Summary
Postdischarge readmission and outpatient illnesses are frequent in children with severe malarial anemia or cerebral malaria. Trials of postdischarge malaria prophylaxis in children with severe malaria should be considered.
Keywords: cerebral malaria, incidence, readmission, severe malarial anemia
Abstract
Background
Cerebral malaria (CM) and severe malarial anemia (SMA) account for a substantial proportion of malaria-related deaths in sub-Saharan Africa. However, postdischarge morbidity in children with CM or SMA has not been well established.
Methods
Children 18 months to 12 years of age, enrolled on admission to Mulago National Referral Hospital in Kampala, Uganda (CM, n = 162; SMA, n = 138), and healthy children recruited from the community (CC) (n = 133) were followed up for 6 months. The incidences of hospitalizations and outpatient clinic visits for illness during the follow-up period were compared between children with CM or SMA and the CC.
Results
After adjustment for age, sex, and nutritional status, children with SMA had a higher incidence rate ratio (IRR) than CC for hospitalization (95% confidence interval [CI], 20.81 [2.48–174.68]), hospitalization with malaria (17.29 [95% CI, 2.02–148.35]), and clinic visits for any illness (95% CI, 2.35 [1.22–4.51]). Adjusted IRRs for children with CM were also increased for all measures compared with those for CC, but they achieved statistical significance only for clinic visits for any illness (2.24 [95% CI, 1.20–4.15]). In both groups, the primary reason for the clinic visits and hospitalizations was malaria.
Conclusions
In the 6 months after initial hospitalization, children with SMA have an increased risk of repeated hospitalization, and children with CM or SMA have an increased risk of outpatient illness. Malaria is the main cause of inpatient and outpatient morbidity. Malaria prophylaxis has the potential to decrease postdischarge morbidity rates in children with SMA or CM.
INTRODUCTION
Cerebral malaria (CM) and severe malarial anemia (SMA) are important manifestations of severe malaria. It is estimated that 6 million African children per year develop CM or SMA [1]. Cerebral malaria is the most lethal manifestation of severe malaria; it causes an inpatient case-fatality rate of 15% to 20% [2]. However, SMA is the most common presentation and affects 7.5% to 34% of children who acquire severe malaria [3–5]. Estimates of deaths caused by CM or SMA are imprecise, but they might account for more than half of all malaria-related deaths in malaria-endemic regions of sub-Saharan Africa [1, 6].
Although their clinical manifestations differ, survivors of CM and SMA both suffer significant postdischarge morbidity. Of children with CM, 11% to 26% are discharged with gross neurological deficits [7–9], and another 25% develop long-term cognitive, motor, or behavior impairments [2, 10, 11]. Similarly 10% to 16% of SMA survivors die or are readmitted 3 to 6 months after discharge from a hospital [12–14], and the long-term overall cognitive impairment in children with SMA approaches that of children with CM [15].
Studies of severe anemia in African children, which is caused most often by malaria, have found higher rates of readmission and death in these children than in nonanemic children [4, 12, 13, 16]. However, there are few data on rates of postdischarge sick visits, outpatient malaria, readmission, and death in children with CM or specifically with SMA (as opposed to all-cause severe anemia). In this study, we aimed to characterize and compare rates of outpatient sick visits and hospitalizations, including sick visits or hospitalizations that resulted from malaria, in children with SMA or CM in the first 6 months after discharge and in healthy children recruited from the community (CC).
METHODS
Study Participants
This study was performed at Mulago National Referral Hospital, Kampala, Uganda, as part of a larger study to assess neurodevelopmental impairment in children with CM or SMA and in CC. Participants were enrolled in the primary study between November 2008 and October 2013. In the primary study, children with CM or SMA and CC were enrolled if they were between 18 months and 12 years of age. Cerebral malaria was defined per World Health Organization (WHO) criteria, which include coma (Blantyre Coma Score, ≤2), blood smear–positive test for Plasmodium falciparum, and no other known cause of the coma (e.g., meningitis, prolonged postictal state, or hypoglycemia-associated coma reversed by glucose infusion). Malarial retinopathy was assessed in 155 of the 162 children with CM and was present in 61% of them. Because the current WHO definition of CM does not include retinopathy as a criterion, all children with WHO-defined CM were included in our analysis. Severe malarial anemia was defined as the presence of P falciparum on a blood smear in children with a hemoglobin level of ≤5 g/dL. Children with CM or SMA were managed according to the Ugandan Ministry of Health treatment guidelines current at the time of the study. These guidelines included intravenous quinine treatment followed by oral quinine for severe malaria while admitted and artemisinin combination therapy for outpatient follow-up therapy. All children with a hemoglobin level of ≤5 g/dL received a blood transfusion.
