Abstract
Background
Epithelial-mesenchymal transition (EMT) is responsible for metastasis of cancers, and NF-κB can promote tumor progression. Ezrin is an important molecule participating in EMT. However, whether Ezrin mediates NF-κB in EGF-induced osteosarcoma is unknown.
Material/Methods
Ezrin phosphorylation, NF-κB activation, and EGF-induced EMT were studied in MG63 and U20S cells with NF-κB inhibition, silencing, or over-expressing Ezrin. Cell morphology, proliferation, migration, and motility were analyzed. An osteosarcoma model was established in mice by injecting MG63 and U20S and reducing Ezrin.
Results
With EGF induction in vitro, Ezrin Tyr353 and Thr567 were phosphorylated, and EMT, proliferation, migration, and motility of osteosarcoma cells were promoted. Silencing Ezrin suppressed and over-expressing Ezrin promoted the nuclear translocation of p65 and phosphorylated IκBα (p-IκBα) in EGF-induced osteosarcoma cells. NF-κB inhibitor blocked EGF-induced EMT in both cell types, as well as reserving cell morphology and suppressing proliferation, migration, and motility. In vivo, reducing Ezrin significantly suppressed metastasis of osteosarcoma xenografts, increased liver and lung weights, and activated NF-κB, which were both induced by EGF.
Conclusions
Ezrin/NF-κB regulated EGF-induced EMT, as well as progression and metastasis of osteosarcoma in vivo and in vitro. Ezrin/NF-κB may be a new therapeutic target to prevent osteosarcoma from deterioration.
MeSH Keywords: Disease Progression, Epithelial-Mesenchymal Transition, Lymphatic Metastasis, Neurofibromin 2, Osteosarcoma, Receptor Activator of Nuclear Factor-kappa B
Background
Osteosarcoma is one of the most common malignant cancers, and is the most common primary malignant neoplasm of bone, with rapid progression and poor prognosis clinically [1,2]. Surgery is the primary treatment for osteosarcoma, and multi-agent chemotherapy and postoperative chemotherapy are adjuvant therapies [3].
Previous studies demonstrated that tumor metastasis is associated with EMT as the biological process in which epithelial cells lose cell-cell contact, with enhanced E-cadherin expression, reduced vimentin, and developing mesenchymal properties, leading to promotion of proliferation, invasion, and viability [4,5]. During the pathogenesis and progression of malignant tumors, many signaling pathways are involved as the regulators triggered by various members of the transforming growth factor-β (TGF-β) superfamily [4,6]. NF-κB is one of the transcription factors demonstrated to suppress EMT and metastasis by blocking its activation and suppressing the nuclear translocation of p65 [7,8]. In addition, activation of NF-κB stimulated by inflammatory cytokines was demonstrated in EMT, motility, and invasion of tumor cells [9].
Studies showed the abnormal level of special proteins regulating metastasis of osteosarcoma, such as P15 [10], hector battifora mesothelial-1 (HBME-1)[11], and epithelial cell transforming sequence 2 (ECT2)[12]. Ezrin is one of the most important members of the ERM family (Ezrin/Radixin/Moesin). Not only is it involved in cytoskeleton organization, Ezrin is also active in transmission of signals in responses to extracellular factors [13,14]. Ezrin has many functions in tumor metastasis [15], including modulating the formation of microvilli [16], maintaining cell shape [17], cell-cell adhesion [18], cell motility [19], and invasion [20]. EGF can stimulate Ezrin activation in tumor cells, which can result in tumor metastasis [17].
According to Gavert et al. [21], NF-κB and Ezrin are both essential for L1-mediated metastasis of colon cancer cells. Lim et al. [22] suggested that NF-κB activity is responsible for Ezrin-induced actin polymerization. Tang et al. [23] found that Ezrin interacts with p65 in breast cancer cells, and could be a promoter in tumor metastasis and proliferation. Increasing evidence suggests that Ezrin is important in EMT by activating NF-κB. However, whether Ezrin can mediate NF-κB activation in EGF-induced osteosarcoma cells is still obscure.
In the present study, we investigated the role of Ezrin in regulating EGF-induced EMT in MG63 and U20S cell lines in vitro. Tumor xenografts were constructed from mouse models of osteosarcoma. The phosphorylation of Ezrin in response to EGF and the effect on the activation of NF-κB were studied to assess the role of Ezrin/NF-κB regulation in EMT and metastasis of osteosarcoma.
Material and Methods
Cell culture and EGF induction
Human osteosarcoma cell lines MG63 and U20S were purchased from American Type Culture Collection (ATCC, Rockville, MD, USA). MG63 and U20S were cultured in Dulbecco’s minimum essential medium (DMEM, Thermo Fisher Scientific, Inc. Shanghai, China) containing 10% fetal bovine serum (FBS, Thermo), 100 U/ml penicillin, and 1% streptomycin (Sigma Aldrich, Co. LLC, Shanghai, China). All the cells were maintained at 37°, 5% CO2, and saturated humidity. When they reached 90% confluence, the cells were digested with 0.25% trypsin-EDTA (Thermo) and sub-cultured (1: 3).
For inhibiting NF-κB, inhibitor of cytokine-induced IκBα phosphorylation (BAY 11–7082, 10μM, Sigma) was used to treat the 90% confluent cells. For EGF induction, 90% confluent cells were starved for 4 h and then stimulated by EGF (25 nM) for 30 min.
