In this cohort of nearly 15000 11-year-old girls with insurance (commercial or Medicaid), the human papillomavirus vaccine was administered at only 1 in 4 well-adolescent visits and approximately one-third of vaccine-related visits, which suggests a substantial number of missed opportunities.
Keywords: human papillomavirus, insured population, missed opportunities, vaccine, vaccination rates
Abstract
Background
This study assessed the initiation of HPV vaccination in insured adolescent females in relation to physician visits and receipt of other vaccines routinely given at the same age.
Methods
January 1, 2010, and September 31, 2015. Vaccination administration was determined by using Current Procedural Terminology codes. A missed opportunity was defined as the absence of an HPV vaccine at the following encounter types: visits with a 4-valent meningococcal conjugate vaccine (MenACWY) or tetanus, diphtheria, and acellular pertussis (Tdap) vaccine claim; well adolescent visits; or any encounter with a primary care provider (PCP). Missed opportunities were stratified by type of provider (pediatrician or nonpediatrician).
Results
Among 14588 adolescent girls, only 6098 (41.8%) initiated the HPV vaccine series. HPV vaccine was given at 37.1% of visits when a Tdap or MenACWY vaccine was administered, 26.0% of well adolescent visits and 41.8% of PCP visits. Pediatricians had fewer missed opportunities than nonpediatricians to administer HPV (50.7% vs 60.8%), as well as Tdap, although the difference was larger for Tdap (7.0% vs 29.6%).
Conclusions
These data indicate that pediatricians and nonpediatricians alike are missing opportunities to administer the HPV vaccine when other adolescent vaccines are given. Efforts should be focused on converting these missed vaccination opportunities into cancer-prevention visits.
Human papillomavirus (HPV) causes cervical, vulvar, vaginal, anal, oral, and penile cancers, among others. Approximately 31000 new HPV-attributable cancers occur in the United States (U.S.) every year; almost two-thirds occur among females, the most common being cervical cancer, which affects about 12000 women [1]. Many HPV types can cause cancer, and the associations between virus and cancer types vary according to anatomic site. It is notable that approximately 70% of cervical cancers are caused by HPV type 16 or 18. Highly effective vaccines against these HPV types have been available in the United States since 2006 [2–10], and we now have strong evidence of their effectiveness at the population level, including evidence for herd effects and cross-protection [4].
Routine vaccination of females has been recommended in the U.S. for a decade, and in males since 2009 [11]. The age for initiation of the vaccine series is 11 to 12 years of age, but it can be administered as early as 9 years of age, with catch-up from 13 to 26 years in females [3]. Virtually all authoritative and professional bodies, including the American Cancer Society, the American Academy of Pediatrics, the US Preventive Services Task Force, the American Academy of Family Physicians, and the American College of Obstetricians and Gynecologists, have endorsed the standing recommendation issued by the Advisory Committee on Immunization Practices. Until recently, completion of the HPV vaccine series necessitated 3 doses at 0, 1 to 2, and 6 months. A 2-dose series (0 and 6 to 12 months) was recently approved for healthy persons 9 to 14 years of age [12, 13].
Despite long-standing recommendations and obvious public health benefits, HPV vaccination initiation rates remain low in the United States (63% in girls), which is in stark contrast to the initiation rates for other routinely recommended adolescent vaccines, such as the 4-valent meningococcal conjugate vaccine (MenACWY) and tetanus, diphtheria, and acellular pertussis (Tdap) vaccine [14]. Identifying missed opportunities for HPV vaccination, particularly those linked to the administration of other adolescent vaccines, could lead to strategies to improve HPV vaccination rates [15]. To that end, in this observational study, we assessed the initiation of HPV vaccination among insured girls aged 11 to 12 years in relation to physician visits and receipt of other vaccines routinely given at the same age in an insured population with at least 2 years of continuous enrollment. Other studies have examined missed opportunities for HPV vaccination, but our study is unique in that, because every subject was enrolled in a health care plan, lack of access to health care was minimized as a factor affecting vaccination rates.
