Abstract
Extraribosomal functions of ribosomal proteins (RPs) have gained much attention for their implications in tumorigenesis and progression. However, the regulations for transition between the ribosomal and extraribosomal functions of RPs are rarely reported. Herein, we identified a ribosomal protein S7-interacting partner, BCCIPβ, which modulates the functional conversion of S7. Through the N-terminal acidic domain, BCCIPβ interacts with the central basic region in S7 and regulates the extraribosomal distribution of S7. BCCIPβ deficiency abrogates the ribosomal accumulation but enhances the ribosome-free location of S7. This translocation further impairs protein synthesis and triggers ribosomal stress. Consequently, BCCIPβ deficiency suppresses the ribosomal function and initiates the extraribosomal function of S7, resulting in restriction of cell proliferation. Moreover, clinically relevant S7 mutations were found to dampen the interaction with BCCIPβ and facilitate the functional transition of S7. In conclusion, BCCIPβ, as a S7 modulator, contributes to the regulation of ribosomal and extraribosomal functions of S7 and has implications in cell growth and tumor development.
Keywords: BCCIPβ, ribosomal protein S7, translation, ribosomal stress, tumor suppressor
Introduction
Ribosomes, which are composed of two subunits consisting of ribosomal RNAs (rRNAs) and ribosomal proteins (RPs) (Melnikov et al., 2012), are cellular machines essential for the translation of genetic information from mRNA to protein. In cells, the biogenesis of ribosomes is an energy-consuming process. Both ribosome biogenesis and translation are highly coordinated, multistep processes that are necessary for sustained cell growth. Due to the deregulation of protein synthesis in cancer cells, the translation is considered as a potential antineoplastic target (Malina et al., 2011).
In metabolically active cells, most RPs are expressed abundantly to meet the high demand for protein synthesis. Physiologically, in normal cells under stimuli such as growth factors and nutrients, the productions of RPs and rRNAs are coordinated to balance for ribosome assembly and translation (Warner, 1999), thus maintaining proper cell growth and proliferation. Once the balance between RPs and rRNAs or the balance among different RPs is lost, ribosome biogenesis may malfunction, and the excess RPs may exert extraribosomal functions, such as induction of ribosomal stress (Warner and McIntosh, 2009). More than 17% of RPs, including L5, L11, L23, L26, S3, S7, S14, and S25 (Lohrum et al., 2003; Dai and Lu, 2004; Dai et al., 2004; Jin et al., 2004; Chen et al., 2007; Yadavilli et al., 2009; Zhu et al., 2009; Zhang et al., 2010, 2013; Zhou et al., 2013), trigger ribosomal stress through directly interacting with MDM2, inhibiting MDM2-mediated ubiquitination of p53, subsequently causing p53 accumulation, and ultimately resulting in cell cycle arrest or apoptosis (Kim et al., 2014). Through the variation of nucleolar integrity, RPs can be released from nucleolus to nucleoplasm and perform the extraribosomal functions, thus responding to diverse types of cellular stress (Rubbi and Milner, 2003).
Deregulation of RPs, which are caretakers for cellular stress and growth, increases cancer risk and plays important roles in cancer development (Goudarzi and Lindstrom, 2016). Abnormal expressions or mutations in RP genes are observed in human malignancies (de Las Heras-Rubio et al., 2014). However, the identities of regulators and the mechanism for the transition between ribosomal and extraribosomal function of RPs remain largely unclear. In the present study, we have found that BCCIPβ, a tumor suppressor involved in the maintenance of genomic integrity, binds to and modulates the function of ribosomal protein S7.
BCCIP is a tumor suppressor originally identified as a protein interacting with BRCA2 and p21CDKN1A/CIP1 (Ono et al., 2000; Liu et al., 2001). Downregulation of BCCIP has been observed in several types of cancer (Roversi et al., 2006; Liu et al., 2009, 2013). BCCIP deficiency causes homologous recombination defects, spontaneous chromatid aberrations, cytokinesis failure, and cell cycle dysregulation (Meng et al., 2004, 2007; Lu et al., 2007, 2011). Due to the alternative pre-mRNA splicing, two major isoforms BCCIPα and BCCIPβ are expressed in human cells (Meng et al., 2003). Only BCCIPβ exists in mouse tissues (Fan et al., 2009). The two isoforms share the identical N-terminal (amino acids 1−258) and differ in the C-terminal domains, comprising 64 and 56 amino acids in BCCIPα and BCCIPβ, respectively (Meng et al., 2003). Both BCCIPα and BCCIPβ localize in the cell nucleus (Ono et al., 2000). Although the two isoforms have much in common in terms of the biochemical characteristics, they perform some preferential functions (Meng et al., 2004; Phillips-Mason et al., 2008; Wyler et al., 2014).
In the present study, we discovered that BCCIPβ, but not BCCIPα, binds to ribosomal protein S7 through electrostatic interactions. Further analysis revealed that BCCIPβ modulates the ribosomal and extraribosomal functions by affecting the ribosome association and nonribosomal localization of S7. These findings suggest a novel regulatory role of BCCIPβ in the activity shift of RPs during the processes of translation and ribosomal stress.
Results
The interaction between BCCIPβ and S7
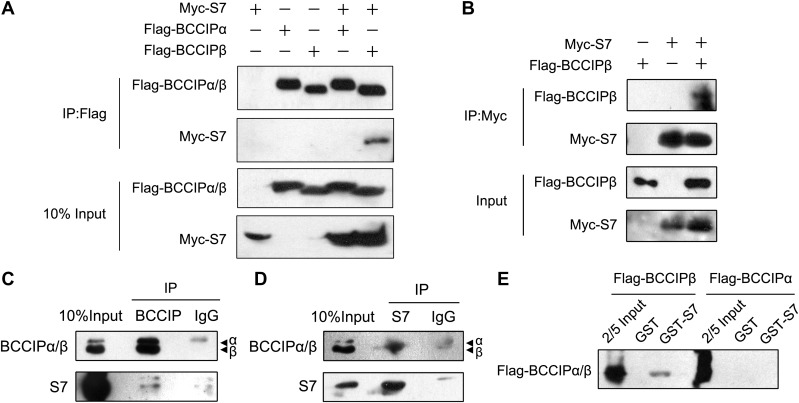
To investigate the potential interaction between BCCIP and S7, we performed co-immunoprecipitation experiments in HEK293T cells by coexpressing Flag-BCCIPα/β and Myc-S7 proteins. Although sharing most protein sequences, BCCIPα and BCCIPβ exhibited different properties in protein interaction. Exogenously expressed BCCIPβ, but not BCCIPα, pulled down S7 from the protein supernatant (Figure 1A), indicating that S7 binds specifically to BCCIPβ. Similarly, BCCIPβ was immunoprecipitated by exogenous S7 (Figure 1B). The interaction between BCCIPβ and S7 was confirmed by endogenous co-immunoprecipitation. In MCF7 cells, both endogenous BCCIPβ and S7 pulled down each other mutually (Figure 1C and D). To determine whether BCCIPβ binds to S7 directly, purified GST-S7 was incubated with Flag-BCCIPα/β proteins, which were translated in vitro, and the GST-pulldown assay was performed. Consistently, BCCIPβ, but not BCCIPα, was pulled down by GST-S7 (Figure 1E). This result demonstrates different S7-binding capacities between BCCIPα and BCCIPβ and shows that BCCIPβ binds to S7 directly.
