Abstract
Background
Jaundice as a presenting symptom of gallbladder cancer has traditionally been considered to be a sign of advanced disease, inoperability, and poor outcome. However, recent studies have demonstrated that a small subset of these patients can undergo resection with curative intent.
Methods
Patients with gallbladder cancer managed surgically from 2000–2014 in 10 US academic institutions were stratified based on the presence of jaundice at presentation (defined as bilirubin ≥ 4 mg/ml or requiring preoperative biliary drainage). Perioperative morbidity, mortality, and overall survival were compared between jaundiced and non-jaundiced patients.
Results
Of 400 gallbladder cancer patients with available preoperative data, 108 (27%) presented with jaundice while 292 (73%) did not. The fraction of patients who eventually underwent curative-intent resection was much lower in the presence of jaundice (n=33, 30%) than not (n=218, 75%, P<0.001). Jaundiced patients experienced higher perioperative morbidity (69% vs. 38%, P=0.002), including a much higher need for reoperation (12% vs. 1%, P=0.003). However, 90-day mortality (6.5% vs. 3.6%, P=0.35) was not significantly higher. Overall survival after resection was worse in jaundiced patients (median 14 vs. 32 months, P<0.001). Further subgroup analysis within the jaundiced patients revealed a more favorable survival after resection in the presence of low CA19-9 < 50 (median 40 vs. 12 months, P=0.003) and in the absence of lymphovascular invasion (40 vs. 14 months; P=0.014).
Conclusion
Jaundice is a powerful preoperative clinical sign of inoperability and poor outcome among gallbladder cancer patients. However, some of these patients may still achieve long-term survival after resection, especially when preoperative CA19-9 levels are low and no lymphovascular invasion is noted pathologically.
Keywords: Gallbladder cancer, jaundice, hyperbilirubinemia, biliary obstruction, resectability
INTRODUCTION
Among patients with gallbladder cancer (GBC) the presence of jaundice has historically been considered a contraindication to surgical exploration due to its association with locally advanced and/or metastatic disease.1, 2,3 Obstructive jaundice in the setting of GBC can be related to direct tumor infiltration into the porta hepatis, lymph node involvement in the hepatoduodenal ligament, or intraluminal tumor thrombus within the biliary system.4–7 Furthermore, obstructive jaundice can have major systemic consequences including impairment of cellular immunity,8–10 reduction of sinusoidal blood flow within the liver,9 alteration in the gut barrier, and up-regulation of inflammatory cytokines.11 Although the mechanism is not entirely clear, the presence of jaundice has also been demonstrated to promote tumor growth and liver metastasis in patients with gastrointestinal malignancies.10, 12
Approximately a quarter to a third of patients with GBC present with obstructive jaundice,3, 6, 13 and resectability rates among jaundiced patients has been reported to be anywhere between 7% and 49%.14, 3, 15, 6 While surgical resection is the single potentially curative option in this setting, there is no firm consensus on the appropriateness of surgery for jaundiced patients with GBC. In one of the first US series consisting of 82 patients with preoperative jaundice, only 6 patients (7%) eventually underwent potentially curative resection, 2 of whom died perioperatively and the remaining 4 experienced disease recurrence within 6 months after surgery.3 Consequently, the authors advised against routine surgical exploration in jaundiced patients with GBC. However, in an updated report from the same institution, a median disease-specific survival of 19 months was reported after extensive resections for GBC patients with clinical involvement of the common bile duct mandating its resection, due to the inability to obtain a negative cystic duct margin, the presence of nodal involvement of the porta hepatis adherent to the common bile duct or adhesions involving the common bile duct that could not be differentiated from the tumor.16 Along the same lines, subsequent studies from other institutions around the world have reproduced these modestly improved survival outcomes after surgical resection of GBC in the setting of jaundice, including studies from Japan (reporting a median survival of 18 months),15 France (11 months),6 India (26 months),14 and China (14 months).13
The objective of this study was to evaluate the prognostic implication of preoperative jaundice on postoperative morbidity and long-term survival following curative-intent resection for GBC utilizing a modern, multi-institutional cohort of patients treated in the US.
METHODS
Data Collection
Patients who underwent surgery for GBC between January 2000 to December 2014 at one of ten academic institutions participating in the US Extrahepatic Biliary Malignancy Consortium (USEBMC) were retrospectively identified (John Hopkins Hospital, Baltimore, MD; Emory University, Atlanta, GA; Stanford University, Palo Alto, CA; University of Wisconsin, Milwaukee, WI; Ohio State University, Columbus, OH; Washington University, St. Louis, MO; Vanderbilt University, Nashville, TN; New York University, New York, NY; University of Louisville, Louisville, KY; Wake Forest University, Winston-Salem, NC). Patients were classified into two groups based on presence of jaundice, which was defined as preoperative serum bilirubin ≥4 mg/dL or need for preoperative biliary drainage (endoscopic or percutaneous). Institutional Review Board approval was obtained by each of the participating institutions.