Transfection
Small interfering RNA (siRNA) silencing Ezrin (5′-GUG GGA UGC UCA AAG AUA ATT-3′) was designed and synthesized by GenePharma (Shanghai, China). An expression construction with Ezrin was a kind gift from Prof. Sanbao Hu. Lipofectamine® 3000 transfection reagents (Thermo) was used to transfect cells with Opti-MEM™ reduced serum media (Thermo) without penicillin- streptomycin for 48 h, according to the manufacturer’s specifications.
Western blotting (WB)
Proteins were extracted from cell lysate with nuclear and cytoplasmic protein extraction kit (Beyotime Biotechnology, Shanghai, China) and then quantified with BCA protein assay kit (Beyotime). Proteins were analyzed by 10% sodium dodecyl sulfate (SDS) polyacrylamide gel and 5% stocking gel, and then transferred to polyvinylidene difluoride (PVDF) membranes (Merck Millipore Corporation, Shanghai, China). Protein bands were incubated with E-cadherin (1: 1000, Proteintech Group, Inc. Wuhan, China), vimentin (1: 1000, Proteintech), Ezrin (1: 1000, Proteintech), p-Ezrin Tyr353 (1: 1000, Abcam, Shanghai, China), p-Ezrin Thr567 (1: 1000, Abcam), NF-κB p65 (1: 3000, Abcam), p-IκBα Ser32/36 (1: 1000, CST), and GAPDH (1: 10000, Proteintech) primary antibodies at 4°C overnight, and then incubated with goat anti-rabbit and goat anti-mouse secondary antibodies (1: 3000, Jackson ImmunoResearch Laboratories, Inc. West Grove, PA, USA) at 25°C for 1 h. An electrochemiluminescence (ECL, Millipore) system was used to expose proteins.
Proliferation assay
Cells were cultured in a 96-well plate (Corning, New York, USA) for 72 h. We used the Cell Counting Kit-8 (CCK-8) liquid (Thermo) and incubated cells for 20 min. Optical density (OD) value was determined by use of a Synergy NEO Microplate Spectrophotometer (BioTek instruments, USA).
Transwell assay
A total of 1×105 cells were cultured in the upper chamber of the polycarbonate Transwell filter chamber (Corning) containing Matrigel (Becton, Dickinson and Company, Shanghai, China) and incubated for 16 h. Cells on the lower surface of the chamber were fixed with 4% paraformaldehyde and stained with 0.25% crystal violet (Solarbio Technology Co., Ltd. Beijing, China). Cells on the upper surface were cleaned. Cells were observed and counted in 5 random 100×fields per well using a microscope (Olympus Corporation, Beijing, China).
Wound-healing assay
A total of 1×106 cells were cultured in a 6-well plate (Corning) until confluence reached 90%. Linear wounds were created with pipette tips and the cells were cultured without serum. Cells were cultured for 10 h and we observed the cells under a microscope (Olympus).
Mouse models of osteosarcoma
From Shanghai Jia Ke Biotechnology Co., Ltd. (China), we obtained 40 male BALB/c-nu mice, age 4 to 6 weeks, weight 14~20 g, and used them to construct an osteosarcoma model (n=5 each group). Mice were kept in a specific pathogen-free (SPF) animal room maintained at 25°C and 60~70% humidity. Mice ate and drank freely. Animal experiment protocols were according to guidelines of Ethical Issues in Animal Experimentation [24] and were approved by the Ethics Committee of Beijing Anzhen Hospital. MG63 and U20S cells with silencing Ezrin were injected subcutaneously into the armpits of the mice according to the method described in a previous report [25]. When the mice developed palpable xenografts (more than 0.5 cm diameter), we injected the tumor with phosphate-buffered solution (PBS) (Beyotime) or EGF (0.02 mg/kg) twice a week for 5 weeks. Eight weeks later, tumor xenografts were extracted, weighed, measured, and frozen in −178°C liquid nitrogen or harvested with tissue lysate buffer (Beyotime). Lung and liver tissues were separated to evaluate metastasis, and hypoxanthine guanine phosphoribosyl transferase (HPRT) was analyzed by quantitative real-time PCR (qRT-PCR) with a Roche-LC96 RT-PCR device (Roche R & D Co., Ltd. Shanghai, China), according to the method described by Sun et al. [26].
Statistical analysis
All data analyses were performed using SPSS 21.0 software. Data are expressed as mean ± standard deviations (X±SDs). All experiments were performed at least in triplicate. One-way ANOVA or t test were used to analyze differences among groups. Levene test was used for homogeneity test of variance, and the LDS was performed for homogenous data, while Dunnett’s T3 was performed for heterogeneous data. P<0.05 was considered a significant difference.
Results
EGF promoted Ezrin phosphorylation and EMT, while silencing Ezrin suppressed EGF-induced EMT in osteosarcoma cells
To investigate the effects of EGF on Ezrin phosphorylation and EMT, we used EGF to induce MG63 and U20S cell lines and then detected Ezrin Tyr353, Ezrin Thr567, E-cadherin, and vimentin.