Figure 1.
BCCIPβ interacts with S7 in vitro and in vivo. (A and B) HEK293T cells were transfected with plasmids expressing Myc-S7, Flag-BCCIPβ, or Flag-BCCIPα for 48 h. Cell lysates were then immunoprecipitated with Flag or Myc antibodies, and the bound proteins were detected by western blotting. (C and D) The lysates of MCF7 cells were immunoprecipitated with nonimmune IgG or an antibody against BCCIP (C) or S7 (D), and the bound proteins were detected by western blotting. (E) GST-S7 was incubated with in vitro-translated Flag-BCCIPα or Flag-BCCIPβ at 4°C overnight. The potential protein complex was separated using GST beads and detected by western blotting with Flag antibody.
Identification of the binding domains and critical residues
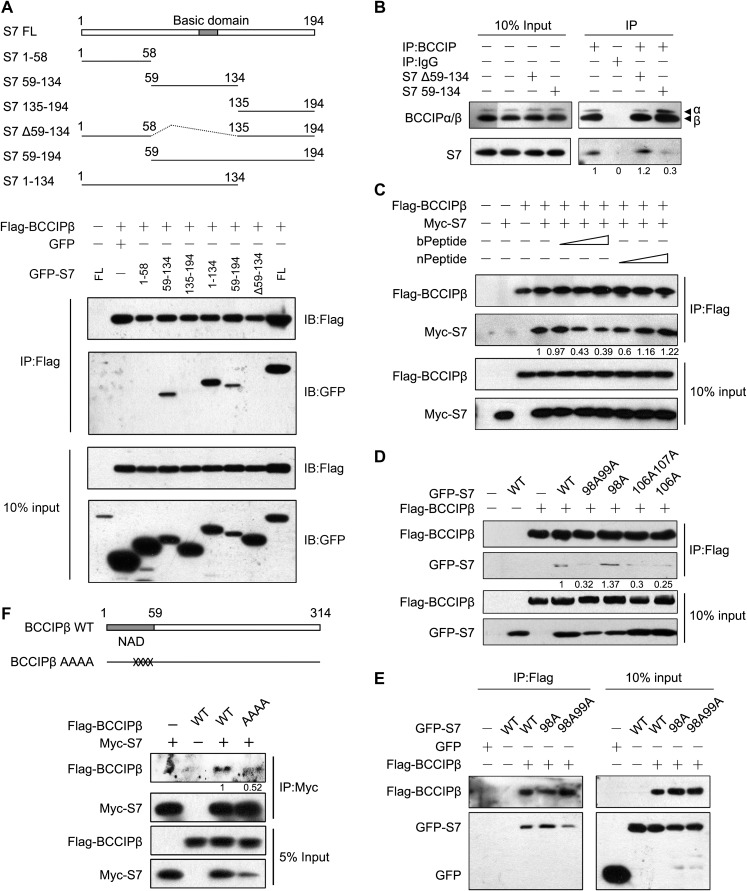
To identify the BCCIPβ-binding domains in S7, a panel of truncated fragments of S7 was constructed and co-immunoprecipitated with BCCIPβ. As shown in Figure 2A, Flag-BCCIPβ co-precipitates all GFP-S7 fragments on the panel, except S7(1–58), S7(135–194), and S7(Δ59–134), suggesting that amino acids 59−134 (the central region) of S7 are critical for the binding to BCCIPβ. To confirm this, S7(59–134) and S7(Δ59-134) fragments were overexpressed in MCF7 cells, and their potential interference with the interaction between endogenous BCCIPβ and S7 was assessed. As shown in Figure 2B, the expression of S7(Δ59-134) did not affect the binding between endogenous proteins, but S7(59-134) reduced the amount of endogenous S7 co-precipitated with BCCIPβ, supporting the concept that the sequence including amino acids 59−134 is involved in the interaction.
Figure 2.
Mapping the binding domains and critical sites in BCCIPβ and S7. (A) HEK293T cells were co-transfected with Flag-BCCIPβ and GFP-tagged full-length and truncated S7 plasmids for 48 h. Whole-cell lysates were subjected to immunoprecipitation with Flag antibody followed by western blotting. (B) MCF7 cells were transfected with GFP-S7 truncated mutants for 48 h, and the endogenous BCCIPβ−S7 interactions were determined by immunoprecipitation with BCCIP antibody. (C) The cell lysates from HEK293T cells transfected with Flag-BCCIPβ and Myc-S7 were subjected to immunoprecipitation in the presence of bPeptide or nPeptide. (D and E) Flag-BCCIPβ with wild-type or mutant GFP-S7 was expressed in HEK293T cells, and the binding capacity of S7 mutants was determined by sequential immunoprecipitation and western blotting. (F) Myc-S7 with wild-type or mutant Flag-BCCIPβ was expressed in HEK293T cells, and the S7-binding proteins were determined by immunoprecipitation with Myc antibody.