METHODS
This study was conducted using Humana’s research database, a deidentified data set that contains medical, pharmacy, and enrollment data for current and previously insured individuals eligible for participation in retrospective research studies. Data from January 1, 2010, through August 31, 2015, were used.
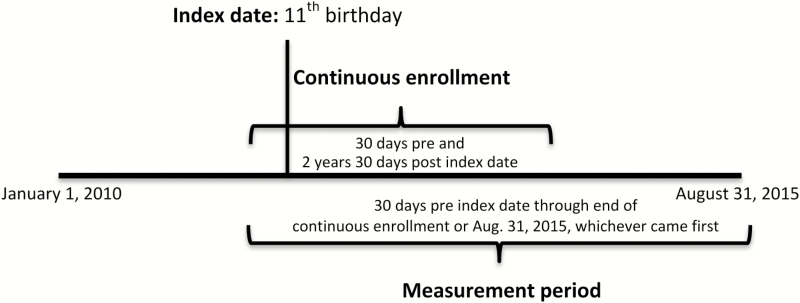
All females in a fully insured commercial or Medicaid plan with continuous enrollment beginning 30 days before their 11th birthday (index date; recommended age of initiation) until at least 30 days after their 13th birthday (recommended age catch-up begins and to capture early visits) and at least one healthcare provider visit were included (Figure 1) [16–18]. The 30-day windows were chosen because a 1-month catch-up period after the recommended start age or interval is allowed for before being considered in the catch-up schedule. Subjects were followed over time as long as they continued to be enrolled in a Humana plan or until August 31, 2015, whichever came first. Thus, the length of follow-up after the index date (11th birthday) was variable for each subject, with a minimum of 2 years and 30 days. The measurement period spanned from 30 days prior to the index date until the end of follow-up for each subject. The median length of follow up was 2.7 years (interquartile range, 2.36–3.24 years) for the entire cohort. Because the study cohort identified subjects on their 11th birthday, girls vaccinated prior to 30 days before their 11th birthday were not assessed as a part of this study.
Figure 1.
Study period.
Initiation of the HPV vaccine series was defined as receipt of at least 1 dose of HPV vaccine during the measurement period. HPV vaccination was determined by the presence of a claim that contained Current Procedural Terminology (CPT) code 90649 (Gardasil) or 90650 (Cervarix). Demographic characteristics and receipt of other adolescent vaccines (≥1 Tdap vaccine [CPT code 90715, Boostrix or Adacel] and/or ≥1 MenACWY vaccine [CPT code 90734, Menactra or Menveo]) were compared between HPV vaccine initiators and noninitiators. Among initiators, age at initiation and completion rate by age at initiation were described. Completion was defined as receipt of ≥3 HPV vaccine doses within the measurement period.
A missed opportunity was defined as the absence of an HPV vaccine dose administered during any visit with a Tdap or MenACWY vaccine claim, any well-adolescent visit (CPT code 99394), or any encounter with a primary care provider (PCP), regardless of visit type, during the measurement period. These encounters were not mutually exclusive. The proportions of the 3 different encounter types with and those without a concomitant HPV vaccine dose administered were determined. Similarly, Tdap and MenACWY missed opportunities were defined as the presence of claims for other study vaccines in the absence of Tdap or MenACWY. Missed opportunities were also identified by provider specialty among girls who received at least 1 dose of HPV, Tdap, or MenACWY vaccine. Specifically, the percentages of girls with any adolescent vaccine who did not receive 1 of the other adolescent vaccines were reported among pediatricians and nonpediatricians. Providers were classified as pediatricians if they were identified as such in the claim; they were classified as nonpediatricians if they were identified as family physicians, internists, nurse practitioners, physician assistants or other nonpediatric practitioners as recorded on the claim submissions.
Descriptive statistics were used to describe baseline demographics, other adolescent vaccine use, and missed opportunities. Kaplan-Meier curves were created to describe age at initiation. The χ2 test was used to analyze categorical variables. All analyses were conducted using SAS Enterprise Guide version 7.1; an a priori α value of 0.05 established statistical significance.
