Abstract
Background
L-theanine is a non-protein amino acid in green tea, and its hepatoprotection and neuroprotection have been verified. However, whether L-theanine can prevent cardiomyocytes from apoptosis is unclear yet. This study evaluated the protective effects of L-theanine on H2O2-induced heart injury in vitro.
Material/Methods
The certified H9C2 cells were pretreated with L-theanine (0 mM, 4 mM, 8 mM, and 16 mM) for 24 h, followed by 160 μM H2O2 solution for 4 h. The cell viability and antioxidant indices were assayed. Quantitative evaluation of apoptosis was performed by flow cytometric analysis. Nuclear morphology of the cells was monitored by 4′,6-diamidino-2-phenylindole staining. Expression of Caspase-3, poly ADP-ribose polymerase (PARP), c-Jun N-terminal kinase (JNK), and mitogen-activated protein kinase p38 was assayed by Western blot.
Results
Compared to the H2O2 treatment, all doses of L-theanine treatments increased the cell viability, glutathione level, and the activities of glutathione peroxidase and superoxide dismutase (P<0.001). The contents of reactive oxygen species, nitric oxide, and oxidized glutathione were decreased by L-theanine treatments (P<0.001). Meanwhile, L-theanine treatments decreased the apoptosis ratio of H2O2-induced H9C2 cells (P<0.001). Pro-Caspase-3 expression was upregulated and cleavaged-PARP expression was inhibited by L-theanine (P<0.001). However, the phosphorylation of JNK and p38 was not affected by L-theanine treatments (P>0.05).
Conclusions
These data indicate that L-theanine pretreatment prevents H2O2-induced apoptosis in H9C2 cells, probably via antioxidant capacity improvement. Therefore, it might be a promising potential drug candidate for prophylaxis of ischemia/reperfusion-induced heart diseases.
MeSH Keywords: Antioxidants, Apoptosis, Caspase 3, JNK Mitogen-Activated Protein Kinases, p38 Mitogen-Activated Protein Kinases, Poly(ADP-ribose) Polymerases
Background
H9C2 is a clonal cell line derived from rat heart and exhibits many of the properties of skeletal muscle. H9C2 cardiomyocytes are adherent cells and have been used as an ideal cell model for the study of cardiovascular diseases, which are the main cause of mortality and morbidity in the world [1]. Oxidative damage caused by reactive oxygen species (ROS) accumulation has been implicated as a critical pathophysiological mechanism in the progression of heart diseases, including ischemia/reperfusion injury [2,3]. The ROS are capable of attacking lipid membrane, proteins, and DNA, initiating lipid peroxidation and causing a depletion or inactivation of the antioxidant enzymes in myocardial cells, then resulting in cell injury and even apoptosis [4,5]. Fortunately, the attenuation of oxidative damage can ultimately protect myocardial cells from apoptosis [6,7]. Therefore, an increasing number of studies have been focused on the pathogenesis of oxidative stress in heart injury as well as therapeutic intervention with antioxidants.
L-theanine, namely N-ethyl-γ-glutamine, is a unique non-protein amino acid in tea [8] that can be degraded by glutaminase to glutamate and ethylamine in vivo [9]. It has many physiological functions, such as anti-oxidative stress [10], regulating immune response [11], and relaxing neural tension [12]. L-theanine alone or in combination with L-cystine administration can restore reduced glutathione (GSH) synthesis in mammals [13], further enhancing the antioxidant capacity [14]. L-theanine can normalize alcohol or doxorubicin-induced increase of lipid peroxide and reduction of GSH in mice [15–18]. Deng et al. [19] also reported that L-theanine decreased oxidative stress in Escherichia coli-infected mice. These studies indicate that L-theanine is an effective antioxidant in vivo. However, whether L-theanine can protect rat cardiomyocytes against oxidative injury is not clear.
Fewer studies reported that L-theanine protects neurocytes and hepatocytes from apoptosis. L-theanine can penetrate the blood-brain barrier, competitively inhibit glutamate receptors, and protect hippocampus neurocytes against ischemia/reperfusion-induced apoptosis by inhibiting JNK signaling pathway [20,21]. L-theanine also decreases alcohol- and carbon tetrachloride-induced apoptosis of hepatocytes [16,22], attenuate ethanol- and H2O2-induced nuclear morphological damages, and decrease the apoptotic level of normal human liver cells [16,23].
Although many studies have demonstrated the antioxidant effects of L-theanine, the anti-apoptotic mechanism of L-theanine is not entirely clear, especially for myocardial cells under oxidative stress. Our recent study [24] showed that L-theanine administration increased the activities of superoxide dismutase (SOD) and catalase, and decreased the level of nitric oxide (NO) by upregulating the mRNA expression of SOD2 in the rat heart. Hence, in this study, on the premise that L-theanine has the antioxidant capacity of protecting rat H9C2 cardiomyocytes against H2O2-induced injury, we further hypothesized that L-theanine could protect H9C2 cells against H2O2-induced apoptosis via suppressing JNK or p38 signaling pathway. The link between its antioxidant effects and anti-apoptotic mechanism will be explored. This study was aimed at providing a new preventive treatment for oxidative stress-induced heart diseases.
Material and Methods
This experiment was conducted according to the animal care guidelines of the Animal Care Committee, Institute of Subtropical Agriculture, the Chinese Academy of Sciences, Changsha city, Hunan province, China (no. KYNEAAM-2013-0009). All efforts were made to minimize suffering of experimental animals.
