Abstract
Cardiovascular diseases (CVDs) are major causes of death worldwide. Identification of promising targets for prevention and treatment of CVDs is paramount in the cardiovascular field. Numerous transcription factors regulate cellular function through modulation of specific genes and thereby are involved in the physiological and pathophysiological processes of CVDs. Although Krüppel-like factors (KLFs) have a similar protein structure with a conserved zinc finger domain, they possess distinct tissue and cell distribution patterns as well as biological functions. In the vascular system, KLF activities are regulated at both transcriptional and posttranscriptional levels. Growing in vitro, in vivo, and genetic epidemiology studies suggest that specific KLFs play important roles in vascular wall biology, which further affect vascular diseases. KLFs regulate various functional aspects such as cell growth, differentiation, activation, and development through controlling a whole cluster of functionally related genes and modulating various signaling pathways in response to pathological conditions. Therapeutic targeting of selective KLF family members may be desirable to achieve distinct treatment effects in the context of various vascular diseases. Further elucidation of the association of KLFs with human CVDs, their underlying molecular mechanisms, and precise protein structure studies will be essential to define KLFs as promising targets for therapeutic interventions in CVDs.
Keywords: endothelial cells, vascular smooth muscle cells, inflammation, shear stress, atherosclerosis, vascular injury, drug development
Introduction
Cardiovascular disease (CVD) ranks the number 1 cause of death worldwide with an estimated 17.5 million people dying from CVD in 2012, according to the World Health Organization. The presence of one or more high risk factors, such as hypertension, diabetes, hyperlipidemia, and smoking, contributes to the pathophysiological process of CVDs. CVDs are complicated genetic and environmental factors-driven diseases. Identification of promising targets for prevention and treatment of CVDs is assiduously pursued in the cardiovascular field. Vascular wall cells, such as endothelial cells (ECs) and vascular smooth muscle cells (VSMCs), are strictly regulated to maintain blood vessel structure and functions. Injury or inflammatory mediators destroy blood vessel homeostasis leading to vascular cell dysfunction and resulting in blood vessels narrowing (e.g. atherosclerosis, restenosis), blockage (thrombosis), regeneration (angiogenesis), stiffening (hypertension), or expansion and rupture (aneurysms). Recent studies suggest that the Krüppel-like factor (KLF) family plays a critical role in the maintenance of body homeostasis including cardiovascular, immune, digestive, respiratory, and hematopoietic systems.
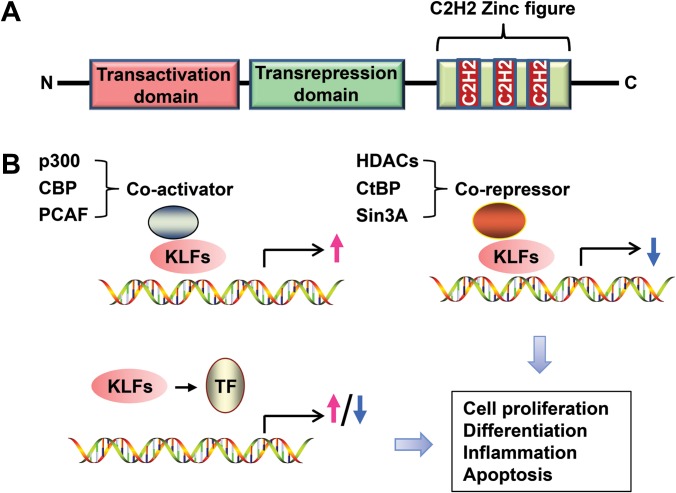
The first member of KLFs, KLF1 (EKLF), was identified in 1993 in erythroid cells, where KLF1 binds to the β-globin promoter (Miller and Bieker, 1993). To date, 18 KLFs (from KLF1 to KLF18) have been identified with distinct tissue distribution patterns and functions (McConnell and Yang, 2010; Pei and Grishin, 2013). KLFs have a conserved protein structure among human, mouse, and rat and amongst themselves. Three conserved Cys2His2-type zinc fingers in the carboxy-terminus of the KLF proteins bind to GC-rich sites in the promoter of target genes. Transactivation domain and transrepression domain are located at the amino-terminus of KLF proteins (Figure 1A) (McConnell and Yang, 2010). Although the similarity in DNA-binding ability leads to overlapping regulation of genes, KLF members have different biological functions and exhibit distinct phenotypes in various diseases, mostly resulting from their N-terminal sequences, which provide unique protein interaction motifs and posttranslational modification sites (McConnell and Yang, 2010). By regulation of gene expression, KLFs are involved in numerous biological processes (Figure 1B). Based on the published HPA RNA-seq from 95 human individuals representing 27 normal different tissues (Fagerberg et al., 2014), we performed additional analysis to identify the top KLF-expressing tissues, and summarized the findings in Table 1.
Figure 1.
Schematic representation of KLF functional domains and gene regulation. (A) The transactivation and transrepression domains are located at the N-terminus of KLF proteins. Three consecutive zinc finger motifs are located at the C-terminus. (B) Diagram illustrating the regulatory patterns for KLFs in gene transcription. KLFs induce or repress gene expression in cooperation with co-activators or co-repressors or through interaction with other specific transcription factors. TF, transcription factor; CBP, CREB-binding protein; PCAF, p300/CBP-associated factor; HDACs, histone deacetylases; CtBP, C-terminal-binding protein; Sin3A, SIN3 transcription regulator family member A.
Table 1.
Tissue distribution of KLFs.
| Human KLFs | Top 3 expressing tissues (RPKM) | Heart (RPKM) |
|---|---|---|
| KLF1 | Bone marrow, placenta, spleen | N/A |
| KLF2 | Fat, ovary, bone marrow | 4.09 |
| KLF3 | Colon, gall bladder, esophagus | 4.69 |
| KLF4 | Colon, esophagus, skin | 9.10 |
| KLF5 | Skin, esophagus, colon | 0.79 |
| KLF6 | Bone marrow, gall bladder, esophagus | 13.44 |
| KLF7 | Bone marrow, endometrium, fat | 1.51 |
| KLF8 | Skin, fat, esophagus | 0.54 |
| KLF9 | Fat, gall bladder, liver | 16.83 |
| KLF10 | Bone marrow, gall bladder, lung | 10.54 |
| KLF11 | Testis, lung, fat | 6.47 |
| KLF12 | Lymph node, spleen, brain | 2.17 |
| KLF13 | Bone marrow, lung, thyroid | 11.78 |
| KLF14 | Testis, adrenal gland, fat | N/A |
| KLF15 | Fat, ovary, kidney | 2.13 |
| KLF16 | Brain, colon, spleen | 0.73 |
| KLF17 | Testis, endometrium, esophagus | 0.003 |
Summary of the KLF abundance as calculated here from the HPA RNA-seq analysis of normal tissue samples from 95 human individuals representing 27 different tissues (Fagerberg et al., 2014). The reads per kilobase per million mapped reads (RPKM) for KLFs in the top 3 KLF-expressing tissues and heart are listed in the table. N/A, not available.
KLFs are critical regulators of vascular homeostasis, and some KLFs exhibit beneficial effects on prevention or inhibition of vascular diseases. Dysfunction of ECs and VSMCs induced by pathologic stimuli initiates or exacerbates various vascular diseases. To further underscore the significance of KLFs in these cells, we determined the mRNA abundance of KLFs in human coronary artery endothelial cells (HCAECs) and human aortic smooth muscle cells (HASMCs), introduced in Table 2. Herein, we summarize and discuss evidence underscoring the essential role of the KLF family in the regulation of vascular wall biology, specifically in ECs and VSCMs, and underlying mechanisms of KLF functions in vascular diseases and highlight the potential for KLFs as therapeutic targets in prevention and treatment of vascular diseases.
Table 2.
KLF abundance in HCAECs and HASMCs.
| Human KLFs | (KLF/GAPDH) × 104 in HCAECs | (KLF/GAPDH) × 104 in HASMCs |
|---|---|---|
| KLF1 | N/A | N/A |
| KLF2 | 93.46 | 25.14 |
| KLF3 | 89.76 | 357.97 |
| KLF4 | 0.79 | 184.43 |
| KLF5 | 3.42 | 51.33 |
| KLF6 | 549.30 | 424.61 |
| KLF7 | 41.39 | 176.19 |
| KLF8 | 0.49 | 2.75 |
| KLF9 | 49.33 | 559.83 |
| KLF10 | 167.61 | 176.32 |
| KLF11 | 2.00 | 63.30 |
| KLF12 | 30.91 | 226.97 |
| KLF13 | 28.89 | 368.51 |
| KLF14 | 0.11 | N/A |
| KLF15 | 2.42 | 3.27 |
| KLF16 | 95.04 | 58.03 |
| KLF17 | N/A | 0.72 |
Cultured human coronary artery endothelial cells (HCAECs) and human aortic smooth muscle cells (HASMCs) were subjected to RNA-sequencing analysis when grown at 90% confluence. The fragments per kilobase of transcript per million mapped reads (FPKM) for KLFs were normalized with those of GAPDH. N/A, not available.
Roles of KLFs in ECs
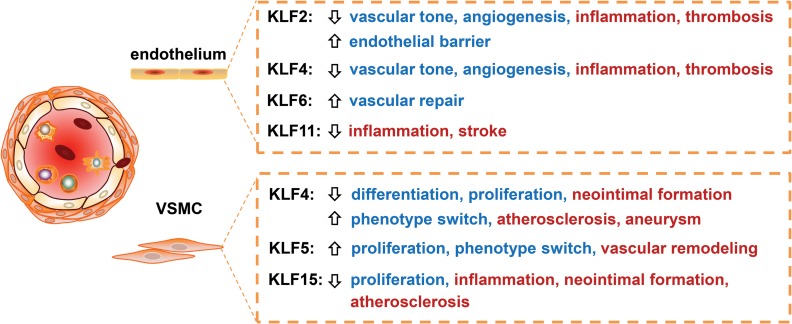
Healthy endothelium is critical to maintain normal vascular homeostasis. Vascular ECs can respond to various physiological or pathophysiological signals by dynamic changes in a wide range of factors that regulate cellular adhesion, vascular inflammation, thromboresistance, vascular tone, and EC-dependent regulation of smooth muscle cell homeostasis. Prolonged and/or repeated exposure to CVD risk factors ultimately leads to EC dysfunction. KLFs, such as KLF2, KLF4, KLF6, and KLF11, exert distinct biological functions in ECs (Figure 2).
