Abstract
Insulin can stimulate hepatic expression of carbohydrate-responsive element-binding protein (ChREBP). As recent studies revealed potential metabolic beneficial effects of ChREBP, we asked whether its expression can also be regulated by the dietary polyphenol curcumin. We also aimed to determine mechanisms underlying ChREBP stimulation by insulin and curcumin. The effect of insulin on ChREBP expression was assessed in mouse hepatocytes, while the effect of curcumin was assessed in mouse hepatocytes and with curcumin gavage in mice. Chemical inhibitors for insulin signaling molecules were utilized to identify involved signaling molecules, and the involvement of p21-activated protein kinase 1 (Pak1) was determined with its chemical inhibitor and Pak1−/− hepatocytes. We found that both insulin and curcumin-stimulated ChREBP expression in Akt-independent but MEK/ERK-dependent manner, involving the inactivation of the transcriptional repressor Oct-1. Aged Pak1−/− mice showed reduced body fat volume. Pak1 inhibition or its genetic deletion attenuated the stimulatory effect of insulin or curcumin on ChREBP expression. Our study hence suggests the existence of a novel signaling cascade Pak1/MEK/ERK/Oct-1 for both insulin and curcumin in exerting their glucose-lowering effect via promoting hepatic ChREBP production, supports the recognition of beneficial functions of ChREBP, and brings us a new overview on dietary polyphenols.
Keywords: Akt, ChREBP, curcumin, dietary polyphenol intervention, insulin, Oct-1, Pak1
Introduction
Acute elevation of plasma insulin level constitutes a major physiological response to counteract postprandial rise in plasma glucose levels. This is achieved by mechanisms including the reduction of hepatic gluconeogenesis, the facilitation of glucose uptake, and the activation of hepatic lipogenesis. As a key lipogenic transcription factor, carbohydrate-responsive element-binding protein (ChREBP), also known as MLX-interacting protein-like (MLXIPL), binds to and activates carbohydrate response element (ChoRE) motifs in the promoters of a battery of lipogenic genes (Kawaguchi et al., 2001). Thus, hepatic ChREBP, via its downstream enzymatic targets, channels the glycolytic end-products into the lipogenic process (Postic et al., 2007).
In addition to ChREBPα, a recent study in adipose tissues revealed the existence of the isoform ChREBPβ, of which the expression is driven by a different promoter (Herman et al., 2012). ChREBPβ is also expressed in the liver (Herman et al., 2012; Eissing et al., 2013) and elsewhere (Li et al., 2006; Poungvarin et al., 2012; Sae-Lee et al., 2016), and its expression can be transcriptionally activated by ChREBPα (Herman et al., 2012).
Although the function of ChREBP is mainly activated by postprandial glucose elevation, we have demonstrated previously that insulin can stimulate ChREBPα transcription, achieved by reducing the binding of the transcriptional repressor, the POU homeodomain (HD) protein Oct-1, to the proximal promoter region of ChREBPα (Sirek et al., 2009).
Curcumin is the principal curcuminoid of turmeric. Pre-clinical and clinical investigations have demonstrated the capability of dietary curcumin intervention in mitigating high-fat diet (HFD) induced insulin resistance and obesity or in preventing diabetes development in pre-diabetic human subjects (Weisberg et al., 2008; Chuengsamarn et al., 2012; Shao et al., 2012; Zeng et al., 2017). We found recently that 6-day curcumin gavage improved insulin sensitivity and glucose disposal in dexamethasone injection induced insulin resistant C57BL/6J mouse model, suggesting that the in vivo insulin signaling improvement effect of curcumin can be dissociated from its anti-inflammation and body weight lowering effects (Tian et al., 2015). The insulin sensitization effect of curcumin was also demonstrated in primary hepatocytes (Tian et al., 2015). Unexpectedly, this 6-day short-term curcumin administration protocol led to increased hepatic ChREBP mRNA expression, in contrast to the inhibitory effect on HFD-induced lipogenesis and liver steatosis with long-term dietary curcumin intervention (Weisberg et al., 2008; Shao et al., 2012; Tian et al., 2015). These serendipitous observations prompted us to ask whether curcumin shares a similar signaling cascade with insulin in stimulating ChREBP expression, how to interpret the absolutely opposite effects of long-term versus short-term curcumin administration on hepatic lipogenic gene expression, and whether the interpretation enriches our understanding on the paradoxical observations on hepatic function of insulin in regulating glucose versus lipid homeostasis in health and during insulin resistance (Li et al., 2010; Owen et al., 2012; Vatner et al., 2015).
Results
Both insulin and curcumin stimulate hepatic ChREBP expression
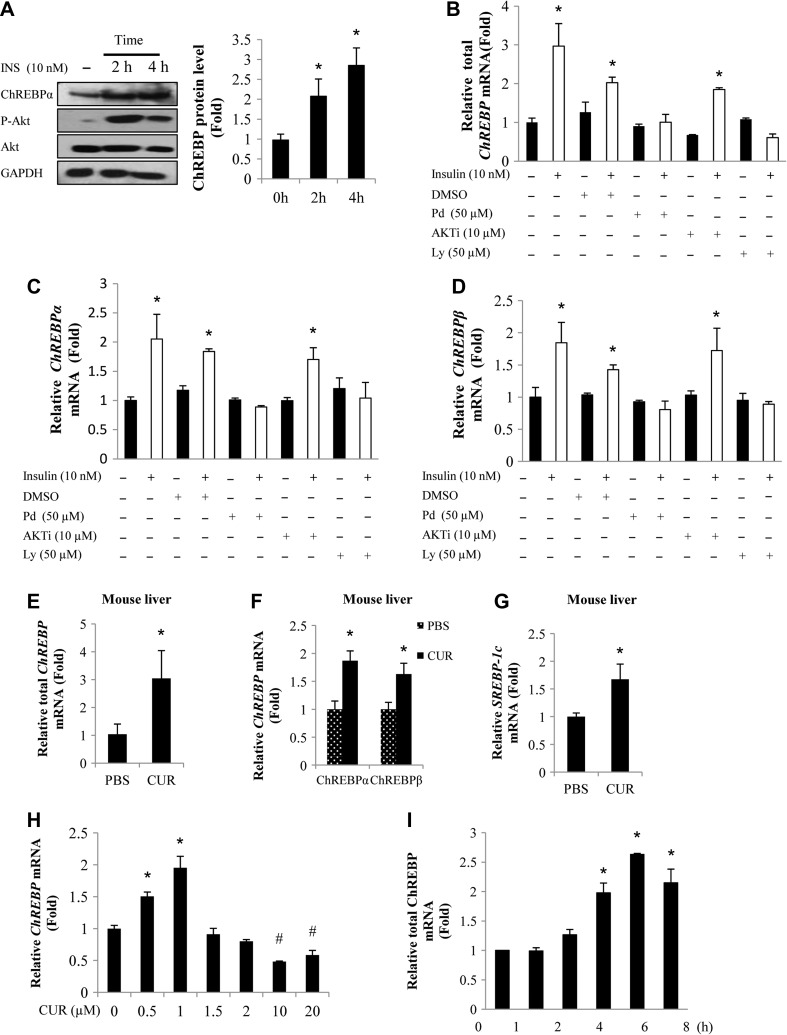
We have reported previously that insulin can stimulate ChREBPα transcription in the human HepG2 cell line and in hamster hepatocytes, involving the inactivation of the transcriptional repressor, the POU homeodomain protein Oct-1 (Sirek et al., 2009). Here we have first of all expanded the investigation into the mouse primary hepatocytes at the ChREBP protein level, showing that 2 or 4 h insulin treatment (10 nM) increased ChREBPα protein expression (Figure 1A). Since there is no available antibody against the new isoform ChREBPβ, we utilized real-time RT-PCR (qRT-PCR) to determine the effect of insulin as well as curcumin treatment on ChREBPβ mRNA expression whenever it is required.