Standard demographics, pathologic, and treatment characteristics were retrospectively reviewed. Operative data collected included type of preoperative biliary drainage procedures, need for common bile duct resection, extent of resection into adjacent organs, intraoperative blood transfusion, and estimated blood loss (EBL). The seventh edition of the AJCC staging manual was used to determine stage.17 Postoperative morbidity was graded using the modified Clavien-Dindo classification of surgical complications.18
Statistical Analysis
Continuous variables were presented as medians with interquartile range (IQR) and compared with Student’s t test and Mann-Whitney test, where applicable. Categorical variables were presented as frequency and percentages and compared using Fisher’s exact test or chi-square, where appropriate. Overall survival (OS) was estimated using Kaplan Meier method and compared using log-rank test. Univariate and multivariate survival analyses were performed using Cox proportional hazards model. Variables with p value of < 0.1 in univariate analysis were incorporated in the multivariate model. Statistical analyses were performed using the STATA 13.0 statistical software package (STATA, College Station, TX, USA) and SPSS 23.0 (IBM, Chicago, IL, USA). Significance was set at a P value of <0.05 (two-tailed).
RESULTS
Study Cohort
Of the 449 patients with GBC in the USEBMC database, 41 patients had insufficient data to determine the presence of preoperative jaundice and 8 patients with in situ disease and were therefore excluded. Of the 292 patients without jaundice, 16 patients with R2 resection and 58 patients with metastatic disease were excluded. Among the 108 patients with jaundice, 2 patients were excluded due to choledocholithiasis, 28 due to R2 resection, and 45 due to presence of distant metastases. The final cohort consisted of 33 patients with jaundice and 218 patients without jaundice who underwent curative-intent resection for GBC.
Clinicopathologic Characteristics
Table 1 details patient characteristics based on the presence or absence of preoperative jaundice. Patient demographics and comorbidities were generally similar between the two groups. The cohort predominantly consisted of females (67%) and the median age was 66 years. The median peak preoperative bilirubin was 9.5 mg/dL in the jaundice group. Patients presenting with jaundice tended to have lower preoperative albumin (3.3 vs. 3.9 g/L, P<0.0001) and higher CA19-9 (187 vs. 15 U/mL, P=0.0005). Among the 33 patients with jaundice, 8 (24.2%) did not undergo preoperative biliary drainage, 48.5% underwent preoperative endoscopic stenting and 18.2% had percutaneous transhepatic biliary drainage. Three (9.1%) patients with jaundice required both endoscopic and percutaneous drainage procedures for biliary decompression.
Table 1.
Clinicopathologic characteristics based on presence of jaundice
| PREOPERATIVE CHARACTERISTICS | No Jaundice (n=218) |
Jaundice (n=33) |
P value |
|---|---|---|---|
|
| |||
| Age (median, IQR) | 67.3 (56.3–73.8) | 64.2 (56.3–69.5) | 0.1575 |
|
| |||
| Male Gender | 73 (33.3) | 9 (27.3) | 0.612 |
|
| |||
| Race (n=241) | |||
| White | 150 (71.8) | 26 (81.3) | 0.552 |
| Black | 29 (13.9) | 1 (3.1) | |
| Asian | 15 (7.1) | 3 (9.4) | |
| Latino | 10 (5.8) | 2 (6.3) | |
| Other | 5 (2.4) | 0 (0) | |
|
| |||
| ASA Class > 3 (n=177) | 87 (57.6) | 16 (61.5) | 0.83 |
|
| |||
| HTN (n=224) | 119 (60.4) | 14 (51.9) | 0.41 |
|
| |||
| Diabetes (n=224) | 49 (24.8) | 1 (3.7) | 0.012 |
|
| |||
| Prior Cardiac Event (n=224) | 25 (12.7) | 0 (0) | 0.051 |
|
| |||
| CHF (n=224) | 8 (4.1) | 1(3.7) | 1.000 |
|
| |||
| Smoking history (n=224) | 32 (16.2) | 8 (29.6) | 0.089 |
|
| |||
| COPD (n=224) | 6 (3.0) | 1 (3.7) | 0.598 |
|
| |||
| Preoperative Sepsis | 3 (1.4) | 1 (1.3) | 0.424 |
|
| |||
| Peak Bilirubin (mg/dL) | 0.6 (0.4–0.9) | 9.55 (4.2–15.9) | <0.001 |
|
| |||
| Last bilirubin (mg/dL) | 0.5 (0.4–0.7) | 1.5 (0.75–2.85) | <0.001 |
|
| |||
| Creatinine (mg/dL) | 0.8 (0.8–1) | 0.8 (0.7–0.9) | 0.0754 |
