Abstract
Background.
We assessed the incidence of tuberculosis, risk factors for tuberculosis, and the contribution of tuberculosis on mortality in a large cohort of human immunodeficiency virus (HIV)-infected children <15 years of age initiating first-line antiretroviral therapy (ART) between 1999 and 2012 in Thailand, one of the 22 high tuberculosis burden countries.
Methods.
A physician reviewed and classified tuberculosis cases. Incidence was the number of children with incident tuberculosis, defined as a first or recurrent tuberculosis diagnosis >30 days after ART initiation, divided by the total person-years of follow-up (PYFU). Risk factors for incident tuberculosis were identified using Fine and Gray’s competing risks models, with death from other causes treated as a competing event, and risk factors for death were identified using Cox models.
Results.
At ART initiation, 670 children (55% female) had a median age of 6.4 years (interquartile range, 2.0–9.6), body mass index-for-age z-score −0.8 (−1.9 to 0.0), HIV ribonucleic acid viral load 5.1 log10 copies/mL (4.6–5.6), and CD4 9% (3–17). Median duration of follow-up was 7.7 years. Tuberculosis incidence was 7 per 1000 PYFU (95% confidence interval [CI], 5–11) and decreased with ART duration. Lower age-adjusted hemoglobin, hematocrit, and CD4 at ART initiation were associated with a higher risk of incident tuberculosis. Of the 30 incident tuberculosis cases, 9 died. Diagnosis of incident tuberculosis was associated with mortality (unadjusted hazard ratio = 10.2, 95% CI = 4.8–21.5, P < .001 and adjusted hazard ratio = 5.4, 95% CI = 2.5–11.7, P < .001).
Conclusions.
Incident tuberculosis was strongly associated with mortality. CD4 counts or hemoglobin or hematocrit levels may prompt clinicians to consider a possible tuberculosis infection.
Keywords: children, HIV, incidence, mortality, tuberculosis.
In 2014, 9.6 million people developed active tuberculosis worldwide, including an estimated 1.2 million living with human immunodeficiency virus (HIV) [1]. Tuberculosis remains the primary cause of death among HIV-infected people (390 000 deaths) [1, 2]. Thailand is one of the 22 high tuberculosis burden countries listed by the World Health Organization (WHO), with an incidence of 171 per 100 000 in the general population in 2014, whereas the incidence reported in the HIV-infected population is more than 20 times higher [1, 3]. Approximately half of the 12 000 people who died of tuberculosis in Thailand in 2014 were infected with HIV [1].
In Thailand, data on tuberculosis incidence and associated mortality are limited, particularly in children living with HIV [4–6], and the long-term risk of tuberculosis in children on antiretroviral therapy (ART) is unknown. Within a large multicenter cohort of HIV-infected children on ART in Thailand, we estimated tuberculosis incidence, the impact of different risk factors on incident tuberculosis, and the contribution of tuberculosis on the risk of death.
METHODS
Study Population
This analysis included all HIV-infected children less than 15 years of age who initiated first-line ART between January 1, 1999 and December 31, 2012 and were followed in the Program for HIV Prevention and Treatment (PHPT) cohort (ClinicalTrials.gov: NCT00433030) in a network of public hospitals throughout the country, as previously described [7].
Baseline Data and Follow-Up
Children were followed-up at 2 weeks, 1 month, and 3 months after initiating ART and every 3 months thereafter for physical examination, complete blood count (hemoglobin, hematocrit, white blood count, red blood count, platelets, lymphocytes, neutrophils), clinical chemistries (plasma glucose, alanine transaminase, creatinine), drug refills, and adherence counseling. CD4 percentage and HIV ribonucleic acid (RNA) viral load were measured 3 and 6 months after ART initiation and every 6 months thereafter. The cohort study protocol included screening for tuberculosis before initiating ART and during follow-up in case of presumptive symptoms, based on interview, medical history, clinical examination, chest x-ray, tuberculin skin test, sputum acid-fast bacillus smear and/or gastric aspirate if indicated.
Children who never came back for follow-up during the study period were considered lost to follow-up. In the event of a participant’s death, the immediate cause and underlying conditions were reported by the attending physicians. All death reports were reviewed, and causes of death were classified by 2 independent physicians based on the International Classification of Diseases [8].
Tuberculosis Case Definition
A physician (C. D.) reviewed all available records of cases with suspected tuberculosis infection and classified each tuberculosis case as clinically diagnosed with or without bacteriologic confirmation, following the WHO reporting guidelines [9].
