Over the past decade, there have been unrecognized increases in the number of infections with highly drug-resistant Pseudomonas aeruginosa occurring in children. This increase in drug resistance in P aeruginosa involves multiple antibiotic classes including our last line of defense therapy, the carbapenems. The implementation of effective preventative strategies is critical.
Keywords: carbapenems, child, drug resistance, epidemiology, Pseudomonas aeruginosa
Abstract
Background
Pseudomonas aeruginosa is a common cause of healthcare-associated infection. Multidrug-resistant (MDR) (>3 classes) and carbapenem-resistant (CR) P aeruginosa are significant threats globally. We used a large reference-laboratory database to study the epidemiology of P aeruginosa in children in the United States.
Methods
Antimicrobial susceptibility data from the Surveillance Network were used to phenotypically identify MDR and CR P aeruginosa isolates in children aged 1 to 17 years between January 1999 and July 2012. Logistic regression analysis was used to calculate trends in the prevalence of MDR and CR P aeruginosa. Isolates from infants (<1 year old) and patients with cystic fibrosis were excluded.
Results
Among the isolates tested, the crude proportion of MDR P aeruginosa increased from 15.4% in 1999 to 26% in 2012, and the proportion of CR P aeruginosa increased from 9.4% in 1999 to 20% in 2012. The proportion of both MDR and CR P aeruginosa increased each year by 4% (odds ratio [OR], 1.04 [95% confidence interval (CI), 1.03–1.04] and 1.04 [95% CI, 1.04–1.05], respectively). In multivariable analysis, both MDR and CR P aeruginosa were more common in the intensive care setting, among children aged 13 to 17 years, in respiratory specimens, and in the West North Central region. In addition, resistance to other antibiotic classes (aminoglycosides, fluoroquinolones, cephalosporins, and piperacillin-tazobactam) often used to treat P aeruginosa increased.
Conclusions
Rates of MDR and CR P aeruginosa infection in children are rising nationally. Aggressive prevention strategies, including instituting antimicrobial stewardship programs in pediatric settings, are essential for combating antimicrobial resistance.
Pseudomonas aeruginosa is a Gram-negative bacterium that causes infections that can lead to significant morbidity and death. It is a complex and formidable adversary that has great genetic “plasticity,” which facilitates development of antibiotic-resistance mutations, and striking survival rates in the most diverse environments because of its minimal nutritional requirements and ability to use a variety of carbon sources for energy [1]. The severity of such infections is attributable to the broad range of virulence factors carried by the bacterium, including the type III secretion system, which helps establish infection by injecting effector proteins into host cells and thereby enhancing disease severity [2, 3].
Potentially even more multifaceted are the mechanisms of antibiotic resistance attributed to P aeruginosa. Foremost have been enzymes that are able to inactivate β-lactam antibiotics; these intrinsic and acquired β-lactamases include cephalosporinases (which produce extended-spectrum cephalosporin resistance) and carbapenemases (which produce carbapenem resistance). Additional resistance mechanisms rely on enzymatic and target-site modifications (aminoglycoside and fluoroquinolone resistance), alterations in outer membrane permeability, and multidrug efflux pumps. The latter 2 mechanisms often render organisms multidrug resistant (MDR) and leave few treatment options against them [4]. The US Centers for Disease Control and Prevention estimates that each year, 51000 healthcare-associated P aeruginosa infections occur in adults and children, of which 13% (>6000) are MDR and account for 400 deaths [5].
Infections with P aeruginosa in children are reported most often in association with pulmonary disease in patients with cystic fibrosis (CF) [6]. In addition, healthy children can experience a variety of P aeruginosa infection types, particularly infection of the urinary tract, ear, sinuses, wounds, skin, and connective tissues (cartilage and bone) [7–12]. However, as in adults, serious and systemic, often opportunistic, infections are substantial in children with critical illness, in those who require ventilator support, and in patients who are immunocompromised and/or have a hematologic-oncologic disease [7, 8, 13–18].
The majority of P aeruginosa studies have examined the effect of these infections on patients with CF. Despite the annual numbers of infection and rising rates of antibiotic resistance, no pediatric studies have assessed national or regional trends in MDR or carbapenem-resistant (CR) P aeruginosa. In this study, we investigated the epidemiology of P aeruginosa isolates from children, excluding those with CF, and explored trends in antibiotic resistance over a period of 13.5 years.
METHODS
Antibiotic-susceptibility data were obtained from the Surveillance Network (TSN) Database–USA (Eurofin-Medinet, Herndon, VA). The database has been used extensively to characterize national antibiotic-susceptibility trends [19–22]. The network included clinical microbiology laboratories that served approximately 300 hospitals that were selected by the 9 US Census Bureau regional divisions to be geographically representative and representative of hospital size and patient population. Laboratories included in the TSN database were required to submit results from all routine antimicrobial-susceptibility testing performed on site. Categorical result interpretations were based on Clinical Laboratory Standards Institute (CLSI) criteria adopted by the reporting facilities at the time of testing and reflect susceptibilities as reported to clinicians [23]. Individual laboratory data were validated electronically and merged into a central TSN database.
The database includes records with the following information: the identified organism; the tested drug and susceptibility result (susceptible, intermediate-resistant, or resistant); the source of the isolate (blood, urine, wound, respiratory tract, or skin); patient characteristics (age and sex); the healthcare setting in which the patient sample was collected (outpatient [ambulatory], inpatient intensive care unit [ICU], inpatient [non-ICU], or long-term care setting); the geographical location of the facility in which the specimen was collected; and the date of the drug-susceptibility test.
Our analysis considered P aeruginosa isolates obtained from all pediatric patients (aged 1–17) who were in an outpatient (ambulatory), inpatient ICU, inpatient non-ICU, or long-term care setting between January 1, 1999, and June 30, 2012. To improve applicability to the general pediatric population, isolates from patients with CF were excluded from the analysis. We also excluded isolates obtained from patients <1 year old, because the epidemiology of MDR and CR P aeruginosa in neonates (in particular, infants cared for in the neonatal ICU) likely differs from that in older children and should be analyzed separately. MDR P aeruginosa was defined using US Centers for Disease Control and Prevention criteria [24], which included nonsusceptibility to agent(s) in at least 3 of the following 5 antimicrobial classes: cephalosporins (cefepime, ceftazidime), β-lactam/β-lactamase-inhibitor combination (piperacillin, piperacillin-tazobactam), carbapenems (imipenem, meropenem, doripenem), fluoroquinolones (ciprofloxacin, levofloxacin), and aminoglycosides (gentamicin, tobramycin, amikacin). An isolate was deemed CR P aeruginosa if it was nonsusceptible to at least 1 of the 3 agents in the carbapenem class (imipenem, meropenem, or doripenem). Only isolates that were tested for all 5 antibiotic classes, including carbapenems, were included in the final analysis.