|
| |||
| Albumin (g/L) | 3.9 (3.5–4.3) | 3.3 (2.7–3.6) | <0.0001 |
|
| |||
| CA19-9 | 15 (11–32) | 187 (25–941) | 0.0005 |
|
| |||
| Preoperative Biliary intervention | |||
| None | 212 (98.6) | 8 (24.2) | <0.001 |
| Endoscopic | 3 (1.4) | 16 (48.5) | |
| Percutaneous | 0 (0) | 6 (18.2) | |
| Both | 0 (0) | 3 (9.1) | |
|
| |||
| Neoadjuvant chemotherapy (n=247) | 8 (3.7) | 2 (6.3) | 0.623 |
|
| |||
| Neoadjuvant radiotherapy (n=242) | 3 (1.4) | 0 (0) | 1.000 |
|
| |||
| OPERATIVE RESULTS | |||
|
| |||
| CBD resection | 64 (29.8) | 29 (87.9) | <0.001 |
|
| |||
| Extent of resection | |||
| Simple cholecystectomy | 21 (9.6) | 3 (9.1) | <0.001 |
| Radical cholecystectomy (Segments 4b/5) | 182 (83.5) | 17 (51.5) | |
| Trisectionectomy | 15 (6.9) | 13 (39.4) | |
|
| |||
| Portal Vein Resection | 1 (0.46) | 4 (12.1) | <0.001 |
|
| |||
| PATHOLOGY | |||
|
| |||
| R1 margins (vs. R0) | 19 (8.8) | 16 (48.5) | <0.001 |
|
| |||
| Grade (n=213) | |||
| Low | 23 (12.6) | 2 (6.7) | 0.349 |
| Moderate | 97 (53) | 20 (66.7) | |
| Poor | 63 (34.4) | 8 (26.7) | |
|
| |||
| AJCC Stage | |||
| I | 19 (9.3) | 0 (0) | 0.072 |
| II | 64 (31.2) | 5 (16.1) | |
| IIIA | 38 (18.5) | 8(25.8) | |
| IIIB | 63 (30.7) | 15 (48.4) | |
| IV* | 21 (10.2) | 3 (9.7) | |
|
| |||
| T-stage | |||
| T1 | 21 (10.2) | 0 (0) | 0.005 |
| T2 | 98 (47.8) | 8 (25.5) | |
| T3 | 73 (35.6) | 20 (64.5) | |
| T4 | 13 (6.3) | 3 (9.7) | |
|
| |||
| N-stage | |||
| N0 | 113 (51.8) | 10 (30.3) | 0.026 |
| N1 | 71 (32.6) | 19 (57.6) | |
| N2 | 11 (5) | 0 (0) | |
| Nx | 23 (10.6) | 4 (12.1) | |
|
| |||
| Papillary variant (n=86) | 17 (22.7) | 4 (36.4) | 0.451 |
|
| |||
| PNI (n=142) | 60 (50.4) | 20 (87) | 0.001 |
|
| |||
| LVI (n=141) | 48 (41) | 17 (70.8) | 0.012 |
Abbreviations: LVI= lymphovascular invasion; PNI= perineural invasion; AJCC= American Joint Commission on Cancer; CBD= common bile duct; COPD= chronic obstructive pulmonary disease; CHF= congestive heart failure; HTN= hypertension; ASA=American Society of Anesthesiologist; IQR= interquartile range
Stage IV includes M0 patients with T1-3N2 and T4N0-2 disease.
The most common operations performed for patients with jaundice were radical cholecystectomy (segment 4b/5 resection, 51.5%) and extended hemi-hepatectomy (39.4%). Only 3 (9.1%) patients with jaundice were treated with simple cholecystectomy, all of whom had papillary carcinoma of the gallbladder. Patients with jaundice had higher rates of extrahepatic bile duct resection compared with those patients without jaundice (87.9 vs. 29.9%, P<0.001), portal vein resection and reconstruction (12% vs. 0.46%, P<0.001), and R1 margins (48.5% vs. 8.8%, P<0.001).
Advanced T stage and N-stage were more commonly associated with jaundice, as were lymphovascular (70.8% vs. 41%, P=0.012) and perineural invasion (87% vs 50.4%,P=0.001). The prevalence of papillary carcinoma (prone to extend intraluminal and occasionally obstruct the biliary system) was not different between the two groups (36.4 vs. 22.7%, P=0.451). Similarly, tumor grade was comparable between the two groups.
Perioperative and Postoperative Outcomes
Perioperative and postoperative outcomes are shown in Table 2. The presence of jaundice was associated with higher operative blood loss (550 vs. 300 mL, P=0.006) and need for intraoperative blood transfusion (37.9% vs. 10.2%, P<0.001). Patients with jaundice experienced higher overall morbidity (68.8% vs. 38.3%, P=0.002), including intra-abdominal abscess (19.4% vs. 7.1%, P=0.038), bile leak (16.1% vs. 5.1%, P=0.038), and new postoperative ascites (12.9% vs. 0.5%, P=0.001). More patients with jaundice required postoperative drainage procedures (18.2% vs. 6.6%, P=0.036) and reoperation (12.1% vs. 0.9%, P=0.003). As a result, hospital length of stay was longer (median 8 vs. 6 days, P<0.001) and the incidence of 90-day readmission (33.3% vs. 15.2%, P=0.024) was higher in patients with jaundice. However, 30- and 90-day mortality rates were not significantly higher in patients with jaundice.