Chemicals and reagents
L-theanine (purity ≥99.2%) was purchased from Hongya Yaxing Biotechnology Co., Ltd (Meishan, Sichuan, China). H9C2 cells were obtained from Shanghai Institutes for Biological Sciences, the Chinese Academy of Sciences (Shanghai, China). The medium was Dulbecco’s modified Eagle’s medium which contained 4.5 g/L glucose (HG-DMEM) (Gibco, Carlsbad, CA, USA). The fetal bovine serum (FBS) was purchased from Hyclone (Hyclone, Logan, Utah, USA). Cell Counting Kit-8 (CCK-8) and 4′,6′-diamidino-2-phenylindole (DAPI) were purchased from Dojindo (Dojindo, China) and Sigma Chemical Co. (MO, USA), respectively. The antibodies to pro-Caspase-3, poly-adenosine diphosphate-ribose polymerase (PARP), and polyclonal antibodies to p38, JNK, β-actin, and anti-phosphorylated JNK (Thr183/Tyr185, p-JNK) and p38 (Thr180/Tyr182, p-p38) were purchased from Cell Signaling Technology (Beverly, MA, USA). All secondary antibodies used for Western blot were obtained from Calbiochem (La Jolla, CA, USA). All assay kits of TBARS, NO, ROS, GSH, oxidized glutathione (GSSG), SOD, and GSH-Px (Lot numbers A003-1, A012, E004, A006-2, A061-1, A001-3, and A005, respectively) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China). All the other reagents used in this study were of analytical grade.
Cell culture
H9C2 cells were cultured in HG-DMEM supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a 5% CO2 humidified environment. L-theanine was dissolved in HG-DMEM, filtered through a 0.22-micron filter, and then stored at 4°C before use. The culture medium was changed to HG-DMEM medium without FBS for 24 h to reduce the serum effect before any treatment. When indicated, L-theanine was added 24 h prior to the treatment of H2O2.
Cell viability assay and cell treatment
Firstly, H9C2 cells were seeded into 96-well plates (5×103 cells/well) for 24 h and treated by different concentrations of H2O2 (100~600 μM) for 4 h or L-theanine (1~32 mM) for 24 h. Cell viability was determined according to the protocols of the CCK-8 kit. Data are expressed as the percentage of cellular viability relative to the control culture (assuming 100%). As a result, 160 μM H2O2 caused about 50% cell viability loss and 4~16 mM L-theanine increased the cell viability compared to the control group. Therefore, in the later experiments, H9C2 cells were pretreated with various concentrations (0 mM, 4 mM, 8 mM, and 16 mM) of L-theanine for 24 h, followed by H2O2 (160 μM) for 4 h.
Antioxidant indices assay
Cells were harvested in a lysate buffer containing 0.1% Triton X-100, then centrifuged at 12 000 rpm/min at 4oC for 15 min to remove the supernatant for assay. The contents of thiobarbituric acid reactive species (TBARS), ROS, NO, GSSG, GSH, and the activities of SOD and GSH-Px in the cell homogenates were determined using analysis kits according to the manufacturer’ instructions.
Nuclear morphological assay
H9C2 cells cultured in 12-well plates at a seeding density of 2×105 cells per well were stained with DAPI to evaluate nuclear morphology. Cells were fixed with 5% formaldehyde diluted in PBS for 30 min at room temperature. Fixed cells were permeabilized with 0.02% Triton in PBS for 5 min. Following incubation with 1 mL (l μg/mL) DAPI for 10 min at 37°C, the cells were washed again with PBS and examined by use of an Olympus fluorescence microscope (Olympus, Tokyo, Japan).
Flow cytometric analysis
Quantitative evaluation of apoptosis was determined using Annexin V-fluoresein-5-isothiocyanate (FITC) and propidine iodide (PI) double-staining flow cytometry. Cells were harvested, washed with PBS twice, trypsinized, and collected by centrifugation at 1000×g for 5 min. Then cells were stained with 1 mg/ml AnnexinV-FITC and 10 mg/ml PI in the dark for 10 min at room temperature. Flow cytometric analysis was performed with FACScan (Becton-Dickinson, Mountainview, CA, USA) with the Cell Quest program. For each sample, 5000 cells were analyzed. Both early apoptotic cells (localized in the lower right quadrant) and late apoptotic cells (upper right quadrant) were regarded as apoptotic cells.
Western blot analysis
The cells cultured in 10 mm culture dishes (2×107 cells) were washed twice with ice-cold PBS and lysed in RIPA buffer (20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.1% SDS, 1 mM EDTA, 1 mM EGTA, 1 mM β-glycerophosphate, 2.5 mM sodium pyrophosphate, 1% Triton X-100, 1 mM sodium orthovanadate, 10 μg/mL aprotinin, 10 μg/mL leupeptin, and 1 mM phenylmethylsulfonyl fluoride (PMSF) supplemented with protease inhibitors for 30 min on ice. Lysates were centrifuged at 12 000 rpm/min for 15 min at 4oC, and the concentration of protein in the supernatant was determined by BCA assay. The electrophoresis, transformation, and blocking of total proteins of cells were the same as the procedures of Yan et al. [25]. The anti-rat primary antibodies (pro-Caspase-3, 1: 200; PARP, 1: 500; Cleaved-PARP, 1: 500; p-JNK, 1: 1000; JNK, 1: 500; p-p38, 1: 500; p38, 1: 500; β-actin, 1: 4000) were diluted in Can Get Signal™ Solution 1 (Toyobo Co., Osaka, Japan) and incubated for 24 h at 4°C. After washing with PBS-Tween 20 buffer, membranes were incubated with horseradish peroxidase-conjugated goat anti-mouse secondary antibodies diluted 1: 3000 in Can Get Signal™ Solution 2 (Toyobo Co., Osaka, Japan) for 3 h at room temperature. Enzymatic detection of horseradish peroxidase was carried out by a Super ECL Detection Kit (KeyGEN Biotech, Nanjing, China). Densitometric signals were obtained after exposure to an X-ray Kodak film (Eastman Kodak Co., Rochester, New York, USA). Band intensities were quantified by Quantity One software (Bio-Rad) and normalized to β-actin as an internal control for total protein loading. Aliquots of whole-cell lysates were subjected to immunoblotting analysis to confirm appropriate expression of proteins.