When duplicate records (with the same patient identification number, drug-susceptibility test, and specimen source) were identified, only the first isolate was included in the analysis. The frequency of MDR and CR P aeruginosa is reported as the number of isolates that tested positive divided by the total number of tested isolates included in the analysis. Individual susceptibility results were stratified according to location (ICU, inpatient non-ICU, outpatient, and long-term care facilities), age (1–5, 6–12, and 13–17 years), sex, isolate source (blood, urine, wound, respiratory tract, and skin), annual intervals, and geographic region based on the location of the healthcare facility (East North Central, East South Central, Mid-Atlantic, Mountain, New England, Pacific, South Atlantic, West North Central, and West South Central). Because the antibiotic-susceptibility patterns differed among hospitalized and nonhospitalized patients, we examined the distributions of MDR and CR P aeruginosa isolates among those obtained from patients in inpatient (ICU or inpatient non-ICU) and outpatient (ambulatory) facilities. In addition, we examined the susceptibility of P aeruginosa isolates to all agents in each of the 5 antibiotic classes among the inpatients and outpatients.
Unadjusted trends in the prevalences of MDR and CR P aeruginosa were calculated using logistic regression. Multivariable logistic regression analysis was used to estimate the prevalences of MDR and CR P aeruginosa, which was adjusted for time (year) and patient characteristics, including age, sex, isolate source, patient location, and geographic region. Confidence intervals (CIs) were calculated using a Wilson score method. A P value of <.05 was considered statistically significant. Data were analyzed using the R programming language.
RESULTS
Overall, 87613 pediatric P aeruginosa isolates were identified in the database between 1999 and 2012, of which 77349 isolates were tested against 5 antibiotic classes, including carbapenems. Of these 77349 P aeruginosa isolates, 15653 (20.2%) isolates were MDR, 8763 (11.3%) were CR, and 6510 (8.4%) were both MDR and CR. Overall, the isolates were most often from outpatients (44158 isolates [57.0%]), a respiratory tract source (47564 isolates [61.4%]), and 1- to 5-year-old patients (28879 isolates [37.3%]) (Table 1). The distributions of isolates among male and female patients were similar (38526 [49.8%] vs 37555 [48.5%], respectively, excluding isolates from patients with unreported sex). Of the 9 regions in the data set, the largest number of isolates came from the South Atlantic region (13559 [17.5%]) (Table 1).
Table 1.
Distribution of MDR and CR Pseudomonas aeruginosa Isolates in Children Aged 1 to 17 Years, 1999–2012*
| Characteristic | Total No. of Isolates (N = 77 349) | No. (%) of MDR† Isolates (N = 15 653) | No. (%) of CR‡ Isolates (N = 8763) |
|---|---|---|---|
| Patient setting | |||
| Inpatient | 24 234 | 5123 (32.7) | 3317 (37.9) |
| Inpatient, ICU | 7893 | 2075 (13.3) | 1458 (16.6) |
| Long-term care | 332 | 107 (0.7) | 49 (0.6) |
| Outpatient | 44 158 | 8267 (52.8) | 3868 (44.1) |
| Unknown | 732 | 81 (0.5) | 71 (0.8) |
| Patient sex | |||
| Female | 37 555 | 7639 (48.8) | 4192 (47.8) |
| Male | 38 526 | 7819 (50.0) | 4393 (50.1) |
| Unknown | 1268 | 195 (1.2) | 178 (2.0) |
| Specimen source | |||
| Blood | 2093 | 323 (2.1) | 263 (3.0) |
| Respiratory tract | 47 564 | 12 117 (77.4) | 6234 (71.1) |
| Urine | 15 364 | 1989 (12.7) | 1288 (14.7) |
| Wound | 11 870 | 1196 (7.6) | 951 (10.9) |
| Skin | 458 | 28 (0.2) | 27 (0.3) |
| Age group | |||
| 1–5 y | 28 879 | 3657 (23.4) | 2541 (29.0) |
| 6–12 y | 25 158 | 4848 (31.0) | 2517 (28.7) |
| 13–17 y | 23 312 | 7148 (45.7) | 3705 (42.3) |
| Region§ | |||
| East North Central | 13 231 | 2514 (16.1) | 1478 (16.9) |
| East South Central | 1886 | 233 (1.5) | 121 (1.4) |
| Mid-Atlantic | 7721 | 1473 (9.4) | 923 (10.5) |
| Mountain | 5299 | 1026 (6.6) | 502 (5.7) |
| New England | 2295 | 445 (2.8) | 273 (3.1) |
| Pacific | 9014 | 1264 (8.1) | 763 (8.7) |
| South Atlantic | 13 559 | 2488 (15.9) | 1370 (15.6) |
| West North Central | 12 465 | 3888 (24.8) | 2067 (23.6) |
| West South Central | 11 879 | 2322 (14.8) | 1266 (14.4) |
Abbreviations: CR, carbapenem resistant; ICU, intensive care unit; MDR, multidrug resistant.
*Data source was the Surveillance Network Database–USA.
†Nonsusceptible to at least 1 agent in at least 3 of the following 5 antimicrobial classes: cephalosporins (cefepime, ceftazidime), β-lactam/β-lactamase-inhibitor combination (piperacillin, piperacillin-tazobactam), carbapenems (imipenem, meropenem, doripenem), fluoroquinolones (ciprofloxacin, levofloxacin), and aminoglycosides (gentamicin, tobramycin, amikacin).
‡Nonsusceptible to at least 1 of the 3 agents in the carbapenem class (imipenem, meropenem, doripenem).
§The East North Central region includes Illinois, Indiana, Michigan, Ohio, and Wisconsin; the East South Central region includes Alabama, Kentucky, Mississippi, and Tennessee; the Mid-Atlantic region includes New Jersey, New York, and Pennsylvania; the Mountain region includes Arizona, Colorado, Idaho, Montana, New Mexico, Nevada, Utah, and Wyoming; the New England region includes Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont; the Pacific region includes Alaska, California, Hawaii, Oregon, and Washington; the South Atlantic region includes Delaware, Florida, Georgia, Maryland, North Carolina, South Carolina, Virginia, and West Virginia; the West North Central region includes Iowa, Kansas, Minnesota, Missouri, North Dakota, Nebraska, and South Dakota; and the West South Central region includes Arkansas, Louisiana, Oklahoma, and Texas.