RESULTS
The study cohort included 14588 adolescent girls. The vast majority of them had commercial insurance (93.8%), lived in an urban area (82.8%), and were geographically located in the southern part of the country (64.1%) (Table 1). This geographic distribution generally reflects Humana’s territorial distribution.
Table 1.
Demographics of Female Adolescent HPV Vaccine Initiators and Noninitiators
| Demographic | Total (N = 14588) (n [%]) | Vaccine Initiators (n = 6098) (n [%]) | Vaccine Noninitiators (n = 8490) (n [%]) | P (Vaccine Initiators vs Noninitiators)a |
|---|---|---|---|---|
| Plan type | <.0001 | |||
| Commercial | 13677 (93.76) | 5441 (89.23) | 8236 (97.01) | |
| Medicaid | 911 (6.24) | 657 (10.77) | 254 (2.99) | |
| Geographic region | <.0001 | |||
| Midwest | 3687 (25.27) | 1392 (22.83) | 2295 (27.03) | |
| Northeast | 16 (0.11) | 8 (0.13) | 8 (0.09) | |
| South | 9349 (64.09) | 4083 (66.96) | 5266 (62.03) | |
| West | 1536 (10.53) | 615 (10.09) | 921 (10.85) | |
| Location | <.0001 | |||
| Rural | 633 (4.54) | 182 (2.98) | 481 (5.67) | |
| Suburban | 1852 (12.70) | 640 (10.50) | 1212 (14.28) | |
| Urban | 12071 (82.75) | 5276 (86.52) | 6795 (80.04) | |
| Unknown | 2 (0.01) | 0 (0.00) | 2 (0.02) | |
| ≥1 dose of MenACWY vaccine | <.0001 | |||
| Yes | 11433 (78.37) | 5239 (85.91) | 5449 (64.18) | |
| No | 3155 (21.63) | 859 (14.09) | 3041 (35.82) | |
| ≥1 dose of Tdap vaccine | <.0001 | |||
| Yes | 10688 (73.27) | 5241 (85.95) | 6192 (72.93) | |
| No | 3900 (26.73) | 857 (14.05) | 2298 (27.07) |
Abbreviations: HPV, human papillomavirus; MenACWY, meningococcal ACWY; Tdap, tetanus, diphtheria, and acellular pertussis.
aAccording to the χ2 test for categorical variables.
HPV Vaccine Series Initiation
Only 6098 (41.8%) eligible girls initiated the HPV vaccine series (Table 1). Compared to noninitiators, a higher proportion of HPV vaccine initiators were insured through managed Medicaid plans and lived in the South and in an urban area. Girls who initiated HPV vaccination were more likely to receive the MenACWY (85.9% vs 64.2%, respectively; P < .0001) and Tdap (86.0% vs 72.9%, respectively; P < .0001) vaccines.
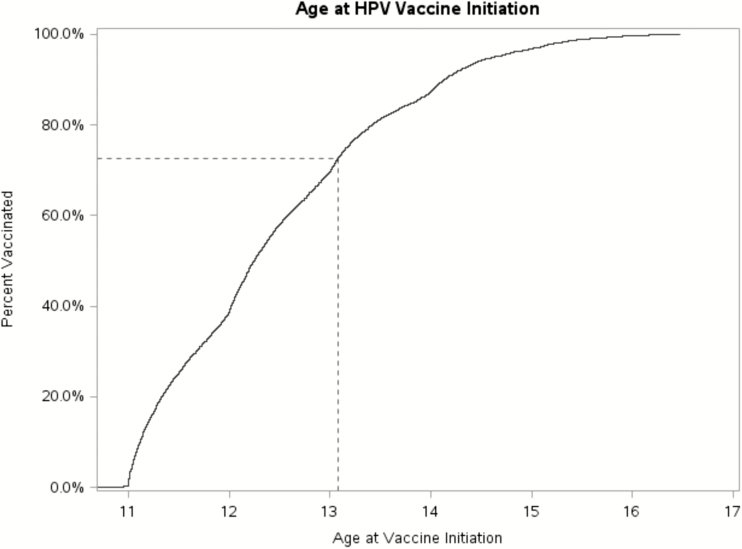
The majority (72.7%) of initiators received their first HPV vaccine on or before their 13th birthday plus 30 days (Figure 2). The majority (67.8%) of girls who initiated the HPV vaccine series at 11 years of age (11 to <12 years; n = 2348) completed 3 doses during the measurement period. For those who initiated at 12 years of age (12 to <13 years; n = 1890), the completion rate was 52.2%.