CC were recruited from the nuclear family, extended family, or household compound of children with CM or SMA. Eligible CC were 18 months to 12 years of age and healthy at the time of enrollment. To recruit CC, parents of children with CM or SMA were provided information about the study and asked to bring any eligible children to the clinic for evaluation. Parents of children in the household compound of a child with CM or SMA were also notified about the study during a home visit. During home visits, study personnel verified that all CC were from the family or household compound as an original child with CM or SMA. Children were enrolled if they met the inclusion criteria and did not meet exclusion criteria. Exclusion criteria for children with CM included having a known chronic illness that required medical care, a known developmental delay, or a previous history of coma, head trauma, hospitalization for malnutrition, or cerebral palsy. In addition, children with SMA were excluded if they had impaired consciousness on physical examination, other clinical evidence of central nervous system disease, or >1 seizure before admission. Exclusion criteria for the CC included having an illness that required medical care within the previous 4 weeks or major medical or neurological abnormalities on screening physical examination.
A subgroup of participants in the primary study was enrolled in a study of acute or delayed iron treatment for children with severe malaria and iron deficiency. Because iron treatment can affect risk of clinical malaria [17], these children were excluded from this study. Children in this study who had a hemoglobin level of <7 g/dL at discharge were prescribed iron treatment for 1 month, but whether they purchased and/or consumed the recommended iron treatment was not known. Children with sickle cell disease (confirmed by genotyping) were also excluded from this study, because they are known to have a higher risk of readmission than children without sickle cell disease.
Clinical and Demographic Assessment
Each child underwent a medical history and physical examination. Children with CM were assessed by indirect ophthalmoscopy for malarial retinopathy [18]. Their nutrition was assessed by height- and weight-for-age z scores (Epi Info 3.5.3, Centers for Disease Control and Prevention, Atlanta, Georgia). Emotional stimulation in the home was measured by age-appropriate versions of the Home Observation for the Measurement of the Environment measure [19]. Their socioeconomic status was measured using a previously described scoring system in which lower scores have been associated with worse cognitive functioning in healthy Ugandan children ≥5 years old [20]. From 2011 onward, a Garmin global positioning system (GPS) was used to locate the homes of study participants and determine their distance from the hospital.
Follow-Up
All study children received an insecticide-treated bednet at the start of the study. All study children were asked to return to the hospital for a follow-up visit in 6 months, and caregivers of all study children were asked to bring the children back to Mulago National Referral Hospital whenever they became sick in the follow-up period. During each hospital visit, children were assessed and managed by study clinicians per national guidelines. All hospital visits and admissions to Mulago National Referral Hospital were recorded, and a blood smear was performed to test for malaria in children who presented with a history of fever or had fever on examination.
Statistical Analysis