In the sequence including amino acids 59−134 in S7, there is an Arg/Lys-rich basic domain (a nucleolar localization sequence, NoLS). We suspected that the basic domain in S7 participates in its interaction with BCCIPβ. Thus, we synthesized a basic-amino-acid-rich peptide (bPeptide) and a control peptide with the basic residues mutated to alanine (nPeptide), and then determined their inhibitory effects on BCCIPβ−S7 interaction. The bPeptide weakened the binding between BCCIPβ and S7, whereas the nPeptide showed little effect (Figure 2C), suggesting that the central basic domain in S7 likely mediates the interaction.
To explore the binding mode in more detail, we utilized a structural simulation (Roy et al., 2010) to yield potential three-dimensional structures of S7 and BCCIPβ. In the central domain of S7 protein, an extension arm with positive charge was identified (Supplementary Figure S1A), which located in the BCCIPβ-binding region. There was a groove domain with negative charge in the N-terminal of BCCIPβ protein (Supplementary Figure S1A). Interestingly, the protein docking model predicted that the positively charged S7 arm could bind with the negatively charged BCCIPβ groove (Supplementary Figure S1B).
This information was utilized as guidance to identify the critical residues involved in BCCIPβ−S7 interaction. Based on the simulated docking interface, we predicted the amino acids that are critical for the interaction and locate in the central basic domain of S7 or the N-terminus acidic domain (NAD) of BCCIPβ (Supplementary Figure S1C). Accordingly, several site-specific S7 and BCCIPβ mutants were constructed, and their binding capacities were determined. The R106A and K107A mutations in S7 decreased the binding with BCCIPβ, and the R98A mutant had a slightly enhanced interaction (Figure 2D and E). For the BCCIPβ protein, the mutation of four acidic amino acids (DEED, 47−50) to alanine (AAAA) reduced the binding between BCCIPβ and S7 (Figure 2F). These results suggest that the positively charged central region in S7 and the negatively charged N-terminal of BCCIPβ are responsible for their interaction.
BCCIPβ deficiency suppresses ribosomal localization of S7 and the translation process
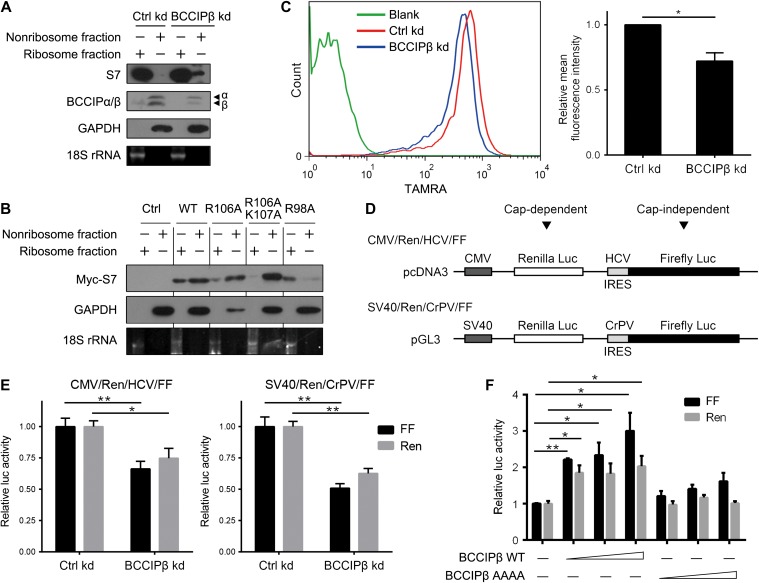
With a lentiviral shRNA expression system, a stable BCCIPβ knockdown (BCCIPβ kd) was constructed in U2OS cells. After BCCIPβ was knocked down, there was an increase in ribosome-free level of endogenous S7, which was unbound with the small subunit (Figure 3A). For the S7 mutants with weaker BCCIPβ interaction, such as R106A and R106AK107A, the amount of S7 in the nonribosome fraction was enhanced, and that in ribosomes was diminished (Figure 3B). However, the S7 R98A mutant, which bound more strongly to BCCIPβ, displayed predominant localization in the ribosome fraction whereas less in the nonribosome fraction compared with wild-type S7 (Figure 3B). These results indicate that the interaction with BCCIPβ is required for ribosomal localization of S7.
Figure 3.
BCCIPβ is required for the ribosomal localization of S7 and cap-dependent/independent translations. (A) Cell extracts from control and BCCIPβ kd U2OS cells were fractionated through sucrose (20%) centrifugation. The proteins (S7, BCCIPα/β, and GAPDH) in nonribosome and ribosome fractions were detected by western blotting, and 18S rRNA was detected by electrophoresis. (B) HEK293T cells were transfected with wild-type or mutant Myc-S7 for 24 h. The proteins in nonribosome and ribosome fractions were separated and subjected to western blotting. (C) The newly synthesized proteins in control and BCCIPβ kd U2OS cells were labeled with AHA, click tagged with TAMRA, and analyzed by flow cytometry (left panel). The relative fluorescent intensity was determined (right panel). (D) Schematic representation of bicistronic reporter constructs with the indicated elements. (E) Control and BCCIPβ kd U2OS cells were transfected with the indicated bicistronic reporter plasmids, and Renilla and firefly luciferase activities in cell lysates were measured. (F) MCF7 cells were transfected with the bicistronic reporter construct (SV40/Ren/CrPV/FF) and BCCIPβ wild-type or mutant expression plasmids for 24 h, and Renilla and firefly luciferase activities in cell lysates were measured. The results are representative of at least three different experiments. *P < 0.05; **P < 0.01.
Considering the role of ribosomes in the process of translation, we examined the influences of BCCIPβ knockdown on global protein synthesis. A Click-iT® protein reaction system was applied to quantify the activity of nascent protein synthesis in control and BCCIPβ-deficient cells. Compared with the control, the amount of nascent protein was reduced in BCCIPβ kd cells (Figure 3C). Moreover, two kinds of bicistronic luciferase reporter plasmids were employed to assess the cap-dependent and independent translation (Figure 3D) (Bordeleau et al., 2008). In BCCIPβ kd cells, both the promoter-mediated Renilla luciferase and the internal ribosome entry site (IRES)-mediated firefly luciferase productions were inhibited (Figure 3E). This observation reflected the translation activity, since there were no changes in luciferase mRNA levels (Supplementary Figure S2A) or total cellular proteins (Supplementary Figure S2B). Further, overexpression of BCCIPβ enhanced cap-dependent and independent protein productions (Figure 3F). Interestingly, overexpression of mutated BCCIPβ with compromised S7-binding capacity (AAAA) showed little effect on translation (Figure 3F), suggesting that BCCIPβ upregulates protein synthesis through interaction with S7.
