Abstract
Background
Cytomegalovirus (CMV) is the most common infectious cause of fetal malformations and childhood hearing loss. CMV is more common among socially disadvantaged groups, and it clusters geographically in poor communities. We conducted a geospatial analysis of chronic and primary CMV infection among pregnant women around Durham, NC.
Methods
We performed a geospatial analysis of subjects from an ongoing study of CMV infection among pregnant women using geographic information systems and spatial statistics. Subjects were categorized on the basis of results of their CMV immunoglobulin G avidity testing as seronegative, seropositive, or primary infection. We used generalized additive models to analyze the spatial distributions of individuals who fell into each category and to control for confounders such as race and age. We used a generalized estimating equation to correlate community-level variables with CMV status.
Results
Of 3527 pregnant women aged 15 to 59 years, 93.4% were either white or black. CMV seropositivity was significantly more common among non-Hispanic white subjects than among minority subjects (odds ratio, 3.76 [95% confidence interval, 3.25–4.34]). We identified a cluster in which women had elevated odds of CMV seropositivity in the urban neighborhoods of Durham. Cases of primary CMV infection were more common in areas with higher-than-average CMV seroprevalence. Neighborhood median family income was associated inversely with the prevalence of chronic CMV.
Conclusions
We found a high prevalence of CMV seropositivity in urban low-income neighborhoods among pregnant women, particularly among racial and ethnic minorities. Seronegative pregnant women from these communities might be at heightened risk for primary CMV infection.
Keywords: African American, black, cluster, cytomegalovirus, disparity, epidemiology, generalized additive model, geographic information system (GIS), poverty, segregation, serology, spatial epidemiology
Congenital cytomegalovirus (CMV) infection is the leading infectious cause of neurologic deficits and hearing loss in infants and results in more long-term pediatric disabilities than Down syndrome and spina bifida [1]. Previous studies in the United States found that CMV is more common among socially disadvantaged groups and nonwhite minorities. We recently completed a geospatial analysis of CMV-tested patients in our health system and found that CMV immunoglobulin G (IgG) seropositivity was nearly twice as common among black than among white patients and that seropositivity was clustered heavily in urban communities [2]. White patients living in these communities had a greater risk of CMV seropositivity than those living elsewhere, which suggests that sociodemographic factors independent of race lead to its concentration in urban centers.
This pattern raised the hypothesis that CMV infection among women of childbearing age also concentrates in these geographic areas, which could create communities in which there is a disproportionately high risk of primary maternal CMV acquisition and congenital CMV infection, and in these areas infants and children experience a higher prevalence of CMV-associated morbidity. As we model preventive strategies such as infant CMV testing and maternal CMV vaccination, we might underestimate the benefit to these communities when their disease burden is averaged with that of lower-risk communities.
For this study, we undertook a geospatial analysis of serologic CMV status among women tested in early pregnancy. Geospatial analyses of health data include quantitative methods for identifying how disease risk varies geographically and what variables influence geographic patterns. These techniques are used frequently in studies of environmental health, health demographics, and the spatial epidemiology of emerging diseases. Our goal in this study was to identify communities with high rates of either primary CMV infection or high CMV seroprevalence among pregnant women and to identify both individual- and neighborhood-level variables associated with this risk.
METHODS
Design
This was a retrospective cross-sectional case-control study in which electronic health records and CMV testing data were used.
Patient Cohort
Duke University is a participating site in the Eunice Kennedy Shriver National Institute of Child Health and Human Development–sponsored multicenter randomized placebo-controlled trial of CMV hyperimmunoglobulin in pregnant women to prevent perinatal transmission of or congenital CMV infection (ClinicalTrials.gov identifier 01376778). As of December 31, 2015, 6396 pregnant women who were receiving prenatal care within the Duke University Health System had been recruited for the screening phase of this ongoing study. CMV serologic status is determined by IgM and IgG quantitation and reflex IgG avidity testing if both IgM69 and IgG seropositive, with low avidity IgG indicating recent primary infection [3–5]. Women were classified as CMV seropositive if they were IgG positive and IgM negative or, in the case of IgG and IgM seropositivity, had high-avidity IgG. Laboratory testing was performed at a single central laboratory. All women in this study cohort were human immunodeficiency virus negative. We used data collected for the trial and an electronic query of the electronic health records to obtain each subject’s age, race, ethnicity, and home address. Because we were interested in associations between race or ethnicity and CMV status, we included only subjects for whom race and ethnicity were recorded within the electronic health records.