Statistical analysis
Statistical analyses were conducted by one-way analysis of variance (ANOVA) using the Mixed Proc of SAS (version 8.2, SAS Institute, Cary, NC, USA). When indicated by ANOVA, means were separated using least significant differences. All data are expressed as means ±SD. Significance was declared at P<0.05 or P<0.01.
Results
Cell viability
As shown in Figure 1A, H2O2 significantly inhibited H9C2 cell viability in a dose-dependent manner. It was observed that H2O2 at 160 μM reduced (P<0.001) the cell survival to 50.58±2.24%. This concentration (160 μM) of H2O2 was thereby used for subsequent experiments. As shown in Figure 1B, L-theanine within 2~32 mM range (except for 4 mM) increased (P<0.05) the cell viability to different extents. Due to its proliferation effect, 4 mM, 8 mM, and 16 mM of L-theanine was thereby chosen for subsequent experiments. To evaluate the effect of L-theanine on H2O2-inducedcell viability loss, H9C2 cells were pretreated with L-theanine at different concentrations (4 mM, 8 mM, and 16 mM) for 24 h, followed by 160 μM H2O2 treatment for 4 h. As shown in Figure 2, H2O2 treatment decreased (P<0.001) the cell viability by about 40% compared to the control group. The decrease of cell viability induced by H2O2 was suppressed (P<0.001) by L-theanine treatments, with 4 mM L-theanine showing the greatest inhibitory effect.
Figure 1.
Effects of H2O2 and L-theanine on the cell viability of H9C2 cells. H9C2 cells were treated with various concentrations of (A) H2O2 for 4 h or (B) L-theanine for 24 h respectively, then measured by CCK-8 analysis. Values were representd as means ±SD (n=6); Bars not sharing a common letter differ (P<0.05).
Figure 2.
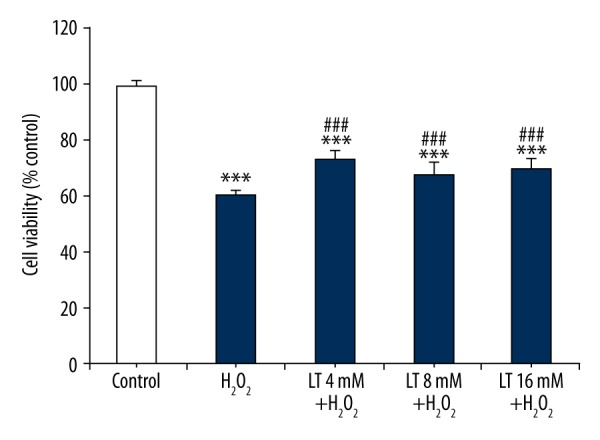
Effects of L-theanine on H9C2 cells against H2O2-induced cell injury. H9C2 cells were pretreated with or without L-theanine for 24 h, followed by 160 μM H2O2 treatment for 4 h, then cell viability was measured by CCK-8 analysis. *** P<0.001, compared with control. ### P<0.001, compared with the group of H2O2-treated H9C2 cells. LT – L-theanine.
Antioxidant indices
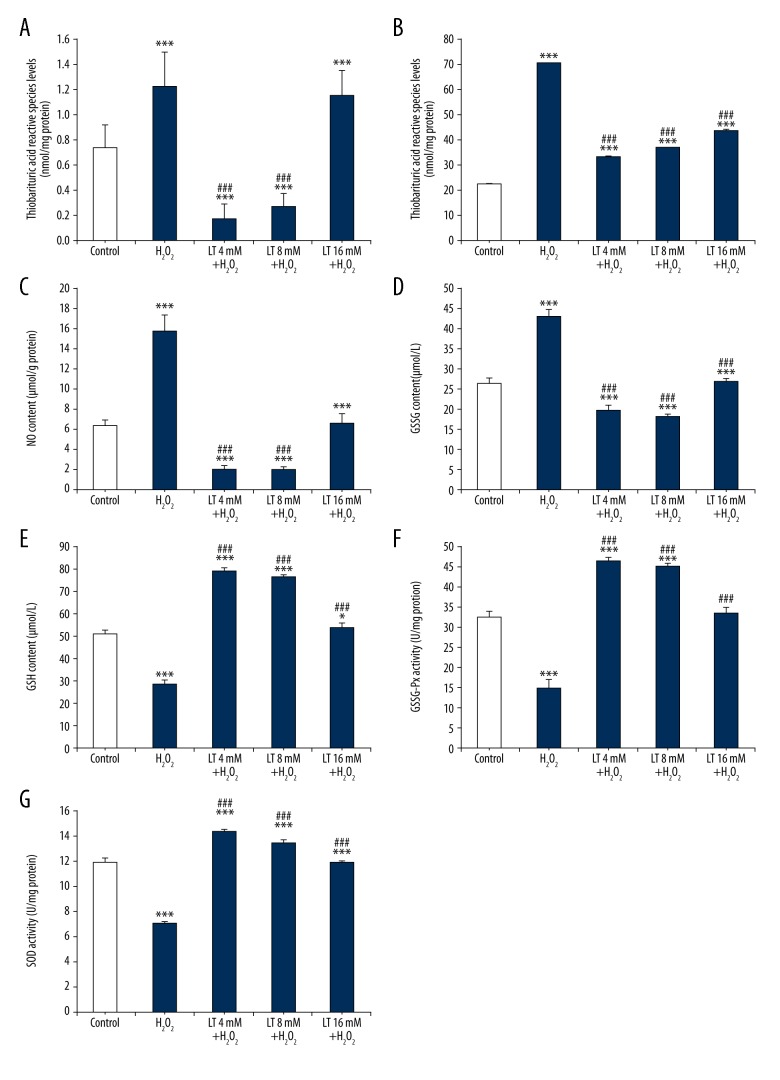
As shown in Figure 3, compared with the control group, H2O2 treatment increased the contents of TBARS, ROS, NO, and GSSG, and decreased the GSH content and the activities of GSH-Px and SOD in the H9C2 cells (P<0.001). L-theanine addition at 4, 8, and 16 mM decreased (P<0.001) the contents of TBARS, ROS, NO, and GSSG, but increased (P<0.001) the content of GSH and the activities of GSH-Px and SOD in the H2O2-induced H9C2 cells (except for the TBARS content at 16 mM). Furthermore, compared with the H2O2 treatment, low and middle doses of L-theanine decreased (P<0.001) the content of TBARS, but the high dose did not. All doses of L-theanine increased (P<0.001) ROS, NO, GSSG, and GSH production and the activities of GSH-Px and SOD compared to the H2O2 treatment group. As depicted in the graphs, 4 mM and 8 mM of L-theanine pretreatment had significant antioxidant effect, with the greatest effect at the dose of 4 mM.