Figure 2.
The roles of KLFs in vascular wall biology. KLFs regulate inflammation, proliferation and differentiation in ECs and VSMCs, and are further involved in various vascular diseases, underscoring an important role of KLFs in maintaining vascular homeostasis.
KLF2 and ECs
The last decades have witnessed growing research on KLF2 in relation to EC biology. KLF2 was first identified in 1995, using the zinc finger region of erythroid Krüppel-like factor (EKLF, KLF1) as the hybridization probe (Anderson et al., 1995), and first termed as lung Krüppel-like factor (LKLF), since it was found to be primarily expressed in lung (Anderson et al., 1995). Nonetheless, as early as E9.5, KLF2 is expressed in the mouse embryo vascular ECs. Klf2–/– mice die on E12.5 to E14.5 because of intra-embryonic hemorrhages (Kuo et al., 1997). Despite normal vascularization and angiogenesis, Klf2–/– mice embryos display impaired smooth muscle cell migration and blood vessel maturation (Wu et al., 2008). Mouse embryos with EC-specific loss of KLF2 die due to loss of normal vessel tone and lethal heart failure (Lee et al., 2006). Mice with KLF2 hemizygous deficiency (Klf2+/–) on an ApoE–/– background exhibit augmented atherosclerosis, attributed mostly to enhanced macrophage lipid uptake (Atkins et al., 2008).
KLF2 and inflammation
EC activation is an early stage in atherosclerosis, the major cause of heart attacks, strokes, and peripheral vascular disease (Davignon and Ganz, 2004). Microarray profiling shows that KLF2 overexpression confers anti-inflammatory and anti-thrombosis properties in ECs (Parmar et al., 2006). KLF2 induces endothelial nitric oxide synthase (eNOS) and simultaneously inhibits proinflammatory cytokine-induced adhesion molecules (E-selectin and VCAM1) through recruitment of co-activator p300/cyclic AMP response element-binding protein (CBP) to reduce NF-κB activity (SenBanerjee et al., 2004). Besides the NF-κB pathway, KLF2 could suppress constitutive proinflammatory transcription via inhibition of ATF2 phosphorylation (Fledderus et al., 2007) and Jun NH2-terminal kinase (JNK) (Boon et al., 2010), thus controlling the contribution of cytoskeletal rearrangements to EC inflammation. In addition, KLF2 inhibits inflammation through upregulation of anti-inflammatory genes in a laminar flow-dependent fashion. KLF2 mediates laminar flow-dependent upregulation of ENTPD1, which protects ApoE–/– mice from atherosclerosis (Kanthi et al., 2015). KLF2 upregulates PPAP2B, a laminar flow sensitive and anti-inflammatory gene in ECs that preserves integrity of the endothelial layer (Wu et al., 2015). The presence of antiphospholipid antibody is the marker for the antiphospholipid syndrome Lupus, characterized by high risk of thrombosis. Antiphospholipid antibody activates EC via inhibition of KLF2 and KLF4, with ensuing NF-κB activation (Allen et al., 2011).
KLF2 and vascular tone
EC is the key cell type controlling vascular tone in vivo. The KLF2–eNOS pathway mediates amelioration of vasospasm after subarachnoid hemorrhage (SAH) in rats treated with scutellarin, a flavonoid extracted from the traditional Chinese herb Erigeron breviscapus (Li et al., 2016a), providing an experimental basis for clinical use of scutellarin treatment in SAH patients. In the apelin-null mice, which develop more severe pulmonary artery hypertension (PAH), KLF2 and eNOS were decreased in the lung, implicating the KLF2–eNOS pathway in the pathological progression of PAH (Chandra et al., 2011).
KLF2 and angiogenesis
Endothelial survival, permeability, migration, and proliferation contribute to angiogenesis. KLF2 inhibits angiogenesis by multiple signaling pathways such as inhibition of transcription of VEGF receptor 2 (VEGFR2) (Bhattacharya et al., 2005) and hypoxia-inducible factor 1 (HIF-1) (Kawanami et al., 2009). KLF2 inhibits proliferation, migration, and tube formation in human liver sinusoidal ECs via suppression of ERK1/2 pathway (Zeng et al., 2015). Consistent with in vitro data, KLF2 heterozygous mice (Klf2+/−) show increased microvessel density in the brain (Kawanami et al., 2009). Vascular ECs are highly glycolytic and have relatively low oxygen demand. During angiogenesis, EC changes from a quiescent to a metabolically active phenotype, relying on glycolysis as energy source (De Bock et al., 2013). KLF2 inhibits glycolysis by downregulation of key glycolytic enzymes such as 6-phosphofructo-2-kinase/fructose-2, 6-biphosphatase-3 (PFKFB3), phosphofructokinase-1, and hexokinase 2 (Doddaballapur et al., 2015), thus further inhibiting angiogenesis. However, in the zebrafish model, KLF2 is required for angiogenesis during aortic arch development (Meadows et al., 2009; Renz et al., 2015), indicating a distinct role of KLF2 in vascular development and postnatal regeneration.
KLF2 and thrombosis
The endothelium is an important barrier to maintain blood fluidity. In ECs, KLF2 inhibits pro-thrombotic factors such as plasminogen activator inhibitor 1 (PAI-1) and tissue factor (TF), and upregulates anti-thrombotic factor thrombomodulin (TM) under inflammatory conditions (Lin et al., 2005; Boon et al., 2007). Inhibition of the inhibitory kappa-B kinase-β (IKKβ) increases thrombomodulin (TM) expression via induction of KLF2 (Pathak et al., 2014). Patients with multiple myeloma, receiving proteasome inhibitors as a part of the chemotherapeutic regimen, appear to be at lower risk for thromboembolic events (Musallam et al., 2009). Mechanistically, proteasome inhibitors increase TM by inducing KLF2 and KLF4, independently of the NF-κB pathway (Hiroi et al., 2009; Nayak et al., 2014), making KLF2 and KLF4 potential targets for inhibition of thrombosis.
KLF2 and endothelial barrier
The pathological process in ischemic stroke shares many similarities with ischemic heart attack. KLF2 reduces infarction size by improving blood–brain barrier function in the focal cerebral ischemia mouse model (Shi et al., 2013). Indeed, KLF2 increases the expression of the key junction protein occludin and phosphorylation of myosin light chain, to preserve endothelial barrier and vascular integrity (Lin et al., 2010).
Other functions of KLF2 in ECs
Autophagy is of importance in maintaining cellular homeostasis. A positive loop between autophagy and KLF2 in ECs has been discovered, in which KLF2 improves microvascular function after acute liver injury induced by cold storage and warm reperfusion (Guixe-Muntet et al., 2017). In addition, KLF2 inhibits complement-mediated lysis (Kinderlerer et al., 2008) and suppresses oxidized LDL-induced apoptosis (Wang et al., 2006), indicating a protective role of KLF2 in ECs. A recent study reported that gain of MEKK3–KLF2/4 function causes cerebral cavernous malformations (CCM) in a neonatal mouse model of CCM disease (Zhou et al., 2016), suggesting that MEKK3–KLF2/4 signaling is a causal mechanism for CCM pathogenesis. These studies implicate a distinct role of KLF2 in vascular and cerebrovascular development.
KLF4 and ECs
KLF4 was first identified as gut-enriched Krüppel-like factor (GKLF) (Yet et al., 1998). KLF4 mRNA is abundant in mouse gastrointestinal and skin epithelial cells and induces growth arrest in numerous cell types (Shields et al., 1996). KLF4-deficient mice exhibit neonatal death due to loss of the skin barrier function (Segre et al., 1999). However, KLF4 is also expressed in the vascular wall, including ECs and VSMCs, and plays a critical role in vascular wall biology. Noteworthy, KLF4 is one of the ‘Yamanaka factors’ (along with Oct3/4, Sox2, and c-Myc) used to induce pluripotency in both mouse and human somatic cells (Takahashi and Yamanaka, 2006).
KLF4 and vascular protection
Similar to KLF2, KLF4 confers an anti-inflammatory and vasoprotective phenotype in ECs by inhibiting NF-κB activation (Hamik et al., 2007), and inducing eNOS expression (Shen et al., 2009; Mun and Boo, 2012). EC-specific Klf4–/– mice exhibit increased atherosclerosis and thrombosis (Yoshida et al., 2014), aggravated LPS-induced lung injury and pulmonary edema through impaired endothelial barrier (Cowan et al., 2010), and deleterious PAH from increased endothelin-1 and decreased eNOS (Shatat et al., 2014).
KLF4 and angiogenesis
KLF4 inhibits angiogenesis and endothelial proliferation via increasing miR-15a in both ECs and VSMCs (Zheng et al., 2013). However, sustained expression of KLF4 promotes ineffective angiogenesis leading to impaired tumor growth by activating the Notch signaling pathway (Hale et al., 2014). In retinal microvascular ECs, KLF4 promotes angiogenesis via activation of VEGF signaling (Wang et al., 2015). Thus, KLF4 may regulate angiogenesis in a cell type- and expression level-dependent manner.
KLF4 and thrombosis
KLF4 has protective effects on thrombosis through transcriptional regulation of pro- and anti-thrombotic genes. Overexpression of KLF4 increases TM and eNOS expression but suppresses PAI-1 and TF expression under inflammatory conditions (Zhou et al., 2012; Stavrou et al., 2015). Mechanistically, KLF4 works synergistically with p300 to activate the TM promoter and, thereby, increasing transcription (Zhou et al., 2012). In addition to KLF2 and KLF4, whether other KLFs are involved in thrombosis remains to be investigated.