Figure 1.
Both insulin and curcumin stimulate hepatic ChREBP expression. (A) Insulin (INS) treatment increased ChREBPα protein (~95 kDa) levels in mouse primary hepatocytes. Left panel is a representative blot (n = 3), while right panel shows the densitometric quantification results. (B–D) qRT-PCR show that insulin-stimulated ChREBP expression can be blocked by the MEK/ERK inhibitor PD98059 or the PI3K inhibitor Ly294002 but not the Akt inhibitor Akti in mouse primary hepatocytes. (E–G) Hepatic ChREBP and SREBP-1c mRNA levels were increased in C57BL/6J mice received 6 day curcumin gavage, assessed by qRT-PCR. (H and I) Curcumin treatment (0.5 or 1 μM) increased ChREBP mRNA levels in mouse hepatocytes (H) and the stimulation with 0.5 μM curcumin was not observed with the incubation time less than 4 h (I). CUR, curcumin; Pd, PD98059; Ly, Ly294002. n ≥ 3 for B–I. * P < 0.05, #P < 0.05 vs. the corresponding control.
As insulin may regulate gene expression in Akt-dependent or independent manner (Jin et al., 2008), we tested the effect of Akt or MEK/ERK inhibition on insulin-stimulated hepatic ChREBP mRNA expression. Figure 1B–D shows that in mouse primary hepatocytes, MEK or PI3K inhibition, but not Akt inhibition blocked the stimulatory effect of insulin on ChREBP (total), ChREBPα, and ChREBPβ expression, suggesting that insulin may stimulate ChREBP expression in an Akt-independent but MEK/ERK-dependent manner. Supplementary Figure S1 shows that in the human HepG2 cell line, insulin-stimulated ChREBP expression was also inhibited by MEK or PI3K inhibition but not by Akt inhibition.
We reported recently that 6-day curcumin gavage improved dexamethasone injection-induced impairment on insulin tolerance in C57BL/6J mice (Tian et al., 2015). In the absence of dexamethasone injection, 6-day curcumin gavage generated no further improvement on insulin tolerance but reduced hepatic G6pase expression and ambient plasma insulin levels (Tian et al., 2015). We then took the liver tissues from those mice for qRT-PCR analyses. Figure 1E–G shows that short-term curcumin gavage increased hepatic ChREBP (both ChREBPα and ChREBPβ) as well as SREBP-1c/Srebf1 mRNA levels.
Mouse primary hepatocytes were then utilized to test the effect of in vitro curcumin treatment on ChREBP mRNA expression. As shown, treating mouse primary hepatocytes with 0.5 or 1 μM curcumin for 4 h increased ChREBP (total) mRNA levels (Figure 1H). The activation, however, was not observed when the curcumin dosage was raised to 1.5 μM or higher (Figure 1H). When mouse primary hepatocytes were treated with 0.5 μM curcumin, the activation was not observed when the incubation time was less than 4 h (Figure 1I), suggesting that it is unlikely that the stimulation is a result of reduced ChREBP mRNA stability.
Elevated ChREBP expression in response to curcumin treatment is associated with elevated expression of its hepatic downstream targets
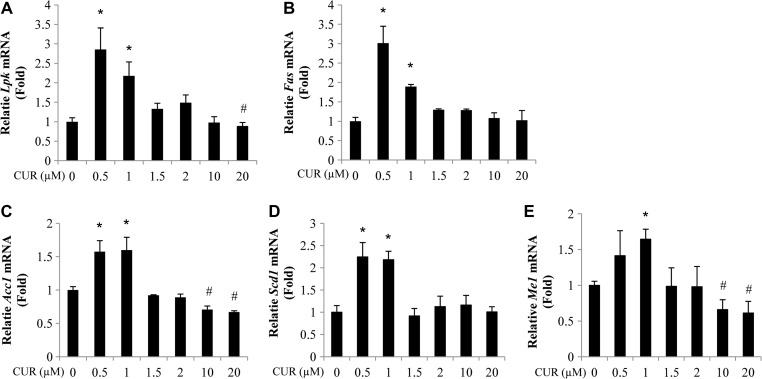
To initiate the assessment on the physiological relevance of the stimulation of ChREBP expression by curcumin treatment, we then verified the stimulatory effect of curcumin treatment on the expression of ChREBP downstream targets in the liver. As shown, in mouse hepatocytes treated with 0.5 or 1.0 μM curcumin, the expression of a battery of genes that encode lipogenic enzymes was increased. The list includes the genes that encode L-type pyruvate kinase (Lpk/Pklr), fatty acid (FA) synthase (Fas/Fasn), acetyl-CoA carboxylase (Acc1/Acaca), stearoyl-CoA desaturase-1 (Scd1), and cytosolic malic enzyme 1 (Me1) (Figure 2A–E).
Figure 2.
Elevated ChREBP expression in response to curcumin treatment is associated with elevated expression of its downstream targets. (A–E) Four-hour curcumin treatment (0.5–1.0 μM) increased the expression of Lpk (A), Fas (B), Acc1 (C), Scd1 (D), and Me1 (E) levels in mouse hepatocytes. n ≥ 3 for A–E. * P < 0.05, #P < 0.05 vs. the corresponding control.