Children were classified in 4 groups according to their tuberculosis infection status: “prevalent tuberculosis” for children with ongoing tuberculosis treatment at the time of ART initiation or starting within the following 30 days; “incident tuberculosis” for children with a first or recurrent tuberculosis diagnosis at least 30 days after ART initiation; “history of treated tuberculosis” for children who had recovered from tuberculosis before ART initiation; and “no tuberculosis” for children who were not diagnosed with tuberculosis before ART initiation or during follow-up. The choice of the cutoff (30 days) to define “prevalent” and “incident” tuberculosis was driven by the literature [10, 11], although it is likely that not all tuberculosis infections already established at time of ART initiation were diagnosed within 30 days.
Statistical Analysis
The follow-up period was from the date of ART initiation to the date of death, date of last clinic visit, or December 31, 2013, whichever occurred first. Children with a history of treated tuberculosis at time of ART initiation were included in the incidence and risk factor analyses, but those with prevalent tuberculosis were excluded.
The incidence of tuberculosis infections diagnosed during follow-up was calculated using person-time incidence rates. Overall and stratified incidence rates were defined as the number of incident tuberculosis cases divided by the number of person-years of follow-up (PYFU). The 95% confidence intervals (CIs) of the incidence rates were calculated based on the Poisson distribution.
Risk factors for incident tuberculosis were identified using univariable and multivariable Fine and Gray’s competing risks proportional subhazards models [12], with death from other causes treated as a competing event. The association between diagnosis of incident tuberculosis and mortality was evaluated using univariable and multivariable Cox proportional hazards regression models, tuberculosis diagnosis being dichotomized and treated as a time-dependent variable (never or ever been diagnosed with tuberculosis).
Potential risk factors for incident tuberculosis and mortality assessed at ART initiation were as follows: sex, age, body mass index (BMI)-for-age [13, 14], calendar year of enrollment, and age-adjusted HIV RNA viral load, CD4 percentage, hemoglobin, hematocrit, neutrophils, and lymphocytes. Missing values at ART initiation were imputed using multiple imputation by chained equations with predictive mean matching (10 imputations) [15]. Except for BMI-for-age and calendar year of enrollment, all continuous variables were first categorized into quartiles rounded to the nearest clinically meaningful values and then put into smaller groups determined by the magnitude of the model coefficients in the univariable analyses. Multivariable models included all variables with P < .25 in the univariable analyses, and a forward selection procedure was used to identify risk factors independently associated with incident tuberculosis and mortality. The proportional hazards assumption of the models was tested using an interaction term between independent variables and time. Data were censored at the date of last clinic visit or December 31, 2013, whichever occurred first.
All reported P values are 2-sided, and P < .05 was considered statistically significant. Missing data imputation and statistical analyses were performed using Stata 13.0 (StataCorp, College Station, TX).
Ethical Considerations
The PHPT Cohort protocol and subsequent amendments were approved by the Ethics Committees of the Thai Ministry of Public Health, the Faculty of Associated Medical Sciences at Chiang Mai University, and local hospital ethics committees.
RESULTS
Study Population Characteristics
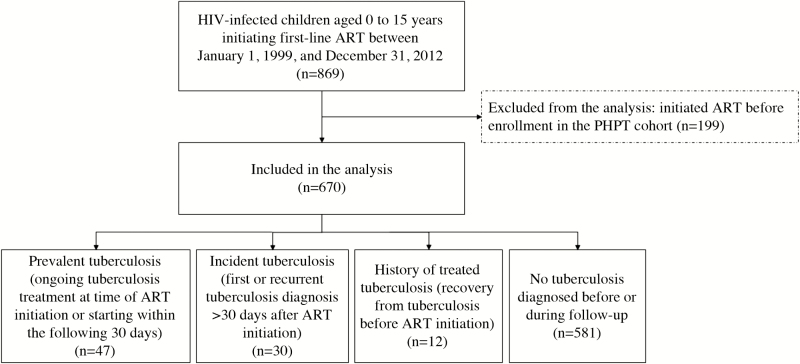
A total of 670 children were eligible for inclusion (Figure 1), including 367 (55%) females. At ART initiation, median age was 6.4 years (interquartile range [IQR], 2.0 to 9.6), BMI-for-age z-score was −0.8 (IQR, −1.9 to 0.0), HIV RNA viral load was 5.1 log10 copies/mL (IQR, 4.6 to 5.6), and CD4 was 9% (IQR, 3 to 17) (Table 1). Fifty-five children (8%) received isoniazid preventive therapy at some point during the study period. The median duration of follow-up from ART initiation was 7.7 years (IQR, 3.6 to 9.8). Of the 670 children, 14 had a history of treated tuberculosis. At the end of the study, 377 (56%) children were no longer on follow-up: 161 voluntarily withdrew from the study, 160 were lost to follow-up, and 56 died.