BCCIPβ deficiency triggers ribosomal stress and induces p53 signaling
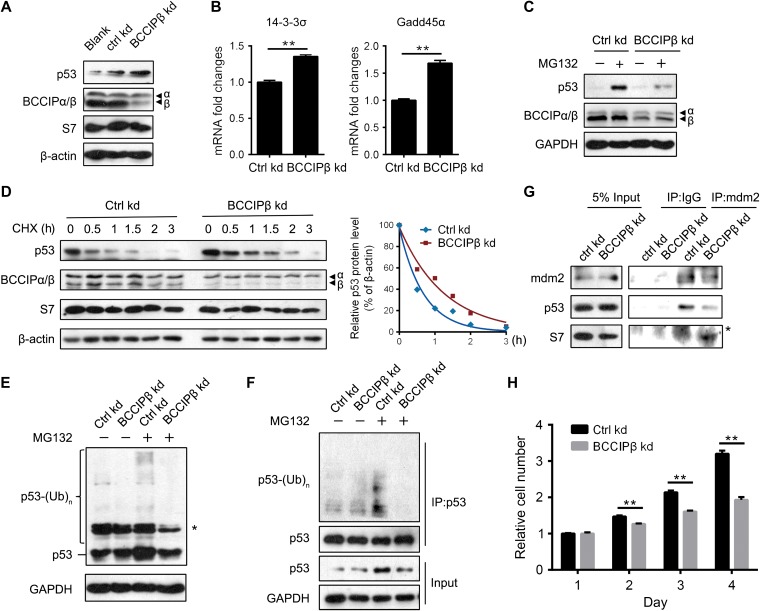
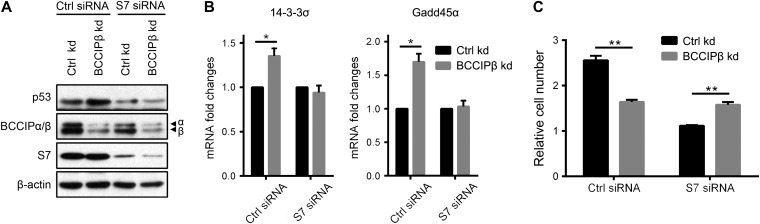
Extraribosomal S7 mediates ribosomal stress through binding to MDM2 and inhibiting the degradation of p53 (Chen et al., 2007; Zhu et al., 2009). We next examined whether the BCCIPβ deficiency-induced increase in free S7 triggers ribosomal stress. After BCCIPβ was knocked down, the protein level of p53 was elevated, and expressions of its target genes, 14-3-3σ and Gadd45α, were also enhanced (Figure 4A and B), indicating that p53 signaling is activated in BCCIPβ-deficient cells. In control cells, blockage of p53 degradation by the proteasome inhibitor MG132 raised the level of p53; however, in BCCIPβ kd cells, MG132 induced a smaller increase in p53 (Figure 4C), suggesting that less degradation of p53 was existed and thus blocked. Further, in BCCIPβ kd cells, the half-life of the p53 protein was longer (Figure 4D). These results indicate that BCCIPβ deficiency-elevated p53 expression is attributed to suppressed degradation and enhanced stability. Moreover, after BCCIPβ knockdown, the ubiquitination of p53 was inhibited, as detected by both direct immunoblotting (Figure 4E) and immunoprecipitation (Figure 4F). With depletion of BCCIPβ, the binding between p53 and its E3 ligase, MDM2, was weakened, whereas the binding between MDM2 and S7 was enhanced (Figure 4G). These results demonstrate that BCCIPβ deficiency inhibits MDM2-mediated p53 ubiquitination and degradation. Furthermore, the influence of BCCIPβ depletion on cell growth was determined. Consistently, the proliferation of BCCIPβ-deficient cells was slower compared with the control (Figure 4H).
Figure 4.
BCCIPβ depletion triggers ribosomal stress. (A) Total proteins from control and BCCIPβ kd cells were extracted and subjected to western blotting. (B) Total mRNA levels of p53 target genes in control and BCCIPβ kd cells were determined by real-time PCR. (C) After 4 h incubation with DMSO or MG132 (50 μM), control and BCCIPβ kd cells were harvested, and target proteins were detected by western blotting. (D) Control and BCCIPβ kd cells were exposed to the protein synthesis inhibitor CHX (10 mg/ml) for various times, and target proteins were detected by western blotting (left panel). Relative p53 levels at different time points were plotted (right panel). (E and F) Ubiquitinated p53 in control and BCCIPβ kd cells was detected either directly by western blotting with p53 antibody (E) or separated by immunoprecipitation with p53 antibody, followed by detection via western blotting with ubiquitin antibody (F). Asterisk indicates nonspecific band. (G) Cell lysates from control and BCCIPβ kd cells were immunoprecipitated with mouse IgG or MDM2 antibody, followed by western blotting. Asterisk indicates nonspecific band. (H) The proliferation of control and BCCIPβ kd U2OS cells was examined by CCK-8. The results are representative of three different experiments. **P < 0.01.
The above data suggest that the free form of S7 induced by BCCIPβ depletion is responsible for ribosome stress. To validate the role of S7 in BCCIPβ deficiency-induced p53 activation and cell growth suppression, we simultaneously interfered with S7 expression and found that S7 knockdown attenuated the influence of BCCIPβ on p53 protein and its target genes (Figure 5A and B). Similarly, when the expression of S7 was inhibited, BCCIPβ depletion no longer suppressed the growth of cells (Figure 5C), indicating that S7 mediates the effect of BCCIPβ deficiency on p53 signaling and cell growth.
Figure 5.
S7 knockdown rescues the effect of BCCIPβ depletion on p53 signaling and cell growth. (A) Control and BCCIPβ kd cells were transfected with control siRNA or S7 siRNA for 48 h, and target proteins were detected by western blotting. (B) Control and BCCIPβ kd cells were transfected with control siRNA or S7 siRNA for 48 h, and mRNA levels of target genes were determined by real-time PCR. (C) Control and BCCIPβ kd cells were transfected with control siRNA or S7 siRNA, and cell growth after 3 days was examined by CCK-8. The results are representative of three different experiments. *P < 0.05; **P < 0.01.