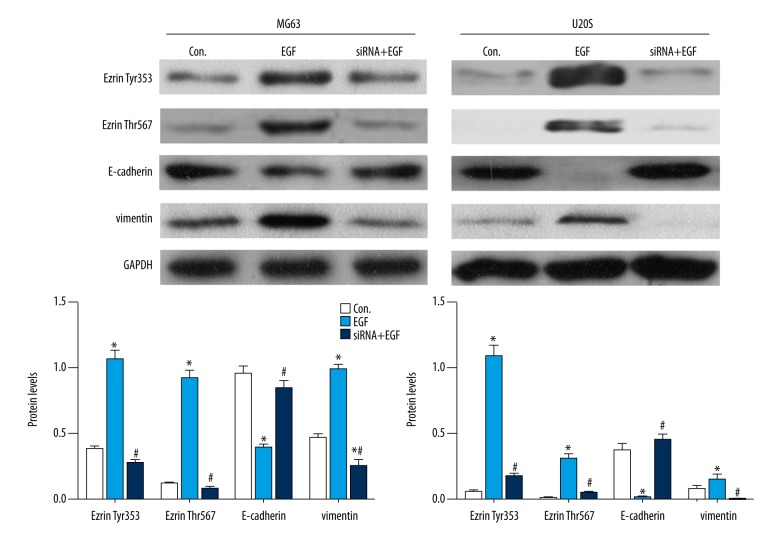
As shown in Figure 1, with EGF induction, Ezrin Tyr353 and Ezrin Thr567 were both significantly (P<0.05) elevated in MG63 and U20S cells compared to control. These results indicated the promotion of EGF on Ezrin phosphorylation. In addition, E-cadherin decreased with EGF while vimentin significantly (P<0.05) increased in MG63 and U20S cells comparing to the control, which suggested that EMT of osteosarcoma cells was promoted by EGF induction.
Figure 1.
EGF promoted Ezrin phosphorylation and EMT, and silencing Ezrin suppressed EGF-induced EMT in osteosarcoma cells. GAPDH was used as internal control (* compared to the Con., P<0.05; # compared to EGF, P<0.05).
To investigate the effects of Ezrin on EMT, we silenced Ezrin in MG63 and U20S cell lines and then detected E-cadherin and vimentin. By silencing Ezrin, on the one hand, Ezrin Tyr353 and Ezrin Thr567 both significantly (P<0.05) decreased in MG63 and U20S cells compared to the EGF treatment. On the other hand, E-cadherin significantly (P<0.05) increased while vimentin decreased in MG63 and U20S cells compared to the EGF treatment.
These results suggest that EGF promoted EMT while silencing Ezrin inhibited EGF-induced EMT in osteosarcoma cells.
EGF induced morphological change and promoted proliferation, migration, and motility of osteosarcoma cells
To investigate the effects of EGF on proliferation, migration, and motility of osteosarcoma cells, we used EGF to treat MG63 and U20S cell lines. Cell morphology was observed and migration and motility were detected by Transwell and wound-healing assays.
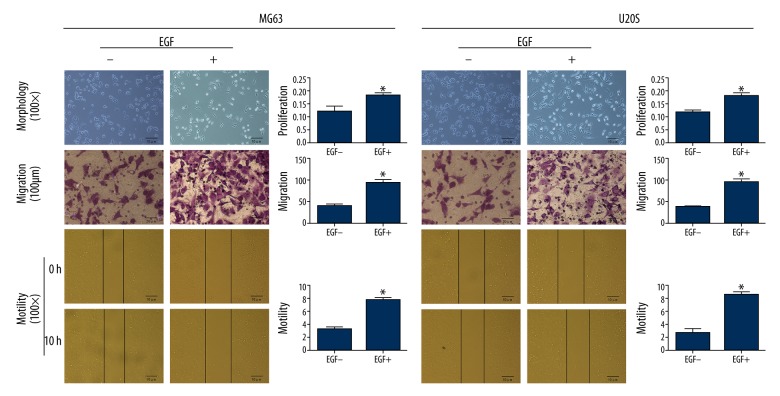
As shown in Figure 2 and Table 1, without EGF induction, MG63 and U20S were round in shape with tight cell-cell junctions, whereas both of them induced with EGF displayed spindle shape and were separated from one another (Figure 2-Morphology).
Figure 2.
EGF induced morphological change and promoted proliferation, migration, and motility of osteosarcoma cells (* comparing to the Con., P<0.05).
Table 1.
Proliferation, migration and motility of MG63 and U20S with EGF treatment (X±SDs).
| EGF | MG63 | U20S | |
|---|---|---|---|
| Proliferation (OD value) | − | 0.121±0.018 | 0.120±0.007 |
| + | 0.185±0.007* | 0.182±0.012* | |
| F | 33.241 | 61.892 | |
| P | 0.004 | 0.001 | |
| Migration (%) | − | 40.827±3.857 | 38.937±1.730 |
| + | 95.470±6.477* | 95.727±6.900* | |
| F | 157.640 | 191.214 | |
| P | <0.001 | <0.001 | |
| Motility (%) | − | 3.177±0.293 | 2.770±0.543 |
| + | 7.803±0.315* | 8.617±0.440* | |
| F | 347.000 | 209.958 | |
| P | <0.001 | <0.001 |
Comparing to the “−”, P<0.05
Without EGF induction, CCK-8 assay demonstrated that the proliferation of MG63 and U20S cells were significantly (P<0.05) lower than those with EGF induction (Figure 2-Proliferation). Transwell assay demonstrated that the invasive ability of MG63 and U20S cells with EGF stimulation were significantly (P<0.05) enhanced, appearing as more cells on the lower surface of the membrane, comparing to that without EGF stimulation (Figure 2-Migration). With EGF treatment, the motility of MG63 and U20S cells was significantly (P<0.05) strengthened compared to those without EGF treatment (Figure 2-Motility). These data suggest that the proliferation, migration, and motility of osteosarcoma cells were promoted by EGF induction.