Figure 1.
Study population flowchart. ART, antiretroviral therapy; HIV, human immunodeficiency virus; PHPT, Program for HIV Prevention and Treatment.
Table 1.
Study Population Characteristics at ART Initiation
| Characteristics at ART Initiation | Prevalent Tuberculosis (n = 47a) n (%) | Incident Tuberculosis (n = 30a) n (%) | No Tuberculosis (n = 593a) n (%) | Overall (n = 670a) n (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Female sex | 26 | (55) | 23 | (77) | 318 | (54) | 367 | (55) |
| Age | ||||||||
| <2 years | 6 | (13) | 7 | (23) | 153 | (26) | 166 | (25) |
| 2 to 6 years | 10 | (21) | 4 | (13) | 138 | (23) | 152 | (23) |
| 6 to 10 years | 19 | (40) | 8 | (27) | 180 | (30) | 207 | (31) |
| ≥10 years | 12 | (26) | 11 | (37) | 122 | (21) | 145 | (22) |
| BMI-for-age | (n = 43) | (n = 27) | (n = 527) | (n = 597) | ||||
| z-score < −2 | 16 | (37) | 9 | (33) | 131 | (25) | 156 | (26) |
| z-score between −2 and +1 | 24 | (56) | 17 | (63) | 347 | (66) | 388 | (65) |
| z-score ≥ +1 | 3 | (7) | 1 | (4) | 49 | (9) | 53 | (9) |
| Calendar Year of Enrollment | ||||||||
| 1999 to 2002 | 5 | (11) | 4 | (13) | 111 | (19) | 120 | (18) |
| 2003 to 2005 | 33 | (70) | 22 | (73) | 331 | (56) | 386 | (58) |
| 2006 to 2012 | 9 | (19) | 4 | (13) | 151 | (25) | 164 | (24) |
| HIV RNA viral load | (n = 37) | (n = 23) | (n = 460) | (n = 520) | ||||
| <4.5 log10 copies/mL | 2 | (5) | 4 | (17) | 91 | (20) | 97 | (19) |
| 4.5 to 5.0 log10 copies/mL | 7 | (19) | 4 | (17) | 88 | (19) | 99 | (19) |
| 5.0 to 5.5 log10 copies/mL | 13 | (35) | 5 | (22) | 131 | (28) | 149 | (29) |
| ≥5.5 log10 copies/mL | 15 | (41) | 10 | (43) | 150 | (33) | 175 | (34) |
| CD4 | (n = 45) | (n = 29) | (n = 562) | (n = 636) | ||||
| <5% | 19 | (42) | 18 | (62) | 192 | (34) | 229 | (36) |
| 5% to 10% | 9 | (20) | 2 | (7) | 100 | (18) | 111 | (17) |
| 10% to 15% | 12 | (27) | 3 | (10) | 95 | (17) | 110 | (17) |
| ≥15% | 5 | (11) | 6 | (21) | 175 | (31) | 186 | (29) |
| Hemoglobin | (n = 43) | (n = 28) | (n = 529) | (n = 600) | ||||
| <9 g/dL | 7 | (16) | 7 | (25) | 83 | (16) | 97 | (16) |
| 9 to 10 g/dL | 8 | (19) | 8 | (29) | 102 | (19) | 118 | (20) |
| 10 to 11 g/dL | 9 | (21) | 7 | (25) | 135 | (26) | 151 | (25) |
| ≥11 g/dL | 19 | (44) | 6 | (21) | 209 | (40) | 234 | (39) |
| Hematocrit | (n = 44) | (n = 28) | (n = 539) | (n = 611) | ||||
| <30% | 9 | (20) | 12 | (43) | 146 | (27) | 167 | (27) |
| 30% to 32% | 7 | (16) | 6 | (21) | 84 | (16) | 97 | (16) |
| 32% to 34% | 10 | (23) | 4 | (14) | 110 | (20) | 124 | (20) |
| ≥34% | 18 | (41) | 6 | (21) | 199 | (37) | 223 | (36) |
| Neutrophils | (n = 43) | (n = 28) | (n = 509) | (n = 580) | ||||
| <3000 cells/mm3 | 28 | (65) | 7 | (25) | 222 | (44) | 257 | (44) |
| 3000 to 4000 cells/mm3 | 3 | (7) | 8 | (29) | 117 | (23) | 128 | (22) |
| 4000 to 5000 cells/mm3 | 4 | (9) | 3 | (11) | 59 | (12) | 66 | (11) |
| ≥5000 cells/mm3 | 8 | (19) | 10 | (36) | 111 | (22) | 129 | (22) |
| Lymphocytes | (n = 43) | (n = 28) | (n = 509) | (n = 580) | ||||
| <2000 cells/mm3 | 22 | (51) | 18 | (64) | 191 | (38) | 231 | (40) |
| 2000 to 3000 cells/mm3 | 7 | (16) | 3 | (11) | 115 | (23) | 125 | (22) |
| 3000 to 4000 cells/mm3 | 7 | (16) | 4 | (14) | 56 | (11) | 67 | (12) |
| ≥4000 cells/mm3 | 7 | (16) | 3 | (11) | 147 | (29) | 157 | (27) |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; HIV, human immunodeficiency virus; RNA, ribonucleic acid.