Among MDR and CR isolates, the largest numbers were from respiratory sources (12117 [77.4%] and 6234 [71.1%], respectively), children aged 13 to 17 years (7148 [45.7%] and 3705 [42.3%], respectively), male patients (7819 [50.0%] and 4393 [50.1%], respectively), an outpatient setting (8267 [52.8%] and 3868 [44.1%], respectively), and the West North Central region (3888 [24.8%] and 2067 [23.6%], respectively) (Table 1).
Overall, among the various patient and isolate characteristics, the proportion of MDR and CR P aeruginosa isolates was higher in inpatients than in outpatients (Table 2). Among them, the highest proportions of MDR and CR isolates were from lower respiratory sources (28.0% and 17.5% [inpatients] and 23.8% and 10.1% [outpatients], respectively), children aged 13 to 17 years (32.4% and 20.3% [inpatients] and 29.5% and 12.9% [outpatients]), and the West North Central region (33.9% and 24.3% [inpatients] and 29.6% and 12.1% [outpatients]) (Table 2).
Table 2.
Proportion of MDR and CR Pseudomonas aeruginosa Isolates in Inpatient and Outpatient Children Aged 1 to 17 Years, 1999–2012*
| Characteristic | Inpatient† | Outpatient | ||||
|---|---|---|---|---|---|---|
| Total No. of Isolates (N = 32 127) |
No. (%) of MDR‡ Isolates (N = 7198) |
No. (%) of CR§ Isolates (N = 4775) |
Total No. of Isolates (N = 44 158) | No. (%) of MDR‡ Isolates (N = 8267) |
No. (%) of CR§ Isolates (N = 3868) |
|
| Patient sex | ||||||
| Female | 14 811 | 3329 (22.5) | 2149 (14.5) | 22 271 | 4238 (19.0) | 1990 (8.9) |
| Male | 16 826 | 3763 (22.4) | 2529 (15.0) | 21 120 | 3941 (18.7) | 1798 (8.5) |
| Unknown | 490 | 106 (21.6) | 97 (19.8) | 767 | 88 (11.5) | 80 (10.4) |
| Specimen source | ||||||
| Blood | 1575 | 269 (17.1) | 225 (14.3) | 495 | 52 (10.5) | 37 (7.5) |
| Respiratory tract | 18 755 | 5247 (28.0) | 3287 (17.5) | 28 214 | 6729 (23.8) | 2863 (10.1) |
| Urine | 5325 | 824 (15.5) | 594 (11.2) | 9760 | 1129 (11.6) | 670 (6.9) |
| Wound | 6319 | 845 (13.4) | 659 (10.4) | 5391 | 343 (6.4) | 283 (5.2) |
| Skin | 153 | 13 (8.5) | 10 (6.5) | 298 | 14 (4.7) | 15 (5.0) |
| Age group | ||||||
| 1–5 y | 13 869 | 2298 (16.6) | 1658 (12.0) | 14 573 | 1293 (8.9) | 839 (5.8) |
| 6–12 y | 8988 | 1893 (21.1) | 1239 (13.8) | 15 832 | 2914 (18.4) | 1257 (7.9) |
| 13–17 y | 9270 | 3007 (32.4) | 1878 (20.3) | 13 753 | 4060 (29.5) | 1772 (12.9) |
| Region | ||||||
| East North Central | 5030 | 1162 (23.1) | 803 (16.0) | 8048 | 1388 (16.6) | 661 (8.2) |
| East South Central | 1166 | 157 (13.5) | 85 (7.3) | 678 | 75 (11.1) | 34 (5.0) |
| Mid-Atlantic | 2950 | 738 (25.0) | 498 (16.9) | 4605 | 696 (15.1) | 401 (8.7) |
| Mountain | 2058 | 415 (20.2) | 207 (10.1) | 3238 | 610 (18.8) | 295 (9.1) |
| New England | 561 | 107 (19.1) | 85 (15.2) | 1727 | 338 (19.6) | 188 (10.9) |
| Pacific | 4283 | 741 (17.3) | 479 (11.2) | 4352 | 484 (11.1) | 254 (5.1) |
| South Atlantic | 5298 | 1006 (19.0) | 704 (13.3) | 8122 | 1459 (18.0) | 650 (8.0) |
| West North Central | 4564 | 1549 (33.9) | 1110 (24.3) | 7898 | 2339 (29.6) | 957 (12.1) |
| West South Central | 6217 | 1323 (21.3) | 804 (12.9) | 5490 | 928 (16.9) | 428 (7.8) |
Abbreviations: CR, carbapenem resistant; MDR, multidrug resistant.
*Data source was the Surveillance Network Database–USA.
†Includes inpatient and inpatient intensive care unit locations.
‡Nonsusceptible to at least 1 agent in at least 3 of the following 5 antimicrobial classes: cephalosporins (cefepime, ceftazidime), β-lactam/β-lactamase-inhibitor combination (piperacillin, piperacillin-tazobactam), carbapenems (imipenem, meropenem, doripenem), fluoroquinolones (ciprofloxacin, levofloxacin), and aminoglycosides (gentamicin, tobramycin, amikacin).
§Nonsusceptible to at least 1 of the 3 agents in the carbapenem class (imipenem, meropenem, doripenem).