Table 2.
Perioperative outcomes based on presence of jaundice
| No Jaundice (n=218) | Jaundice (n=33) | P value | |
|---|---|---|---|
| Intraoperative blood transfusion (n=235) | 21 (10.2) | 11 (37.9) | <0.001 |
| EBL (mL) | 300 (150–500) | 550 (200–750) | 0.0006 |
| Any complication (n=233) | 77 (38.3) | 22 (68.8) | 0.002 |
| Grade > 3 complication (n=233) | 18 (9) | 4 (12.5) | 0.356 |
| Wound infection (n=227) | 7 (3.6) | 3 (9.7) | 0.142 |
| Intra-abdominal abscess (n=227) | 14 (7.1) | 6 (19.4) | 0.038 |
| Bleeding (n=227) | 4 (2) | 3 (9.7) | 0.055 |
| Bile leak (n=227) | 10 (5.1) | 5 (16.1) | 0.038 |
| Anastomotic leak (n=226) | 3 (1.5) | 3 (9.7) | 0.035 |
| Postoperative drainage procedure (n=245) | 14 (6.6) | 6 (18.2) | 0.036 |
| Postoperative ascites (n=228) | 1 (0.5) | 4 (12.9) | 0.001 |
| Postoperative liver failure | 0 (0) | 1 (3.2) | 0.136 |
| Reoperation (n=247) | 2 (0.9) | 4 (12.1) | 0.003 |
| In hospital mortality | 10 (4.6) | 2 (6.1) | 0.662 |
| Death within 30 days | 4 (1.9) | 1 (3.0) | 0.521 |
| Death within 90 days | 7 (3.6) | 2 (6.5) | 0.352 |
| LOS (median, IQR) | 6 (4–7) | 8 (6–16) | <0.0001 |
| Readmission (n=243) | 32 (15.2) | 11 (33.3) | 0.024 |
| Adjuvant chemotherapy (n=207) | 82 (45.6) | 11 (40.7) | 0.683 |
| Adjuvant radiotherapy (n=194) | 36 (21.4) | 9 (34.6) | 0.142 |
Abbreviation: EBL= estimated blood loss; LOS= length of stay; IQR=interquartile range
Survival
Median follow-up for the entire study population was 17 months (IQR 7.8 – 32.4 months) and for patients alive at last follow-up was 18 months (IQR 4.8 – 44.5 months). OS was worse in patients with jaundice compared with those without jaundice (Figure 1: 3–year 23.5% vs. 47.2%; 5-year 9.4% vs. 39.2%; median 13.9 vs. 32.4 months; P<0.001). Stratifying by stage (III & IV), patients with jaundice had a lower median survival compared with patients who did not have jaundice in Stages IIIb (11.6 vs. 27.6 months, P=0.006) and IV (13.9 vs. 20 months, P=0.025), but not in Stage IIIa (16.4 vs. 17.2 months, P=0.562). The number of jaundiced patients with earlier stages of disease was not sufficient for further stage-by-stage analysis.
Figure 1.
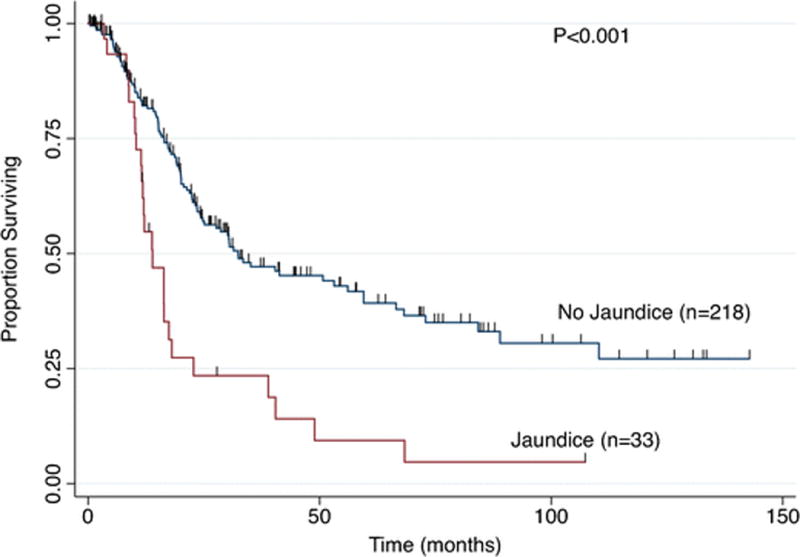
Overall Survival based on presence of jaundice
Univariate and multivariate survival analyses are presented in Table 3. While preoperative jaundice was a significant predictor of poor survival on univariate analysis, multivariate cox regression revealed that advanced stage and blood transfusion, but not jaundice, were independent predictors of poor survival.
Table 3.