Demographics among the 3 groups were compared using the Pearson χ2 and Wilcoxon rank-sum tests. The incidence of clinic events (outpatient clinic visits, hospitalization, or deaths) was calculated and compared to the incidence in the CC by negative binomial regression, with adjustment for age, sex, and weight-for-age z scores, which differed significantly between the study groups. Negative binomial regression was used because of significant overdispersion in the incidence rates. Hazard ratios (HRs), adjusted for age, sex, and weight-for-age z scores using Cox regression, were calculated. Incidence rate ratios (IRRs) and HRs cannot be computed when there are no events in 1 of the groups. Because there were no readmissions in the CC groups, we calculated the IRRs and HRs for readmissions comparing children with CM or SMA with CC by imputing 1 admission to the CC group.
Ethical Review
Written informed consent was obtained from the parents or guardians of study participants. Ethical approval was granted by the institutional review boards for human studies at Makerere University and the University of Minnesota.
RESULTS
Demographic and Clinical Characteristics
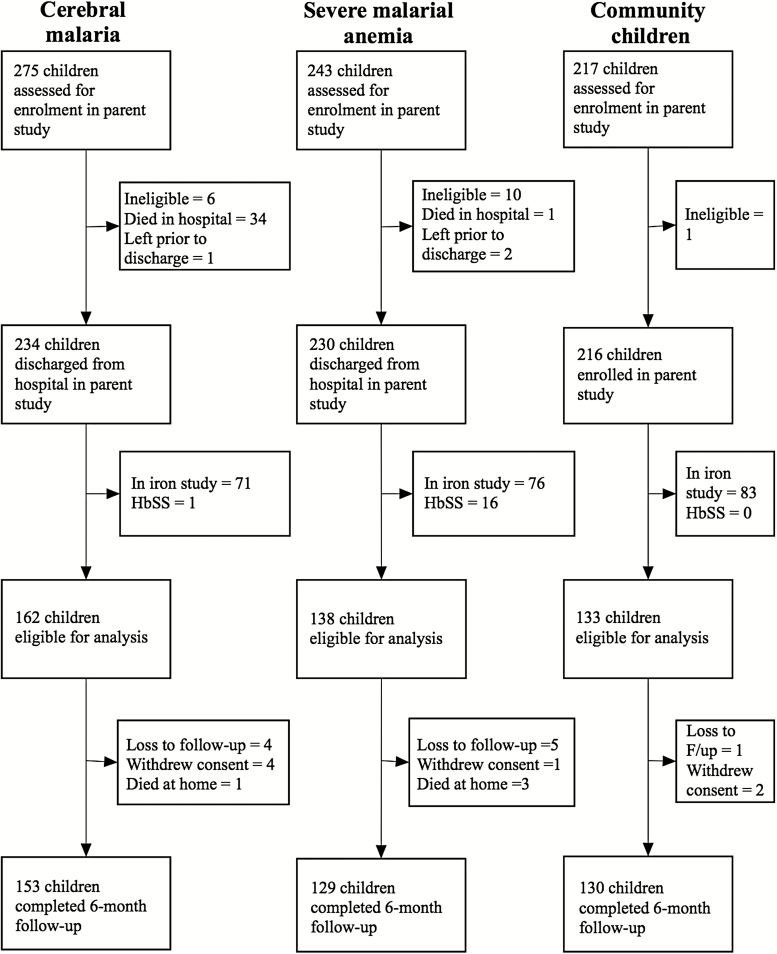
A total of 433 children were discharged and eligible for analysis in this study. Of these children, 412 (95.2% [153 CM, 129 SMA, and 130 CC]) completed a 6-month follow-up examination (Figure 1). Twenty-six percent (42 of 162) of the children with CM had concurrent SMA. Concurrent SMA did not modify the effect of CM on any outcome, so the children with CM were assessed as a single group regardless of whether they had concurrent SMA. The children with SMA were younger than the children with CM or the CC, whereas children with CM were more likely to be male, and the children with CM or SMA had lower weight-for-age z scores, which is a marker of wasting and malnutrition (Table 1). Overall, 5.3% of the children were carriers of the sickle cell gene (HbAS). The CC were more likely to be carriers of the sickle cell gene than children with CM or SMA, which confirms the protective nature of the sickle cell carrier state against severe malaria (Table 1). Blood cultures were performed for 275 children, including almost all the children with CM (n = 156) and the children with SMA who had clinical signs that suggested pneumonia or sepsis (n = 119). The results of 24 (8.7% [16 children with CM, 8 children with SMA]) of the 275 blood cultures performed were positive, primarily for Staphylococcus aureus. Distance from the child's household to Mulago National Referral Hospital was calculated for 177 children, and distances from the hospital were similar among the 3 groups (P > .5 for all comparisons).
Figure 1.
Study enrollment and follow-up.
Table 1.
Baseline Characteristics of the Study Cohort
| Characteristic | CM (n = 162) | SMA (n = 138) | CC (n = 133) | CM vs CC (P) | SMA vs CC (P) |
|---|---|---|---|---|---|
| Age (median [25%, 75%]) | 3.9 (2.7, 6.0) | 2.8 (2.1, 4.6) | 4.0 (3.0, 5.7) | .43a | <.001a |
| Sex (n [%] female) | 57 (35.2) | 55 (39.9) | 64 (48.1) | .03b | .17b |
| Height-for-age z score (median [25%, 75%]) | −0.35 (−1.25, 0.53) | −0.90 (−1.7, −0.24) | −0.89 (−1.8, −0.17) | <.001a | .61a |
| Weight-for-age z score (median [25%, 75%]) | −1.2 (−1.9, −0.40) | −1.6 (−2.5, −0.63) | −0.64 (−1.3, 0.10) | <.001a | <.001a |
| Total SES (median [25%, 75%]) | 9.0 (8.0, 10.0) | 8.5 (7.0, 11.0) | 9.0 (7.0, 11.0) | .85a | .88a |
| HIV infection (n [% positive]) | 4 (2.5) | 4 (2.9) | 1 (0.8) | .25b | .19b |
| Mother is primary caregiver (n [%]) | 110 (67.9) | 106 (76.8) | 81 (60.1) | .21b | .005b |
| Maternal education level (n [%] secondary education) | 63 (38.9) | 60 (43.5) | 55 (41.3) | .40b | .99b |
| Possesses sickle cell trait (HbAS) (n [%]) | 2 (1.2) | 2 (1.4) | 19 (14.3) | <.001b | <.001b |
Abbreviations: CC, healthy children recruited from the community; CM, cerebral malaria; HIV, human immunodeficiency virus; SES, socioeconomic status; SMA, severe malarial anemia.
aAnalyzed using the Wilcoxon rank-sum test.
bAnalyzed using the Pearson χ2 test.
Deaths During the Follow-Up Period
Four children died during the 6 months of follow-up, including 1 (0.6%) of the 162 children with CM, 3 (2.2%) of the 138 children with SMA, and none of the 132 CC (Figure 1).