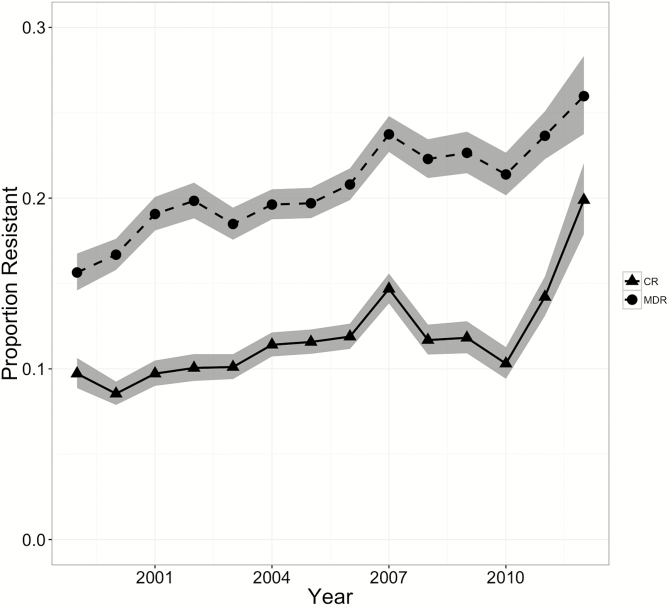
An analysis of linear trends using logistic regression revealed a significant increase in the proportion of both MDR (15.4%–26.0%; P < .001) and CR (9.4%–20.0%; P < .001) P aeruginosa isolates between 1999 and 2012 (Figure 1). With each year, the proportions of MDR and CR P aeruginosa isolates increased by 4% (odds ratios [ORs], 1.04 [95% CI, 1.03–1.04] and 1.04 [95% CI, 1.04–1.05], respectively). The proportions of both MDR and CR P aeruginosa isolates increased significantly in all age categories and patient locations except among children aged 13 to 17 years in an inpatient setting (Supplementary Table 1). After the data were adjusted for year, patient, and isolate characteristics, patients who were 13 to 17 years old had the highest prevalences of MDR and CR P aeruginosa, which were 3 times (adjusted ORs [aORs], 3.0 [95% CI, 2.8–3.1]) and 2 times (aOR, 1.96 [95% CI, 1.8–2.0]) higher than those in 1- to 5-year-old children (Table 3). Among patient locations, long-term care residents had the highest prevalence of MDR P aeruginosa, 2.3 times (aOR, 2.3 [95% CI, 1.8–2.9]) higher than that in the outpatient locations, whereas the CR P aeruginosa prevalence was highest in ICUs, 2.6 times (95% CI, 2.4–2.8] higher than that in outpatient locations (Table 3). Among specimen sources, respiratory specimens had the highest prevalences of MDR and CR P aeruginosa, 1.8 times (aOR, 1.8 [95% CI, 1.6–2.1]) and 1.2 times (aOR, 1.2 [95% CI, 1.0–1.3]) higher than that of specimens obtained from blood. Among the geographic regions, MDR and CR P aeruginosa isolates were most prevalent in the West North Central Region, 1.8 times (aOR, 1.8 [95% CI, 1.7–1.9]) and 1.5 times (aOR, 1.5 [95% CI, 1.3–1.6]) higher than that in the East North Central region of United States.
Figure 1.
National trends in prevalence of carbapenem-resistant (CR) and multidrug-resistant (MDR) Pseudomonas aeruginosa isolates from children >1 year old (data are from the Surveillance Network Database–USA database, 1999–2012). The proportion of MDR and CR P aeruginosa isolates increased significantly by 4% every year (odds ratios, 1.04 [95% confidence interval (CI), 1.03–1.04] [P for trend < .001] and 1.04 [95% CI, 1.04–1.05] [P for trend < .001], respectively).
Table 3.
Logistic Model of Multidrug- and Carbapenem-Resistant Pseudomonas aeruginosa Adjusted for Patient and Isolate Characteristics in Children Aged 1–17 Years, 1999–2012
| Isolate Characteristic* | MDR P aeruginosa (N = 77 439) | CR P aeruginosa (N = 77 439) | ||
|---|---|---|---|---|
| aOR (95% CI) | P | aOR (95% CI) | P | |
| Linear trend, 1999–2012 | 1.031 (1.025–1.036) | <.001 | 1.037(1.030–1.044) | <.001 |
| Age (reference, 1–5 y) | ||||
| 6–12 y | 1.709 (1.628–1.794) | <.001 | 1.243 (1.171–1.319) | <.001 |
| 13–17 y | 3.084 (2.945–3.230) | <.001 | 2.034 (1.925–2.150) | <.001 |
| Patient location (reference, outpatient) | ||||
| Inpatient | 1.442 (1.382–1.503) | <.001 | 1.908 (1.813–2.009) | <.001 |
| Inpatient, ICU | 1.790 (1.687–1.899) | <.001 | 2.589 (2.418–2.772) | <.001 |
| Long-term care | 2.322 (1.817–2.949) | <.001 | 1.881 (1.364–2.539) | <.001 |
| Unknown | 0.932 (0.728–1.178) | .567 | 1.765 (1.360–2.255) | <.001 |
| Specimen source (reference, blood) | ||||
| Respiratory tract | 1.818 (1.608–2.062) | <.001 | 1.157 (1.013–1.327) | .035 |
| Skin | 0.399 (0.261–0.589) | <.001 | 0.572 (0.370–0.851) | .008 |
| Urine | 0.789 (0.693–0.902) | <.001 | 0.734 (0.636–0.850) | <.001 |
| Wound | 0.593 (0.518–0.681) | <.001 | 0.661 (0.571–0.767) | <.001 |
| Region (reference, East North Central) | ||||
| East South Central | 0.676 (0.581–0.783) | <.001 | 0.531 (0.435–0.643) | <.001 |
| Mid-Atlantic | 0.973 (0.903–1.048) | .468 | 1.045 (0.956–1.143) | .332 |
| Mountain | 1.107 (1.107–1.204) | .019 | 0.900 (0.807–1.003) | .059 |
| New England | 0.818 (0.727–0.918) | .001 | 0.985 (0.854–1.133) | .836 |
| Pacific | 0.693 (0.641–0.748) | <.001 | 0.663 (0.603–0.728) | <.001 |
| South Atlantic | 0.917 (0.860–0.978) | .009 | 0.853 (0.787–0.924) | <.001 |
| West North Central | 1.830 (1.721–1.947) | <.001 | 1.454 (1.349–1.568) | <.001 |
| West South Central | 1.031 (0.965–1.101) | .370 | 0.874 (0.805–0.948) | .001 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; CR, carbapenem resistant; ICU, intensive care unit; MDR, multidrug resistant.
*Sex was not considered, because no significant differences in the distributions of isolates between male and female patients were found.
P aeruginosa resistance to other frequently used antibiotic classes was higher among inpatient isolates than among outpatient isolates with the exception of aminoglycosides (amikacin, gentamicin, and tobramycin) (Table 4 and Supplementary Table 2). Resistance to gentamicin was high among both inpatients and outpatients over the study period. Although doripenem resistance among inpatients was highest, only 87 isolates were tested between 2008 and 2012 (Table 4). In addition, gentamicin resistance increased over the study period among both the inpatient (23.8% in 1999–2003 to 26.1% in 2008–2012) and outpatient (25.7% in 1999–2003 to 28.4% in 2008–2012) isolates. Among the antibiotics, piperacillin-tazobactam had the least resistance in the inpatient and outpatient isolates. However, resistance to piperacillin-tazobactam increased over the study period among both the inpatient (7.9% in 1999–2003 to 12.5% in 2008–2012) and outpatient (3.2% in 1999–2003 vs 4.4% in 2008–2012) isolates (Table 4).