BCCIPβ−S7 interaction in cancer development
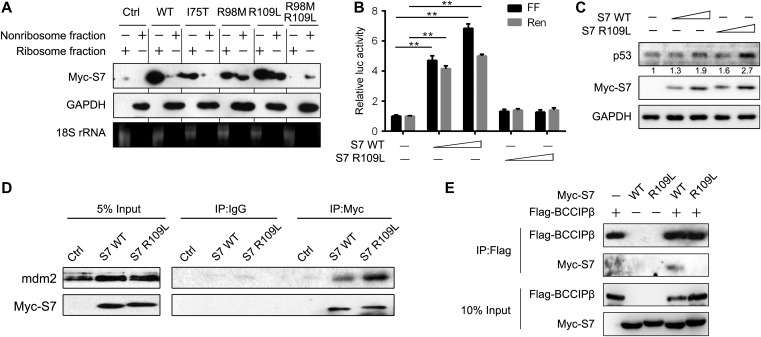
To explore the clinical relevance of BCCIPβ−S7 interaction, we determined the influence of several site mutations of S7, which were identified as somatic mutations in clinical cancer samples by the Catalogue of Somatic Mutations in Cancer (COSMIC) database, on BCCIPβ−S7 interaction, translation, and ribosomal stress. As shown in Figure 6A, mutations of basic amino acids (R98M and R109L) increased nonribosomal levels of mutated S7 protein. The R109L mutant was further analyzed due to the comparative protein expression level with wild-type S7. Consistent with the alteration of ribosomal localization, the R109L mutant only weakly prompted the protein synthesis (Figure 6B) but induced more accumulation of p53 (Figure 6C). The R109L mutant bound more readily to MDM2 compared with the wild-type protein (Figure 6D), suggesting that the S7 R109L mutant bears a stronger triggering capacity on ribosomal stress. Such weak translation-promoting and strong ribosomal stress-inducing effects were similar to that of BCCIPβ deficiency. As expected, the binding capacity of the S7 R109L mutant to BCCIPβ was reduced (Figure 6E), further implicating a role of BCCIPβ−S7 interaction in the functional transition of S7 during cancer development.
Figure 6.
The clinically identified mutation in S7 and BCCIPβ−S7 interaction. (A) HEK293T cells were transfected with wild-type and mutant Myc-S7 for 24 h. The proteins in nonribosome and ribosome fractions were separated and subjected to western blotting, and 18S rRNA was detected by electrophoresis. (B) MCF7 cells were transfected with the bicistronic reporter construct (SV40/Ren/CrPV/FF) and S7 wild-type or mutant expression plasmids for 24 h, and Renilla and firefly luciferase activities in cell lysates were measured. The results are representative of three different experiments. **P < 0.01. (C) MCF7 cells were transfected with wild-type or mutant S7 plasmids, and target proteins were detected by western blotting. (D) MCF7 cells were transfected with wild-type or mutant S7 plasmids, and cell lysates were immunoprecipitated with mouse IgG or Myc antibody followed by western blotting. (E) Flag-BCCIPβ with wild-type or mutant Myc-S7 were expressed in HEK293T cells, and binding proteins were determined by immunoprecipitation with Flag antibody.
In addition, we observed a lower expression of BCCIPβ protein in liver cancer tissues compared with adjacent tissues (Supplementary Figure S3). Furthermore, exogenous expression of CagA, the virulence factor of Helicobacter pylori, in human gastric epithelium cells decreased the level of BCCIPβ (Supplementary Figure S4A). Exogenous expression of HBx, the viral protein of Hepatitis B virus, caused a reduction in BCCIPβ expression in human liver cells (Supplementary Figure S4B). Consistently, the nonribosomal fraction of S7 was increased after CagA and HBx overexpression (Supplementary Figure S4C and D). These results suggest that BCCIPβ and its interaction with S7 may be involved in carcinogenesis and development.
Discussion
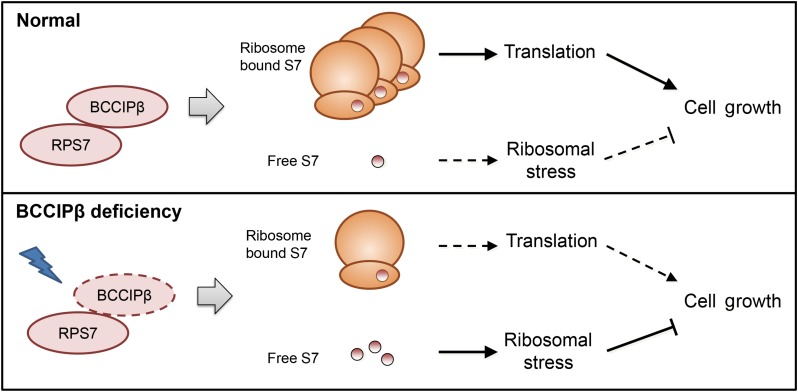
RPs, which maintain a balance between protein synthesis and other functions, are involved in diverse cellular physiological processes. In the present study, we identified a novel S7-interacting protein, BCCIPβ, which regulates the ribosomal localization of S7 and modulates its ribosomal and extraribosomal functions. Under normal physiological conditions, S7 localizes mainly in ribosomes and participates in the translation process to support the cell growth. Under the condition of insufficient BCCIPβ, which can be induced by various stimuli, the ribosome-associated S7 was reduced and the extraribosomal S7 was increased. Such a change compromises the translation process, elicits the ribosomal stress, and subsequently results in blockage of cell growth (Figure 7).
Figure 7.
Proposed mode of action for BCCIPβ−S7 interaction. BCCIPβ acts as an S7-interacting protein to regulate the ribosome association and extraribosomal distribution, to modulate the balance between ribosomal and extraribosomal functions of S7, and to support cell growth.