Incidence and Risk of Hospitalization and Outpatient Clinic Visits
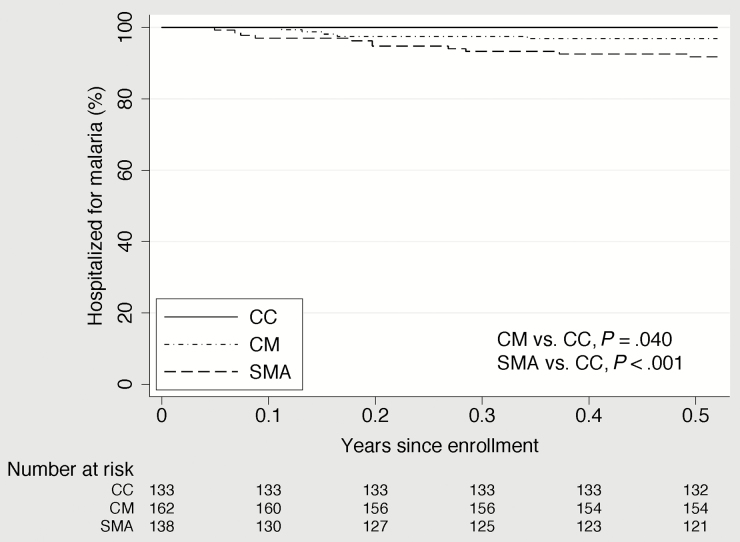
A total of 18 children (5 with CM, 13 with SMA) were hospitalized during the study period; 15 of these children were hospitalized once, 2 were hospitalized twice, and 1 were hospitalized thrice. Eighty children (35 with CM, 33 with SMA, and 12 CC) made clinic visits; 63 of them made 1 clinic visit, 16 made 2 visits, and 1 made 3 visits. The unadjusted incidence of all-cause hospitalizations, hospitalizations that resulted from malaria, and all-cause clinic visits were each significantly higher in children with SMA or CM than in the CC (Table 2). The children with SMA or CM had 24.4 and 6.0 hospitalizations per 100 person-years, respectively, whereas the CC had no hospitalizations in the entire 6-month follow-up period (P < .001 for children with SMA compared with CC, P = .049 for children with CM compared with CC). The incidence of clinic visits because of malaria was significantly higher in children with SMA than in the CC but not in children with CM than in the CC (Table 2). Malaria was the most common reason for the hospitalizations (16 of 18 [88.9%]) and clinic visits (47 of 80 [58.8%]).
Table 2.
Incidence of Hospitalizations and Outpatient Clinic Visits for Children With Cerebral Malaria or Severe Malarial Anemia and Community Children
| Clinical Outcome | CM (n = 162) | SMA (n = 138) | CC (n = 133) | CM vs CC (P) | SMA vs CC (P) |
|---|---|---|---|---|---|
| Children hospitalized (n [%]) | 5 (3.1) | 13 (9.4) | 0 (0) | .07a | <.001a |
| Hospitalizations per 100 person-years | 6.0 | 24.4 | 0 | .049 | <.001 |
| Children hospitalized for malaria (n [%]) | 5 (3.1) | 11 (8.0) | 0 (0) | .07a | .001a |
| Hospitalizations for malaria per 100 person-years | 6.3 | 20.1 | 0 | .049 | .001 |
| Children seen in clinic for any illness (n [%]) | 35 (21.6) | 33 (23.9) | 12 (9.0) | .003b | .001b |
| Clinic visits per 100 person-years | 52.9 | 56.1 | 21.7 | .002 | .001 |
| Children seen in clinic for malaria (n [%]) | 18 (11.1) | 21 (15.2) | 8 (6.0) | .12b | .01b |
| Clinic visits for malaria per 100 person-years | 26.5 | 31.6 | 14.5 | .11 | .04 |
Abbreviations: CC, healthy children recruited from the community; CM, cerebral malaria; SMA, severe malarial anemia.
aAnalyzed using the Fisher exact test.
bAnalyzed using the Pearson χ2 test.
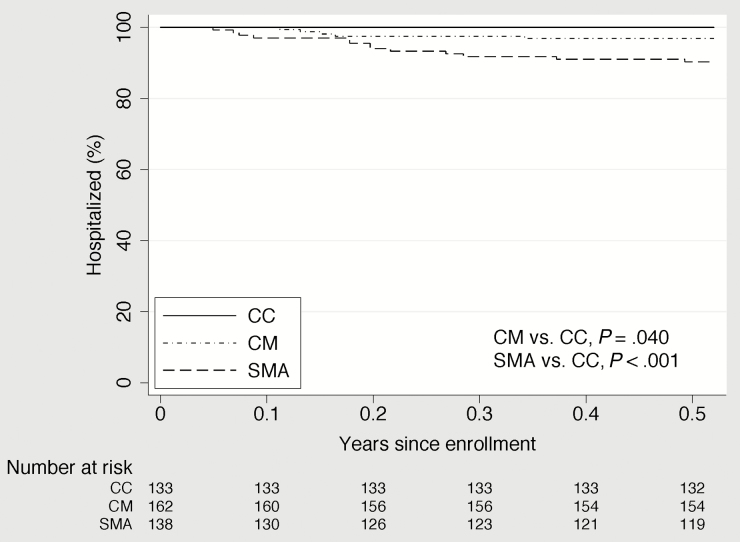
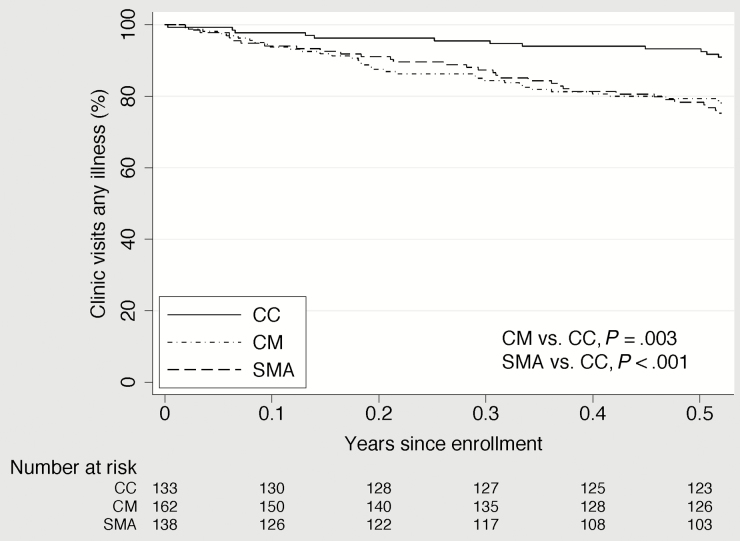
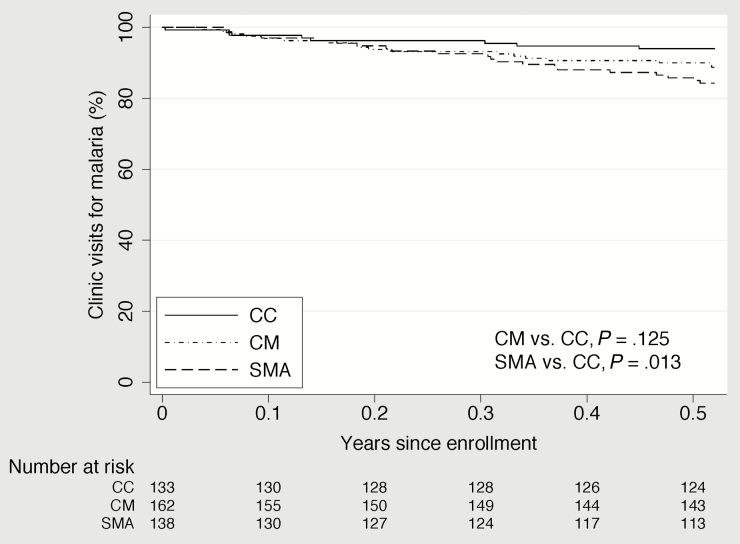
In unadjusted analysis, children with CM or SMA had a statistically significantly shorter time to their first clinic visit for any cause than the CC and a shorter time to their first hospitalization for malaria than the CC (Figures 2–4). Compared to the CC, time to first clinic visit for malaria was statistically significantly shorter in children with SMA but not in those with CM (Figure 5).