Univariate and Multivariate Cox Regression Predicting Overall Survival
| Univariate | Multivariate | |||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | P value | HR 95% CI | P value | |
|
| ||||
| Age (per year) | 1.014 (0.999–1.029) | 0.066 | — | |
|
| ||||
| Non-white race | 0.767 (0.4967–1.186) | 0.234 | — | |
|
| ||||
| CA19-9 > 50 | 1.205 (0.832–1.746) | 0.323 | — | |
|
| ||||
| Preoperative Jaundice | 3.164 (2.006–4.991) | <0.001 | 0.726 (0.273–1.929) | 0.522 |
|
| ||||
| Extent of Resection | ||||
| Simple cholecystectomy | Reference | Reference | ||
| Radical cholecystectomy | 1.785 (0.829–3.842) | 0.138 | 0.788 (0.173–3.729) | 0.758 |
| Extended hepatectomy | 3.429 (1.445–8.136) | 0.005 | 1.251 (0.224–6.991) | 0.799 |
|
| ||||
| Intraoperative Blood transfusion | 2.423 (1.616–3.633) | <0.001 | 2.255 (1.042–4.876) | 0.039 |
|
| ||||
| Positive margins (R1/R0) | 2.941 (1.965–4.402) | <0.001 | 1.435 (0.652–3.157) | 0.369 |
|
| ||||
| Poor Grade | 1.636 (1.122–2.385) | 0.010 | 0.955 (0.522–1.745) | 0.881 |
|
| ||||
| AJCC Stage | ||||
| I | Reference | Reference | ||
| II | 1.927 (0.481–7.709) | 0.354 | 3.698 (0.330–41.417) | 0.289 |
| III | 5.592 (1.761–17.752) | 0.003 | 13.88 (1.807–106.73) | 0.011 |
| IV* | 7.916 (2.334–26.843) | 0.001 | 19.443(2.075–182.13) | 0.009 |
|
| ||||
| LVI | 1.900 (1.187–3.042) | 0.007 | 1.430 (0.749–2.729) | 0.278 |
Abbreviations: HR= hazard ratio; CI= confidence interval; LVI= lymphovascular invasion; AJCC=American Joint Commission on Cancer; CA=carcinoembryonic antigen
Stage IV includes M0 patients with T1-3N2 and T4N0-2 disease.
To further understand the value of surgery in patients with jaundice, subgroup survival analyses of the 33 jaundiced patients were performed; the absence of lymphovascular invasion (median 40.5 vs. 13.8 months; P=0.014, Figure 2A) and CA19-9 < 50 U/mL (median 40.4 vs. 12.1 months; P=0.0034, Figure 2B) were associated with substantially improved survival. There were no differences in median OS within the jaundice group based on margin status (R0 and R1: 16.3 vs. 12.1 months, respectively; P=0.284), tumor grade (well-moderate and poor grade: 16.3 vs. 13.8 months, respectively; P=0.950) and lymph node involvement (node negative and positive: 13.9 vs. 12.1 months, respectively; P=0.755). Furthermore, of the 12 jaundice patients with available data on location of positive margins, 7 patients had microscopic residual disease at the bile duct while 5 patients had microscopic disease at the liver parenchyma. OS was similar between those with positive margins at the bile duct and liver parenchyma (median 13.9 vs. 8.7 months, P=0.16). However, RFS was significantly better among those with R1 margins at the bile duct compared with liver parenchyma (median 18.8 vs. 6.2 months, P=0.003).
Figure 2.
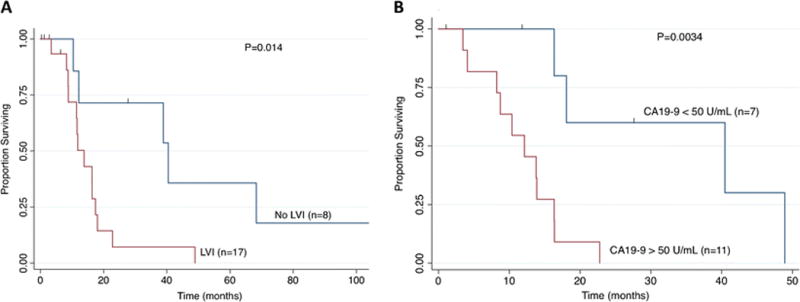
Survival analysis of patients with jaundice based on (A) presence of lymphovascular invasion and (B) preoperative CA19-9.
Clinicopathologic Characteristics of Long-Term Survivors with Jaundice
Table 4 shows the clinicopathologic characteristics of the five actual 3-year survivors among the 33 patients with jaundice who underwent curative resection. Only 2 patients reached the 5-year survival milestone and 1 patient had no evidence of disease at the time of last follow-up. Extended hepatectomy with bile duct resection was performed in 1 patient. None of the patients who survived for three or more years underwent portal vein resection and none had T4 disease. Four out of five 3-year survivors did not have evidence of lymphovascular invasion.
Table 4.