aUnless otherwise specified.
Description of Tuberculosis Cases
Of the 670 children, 77 (11%) experienced tuberculosis. Of these 77 cases, 47 were prevalent (none of them experienced recurrent tuberculosis after ART initiation) and 30 incident (including 2 with a history of treated tuberculosis before ART initiation and 1 with 2 tuberculosis episodes after ART initiation). Eight were bacteriologically confirmed and 69 were clinically diagnosed. There were 56 pulmonary, 12 lymphadenitis, 2 meningitis, 2 abdominal, 1 pulmonary plus meningitis, 1 parotid and 1 cutaneous tuberculosis, and 2 unspecified tuberculosis sites.
During tuberculosis treatment, 54 received an efavirenz-based ART regimen, 8 received a nevirapine-based regimen, 7 received a protease inhibitor-based regimen, and 4 received a zidovudine plus didanosine regimen; 3 discontinued ART before tuberculosis treatment; and 1 died before starting tuberculosis treatment. Based on the data available, which were mostly collected before consensus definitions of immune reconstitution inflammatory syndrome (IRIS) were proposed [16], the physician in charge of the retrospective review suspected that 8 children (3 with a prevalent tuberculosis and 5 with an incident tuberculosis) developed an IRIS between 10 days and 4 months after ART initiation. However, the information available was not sufficient to definitely classify these cases.
Tuberculosis Incidence
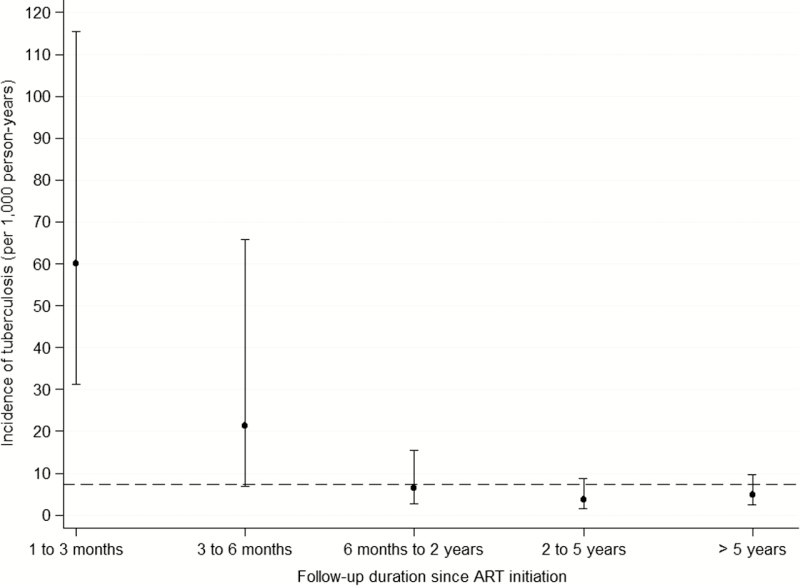
After exclusion of the 47 prevalent tuberculosis cases, 623 children contributed to 4068 PYFU. There were 30 incident tuberculosis cases, leading to an overall crude tuberculosis incidence rate of 7 per 1000 PYFU (95% CI, 5–11) (when including the 47 prevalent tuberculosis cases in the calculation, the incidence was similar: 7 per 1 000 PYFU [95% CI, 5–10]). The incidence rate was 4 per 1000 PYFU in males (95% CI, 2–8) and 10 per 1000 PYFU in females (95% CI, 7–16). Median duration of follow-up between ART initiation and tuberculosis diagnosis was 15 months (IQR, 3–65). Incidence rates sharply decreased with the duration of follow-up, from 60 per 1000 PYFU (95% CI, 31–115) from 1 to 3 months after ART initiation to 5 per 1000 PYFU (95% CI, 2–10) after 5 years (Figure 2).
Figure 2.