We found that BCCIPβ bound to S7 through electrostatic interactions between the basic region in S7 and the NAD region in BCCIPβ. Considering the prevalence of basic regions in RPs (NoLS sequences), the interaction between BCCIPβ and RPs is likely not rare. Thus, BCCIPβ may be involved in regulating the conversion of ribosomal and extraribosomal functions through binding to various RPs. This would allow exploration of the regulatory mechanism for ribosome functions and merits further investigations. The ribosomal stress mediator, MDM2, which has a central acidic domain, also binds to RPs through the electrostatic interactions. Seventeen percent of RPs interact with MDM2 (Kim et al., 2014), suggesting that electrostatic interaction might be an important factor in regulation of RPs. Unlike MDM2, which is inhibited by RPs to activate p53 and thereby mediates downstream effects in the process of ribosomal stress, BCCIPβ elicits the upstream signaling of RPs. Sensing external stimuli, BCCIPβ could alter its expression levels and fine tune the balance of RPs with a different distribution. On the other hand, MDM2 has a feedback effect to ubiquitinate several RPs, including S7, L26, S27L, and S27a (Ofir-Rosenfeld et al., 2008; Zhu et al., 2009; Sun et al., 2011; Xiong et al., 2011). The similar binding mode of BCCIPβ and MDM2 to RPs raises the possibility that BCCIPβ protects ribosome-free S7 from MDM2-mediated ubiquitination and degradation. The stability of extraribosomal L23 can be enhanced by BCCIPβ (Wyler et al., 2014); nevertheless, the involvement of MDM2 in this process and the similar effect of BCCIPβ on other RPs, such as S7 and L26, should be explored.
The interaction between BCCIPβ and L23 has been described. BCCIPβ could stabilize overexpressed L23, and BCCIPβ depletion destabilizes extraribosomal L23 (Wyler et al., 2014). However, in our study, BCCIPβ depletion increased extraribosomal S7 and induced ribosomal stress, suggesting different effects of BCCIPβ on L23 and S7. Further, the binding region of L23 in BCCIPβ is the C-terminal domain, which is different from that of S7. We also found that the BCCIPβ mutant with low S7-binding capacity (AAAA) showed a similar interaction with L23 (data not shown). We did not observe the binding between BCCIPβ and L11. When we interfered with the expression of L11 or L23, we found that L11 knockdown, but not L23 knockdown, attenuated the influence of BCCIPβ on cell growth (data not shown), which may be due to the important role of L11 in ribosomal stress. These results suggest that BCCIPβ is likely to bind to various RPs; however, the binding strength, binding domain, and function may be different.
BCCIPα/β has paradoxical roles in tumorigenesis and progression (Huang et al., 2013). On one hand, BCCIP maintains genome integrity and functions as a caretaker to suppress tumor initiation. On the other hand, BCCIP is required for the growth of transformed malignant cells. This manifests a reduced function of BCCIP in early-stage tumors, which is restored or even enhanced in fully developed tumors (Huang et al., 2013). This phenomenon can also be observed in RPs. Various RPs show elevated expressions in different types of tumors; some of them, including L5, S3, and S14, act as tumor suppressors by mediating ribosomal stress (Wang et al., 2009; Warner and McIntosh, 2009). In regard to S7, it binds to MDM2, stabilizes p53, and activates the ribosomal stress process, thus acting as a tumor suppressor. However, we have found that the expression of S7 is elevated in breast and gastric cancers (data not shown). These similarities imply that there are links between BCCIP and S7. The current study shows that BCCIPβ binds to and regulates ribosomal protein S7, which provides a possible explanation for previous observations.
For most part, BCCIPα and BCCIPβ share a common amino acid sequence except for the C-terminal. The S7-binding domain in BCCIPβ is found in the NAD region, which possesses an amino acid sequence identical to that in BCCIPα. However, only BCCIPβ interacts with S7. Consistently with our results, different interactions of BCCIPα and BCCIPβ with other proteins have been observed (Meng et al., 2004; Phillips-Mason et al., 2008; Wyler et al., 2014). These findings suggest that the stereostructures of BCCIPα and BCCIPβ may be distinct, which is consistent with our simulation results (see Supplementary Results and Figure S1A), and thus perform their specific functions. In mice, the BCCIP protein appears in only one isoform, BCCIPβ, suggesting a more conserved function of this protein. Yet most functional studies on BCCIP cannot differentiate the two isoforms. Our results demonstrate that BCCIPβ, but not BCCIPα, is involved in the functional modulation of ribosomes.
In summary, our results show that BCCIPβ binds to the central region of S7, maintains the ribosome association of S7, modulates the transition between ribosomal and extraribosomal functions, and supports cell growth. Acting as a modulator of RPs, BCCIPβ may be involved in determination of cell proliferation under normal physiological conditions or growth arrest in unfavorable or abnormal environments. The present findings provide a better understanding of the contribution of BCCIPβ and S7 to tumorigenesis and progression.
Materials and methods
Cell culture and reagents
All cells were obtained from the Cell Bank of the Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 100 μg/ml penicillin, and 100 μg/ml streptomycin and maintained in a humidified incubator at 37°C and 5% CO2. Cell growth was determined using Cell Counting Kit-8 (CCK-8, Dojindo). MG132 and cycloheximide (CHX) were purchased from Sigma-Aldrich, Inc.
Plasmids and siRNA transfection
Cells were transfected with the indicated plasmids by FuGene HD or siRNA by Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's instructions. BCCIPβ and S7 expression plasmids were described previously (Chen et al., 2007; Lu et al., 2007). Site mutants of BCCIPβ and S7 were made using the Fast Mutagenesis System (TransGene). Bicistronic luciferase reporter plasmids pGL3-Ren-CrPV-FF (SV40/Ren/CrPV/FF) and pcDNA3-Ren-HCV-FF (CMV/Ren/HCV/FF) were kindly provided by Dr Jing Fang (Bordeleau et al., 2008; Zhao et al., 2013). The siRNA was purchased from Invitrogen and the sequences were: Ctrl siRNA, forward strand: UUCUCCGAACGUGUCACGUtt, reverse strand: ACGUGACACGUUCGGAGAAtt; S7 siRNA, forward strand: UGCUCUCGCGAGAUUUGGGUCUCUUtt, reverse strand: AAGAGACCCAAAUCUCGCGAGAGCAtt.