Ezrin was responsible for NF-κB activity in osteosarcoma cells
To investigate the role of Ezrin in regulating NF-κB activity in osteosarcoma, we silenced Ezrin by RNA interfering (RNAi) assay or over-expressed Ezrin with expression construction, both with EGF induction, in MG63 and U20S cell lines. The protein levels of nuclear p65 and p- IκBα were detected.
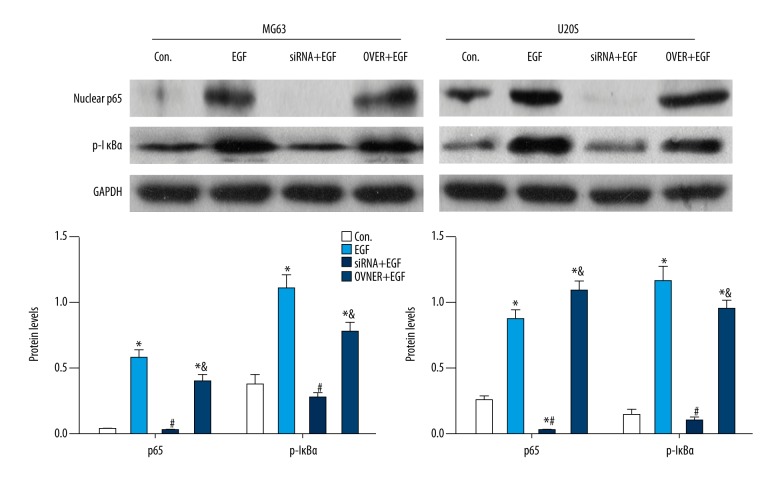
As shown in Figure 3, WB assay showed that with EGF treatment, nuclear p65 and p- IκBα levels both significantly (P<0.05) increased compared to those without EGF induction. After silencing Ezrin with siRNA, MG63 and U20S cells were induced with EGF. The results showed that nuclear p65 and p- IκBα levels were both significantly (P<0.05) suppressed compared to EGF stimulation only. With over-expressing Ezrin and EGF induction, nuclear p65 and p- IκBα levels were both significantly (P<0.05) promoted compared to the control, but without significance compared to EGF induction. These data indicate that Ezrin was responsible for translocation of nuclear p65, suggesting the activation of NF-κB in EGF-induced osteosarcoma cells.
Figure 3.
Ezrin was responsible for NF-κB activity in osteosarcoma cells. GAPDH was used as internal control (* comparing to the Con., P<0.05; # compared to EGF, P<0.05; & compared to siRNA+EGF, P<0.05).
EGF induced EMT by Ezrin/NF-κB in osteosarcoma cells
To study the role of Ezrin/NF-κB in EMT in osteosarcoma, we used IκBα phosphorylation inhibitor (BAY) to suppress NF-κB activity, as well as silencing Ezrin in EGF-induced MG63 and U20S. E-cadherin and vimentin levels were detected.
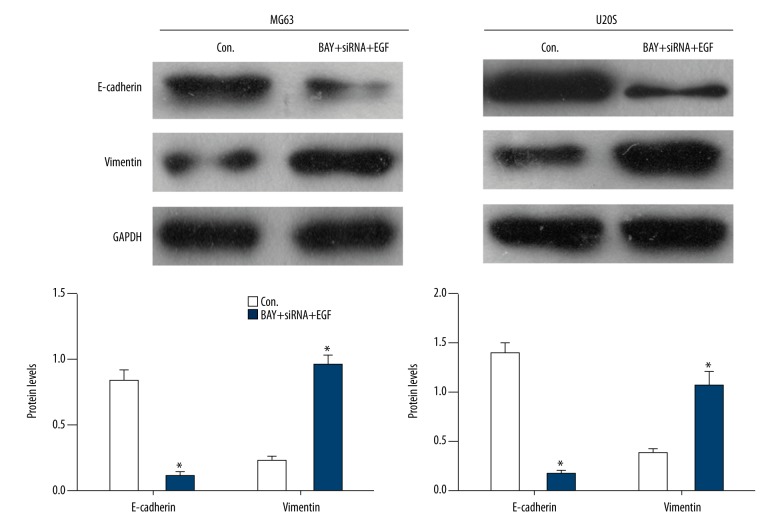
As shown in Figure 4 and Table 2, with inhibiting NF-κB activation and silencing Ezrin, E-cadherin significantly (P<0.05) decreased, while vimentin increased in EGF-induced MG63 and U20S cells compared to the control, suggesting the inhibition of EMT by MG63 and U20S.
Figure 4.
EGF induced EMT by Ezrin/NF-κB in osteosarcoma cells (* compared to the Con., P<0.05).
Table 2.