KLF6 and ECs
KLF6 transactivates the expression of a considerable number of genes involved in the TGFβ pathway, e.g. endoglin, collagen 1, urokinase-type plasminogen activator, TGFβ receptor type 1, and MMP14 (Botella et al., 2002; Gallardo-Vara et al., 2016), and colocalizes with endoglin in the vascular endothelium following carotid balloon injury in rats (Botella et al., 2002). Interestingly, KLF6 interacts with specificity protein 1 (Sp1) to cooperatively transactivate common target genes. Vascular injury triggers KLF6 nuclear translocation and cooperation with Sp1 to upregulate activin receptor-like kinase 1 (ALK1) and consequently induce endothelial activation (Garrido-Martín et al., 2013). TGFβ, in turn, enhances the interaction between KLF6 and Sp1 by inhibiting KLF6 RNA alternative splicing that functionally antagonizes full-length KLF6 (Botella et al., 2009), leading to an increase in growth-inhibitory KLF6 activity. In addition, disruption of Sp2/KLF6 repression complex is required for farnesoid X receptor to increase EC migration (Das et al., 2006).
KLF11 and ECs
KLF11 was cloned as a Sp1-like transcription factor and is involved in cell growth and differentiation (Cook et al., 1998). In population studies, KLF11 mutation causes maturity-onset diabetes of the young type 7 (MODY7) (Neve et al., 2005). Indeed, recent studies by others and us provide evidence on the critical role of KLF11 in EC homeostasis. Proinflammatory stimuli, like TNFα and LPS, increase KLF11 expression in vascular ECs (Fan et al., 2012). KLF11 potently inhibits inflammation by suppressing the NF-κB pathway (Fan et al., 2012) and downregulating endothelin-1 in ECs (Glineur et al., 2013). Klf11–/– mice display exacerbated endothelial inflammation represented by increased leukocyte–endothelial recruitment and upregulated proinflammatory adhesion molecules (Fan et al., 2012). We also found that KLF11 deficiency aggravates ischemic stroke in a mouse middle cerebral artery occlusion model. KLF11 facilitates PPARγ-mediated inhibition of pro-apoptotic miR-15a in cerebral vascular ECs (Yin et al., 2013). In addition, KLF11 antagonizes caveolin-1 transcription induced by Sp1/sterol-responsive element-binding protein (SREBP) during cholesterol depletion in ECs, suggesting a role of KLF11 in cholesterol metabolism in ECs (Cao et al., 2005).
Roles of KLFs in VSMCs
VSMC is the major cell type in the vascular wall, controlling vascular tone and maintaining vascular wall homeostasis. It displays plasticity, switching from a quiescent, contractile phenotype to a secretory, proliferative phenotype, during vascular inflammation or injury. KLFs appear to regulate phenotypic switch, proliferation, migration, apoptosis, and inflammation in VSMCs. Accordingly, studies indicate that KLFs in VSMCs are involved in vascular diseases such as restenosis, atherosclerosis, and aneurysm (Figure 2).
KLF4 and VSMCs
KLF4 and VSMC phenotypic switch
KLF4 deficiency in smooth and cardiac muscles in mice results in postnatal death and growth restriction, underscoring its importance in cardiovascular development (Yoshida et al., 2010). KLF4 is upregulated by platelet-derived growth factor BB (PDGF-BB) via transcription factor Sp1, a potent inhibitor of VSMC differentiation (Deaton et al., 2009). In turn, KLF4 overexpression inhibits the expression of VSMC contractile markers, including myocardin (Turner et al., 2013), and mediates the elongation of long-chain fatty acid family member 6 (Elovl6)-induced VSMC phenotypic switch (Sunaga et al., 2016). Therefore, KLF4 is a critical driver of VSMC phenotypic switch from a contractile to a secretory phenotype. In contrast, TGF-β, a positive regulator of VSMC differentiation from secretory to contractile phenotype, reduces KLF4 expression through miR-143/145 in VSMCs (Davis-Dusenbery et al., 2011). Furthermore, bone morphogenetic proteins (BMP) 2, 4, 6 and TGF-β share KLF4 as a common downstream molecule in maintaining the VSMC contractile phenotype (King et al., 2003). All-trans retinoic acid (ATRA) induces VSMC differentiation and inhibits proliferation via downregulation of KLF4 (Wang et al., 2008; Yu et al., 2011). Mechanistically, KLF4 inhibits myocardin, a co-activator of serum response factor (SRF) essential to maintain VSMC differentiation and contractile phenotype (Owens et al., 2004; Turner et al., 2013).
Additional mechanisms could mediate KLF4 regulation of VSMC phenotypic switch. KLF4 cooperates with ELK-1 (a co-repressor of SRF) and HDAC2 to suppress VSMC differentiation markers in vitro and in vivo (Salmon et al., 2012). Some evidence indicates that KLF4 promotes VSMC differentiation by Smad and p38 MAPK pathway (Li et al., 2010). Additionally, high phosphate induces VSMC switch to an osteogenic phenotype via upregulation of KLF4 (Yoshida et al., 2012), suggesting a positive and complex effect of KLF4 on VSMC dedifferentiation and osteogenic differentiation.
KLF4 and vascular injury
KLF4 is barely expressed in normal, contractile VSMCs in vivo, but is induced upon vascular injury (Liu et al., 2005). KLF4 inhibits VSMC proliferation and neointimal formation (Zheng et al., 2009; Wang et al., 2012b). Conditional knockout of Klf4 delays VSMC dedifferentiation, but increases VSMC proliferation by removing KLF4-dependent upregulation of p21, resulting in exacerbated neointimal formation after vascular injury in mice (Yoshida et al., 2008). A recent study demonstrated that SMC-specific KLF4 deficiency reduces the numbers of SMC-derived adventitial progenitors, which have the potential to differentiate into multiple lineages, including mature SMCs, resident macrophages, and endothelial-like cells, potentially contributing to intimal lesions in vivo (Majesky et al., 2017).
KLF4 and atherosclerosis
Many studies have shown that VSMC plasticity contributes to the development of atherosclerosis (Gomez and Owens, 2012). SMC-specific knockout of Klf4 results in reduced numbers of SMC-derived macrophage and mesenchymal stem cell-like cells, a marked reduction in atherosclerosis and an increase in plaque stability, compared to wild-type controls, reinforcing the contribution of SMCs to atherosclerotic plaques (Shankman et al., 2015).
KLF4 and aneurysm
At the histological level, inflammation, VSMC apoptosis, extracellular matrix degradation, and oxidative stress have been recognized as visible hallmarks of aortic aneurysm pathogenesis (Kuivaniemi et al., 2015). KLF4 expression is progressively increased in the vessel wall after aortic elastase perfusion in a mouse model of aneurysm. Conditional KLF4 deletion attenuates abdominal aortic aneurysm in both elastase perfusion and angiotensin II infusion-induced mouse models of aneurysm (Salmon et al., 2013). KLF4 knockdown also attenuates the TNFα-induced phenotypic switch from contractile phenotype to secretory phenotype in cerebral SMCs (Ali et al., 2013). These findings underscore the importance of KLF4 in the mechanisms behind intracranial and aortic aneurysms.
KLF5 and VSMCs
Like KLF4, KLF5 is also induced in VSMCs after vascular injury (Watanabe et al., 1999). However, unlike KLF4, KLF5 promotes VSMC proliferation and aggravates neointimal formation after carotid balloon injury in rat (Suzuki et al., 2009; Shi et al., 2012). KLF5 heterozygous deficient mice (KLF5+/−) exhibited reduced vascular injury response, vascular remodeling, angiogenesis, and cardiac hypertrophy and fibrosis (Shindo et al., 2002). Angiotensin II increases KLF5 in a PKC, p38 MAPK, and NADH/NADPH oxidase-dependent pathway (Gao et al., 2006). In turn, KLF5 mediates the pro-proliferative effect of angiotensin II (AngII) via interaction with c-Jun in VSMCs (Liu et al., 2010), thus creating a positive feedback loop to regulate VSMC response to AngII. KLF5 increases proliferation and decreases apoptosis in pulmonary artery SMCs, of relevance to development of PAH (Courboulin et al., 2011). Indeed, periostin-positive cells (both VSMC and fibroblast)-specific KLF5 deficiency attenuates vascular remodeling in deoxycorticosteroneacetate (DOCA) salt-induced mouse hypertension model (Zempo et al., 2016). Short-hairpin RNA (shRNA)-mediated KLF5 knockdown attenuates pulmonary artery remodeling in a rat PAH model (Li et al., 2016b). Beyond those roles, clinical evidence demonstrates that KLF5 is highly expressed in large and giant unruptured cerebral aneurysms in human samples, although the specific role of KLF5 and the mechanism underlying its potential role in cerebral aneurysms remain unclear (Nakajima et al., 2012).
KLF15 and VSMCs
KLF15 is expressed in quiescent VSMCs and decreased upon PDGF-BB in vitro or after vascular injury in vivo. Klf15–/– mice exhibited increased neointimal formation after vascular injury and Klf15 deficiency enhanced VSMC proliferation and migration (Lu et al., 2010). Furthermore, KLF15 is downregulated in human atherosclerotic lesion and SMC-specific KLF15 deficiency aggravates atherosclerosis development in ApoE–/– mice through increased proinflammatory activation of VSMCs (Lu et al., 2013), suggesting that KLF15 has a protective effect on vascular inflammatory diseases.
The regulation of KLFs in the vascular system
The transcriptional and posttranscriptional regulation of KLFs is a critical way for cells to adapt to changes under both physiological and pathophysiological conditions (McConnell and Yang, 2010). Further understanding of KLFs regulation will provide new avenues to modulate their activity according to their roles in the context of cellular process. Here, we briefly discuss key emerging aspects of the regulation of KLFs at both transcriptional and posttranscriptional levels in the vascular system.
DNA methylation is the most stable epigenetic hallmark that confers persistent changes in gene expression and plays a key role in maintaining endothelial cell homeostasis and participates in vascular disease development. Methylation in the KLFs promoters changes the transcription level of KLFs. Disturbed oscillatory blood flow upregulates expression of DNA methyltransferases (DNMTs) both in vitro and in vivo, which alters genome-wide DNA methylation and global gene expression (Dunn et al., 2015). Upon disturbed hemodynamics, CpG islands within the KLF4 promoter are hypermethylated in a DNMT-dependent fashion in ECs (Dunn et al., 2015), which significantly represses KLF4 transcription, while DNMT inhibitors and knockdown of DNMT3A rescue such epigenetic silencing (Jiang et al., 2014). Low-density lipoprotein (LDL) cholesterol induces endothelial dysfunction and is a major risk factor for coronary heart disease. Pharmacological inhibition or genetic inactivation of DNMTs prevents LDL-induced downregulation of KLF2 in ECs (Kumar et al., 2013). Altogether, these data suggest that DNA methylation may directly affect KLF expression in ECs in a context-dependent manner.