Figure 2.
Time to hospitalization for any illness in community children (CC), children with cerebral malaria (CM), and children with severe malarial anemia (SMA).
Figure 4.
Time to clinic visit for any illness in community children (CC), children with cerebral malaria (CM), and children with severe malarial anemia (SMA).
Figure 5.
Time to clinic visit for malaria in community children (CC), children with cerebral malaria (CM), and children with severe malarial anemia (SMA).
Figure 3.
Time to hospitalization for malaria in community children (CC), children with cerebral malaria (CM), and children with severe malarial anemia (SMA).
Because the CC had no hospitalizations, HRs and IRRs were calculated by imputing 1 hospitalization to the CC group. Adjusted HRs for hospitalization or hospitalization that resulted from malaria were higher in the children with CM or SMA than in the CC, but the difference achieved statistical significance only in children with SMA (Table 3). The adjusted HR of a clinic visit for any cause remained significantly higher in children with CM or SMA than in the CC, but the adjusted risk of a clinic visit for malaria was higher only in children with SMA than in the CC (Table 3).
Table 3.
Hazard Ratios for Risk of First Hospitalization or First Outpatient Clinic Visit in Children With Cerebral Malaria or Severe Malarial Anemia Compared With Those of Community Children
| Clinical Outcome | Crude HR (95% CI) | P | Adjusted HR (95% CI)a | P |
|---|---|---|---|---|
| Hospitalizationb | ||||
| CC | 1.0 (reference) | |||
| SMA | 13.57 (1.77–103.71) | .01 | 16.26 (2.03–130.34) | .01 |
| CM | 4.23 (.49–36.17) | .19 | 4.45 (.51–38.55) | .18 |
| Hospitalization for malariab | ||||
| CC | 1.0 (reference) | |||
| SMA | 12.70 (1.64–98.38) | .02 | 15.77 (1.91–130.54) | .01 |
| CM | 4.37 (.51–37.41) | .18 | 4.69 (.54–40.90) | .16 |
| Clinic visit for any illness | ||||
| CC | 1.0 (reference) | 1.0 (reference) | ||
| SMA | 3.24 (1.64–6.40) | .001 | 2.99 (1.46–6.10) | .003 |
| CM | 2.89 (1.47–5.69) | .002 | 2.65 (1.33–5.26) | .005 |
| Clinic visit for malaria | ||||
| CC | 1.0 (reference) | 1.0 (reference) | ||
| SMA | 3.09 (1.31–7.27) | .01 | 2.82 (1.15–6.91) | .02 |
| CM | 2.18 (.91–5.23) | .08 | 2.15 (.89–5.21) | .09 |
Abbreviations: CC, healthy children recruited from the community; CI, confidence interval; CM, cerebral malaria; HR, hazard ratio; SMA, severe malarial anemia.
aAdjusted for age, sex, and weight-for-age z score using Cox regression.
bCC had no hospitalizations within 6 months of follow-up, but the HRs were calculated as if 1 CC was hospitalized.