Table 4.
Pseudomonas aeruginosa Antibiotic Susceptibilities in Inpatient and Outpatient Children Aged 1–17 Years, 1999–2012*
| Antibiotic | % Resistant Isolates (n)† | ||
|---|---|---|---|
| 1999–2003 | 2004–2007 | 2008–2012 | |
| Inpatients | |||
| Amikacin | 13.7 (12 928) | 12.9 (10 990) | 13.2 (7079) |
| Cefepime | 16.5 (10 747) | 18.5 (10 809) | 19.7 (7119) |
| Ceftazidime | 15.7 (14 239) | 19.1 (12 112) | 18.0 (7398) |
| Ciprofloxacin | 13.4 (13 972) | 15.4 (12 231) | 16.2 (7793) |
| Doripenem | 0 (0) | 0 (0) | 27.6 (87) |
| Gentamicin | 23.8 (15 367) | 24.4 (12 803) | 26.1 (7845) |
| Imipenem | 11.7 (13 128) | 15.4 (10 612) | 15.7 (6185) |
| Levofloxacin | 12.4 (9967) | 14.2 (9377) | 16.9 (5221) |
| Meropenem | 10.5 (5539) | 15.5 (7965) | 16.1 (5622) |
| Piperacillin | 12.9 (11 560) | 13.9 (7596) | 16.4 (3164) |
| Piperacillin-tazobactam | 7.9 (9497) | 8.9 (11 180) | 12.5 (7393) |
| Tobramycin | 10.9 (14 430) | 12.5 (12 389) | 14.2 (7640) |
| Outpatients | |||
| Amikacin | 18.4 (15 710) | 20.5 (15 696) | 21.8 (11 648) |
| Cefepime | 11.1 (13 215) | 11.7 (14 692) | 11.9 (10 822) |
| Ceftazidime | 7.3 (17 465) | 8.9 (16 432) | 7.7 (11 816) |
| Ciprofloxacin | 12.8 (17 472) | 14.8 (17 132) | 13.6 (12 687) |
| Doripenem | 0 (0) | 0 (0) | 12.5 (104) |
| Gentamicin | 25.7 (19 616) | 27.0 (171 719) | 28.4 (12 184) |
| Imipenem | 7.3 (15 687) | 9.5 (14 032) | 10.0 (10 503) |
| Levofloxacin | 13.6 (11 037) | 14.2 (11 243) | 13.1 (8252) |
| Meropenem | 5.8 (6491) | 7.5 (10 743) | 7.4 (9342) |
| Piperacillin | 5.9 (14 547) | 7.1 (9040) | 7.4 (4365) |
| Piperacillin-tazobactam | 3.2 (10 975) | 4.2 (15 188) | 4.4 (10 876) |
| Tobramycin | 11.2 (18 316) | 14.9 (16 996) | 17.3 (12 103) |
*Data source was the Surveillance Network Database–USA.
†n indicates the number of isolates tested.
DISCUSSION
We observed significant increases in the prevalences of MDR and CR P aeruginosa isolates from US children between 1999 and 2012. Children admitted to an ICU, with a respiratory tract infection, between the ages of 13 and 17 years, and in the West North Central region of the United States had higher prevalences of MDR and CR P aeruginosa. Although the prevalence of MDR P aeruginosa in long-term care settings was highest, overall, there were not enough isolates from this setting in our data set to derive a robust conclusion.
Antibiotic resistance in P aeruginosa, arguably the most resistance-prone healthcare pathogen, is not a new phenomenon; however, antibiotic-resistant infections continue to become more complex and difficult to treat and are associated with significant hospital and societal costs [25]. P aeruginosa has a unique ability to survive in harsh environments; mechanisms that protect this bacterium from the actions of antibiotics include intrinsic resistance associated with chromosomally encoded β-lactamase enzymes; quorum-sensing proteins that let the bacteria communicate, which facilitates the formation of protective biofilms; efflux pumps that remove threats from the bacterial cytoplasm; structural topoisomerase mutations that deactivate fluoroquinolones; and cell wall porin alterations and outer membrane changes that prevent entry of antibiotics or other threats to bacterial survival and that work in concert to prevent antibiotics from eradicating it [4, 26]. The acquisition of mobile genetic elements, such as plasmids and transposons that harbor genes encoding for carbapenemases, aminoglycoside-modifying enzymes, and fluoroquinolone resistance determinants, contribute to high levels of resistance to last-line antibiotics such as the carbapenems, (imipenem, meropenem, and doripenem) and to the expression of MDR phenotypes among isolates [4, 27, 28].
Because ICUs harbor critically ill and immunocompromised children and involve high antibiotic and device usage rates, it is not surprising that we found high prevalences of MDR and CR P aeruginosa isolates in ICU patients [29]. High prevalences of MDR and CR P aeruginosa isolates among older children might relate to an increasing number of children with a medically complex condition who have frequent exposure to the healthcare environment [30].
With increasing drug resistance, the antibiotics used for effective treatment have expanded, and the selection pressure for the MDR and CR phenotypes continues to grow; institutional pan-drug-resistant P aeruginosa outbreaks in the United States have been described [31]. Colonization of P aeruginosa is associated with a polyclonal endemicity in hospitalized patients [32], and for decades, traditional infection-control practices have failed to control it [33]. The ubiquitous presence of P aeruginosa in the environment, water sources, and healthcare settings has prevented control of its acquisition among our most vulnerable patients, including critically ill patients, burn victims, immunocompromised patients, and children [29, 34, 35]. However, there have been notable infection-control successes in decreasing organism burdens, particularly with regard to catheter-associated infections [36, 37]. P aeruginosa is a common cause of catheter-associated urinary tract infection (CAUTI) and is associated with the formation of antibiotic- resistant biofilms and subsequent risk for severe infections such as bacteremia [38]. National efforts to reduce CAUTIs through detection and prevention programs include a multifaceted approach that involves provider education, microbial surveillance, performance measures, and bundled interventions; successful program implementations have been associated with improved patient outcomes [36, 37].
The increase in MDR P aeruginosa strains in children during the study period also might relate to trends in antibiotic-prescribing practices in the United States. Between 2000 and 2010, prescriptions of third- and fourth-generation cephalosporins and other broad-spectrum agents, including fluoroquinolones, increased in children in outpatient settings [39, 40]. Similarly, recent antibiotic-prescribing data from inpatient studies revealed similar findings, indicating increased selection pressure for colonization with MDR strains [41]. High prevalences of MDR and CR P aeruginosa isolates in the West North Central region might be associated with the reported high antibiotic consumption in this region [42, 43]. The highest rates of other MDR Gram-negative bacilli in children, such as CR Enterobacteriaceae, were reported in this region also [21].