Acetylation is important for KLF4-mediated transactivation. ATRA increases KLF4 acetylation by inducing HDAC2 phosphorylation and further promotes VSMC secretory phenotype (Meng et al., 2009). Endogenous KLF4 is acetylated by p300/CBP and mutagenesis of the acetylated lysines in KLF4 protein results in a decreased ability of KLF4 to activate target genes (Evans et al., 2007). The deacetylase HDAC1 can negatively regulate KLF5 activity through direct interaction with the first zinc finger of KLF5, inhibiting the binding of p300 to the same region of KLF5 (Matsumura et al., 2005). Additionally, SUMOylation can alter protein activity and stability. In the presence of PDGF-BB, KLF4 SUMOylation leads to release of the p300 co-activator required for p21 expression, recruitment of co-suppressors, and downregulation of p21 expression with the ensuing increase of VSMC proliferation (Nie et al., 2016).
Interaction with an extensive network of co-regulators modulates the transcriptional activities of KLFs, which cannot be covered in depth here. Briefly, besides CBP and p300, some KLFs (e.g. KLF3, 8, and 12) bind to transcriptional regulator C-terminal-binding protein 1 (CtBP) through a consensus-binding element in their N-terminal regions to recruit histone deacetylases and histone methyltransferases to transcriptional complexes, thus affecting chromatin remodeling of the target genes (Turner and Crossley, 1998). Furthermore, KLF9, 10, 11, 13, 14, and 16 recruit the transcriptional repressor Sin3A, which binds to HDAC1 and HDAC2 to modify chromatin conformation (McConnell and Yang, 2010). Heterochromatin protein 1 (HP1) interacts with KLF11 to compact chromatin and silence gene expression (Lomberk et al., 2012). Additionally, KLFs can interact with nuclear receptors to further modulate their functions. Upon agonist stimulation of PPARδ, KLF5 is deSUMOylated and binds to transcriptional activation complexes containing both ligand-bound PPARδ and CBP to cooperatively regulate transcriptional pathways of lipid metabolism in C2C12 cells, an immortalized mouse myoblast cell line (Oishi et al., 2008). Through a genome-wide and high-throughput co-activation screening, KLF11 was demonstrated to interact with PPARγ to attenuate mouse cerebral vascular EC dysfunction (Yin et al., 2013).
Hemodynamic flow and KLFs
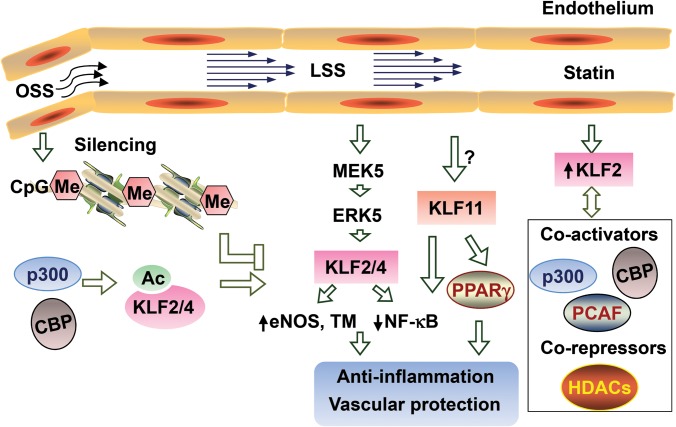
Hemodynamic flow-induced mechanotransduction regulates vascular cell homeostasis, including inflammation, proliferation, survival, metabolism, and cytoskeletal reorganization. Steady laminar flow (atheroprotective) maintains EC homeostasis, while disturbed flow (athero-prone) induces an activated and proinflammatory phenotype in ECs (Chiu and Chien, 2011). KLF2 is well-recognized as upregulated by laminar shear stress (LSS), exhibiting anti-inflammatory, anti-proliferative, and anti-thrombotic effects in vascular ECs (Dekker et al., 2002). Transcriptome analysis reveals that KLF2 and Nrf2 upregulation accounts for most of the LSS-induced gene expression (Fledderus et al., 2007, 2008). Besides driving epigenetic control of KLF2 transcription, LSS further increases KLF2 mRNA abundance though multiple signaling pathways, including enhanced MEK5/ERK5/myocyte enhancing factor-2A (MEF2) activity (Parmar et al., 2006). Additionally, LSS stimulates HDAC5 phosphorylation and nuclear translocation, further enhancing MEF2 transcriptional activity, and hence inducing the expression of KLF2 (Wang et al., 2010; Kwon et al., 2014). Under LSS, several transcription factors, including heterogeneous nuclear ribonucleoprotein D (hnRNP-D) and nucleolin, have been identified to induce histone H3 and H4 acetylation-related chromatin remodeling in the KLF2 promoter in a PI3K-dependent manner (Huddleson et al., 2005, 2006). TGFβ/activin receptor-like kinase 5 (Alk5) signaling (Walshe et al., 2013) and AMP-activated protein kinase (Young et al., 2009) are required for the LSS induction of KLF2 in ECs. Beside this complex transcriptional regulation, prolonged LSS stabilizes KLF2 mRNA, also in a PI3K-dependent fashion (van Thienen et al., 2006). Conversely, disturbed oscillatory shear stress (OSS) decreases KLF2 expression through multiple pathways. OSS inhibits KLF2 transcription through MEF2 deacetylation mediated by class I HDAC (HDAC3/5/7) (Lee et al., 2012). Non-receptor tyrosine kinase Src (Wang et al., 2006) and thioredoxin-interacting protein (TXNIP) (Wang et al., 2012a) also mediate the inhibitory effect of OSS on KLF2 expression. Furthermore, KLF2 mRNA is directly downregulated by miR-92a, which, in turn, is decreased by atheroprotective laminar flow in ECs (Wu et al., 2011; Fang and Davies, 2012). The pathways that modulate KLF2, KLF4, and KLF11 activity and expression levels in ECs are summarized in Figure 3. Unlike KLF2, which is suppressed by proinflammatory cytokines such as TNFα and IL1β in ECs (Cunningham and Gotlieb, 2004; SenBanerjee et al., 2004), basal expression of KLF4 is low, but KLF4 can be induced by TNFα, IL-1β, and interferon γ (Hamik et al., 2007). Therefore, KLF2 may be constitutive in basal conditions, while KLF4 is adaptive in ECs under different stimuli. KLF4 is upregulated by LSS in human ECs (McCormick et al., 2001; Methe et al., 2007), whereas OSS induces DNA methylation of the KLF4 promoter, resulting in decreased KLF4 transcription (Jiang et al., 2014). Like KLF2, KLF4 is also downregulated by miR-92a (Fang and Davies, 2012). In addition, KLF4 protein stability is increased by PPARγ via activation of Akt signaling and reduction of KLF4 ubiquitination (Sun et al., 2014). The basal expression level of KLF11 in human coronary artery ECs (HCAECs) is as low as that of KLF4, but RNA-sequencing analysis shows that KLF11 is upregulated by LSS (Qiao et al., 2016) and increased by TNFα in a dose-dependent manner in ECs (Fan et al., 2012). As a whole, these findings indicate that KLFs are key downstream effectors of mechanotransduction in vascular cells. Further understanding of the specific roles of individual KLFs and their complex network of interactions will likely accelerate finding interventions to address the multifaceted and deleterious effects of hemodynamic flow changes leading to CVDs.
Figure 3.
The regulation of KLFs in ECs. In ECs, laminar shear stress (LSS) upregulates, while oscillatory shear stress (OSS) downregulates KLF2 and KLF4. Epigenetic regulation (DNA methylation or histone acetylation) and MEK5–ERK5 signaling mediate the shear stress-dependent regulation of KLF2 and KLF4. KLF2, KLF4, and KLF11 potently inhibit inflammation through suppression of the NF-κB pathway. KLF11 facilitates PPARγ protective effect on ECs, while KLF2 dynamically interacts with co-activators or co-repressors to modulate cell function. Statins upregulate KLF2 in ECs.
Clinical perspective: modulation of KLFs activity in CVDs?
Recent advances in the understanding of KLFs biology and the increasingly recognized importance of KLFs to CVD, cancer, and digestive disease have stimulated researchers’ fervor towards development of drugs targeting KLFs, in an increased effort to modulate KLF activity (upregulation or downregulation) on the basis of their roles in the context of specific diseases (Bialkowska et al., 2009; Guo et al., 2015; Khedkar et al., 2015; Ruiz de Sabando et al., 2016). KLFs regulate various cellular functions such as growth and differentiation, activation, and development, and may control a whole cluster of functionally related genes in response to physiological and pathological conditions. Therefore, therapeutic targeting of a selective KLF might achieve desirable biological effects in specific diseases. Unfortunately, there is high sequence and structural conservation in the KLF family and possible functional redundancy among some KLFs. Furthermore, unlike nuclear receptors, KLFs lack clear molecule ‘pockets’, thus thwarting efforts to find small therapeutic molecules. However, alternative strategies have been employed to achieve this goal.
Drugs that regulate KLF expression
Some drugs or chemical compounds have been identified to regulate KLF expression. Statins, the most widely used drugs for lipid-lowering and prevention of CVDs, effectively reduce clinical cardiovascular events in a variety of patients, not only in those with established CVD but also those who are at risk for CVD (Heart Protection Study Collaborative, 2002). Several studies demonstrated that statins increase KLF2 transcription in ECs (Parmar et al., 2005; Sen-Banerjee et al., 2005), while siRNA-mediated KLF2 knockdown abolishes the regulatory effect of statins on eNOS, TM, and prostaglandin D2 synthase (Parmar et al., 2005; Sen-Banerjee et al., 2005), indicating a critical role of KLF2 in mediating the atheroprotective role of statins in ECs. Resveratrol, an NAD-dependent deacetylase sirtuin-1 (SIRT1) activator, induces the expression of KLF2 and KLF2-dependent atheroprotective genes in ECs (Gracia-Sancho et al., 2010). Furthermore, KLF4 is upregulated by simvastatin and resveratrol in ECs (Villarreal et al., 2010), and increased by rapamycin in VSMCs (Wang et al., 2012b).