Clinicopathologic Features of Five Actual 3-Year Survivors with Jaundice
| Age/ Sex |
Peak Bili |
CA 19-9 |
Preoperative Biliary Drainage |
Type of Resection |
Margin | Grade | Stage | LVI | PNI | DFI (mo) |
Adjuvant therapy |
OS (mo) |
Vital Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 52/M | 14.3 | NR | None | Radical Cholecystectomy | R0 | Moderate | T2NX | No | No | 107 | No | 107 | NED |
| 25/M | NR | NR | Percutaneous | Cholecystectomy + CBD resection | R1 | Moderate | TxN1 | No | Yes | 49 | NR | 68.3 | DOD |
| 64/F | NR | 24 | Endoscopic | Radical Cholecystectomy + CBD resection | R1 | Moderate | T3N1 | Yes | No | 18.8 | Yes | 48.9 | DOD |
| 48/M | 6.5 | 31 | Endoscopic | Radical Cholecystectomy | R0 | Poor | T3N1 | No | Yes | 18.2 | No | 40.5 | DOD |
| 67/F | 4.9 | NR | None | Extended Major Hepatectomy + CBD resection | R1 | Poor | T3N1 | No | Yes | 10 | Yes | 38.9 | DOD |
Abbreviations: M=male; F=female; NR=not recorded; CBD= common bile duct; Bili= bilirubin; LVI= lymphovascular invasion; PNI=perineural invasion; DFI= disease free interval; mo=months; Chemo=chemotherapy; RT=radiotherapy; OS=overall survival; CA=carcinoembryonic antigen
DISCUSSION
Preoperative jaundice is often an indicator of advanced GBC and the value of surgery in these patients remains controversial. In this study, preoperative jaundice was indeed associated with a lower likelihood of curative resection, higher operative morbidity and shorter long-term survival. However, jaundice was not an independent predictor of survival after controlling for advanced stage and intraoperative blood transfusion. A subset of jaundiced GBC patients did achieve long-term survival after resection, and further analysis revealed that low CA19-9 levels and the absence of lymphovascular invasion on pathology could help identify patients who benefitted the most from surgical resection.
A handful of studies over the last decade have reported on actuarial and actual 5-year survivors among patients with GBC and common bile duct involvement undergoing surgical resection. In 2007, a study from New Delhi, India described 14 GBC patients with jaundice who underwent margin-negative resection. Seven patients survived for more than 2 years and one patient (7%) survived beyond 5 years.14 In 2009, an updated report from Memorial Sloan Kettering Cancer Center (MSKCC) described 36 GBC patients with bile duct involvement necessitating biliary resection and reconstruction achieving a 5-year disease-specific actuarial survival rate of 21%.16 It should be noted that this study excluded 5 patients (12%) who died perioperatively from the survival analysis. Had these patients been included in the analysis, the resulting overall survival rate would have been significantly lower. In 2011, a multi-institutional study from France reported 4 (8%) actual 5-year survivors out of 50 GBC patients with jaundice who underwent curative resection.6 Concurrently, the hepatobiliary surgical unit from Nagoya University in Japan published their extensive experience with surgical resection of 73 GBC patients with pathologic extrahepatic bile duct invasion and noted a 23% 5-yr disease-specific survival.15 Again, had these authors included the 11% of patients who died perioperatively as events in their survival analysis, the resulting overall survival rate would have been about half of the reported disease-specific survival rate. More recently, in 2014, investigators from Shanghai, China reported a 5-year survival of 6% in 47 patients with GBC and jaundice undergoing surgical resection.13 Taken together, the results of these 5 previous studies appear to be in agreement with our study, where the actuarial 5-year survival for jaundiced GBC patients undergoing resection was noted to be 9%.
Despite the overall poor prognosis, it is clear from the aforementioned studies that surgery can provide a survival benefit for a small fraction of jaundiced GBC patients. To further clarify the value of surgery in this setting, we performed a subset analysis, which revealed the absence of lymphovascular invasion (median 40.5 vs. 13.8 months; P=0.014) and lower CA19-9 levels (median 40.4 vs. 12.1 months; P=0.0034) to be associated with better OS. These findings may have important implications in selecting jaundiced GBC patients for aggressive resection. For instance, CA19-9 levels have been shown to correlate with metastatic disease and poor survival in patients with pancreaticobiliary malignancy,19, 20 therefore one can infer that jaundiced GBC patients with higher CA19-9 are more likely to have occult micrometastatic disease that may not be obvious with current preoperative staging studies or during surgical exploration. Furthermore, we noted that the presence of lymphovascular invasion on pathology was associated with worse overall survival. Lymphovascular invasion has been shown to be a surrogate for an aggressive malignant phenotype in GBC.21, 22 Because lymphovascular invasion can be pathologically examined following cholecystectomy or with a preoperative (laparoscopic, endoscopic, or percutaneous) biopsy, it may be a useful tool in the selection of jaundiced GBC patients for radical surgery.