Figure 2.
Kaplan-Meier curve for age at first HPV vaccination among girls who received at least 1 HPV vaccine dose.
Missed Opportunities
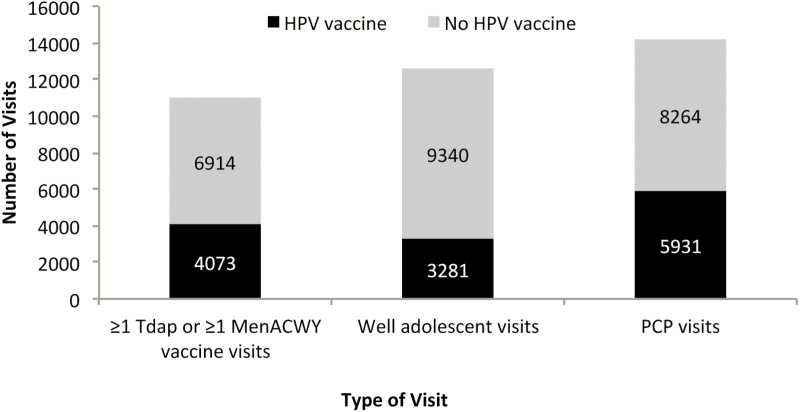
Among 10987 visits in which a Tdap or MenACWY vaccine dose was administered, HPV vaccine was concomitantly administered only 37.1% (n = 4073) of the time (Figure 3). Similarly, HPV vaccine was given at only 26.0% (3281 of 12621) of well-adolescent visits and 41.8% (5931 of 14195) of PCP visits.
Figure 3.
Missed opportunities for HPV vaccination according to type of visit.
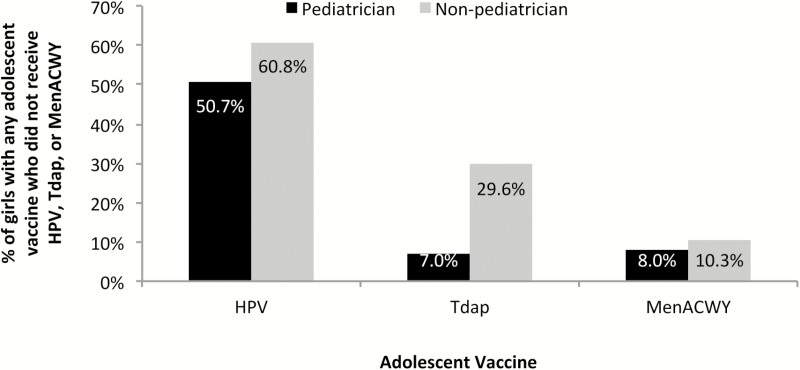
A total of 12940 (88.7%) girls in the cohort received at least 1 dose of the HPV, Tdap, or MenACWY vaccine. Among those girls, pediatricians administered the HPV, Tdap, and MenACWY vaccine 78.3%, 78.4%, and 78.7% of the time, respectively. When pediatricians administered these vaccines, they did not administer HPV, Tdap, or MenACWY vaccine to 50.7%, 7.0%, and 8.0% of the girls, respectively. Similarly, nonpediatricians missed opportunities for vaccination against HPV, Tdap, and MenACWY vaccines in 60.8%, 29.6%, and 10.3% of the girls, respectively (Figure 4). In the nonpediatrician group, 88.3% of the providers were identified as a family physician, internist, public health physician, obstetrician/gynecologist, general practitioner, Medicaid provider, nurse practitioner, or physician assistant.