A third-generation small molecule compound, ML264, derived from ultrahigh-throughput screenings, potently inhibited KLF5 expression in colorectal cancer models (Ruiz de Sabando et al., 2016) but remains to be tested in the context of CVD. Recently, through a high-throughput compound screening based on KLF14 promoter-reporter assays, our lab demonstrated that perhexiline, an FDA-approved drug for chronic heart failure and refractory angina, transcriptionally upregulated KLF14 expression and further increased ApoA1 level. Perhexiline treatment, consequently and significantly, inhibits atherosclerosis in ApoE–/– mice (Guo et al., 2015).
Small molecule compounds that disrupt DNA-binding activity of KLFs
A large, ‘shallow’ pocket was identified within the middle zinc finger region of KLF10 leading to identification of small molecule compounds that bind in this pocket and inhibit the KLF10–DNA interaction interface, using computer-aided drug design screening of chemical libraries (Khedkar et al., 2015). This study provides optimism regarding the feasibility to identify small molecules that directly target KLFs. However, due to the conserved protein structure of the zinc finger region among KLF members, the selectivity of these compounds remains unclear.
Nucleic acid-based gene regulation of KLFs
Because of its specificity and efficacy, RNA interference (RNAi)-mediated gene silencing using siRNA or short hairpin RNA (shRNA) is a particularly promising approach to decrease expression of target genes, especially genes whose products are not considered to be practical drug targets (e.g. transcription factors). Liposome–siRNA complexes or polymer-based RNA complexes have been often used in preclinical studies for cardiovascular disorders. MicroRNAs (miRNAs) are critical to the regulation of vascular function through posttranscriptional modification or translational repression of target genes. It has been demonstrated that miR-143/145 targets KLF4, leading to decreased expression of KLF4 in vascular smooth muscle cells (Cordes et al., 2009; Davis-Dusenbery et al., 2011), while miR-92a decreases the expression of KLF2 and KLF4 in ECs (Wu et al., 2011; Fang and Davies, 2012). miRNA mimics and antagonists such as antisense oligonucleotides, anti-miRNA oligonucleotides, and aptamers targeting specific KLFs may provide specific tools for treatment of CVD (Deshpande et al., 2016). The rapidly developing gene editing technologies, such as transcription activator-like effector nucleases (TALEN), Zinc Finger Nuclease (ZNF), and CRISPR/Cas9, make it possible to regulate gene expression and could be eventually applied to treat human diseases (Hsu et al., 2014).
Conclusions and future perspectives
Although the KLF family shares a similar protein structure with conserved zinc finger domain, members have distinct tissue distribution patterns and depict different biological functions in various CVDs. The differential expression of KLF members is dynamically changed in response to specific pathophysiological processes through transcriptional regulation and posttranscriptional modifications and dynamic interactions with co-regulators.
Essential roles of KLFs in vascular biology have been extensively demonstrated, especially in the vascular wall cells: ECs and VSMCs. Nonetheless, key aspects of their involvement and mechanisms of action still remain obscure. The plethora of findings on relative alterations in KLF gene expression in CVDs opens the question on whether there will be a compensatory effects among KLFs when enhancing or decreasing a given KLF member by specific targeting. It also remains to be investigated whether there is a cooperative or independent effect among KLFs in the same cell/tissue and disease manifestation. For instance, vascular integrity is broken and gene expression of VEGF receptor 2, eNOS, and occludin is reduced in E9.5 Klf2–/–/Klf4–/– double knockout (EC-DKO) compared to Klf2–/– embryos (Chiplunkar et al., 2013). Moreover, a recent study, using inducible endothelial-specific deletion of Klf2 and/or Klf4 mouse models, revealed that Klf2 and Klf4 EC-DKO leads to acute death from myocardial infarction, heart failure, and stroke. EC-DKO mice also exhibit impaired vascular integrity and coagulation (Sangwung et al., 2017). Collectively, these studies establish a requirement for both KLF2 and KLF4 for maintenance of vascular integrity in both embryo development and the adult animal. However, whether KLF2 and KLF4 regulate EC functions synergistically through common mechanisms or individually through distinct mechanisms, with the ensuing implications for therapeutic targeting, is a question for future studies.
The newly found genetic associations of KLFs with CVD are a new and promising area of research. Recent genetic studies have revealed the associations of KLFs with human CVDs. Missense mutations in the KLF10 gene were identified to be positively associated with hypertrophic cardiomyopathy (Bos et al., 2012). In addition, a single nucleotide polymorphism located at −1282 bp within the KLF5 locus is associated with an increased risk of hypertension (Oishi et al., 2010). Recently, a novel missense mutation (p.H288Y) located in the zinc finger domain of KLF2, which is a recurrent somatic mutation in B-cell lymphoma, was found to likely disrupt gene function and lead to heritable pulmonary arterial hypertension (HPAH) (Eichstaedt et al., 2017). Considering the multiple homeostatic roles of this extensive family, it is likely that, as further targeted genetic studies are conducted, novel variants in KLF genes or their regulatory regions that affect KLF expression/activity in the cardiovascular system may be linked to CVD development. In spite of its essential involvement in CVD, a genetic association between KLF4 with human CVDs is yet to be found. Conversely, human genetic studies revealed an association between KLF11 and diabetes (Neve et al., 2005), but it is still unknown whether KLF11 plays an important role in diabetes-associated CVD. The answers to these important questions will facilitate our understanding of the roles of KLFs in human CVDs and inform new therapeutic strategies.
Strategies to utilize chemical compounds and nucleotides as therapeutic agents require efficient delivery systems. Since the KLFs have distinct and complicated functions in different tissues and are involved in various diseases, cell or tissue-specific delivery of therapeutic agents and optimized dosage are a pre-requisite to avoid side effects. Drug delivery systems (DDS) are always critical to enhance the efficacy and safety of therapeutic agents and overcome their limitations, such as poor organ specificity, toxicity, low water solubility, and low bioavailability. Nanoparticle-mediated DDS (nano-DDS) modify the in vivo kinetics of therapeutic agents and are superior in that drug targeting can leverage physiologic and pathophysiological properties specific to certain disease conditions. Two polymers, polylactide (PLA) and poly (lactide-co-glycolide) (PLGA), are been extensively used for the synthesis of polymeric biodegradable nano-DDS (Matoba and Egashira, 2014). For ECs, specific cell surface molecular determinants, such as membrane receptors and adhesion molecules, can be targets for the delivery of a variety of agents-loaded pharmaceutical carriers (Koren and Torchilin, 2011). Therapeutic agents targeting specific KLF combined with optimal advanced nano-DDS may represent a promising approach to treat or prevent vascular diseases.
Although the high similarity of protein structure among the large number of KLF members, along with the scarcity of drug targetable pockets largely hindering the development of KLFs as therapeutic agents, the critical functions of KLFs have provided new insights for novel pharmacological perspectives. Further elucidation of the association of KLFs with human diseases, the KLF biological functions, molecular mechanisms and structure analysis, and drug development will be essential for the ascension of KLFs to the clinical arena in cardiovascular medicine.
Funding
This work was supported, in whole or in part, by the National Institutes of Health grants (HL068878, HL105114, and HL088391 to Y.E.C.) and American Heart Association grants (14SDG19880014 to Y.F. and 17PRE33400179 to H.L.).
Conflict of interest: none declared.
Acknowledgements
We would like to acknowledge all the other studies in this field that were not discussed or cited solely due to space limitations and Dr Minerva Garcia-Barrio (Cardiovascular Research Institute, Morehouse School of Medicine) for feedback on the manuscript.