Figure 3.
Effects of L-theanine on antioxidant indices of H2O2-induced H9C2 cells. H9C2 cells were pretreated with or without L-theanine for 24 h, followed by 160 μM H2O2 treatment for 4 h, then the antioxidant indices were assayed. (A–G) Represented the content of thiobarbituric acid reactive species (malondialdehyde equivalents), ROS, NO, GSSG, GSH and the activities of GSH-Px, SOD respectively. * P<0.05 and *** P<0.001, compared with control. ### P<0.001, compared with the group of H2O2-treated H9C2 cells. LT – L-theanine.
Nuclear morphology
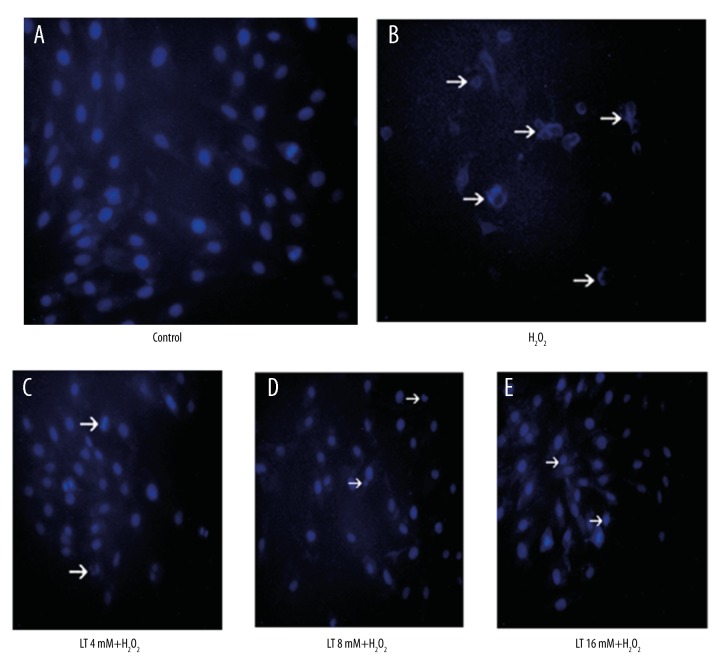
The nuclear morphological changes of H9C2 cells were evaluated by fluorescence microscopy after DAPI staining. As shown in Figure 4, characteristics of apoptosis of nuclear condensation and fragmentation were detected in the cells treated with H2O2, but concentrations of L-theanine pretreatment significantly attenuated H2O2-induced nuclear morphological damage.
Figure 4.
Effects of L-theanine on the nuclear morphology of H2O2-induced H9C2 cells. H9C2 cells were pretreated with or without L-theanine for 24 h then treated with 160 μM H2O2 for 4 h. The nuclear morphological analysis was performed by DAPI staining under an Olympus fluorescence microscopy (magnification ×200). Arrows indicated nuclear condensation and nuclear fragmentation. LT – L-theanine.
Apoptosis
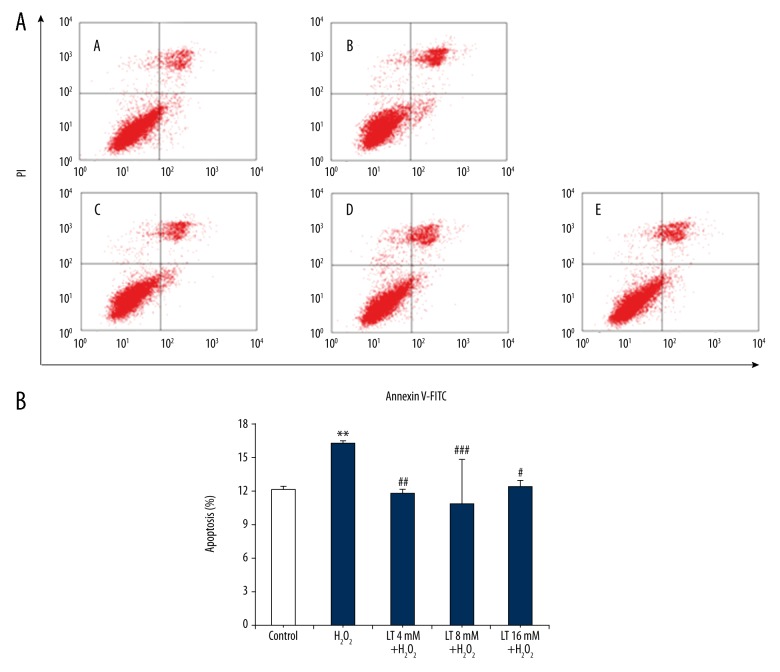
To further quantify the effect of L-theanine conferring protection against H2O2-induced apoptosis, Annexin-V-FITC and PI staining were performed by flow cytometry. As shown in Figure 5, exposure of H9C2 cells to 160 μM H2O2 for 4 h caused the occurrence of apoptosis (P<0.001) and there were about 16.30±0.18% of apoptotic cells. In contrast, pretreatment with L-theanine at concentration of 4, 8, and 16 mM remarkably attenuated H2O2-induced apoptosis (P<0.01, P<0.001, and P<0.05, respectively), with the minimal apoptotic ratio at 8 mM.