Although the present study focused on jaundice as a physical sign, it is worth noting that extrahepatic bile duct invasion and jaundice are closely related, but not a single and indistinguishable entity. In fact, the definition of “jaundice” among the aforementioned studies has been very variable. Most studies used clinically evident jaundice as their inclusion criterion,14, 6 along with elevated bilirubin levels (ranging from > 2 to >8 mg/dL),3, 13 and one study simply included patients if “clinical involvement of the bile duct mandating resection” was noted.16 Our study used clinically evident jaundice, a bilirubin of > 4 mg/dL, and/or the need for preoperative biliary drainage as inclusion criteria. Within this cohort, resection of the common bile duct was necessary in 88% of patients. Conversely, in the study from Japan, the authors used pathologic extrahepatic bile duct invasion as the variable to stratify patients and noted that only 78% of them had preoperative jaundice.15 These observations reflect the fact that the process leading to jaundice in locally advanced GBC can be related to a variety of factors, including direct mass effect and compression of the bile duct, true pathologic invasion, intraluminal tumor extension, and peribiliary lymphatic infiltration. The multifactorial nature of this process may partly explain the variable perioperative outcomes reported for this patient population.
There are several important limitations when interpreting the current study. First, selection bias could not be controlled for given the retrospective nature of the study. Second, clinical practices may vary in the 10 academic institutions participating in this study. Third, the total number of patients undergoing resection for gallbladder cancer with preoperative jaundice was small, underscoring the fact that these patients are rarely candidates for curative resection. Therefore, survival analyses within thus subgroup were likely underpowered due to the small sample size. Last, while we suggested that CA19-9 levels may help in identifying jaundiced patients who may benefit from surgery, elevated CA19-9 may also be a manifestation of biliary obstruction. Furthermore, this serum tumor marker may not be secreted in 10% of the Caucasian population who lack the Lewis blood type antigen in their red blood cells.23 Despite these limitations, this multi-institutional study adds generalizable findings to this challenging clinical scenario where data are already limited.
In conclusion, the presence of jaundice in GBC was strongly associated with inoperability, advanced disease and higher operative mortality. Although survival even after curative-intent resection remains dismal, a small subset of patients can still achieve long-term survival after surgery. Future studies should focus on how to reliably identify this subset of patients. Serum CA19-9 levels and preoperative biopsy looking for lymphovascular invasion may be helpful in this direction. Although jaundice is a very alarming clinical sign in patients with GBC, it should not be considered an absolute contraindication to surgical exploration, but expectations should be carefully set and patient selection should be very cautious.
Acknowledgments
Funding/Support: None
Footnotes
Author Contributions:
Study conception and design: Tran, Poultsides, Norton, Maithel, Hawkins
Acquisition of data: Tran, Ethun, Beal, Buettner, Krasnick, Salem, Martin, Mogal, Isom, Shenoy
Analysis and interpretation of data: Tran, Poultsides, Maithel, Norton, Schmidt, Pawlik, Hawkins, Fields, Weber, Scoggins, Shen, Idrees, Hatzaras
Drafting of manuscript: Tran, Poultsides
Critical revision: Tran, Poultsides, Maithel, Norton, Schmidt, Pawlik, Hawkins, Fields, Weber, Scoggins, Shen, Idrees, Hatzaras, Ethun, Beal, Buettner, Krasnick, Salem, Martin, Mogal, Isom, Shenoy
Poster Presentation at the Society of Surgical Oncology, March 3–5, 2016 in Boston, MA and at the American Society of Clinical Oncology Gastrointestinal Cancers Symposium January 21–23, 2016 in San Francisco, CA.
References
- 1.Jarnagin WR, Ruo L, Little SA, Klimstra D, D’Angelica M, DeMatteo RP, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003;98(8):1689–700. doi: 10.1002/cncr.11699. [DOI] [PubMed] [Google Scholar]
- 2.Wernberg JA, Lucarelli DD. Gallbladder cancer. Surg Clin North Am. 2014;94(2):343–60. doi: 10.1016/j.suc.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Hawkins WG, DeMatteo RP, Jarnagin WR, Ben-Porat L, Blumgart LH, Fong Y. Jaundice predicts advanced disease and early mortality in patients with gallbladder cancer. Ann Surg Oncol. 2004;11(3):310–5. doi: 10.1245/aso.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Rau C, Marec F, Vibert E, Geslin G, Yzet T, Joly JP, et al. Gallbladder cancer revealed by a jaundice caused by an endobiliary tumor thrombus. Ann Chir. 2004;129(6–7):368–71. doi: 10.1016/j.anchir.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Redaelli CA, Buchler MW, Schilling MK, Krahenbuhl L, Ruchti C, Blumgart LH, et al. High coincidence of Mirizzi syndrome and gallbladder carcinoma. Surgery. 1997;121(1):58–63. doi: 10.1016/s0039-6060(97)90183-5. [DOI] [PubMed] [Google Scholar]