References
- Ali M.S., Starke R.M., Jabbour P.M., et al. (2013). TNF-α induces phenotypic modulation in cerebral vascular smooth muscle cells: implications for cerebral aneurysm pathology. J. Cereb. Blood Flow Metab. 33, 1564–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen K.L., Hamik A., Jain M.K., et al. (2011). Endothelial cell activation by antiphospholipid antibodies is modulated by Krüppel-like transcription factors. Blood 117, 6383–6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K.P., Kern C.B., Crable S.C., et al. (1995). Isolation of a gene encoding a functional zinc finger protein homologous to erythroid Krüppel-like factor: identification of a new multigene family. Mol. Cell. Biol. 15, 5957–5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins G.B., Wang Y., Mahabeleshwar G.H., et al. (2008). Hemizygous deficiency of Krüppel-like factor 2 augments experimental atherosclerosis. Circ. Res. 103, 690–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya R., SenBanerjee S., Lin Z., et al. (2005). Inhibition of vascular permeability factor/vascular endothelial growth factor-mediated angiogenesis by the Kruppel-like factor KLF2. J. Biol. Chem. 280, 28848–28851. [DOI] [PubMed] [Google Scholar]
- Bialkowska A.B., Du Y., Fu H., et al. (2009). Identification of novel small-molecule compounds that inhibit the proproliferative Kruppel-like factor 5 in colorectal cancer cells by high-throughput screening. Mol. Cancer Ther. 8, 563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon R.A., Fledderus J.O., Volger O.L., et al. (2007). KLF2 suppresses TGF-β signaling in endothelium through induction of Smad7 and inhibition of AP-1. Arterioscler. Thromb. Vasc. Biol. 27, 532–539. [DOI] [PubMed] [Google Scholar]
- Boon R.A., Leyen T.A., Fontijn R.D., et al. (2010). KLF2-induced actin shear fibers control both alignment to flow and JNK signaling in vascular endothelium. Blood 115, 2533–2542. [DOI] [PubMed] [Google Scholar]
- Bos J.M., Subramaniam M., Hawse J.R., et al. (2012). TGFβ-inducible early gene-1 (TIEG1) mutations in hypertrophic cardiomyopathy. J. Cell. Biochem. 113, 1896–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botella L.M., Sánchez-Elsner T., Sanz-Rodriguez F., et al. (2002). Transcriptional activation of endoglin and transforming growth factor-β signaling components by cooperative interaction between Sp1 and KLF6: their potential role in the response to vascular injury. Blood 100, 4001–4010. [DOI] [PubMed] [Google Scholar]
- Botella L.M., Sanz-Rodriguez F., Komi Y., et al. (2009). TGF-β regulates the expression of transcription factor KLF6 and its splice variants and promotes co-operative transactivation of common target genes through a Smad3–Sp1–KLF6 interaction. Biochem. J. 419, 485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S., Fernandez-Zapico M.E., Jin D., et al. (2005). KLF11-mediated repression antagonizes Sp1/sterol-responsive element-binding protein-induced transcriptional activation of caveolin-1 in response to cholesterol signaling. J. Biol. Chem. 280, 1901–1910. [DOI] [PubMed] [Google Scholar]
- Chandra S.M., Razavi H., Kim J., et al. (2011). Disruption of the apelin-APJ system worsens hypoxia-induced pulmonary hypertension. Arterioscler. Thromb. Vasc. Biol. 31, 814–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiplunkar A.R., Curtis B.C., Eades G.L., et al. (2013). The Kruppel-like factor 2 and Kruppel-like factor 4 genes interact to maintain endothelial integrity in mouse embryonic vasculogenesis. BMC Dev. Biol. 13, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu J.J., and Chien S. (2011). Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol. Rev. 91, 327–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook T., Gebelein B., Mesa K., et al. (1998). Molecular cloning and characterization of TIEG2 reveals a new subfamily of transforming growth factor-β-inducible Sp1-like zinc finger-encoding genes involved in the regulation of cell growth. J. Biol. Chem. 273, 25929–25936. [DOI] [PubMed] [Google Scholar]
- Cordes K.R., Sheehy N.T., White M.P., et al. (2009). miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 460, 705–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courboulin A., Tremblay V.L., Barrier M., et al. (2011). Krüppel-like factor 5 contributes to pulmonary artery smooth muscle proliferation and resistance to apoptosis in human pulmonary arterial hypertension. Respir. Res. 12, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan C.E., Kohler E.E., Dugan T.A., et al. (2010). Kruppel-like factor-4 transcriptionally regulates VE-cadherin expression and endothelial barrier function. Circ. Res. 107, 959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham K.S., and Gotlieb A.I. (2004). The role of shear stress in the pathogenesis of atherosclerosis. Lab. Invest. 85, 9–23. [DOI] [PubMed] [Google Scholar]
- Das A., Fernandez-Zapico M.E., Cao S., et al. (2006). Disruption of an SP2/KLF6 repression complex by SHP is required for farnesoid X receptor-induced endothelial cell migration. J. Biol. Chem. 281, 39105–39113. [DOI] [PubMed] [Google Scholar]
- Davignon J., and Ganz P. (2004). Role of endothelial dysfunction in atherosclerosis. Circulation 109, III-27–III-32. [DOI] [PubMed] [Google Scholar]
- Davis-Dusenbery B.N., Chan M.C., Reno K.E., et al. (2011). down-regulation of Kruppel-like factor-4 (KLF4) by microRNA-143/145 is critical for modulation of vascular smooth muscle cell phenotype by transforming growth factor-β and bone morphogenetic protein 4. J. Biol. Chem. 286, 28097–28110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bock K., Georgiadou M., Schoors S., et al. (2013). Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 154, 651–663. [DOI] [PubMed] [Google Scholar]
- Deaton R.A., Gan Q., and Owens G.K. (2009). Sp1-dependent activation of KLF4 is required for PDGF-BB-induced phenotypic modulation of smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 296, H1027–H1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker R.J., van Soest S., Fontijn R.D., et al. (2002). Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Krüppel-like factor (KLF2). Blood 100, 1689–1698. [DOI] [PubMed] [Google Scholar]
- Deshpande D., Janero D.R., Segura-Ibarra V., et al. (2016). Nucleic acid delivery for endothelial dysfunction in cardiovascular diseases. Methodist Debakey Cardiovasc. J. 12, 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doddaballapur A., Michalik K.M., Manavski Y., et al. (2015). Laminar shear stress inhibits endothelial cell metabolism via KLF2-mediated repression of PFKFB3. Arterioscler. Thromb. Vasc. Biol. 35, 137–145. [DOI] [PubMed] [Google Scholar]
- Dunn J., Thabet S., and Jo H. (2015). Flow-dependent epigenetic DNA methylation in endothelial gene expression and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 35, 1562–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichstaedt C.A., Song J., Rodriguez Viales R., et al. (2017). First identification of Kruppel-like factor 2 mutation in heritable pulmonary arterial hypertension. Clin. Sci. 131, 689–698. [DOI] [PubMed] [Google Scholar]
- Evans P.M., Zhang W., Chen X., et al. (2007). Kruppel-like factor 4 is acetylated by p300 and regulates gene transcription via modulation of histone acetylation. J. Biol. Chem. 282, 33994–34002. [DOI] [PubMed] [Google Scholar]
- Fagerberg L., Hallstrom B.M., Oksvold P., et al. (2014). Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteomics 13, 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Guo Y., Zhang J., et al. (2012). Kruppel-like factor-11, a transcription factor involved in diabetes mellitus, suppresses endothelial cell activation via the nuclear factor-κB signaling pathway. Arterioscler. Thromb. Vasc. Biol. 32, 2981–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y., and Davies P.F. (2012). Site-specific microRNA-92a regulation of Krüppel-like factors 4 and 2 in atherosusceptible endothelium. Arterioscler. Thromb. Vasc. Biol. 32, 979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fledderus J.O., Boon R.A., Volger O.L., et al. (2008). KLF2 primes the antioxidant transcription factor Nrf2 for activation in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 28, 1339–1346. [DOI] [PubMed] [Google Scholar]
- Fledderus J.O., van Thienen J.V., Boon R.A., et al. (2007). Prolonged shear stress and KLF2 suppress constitutive proinflammatory transcription through inhibition of ATF2. Blood 109, 4249–4257. [DOI] [PubMed] [Google Scholar]
- Gallardo-Vara E., Blanco F.J., Roqué M., et al. (2016). Transcription factor KLF6 upregulates expression of metalloprotease MMP14 and subsequent release of soluble endoglin during vascular injury. Angiogenesis 19, 155–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D., Niu X., Ning N., et al. (2006). Regulation of angiotensin II-Induced Kruppel-like factor 5 expression in vascular smooth muscle cells. Biol. Pharm. Bull. 29, 2004–2008. [DOI] [PubMed] [Google Scholar]
- Garrido-Martín E.M., Blanco F.J., Roquè M., et al. (2013). Vascular injury triggers Krüppel-like factor 6 mobilization and cooperation with specificity protein 1 to promote endothelial activation through upregulation of the activin receptor-like kinase 1 gene. Circ. Res. 112, 113–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glineur C., Gross B., Neve B., et al. (2013). Fenofibrate inhibits endothelin-1 expression by peroxisome proliferator-activated receptor α-dependent and independent mechanisms in human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 33, 621–628. [DOI] [PubMed] [Google Scholar]
- Gomez D., and Owens G.K. (2012). Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc. Res. 95, 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracia-Sancho J., Villarreal G., Zhang Y., et al. (2010). Activation of SIRT1 by resveratrol induces KLF2 expression conferring an endothelial vasoprotective phenotype. Cardiovasc. Res. 85, 514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guixe-Muntet S., de Mesquita F.C., Vila S., et al. (2017). Cross-talk between autophagy and KLF2 determines endothelial cell phenotype and microvascular function in acute liver injury. J. Hepatol. 66, 86–94. [DOI] [PubMed] [Google Scholar]
- Guo Y., Fan Y., Zhang J., et al. (2015). Perhexiline activates KLF14 and reduces atherosclerosis by modulating ApoA-I production. J. Clin. Invest. 125, 3819–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale A.T., Tian H., Anih E., et al. (2014). Endothelial Kruppel-like factor 4 regulates angiogenesis and the Notch signaling pathway. J. Biol. Chem. 289, 12016–12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamik A., Lin Z., Kumar A., et al. (2007). Kruppel-like factor 4 regulates endothelial inflammation. J. Biol. Chem. 282, 13769–13779. [DOI] [PubMed] [Google Scholar]
- Heart Protection Study Collaborative, G. (2002). MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 360, 7–22. [DOI] [PubMed] [Google Scholar]