Figure 5.
Effects of L-theanine on the apoptosis ratio of H2O2-induced H9C2 cells. After treated with L-theanine or H2O2 for indicated concentration and time, H9C2 cells were dyed with both of Annexin V-FITC and propidine iodide (PI). (A) Flow cytometric analysis was performed with FACScan with the Cell Quest program: A – Control; B – H2O2 160 μM; C – L-theanine 4mM + H2O2 160 μM; D – L-theanine 8 mM + H2O2 160 μM; E – L-theanine 16 mM + H2O2 160 μM. (B) Percentage of apoptotic cells, as determined by Annexin V-FITC and PI staining followed by the fluorescence-activated cell sorting using flow cytometry. Data are expressed as mean ±SD (n=4). ** P<0.01, compared with control. ## P<0.01 and ### P<0.001, compared with the group of H2O2-treated H9C2 cells. LT, L-theanine.
Pro-Caspase-3 and PARP expression
Western blot analysis was used to examine the expression of pro-Caspase-3 and PARP. As shown in Figure 6, pro-Caspase-3 protein was downregulated and PARP was cleaved after H2O2 treatment in H9C2 cells. The proteolytic cleavage of PARP resulted in the characteristic shift of the protein from 116 to 89 kDa, showing that H2O2-induced cells were undergoing apoptosis. Fortunately, L-theanine pretreatment inhibited the reduction of pro-Caspase-3 and the increment of cleavage of PARP induced by H2O2 (P<0.001). Moreover, all doses of L-theanine pretreatment had the same dramatic anti-apoptotic effect (P<0.001).
Figure 6.
Effects of L-theanine on the expression of pro-caspase 3 and PARP in H2O2-induced H9C2 cells. H9C2 cells were pretreated with or without 4, 8 and 16 mM L-theanine for 24 h, followed by 160 μM H2O2 treatment for 4 h. Then, the expression of pro-caspase 3 and PARP were tested by Western blot. β-actin was used to confirm that equal cell equivalents were loaded. A representative Western blot for each treatment from three independent experiments was shown. Data are expressed as means ± SD. *** P<0.001, compared with control. ### P<0.001, compared with the group of H2O2-treated H9C2 cells.
P38 and JNK signaling pathways
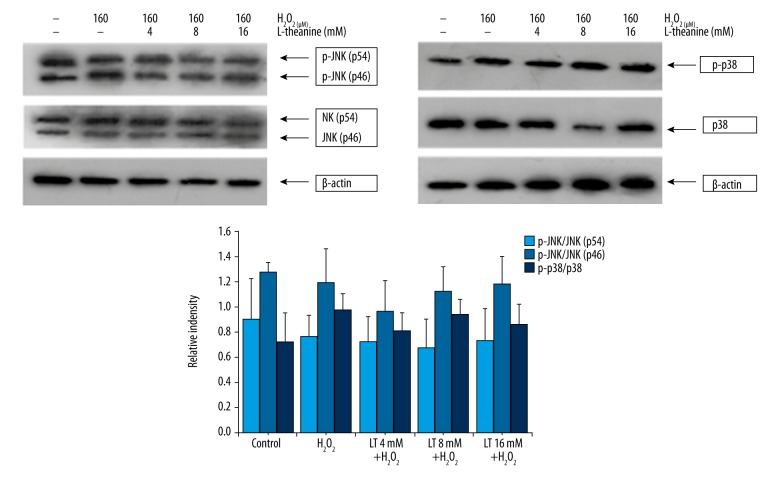
Western blot analysis was used to examine whether L-theanine affected H2O2-induced activation of p38 and JNK. As shown in Figure 7, H2O2 and L-theanine treatments did not influence the levels of total JNK protein and phosphorylation of JNK (p46 and p56, P>0.05). Expression of total p38 and its phosphorylation were also not affected by H2O2 or L-theanine (P>0.05).
Figure 7.
Effects of L-theanine on the caspase pathway of JNK and p38 MAPKs in the H2O2-induced H9C2 cells. H9C2 cells were pretreated with or without L-theanine for 24 h, followed by 160 μM H2O2 treatment for 4 h. Then, the expression of p38, p-p38, JNK, and p-JNK were tested by Western blot. β-actin was used to confirm that equal cell equivalents were loaded. A representative Western blot for each treatment from four independent experiments was shown. The ratios of (p-p54/β-actin)/(p54/β-actin), (p-p46/β-actin)/(p46/β-actin), (p-p38/β-actin)/(p38/β-actin) were used for statistical analysis. Data are expressed as means ±SD (n=4).