Western blotting, immunoprecipitation, and GST-pulldown
Western blotting, immunoprecipitation, and GST-pulldown assays were performed as described previously (Chen et al., 2007). Myc and Flag antibodies were obtained from Cell Signaling Technology Inc. Anti-p53 and anti-MDM2 were from Calbiochem. Anti-human β-actin was from Sigma-Aldrich, Inc. Anti-BCCIP was from BD PharMingen, Anti-BCCIPβ was from Santa Cruz Biotechnology. The antibodies against S7, GFP, ubiquitin, and GAPDH, normal mouse IgG, normal rabbit IgG, and Glutathione-Agarose were purchased from Santa Cruz Biotechnology. Protein G Sepharose was from GE Healthcare. Anti-Flag M2 Affinity Gel was from Sigma-Aldrich, Inc. The peptides were synthesized by GeneScript and the sequences were as follows: bPeptide, TKKRKMVEMLEKKRKKKKIK; nPeptide, TKAAAMVEMLEKKRKAAAIK.
Ribosome fractionation
Ribosome and nonribosome fractions were collected as described (Mazumder et al., 2003). In brief, 5×108 cells were lysed in 5 ml PB buffer (20 mM Tris–HCl, pH 7.4, 10 mM MgCl2, 300 mM KCl, 0.5% NP-40, 10 mM DTT, 100 U/ml RNasin, 100 μg/ml CHX, and protease inhibitor cocktail) and centrifuged at 10000× g for 15 min to remove mitochondria and debris. The supernatant was loaded on a 20% (w/v) sucrose cushion in PB buffer and centrifuged at 149000× g for 2 h. The ribosome pellet was collected, and the nonribosome supernatant was subjected to a second centrifugation to remove ribosome contaminants. For protein analysis, ribosome pellets and concentrated nonribosome fractions by trichloroacetic acid precipitation were suspended in Laemmli's buffer and subjected to western blotting. For RNA analysis, total RNA in both fractions was extracted by Trizol, and the 18S rRNA was resolved by electrophoresis.
Newly synthesized protein analysis
Nascent protein synthesis was measured by Click-iT® protein reaction system (Invitrogen) with L-azidohomoalanine (AHA) as the metabolic labeling regent and tetramethylrhodamine (TAMRA) as the detection regent according to the manufacturer's protocol. Briefly, cells were washed with warm phosphate-buffered saline, incubated with methionine-free DMEM for 30 min to deplete the methionine reserves, and labeled with AHA (25 μM) at 37°C, 5% CO2 for 1 h. The cells were then harvested, fixed with 4% paraformaldehyde, permeabilized with 0.25% Triton X-100, and subjected to the Click reaction using TAMRA as the detection reagent. The nascent protein levels were determined by flow cytometry.
Cap-dependent and independent translation assay
Cap-dependent and cap-independent translations were determined by luciferase reporter assay. Cells were seeded at a sub-confluent density and transfected with the bicistronic luciferase reporter plasmids. After 24 h, cells were harvested and lysed. The luciferase light units were measured with the Dual-Luciferase Reporter Assay System according to the manufacturer's protocol. The total protein levels and the luciferase mRNA levels were also measured as internal controls for transfection efficiency.
Real-time polymerase chain reaction
Total RNA was extracted using Trizol Reagent (Invitrogen) and reverse-transcribed by the PrimeScriptTM RT Reagent Kit. Real-time polymerase chain reaction (PCR) was performed using the CFX96TM Real-Time PCR Detection System (Bio-Rad). The dissociation curve was obtained to verify the specificity of the amplification. The primer sequences for real-time PCR were as follows: 14-3-3σ, forward: 5′-CCAGGCTACTTCTCCCCTC-3′, reverse: 5′-CTGTCCAGTTCTCAGCCACA-3′; Gadd45α, forward: 5′-AGGAAGTGCTCAGCAAAGCC-3′, reverse: 5′-GCACAACACCACGTTATCGG-3′; β-actin, forward: 5′-GGCGGCACCACCATGTACCCT-3′, reverse: 5′-AGGGGCCGGACTCGTCATACT-3′.
Statistical analysis
All data are presented as mean ± SEM. The statistical significance of differences was examined using ANOVA or Student's t-test. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgements
The authors thank Dr Donald L. Hill (Comprehensive Cancer Center, University of Alabama, Birmingham) for kind assistance in editing this manuscript.
Supplementary material
Supplementary material is available at Journal of Molecular Cell Biology online.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81573161, 81630086, and 81427805), the Key Research Program (ZDRW-ZS-2017-1), CAS/SAFEA International Partnership Program for Creative Research Teams of the Chinese Academy of Sciences, the Science and Technology Commission of Shanghai Municipality (16391903700), and the National Institutes of Health (NIH) (R01 CA156706 and R01 CA195612).
Conflict of interest: none declared.