- Hiroi T., Deming C.B., Zhao H., et al. (2009). Proteasome inhibitors enhance endothelial thrombomodulin expression via induction of Kruppel-like transcription factors. Arterioscler. Thromb. Vasc. Biol. 29, 1587–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P.D., Lander E.S., and Zhang F. (2014). Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huddleson J.P., Ahmad N., and Lingrel J.B. (2006). Up-regulation of the KLF2 transcription factor by fluid shear stress requires nucleolin. J. Biol. Chem. 281, 15121–15128. [DOI] [PubMed] [Google Scholar]
- Huddleson J.P., Ahmad N., Srinivasan S., et al. (2005). Induction of KLF2 by fluid shear stress requires a novel promoter element activated by a phosphatidylinositol 3-kinase-dependent chromatin-remodeling pathway. J. Biol. Chem. 280, 23371–23379. [DOI] [PubMed] [Google Scholar]
- Jiang Y.Z., Jimenez J.M., Ou K., et al. (2014). Hemodynamic disturbed flow induces differential DNA methylation of endothelial Kruppel-like factor 4 promoter in vitro and in vivo. Circ. Res. 115, 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanthi Y., Hyman M.C., Liao H., et al. (2015). Flow-dependent expression of ectonucleotide tri(di)phosphohydrolase-1 and suppression of atherosclerosis. J. Clin. Invest. 125, 3027–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanami D., Mahabeleshwar G.H., Lin Z., et al. (2009). Kruppel-like factor 2 inhibits hypoxia-inducible factor 1α expression and function in the endothelium. J. Biol. Chem. 284, 20522–20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedkar S.A., Sun X., Rigby A.C., et al. (2015). Discovery of small molecule inhibitors to Kruppel-like factor 10 (KLF10): implications for modulation of T regulatory cell differentiation. J. Med. Chem. 58, 1466–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinderlerer A.R., Ali F., Johns M., et al. (2008). KLF2-dependent, shear stress-induced expression of CD59: a novel cytoprotective mechanism against complement-mediated injury in the vasculature. J. Biol. Chem. 283, 14636–14644. [DOI] [PubMed] [Google Scholar]
- King K.E., Iyemere V.P., Weissberg P.L., et al. (2003). Krüppel-like factor 4 (KLF4/GKLF) is a target of bone morphogenetic proteins and transforming growth factor β1 in the regulation of vascular smooth muscle cell phenotype. J. Biol. Chem. 278, 11661–11669. [DOI] [PubMed] [Google Scholar]
- Koren E., and Torchilin V.P. (2011). Drug carriers for vascular drug delivery. IUBMB Life 63, 586–595. [DOI] [PubMed] [Google Scholar]
- Kuivaniemi H., Ryer E.J., Elmore J.R., et al. (2015). Understanding the pathogenesis of abdominal aortic aneurysms. Expert Rev. Cardiovasc. Ther. 13, 975–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Kumar S., Vikram A., et al. (2013). Histone and DNA methylation-mediated epigenetic downregulation of endothelial Kruppel-like factor 2 by low-density lipoprotein cholesterol. Arterioscler. Thromb. Vasc. Biol. 33, 1936–1942. [DOI] [PubMed] [Google Scholar]
- Kuo C.T., Veselits M.L., Barton K.P., et al. (1997). The LKLF transcription factor is required for normal tunica media formation and blood vessel stabilization during murine embryogenesis. Genes Dev. 11, 2996–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon I.S., Wang W., Xu S., et al. (2014). Histone deacetylase 5 interacts with Kruppel-like factor 2 and inhibits its transcriptional activity in endothelium. Cardiovasc. Res. 104, 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.-Y., Lee C.-I., Lin T.-E., et al. (2012). Role of histone deacetylases in transcription factor regulation and cell cycle modulation in endothelial cells in response to disturbed flow. Proc. Natl Acad. Sci. USA 109, 1967–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.S., Yu Q., Shin J.T., et al. (2006). Klf2 is an essential regulator of vascular hemodynamic forces in vivo. Dev. Cell 11, 845–857. [DOI] [PubMed] [Google Scholar]
- Li H.-x., Han M., Bernier M., et al. (2010). Krüppel-like factor 4 promotes differentiation by transforming growth factor-β receptor-mediated Smad and p38 MAPK signaling in vascular smooth muscle cells. J. Biol. Chem. 285, 17846–17856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Chen Y., Zhang X., et al. (2016. a). Scutellarin attenuates vasospasm through the Erk5-KLF2-eNOS pathway after subarachnoid hemorrhage in rats. J. Clin. Neurosci. 34, 264–270. [DOI] [PubMed] [Google Scholar]
- Li X., He Y., Xu Y., et al. (2016. b). KLF5 mediates vascular remodeling via HIF-1α in hypoxic pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 310, L299–L310. [DOI] [PubMed] [Google Scholar]
- Lin Z., Kumar A., SenBanerjee S., et al. (2005). Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circ. Res. 96, e48–e57. [DOI] [PubMed] [Google Scholar]
- Lin Z., Natesan V., Shi H., et al. (2010). Kruppel-like factor 2 regulates endothelial barrier function. Arterioscler. Thromb. Vasc. Biol. 30, 1952–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Sinha S., McDonald O.G., et al. (2005). Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J. Biol. Chem. 280, 9719–9727. [DOI] [PubMed] [Google Scholar]
- Liu Y., Wen J.-k., Dong L.-h., et al. (2010). Krüppel-like factor (KLF) 5 mediates cyclin D1 expression and cell proliferation via interaction with c-Jun in Ang II-induced VSMCs. Acta Pharmacol. Sin. 31, 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomberk G., Mathison A.J., Grzenda A., et al. (2012). Sequence-specific recruitment of heterochromatin protein 1 via interaction with Kruppel-like factor 11, a human transcription factor involved in tumor suppression and metabolic diseases. J. Biol. Chem. 287, 13026–13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Haldar S., Croce K., et al. (2010). Kruppel-like factor 15 regulates smooth muscle response to vascular injury—brief report. Arterioscler. Thromb. Vasc. Biol. 30, 1550–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Zhang L., Liao X., et al. (2013). Kruppel-like factor 15 is critical for vascular inflammation. J. Clin. Invest. 123, 4232–4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M.W., Horita H., Ostriker A., et al. (2017). Differentiated smooth muscle cells generate a subpopulation of resident vascular progenitor cells in the adventitia regulated by KLF4. Circ. Res. 120, 296–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba T., and Egashira K. (2014). Nanoparticle-mediated drug delivery system for cardiovascular disease. Int. Heart J. 55, 281–286. [DOI] [PubMed] [Google Scholar]
- Matsumura T., Suzuki T., Aizawa K., et al. (2005). The deacetylase HDAC1 negatively regulates the cardiovascular transcription factor Kruppel-like factor 5 through direct interaction. J. Biol. Chem. 280, 12123–12129. [DOI] [PubMed] [Google Scholar]
- McConnell B.B., and Yang V.W. (2010). Mammalian Kruppel-like factors in health and diseases. Physiol. Rev. 90, 1337–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick S.M., Eskin S.G., McIntire L.V., et al. (2001). DNA microarray reveals changes in gene expression of shear stressed human umbilical vein endothelial cells. Proc. Natl Acad. Sci. USA 98, 8955–8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows S.M., Salanga M.C., and Krieg P.A. (2009). Krüppel-like factor 2 cooperates with the ETS family protein ERG to activate Flk1 expression during vascular development. Development 136, 1115–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F., Han M., Zheng B., et al. (2009). All-trans retinoic acid increases KLF4 acetylation by inducing HDAC2 phosphorylation and its dissociation from KLF4 in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 387, 13–18. [DOI] [PubMed] [Google Scholar]
- Methe H., Balcells M., del Carmen Alegret M., et al. (2007). Vascular bed origin dictates flow pattern regulation of endothelial adhesion molecule expression. Am. J. Physiol. Heart Circ. Physiol. 292, H2167–H2175. [DOI] [PubMed] [Google Scholar]
- Miller I.J., and Bieker J.J. (1993). A novel, erythroid cell-specific murine transcription factor that binds to the CACCC element and is related to the Kruppel family of nuclear proteins. Mol. Cell. Biol. 13, 2776–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun G.I., and Boo Y.C. (2012). A regulatory role of Kruppel-like factor 4 in endothelial argininosuccinate synthetase 1 expression in response to laminar shear stress. Biochem. Biophys. Res. Commun. 420, 450–455. [DOI] [PubMed] [Google Scholar]
- Musallam K.M., Dahdaleh F.S., Shamseddine A.I., et al. (2009). Incidence and prophylaxis of venous thromboembolic events in multiple myeloma patients receiving immunomodulatory therapy. Thromb. Res. 123, 679–686. [DOI] [PubMed] [Google Scholar]
- Nakajima N., Nagahiro S., Sano T., et al. (2012). Kruppel-like zinc-finger transcription factor 5 (KLF5) is highly expressed in large and giant unruptured cerebral aneurysms. World Neurosurg. 78, 114–121. [DOI] [PubMed] [Google Scholar]
- Nayak L., Shi H., Atkins G.B., et al. (2014). The thromboprotective effect of bortezomib is dependent on the transcription factor Kruppel-like factor 2 (KLF2). Blood 123, 3828–3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve B., Fernandez-Zapico M.E., Ashkenazi-Katalan V., et al. (2005). Role of transcription factor KLF11 and its diabetes-associated gene variants in pancreatic β cell function. Proc. Natl Acad. Sci. USA 102, 4807–4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie C.-J., Hui Li Y., Zhang X.-H., et al. (2016). SUMOylation of KLF4 acts as a switch in transcriptional programs that control VSMC proliferation. Exp. Cell Res. 342, 20–31. [DOI] [PubMed] [Google Scholar]
- Oishi Y., Manabe I., Imai Y., et al. (2010). Regulatory polymorphism in transcription factor KLF5 at the MEF2 element alters the response to angiotensin II and is associated with human hypertension. FASEB J. 24, 1780–1788. [DOI] [PubMed] [Google Scholar]
- Oishi Y., Manabe I., Tobe K., et al. (2008). SUMOylation of Kruppel-like transcription factor 5 acts as a molecular switch in transcriptional programs of lipid metabolism involving PPAR-delta. Nat. Med. 14, 656–666. [DOI] [PubMed] [Google Scholar]
- Owens G.K., Kumar M.S., and Wamhoff B.R. (2004). Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 84, 767–801. [DOI] [PubMed] [Google Scholar]
- Parmar K.M., Larman H.B., Dai G., et al. (2006). Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J. Clin. Invest. 116, 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar K.M., Nambudiri V., Dai G., et al. (2005). Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J. Biol. Chem. 280, 26714–26719. [DOI] [PubMed] [Google Scholar]
- Pathak R., Shao L., Chafekar S.M., et al. (2014). IKKβ regulates endothelial thrombomodulin in a Klf2-dependent manner. J. Thromb. Haemost. 12, 1533–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei J., and Grishin N.V. (2013). A new family of predicted Kruppel-like factor genes and pseudogenes in placental mammals. PLoS One 8, e81109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao C., Meng F., Jang I., et al. (2016). Deep transcriptomic profiling reveals the similarity between endothelial cells cultured under static and oscillatory shear stress conditions. Physiol. Genomics 48, 660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz M., Otten C., Faurobert E., et al. (2015). Regulation of β1 integrin-Klf2-mediated angiogenesis by CCM proteins. Dev. Cell 32, 181–190. [DOI] [PubMed] [Google Scholar]