Incidence rates of tuberculosis after antiretroviral therapy (ART) initiation, stratified by follow-up duration. Circles: estimated incidence rates per stratum of follow-up duration since ART initiation. Segments: 95% confidence intervals of the incidence rates per stratum, calculated based on the Poisson distribution. Dotted line: overall incidence rate.
Risk Factors for Incident Tuberculosis
In the univariable analysis to identify risk factors for incident tuberculosis, female sex (subhazard ratio [SHR] = 2.7, P = .02) and age-adjusted CD4 <5% (SHR = 3.5, P = .01), hemoglobin <9 g/dL (SHR = 2.7, P = .03), hematocrit <30% (SHR 2.5, P = .02), and lymphocytes <2000 cells/mm3 (SHR = 3.2, P = .04) at ART initiation were associated with a higher risk of incident tuberculosis (Table 2). In the multivariable analysis, female sex (adjusted SHR [aSHR] = 2.9, 95% CI = 1.3–6.8, P = .01) and age-adjusted CD4 <5% at ART initiation (aSHR = 3.9, 95% CI = 1.5–10.1, P = .006) were independently associated with a higher risk of incident tuberculosis. The same factors were found associated with incident tuberculosis when the analysis was restricted to children with complete data (female sex: aSHR = 2.8, 95% CI = 1.2–6.6, P = .02, and age-adjusted CD4 <5%: aSHR = 3.8, 95% CI = 1.5–10.0, P = .006).
Table 2.
Association Between Characteristics at ART Initiation and Diagnosis of Incident Tuberculosis
| Characteristics at ART Initiation | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| SHR (95% CI) | P Value | aSHR (95% CI) | P Value | |||
| Female sex | 2.7 (1.2 to 6.4) | .02 | 2.9 (1.3 to 6.8) | .01 | ||
| Age (ref: 2 to 6 years) | .16 | |||||
| <2 years | 1.6 (0.5 to 5.5) | |||||
| 6 to 10 years | 1.5 (0.5 to 5.1) | |||||
| ≥10 years | 3.2 (1.0 to 9.9) | |||||
| BMI-for-age z-scorea < −2 | 1.3 (0.6 to 2.9) | .49 | ||||
| Calendar year of enrollment (ref: 1999 to 2002) | .22 | |||||
| 2003 to 2005 | 2.3 (0.7 to 7.1) | |||||
| 2006 to 2012 | 1.2 (0.3 to 5.1) | |||||
| HIV RNA viral loada,b ≥5.5 log10 copies/mL | 2.5 (0.9 to 7.5) | .09 | ||||
| CD4a,b <5% | 3.5 (1.3 to 9.2) | .01 | 3.9 (1.5 to 10.1) | .006 | ||
| Hemoglobina,b <9 g/dL | 2.7 (1.1 to 6.7) | .03 | ||||
| Hematocrita,b <30% | 2.5 (1.2 to 5.5) | .02 | ||||
| Neutrophilsa,b ≥3000 cells/mm3 | 2.1 (0.9 to 5.1) | .09 | ||||
| Lymphocytesa,b <2000 cells/mm3 | 3.2 (1.1 to 9.5) | .04 | ||||
Abbreviations: ART, antiretroviral therapy; aSHR, adjusted subhazard ratio; BMI, body mass index; CI, confidence interval; HIV, human immunodeficiency virus; ref, reference; RNA, ribonucleic acid; SHR, subhazard ratio.
aMissing values were imputed using multiple imputation by chained equations with predictive mean matching.
bAdjusted for age.
Mortality
Diagnosis of incident tuberculosis was associated with a higher risk of mortality in both the univariable analysis (hazard ratio [HR] = 10.2, 95% CI = 4.8–21.5, P < .001) and the multivariable analysis adjusting for age, BMI-for-age, CD4 percentage, and hemoglobin levels at ART initiation (adjusted HR = 5.4, 95% CI = 2.5–11.7, P < .001) (Table 3).
Table 3.