The stimulation of ChREBP expression by curcumin treatment also involves Oct-1 attenuation and it is an Akt-independent event
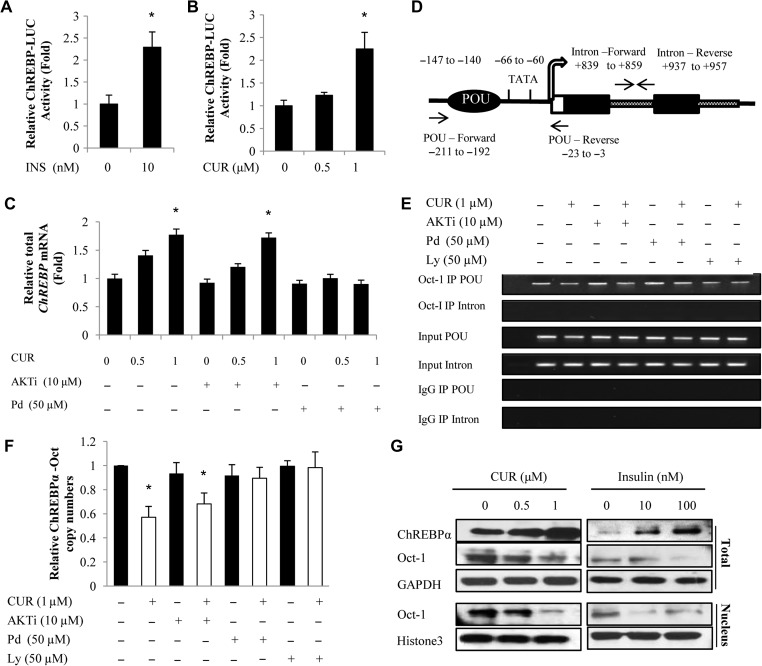
We reported previously that insulin stimulates ChREBP expression at the transcription level (Sirek et al., 2009). To further determine whether curcumin treatment also stimulates ChREBP expression at the transcription level, we then transiently transfected the ChREBPα-LUC fusion gene construct, generated in our previous study (Sirek et al., 2009), into the mouse primary hepatocytes. As shown in Figure 3A and B, both insulin (10 nM) and curcumin (at 1 μM) treatment resulted in stimulated ChREBPα-LUC activity. Figure 3C shows that curcumin-stimulated ChREBP mRNA expression can be blocked by MEK/ERK inhibition but not by Akt inhibition.
Figure 3.
The stimulation on ChREBP expression by curcumin treatment also involves Oct-1 attenuation and it is Akt-independent. (A and B) Four-hour insulin (INS) or curcumin (CUR) treatment stimulated the activity of the 1.4 kb-ChREBPα-LUC reporter when it was transfected into mouse primary hepatocytes. (C) Curcumin-stimulated ChREBP expression in mouse primary hepatocytes was blocked by 45 min pre-incubation with the MEK inhibitor PD98059 (Pd) but not the Akt inhibitor Akti. (D) Overall organization of the mouse ChREBPα gene proximal 5′ flanking region and the positions of primers utilized for ChIP and qChIP. (E and F) ChIP (E) and qChIP (F) show that curcumin attenuated Oct-1 binding to ChREBPα promoter in mouse primary hepatocytes can be blocked by MEK/ERK or PI3K inhibition but not by Akt inhibition. (G) Increased ChREBPα levels in mouse hepatocytes in response to curcumin or insulin treatment (4 h with indicated doses) were associated with reduced Oct-1 levels. n ≥ 3 for A–C and F. * P < 0.05 vs. the corresponding control. Representative blots of three independent experiments are shown in G.
A typical OCT binding motif was located within ChREBPα promoters (Sirek et al., 2009), but not within that of ChREBPβ. We then established the chromatin immunoprecipitation (ChIP) method for mouse primary hepatocytes. Figure 3D shows the overall organization of the mouse ChREBPα gene 5′ flanking region as well as positions of PCR primers utilized in the ChIP (Figure 3E) and quantitative ChIP (qChIP) (Figure 3F) assays. Curcumin treatment attenuated the binding of Oct-1 to the mouse ChREBPα gene promoter. Again, the attenuation was not blocked by Akt inhibition with Akti pre-treatment but was blocked by MEK/ERK inhibition with PD98059 pre-treatment or by PI3K inhibition with LY294002 pre-treatment (Figures 3E and F). Similar observations were then obtained in the human HepG2 cell line in response to insulin treatment (Supplementary Figure S2). Figure 3G shows that increased ChREPBα protein level in response to either curcumin or insulin treatment was associated with reduced nuclear and cytoplasmic Oct-1 levels. The above observations collectively suggest that curcumin treatment mimics the effect of insulin treatment on stimulating ChREBPα expression via inactivating the transcriptional repressor Oct-1, in Akt-independent but MEK/ERK-dependent manner.
Pak1 is among the mediators of both curcumin and insulin in stimulating ChREBP expression
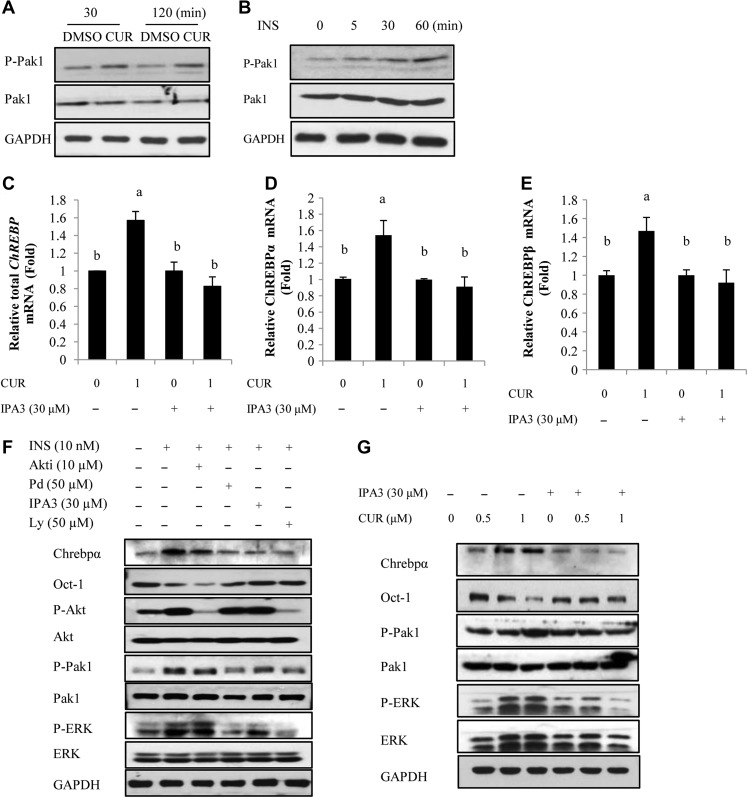
In addition to Akt, insulin was shown to activate Pak1 in a number of cell lineages including hepatocytes, demonstrated by our team and by others (Tsakiridis et al., 1996; Sun et al., 2009; Rudich and Klip, 2013; Chiang and Jin, 2014). Such activation may or may not involve the function of Akt (Sun et al., 2009; Rudich and Klip, 2013). We show here that both insulin and curcumin can stimulate Pak1 via increasing its Thr423 phosphorylation in mouse primary hepatocytes (Figure 4A and B) and in the human HepG2 cell line (Supplementary Figure S3).