References
- Bordeleau M.E., Robert F., Gerard B., et al. (2008). Therapeutic suppression of translation initiation modulates chemosensitivity in a mouse lymphoma model. J. Clin. Invest. 118, 2651–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Zhang Z., Li M., et al. (2007). Ribosomal protein S7 as a novel modulator of p53-MDM2 interaction: binding to MDM2, stabilization of p53 protein, and activation of p53 function. Oncogene 26, 5029–5037. [DOI] [PubMed] [Google Scholar]
- Dai M.S., and Lu H. (2004). Inhibition of MDM2-mediated p53 ubiquitination and degradation by ribosomal protein L5. J. Biol. Chem. 279, 44475–44482. [DOI] [PubMed] [Google Scholar]
- Dai M.S., Zeng S.X., Jin Y., et al. (2004). Ribosomal protein L23 activates p53 by inhibiting MDM2 function in response to ribosomal perturbation but not to translation inhibition. Mol. Cell. Biol. 24, 7654–7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Las Heras-Rubio A., Perucho L., Paciucci R., et al. (2014). Ribosomal proteins as novel players in tumorigenesis. Cancer Metastasis Rev. 33, 115–141. [DOI] [PubMed] [Google Scholar]
- Fan J., Wray J., Meng X., et al. (2009). BCCIP is required for the nuclear localization of the p21 protein. Cell Cycle 8, 3019–3024. [PMC free article] [PubMed] [Google Scholar]
- Goudarzi K.M., and Lindstrom M.S. (2016). Role of ribosomal protein mutations in tumor development (Review). Int. J. Oncol. 48, 1313–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.Y., Dai L., Gaines D., et al. (2013). BCCIP suppresses tumor initiation but is required for tumor progression. Cancer Res. 73, 7122–7133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin A., Itahana K., O'Keefe K., et al. (2004). Inhibition of HDM2 and activation of p53 by ribosomal protein L23. Mol. Cell. Biol. 24, 7669–7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.H., Leslie P., and Zhang Y. (2014). Ribosomal proteins as unrevealed caretakers for cellular stress and genomic instability. Oncotarget 5, 860–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Lu H., Ohgaki H., et al. (2009). Alterations of BCCIP, a BRCA2 interacting protein, in astrocytomas. BMC Cancer 9, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Yuan Y., Huan J., et al. (2001). Inhibition of breast and brain cancer cell growth by BCCIPalpha, an evolutionarily conserved nuclear protein that interacts with BRCA2. Oncogene 20, 336–345. [DOI] [PubMed] [Google Scholar]
- Liu X., Cao L., Ni J., et al. (2013). Differential BCCIP gene expression in primary human ovarian cancer, renal cell carcinoma and colorectal cancer tissues. Int. J. Oncol. 43, 1925–1934. [DOI] [PubMed] [Google Scholar]
- Lohrum M.A., Ludwig R.L., Kubbutat M.H., et al. (2003). Regulation of HDM2 activity by the ribosomal protein L11. Cancer Cell 3, 577–587. [DOI] [PubMed] [Google Scholar]
- Lu H., Huang Y.Y., Mehrotra S., et al. (2011). Essential roles of BCCIP in mouse embryonic development and structural stability of chromosomes. PLoS Genet. 7, e1002291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Yue J., Meng X., et al. (2007). BCCIP regulates homologous recombination by distinct domains and suppresses spontaneous DNA damage. Nucleic Acids Res. 35, 7160–7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malina A., Cencic R., and Pelletier J. (2011). Targeting translation dependence in cancer. Oncotarget 2, 76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder B., Sampath P., Seshadri V., et al. (2003). Regulated release of L13a from the 60 S ribosomal subunit as a mechanism of transcript-specific translational control. Cell 115, 187–198. [DOI] [PubMed] [Google Scholar]
- Melnikov S., Ben-Shem A., Garreau de Loubresse N., et al. (2012). One core, two shells: bacterial and eukaryotic ribosomes. Nat. Struct. Mol. Biol. 19, 560–567. [DOI] [PubMed] [Google Scholar]
- Meng X., Fan J., and Shen Z. (2007). Roles of BCCIP in chromosome stability and cytokinesis. Oncogene 26, 6253–6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X., Liu J., and Shen Z. (2003). Genomic structure of the human BCCIP gene and its expression in cancer. Gene 302, 139–146. [DOI] [PubMed] [Google Scholar]
- Meng X., Liu J., and Shen Z. (2004). Inhibition of G1 to S cell cycle progression by BCCIP beta. Cell Cycle 3, 343–348. [PubMed] [Google Scholar]
- Ofir-Rosenfeld Y., Boggs K., Michael D., et al. (2008). Mdm2 regulates p53 mRNA translation through inhibitory interactions with ribosomal protein L26. Mol. Cell 32, 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T., Kitaura H., Ugai H., et al. (2000). TOK-1, a novel p21Cip1-binding protein that cooperatively enhances p21-dependent inhibitory activity toward CDK2 kinase. J. Biol. Chem. 275, 31145–31154. [DOI] [PubMed] [Google Scholar]
- Phillips-Mason P.J., Mourton T., Major D.L., et al. (2008). BCCIP associates with the receptor protein tyrosine phosphatase PTPmu. J. Cell. Biochem. 105, 1059–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roversi G., Pfundt R., Moroni R.F., et al. (2006). Identification of novel genomic markers related to progression to glioblastoma through genomic profiling of 25 primary glioma cell lines. Oncogene 25, 1571–1583. [DOI] [PubMed] [Google Scholar]
- Roy A., Kucukural A., and Zhang Y. (2010). I-TASSER: a unified platform for automated protein structure and function prediction. Nat. Protoc. 5, 725–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubbi C.P., and Milner J. (2003). Disruption of the nucleolus mediates stabilization of p53 in response to DNA damage and other stresses. EMBO J. 22, 6068–6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X.X., DeVine T., Challagundla K.B., et al. (2011). Interplay between ribosomal protein S27a and MDM2 protein in p53 activation in response to ribosomal stress. J. Biol. Chem. 286, 22730–22741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Hu Y., and Stearns M.E. (2009). RPS2: a novel therapeutic target in prostate cancer. J. Exp. Clin. Cancer Res. 28, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J.R. (1999). The economics of ribosome biosynthesis in yeast. Trends Biochem. Sci. 24, 437–440. [DOI] [PubMed] [Google Scholar]
- Warner J.R., and McIntosh K.B. (2009). How common are extraribosomal functions of ribosomal proteins. Mol. Cell 34, 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyler E., Wandrey F., Badertscher L., et al. (2014). The beta-isoform of the BRCA2 and CDKN1A(p21)-interacting protein (BCCIP) stabilizes nuclear RPL23/uL14. FEBS Lett. 588, 3685–3691. [DOI] [PubMed] [Google Scholar]
- Xiong X., Zhao Y., He H., et al. (2011). Ribosomal protein S27-like and S27 interplay with p53-MDM2 axis as a target, a substrate and a regulator. Oncogene 30, 1798–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadavilli S., Mayo L.D., Higgins M., et al. (2009). Ribosomal protein S3: A multi-functional protein that interacts with both p53 and MDM2 through its KH domain. DNA Repair 8, 1215–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Wang W., Wang H., et al. (2013). Identification of ribosomal protein S25 (RPS25)-MDM2-p53 regulatory feedback loop. Oncogene 32, 2782–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wang J., Yuan Y., et al. (2010). Negative regulation of HDM2 to attenuate p53 degradation by ribosomal protein L26. Nucleic Acids Res. 38, 6544–6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W.T., Zhou C.F., Li X.B., et al. (2013). The von Hippel-Lindau protein pVHL inhibits ribosome biogenesis and protein synthesis. J. Biol. Chem. 288, 16588–16597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Hao Q., Liao J., et al. (2013). Ribosomal protein S14 unties the MDM2-p53 loop upon ribosomal stress. Oncogene 32, 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Poyurovsky M.V., Li Y., et al. (2009). Ribosomal protein S7 is both a regulator and a substrate of MDM2. Mol. Cell 35, 316–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.