Similar results were found for IRRs; adjusted IRRs for hospitalization and hospitalization with malaria were higher in the children with CM or SMA than in the CC but were statistically significant only in children with SMA. The adjusted IRRs for all-cause clinic visits were increased significantly in children with CM or SMA compared with that in the CC, but clinic visits that resulted from malaria were not increased significantly for children with CM or SMA (Table 4). Incidence, risk, and unadjusted and adjusted IRRs or HRs for hospitalizations and clinic visits did not differ significantly among the children with CM and those with SMA (data not shown).
Table 4.
Incidence Rate Ratios for Hospitalizations and Outpatient Clinic Visits for Illness in Children With Cerebral Malaria or Severe Malarial Anemia (SMA) Compared to Those for Community Children
| Clinical Outcome | Crude IRR (95% CI) | P | Adjusted IRR (95% CI)a | P |
|---|---|---|---|---|
| All hospitalizationsb | ||||
| CC | 1.0 (reference) | |||
| SMA | 16.88 (2.14–133.21) | .007 | 20.81 (2.48–174.68) | .005 |
| CM | 4.15 (.47–37.11) | .20 | 4.59 (.50–41.80) | .18 |
| Hospitalizations for malariab | ||||
| CC | 1.0 (reference) | |||
| SMA | 13.90 (1.74–110.98) | .01 | 17.29 (2.02–148.35) | .009 |
| CM | 4.15 (.47–37.10) | .20 | 4.51 (.49–41.26) | .18 |
| Clinic visits for any illness | ||||
| CC | 1.0 (reference) | 1.0 (reference) | ||
| SMA | 2.58 (1.38–4.83) | .003 | 2.35 (1.22–4.51) | .01 |
| CM | 2.44 (1.32–4.50) | .004 | 2.24 (1.20–4.15) | .01 |
| Clinic visits for malaria | ||||
| CC | 1.0 (reference) | 1.0 (reference) | ||
| SMA | 2.19 (.99–4.81) | .05 | 1.92 (.84–4.40) | .12 |
| CM | 1.83 (.83–4.01) | .13 | 1.78 (.80–3.93) | .16 |
Abbreviations: CC, healthy children recruited from the community; CI, confidence interval; CM, cerebral malaria; IRR, incidence rate ratio; SMA, severe malarial anemia.
aAdjusted for age, sex, and weight-for-age z score using negative binomial regression.
bCC had no hospitalizations within 6 months of follow-up, but the IRRs were calculated as if 1 CC was hospitalized.
DISCUSSION
In this study, we found that children with SMA are at an increased risk of all-cause and malaria-specific hospitalization and outpatient sick visits in the first 6 months after discharge and that children with CM have the same trends in increased risk of hospitalization and have a significantly increased risk of outpatient sick visits. Our study findings establish that, compared with CC, children with SMA have a particularly high risk of postdischarge readmission, and children with CM are at a higher risk of postdischarge outpatient illness and also might have a higher risk of readmission.
None of the CC required readmission, so we had to artificially increase the number of readmissions to 1 in the CC group to calculate HRs and IRRs. This adjustment moved comparisons between disease groups and CC toward the null hypothesis, so differences in the adjusted analyses were likely more pronounced for children with CM or SMA than the differences in our analysis. Ninety percent of hospitalizations and almost 60% of the outpatient visits were a result of malaria. Together, these findings support the use of malaria prophylaxis in children with SMA to reduce readmissions and outpatient illness caused by malaria and suggest that malaria prophylaxis might be useful in other forms of severe malaria, such as CM, to prevent outpatient illness and readmission caused by malaria.
Studies in Malawi [12] and Kenya [13, 14] also found increased mortality rates in children with all-cause severe anemia. The mortality rate in children with SMA in our study over 6 months of follow-up was lower (2.1%) than those in the other studies, although those studies had different follow-up times (Kenya, 13% over 8 weeks; Malawi 12.6% over 18 months). The use of artemisinin combination therapy in this study as standard-of-care first-line treatment for malaria likely contributed to some of the difference in mortality findings from these earlier studies; a subsequent study in Malawi of malaria prophylaxis for severe malaria [21] found a 2% mortality rate in the placebo group, a rate similar to that seen in our study.
It was critical for the study to have CC with malaria exposure that was similar to that of the children with CM or SMA, so children were recruited from the family or extended household of a child with CM or SMA. A 1:1 matched design in which each CC was age-matched to each child with CM or SMA would have helped us to control more definitively for exposure, but matching according to age range was necessary, because it was not possible to find age-matched children in the same household as each index case child, and it was not possible within funding constraints to recruit a control for each child with CM or SMA. Instead, we recruited a total number of CC that was similar to the total with CM or SMA. However, the CC were recruited equally from the households of children with CM and those with SMA, so it is likely that overall malaria exposure in the CC was similar that of the CM and SMA groups.