Figure 4.
Pak1 is among mediators of both curcumin and insulin in stimulating ChREBP expression. (A and B) Pak1 Thr423 phosphorylation in mouse hepatocytes was stimulated by curcumin (1 μM) or insulin (10 nM) at indicated time intervals. (C–E) qRT-PCR shows that in mouse primary hepatocytes, curcumin-stimulated ChREBP (total as well as α and β isoforms) expression was blocked by the Pak inhibitor IPA3. (F and G) Western blotting show the effect of IPA3 pre-treatment on insulin (F) or curcumin (G)-stimulated ChREBP expression, Oct-1 content, Pak1 as well as ERK phosphorylation. Panels F and G are representative blot of three independent experiments. n ≥ 3 for C–E, where level means without a common letter are statistically different.
We then tested the effect of Pak1 inhibition with the chemical inhibitor IPA3 (Chiang et al., 2013) on insulin or curcumin-stimulated ChREBP expression. IPA3 pre-incubation in mouse primary hepatocytes blocked the stimulatory effect of curcumin on ChREBP mRNA expression (Figure 4C–E). Figure 4F shows that the stimulation on ChREBPα protein expression by insulin treatment was also blocked by IPA3 pre-treatment; associated with un-reduced Oct-1 content and attenuated ERK phosphorylation. Figure 4G shows that curcumin treatment stimulated ChREBPα expression, associated with reduced Oct-1 content. The stimulation of Pak1 phosphorylation was observed with 1.0 μM curcumin treatment, while the stimulation was shown to be blocked with IPA3 pre-treatment, associated with reduced ChREBPα protein expression and unchanged Oct-1 content.
Pak1−/− hepatocytes show the loss of response to insulin or curcumin treatment on stimulating ChREBP expression
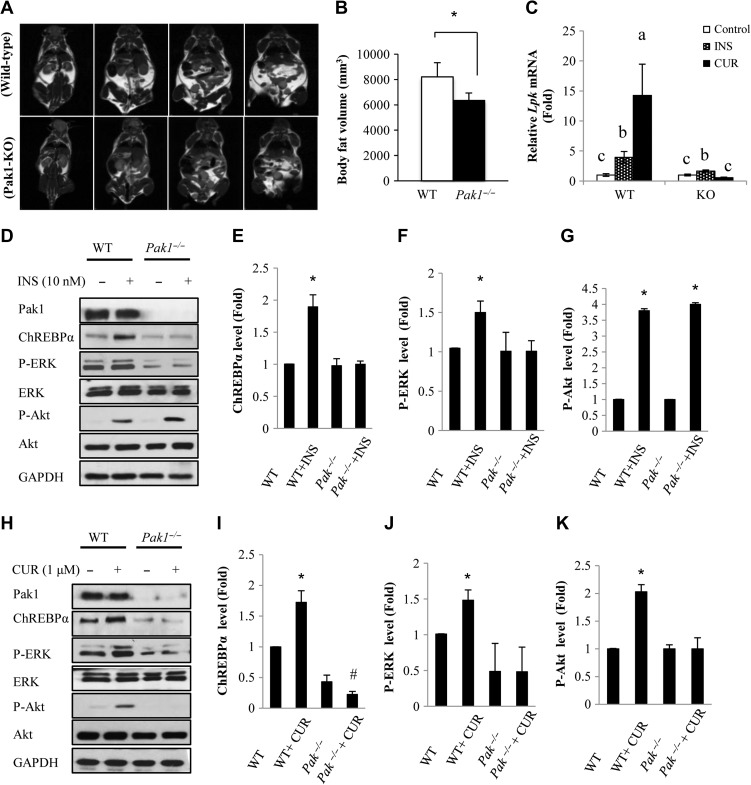
We and others have reported previously that Pak1−/− mice are glucose intolerant and insulin resistance, with reduced insulin secretion and gut incretin hormone glucagon-like peptide-1 (GLP-1) production (Wang et al., 2011; Chiang et al., 2013). Unexpectedly, we found that aged (32 weeks) Pak1−/− mice with regular chow diet feeding exhibited reduced whole-body fat volume, determined by magnetic resonance imaging (MRI) (Figure 5A and B), indicting the dissociation between fat volume and insulin resistance in this transgenic mouse line. We then isolated primary hepatocytes from Pak1−/− mice and wild-type littermate controls for testing their response to insulin or curcumin treatment. Supplementary Figure S4 shows that in Pak1−/− hepatocytes, curcumin cannot stimulate ChREBPα or ChREBPβ mRNA expression. In addition, in Pak1−/− hepatocytes, curcumin cannot stimulate the expression of the three example genes (Scd1, mE1, and Fas) that encode the lipogenic enzymes as efficient as that in wild-type hepatocytes (Supplementary Figure S5). More importantly, Lpk is a downstream target of ChREBP but not SREBP-1c. In Pak1−/− hepatocytes, the stimulatory effects of both insulin and curcumin on Lpk expression were attenuated (Figure 5C). We then compared the stimulatory effect of insulin and curcumin on ChREBP protein expression in Pak1−/− mouse hepatocytes and littermate control mouse hepatocytes. As shown in Figure 5D–G, insulin treatment induced ChREBP expression and ERK activation were both attenuated in Pak1−/− hepatocytes, while insulin was still able to stimulate Akt phosphorylation. Figure 5H–K shows that curcumin treatment induced ChREBP expression, ERK activation, as well as Akt phosphorylation were all attenuated in Pak1−/− hepatocytes. The above observations collectively suggest that Pak1 is an essential mediator that conveys the stimulatory effect of insulin and curcumin on ChREBP expression, involving MEK/ERK activation.
Figure 5.
Curcumin-stimulated ChREBP expression is attenuated in Pak1−/− hepatocytes. (A and B) Aged Pak1−/− mice exhibited reduced whole-body fat. MRI was performed in aged mice (32 weeks). Adipose tissue was measured and was used to calculate whole-body fat volume. Representative serial MRI scans of wild-type and Pak1−/− mice are shown in A. WT, n = 4. KO, n = 5. Panel B shows the quantitative analysis result of Panel A. (C) Pak1−/− hepatocytes show attenuated response to 4 h insulin (10 nM) or curcumin (1 μM) treatment on Lpk expression. (D–G) Western blotting shows the response to insulin treatment (D) on ChREBPα protein expression, as well as Akt and Erk phosphorylation in wild-type and Pak1−/− hepatocytes. Panels E–G are densitometric analysis data of Panel D. (H–K) Western blotting show the response to curcumin treatment (H) on ChREBPα protein expression, as well as Akt and Erk phosphorylation in wild-type and Pak1−/− hepatocytes. Densitometric analysis data of H are shown in I–K. For B, E–G, and I–K, * P < 0.05, #P < 0.05 vs. the correspondent control. For Panel C, level means without a common letter are statistically different.