Risk of
Death After Diagnosis of Incident Tuberculosis
| Characteristics | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | aHR (95% CI) | P Value | |||
| Tuberculosis diagnosis during follow-up (time-dependent variable) | 10.2 (4.8 to 21.5) | <.001 | 5.4 (2.5 to 11.7) | <.001 | ||
| Characteristics at ART initiation | ||||||
| Female sex | 1.1 (0.6 to 1.9) | .71 | ||||
| Age (ref: 2 to 6 years) | .08 | |||||
| <2 years | 3.6 (1.3 to 9.8) | |||||
| 6 to 10 years | 2.5 (0.9 to 6.8) | |||||
| ≥10 years | 3.2 (1.2 to 9.0) | |||||
| BMI-for-age z-scorea< −2 | 3.7 (2.1 to 6.5) | <.001 | 2.6 (1.4 to 4.6) | .002 | ||
| Calendar year of enrollment (ref: 1999 to 2002) | .35 | |||||
| 2003 to 2005 | 0.7 (0.3 to 1.2) | |||||
| 2006 to 2012 | 0.6 (0.3 to 1.4) | |||||
| HIV RNA viral loada,b ≥5.5 log10 copies/mL | 2.4 (1.2 to 4.7) | .01 | ||||
| CD4a,b <5% | 3.5 (1.8 to 6.8) | <.001 | 2.2 (1.1 to 4.6) | .03 | ||
| Hemoglobina,b <9 g/dL | 3.5 (1.6 to 7.5) | .001 | 2.4 (1.1 to 5.2) | .03 | ||
| Hematocrita,b <30% | 2.9 (1.6 to 5.2) | .001 | ||||
| Neutrophilsa,b ≥3000 cells/mm3 | 2.3 (1.2 to 4.4) | .02 | ||||
| Lymphocytesa,b <2000 cells/mm3 | 2.1 (1.1 to 4.1) | .03 | ||||
Abbreviations: aHR, adjusted hazard ratio; ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; HR, hazard ratio; HIV, human immunodeficiency virus; ref, reference; RNA, ribonucleic acid.
aMissing values were imputed using multiple imputation by chained equations with predictive mean matching.
bAdjusted for age.
Of the 30 incident tuberculosis cases, 9 (30%) died: 1 on the day after tuberculosis diagnosis, 3 while still on tuberculosis treatment, and 5 after completion of tuberculosis treatment. In this group of 9 children, 3 had a tuberculosis bacteriologically confirmed (2 pulmonary and 1 cutaneous) and 6 clinically diagnosed (5 pulmonary and 1 lymphadenitis). Ages at death were 1.3, 1.7, 6.5, 10.0, 12.1, 13.9, 14.9, 17.5, and 20.3 years. The median survival time after tuberculosis diagnosis was 5 months, and 2 had a concurrent acquired immune deficiency syndrome-defining event (progressive multifocal leukoencephalopathy and cryptococcal meningitis).
DISCUSSION
In this large prospective cohort of 670 HIV-infected children initiating ART in Thailand and followed for a median of 7.7 years, the overall tuberculosis incidence rate during follow-up was 7 per 1000 PYFU, with a dramatic decrease a few months after ART initiation (Figure 2). The estimated incidence rate in these HIV-infected children was much higher than in the general population of children in Thailand, as previously estimated in 2000 (0.15 per 1000 PYFU) or between 2004 and 2006 (0.14 per 1000 PYFU) [4, 6].
Despite Thailand being on the list of the 22 high tuberculosis burden countries, the incidence rate estimated in this study was similar to that reported from studies of HIV-infected children in the United States, the United Kingdom and Ireland, and China, but it was much lower than in sub-Saharan Africa. The tuberculosis incidence rate was 6 per 1000 PYFU in the United States (approximately 5-year follow-up) [17], 2 per 1000 PYFU in the United Kingdom and Ireland (8 years) [10], and 8 per 1000 PYFU in China (7 years) [18]. In contrast, the tuberculosis incidence rates were 64 per 1000 PYFU in 2 studies in South Africa (14 and 10 months, respectively) [19, 20], 175 per 1000 PYFU in Kenya (8 months) [21], 102 per 1000 PYFU in the Democratic Republic of the Congo (23 months) [22], and 52 per 1000 PYFU in Tanzania (10 months) [23]. This suggests that the tuberculosis incidence in HIV-infected children depends on the extent of the HIV epidemic in the HIV-infected and uninfected adult population; for example, in 2014, the tuberculosis incidence in the whole population was 1.8 per 1000 PYFU in Thailand compared with 8.3 per 1000 PYFU in South Africa [1].
The sharp decrease in tuberculosis incidence observed a few months after ART initiation was similar in previous reports such as in Kenya (3550 per 1000 PYFU within the 2 months after enrollment, then 460 per 1000 PYFU between 2 and 6 months, and 47 per 1000 PYFU after 6 months) [21] and in the Democratic Republic of the Congo (from 189 per 1000 PYFU in the first 6 months to 53 per 1000 PYFU after 12 months) [22]. This is likely the result of immune restoration. However, in our study, the incidence remained much higher than in the HIV-uninfected population: 5 per 1000 PYFU (95% CI, 2–10) after 5 years after ART initiation, compared with an estimated 0.15 per 1000 PYFU in the general pediatric population in Thailand [4, 6]. This may be related to other environmental factors, eg, poverty and social and family variables, which are not modified by ART.