Discussion
H2O2 is usually considered to be the major precursor of highly reactive free radicals such as superoxide anion (O2−) and hydroxyl radical (−OH), ultimately leading to cell apoptosis or necrosis [26]. It has been proven that H2O2 easily induces apoptosis in various cell types, including myocardial cells [27]. It has been reported that L-theanine administration alone or in combination with cysteine can increase GSH synthesis in human macrophages [13,28], which can clear free radicals and lipid peroxidation, then enhance the antioxidation ability of the body [16,23,29–31]. L-theanine pretreatment (750 μM and 1000 μM) can protect L02 cells from ethanol-induced injury by restoration of the GSH level and enhancement of the activity of SOD [16]. Besides, oral administration of L-theanine (2 and 4 mg/kg) attenuates the oxidative damage by recovering Aβ1–42-induced depletion of GSH and the generation of lipid peroxidation in mouse brain tissue [30]. Moreover, L-theanine treatment (50 μg/mL) protects human neuroblastoma cells against oxidative stress injury by restoring Aβ1–42-induced depletion of GSH and suppressing Aβ1–42-induced development of ROS time-dependently in neuroepithelioma cells (nondopaminergic cells) and dose-dependently in neuroepithelioma and neuroblastoma cells (dopaminergic cells) [29]. In this study, the protective effects of L-theanine on H2O2-induced apoptosis in H9C2 cells were evaluated. We found that L-theanine pretreatment effectively decreased the contents of TBARS, ROS, NO, and GSSG, while it increased the content of GSH and the activities of GSH-Px and SOD in the H2O2-induced H9C2 cells. These results suggest that L-theanine can improve the antioxidant protection of H2O2-induced H9C2 cells, which is largely consistent with previous findings [16,29,30].
Previous studies have reported that L-theanine pretreatment (1 mM) reduces the viability loss and the nuclear morphological damage of H2O2-injured L02 cells [23] and rotenone- or dieldrin-induced human neuroblastoma SH-SY5Y cells [31]. The present study noted that L-theanine pretreatment reduced the mortality of H2O2-injured H9C2 cells, and the typical appearance of apoptosis induced by H2O2 (chromatin condensation and fragmented nuclei) was alleviated by L-theanine. These results are completely in agreement with previous findings [23,31], indicating that L-theanine can protect H9C2 cells against injury induced by oxidative stress.
It has been reported [32] that Caspase-3 is the main effector of cell death, involving the cleavage of many apoptosis related proteins such as PARP, which can be cleaved into 2 parts (24 and 89 kDa), serving as an indicator of cells undergoing apoptosis. Previous studies have shown that L-theanine pretreatment (1 mM) diminishes ethanol-induced [16] and H2O2-induced [23] apoptosis of hepatocytes by decreasing the expression of cleaved-PARP and Caspase-3. L-theanine pretreatment (0.5 mM) also depresses cadmium-induced upregulation of Caspase-3 and the cleavage of PARP in pheochromocytoma (PC12) cells [33]. Our data show that H2O2 induced pro-Caspase-3 downregulation and PARP cleavage in H9C2 cells, denoting that the cells were undergoing apoptosis [34], and this result is consistent with the apoptosis ratio of flow cytometric analysis. Fortunately, L-theanine pretreatment effectively attenuated the reduction of pro-Caspase-3 and the increase of cleaved-PARP. This result largely agrees with the previous studies [16,23,31,33] mentioned above, suggesting that L-theanine pretreatment protects H9C2 cells from H2O2-induced apoptosis.
A variety of stresses and proapoptotic signals, such as H2O2, TNF-α, chemotherapeutics, and growth factor deprivation, can preferentially stimulate the MAPKs signaling pathways, which are evolutionarily well-conserved in all eukaryotic cells [35–37]. MAPKs involve cellular apoptosis, proliferation, differentiation, and development [38] and consists of both apoptotic and anti-apoptotic proteins, including JNK and p38. Activation of the JNK and p38 pathways further induce the stimulation of effector caspases, including Caspase-3 [39,40]. Previous studies have also reported that L-theanine exerts anti-apoptosis effects by suppressing the activation of JNK or p38 and NF-κB in H2O2-induced hepatocytes [23], L-glutamate-induced human Amyloid-beta A4 protein (Swedish mutation) transgenic SH-SY5Y cells [41], Aβ1–42-induced human neuroblastoma cells [29], and mouse brain nerve cells [29] due to its ROS-scavenging ability. L-theanine pretreatment also protects PC12 cells from apoptosis by depressing cadmium-induced protein phosphorylation of ERK, JNK, Akt, p38, and the ratio of Bax/Bcl-2 and recovering mitochondrial dysfunction [33]. These studies have demonstrated that L-theanine can protect cells against apoptosis through inhibiting the activation of the apoptotic pathways. Our results show that L-theanine pretreatment did not affect the phosphorylation of JNK and p38, indicating that JNK and p38 are not involved in the regulation of H2O2-induced apoptosis in H9C2 cells. These results are not consistent with previous findings [16,22,23,29,30,33,41] and this discrepancy might be due to the difference in cell models or the dose of L-theanine used. Furthermore, other proteins, such as ERK, NF-κB, and mitochondrial apoptotic pathways, including Bax/Bcl-2 and PI3K/AKT, may also play an anti-apoptosis role. Further investigation is needed to elucidate the antioxidant and anti-apoptotic mechanisms of L-theanine in cardiocytes.
Conclusions
In summary, L-theanine pretreatment within the dose range of 4~16 mM increased the cell viability, GSH content, and activities of GSH-Px and SOD, and decreased levels of TBRAS, ROS, NO, and GSSG in H2O2-induced H9C2 cells. Furthermore, all doses of L-theanine decreased the apoptosis ratio, upregulated pro-Caspase-3 expression, and downregulated cleavaged-PARP expression without the activation of p38 and JNK. Our study indicates that L-theanine pretreatment protects H9C2 cells against H2O2-induced apoptosis, probably via antioxidant capacity improvement. Therefore, it might be a promising potential drug candidate for prophylaxis of oxidative stress-induced heart diseases.