Antimicrobial stewardship programs, which improve prescribing practices and restrict the use of broad-spectrum antimicrobial agents, have been shown to be highly effective in decreasing the incidence of healthcare-acquired infections, including infection with P aeruginosa [44] A 22-hospital survey found that restriction of carbapenem use was successful in reducing the incidence rates of CR P aeruginosa over a 5-year period in adult and pediatric patients [38]. A meta-analysis of MDR infections suggested substantial links to antibiotic usage, and improved antibiotic-prescribing practices resulted in reductions in antibiotic-resistant pathogens [45]. These best practices for decreasing antibiotic consumption have been substantiated in pediatrics and in critical care units, which is relevant to our findings of the most dramatic uptrends occurring in young children and those cared for in an intensive care setting [46, 47].
The importance of these initiatives is underscored not only in our findings of increasing MDR and CR P aeruginosa isolates but also in the notable increases in resistance to other commonly used therapies, including cefepime, piperacillin-tazobactam, fluoroquinolones (ciprofloxacin and levofloxacin), and aminoglycosides (gentamicin, tobramycin, and amikacin). The high rates of fluoroquinolone resistance in P aeruginosa related to the widespread use of this agent (associated with the availability of oral and intravenous formulations) have been well described in adult study reports [48]; however, before this study, fluoroquinolone-resistant P aeruginosa was described recently as having a low prevalence in US children, except in patients with CF [49, 50]. Similarly, the findings of high levels of gentamicin resistance in inpatient and outpatient settings might be reflective of the multiple modes of delivery, including topical, inhalation, and intravenous formulations, available to children. Colonization with gentamicin-resistant organisms has been associated with all formulations, including topical formulations [51, 52].
Increasing trends in P aeruginosa resistance to various antibiotics in ambulatory settings might reflect improvements in outpatient therapeutic approaches for patients who previously required hospitalization (such as in the care of children with neutropenic cancer or in ventilator-dependent children) [53, 54], or alternatively, notable practices of prescribing long-term prophylactic antibiotics and multiple oral treatment courses for children with recurrent urinary tract infections and/or urinary tract abnormalities [55, 56]. The significant number of tracheal aspirates (~12%) obtained from patients in an outpatient setting (Supplementary Table 3) suggests that, in recent decades, more children with a chronic respiratory disease and/or a tracheostomy are being managed at home with ventilator support rather than in a long-term care facility and that these children are often monitored in ambulatory ventilator or pulmonology clinics.
However, the frequent need for hospitalization, concern about morbidity and death that result from sepsis, and subsequent exposure to broad-spectrum intravenous antibiotics remains a burden for all of these children [15, 57, 58]. Revolutionary advances in rapid molecular diagnostics for viral and bacterial pathogens are assisting in the identification of patients who might more definitively benefit from antimicrobial therapy and those for whom antibiotics are not indicated [59–61].
Our study has important limitations. First, because the data stemmed from laboratory surveillance, we were not able to distinguish between colonization and true infection, and we could not account for detailed clinical characteristics of the individual patients. Second, as is common with large databases, some uncertainty was introduced by coding discrepancies, as for cases in which patients were treated in a location other than that recorded at admission. Third, although all TSN laboratories apply CLSI methods, minimum inhibitory concentrations (MICs) were not available, and susceptibility testing was not centralized, which introduced some margin of uncertainty as a result of local discrepancies. Fourth, there was a reduction in the number of laboratories that reported data in the final years of the study, which might have affected variations in carbapenem and multidrug resistance; however, the increasing trends and high levels of statistical significance found throughout the study period lessen this concern. Fifth, we do not have more recent data (beyond 2012), because the TSN database was disbanded after 2012; however, these are the only national-level trend data available. Sixth, CLSI breakpoints for piperacillin-tazobactam were lowered in January 2012 [62], which might have contributed to the higher MDR rates in 2012; however, a sensitivity analysis in which the year 2012 was excluded still showed a significant increase in MDR P aeruginosa trends. Seventh, we included only isolates that were tested against all 5 classes of antibiotics. Although the distribution of excluded isolates according to patient and isolate characteristics was similar to that of the included isolates (Supplementary Table 4), there is a possibility of both underestimation and overestimation of MDR isolates with exclusion of these isolates. Finally, the observed increases in the rates of MDR and CR P aeruginosa over time might partly be a result of clinicians becoming more discerning about which patients to test.
CONCLUSIONS
Infection with MDR or CR P aeruginosa is a significant threat to all patients, including children. The results of our study highlight the need for bacterial surveillance, strategies for implementing effective infection-prevention, and antimicrobial stewardship programs. The institution of rapid molecular diagnostic platforms should be considered by all healthcare facilities to guide antibiotic treatment decisions in an effort to reduce the burden of the persistent and continually evolving global threat of extensively drug-resistant organisms.
Supplementary Data
Supplementary Data Supplementary materials are available at the Journal of the Pediatric Infectious Diseases Society online (http://jpids.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Notes
Acknowledgments. We thank Drs Robert Bonomo, Kenneth Boyer, and Mary Hayden for their thoughtful comments and guidance.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or the Centers for Disease Control and Prevention.
