique—a case study. J Int AIDS Soc. 2010;13:3. doi: 10.1186/1758-2652-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Etienne M, Burrows L, Osotimehin B, Macharia T, Hossain B, Redfield RR, et al. Situational analysis of varying models of adherence support and loss to follow up rates; findings from 27 treatment facilities in eight resource limited countries. Trop Med Int Health. 2010;15(Suppl 1):76–81. doi: 10.1111/j.1365-3156.2010.02513.x. [DOI] [PubMed] [Google Scholar]
- 73.Lamb MR, El-Sadr WM, Geng E, Nash D. Association of adherence support and outreach services with total attrition, loss to follow-up, and death among ART patients in sub-Saharan Africa. PLoS One. 2012;7(6):e38443. doi: 10.1371/journal.pone.0038443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buchacz KA, Parisi MK, Yoshida M, Antunez E, Delgado V, Moss NJ. Using HIV surveillance registry data to re-link patients to care: the RSVP project in San Francisco. Poster presented at: 21st Conference for Retroviruses and Opportunistic Infections March 3–6; Boston, MA. Abstract 978. [Google Scholar]
- 75.Dombrowski J, Hughes JP, Castel AD, Brady KA, Buskin S, Bennett A, et al. A surveillance-based risk-scoring tool to prioritize cases for HIV care re-linkage efforts. Poster presented at: 21st Conference for Retroviruses and Opportunistic Infections 2014 March 3–6; Boston, MA. Abstract 982. [Google Scholar]
- 76.Wispelwey E, Gordy A, Schaefer K, Dillingham R, Ingersoll K. Initiation of a retention in care (RiC) program in a semi-rural clinic in the Southeastern United States. Poster presented at: 8th International Conference on HIV Treatment and Prevention Adherence 2013 June 2–4; Miami, FL. Abstract 208. [Google Scholar]
- 77.Carda-Auten J, Wohl D, Golin C, Groves J, White BL, Pence B, et al. Cell phones to augment study visit retention for HIV+ individuals recently released from prison participating in the imPACT study. Poster presented at: 8th International Conference on Treatment and Prevention Adherence 2013 June 2–4; Miami, FL. Abstract 237. [Google Scholar]
- 78.Watkins K, Grimes R, Yang B, Mcneese M, Wolverton M. Innovative approaches for identifying out-of-care persons for re-linkage to care. Poster presented at: 9th International Conference for Treatment and Prevention Adherence 2014 June 8–10; Miami, FL. Abstract 300. [Google Scholar]
- 79.Keller J, Schafer K, Cox MB, Heine A, Sena A, Wilkin A, et al. Bridging the gaps: the use of health information technology and bridge counseling to improve retention in care in North Carolina. Oral presentation at: 9th International Conference on Treatment and Prevention Adherence 2014 June 8–10; Miami, FL. Abstract 322. [Google Scholar]
- 80.Ostermann J, Whetten K, Reddy E, Pence B, Weinhold A, Itemba D, et al. Treatment retention and care transitions during and after the scale-up of HIV care and treatment in Northern Tanzania. AIDS Care. 2014;26(11):1352–8. doi: 10.1080/09540121.2014.882493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ostermann J, Njau B, Brown DS, Mühlbacher A, Thielman N. Heterogeneous HIV testing preferences in an urban setting in Tanzania: results from a discrete choice experiment. PLoS One. 2014;9(3):e92100. doi: 10.1371/journal.pone.0092100. [DOI] [PMC free article] [PubMed] [Google Scholar]