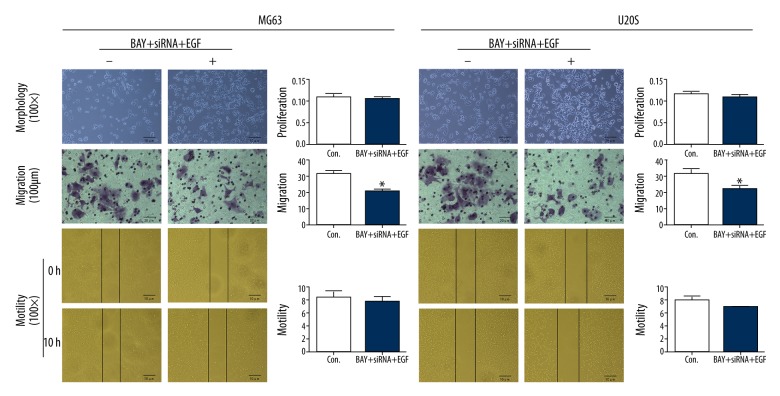
Proliferation, migration and motility of MG63 and U20S with NF-κB inhibitor, silencing Ezrin and EGF treatments (X±SDs).
| BAY+ siRNA +EGF | MG63 | U20S | |
|---|---|---|---|
| Proliferation (OD value) | − | 0.109±0.007 | 0.115±0.005 |
| + | 0.103±0.007 | 0.107±0.006 | |
| F | 1.338 | 3.238 | |
| P | 0.312 | 0.146 | |
| Migration (%) | − | 31.360±2.098 | 30.727±3.252 |
| + | 20.970±1.304* | 21.513±2.214* | |
| F | 53.055 | 16.451 | |
| P | 0.002 | 0.015 | |
| Motility (%) | − | 8.380±0.884 | 7.930±0.665 |
| + | 7.693±0.754 | 6.953±0.085 | |
| F | 1.048 | 6.374 | |
| P | 0.364 | 0.065 |
Comparing to the “−”, P<0.05
Additionally, to investigate the effect of Ezrin/NF-κB on the progression of osteosarcoma cells, we observed the morphology and detected the proliferation, migration, and motility of MG63 and U20S cell lines. As shown in Figure 5, suppressing NF-κB and silencing Ezrin reversed the mesenchymal morphology of EGF-induced MG63 and U20S, appearing rounded, with tight cell-cell junctions, similar to the control. With BAY and siRNA treatments, CCK-8 assay showed that the proliferation of MG63 and U20S cells was not significantly different from the control. The wound-healing assay showed that motility of MG63 and U20S were not significantly different from the control. However, Transwell assay showed that after suppressing NF-κB and silencing Ezrin, the invasive ability of MG63 and U20S cells were significantly (P<0.05) weakened compared to the control.
Figure 5.
Suppressing Ezrin/NF-κB reversed EGF-induced morphological change and promotion of proliferation, migration, and motility of osteosarcoma cells (* compared to the Con., P<0.05).
These data suggest that Ezrin/NF-κB was responsible for the EMT of osteosarcoma, as well as the progression and pathogenesis.
Reduction of Ezrin suppressed metastasis of EGF-induced osteosarcoma xenografts
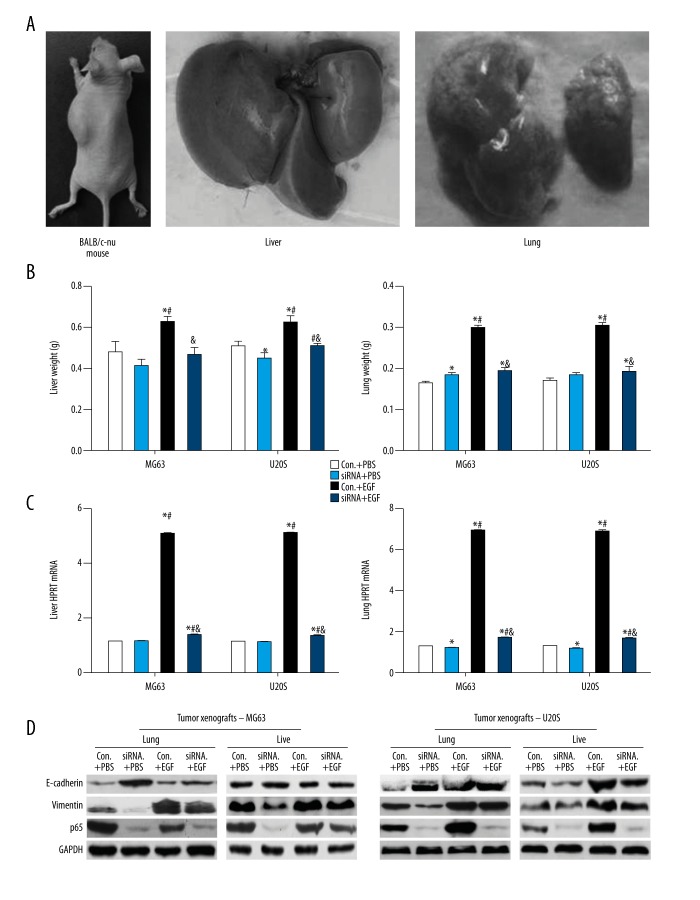
To investigate the effect of reducing Ezrin on metastasis of EGF-induced osteosarcoma xenografts, we injected mice with MG63 and U20S with silencing Ezrin to construct osteosarcoma models (Figure 6A); 40 mice received osteosarcoma xenografts successfully and no mice died.
Figure 6.