Discussion
Although the function of ChREBP is mainly activated by glucose elevation via its cytosol-nuclei translocation, we have reported previously that insulin stimulates ChREBPα transcription, involving Oct-1 inactivation (Sirek et al., 2009). Here we demonstrated that the stimulation is Akt-independent, and that the dietary polyphenol compound curcumin can also stimulate ChREBP expression, mediated by a similar Akt-independent mechanism. The effect of short-term curcumin treatment on ChREBP expression observed in this current study is in contrast with long-term dietary curcumin intervention in HFD fed mice (Weisberg et al., 2008; Shao et al., 2012; Tian et al., 2015; Zeng et al., 2017). We also expanded our investigation into ChREBPβ, a new isoform associated with insulin sensitivity improvement (Herman et al., 2012; Eissing et al., 2013; Kursawe et al., 2013). Furthermore, we identified Pak1 as an upstream component of this novel signaling cascade. Finally, this investigation added to our understanding on the long-term paradoxical observations on hepatic functions of insulin in regulating glucose versus lipid homeostasis, as discussed in below.
In the liver, postprandial insulin elevation leads to accelerated glycogen synthesis, inhibited gluconeogenesis, but stimulated lipogenesis. Paradoxically, subjects with insulin resistance show elevated hepatic gluconeogenesis and lipogenesis, in contrast to the physiological status that insulin exerts opposite effects on lipogenesis and gluconeogenesis. It was postulated that insulin can lose its ability to attenuate glucose production but retain its ability to stimulate lipogenesis (Li et al., 2010; Owen et al., 2012). Underlying mechanisms for such ‘selective insulin resistance/activation’ however need to be further explored. Although our current study cannot resolve this paradox, it supports the recent recognition for the metabolic beneficial effect of ChREBP, added to our understanding on the ‘paradox’. We found exactly that the stimulation on lipogenic gene expression (this study) and the inhibition on glucose production (Tian et al., 2015) in hepatocyte in response to in vitro or short-term in vivo curcumin treatment were in contrast to the attenuation of hepatic steatosis along with improved insulin sensitivity in HFD fed mice with long-term dietary curcumin intervention. A plausible interpretation for these seemly paradoxical observations is that ChREBP mediates the physiological effect of insulin as well as the effect of dietary polyphenols (with curcumin as an example here) in converting the glycolytic end-products into the lipogenic process, which is essential for the long-term beneficial effect of curcumin in maintaining insulin sensitivity and in preventing the development of abnormalities on both glucose and lipid homeostasis. Such explanation would bring us a novel angle in the exploration of mechanisms underlying dietary interventions in metabolic disease prevention or treatment. We suggest that to bring nutrient sensing and dietary intervention into the investigation is essential for eventually resolving the paradox of hepatic function of insulin.
The assessment on ChREBP−/− mice revealed the requirement of ChREBP for basal and carbohydrate-induced expression of hepatic enzymes that are essential for lipid synthesis. ChREBP−/− mice show reduced lipogenesis (Iizuka et al., 2004). In addition, ob/ob/ChREBP−/− mice showed reduced expression of mRNAs for all hepatic lipogenic enzymes, resulting in decreased hepatic FA synthesis, normalized plasma free FA and TG levels, reduced weight gain and decreased adiposity (Iizuka et al., 2006). These observations have prompted some scientists to wonder whether ChREBP inhibition serves as a novel therapeutic tool for metabolic syndromes (Iizuka et al., 2006). A few recent studies, however, brought us the opposite view. Firstly, although adenovirus mediated hepatic expression of active ChREBP in mice led to the induction of the entire lipogenic and esterification program, the mice remained insulin sensitive (Benhamed et al., 2012). More importantly, when ChREBP overexpressing mice were fed with HFD, they showed improved insulin signaling and glucose tolerance, despite persistence of diet-induced hepatic steatosis. Thus, increased hepatic ChREBP expression can dissociate hepatic steatosis from insulin resistance, with the improvement on both glucose and lipid metabolism (Benhamed et al., 2012). Other studies have also revealed the metabolic beneficial effect of ChREBP. ChREBPβ was identified in white adipose tissues and its expression appears to predict insulin sensitivity (Herman et al., 2012). Recently, a study demonstrated that FA binding protein 4 (FABP4)-Cre mediated expression of constitutively active ChREBP in mice improved insulin sensitivity and glucose tolerance in response to HFD challenge (Nuotio-Antar et al., 2015). We show here that ChREBP is among the hepatic targets of the dietary polyphenol curcumin, which is in pace with the current recognition of the metabolic beneficial effects of ChREBP.
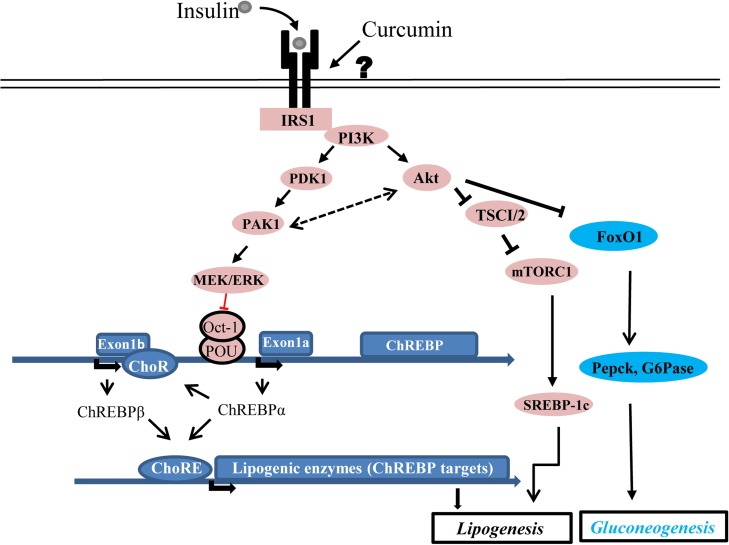
Curcumin, resveratrol and anthocyanin are the three most studied dietary polyphenols (Anhe et al., 2015; Amiot et al., 2016). They share a common feature in targeting multiple cell lineages without a defined receptor. Another common feature for these plant polyphenols is their low absorption rates in the gut (Lao et al., 2006). These characteristics have created certain difficulties in dissecting mechanistic insights of their functions. The recognition of the direct insulin sensitization effect of curcumin, however, has advanced our understanding on its overall hepatic beneficial effect. As illustrated in Figure 6, insulin or curcumin can utilize the Akt-FoxO signaling cascade to repress hepatic gluconeogenesis (Tian et al., 2015). Akt activation can also lead to the stimulation of mTORC via TSC1/2 inactivation, leading to SREBP-1c activation (Horton et al., 2002; Owen et al., 2012), although this can occur in mTORC1-dependent and independent manners (Yecies et al., 2011). Importantly, both insulin and curcumin can utilize an Akt-independent but Pak1/MEK/ERK-dependent signaling cascade to activate ChREBPα transcription, involving the inactivation of the transcriptional repressor Oct-1. As ChREBPβ transcription is not under the control of the promoter with the known OCT binding motif, stimulation on ChREBPβ expression by insulin or curcumin is likely an indirect response, achieved via ChREBPα elevation (Herman et al., 2012). It is necessary to point out that we show here for the first time that short-term curcumin treatment can also lead to increased SREBP-1c expression. The contribution of this stimulation on hepatic metabolic homeostasis is worth to be further investigated.