Figure 4.
Percentage of girls with any adolescent vaccine and missed opportunities for vaccination against HPV, Tdap, and/or MenACWY according to provider specialty (n = 12940 girls with ≥1 HPV, MenACWY, or Tdap vaccine).
DISCUSSION
The Healthy People 2020 target for completed HPV vaccination series by 13 to 15 years of age is 80% [19]. The current rates fall far below this goal; in fact, according to the most recent national data, initiation of HPV vaccination among girls 13 to 17 years of age is only 63% [14]. The initiation rate in our cohort of 11- to 12-year-olds was even lower, and subjects were less likely to receive HPV vaccine than other recommended adolescent vaccines. Missed opportunities for the administration of HPV vaccine were most common among well-adolescent visits, followed by vaccine-related visits and then all-cause PCP visits.
Low HPV vaccination rates are considered a public health problem, and multiple studies have attempted to identify the barriers to vaccination among US adolescents. A systematic review reported that parents’ attitudes toward the vaccine, inadequate insurance coverage and reimbursement, financial concerns, preference for vaccinating older adolescents, and knowledge gaps are the most important factors explaining lack of advocacy towards vaccination from the provider perspective [20]. On the other hand, not receiving a provider’s recommendation, lack of information, concerns about timing of vaccination (child’s age), misconceptions about efficacy and safety, cost and availability were identified as parental barriers for vaccination [20]. In addition, providers’ discomfort talking about a topic related to sexual behavior, lack of time or incentives for parental education, lack of systems to remind regarding eligibility, the 3-dose schedule at the time of the present study, and the fact that HPV vaccine is not mandated for school entry in most states contribute to low vaccination rates [15]. Every effort should be made to overcome those barriers from the provider and parent standpoint to increase HPV vaccination rates.
It stands to reason that “normalization” of HPV vaccination—that is, conceptually bundling it with the other adolescent vaccines and presenting that bundle as a package to families—might increase HPV vaccination rates [21]. The adoption of a 2-dose series for healthy persons in the 11- to 12-year age range also might serve to increase completion rates [22], although a disconnect between the HPV and other adolescent vaccines is still likely. Efforts centered on educating healthcare providers to empower them to strengthen their recommendations and highlight the benefits of vaccination should be prioritized in the immunization agenda to improve rates of HPV vaccination in the future. Focusing on the ability of the vaccine to protect against cancer and its proven efficacy should substantially reduce the number of missed opportunities during clinical encounters with adolescents.
Many studies have shown pediatricians to be stronger at advocating for immunizations than physicians within other specialities [23]. The current study showed fewer missed opportunities for any of the studied vaccines for pediatricians relative to nonpediatric practitioners. Missed opportunities for MenACWY vaccination were low regardless of provider type; however, pediatricians had substantially fewer missed opportunities for Tdap vaccination than nonpediatricians, 7.0% vs 29.6%. However, we noted a smaller difference between pediatricians and nonpediatricians in missed opportunities for HPV vaccination (50.7% vs 60.8%, respectively). In essence, pediatricians might be “equally bad” as other providers at promoting HPV vaccination. Cognitive biases on the part of providers might be partially responsible for this phenomenon and contribute to low vaccination rates [24]. It should be noted that in this study, the nonpediatrician group included any provider who administered a vaccine in the study, not just those for whom vaccinating is in their general scope of practice.
Most HPV vaccine initiators in this cohort received their first dose before 13 years of age, which indicates that, by and large, the recommendation to give the series at 11 to 12 years of age (before the 13th birthday) was being followed. This guideline is important for ensuring immunity well before HPV exposure is likely [8]. Completion rates for the 3-dose series could not be assessed in the entire study cohort because of variability in the duration of follow-up (nationally, the completion rate in 2015 was only 42% [14]). Yet, the differences between 3-dose administration among younger and older girls in this cohort suggest that, as found in other studies, the earlier the vaccine series is started, the higher the completion rate that is achieved [22].