As already known in other parts of the world, tuberculosis during follow-up was the strongest independent risk factor for death, indicating the severity of HIV disease. Simple, inexpensive, and widely available markers, ie, lower hematocrit and hemoglobin levels and lower lymphocyte and CD4 counts, are indicative of HIV severity and should prompt more investigations to detect life-threatening complications.
This study has several limitations. First, we used the WHO recommendations for diagnosis, ie, tuberculosis was not often bacteriologically confirmed. It has to be noted that definitive diagnosis of tuberculosis is now somewhat easier, although still a challenge, than at the beginning of our study due to the increased availability of new diagnostic tools, particularly GeneXpert. Second, the results of the analyses assessing risk factors need to be interpreted with caution due to the relatively small number of incident tuberculosis cases and deaths observed in our cohort. For example, the unexpected association of incident tuberculosis with female sex may be due to sampling.
CONCLUSIONS
In this large cohort of HIV-infected children in Thailand, tuberculosis was mostly diagnosed around the time of ART initiation, and the incidence declined soon after but remained much higher than the national estimate in the general pediatric population. As previously described, incident tuberculosis was strongly associated with the risk of death. Our analysis suggests that CD4 counts or hemoglobin or hematocrit levels, often routinely assessed in HIV treatment programs, may prompt clinicians to consider a possible tuberculosis infection.
Notes
Acknowledgments. We thank all children who participated in the Program for HIV Prevention and Treatment (PHPT) cohort as well as their caregivers; all staff at the study coordination center who contributed to the monitoring and data acquisition and management for this study; S. Chalermpantmetagul for the data management of medical records; L. Decker for the development of study IT systems; and T. Delory for critical review of the manuscript.
The study sites (number of children enrolled at each site) and investigators who contributed to this study were as follows: Chiangrai Prachanukroh (156), R. Hansudewechakul; Nakornping (120), S. Kanjanavanit; Chonburi (48), S. Hongsiriwon; Prapokklao (38), C. Ngampiyaskul; Bhumibol Adulyadej (30), J. Mekmullica, P. Layangool, and S. Phongjitsiri; Phayao Provincial (30), P. Techakunakorn; Samutsakhon (25), P. Thanasiri and S. Krikajornkitti; Kalasin (22), S. Srirojana; Lamphun (20), P. Wannarit, R. Somsamai, and R. Kosonsasitorn; Nakhonpathom (14), S. Bunjongpak; Rayong (14), W. Karnchanamayul; Somdej Prapinklao (14), M. Nantarukchaikul, B. Techasaensiri (Debaval), and N. Kamonpakorn; Mae Chan (13), S. Piyaworawong and S. Buranabanjasatean; Samutprakarn (12), C. Sriwacharakarn and A. Puangsombat; Doi Saket (10), P. Sirichithaporn; Nong Khai (10), S. Potchalongsin; Phaholpolpayuhasaena (9), P. Attavinijtrakarn; Chiang Dao (7), A. Saipanya; Buddhachinaraj (6), W. Ardong and N. Lertpienthum; Mae Sai (6), R. Paramee, S. Nanta, and S. Kunkongkapan; Phan (6), S. Jungpichanvanich and S. Theansavettrakul; Regional Health Promotion Centre 6, Khon Kaen (6), S. Hanpinitsak; Hat Yai (5), B. Warachit and T. Borkird; Queen Sirikit (5), T. Hinjiranandana; Banglamung (4), P. Kanjanavikai; Bhuddasothorn (4), R. Kwanchaipanich; Chiang Kham (4), V. Wanchaitanawong; Mahasarakam (4), S. Na-Rajsima; Sanpatong (4), N. Akarathum; Mae On (3), N. Pattanapornpun; Pranangklao (3), P. Lucksanapisitkul and S. Watanayothin; San Sai (3), W. Cowatcharagul; Sankhampang (3), N. Pipattanawong; Bamrasnaradura (2), S. Sirikwin; Ratchaburi (2), C. Sutthipong; Roi-et (2), P. Ananpatharachai; Srinagarind (2), P. Kosalaraksa; Banchang (1), K. Pattarakunpong and N. Sangwannakul; Health Promotion Center Region 10 (1), W. Jitphiankha; Phayamengrai (1), S. Laopichianpong; Vachira Phuket (1), W. Lawtongkum.