Reducing Ezrin suppressed metastasis of EGF-injected MG63 and U20S xenografts of BALB/c-nu mice. (A) BALB/c-mu mouse with osteosarcoma xenografts. (B) Liver and lung weights of tumorigenic mice. (C) HPRT mRNA level in livers and lungs of tumorigenic mice. (D) E-cadherin, vimentin, and p65 protein levels in livers and lungs of tumorigenic mice. (HPRT, hypoxanthine guanine phosphoribosyl transferase; Con.+PBS, tumor xenografts and PBS injection; siRNA+PBS, tumor xenografts with silencing Ezrin and PBS injection; Con.+EGF, tumor xenografts and EGF injection; siRNA+EGF, tumor xenografts with silencing Ezrin and EGF injection; * compared to Con.+PBS, P<0.05; # compared to siRNA+PBS, P<0.05; & compared to Con.+EGF, P<0.05).
As shown in Table 3 and Figure 6B, with both MG63 or U20S osteosarcoma xenografts, liver weights of the tumorigenic mice with EGF injection increased significantly (P<0.05) compared to tumorigenic mice without EGF induction. Silencing Ezrin obviously and significantly (P<0.05) reduced liver weight of tumorigenic mice with EGF induction, and similar results were found for lung weight.
Table 3.
Liver and lung weight of mice with osteosarcoma (X±SDs) (g).
| Con.+PBS | siRNA+PBS | Con.+EGF | siRNA+EGF | F | P | |
|---|---|---|---|---|---|---|
| Liver | ||||||
| MG63 | 0.479±0.055 | 0.412±0.032 | 0.625±0.029*# | 0.468±0.036& | 16.135 | 0.001 |
| U20S | 0.508±0.023 | 0.448±0.029* | 0.626±0.032*# | 0.510±0.013#& | 26.359 | <0.001 |
| F | 0.697 | |||||
| P | 0.413 | |||||
| Lung | ||||||
| MG63 | 0.164±0.006 | 0.184±0.007* | 0.298±0.007*# | 0.194±0.008*& | 217.230 | <0.001 |
| U20S | 0.171±0.006 | 0.183±0.008 | 0.302±0.010*# | 0.192±0.012*& | 129.802 | <0.001 |
| F | 0.011 | |||||
| P | 0.918 | |||||
Con.+PBS – tumor xenografts and PBS injection; siRNA+PBS – tumor xenografts with silencing Ezrin and PBS injection; Con.+EGF – tumor xenografts and EGF injection; siRNA+EGF – tumor xenografts with silencing Ezrin and EGF injection;
comparing to Con.+PBS, P<0.05;
comparing to siRNA+PBS, P<0.05;
comparing to Con.+EGF, P<0.05.
As shown in Table 4 and Figure 6C, in both MG63 and U20S osteosarcoma xenografts, HPRT in livers and lungs of tumorigenic mice injected with EGF were obviously higher than in the tumorigenic mice without EGF injection, while silencing Ezrin significantly (P<0.05) reduced HPRT in livers and lungs.
Table 4.
Liver and lung HPRT of mice with osteosarcoma (X±SDs).
| Con.+PBS | siRNA+PBS | Con.+EGF | siRNA+EGF | F | P | |
|---|---|---|---|---|---|---|
| Liver | ||||||
| MG63 | 1.125±0.008 | 1.128±0.003 | 5.074±0.034*# | 1.363±0.014*#& | 31367.710 | <0.001 |
| U20S | 1.119±0.014 | 1.125±0.013 | 5.100±0.037*# | 1.348±0.011*#& | 25445.074 | <0.001 |
| F | 0.000 | |||||
| P | 0.000 | |||||
| Lung | ||||||
| MG63 | 1.255±0.013 | 1.177±0.015* | 6.873±0.021*# | 1.668±0.017*#& | 84049.804 | <0.001 |
| U20S | 1.286±0.012 | 1.174±0.027* | 6.812±0.090*# | 1.653±0.032*#& | 8905.229 | <0.001 |
| F | 1.000 | |||||
| P | 0.991 | |||||
HPRT – hypoxanthine guanine phosphoribosyl transferase; Con.+PBS – tumor xenografts and PBS injection; siRNA+PBS – tumor xenografts with silencing Ezrin and PBS injection; Con.+EGF – tumor xenografts and EGF injection; siRNA+EGF – tumor xenografts with silencing Ezrin and EGF injection;
comparing to Con.+PBS, P<0.05;
comparing to siRNA+PBS, P<0.05;
comparing to Con.+EGF, P<0.05.
As shown in Figure 6D, reduction of Ezrin obviously suppressed the down-regulation of E-cadherin and up-regulation of vimentin, as well as the up-regulation of p65 expression. All these results suggest that reducing Ezrin can suppress EMT and metastasis of osteosarcoma by regulating the NF-κB signaling pathway in vivo.
Discussions
In the present study, we demonstrated that Ezrin/NF-κB was responsible for EGF-induced EMT and progression in osteosarcoma. Reducing Ezrin reversed mesenchymal features of EGF-treated osteosarcoma cells and suppressed proliferation, migration, and motility of osteosarcoma cells by inhibiting NF-κB activity, as well as inhibiting osteosarcoma metastasis in mice with tumor xenografts.