The exclusion of children <18 months old from the study might have led to many younger children, who are generally more prone to malarial attacks, particularly SMA, not being included [6, 22]. Also, as part of this study, all the children were provided with insecticide-treated bednets and were facilitated by the study to get medical care whenever they were ill. Given the prevailing low level of net coverage and poor access to care in Uganda [23], under nonstudy conditions, the postdischarge morbidity rate would likely have been higher.
The pathophysiological mechanisms for the high incidence of postdischarge morbidity in survivors of severe malarial forms such as SMA and CM are unclear. It has been reported that children with severe malaria have continued dyserythropoiesis and bone marrow suppression that lasts for several weeks, even after the initial clearance of parasitemia [24, 25]. This bone marrow suppression can impair a child's ability to respond effectively to repeat malarial attacks or other bacterial infections in the postdischarge period, which makes the child more prone to illnesses that require clinic visits and even hospitalizations. It is also possible for malaria to combine with underlying conditions, such as malnutrition or iron and other micronutrient deficiencies, to produce more severe disease. Thus, the cause of illness that leads to readmission in these children might be multifactorial, but with malaria present in ∼90% of readmissions, it is clearly a driving factor in readmissions in these children.
Postdischarge morbidity in survivors of severe malaria might also be attributable to recrudescence of uncleared parasitemia [26], untreated bacteremia during the malaria episode [27], or coinfection with human immunodeficiency virus (HIV). However, none of these problems was likely a major cause of postdischarge morbidity in our study population. In this study, after initial parenteral quinine, all children were put on artemether-lumefantrine, a highly efficacious antimalarial that confers a low risk of recrudescence after treatment [26]. Most children with CM were empirically started on broad-spectrum antibiotics at admission, whereas children with SMA who had clinical signs of pneumonia or sepsis were also treated presumptively with antibiotics, and all children whose blood culture was positive were treated with antibiotics. Therefore, it is unlikely that untreated bacteremia was responsible for postdischarge morbidity in this study. HIV infection has been associated with poor postdischarge outcomes in children with severe malaria [28, 29], but in this study, only 2.1% of all the study children were HIV positive, and none of these children were readmitted.
Regardless of the pathophysiological mechanism involved, the results of this study add to those of previous studies of severe anemia to suggest that postdischarge morbidity in children with severe malaria, not only SMA but also CM, is high. There is an urgent need to develop guidelines for the postdischarge management of survivors of severe malaria. A recent study found that prophylaxis with artemether-lumefantrine in Malawian children with severe anemia decreased deaths or readmissions caused by severe anemia or malaria by 31% [21] and decreased clinic visits for malaria by 26% during 6 months of follow-up. Future studies should assess whether such interventions should be extended to children with CM and other forms of severe malaria.
In conclusion, in the 6 months after discharge, children with SMA or CM are at increased risk for outpatient morbidity that requires medical evaluation, and children with SMA and possibly CM are at an increased risk for readmission. Outpatient and inpatient illness in these children is largely caused by malaria. Postdischarge malaria prophylaxis has the potential to decrease postdischarge morbidity in children with CM or SMA. Additional studies are needed to elucidate what predisposes survivors of severe malaria to continued postdischarge morbidity.
Notes
Acknowledgments. We thank the children and their parents for their participation in this study and the study team for their dedicated efforts in treating the children and collecting the data.
Author contributions. R. O. O., N. B., R. I., P. B., and C. C. J. were involved in the design and conduct of this study; K. E. S. H. conducted the data analysis; and R. O. O. wrote the first draft of the manuscript. All authors commented on and approved the final version of the manuscript.
Disclaimer. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Institute of Neurological Disorders and Stroke and the Fogarty International Center (grants R01NS055349 and D43 NS078280).