Financial support. This work was funded by the following: Global Fund to Fight AIDS, Malaria and Tuberculosis (Grant PR-A-N-008); Oxfam Great Britain, Thailand (Grant THAA51); Ministry of Public Health, Thailand; and Institut de Recherche pour le Développement, France.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global Tuberculosis Report 2015. Geneva: World Health Organization; 2015. [Google Scholar]
- 2. World Health Organization. TB/HIV Facts 2015. Available at: http://www.who.int/hiv/topics/tb/tbhiv_facts_2015/en/ Accessed 5 January 2017. [Google Scholar]
- 3. Thai National AIDS Committee. Thailand AIDS Response Progress Report 2015. Available at: http://www.unaids.org/sites/default/files/country/documents/THA_narrative_report_2015.pdf Accessed 5 January 2017. [Google Scholar]
- 4. Nelson LJ, Wells CD. Global epidemiology of childhood tuberculosis. Int J Tuberc Lung Dis 2004; 8:636–47. [PubMed] [Google Scholar]
- 5. Venturini E, Turkova A, Chiappini E, et al. Tuberculosis and HIV co-infection in children. BMC Infect Dis 2014; 14(Suppl 1):S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lolekha R, Anuwatnonthakate A, Nateniyom S, et al. Childhood TB epidemiology and treatment outcomes in Thailand: a TB active surveillance network, 2004 to 2006. BMC Infect Dis 2008; 8: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Collins IJ, Jourdain G, Hansudewechakul R, et al. Long-term survival of HIV-infected children receiving antiretroviral therapy in Thailand: a 5-year observational cohort study. Clin Infect Dis 2010; 51:1449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th Revision. Available at: http://www.who.int/classifications/icd/ICD10Volume2_en_2010.pdf Accessed 5 January 2017. [Google Scholar]
- 9. World Health Organization. Definitions and Reporting Framework for Tuberculosis, 2013 Revision. Geneva: World Health Organization; 2013. [Google Scholar]
- 10. Turkova A, Chappell E, Judd A, et al. Prevalence, incidence, and associated risk factors of tuberculosis in children with HIV living in the UK and Ireland (CHIPS): a cohort study. Lancet HIV 2015; 2:e530–9. [DOI] [PubMed] [Google Scholar]
- 11. Hasse B, Walker AS, Fehr J, et al. Co-trimoxazole prophylaxis is associated with reduced risk of incident tuberculosis in participants in the Swiss HIV Cohort Study. Antimicrob Agents Chemother 2014; 58:2363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94:496–509. [Google Scholar]
- 13. World Health Organization Multicentre Growth Reference Study Group. WHO Child Growth Standards. Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. Geneva: World Health Organization; 2006. [Google Scholar]
- 14. de Onis M, Onyango AW, Borghi E, et al. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 2007; 85:660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011; 30:377–99. [DOI] [PubMed] [Google Scholar]
- 16. Meintjes G, Lawn SD, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis 2008; 8:516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thomas P, Bornschlegel K, Singh TP, et al. Tuberculosis in human immunodeficiency virus-infected and human immunodeficiency virus-exposed children in New York City. The New York City Pediatric Spectrum of HIV Disease Consortium. Pediatr Infect Dis J 2000; 19:700–6. [DOI] [PubMed] [Google Scholar]
- 18. Mu W, Zhao Y, Sun X, et al. Incidence and associated factors of pulmonary tuberculosis in HIV-infected children after highly active antiretroviral therapy (HAART) in China: a retrospective study. AIDS Care 2014; 26:1127–35. [DOI] [PubMed] [Google Scholar]
- 19. Walters E, Cotton MF, Rabie H, et al. Clinical presentation and outcome of tuberculosis in human immunodeficiency virus infected children on anti-retroviral therapy. BMC Pediatr 2008; 8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martinson NA, Moultrie H, van Niekerk R, et al. HAART and risk of tuberculosis in HIV-infected South African children: a multi-site retrospective cohort. Int J Tuberc Lung Dis 2009; 13:862–7. [PMC free article] [PubMed] [Google Scholar]
- 21. Braitstein P, Nyandiko W, Vreeman R, et al. The clinical burden of tuberculosis among human immunodeficiency virus-infected children in Western Kenya and the impact of combination antiretroviral treatment. Pediatr Infect Dis J 2009; 28:626–32. [DOI] [PubMed] [Google Scholar]
- 22. Edmonds A, Lusiama J, Napravnik S, et al. Anti-retroviral therapy reduces incident tuberculosis in HIV-infected children. Int J Epidemiol 2009; 38:1612–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li N, Manji KP, Spiegelman D, et al. Incident tuberculosis and risk factors among HIV-infected children in Tanzania. AIDS 2013; 27:1273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]