Footnotes
Source of support: The work was supported by the Hunan Provincial Natural Science Foundation of China \(2018JJ5052\), the Hunan Provincial Creation Development Project \(2013TF3006\), the National Natural Science Foundation of China \(31320103917 and 31172234\), and the Youth Innovation Team Project of ISA, CAS \(2017QNCXTD_ZCS\)
Conflicts of interest
None.
References
- 1.Townsend N, Wilson L, Bhatnagar P, et al. Cardiovascular disease in Europe 2016: An epidemiological update. Eur Heart J. 2016;37:3182–83. doi: 10.1093/eurheartj/ehw468. [DOI] [PubMed] [Google Scholar]
- 2.Williams J, Kellett J, Roach P, et al. L-theanine as a functional food additive: Its role in disease prevention and health promotion. Beverages. 2016;2:1–13. [Google Scholar]
- 3.Sun GZ, Ye N, Dai DX, et al. The protective role of the TOPK/PBK pathway in myocardial ischemia/reperfusion and H2O2-induced injury in H9C2 cardiomyocytes. Int J Mol Sci. 2016;17:267. doi: 10.3390/ijms17030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mantzaris MD, Bellou S, Skiada V, et al. Intracellular labile iron determines H2O2-induced apoptotic signaling via sustained activation of ASK1/JNK-p38 axis. Free Radic Biol Med. 2016;97:454–65. doi: 10.1016/j.freeradbiomed.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Kei T, Atsushi M, Takahashi T, et al. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO J. 2001;2:222–28. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bo Y, Meng FS, Yang Y, et al. NOX2 antisense attenuates hypoxia-induced oxidative stress and apoptosis in cardiomyocyte. Int J Med Sci. 2016;13:646–52. doi: 10.7150/ijms.15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Yu P, Gou H, et al. Cardioprotective effects of 20(S)-ginsenoside Rh2 against doxorubicin-induced cardiotoxicity in vitro and in vivo. Evid Based Complement Alternat Med. 2012;2012:506214. doi: 10.1155/2012/506214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai MJ, Gill MS, Hsu WH, Armstrong DW. Pharmacokinetics of theanine enantiomers in rats. Chirality. 2005;17:154–62. doi: 10.1002/chir.20144. [DOI] [PubMed] [Google Scholar]
- 9.Tsuge H, Sano S, Hayakawa T, et al. Theanine, gamma-glutamylethylamide, is metabolized by renal phosphate-independent glutaminase. Biochim Biophys Acta. 2003;1620:47–53. doi: 10.1016/s0304-4165(02)00504-4. [DOI] [PubMed] [Google Scholar]
- 10.Tsai WH, Wu CH, Yu HJ, Chien CT. l-theanine inhibits proinflammatory PKC/ERK/ICAM-1/IL-33 signaling, apoptosis, and autophagy formation in substance P-induced hyperactive bladder in rats. Neurourol Urodyn. 2017;36:297–307. doi: 10.1002/nau.22965. [DOI] [PubMed] [Google Scholar]
- 11.Bukowski JF, Percival SS. L-theanine intervention enhances human γδ T lymphocyte function. Nutr Rev. 2008;66:96–102. doi: 10.1111/j.1753-4887.2007.00013.x. [DOI] [PubMed] [Google Scholar]
- 12.Unno K, Iguchi K, Tanida N, et al. Ingestion of theanine, an amino acid in tea, suppresses psychosocial stress in mice. Exp Physiol. 2013;98:290–303. doi: 10.1113/expphysiol.2012.065532. [DOI] [PubMed] [Google Scholar]
- 13.Kurihara S, Shibahara S, Arisaka H, Akiyama Y. Enhancement of antigen-specific immunoglobulin G production in mice by co-administration of L-cystine and L-theanine. J Vet Med Sci. 2007;69:1263–70. doi: 10.1292/jvms.69.1263. [DOI] [PubMed] [Google Scholar]
- 14.Wen H, Wei SL, Zhang SR, et al. [Research advance in L-theanine in animals immunomodulatory and antioxidation]. Chinese Journal of Animal Science. 2012;48:84–87. [in Chinese] [Google Scholar]
- 15.Sadzuka Y, Inoue C, Hirooka S, et al. Effects of theanine on alcohol metabolism and hepatic toxicity. Biol Pharm Bull. 2005;28:1702–6. doi: 10.1248/bpb.28.1702. [DOI] [PubMed] [Google Scholar]
- 16.Li GL, Ye Y, Kang JJ, et al. L-theanine prevents alcoholic liver injury through enhancing the antioxidant capability of hepatocytes. Food Chem Toxicol. 2012;50:363–72. doi: 10.1016/j.fct.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 17.Sugiyama T, Sadzuka Y. Theanine and glutamate transporter inhibitors enhance the antitumor efficacy of chemotherapeutic agents. Biochim Biophys Acta. 2003;1653:47–59. doi: 10.1016/s0304-419x(03)00031-3. [DOI] [PubMed] [Google Scholar]
- 18.Sadzuka Y, Sugiyama T, Miyagishima A, et al. The effects of theanine, as a novel biochemical modulator, on the antitumor activity of adriamycin. Cancer Lett. 1996;105:203–9. doi: 10.1016/0304-3835(96)04282-6. [DOI] [PubMed] [Google Scholar]
- 19.Deng YL, Xiao WJ, Chen L, et al. In vivo antioxidative effects of l-theanine in the presence or absence of Escherichia coli-induced oxidative stress. Journal of Functional Foods. 2016;24:527–36. [in Chinese] [Google Scholar]
- 20.Egashira N, Ishigami N, Pu FL, et al. Theanine prevents memory impairment induced by repeated cerebral ischemia in rats. Phytother Res. 2008;22:65–68. doi: 10.1002/ptr.2261. [DOI] [PubMed] [Google Scholar]