- 6.Regimbeau JM, Fuks D, Bachellier P, Le Treut YP, Pruvot FR, Navarro F, et al. Prognostic value of jaundice in patients with gallbladder cancer by the AFC-GBC-2009 study group. Eur J Surg Oncol. 2011;37(6):505–12. doi: 10.1016/j.ejso.2011.03.135. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu Y, Ohtsuka M, Ito H, Kimura F, Shimizu H, Togawa A, et al. Should the extrahepatic bile duct be resected for locally advanced gallbladder cancer? Surgery. 2004;136(5):1012–7. doi: 10.1016/j.surg.2004.04.032. discussion 8. [DOI] [PubMed] [Google Scholar]
- 8.Katz SC, Ryan K, Ahmed N, Plitas G, Chaudhry UI, Kingham TP, et al. Obstructive jaundice expands intrahepatic regulatory T cells, which impair liver T lymphocyte function but modulate liver cholestasis and fibrosis. J Immunol. 2011;187(3):1150–6. doi: 10.4049/jimmunol.1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawarabayashi N, Seki S, Hatsuse K, Kinoshita M, Takigawa T, Tsujimoto H, et al. Immunosuppression in the livers of mice with obstructive jaundice participates in their susceptibility to bacterial infection and tumor metastasis. Shock. 2010;33(5):500–6. doi: 10.1097/SHK.0b013e3181c4e44a. [DOI] [PubMed] [Google Scholar]
- 10.Nehez L, Andersson R. Compromise of immune function in obstructive jaundice. Eur J Surg. 2002;168(6):315–28. doi: 10.1080/11024150260284815. [DOI] [PubMed] [Google Scholar]
- 11.Kimmings AN, van Deventer SJ, Obertop H, Rauws EA, Huibregtse K, Gouma DJ. Endotoxin, cytokines, and endotoxin binding proteins in obstructive jaundice and after preoperative biliary drainage. Gut. 2000;46(5):725–31. doi: 10.1136/gut.46.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strasberg SM, Gao F, Sanford D, Linehan DC, Hawkins WG, Fields R, et al. Jaundice: an important, poorly recognized risk factor for diminished survival in patients with adenocarcinoma of the head of the pancreas. HPB (Oxford) 2014;16(2):150–6. doi: 10.1111/hpb.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang XW, Yuan JM, Chen JY, Yang J, Gao QG, Yan XZ, et al. The prognostic importance of jaundice in surgical resection with curative intent for gallbladder cancer. BMC Cancer. 2014;14:652. doi: 10.1186/1471-2407-14-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwal AK, Mandal S, Singh S, Bhojwani R, Sakhuja P, Uppal R. Biliary obstruction in gall bladder cancer is not sine qua non of inoperability. Ann Surg Oncol. 2007;14(10):2831–7. doi: 10.1245/s10434-007-9456-y. [DOI] [PubMed] [Google Scholar]
- 15.Nishio H, Ebata T, Yokoyama Y, Igami T, Sugawara G, Nagino M. Gallbladder cancer involving the extrahepatic bile duct is worthy of resection. Ann Surg. 2011;253(5):953–60. doi: 10.1097/SLA.0b013e318216f5f3. [DOI] [PubMed] [Google Scholar]
- 16.D’Angelica M, Dalal KM, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Analysis of the extent of resection for adenocarcinoma of the gallbladder. Ann Surg Oncol. 2009;16(4):806–16. doi: 10.1245/s10434-008-0189-3. [DOI] [PubMed] [Google Scholar]
- 17.Edge SB, American Joint Committee on Cancer . American Cancer Society AJCC cancer staging manual. 7th. New York; London: Springer; 2010. [Google Scholar]
- 18.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–96. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 19.Kondo N, Murakami Y, Uemura K, Sudo T, Hashimoto Y, Sasaki H, et al. Elevated perioperative serum CA 19-9 levels are independent predictors of poor survival in patients with resectable cholangiocarcinoma. J Surg Oncol. 2014;110(4):422–9. doi: 10.1002/jso.23666. [DOI] [PubMed] [Google Scholar]
- 20.Maithel SK, Maloney S, Winston C, Gonen M, D’Angelica MI, Dematteo RP, et al. Preoperative CA 19-9 and the yield of staging laparoscopy in patients with radiographically resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2008;15(12):3512–20. doi: 10.1245/s10434-008-0134-5. [DOI] [PubMed] [Google Scholar]
- 21.Shibata K, Uchida H, Iwaki K, Kai S, Ohta M, Kitano S. Lymphatic invasion: an important prognostic factor for stages T1b-T3 gallbladder cancer and an indication for additional radical resection of incidental gallbladder cancer. World J Surg. 2009;33(5):1035–41. doi: 10.1007/s00268-009-9950-4. [DOI] [PubMed] [Google Scholar]
- 22.Ethun CG, Postlewait LM, Le N, Pawlik TM, Buettner S, Poultsides G, et al. A Novel Pathology-Based Preoperative Risk Score to Predict Locoregional Residual and Distant Disease and Survival for Incidental Gallbladder Cancer: A 10-Institution Study from the U.S. Extrahepatic Biliary Malignancy Consortium. Ann Surg Oncol. 2016 doi: 10.1245/s10434-016-5637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33(3):266–70. doi: 10.1016/j.ejso.2006.10.004. [DOI] [PubMed] [Google Scholar]