Prior studies in insured populations have demonstrated lower HPV vaccine uptake among commercial health plans compared with Medicaid [25]. In our study, we found a greater association between HPV vaccine initiation and managed Medicaid insurance than between vaccine initiation and private insurance with the same carrier. In as much as Medicaid populations are at higher risk of HPV infection [6, 26, 27], this difference could be driven by parental or provider greater perceived benefit for Medicaid-insured girls and less perceived need in commercially-insured girls. Alternatively, there may be more hesitancy to vaccinate among commercially-insured families.
The results of this study are subject to limitations inherent to the observational nature of the study. Because the administrative claims were collected for payment rather than clinical observation, missing data and incorrect coding can occur. It is not possible to know the extent to which these factors affected the results. This study attempted to characterize missed opportunities for HPV vaccine administration; however, there was no information available in the claims on why the vaccine was not given. Thus, a very small percentage of females with a contraindication to the vaccine, such as the visit being too close to a prior dose, may have been misclassified as a missed opportunity. Some of those missed opportunities might have represented parental hesitancy despite the provider’s advocacy. It is also possible that HPV vaccine may have been administered but a claim was not filed with the insurer (e.g., free public health clinic), although since the entire sample had continuous enrollment for at least 2 years, this is unlikely. It should be noted that we included girls starting on their 11th birthday and thus did not assess vaccines given at 9 or 10 years of age. Last, it is well accepted that vaccine delivery is associated with health insurance coverage and geography [14]. This study of an insured population geographically concentrated in the South might not be generalizable to some populations.
CONCLUSIONS
HPV vaccine initiation among young adolescent girls in this insured population was suboptimal, and opportunities to administer the HPV vaccine were missed, particularly during well-adolescent and vaccine-related visits. Conceptually connecting HPV vaccine with the MenACWY and Tdap vaccines—in essence, presenting the package as the norm for the 11- to 12-year-old visit—could increase HPV vaccination rates. The data also suggest that pediatricians and nonpediatricians alike are missing opportunities to administer the HPV vaccine when other adolescent vaccines are given. Future research should focus on communication strategies that might facilitate the conceptual “bundling” of HPV vaccine with other adolescent vaccines in the provider’s office. Healthcare organizations should develop action plans that help providers avoid missed opportunities, and public health agencies should continue to focus public awareness campaigns on HPV vaccination as a critical element of community cancer prevention strategies.
Notes
Financial support. No external funding was provided for this study. Each of the authors conducted the work within the scope of their normal employment.
Potential conflicts of interest. C. M. E., G. S. M., C. R. W., and M. J. S. are employed by the University of Louisville School of Medicine. Q. M., D. E., I. N., and L. E. H. are employed by Humana. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Viens LJ, Henley SJ, Watson M et al. Human papillomavirus-associated cancers—United States, 2008–2012. MMWR Morb Mortal Wkly Rep 2016; 65:661–6. [DOI] [PubMed] [Google Scholar]
- 2. Saraiya M, Unger ER, Thompson TD et al. ; HPV Typing of Cancers Workgroup US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst 2015; 107:djv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Markowitz LE, Dunne EF, Saraiya M et al. ; Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP) Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56:1–24. [PubMed] [Google Scholar]
- 4. Drolet M, Bénard É, Boily MC et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2015; 15:565–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hariri S, Bennett NM, Niccolai LM et al. ; HPV-IMPACT Working Group Reduction in HPV 16/18-associated high grade cervical lesions following HPV vaccine introduction in the United States—2008–2012. Vaccine 2015; 33:1608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hofstetter AM, Ompad DC, Stockwell MS et al. Human papillomavirus vaccination and cervical cytology outcomes among urban low-income minority females. JAMA Pediatr 2016; 170:445–52. [DOI] [PubMed] [Google Scholar]
- 7. Markowitz LE, Liu G, Hariri S et al. Prevalence of HPV after introduction of the vaccination program in the United States. Pediatrics 2016; 137:e20151968. [DOI] [PubMed] [Google Scholar]
- 8. Smith LM, Strumpf EC, Kaufman JS et al. The early benefits of human papillomavirus vaccination on cervical dysplasia and anogenital warts. Pediatrics 2015; 135:e1131–40. [DOI] [PubMed] [Google Scholar]
- 9. Tabrizi SN, Brotherton JM, Kaldor JM et al. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis 2012; 206: 1645–51. [DOI] [PubMed] [Google Scholar]
- 10. Brotherton JM, Fridman M, May CL et al. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet 2011; 377:2085–92. [DOI] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention. Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011;60:1705–8. [PubMed] [Google Scholar]
- 12. Blomberg M, Dehlendorff C, Sand C, Kjaer SK. Dose-related differences in effectiveness of human papillomavirus vaccination against genital warts: a nationwide study of 550000 young girls. Clin Infect Dis 2015; 61:676–82. [DOI] [PubMed] [Google Scholar]
- 13. Advisory Committee on Immunization Practices. ACIP votes, October 2016 Available at: https://www.cdc.gov/vaccines/acip/index.html. Accessed October 31, 2016.