Financial support. This work was supported directly by The National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grant K08AI112506 to L. K. L.), the Global Antibiotic Resistance Partnership, funded by the Bill and Melinda Gates Foundation (S. G., E. Y. K., J. L., and R. L.), and the Health Grand Challenges Program at Princeton University (R. L.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Kung VL, Ozer EA, Hauser AR. The accessory genome of Pseudomonas aeruginosa. Microbiol Mol Biol Rev 2010; 74:621–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hauser AR. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat Rev Microbiol 2009; 7:654–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Howell HA, Logan LK, Hauser AR. Type III secretion of ExoU is critical during early Pseudomonas aeruginosa pneumonia. mBio 2013; 4:e00032–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonomo RA, Szabo D. Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin Infect Dis 2006; 43(Suppl 2):S49–56. [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States. Atlanta, GA: Center for Disease Control and Prevention; 2013: pp 1–114. [Google Scholar]
- 6. Saiman L, Siegel J. Infection control in cystic fibrosis. Clin Microbiol Rev 2004; 17:57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang MA, Lee J, Choi EH, Lee HJ. Pseudomonas aeruginosa bacteremia in children over ten consecutive years: analysis of clinical characteristics, risk factors of multi-drug resistance and clinical outcomes. J Korean Med Sci 2011; 26:612–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wagner J, Catto-Smith AG, Cameron DJ, Kirkwood CD. Pseudomonas infection in children with early-onset Crohn’s disease: an association with a mutation close to PSMG1. Inflamm Bowel Dis 2013; 19:E58–9. [DOI] [PubMed] [Google Scholar]
- 9. Tramper-Stranders GA, van der Ent CK, Wolfs TF, et al. Pseudomonas aeruginosa diversity in distinct paediatric patient groups. Clin Microbiol Infect 2008; 14:935–41. [DOI] [PubMed] [Google Scholar]
- 10. Sreeramoju PV, Garcia-Houchins S, Bova J, et al. Correlation between respiratory colonization with Gram-negative bacteria and development of Gram-negative bacterial infection after cardiac surgery. Infect Control Hosp Epidemiol 2008; 29:546–8. [DOI] [PubMed] [Google Scholar]
- 11. Santolaya ME, Farfán MJ, De La Maza V, et al. Diagnosis of bacteremia in febrile neutropenic episodes in children with cancer: microbiologic and molecular approach. Pediatr Infect Dis J 2011; 30:957–61. [DOI] [PubMed] [Google Scholar]
- 12. Rao AR, Splaingard MS, Gershan WM, et al. Detection of Pseudomonas aeruginosa type III antibodies in children with tracheostomies. Pediatr Pulmonol 2005; 39:402–7. [DOI] [PubMed] [Google Scholar]
- 13. Olejnickova K, Hola V, Ruzicka F. Catheter-related infections caused by Pseudomonas aeruginosa: virulence factors involved and their relationships. Pathog Dis 2014; 72:87–94. [DOI] [PubMed] [Google Scholar]
- 14. Kralinsky K, Svetlansky I, Kovacicova G, et al. Nosocomial meningitis due to Pseudomonas aeruginosa in children. J Chemother 2000; 12:538–9. [DOI] [PubMed] [Google Scholar]
- 15. Marcus N, Ashkenazi S, Samra Z, et al. Community-acquired Pseudomonas aeruginosa urinary tract infections in children hospitalized in a tertiary center: relative frequency, risk factors, antimicrobial resistance and treatment. Infection 2008; 36:421–6. [DOI] [PubMed] [Google Scholar]
- 16. Butbul-Aviel Y, Miron D, Halevy R, Koren A, Sakran W. Pseudomonas aeruginosa as a leading pathogen. Int J Pediatr Otorhinolaryngol 2003; 67:277–81. [DOI] [PubMed] [Google Scholar]
- 17. Fustes-Morales A, Gutierrez-Castrellon P, Duran-Mckinster C, et al. Necrotizing fasciitis: report of 39 pediatric cases. Arch Dermatol 2002; 138:893–9. [DOI] [PubMed] [Google Scholar]
- 18. Kastner U, Guggenbichler JP. Influence of macrolide antibiotics on promotion of resistance in the oral flora of children. Infection 2001; 29:251–6. [DOI] [PubMed] [Google Scholar]
- 19. Hajjartabar M. Poor-quality water in swimming pools associated with a substantial risk of otitis externa due to Pseudomonas aeruginosa. Water Sci Technol 2004; 50:63–7. [PubMed] [Google Scholar]
- 20. Logan LK, Braykov NP, Weinstein RA, Laxminarayan R. Extended-spectrum β-lactamase-producing and third-generation cephalosporin-resistant Enterobacteriaceae in children: trends in the United States, 1999–2011. J Pediatr Infect Soc. 2014; 3:320–8. [DOI] [PubMed] [Google Scholar]
- 21. Logan L, Renschler J, Gandra S, Weinstein R, Laxminarayan R. Carbapenem-resistant Enterobacteriaceae in children, United States, 1999–2012. Emerg Infect Dis 2015; 15:2014–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Braykov NP, Eber MR, Klein EY, et al. Trends in resistance to carbapenems and third-generation cephalosporins among clinical isolates of Klebsiella pneumoniae in the United States, 1999–2010. Infect Control Hosp Epidemiol 2013; 34:259–68. [DOI] [PubMed] [Google Scholar]
- 23. Gandra S, Braykov NP, Laxminarayan R. East North Central region has the highest prevalence of vancomycin-resistant Enterococcus faecalis in the United States Infect Control Hosp Epidemiol 2013; 34:443–5. [DOI] [PubMed] [Google Scholar]
- 24. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Wayne, PA: Clinical and Laboratory Standards Institute; 2010. [Google Scholar]
- 25. Roberts RR, Hota B, Ahmad I, et al. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis 2009; 49:1175–84. [DOI] [PubMed] [Google Scholar]
- 26. Smith RS, Iglewski BH. Pseudomonas aeruginosa quorum sensing as a potential antimicrobial target. J Clin Invest 2003; 112:1460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ribeiro J, Mendes RE, Domingos R, et al. Microbiological and epidemiological characterization of imipenem-resistant Pseudomonas aeruginosa strains from a Brazilian tertiary hospital: report from the SENTRY Antimicrobial Surveillance Program. J Chemother 2006; 18:461–7. [DOI] [PubMed] [Google Scholar]
- 28. Strateva T, Yordanov D. Pseudomonas aeruginosa—a phenomenon of bacterial resistance. J Med Microbiol 2009; 58:1133–48. [DOI] [PubMed] [Google Scholar]
- 29. Kerr KG, Snelling AM. Pseudomonas aeruginosa: a formidable and ever-present adversary. J Hosp Infect 2009; 73:338–44. [DOI] [PubMed] [Google Scholar]
- 30. Burns KH, Casey PH, Lyle RE, et al. Increasing prevalence of medically complex children in US hospitals. Pediatrics 2010; 126:638–46. [DOI] [PubMed] [Google Scholar]