Silencing Ezrin was demonstrated to suppress EMT [14], while over-expression of Ezrin changed cell morphology, adhesion, metastasis, motility, and apoptosis in different kinds of human tumors [15,16,18,27]. Ezrin phosphorylation was responsible for the activity of many cell lines, especially Ezrin Tyr353 and Ezrin Thr567, as the 2 most common types of activated Ezrin [14]. According to Crepaldi et al. [28], EGF can activate Ezrin by phosphorylation. In our study, we used EGF to induce MG63 and U20S cells. Results showed that Ezrin Tyr353 and Ezrin Thr567 were both activated in vitro. By detecting E-cadherin and vimentin levels, we found that EMT in osteosarcoma cells and xenografts were both promoted by EGF. CCK-8, Transwell, and wound-healing assays showed the promotion of proliferation, migration, and motility in osteosarcoma cells, which was consistent with results of previous studies.
Previous studies [27,29] demonstrated that several signal pathways, such as Akt, ERK1/2, and ROCK1 were related to the activation of Ezrin. Wang et al. [30] showed that the Akt/Ezrin Tyr353/NF-κB pathway regulated EGF-induced EMT and metastasis in tongue squamous cell carcinoma. In our investigations, we silenced Ezrin and found that nuclear p65 and p-IκBα, as the direct activators of NF-κB, were both lower, while over-expression of Ezrin increased nuclear p65 and p-IκBα in vitro. In vivo, reducing Ezrin also suppressed p65 expression in lungs and livers of osteosarcoma mice. All of these results suggest that phosphorylated Ezrin is required for the activation of NF-κB in EGF-induced osteosarcoma.
EMT was demonstrated to be crucial in progression in metastasis of many tumors, and EGF is an important inducer of EMT [31,32]. Gan et al. [33] showed that EGF promoted EMT by activating the Akt pathway. Gupta et al. [34] found that carcinoma metastasis was a major issue in human cancer, and EMT is known to be essential for initiation of metastasis. In our study, we used IκBα inhibitor to suppress NF-κB activation, and then silenced Ezrin in EGF-induced MG63 and U20S cells. Our results suggest that suppressing NF-κB activity and silencing Ezrin can inhibit EMT induced by EGF in osteosarcoma cells. Additionally, suppressing NF-κB activity and silencing Ezrin obviously reversed osteosarcoma cell morphology and motility but weakened cell migration. In vivo, reducing Ezrin suppressed EGF-induced EMT of osteosarcoma xenografts. These results indicate that Ezrin/NF-κB plays a pivotal role in EMT in EGF-induced metastasis of osteosarcoma. The molecular mechanisms we found agree with previous reports.
Cell and animal experiments both confirmed that Ezrin promotes osteosarcoma metastasis by regulating NF-κB pathway activity, which suggests the importance of the relationship between Ezrin and the NF-κB pathway in regulating osteosarcoma metastasis and progression.
Conclusions
Cell and animal experiments both confirmed Ezrin promotes osteosarcoma metastasis by regulating NF-κB pathway activity, which has a critical role in regulating EGF-induced EMT and progression of osteosarcoma in vivo and in vitro. Our study provides clinical treatments and prevention of osteosarcoma using molecular mechanisms and therapeutic targets.
Footnotes
Source of support: Self financed
Conflict of interest
None.
References
- 1.Yang J, Zhang W. New molecular insights into osteosarcoma targeted therapy. Curr Opin Oncol. 2013;25(4):398–406. doi: 10.1097/CCO.0b013e3283622c1b. [DOI] [PubMed] [Google Scholar]
- 2.Kansara M, Teng MW, Smyth MJ, Thomas DM. Translational biology of osteosarcoma. Nat Rev Cancer. 2014;14(11):722–35. doi: 10.1038/nrc3838. [DOI] [PubMed] [Google Scholar]
- 3.Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment – where do we stand? A state of the art review. Cancer Treat Rev. 2014;40(4):523–32. doi: 10.1016/j.ctrv.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 5.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–73. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 6.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–28. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai X, Brophy ML, Hahn S, et al. Abstract P1-05-21: The role of epsin in promoting epithelial-mesenchymal transition and metastasis by activating NF-κB signaling in breast cancer. Cancer Res. 2012;72(24 Suppl) P1–05-21. [Google Scholar]
- 8.Liang F, et al. Overexpression of ILK Promotes EMT in Glioma via NF-κb pathway. Journal of Sun Yat-sen University (Medical Sciences) 2016 [in Chinese] [Google Scholar]
- 9.Wu Y, Deng J, Rychahou PG, et al. Stabilization of Snail by NF-κB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15(5):416–28. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu CS, Wang WB. Relationship between P15 gene mutation and formation and metastasis of malignant osteosarcoma. Med Sci Monit. 2016;22:656–61. doi: 10.12659/MSM.895022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao YX, Gao ST, Wang JQ, et al. Correlations Between Hector Battifora Mesothelial-1 (HBME-1) Expression and Clinical Pathological Characteristics and Prognosis of Osteosarcoma Patients. Med Sci Monit. 2018;24:665–72. doi: 10.12659/MSM.898820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Z, Liu J, Zhang Y. Role of epithelial cell transforming sequence 2 (ECT2) in predicting prognosis of osteosarcoma. Med Sci Monit. 2018;24:3861–68. doi: 10.12659/MSM.905951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louvet-Vallée S. ERM proteins: From cellular architecture to cell signaling. Biol Cell. 2000;92(5):305–16. doi: 10.1016/s0248-4900(00)01078-9. [DOI] [PubMed] [Google Scholar]