- 14. Reagan-Steiner S, Yankey D, Jeyarajah J et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2015. MMWR Morb Mortal Wkly Rep 2016; 65:850–8. [DOI] [PubMed] [Google Scholar]
- 15. President’s Cancer Panel. Accelerating HPV vaccine uptake: urgency for action to prevent cancer. A report to the President of the United States from the President’s Cancer Panel. Bethesda, MD; 2014. Available at: https://deainfo.nci.nih.gov/advisory/pcp/annualReports/HPV/index.htm. Accessed May 25, 2017. [Google Scholar]
- 16. Zickafoose JS, Cohn LM, Clark SJ. Outpatient utilization by infants auto-assigned to Medicaid managed care plans. Matern Child Health J 2014; 18:737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Robinson CL, Romero JR, Kempe A, Pellegrini C; Advisory Committee on Immunization Practices (ACIP) Child/Adolescent Immunization Work Group Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger—United States, 2017. MMWR Morb Mortal Wkly Rep 2017; 66:134–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention. Recommended childhood and adolescent immunization schedule—United States, 2005. MMWR Morb Mortal Wkly Rep 2005;53:Q1–3. [PubMed] [Google Scholar]
- 19. Office of Disease Prevention and Health Promotion. IID-11.4: Increase the vaccination coverage level of 3 doses of human papillomavirus (HPV) vaccine for females by age 13 to 15 years Available at: https://www.healthypeople.gov/node/4657/data_details. Accessed September 20, 2016.
- 20. Holman DM, Benard V, Roland KB et al. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr 2014; 168:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moss JL, Reiter PL, Brewer NT. Concomitant adolescent vaccination in the U.S., 2007–2012. Am J Prev Med 2016;51:693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cloessner EA, Stokley S, Yankey D, Markowitz LE. Timing of HPV vaccine intervals among United States teens with consideration to the current ACIP schedule and the WHO 2-dose schedule. Hum Vaccin Immunother 2016; 12:1375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Allison MA, Hurley LP, Markowitz L et al. Primary care physicians’ perspectives about HPV vaccine. Pediatrics 2016; 137:e20152488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Niccolai LM, Pettigrew MM. The role of cognitive bias in suboptimal HPV vaccine uptake. Pediatrics 2016; 138:pii:e20161537. [DOI] [PubMed] [Google Scholar]
- 25. Ng J, Ye F, Roth L et al. Human papillomavirus vaccination coverage among female adolescents in managed care plans—United States, 2013. MMWR Morb Mortal Wkly Rep 2015; 64:1185–9. [DOI] [PubMed] [Google Scholar]
- 26. Watson M, Saraiya M, Benard V et al. Burden of cervical cancer in the United States, 1998–2003. Cancer 2008; 113:2855–64. [DOI] [PubMed] [Google Scholar]
- 27. Shikary T, Bernstein DI, Jin Y et al. Epidemiology and risk factors for human papillomavirus infection in a diverse sample of low-income young women. J Clin Virol 2009; 46:107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]