Figure 6.
A simplified diagram shows our current understanding of the role of insulin and curcumin on hepatic lipogenesis. Both insulin and curcumin can repress gluconeogenesis via activating the Akt signaling, which inactivates FoxO1 and the hepatic gluconeogenic pathway. Curcumin shares with insulin in using the IRS1/PI3K/Akt/TSC1/2/mTORC1 signaling cascade in activating SREBP-1c, although we are still unclear whether a yet to be defined receptor mediates the function of curcumin (indicated with the question mark). Importantly, both insulin and curcumin utilize the newly defined Pak1/MEK/ERK/Oct-1 signaling cascade in up-regulating ChREBPα transcription, leading to increased hepatic lipogenesis. The stimulation of ChREBPβ expression by insulin or curcumin is likely mediated by ChREBPα. The existence of possible feedback loop between Pak1 and Akt are illustrated with dotted arrows.
As a ubiquitously expressed POU HD protein, the transcriptional repressive feature of Oct-1 has been well documented during the past decade by our team and by others (Schwachtgen et al., 1998; Tantin et al., 2005; Thum and Borlak, 2008; Sirek et al., 2009; Wang et al., 2009; Voleti et al., 2012; Lin et al., 2013). Oct-1 may also serve as a stress sensor (Tantin et al., 2005) or even a global regulator in maintaining the spatial organization of chromosomes (Kim et al., 2014). To determine how MEK/ERK activation leads to reduced Oct-1 content, a systematic assessment of Oct-1 serine and threonine residue phosphorylation is required. Furthermore, it is worth to investigate whether this signaling cascade functions efficiently or differently after the development of insulin resistance.
Although group I Paks were initially recognized as effectors that link the Rho family of GTPases to cytoskeleton reorganization, Pak1 activation by insulin has been previously recognized (Tsakiridis et al., 1996; Sun et al., 2009; Rudich and Klip, 2013). Pak1 exert its metabolic beneficial effects via its function in multiple cell lineages, including pancreatic β-cells, gut endocrine L cells, and skeletal muscles (Wang et al., 2011; Nie et al., 2012; Chiang et al., 2013). With the chemical inhibitor IPA3 and Pak1−/− hepatocytes, we demonstrated its role in mediating the function of insulin and curcumin on stimulating ERK phosphorylation and ChREBP expression. Detailed dissection of in vivo metabolic functions of hepatic Pak1 is desired. However, this requires the generation of liver specific Pak1−/−mice, as Pak1 exerts its function in several metabolic organs (Wang et al., 2011; Nie et al., 2012; Chiang et al., 2013).
Pak1 may possess oncogenic potential while curcumin can repress its function (Cai et al., 2009; Chen et al., 2014). Many cancer cell studies were performed with high dosages of curcumin (>10 μM). In multi-potent neural progenitor cells (NPC), curcumin showed biphasic effects. Low dosages of curcumin-stimulated NPC proliferation while high dosages of curcumin were cytotoxic (Kim et al., 2008). We show here the biphasic effect of curcumin on ChREBP expression (Figure 1H). As dietary bioavailability of curcumin is relatively low, it is unlikely that hepatic curcumin from dietary sources can reach the levels that repress ChREBP expression.
In summary, our current study defined a novel signaling cascade Pak1/MEK/ERK/Oct-1 in mediating the physiological effect of insulin and the dietary polyphenol curcumin in up-regulating ChREBP expression. This investigation further expanded our understanding on the metabolic beneficial effect of curcumin from anti-inflammation to the improvement of glucose disposal via regulating hepatic ChREBP expression, enriched our knowledge on the metabolic regulatory effect of the transcriptional repressor Oct-1, and opened new windows for assessing metabolic functions of Pak1 and dietary polyphenol interventions. This study has also added to our understanding on the paradox of hepatic function of insulin. We suggest that to bring dietary intervention into the investigation is essential for eventually resolving this paradox.
Materials and methods
Animals
Eight week old C57BL/6J male mice were purchased from Charles River Laboratories. The generation, genotyping and characterization of Pak1−/− mice were described previously (Allen et al., 2009; Chiang et al., 2013). The protocol for curcumin gavage and the results of intraperitoneal insulin tolerance assay (IPITT) were presented previously (Tian et al., 2015). Briefly, C57BL/6J mice on regular chow diet feeding were received either curcumin gavage (500 mg/kg body weight per day) or the gavage with the same amount of solvent for curcumin (sesame oil) for 5 days. After one day rest followed by IPITT (on Day 7), a booster curcumin gavage was performed (on Day 8). We define this protocol as 6-day curcumin gavage. The mice were then killed by cervical dislocation on Day 10 for liver tissue collection. The animal protocol including the euthanasia method was approved by the University Health Network Animal Care Committee and was performed in accordance with the guidelines of the Canadian Council of Animal Care.
Cell cultures and chemical reagents
The growth of the human HepG2 cell line has been previously described (Ip et al., 2015). Male C57BL/6J mice at the ages of 8–12 weeks were utilized for mouse primary hepatocytes isolation, as previously described (Ip et al., 2015). The group I Pak inhibitor 2,2′-dihydroxy-1,1′-dinaphthyldisulfide (IPA3) was obtained from Sigma-Aldrich. Curcumin was purchased from Sigma-Aldrich for the cell culture experiments or from Organika Health Products (a 95% standardized curcumin extract) for the in vivo mouse study. The Akt inhibitor Akti1/2, the MEK inhibitor PD98059, and the PI3K inhibitor Ly294002 and palmitic acid were all purchased from Sigma-Aldrich.