- 31. Lolans K, Queenan AM, Bush K, et al. First nosocomial outbreak of Pseudomonas aeruginosa producing an integron-borne metallo-beta-lactamase (VIM-2) in the United States. Antimicrob Agents Chemother 2005; 49:3538–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bonten MJ, Bergmans DC, Speijer H, Stobberingh EE. Characteristics of polyclonal endemicity of Pseudomonas aeruginosa colonization in intensive care units. Implications for infection control. Am J Respir Crit Care Med 1999; 160:1212–9. [DOI] [PubMed] [Google Scholar]
- 33. Weinstein RA. Epidemiology and control of nosocomial infections in adult intensive care units. Am J Med 1991; 91:179–84S. [DOI] [PubMed] [Google Scholar]
- 34. Olson B, Weinstein RA, Nathan C, et al. Epidemiology of endemic Pseudomonas aeruginosa: why infection control efforts have failed. J Infect Dis 1984; 150:808–16. [DOI] [PubMed] [Google Scholar]
- 35. Sydnor ER, Perl TM. Hospital epidemiology and infection control in acute-care settings. Clin Microbiol Rev 2011; 24:141–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lo E, Nicolle L, Classen D, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals. Infect Control Hosp Epidemiol 2008; 29(Suppl 1):S41–50. [DOI] [PubMed] [Google Scholar]
- 37. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014; 35:464–79. [DOI] [PubMed] [Google Scholar]
- 38. Warren JW. Catheter-associated urinary tract infections. Int J Antimicrob Agents 2001; 17:299–303. [DOI] [PubMed] [Google Scholar]
- 39. Vaz LE, Kleinman KP, Raebel MA, et al. Recent trends in outpatient antibiotic use in children. Pediatrics 2014; 133:375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee GC, Reveles KR, Attridge RT, et al. Outpatient antibiotic prescribing in the United States: 2000 to 2010. BMC Med 2014; 12:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Milner TL, McCulloh R, Koster M, et al. Antibiotic prescribing patterns across the continuum of care for children hospitalized with community-acquired pneumonia [published online ahead of print 2015]. Pediatr Emerg Care. doi:10.1097/PEC.0000000000000598. [DOI] [PubMed] [Google Scholar]
- 42. Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis 2015; 60:1308–16. [DOI] [PubMed] [Google Scholar]
- 43. Klein EY, Makowsky M, Orlando M, et al. Influence of provider and urgent care density across different socioeconomic strata on outpatient antibiotic prescribing in the USA. J Antimicrob Chemother 2015; 70:1580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pakyz AL, Oinonen M, Polk RE. Relationship of carbapenem restriction in 22 university teaching hospitals to carbapenem use and carbapenem-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother 2009; 53:1983–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tacconelli E. Antimicrobial use: risk driver of multidrug resistant microorganisms in healthcare settings. Curr Opin Infect Dis 2009; 22:352–8. [DOI] [PubMed] [Google Scholar]
- 46. Di Pentima MC, Chan S, Hossain J. Benefits of a pediatric antimicrobial stewardship program at a children’s hospital. Pediatrics 2011; 128:1062–70. [DOI] [PubMed] [Google Scholar]
- 47. Kaki R, Elligsen M, Walker S, et al. Impact of antimicrobial stewardship in critical care: a systematic review. J Antimicrob Chemother 2011; 66:1223–30. [DOI] [PubMed] [Google Scholar]
- 48. Mesaros N, Nordmann P, Plésiat P, et al. Pseudomonas aeruginosa: resistance and therapeutic options at the turn of the new millennium. Clin Microbiol Infect 2007; 13:560–78. [DOI] [PubMed] [Google Scholar]
- 49. Fedler KA, Jones RN, Sader HS, Fritsche TR. Activity of gatifloxacin tested against isolates from pediatric patients: report from the SENTRY Antimicrobial Surveillance Program (North America, 1998–2003). Diagn Microbiol Infect Dis 2006; 55:157–64. [DOI] [PubMed] [Google Scholar]
- 50. Bradley JS, Jackson MA; Committee on Infectious Diseases, American Academy of Pediatrics The use of systemic and topical fluoroquinolones. Pediatrics 2011; 128:e1034–45. [DOI] [PubMed] [Google Scholar]
- 51. Shulman JA, Terry PM, Hough CE. Colonization with gentamicin-resistant Pseudomonas aeruginosa, pyocine type 5, in a burn unit. J Infect Dis 1971; 124(Suppl):S18–23. [DOI] [PubMed] [Google Scholar]
- 52. Graham DR, Correa-Villasenor A, Anderson RL, et al. Epidemic neonatal gentamicin-methicillin–resistant Staphylococcus aureus infection associated with nonspecific topical use of gentamicin. J Pediatr 1980; 97:972–8. [DOI] [PubMed] [Google Scholar]
- 53. Holdsworth M, Hanrahan J, Albanese B, Frost J. Outpatient management of febrile neutropenia in children with cancer. Paediatr Drugs 2003; 5:443–55. [DOI] [PubMed] [Google Scholar]
- 54. Lewis CW, Carron JD, Perkins JA, et al. Tracheotomy in pediatric patients: a national perspective. Arch Otolaryngol Head Neck Surg 2003; 129:523–9. [DOI] [PubMed] [Google Scholar]
- 55. Bitsori M, Maraki S, Koukouraki S, Galanakis E. Pseudomonas aeruginosa urinary tract infection in children: risk factors and outcomes. J Urol 2012; 187:260–4. [DOI] [PubMed] [Google Scholar]
- 56. Goldman M, Rosenfeld-Yehoshua N, Lerner-Geva L, et al. Clinical features of community-acquired Pseudomonas aeruginosa urinary tract infections in children. Pediatr Nephrol 2008; 23:765–8. [DOI] [PubMed] [Google Scholar]
- 57. Graf JM, Montagnino BA, Hueckel R, McPherson ML. Pediatric tracheostomies: a recent experience from one academic center. Pediatr Crit Care Med 2008; 9:96–100. [DOI] [PubMed] [Google Scholar]
- 58. Kutko MC, Calarco MP, Flaherty MB, et al. Mortality rates in pediatric septic shock with and without multiple organ system failure. Pediatr Crit Care Med 2003; 4:333–7. [DOI] [PubMed] [Google Scholar]
- 59. Nolte FS. Molecular diagnostics for detection of bacterial and viral pathogens in community-acquired pneumonia. Clin Infect Dis 2008; 47(Suppl 3):S123–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dong T, Zhao X. Rapid identification and susceptibility testing of uropathogenic microbes via immunosorbent ATP-bioluminescence assay on a microfluidic simulator for antibiotic therapy. Anal Chem 2015; 87:2410–8. [DOI] [PubMed] [Google Scholar]
- 61. Bauer KA, Perez KK, Forrest GN, Goff DA. Review of rapid diagnostic tests used by antimicrobial stewardship programs. Clin Infect Dis 2014; 59(Suppl 3):S134–45. [DOI] [PubMed] [Google Scholar]
- 62. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 22nd international supplement. Report M100-S22. Wayne, PA: Clinical and Laboratory Standards Institute; 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.