- 14.Arpin M, Chirivino D, Naba A, Zwaenepoel I, et al. Emerging role for ERM proteins in cell adhesion and migration. Cell Adh Migr. 2011;5(2):199–206. doi: 10.4161/cam.5.2.15081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khanna C, Wan X, Bose S, et al. The membrane-cytoskeleton linker Ezrin is necessary for osteosarcoma metastasis. Nat Med. 2004;10(2):182–86. doi: 10.1038/nm982. [DOI] [PubMed] [Google Scholar]
- 16.Chiang Y, Chou CY, Hsu KF, et al. EGF upregulates Na+/H+ exchanger NHE1 by post-translational regulation that is important for cervical cancer cell invasiveness. J Cell Physiol. 2008;214(3):810–19. doi: 10.1002/jcp.21277. [DOI] [PubMed] [Google Scholar]
- 17.Baumgartner M, Sillman AL, Blackwood EM, et al. The Nck-interacting kinase phosphorylates ERM proteins for formation of lamellipodium by growth factors. Proc Natl Acad Sci. 2006;103(36):13391–96. doi: 10.1073/pnas.0605950103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srivastava J, Elliott BE, Louvard D, Arpin M. Src-dependent Ezrin phosphorylation in adhesion-mediated signaling. Mol Biol Cell. 2005;16(3):1481–90. doi: 10.1091/mbc.E04-08-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng S, Huang J, Zhou K, et al. 17β-Estradiol enhances breast cancer cell motility and invasion via extra-nuclear activation of actin-binding protein Ezrin. PLoS One. 2011;6(7):e22439. doi: 10.1371/journal.pone.0022439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chuan YC, Iglesias-Gato D, Fernandez-Perez L, et al. Ezrin mediates c-Myc actions in prostate cancer cell invasion. Oncogene. 2010;29(10):1531–42. doi: 10.1038/onc.2009.442. [DOI] [PubMed] [Google Scholar]
- 21.Gavert N, Ben-Shmuel A, Lemmon V, et al. Nuclear factor-kappaB signaling and Ezrin are essential for L1-mediated metastasis of colon cancer cells. J Cell Sci. 2010;123(12):2135–43. doi: 10.1242/jcs.069542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim S, Ryu J, Shin JA, et al. Tumor necrosis factor-alpha potentiates RhoA-mediated monocyte transmigratory activity in vivo at a picomolar level. Arterioscler Thromb Vasc Biol. 2009;29(12):2138–45. doi: 10.1161/ATVBAHA.109.195735. [DOI] [PubMed] [Google Scholar]
- 23.Tang R, Li FX, Shao WF, et al. Protein-protein interaction between Ezrin and p65 in human breast cancer cells. Gen Mol Res. 2016;15(2) doi: 10.4238/gmr.15028334. [DOI] [PubMed] [Google Scholar]
- 24.Adolphe M, Parodi AL. [Ethical issues in animal experimentation]. Bull Acad Natl Med. 2009;193(8):1803–4. [in French] [PubMed] [Google Scholar]
- 25.Wu LM, Wu SG, Chen WW, Bao B. Construction of nude mouse models bearing subcutaneous tumors: Human osteosarcoma cell lines MG63, U2OS and 143B. Chemosphere. 2015;81(81):430–65. [Google Scholar]
- 26.Sun L, Yao Y, Liu B, et al. MiR-200b and miR-15b regulate chemotherapy-induced epithelial-mesenchymal transition in human tongue cancer cells by targeting BMI1. Oncogene. 2012;31(4):432–45. doi: 10.1038/onc.2011.263. [DOI] [PubMed] [Google Scholar]
- 27.Elliott BE, Meens JA, SenGupta SK, et al. The membrane cytoskeletal crosslinker Ezrin is required for metastasis of breast carcinoma cells. Breast Cancer Res. 2005;7(3):R365–73. doi: 10.1186/bcr1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crepaldi T, Gautreau A, Comoglio PM, et al. Ezrin Is an Effector of Hepatocyte Growth Factor–mediated Migration and Morphogenesis in Epithelial Cells. J Cell Biol. 1997;138(2):423–34. doi: 10.1083/jcb.138.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sizemore S, Cicek M, Sizemore N, et al. Podocalyxin increases the aggressive phenotype of breast and prostate cancer cells in vitro through its interaction with Ezrin. Cancer Res. 2007;67(13):6183–91. doi: 10.1158/0008-5472.CAN-06-3575. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Lin Z, Sun L, et al. Akt/Ezrin Tyr353/NF-κB pathway regulates EGF-induced EMT and metastasis in tongue squamous cell carcinoma. Br J Cancer. 2013;110(3):695–705. doi: 10.1038/bjc.2013.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardy KM, Booth BW, Hendrix MJ, et al. ErbB/EGF Signaling and EMT in Mammary Development and Breast Cancer. J Mammary Gland Biol Neoplasia. 2010;15(2):191–99. doi: 10.1007/s10911-010-9172-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al Moustafa AE, Achkhar A, Yasmeen A. EGF-receptor signaling and epithelial-mesenchymal transition in human carcinomas. Front Biosci. 2012;4(2):671–84. doi: 10.2741/s292. [DOI] [PubMed] [Google Scholar]
- 33.Gan Y, Shi C, Inge L, et al. Differential roles of ERK and Akt pathways in regulation of EGFR-mediated signaling and motility in prostate cancer cells. Oncogene. 2010;29(35):4947–58. doi: 10.1038/onc.2010.240. [DOI] [PubMed] [Google Scholar]
- 34.Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127(4):679–95. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]