Western blotting, RNA extraction, and real-time RT-PCR
Methods for western blotting were previously described (Ip et al., 2015) with indicated antibodies listed in Supplementary Table S1. Total RNA from mouse liver tissue, mouse hepatocytes, or the human HepG2 cell line was isolated utilizing the TRI Reagent (Sigma-Aldrich). Real-time PCR (qPCR) was performed with the use of Power SYBR Green Mix (Applied Biosystems) and a 7900 Fast Real-Time PCR System (Applied Biosystems) with primers presented in Supplementary Table S2. GAPDH was utilized as the house keeping gene in qPCR assays unless specified.
Luciferase assay and ChIP
The fusion gene construct in which the expression of LUC reporter is driven by 1.4-kb human ChREBP gene 5-flanking sequence, designated as 1.4-kb-ChREBP-LUC, was generated previously (Sirek et al., 2009). LUC reporter assay was conducted as previously described (Ip et al., 2015).
The ChIP and qChIP assays in assessing DNA-protein interactions within the intact cells were performed as we have previous reported (Sirek et al., 2009). Briefly, mouse hepatocytes or the human HepG2 cell line were cultured to 90% confluence, pretreated with or without an indicated chemical inhibitor for 1 h, followed by curcumin (1 μM) or insulin (10 nM) treatment for 4 h. Cells were fixed with 1% formaldehyde for 10 min to cross-link chromatin DNA and nuclear proteins. After a neutralization procedure, chromatin DNA was sheared by sonication. The Oct-1 antibody was then used (1:200) to precipitate the sheared chromatin DNA. After washing, elution, reverse cross-linking, and DNA purification, approximately one twentieth of purified DNA (2 μl) was taken for each of the PCR reaction (ChIP) or real-time PCR (qChIP). DNA sequences of the primers used in the ChIP and qChIP assays are listed in Supplementary Table S2.
MRI assessment of total fat mass and lipid content
This was performed by The STTARR Innovation Centre, Radiation Medicine Program, Princess Margaret Hospital, University Health Network, Toronto, ON, Canada, as previously described (Yu et al., 2011).
Statistics
Quantitative results are expressed as mean ± SD. For comparison of two groups, the Student’s t-test was used. Comparisons between groups with one treatment were determined by one-way ANOVA followed by Bonferroni post hoc tests. In instances of multiple means comparisons, two-way ANOVA followed by Bonferroni post hoc tests were used. P < 0.05 was considered to indicate a statistically significant difference.
Supplementary material
Supplementary material is available at Journal of Molecular Cell Biology online.
Funding
This work was supported by an operating grant by the Canadian Institutes of Health Research (MOP 97790, to T.J. and D.S.N.), an National Natural Science Foundation of China (NSFC) and Canadian Institutes of Health Research (CIHR) Joint Operating Grant (81261120565) to J.W., and an operating grant by the National Institutes of Health (NIH) to J.C. (RO1 CA142928). K.Z. is the receipt the Scholarship for International Program for Ph.D. Candidates, Sun Yat-Sen University; L.T. is the recipient of Banting and Best Diabetes Centre Post-Doctoral Fellowship.
Conflict of interest: none declared.
Supplementary Material
Acknowledgements
We thank Ms Jonelle Martineau of University Health Network for help in the preparation of this manuscript.
References
- Allen J.D., Jaffer Z.M., Park S.J., et al. (2009). p21-activated kinase regulates mast cell degranulation via effects on calcium mobilization and cytoskeletal dynamics. Blood 113, 2695–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiot M.J., Riva C., and Vinet A. (2016). Effects of dietary polyphenols on metabolic syndrome features in humans: a systematic review. Obes. Rev. 17, 573–586. [DOI] [PubMed] [Google Scholar]
- Anhe F.F., Roy D., Pilon G., et al. (2015). A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 64, 872–883. [DOI] [PubMed] [Google Scholar]
- Benhamed F., Denechaud P.D., Lemoine M., et al. (2012). The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. J. Clin. Invest. 122, 2176–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X.Z., Wang J., Li X.D., et al. (2009). Curcumin suppresses proliferation and invasion in human gastric cancer cells by downregulation of PAK1 activity and cyclin D1 expression. Cancer Biol. Ther. 8, 1360–1368. [DOI] [PubMed] [Google Scholar]
- Chen Q.Y., Zheng Y., Jiao D.M., et al. (2014). Curcumin inhibits lung cancer cell migration and invasion through Rac1-dependent signaling pathway. J. Nutr. Biochem. 25, 177–185. [DOI] [PubMed] [Google Scholar]
- Chiang Y.A., Shao W., Xu X.X., et al. (2013). P21-activated protein kinase 1 (Pak1) mediates the cross talk between insulin and β-catenin on proglucagon gene expression and its ablation affects glucose homeostasis in male C57BL/6 mice. Endocrinology 154, 77–88. [DOI] [PubMed] [Google Scholar]
- Chiang Y.T., and Jin T. (2014). p21-Activated protein kinases and their emerging roles in glucose homeostasis. Am. J. Physiol. Endocrinol. Metab. 306, E707–E722. [DOI] [PubMed] [Google Scholar]
- Chuengsamarn S., Rattanamongkolgul S., Luechapudiporn R., et al. (2012). Curcumin extract for prevention of type 2 diabetes. Diabetes Care 35, 2121–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissing L., Scherer T., Todter K., et al. (2013). De novo lipogenesis in human fat and liver is linked to ChREBP-β and metabolic health. Nat. Commun. 4, 1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman M.A., Peroni O.D., Villoria J., et al. (2012). A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature 484, 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton J.D., Goldstein J.L., and Brown M.S. (2002). SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 109, 1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka K., Bruick R.K., Liang G., et al. (2004). Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc. Natl Acad. Sci. USA 101, 7281–7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka K., Miller B., and Uyeda K. (2006). Deficiency of carbohydrate-activated transcription factor ChREBP prevents obesity and improves plasma glucose control in leptin-deficient (ob/ob) mice. Am. J. Physiol. Endocrinol. Metab. 291, E358–E364. [DOI] [PubMed] [Google Scholar]
- Ip W., Shao W., Song Z., et al. (2015). Liver-specific expression of dominant-negative transcription factor 7-like 2 causes progressive impairment in glucose homeostasis. Diabetes 64, 1923–1932. [DOI] [PubMed] [Google Scholar]
- Jin T., George Fantus I., and Sun J. (2008). Wnt and beyond Wnt: multiple mechanisms control the transcriptional property of β-catenin. Cell. Signal. 20, 1697–1704. [DOI] [PubMed] [Google Scholar]