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Murphy SC, Breman JG. Gaps in the childhood malaria burden in Africa: cerebral malaria, neurological sequelae, anemia, respiratory distress, hypoglycemia, and complications of pregnancy. Am J Trop Med Hyg 2001; 64(1–2 Suppl):57–67. [DOI] [PubMed] [Google Scholar]
- 2. Idro R, Marsh K, John CC, Newton CR. Cerebral malaria: mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatr Res 2010; 68:267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Obonyo CO, Vulule J, Akhwale WS, Grobbee DE. In-hospital morbidity and mortality due to severe malarial anemia in western Kenya. Am J Trop Med Hyg 2007; 77(6 Suppl):23–8. [PubMed] [Google Scholar]
- 4. Biemba G, Dolmans D, Thuma PE, Weiss G, Gordeuk VR. Severe anaemia in Zambian children with Plasmodium falciparum malaria. Trop Med Int Health 2000; 5:9–16. [DOI] [PubMed] [Google Scholar]
- 5. Taylor T, Olola C, Valim C, et al. Standardized data collection for multi-center clinical studies of severe malaria in African children: establishing the SMAC network. Trans R Soc Trop Med Hyg 2006; 100:615–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Opoka RO, Xia Z, Bangirana P, John CC. Inpatient mortality in children with clinically diagnosed malaria as compared with microscopically confirmed malaria. Pediatr Infect Dis J 2008; 27:319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Newton CR, Krishna S. Severe falciparum malaria in children: current understanding of pathophysiology and supportive treatment. Pharmacol Ther 1998; 79:1–53. [DOI] [PubMed] [Google Scholar]
- 8. van Hensbroek MB, Palmer A, Jaffar S, Schneider G, Kwiatkowski D. Residual neurologic sequelae after childhood cerebral malaria. J Pediatr 1997; 131:125–9. [DOI] [PubMed] [Google Scholar]
- 9. John CC, Bangirana P, Byarugaba J, et al. Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics 2008; 122:e92–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carter JA, Ross AJ, Neville BG, et al. Developmental impairments following severe falciparum malaria in children. Trop Med Int Health 2005; 10:3–10. [DOI] [PubMed] [Google Scholar]
- 11. Ngoungou EB, Preux PM. Cerebral malaria and epilepsy. Epilepsia 2008; 49(Suppl 6):19–24. [DOI] [PubMed] [Google Scholar]
- 12. Phiri KS, Calis JC, Faragher B, et al. Long term outcome of severe anaemia in Malawian children. PloS One 2008; 3:e2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zucker JR, Lackritz EM, Ruebush TK, 2nd, et al. Childhood mortality during and after hospitalization in western Kenya: effect of malaria treatment regimens. Am J Trop Med Hyg 1996; 55:655–60. [DOI] [PubMed] [Google Scholar]
- 14. Lackritz EM, Hightower AW, Zucker JR, et al. Longitudinal evaluation of severely anemic children in Kenya: the effect of transfusion on mortality and hematologic recovery. AIDS (London, England) 1997; 11:1487–94. [DOI] [PubMed] [Google Scholar]
- 15. Bangirana P, Opoka RO, Boivin MJ, et al. Severe malarial anemia is associated with long-term neurocognitive impairment. Clin Infect Dis 2014; 59:336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lackritz EM, Campbell CC, Ruebush TK, 2nd, et al. Effect of blood transfusion on survival among children in a Kenyan hospital. Lancet 1992; 340:524–8. [DOI] [PubMed] [Google Scholar]
- 17. Sazawal S, Black RE, Ramsan M, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet 2006; 367:133–43. [DOI] [PubMed] [Google Scholar]
- 18. Birbeck GL, Beare N, Lewallen S, et al. Identification of malaria retinopathy improves the specificity of the clinical diagnosis of cerebral malaria: findings from a prospective cohort study. Am J Trop Med Hyg 2010; 82:231–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caldwell BM, Bradley RH. Home Inventory Administration Manual, 3rd edition Little Rock, AR: University of Arkansas; 2001. [Google Scholar]
- 20. Bangirana P, John CC, Idro R, et al. Socioeconomic predictors of cognition in Ugandan children: implications for community interventions. PLoS One 2009; 4:e7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Phiri K, Esan M, van Hensbroek MB, Khairallah C, Faragher B, ter Kuile FO. Intermittent preventive therapy for malaria with monthly artemether-lumefantrine for the post-discharge management of severe anaemia in children aged 4–59 months in southern Malawi: a multicentre, randomised, placebo-controlled trial. Lancet Infect Dis 2012; 12:191–200. [DOI] [PubMed] [Google Scholar]
- 22. Roca-Feltrer A, Carneiro I, Smith L, Schellenberg JR, Greenwood B, Schellenberg D. The age patterns of severe malaria syndromes in sub-Saharan Africa across a range of transmission intensities and seasonality settings. Malaria J 2010; 9:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wanzira H, Yeka A, Kigozi R, et al. Long-lasting insecticide-treated bed net ownership and use among children under five years of age following a targeted distribution in central Uganda. Malaria J 2014; 13:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Camacho LH, Gordeuk VR, Wilairatana P, Pootrakul P, Brittenham GM, Looareesuwan S. The course of anaemia after the treatment of acute, falciparum malaria. Ann Trop Med Parasitol 1998; 92:525–37. [DOI] [PubMed] [Google Scholar]
- 25. Kurtzhals JA, Rodrigues O, Addae M, Commey JO, Nkrumah FK, Hviid L. Reversible suppression of bone marrow response to erythropoietin in Plasmodium falciparum malaria. Br J Haematol 1997; 97:169–74. [DOI] [PubMed] [Google Scholar]
- 26. Bukirwa H, Yeka A, Kamya MR, et al. Artemisinin combination therapies for treatment of uncomplicated malaria in Uganda. PLoS Clin Trials 2006; 1:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Church J, Maitland K. Invasive bacterial co-infection in African children with Plasmodium falciparum malaria: a systematic review. BMC Med 2014; 12:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ezeamama AE, Spiegelman D, Hertzmark E, et al. HIV infection and the incidence of malaria among HIV-exposed children from Tanzania. J Infect Dis 2012; 205:1486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malamba S, Hladik W, Reingold A, et al. The effect of HIV on morbidity and mortality in children with severe malarial anaemia. Malaria J 2007; 6:143. [DOI] [PMC free article] [PubMed] [Google Scholar]