- 21.Wang N, Jianrui L, Zhang ZN, Xue RL. [Effect of theanine on JNK signaling pathway and DNA repair in global brain ischemia/reperfusion rats]. Journal of Shanxi Medical University. 2016;47:415–18. [in Chinese] [Google Scholar]
- 22.Jiang W, Gao M, Sun SA, et al. Protective effect of L-theanine on carbon tetrachloride-induced acute liver injury in mice. Biochem Biophys Res Commun. 2012;422:344–50. doi: 10.1016/j.bbrc.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 23.Li GL, Kang JJ, Yao XY, et al. The component of green tea, L-theanine protects human hepatic L02 cells from hydrogen peroxide-induced apoptosis. Europen Food Research & Technology. 2011;233:427–35. [Google Scholar]
- 24.Li CJ, Tong HO, Yan QX, et al. Effects of L-theanine on the antioxidant capacity and related gene mRNA expression in rat visceral tissues. Food Sience. 2017;38:188–94. [Google Scholar]
- 25.Yan QX, Tang SX, Tan ZL, et al. Proteomic analysis of isolated plasma membrane fractions from the mammary gland in lactating cows. J Agr Food Chem. 2015;63:7388–98. doi: 10.1021/acs.jafc.5b02231. [DOI] [PubMed] [Google Scholar]
- 26.Chandra J, Samali A, Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med. 2000;29:323–33. doi: 10.1016/s0891-5849(00)00302-6. [DOI] [PubMed] [Google Scholar]
- 27.Ilavenil S, Kim DH, Jeong YL, et al. Trigonelline protects the cardiocyte from hydrogen peroxide induced apoptosis in H9c2 cells. Asian Pac J Trop Med. 2015;8:263–68. doi: 10.1016/S1995-7645(14)60328-X. [DOI] [PubMed] [Google Scholar]
- 28.Rimaniol AC, Mialocq P, Clayette P, et al. Role of glutamate transporters in the regulation of glutathione levels in human macrophages. Am J Physiol Cell Physiol. 2001;281:C1964–70. doi: 10.1152/ajpcell.2001.281.6.C1964. [DOI] [PubMed] [Google Scholar]
- 29.Mi RJ, Mi HP, Dong YC, et al. Neuroprotective effect of L-theanine on Aβ-induced neurotoxicity through anti-Oxidative mechanisms in SK-N-SH and SK-N-MC cells. Biomolecules & Therapeutics. 2011;19:288–95. [Google Scholar]
- 30.Li J, Herold MJ, Kimmel B, et al. Reduced expression of the mevalonate pathway enzyme farnesyl pyrophosphate synthase unveils recognition of tumor cells by γ2δ2 T cells. J Immunol. 2009;182:8118–24. doi: 10.4049/jimmunol.0900101. [DOI] [PubMed] [Google Scholar]
- 31.Cho HS, Kim S, Lee SY, et al. Protective effect of the green tea component, l-theanine on environmental toxins-induced neuronal cell death. Neurotoxicology. 2008;29:656–62. doi: 10.1016/j.neuro.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Slee EA, Adrain C, Martin SJ. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem. 2001;276:7320–26. doi: 10.1074/jbc.M008363200. [DOI] [PubMed] [Google Scholar]
- 33.Ben PL, Zhang ZP, Xuan CX, et al. Protective effect of L-theanine on cadmium-induced apoptosis in PC12 cells by inhibiting the mitochondria-mediated pathway. Neurochem Res. 2015;40:1661–70. doi: 10.1007/s11064-015-1648-4. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Xue Z, Wang Q, et al. Propofol protects hepatic L02 cells from hydrogen peroxide-induced apoptosis via activation of extracellular signal-regulated kinases pathway. Anesth Analg. 2008;107:534–40. doi: 10.1213/ane.0b013e3181770be9. [DOI] [PubMed] [Google Scholar]
- 35.Ichijo H, Nishida E, Irie KJ, et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 36.Takeda K, Noguchi T, Naguro I, Ichijo H. Apoptosis signal-regulating kinase 1 in stress and immune response. Ann Rev Pharmacol Toxicol. 2008;48:199–225. doi: 10.1146/annurev.pharmtox.48.113006.094606. [DOI] [PubMed] [Google Scholar]
- 37.Kumar S, Boehm J, Lee JC. p38 MAP kinases: Key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2:717–26. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- 38.Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23:2838–49. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- 39.Chaudhary PM, Eby MT, Jasmin A, Hood L. Activation of the c-Jun N-terminal kinase/stress-activated protein kinase pathway by overexpression of caspase-8 and its homologs. J Biol Chem. 1999;274:19211–19. doi: 10.1074/jbc.274.27.19211. [DOI] [PubMed] [Google Scholar]
- 40.Frasch SC, Nick JA, Fadok VA, et al. p38 mitogen-activated protein kinase-dependent and -independent intracellular signal transduction pathways leading to apoptosis in human neutrophils. J Biol Chem. 1998;273:8389–97. doi: 10.1074/jbc.273.14.8389. [DOI] [PubMed] [Google Scholar]
- 41.Di X, Yan J, Zhao Y, et al. L-theanine protects the APP (Swedish mutation) transgenic SH-SY5Y cell against glutamate-induced excitotoxicity via inhibition of the NMDA receptor pathway. Neuroscience. 2010;168:778–86. doi: 10.1016/j.neuroscience.2010.04.019. [DOI] [PubMed] [Google Scholar]