- Kawaguchi T., Takenoshita M., Kabashima T., et al. (2001). Glucose and cAMP regulate the L-type pyruvate kinase gene by phosphorylation/dephosphorylation of the carbohydrate response element binding protein. Proc. Natl Acad. Sci. USA 98, 13710–13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim L.K., Esplugues E., Zorca C.E., et al. (2014). Oct-1 regulates IL-17 expression by directing interchromosomal associations in conjunction with CTCF in T cells. Mol. Cell 54, 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.J., Son T.G., Park H.R., et al. (2008). Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. J. Biol. Chem. 283, 14497–14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kursawe R., Caprio S., Giannini C., et al. (2013). Decreased transcription of ChREBP-α/β isoforms in abdominal subcutaneous adipose tissue of obese adolescents with prediabetes or early type 2 diabetes: associations with insulin resistance and hyperglycemia. Diabetes 62, 837–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao C.D., Ruffin M.T.t., Normolle D., et al. (2006). Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 6, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.V., Chang B., Imamura M., et al. (2006). Glucose-dependent transcriptional regulation by an evolutionarily conserved glucose-sensing module. Diabetes 55, 1179–1189. [DOI] [PubMed] [Google Scholar]
- Li S., Brown M.S., and Goldstein J.L. (2010). Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc. Natl Acad. Sci. USA 107, 3441–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Yang H., Zhang H., et al. (2013). A novel transcription mechanism activated by ethanol: induction of Slc7a11 gene expression via inhibition of the DNA-binding activity of transcriptional repressor octamer-binding transcription factor 1 (OCT-1). J. Biol. Chem. 288, 14815–14823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J., Sun C., Faruque O., et al. (2012). Synapses of amphids defective (SAD-A) kinase promotes glucose-stimulated insulin secretion through activation of p21-activated kinase (PAK1) in pancreatic β-cells. J. Biol. Chem. 287, 26435–26444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuotio-Antar A.M., Poungvarin N., Li M., et al. (2015). FABP4-Cre mediated expression of constitutively active ChREBP protects against obesity, fatty liver, and insulin resistance. Endocrinology 156, 4020–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen J.L., Zhang Y., Bae S.H., et al. (2012). Insulin stimulation of SREBP-1c processing in transgenic rat hepatocytes requires p70 S6-kinase. Proc. Natl Acad. Sci. USA 109, 16184–16189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postic C., Dentin R., Denechaud P.D., et al. (2007). ChREBP, a transcriptional regulator of glucose and lipid metabolism. Annu. Rev. Nutr. 27, 179–192. [DOI] [PubMed] [Google Scholar]
- Poungvarin N., Lee J.K., Yechoor V.K., et al. (2012). Carbohydrate response element-binding protein (ChREBP) plays a pivotal role in β cell glucotoxicity. Diabetologia 55, 1783–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudich A., and Klip A. (2013). Putting Rac1 on the path to glucose uptake. Diabetes 62, 1831–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sae-Lee C., Moolsuwan K., Chan L., et al. (2016). ChREBP regulates itself and metabolic genes implicated in lipid accumulation in β-cell line. PLoS One 11, e0147411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwachtgen J.L., Remacle J.E., Janel N., et al. (1998). Oct-1 is involved in the transcriptional repression of the von willebrand factor gene promoter. Blood 92, 1247–1258. [PubMed] [Google Scholar]
- Shao W., Yu Z., Chiang Y., et al. (2012). Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PLoS One 7, e28784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirek A.S., Liu L., Naples M., et al. (2009). Insulin stimulates the expression of carbohydrate response element binding protein (ChREBP) by attenuating the repressive effect of Pit-1, Oct-1/Oct-2, and Unc-86 homeodomain protein octamer transcription factor-1. Endocrinology 150, 3483–3492. [DOI] [PubMed] [Google Scholar]
- Sun J., Khalid S., Rozakis-Adcock M., et al. (2009). P-21-activated protein kinase-1 functions as a linker between insulin and Wnt signaling pathways in the intestine. Oncogene 28, 3132–3144. [DOI] [PubMed] [Google Scholar]
- Tantin D., Schild-Poulter C., Wang V., et al. (2005). The octamer binding transcription factor Oct-1 is a stress sensor. Cancer Res. 65, 10750–10758. [DOI] [PubMed] [Google Scholar]
- Thum T., and Borlak J. (2008). LOX-1 receptor blockade abrogates oxLDL-induced oxidative DNA damage and prevents activation of the transcriptional repressor Oct-1 in human coronary arterial endothelium. J. Biol. Chem. 283, 19456–19464. [DOI] [PubMed] [Google Scholar]
- Tian L., Zeng K., Shao W., et al. (2015). Short-term curcumin gavage sensitizes insulin signaling in dexamethasone-treated C57BL/6 mice. J. Nutr. 145, 2300–2307. [DOI] [PubMed] [Google Scholar]
- Tsakiridis T., Taha C., Grinstein S., et al. (1996). Insulin activates a p21-activated kinase in muscle cells via phosphatidylinositol 3-kinase. J. Biol. Chem. 271, 19664–19667. [DOI] [PubMed] [Google Scholar]
- Vatner D.F., Majumdar S.K., Kumashiro N., et al. (2015). Insulin-independent regulation of hepatic triglyceride synthesis by fatty acids. Proc. Natl Acad. Sci. USA 112, 1143–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voleti B., Hammond D.J. Jr., Thirumalai A., et al. (2012). Oct-1 acts as a transcriptional repressor on the C-reactive protein promoter. Mol. Immunol. 52, 242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Wang Q., Sun J., et al. (2009). POU homeodomain protein Oct-1 functions as a sensor for cyclic AMP. J. Biol. Chem. 284, 26456–26465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Oh E., Clapp D.W., et al. (2011). Inhibition or ablation of p21-activated kinase (PAK1) disrupts glucose homeostatic mechanisms in vivo. J. Biol. Chem. 286, 41359–41367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg S.P., Leibel R., and Tortoriello D.V. (2008). Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology 149, 3549–3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yecies J.L., Zhang H.H., Menon S., et al. (2011). Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 14, 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z., Shao W., Chiang Y., et al. (2011). Oltipraz upregulates the nuclear factor (erythroid-derived 2)-like 2 [corrected](NRF2) antioxidant system and prevents insulin resistance and obesity induced by a high-fat diet in C57BL/6J mice. Diabetologia 54, 922–934. [DOI] [PubMed] [Google Scholar]
- Zeng K., Tian L., Patel R., et al. (2017). Diet polyphenol curcumin stimulates hepatic Fgf21 production and restores its sensitivity in high-fat-diet-fed male mice. Endocrinology 158, 277–292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.