- Ruiz de Sabando A., Wang C., He Y., et al. (2016). ML264, A novel small-molecule compound that potently inhibits growth of colorectal cancer. Mol. Cancer Ther. 15, 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon M., Gomez D., Greene E., et al. (2012). Cooperative binding of KLF4, pELK-1, and HDAC2 to a G/C repressor element in the SM22α promoter mediates transcriptional silencing during SMC phenotypic switching in vivo. Circ. Res. 111, 685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon M., Johnston W.F., Woo A., et al. (2013). KLF4 regulates abdominal aortic aneurysm morphology and deletion attenuates aneurysm formation. Circulation 128, S163–S174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangwung P., Zhou G., Nayak L., et al. (2017). KLF2 and KLF4 control endothelial identity and vascular integrity. JCI Insight 2, e91700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segre J.A., Bauer C., and Fuchs E. (1999). Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat. Genet. 22, 356–360. [DOI] [PubMed] [Google Scholar]
- Sen-Banerjee S., Mir S., Lin Z., et al. (2005). Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation 112, 720–726. [DOI] [PubMed] [Google Scholar]
- SenBanerjee S., Lin Z., Atkins G.B., et al. (2004). KLF2 is a novel transcriptional regulator of endothelial proinflammatory activation. J. Exp. Med. 199, 1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman L.S., Gomez D., Cherepanova O.A., et al. (2015). KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat. Med. 21, 628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatat M.A., Tian H., Zhang R., et al. (2014). Endothelial Kruppel-like factor 4 modulates pulmonary arterial hypertension. Am. J. Respir. Cell Mol. Biol. 50, 647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B., Smith R.S. Jr, Hsu Y.T., et al. (2009). Kruppel-like factor 4 is a novel mediator of Kallistatin in inhibiting endothelial inflammation via increased endothelial nitric-oxide synthase expression. J. Biol. Chem. 284, 35471–35478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Sheng B., Zhang F., et al. (2013). Kruppel-like factor 2 protects against ischemic stroke by regulating endothelial blood brain barrier function. Am. J. Physiol. Heart Circ. Physiol. 304, H796–H805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H.J., Wen J.K., Miao S.B., et al. (2012). KLF5 and hhLIM cooperatively promote proliferation of vascular smooth muscle cells. Mol. Cell. Biochem. 367, 185–194. [DOI] [PubMed] [Google Scholar]
- Shields J.M., Christy R.J., and Yang V.W. (1996). Identification and characterization of a gene encoding a gut-enriched Krüppel-like factor expressed during growth arrest. J. Biol. Chem. 271, 20009–20017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo T., Manabe I., Fukushima Y., et al. (2002). Krüppel-like zinc-finger transcription factor KLF5/BTEB2 is a target for angiotensin II signaling and an essential regulator of cardiovascular remodeling. Nat. Med. 8, 856–863. [DOI] [PubMed] [Google Scholar]
- Stavrou E.X., Fang C., Merkulova A., et al. (2015). Reduced thrombosis in Klkb1−/− mice is mediated by increased Mas receptor, prostacyclin, Sirt1, and KLF4 and decreased tissue factor. Blood 125, 710–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Zheng B., Zhang X.-H., et al. (2014). PPAR-γ agonist stabilizes KLF4 protein via activating Akt signaling and reducing KLF4 ubiquitination. Biochem. Biophys. Res. Commun. 443, 382–388. [DOI] [PubMed] [Google Scholar]
- Sunaga H., Matsui H., Anjo S., et al. (2016). Elongation of long-chain fatty acid family member 6 (Elovl6)-driven fatty acid metabolism regulates vascular smooth muscle cell phenotype through AMP-activated protein kinase/Krüppel-like factor 4 (AMPK/KLF4) signaling. J. Am. Heart Assoc. 5, e004014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Sawaki D., Aizawa K., et al. (2009). Kruppel-like factor 5 shows proliferation-specific roles in vascular remodeling, direct stimulation of cell growth, and inhibition of apoptosis. J. Biol. Chem. 284, 9549–9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., and Yamanaka S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676. [DOI] [PubMed] [Google Scholar]
- Turner E.C., Huang C.L., Govindarajan K., et al. (2013). Identification of a Klf4-dependent upstream repressor region mediating transcriptional regulation of the myocardin gene in human smooth muscle cells. Biochim. Biophys. Acta 1829, 1191–1201. [DOI] [PubMed] [Google Scholar]
- Turner J., and Crossley M. (1998). Cloning and characterization of mCtBP2, a co-repressor that associates with basic Kruppel-like factor and other mammalian transcriptional regulators. EMBO J. 17, 5129–5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Thienen J.V., Fledderus J.O., Dekker R.J., et al. (2006). Shear stress sustains atheroprotective endothelial KLF2 expression more potently than statins through mRNA stabilization. Cardiovasc. Res. 72, 231–240. [DOI] [PubMed] [Google Scholar]
- Villarreal G. Jr., Zhang Y., Larman H.B., et al. (2010). Defining the regulation of KLF4 expression and its downstream transcriptional targets in vascular endothelial cells. Biochem. Biophys. Res. Commun. 391, 984–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walshe T.E., dela Paz N.G., and D’Amore P.A. (2013). The role of shear-induced transforming growth factor-β signaling in the endothelium. Arterioscler. Thromb. Vasc. Biol. 33, 2608–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Han M., Zhao X.-M., et al. (2008). Krüppel-like factor 4 is required for the expression of vascular smooth muscle cell differentiation marker genes induced by all-trans retinoic acid. J. Biochem. 144, 313–321. [DOI] [PubMed] [Google Scholar]
- Wang N., Miao H., Li Y.S., et al. (2006). Shear stress regulation of Kruppel-like factor 2 expression is flow pattern-specific. Biochem. Biophys. Res. Commun. 341, 1244–1251. [DOI] [PubMed] [Google Scholar]
- Wang W., Ha C.H., Jhun B.S., et al. (2010). Fluid shear stress stimulates phosphorylation-dependent nuclear export of HDAC5 and mediates expression of KLF2 and eNOS. Blood 115, 2971–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.Q., Nigro P., World C., et al. (2012. a). Thioredoxin interacting protein promotes endothelial cell inflammation in response to disturbed flow by increasing leukocyte adhesion and repressing Kruppel-like factor 2. Circ. Res. 110, 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Yang C., Gu Q., et al. (2015). KLF4 promotes angiogenesis by activating VEGF signaling in human retinal microvascular endothelial cells. PLoS One 10, e0130341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhao B., Zhang Y., et al. (2012. b). Kruppel-like factor 4 is induced by rapamycin and mediates the anti-proliferative effect of rapamycin in rat carotid arteries after balloon injury. Br. J. Pharmacol. 165, 2378–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., Kurabayashi M., Shimomura Y., et al. (1999). BTEB2, a Krüppel-like transcription factor, regulates expression of the SMemb/Nonmuscle myosin heavy chain B (SMemb/NMHC-B) gene. Circ. Res. 85, 182–191. [DOI] [PubMed] [Google Scholar]
- Wu C., Huang R.T., Kuo C.H., et al. (2015). Mechanosensitive PPAP2B regulates endothelial responses to atherorelevant hemodynamic forces. Circ. Res. 117, e41–e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Bohanan C.S., Neumann J.C., et al. (2008). KLF2 transcription factor modulates blood vessel maturation through smooth muscle cell migration. J. Biol. Chem. 283, 3942–3950. [DOI] [PubMed] [Google Scholar]
- Wu W., Xiao H., Laguna-Fernandez A., et al. (2011). Flow-dependent regulation of Kruppel-like factor 2 is mediated by microRNA-92a. Circulation 124, 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yet S.-F., McA’Nulty M.M., Folta S.C., et al. (1998). Human EZF, a Krüppel-like Zinc finger protein, is expressed in vascular endothelial cells and contains transcriptional activation and repression domains. J. Biol. Chem. 273, 1026–1031. [DOI] [PubMed] [Google Scholar]
- Yin K.J., Fan Y., Hamblin M., et al. (2013). KLF11 mediates PPARγ cerebrovascular protection in ischaemic stroke. Brain 136, 1274–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Gan Q., Franke A.S., et al. (2010). Smooth and cardiac muscle-selective knock-out of Krüppel-like factor 4 causes postnatal death and growth retardation. J. Biol. Chem. 285, 21175–21184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Kaestner K.H., and Owens G.K. (2008). Conditional deletion of Krüppel-like factor 4 delays downregulation of smooth muscle cell differentiation markers but accelerates neointimal formation following vascular injury. Circ. Res. 102, 1548–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Yamashita M., and Hayashi M. (2012). Krüppel-like factor 4 contributes to high phosphate-induced phenotypic switching of vascular smooth muscle cells into osteogenic cells. J. Biol. Chem. 287, 25706–25714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Yamashita M., Horimai C., et al. (2014). Deletion of Krüppel-like factor 4 in endothelial and hematopoietic cells enhances neointimal formation following vascular injury. J. Am. Heart Assoc. 3, e000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A., Wu W., Sun W., et al. (2009). Flow activation of AMP-activated protein kinase in vascular endothelium leads to Krüppel-like factor 2 expression. Arterioscler. Thromb. Vasc. Biol. 29, 1902–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K., Zheng B., Han M., et al. (2011). ATRA activates and PDGF-BB represses the SM22α promoter through KLF4 binding to, or dissociating from, its cis-DNA elements. Cardiovasc. Res. 90, 464–474. [DOI] [PubMed] [Google Scholar]
- Zempo H., Suzuki J.-i., Ogawa M., et al. (2016). Influence of periostin-positive cell-specific Klf5 deletion on aortic thickening in DOCA-salt hypertensive mice. Hypertens. Res. 39, 764–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X.Q., Li N., Pan D.Y., et al. (2015). Kruppel-like factor 2 inhibit the angiogenesis of cultured human liver sinusoidal endothelial cells through the ERK1/2 signaling pathway. Biochem. Biophys. Res. Commun. 464, 1241–1247. [DOI] [PubMed] [Google Scholar]
- Zheng B., Han M., Bernier M., et al. (2009). Krüppel-like factor 4 inhibits proliferation by platelet-derived growth factor receptor β-mediated, not by retinoic acid receptor α-mediated, phosphatidylinositol 3-kinase and ERK signaling in vascular smooth muscle cells. J. Biol. Chem. 284, 22773–22785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Li A., Zhao L., et al. (2013). Key role of microRNA-15a in the KLF4 suppressions of proliferation and angiogenesis in endothelial and vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 437, 625–631. [DOI] [PubMed] [Google Scholar]
- Zhou G., Hamik A., Nayak L., et al. (2012). Endothelial Kruppel-like factor 4 protects against atherothrombosis in mice. J. Clin. Invest. 122, 4727–4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Tang A.T., Wong W.Y., et al. (2016). Cerebral cavernous malformations arise from endothelial gain of MEKK3-KLF2/4 signalling. Nature 532, 122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]