Geographic Data Management
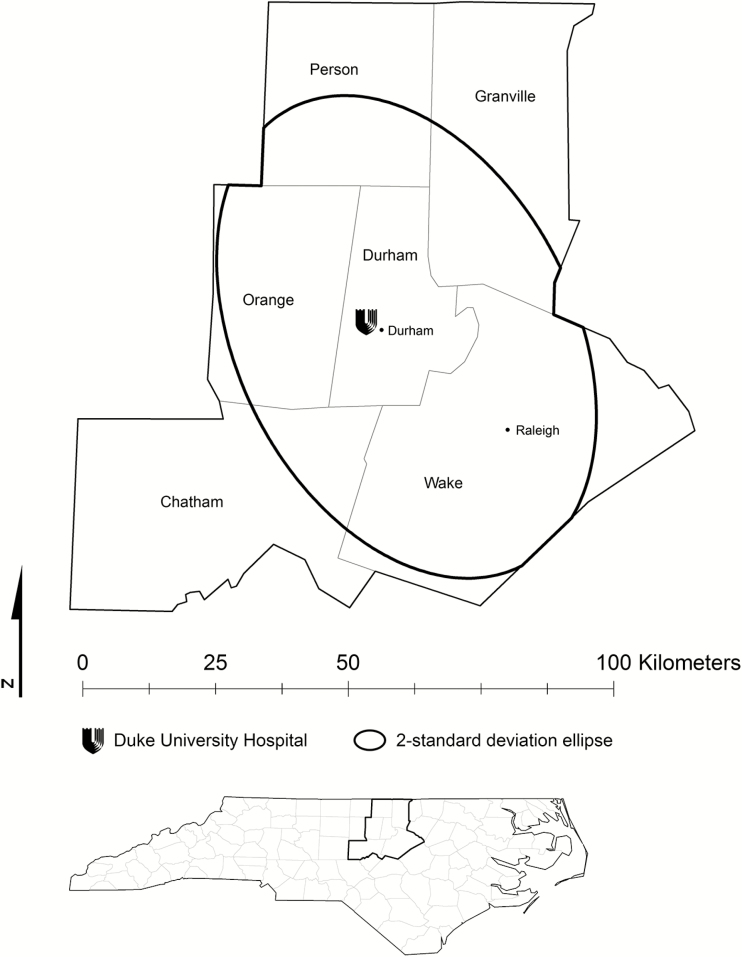
Geocoding of patient residential addresses was performed internally within Duke’s informatics systems, which enabled us to obtain study-subject residential coordinates. These coordinate data were displayed as points in a geographic information system using ArcGIS 10.3.1 (ESRI, Redlands, CA). From this overall cohort we retained only patients whose residential coordinates fell within Durham County, North Carolina, or 1 of its 5 bordering counties (Wake, Person, Chatham, Orange, and Granville counties). Within these 6 counties were some peripheral areas with very few subjects; to maximize spatial sampling density, we calculated a 2-standard deviation ellipse and retained the subjects it contained (Figure 1). This technique computes the smallest ellipse that circumscribes 95% of the subjects.
Figure 1.
Study area. We selected study subjects who lived within Durham County, North Carolina, or 1 of its 5 adjacent counties. To maximize the sampling density of our subjects, we further limited the cohort to an ellipse containing 2 standard deviations (95%) of the subjects.
Statistical Analyses
Nonspatial statistical analyses were performed using the statistical programming language R 3.2.3 (www.r-project.org), including multivariate logistic regression, the Fisher exact test, and the Wilcoxon sign-rank test. For our spatial analyses we used ArcGIS, R, and SAS 9.4 (SAS, Cary, NC).
Our primary spatial analytical technique was a spatial generalized additive model (GAM), for which we used the GAM package in R [6]. The GAM is similar to logistic regression, in which a binary outcome variable (in this case, CMV positivity or negativity) is modeled against predictor variables. In this case, the primary predictor was the 2-dimensional combination of the longitudinal and latitudinal spatial coordinates. Because we could not assume that a linear model would best describe the relationship between disease odds and geography, in the GAM we used nonparametric local regression (“loess”) as a smoothing function for the spatial coordinate pair. The loess smoother incorporated data from nearby data points, weighting closer points more heavily than distant ones, and thereby modeled local (rather than global) variability in the relationship between the outcome variable and geographic space. The optimal bandwidth for the loess function (ie, the proportion of the total data set included in the loess function) was that which produced the best model fit (as defined by the lowest Akaike information criterion value). The model produced log odds of the outcome variable, which can be predicted on each point of a dense grid covering the geographic extent of the study area. Using the average disease odds as a referent group, the odds of disease can be presented as a local odds ratio (OR). A permutation test was then used to determine the significance of OR predictions; a 2-tailed α value of .05 after 1000 permutations is accepted as statistically significant. We used unadjusted models followed by adjusted models that incorporated patient race and age as independent variables. Because we hypothesized that the relationship between age and CMV seropositivity would vary according to race, we also included an interaction term in adjusted models (eg, age × race). Spatial models were used for 2 groups: CMV seropositivity versus seronegativity (excluding cases of primary infection) and primary CMV infection versus CMV seronegativity. For the second case, we felt that CMV-seronegative subjects would be the correct control group, because primary CMV infection would have arisen among previously seronegative subjects.
We also evaluated associations between serologic CMV status and neighborhood demographic variables, including median family income, average household size, population density, and percentage of the population that reported being white. These data were obtained from the US Census (2010) and the American Community Survey (2008–2013 5-year estimates) [7]. These analyses were conducted using a categorized version of each community-level variable (categorized according to quartile). We used generalized estimating equations (GEEs) to investigate associations at both the tract and block-group level. The GEEs enabled us to take into account residual within-group correlation that can occur when group-level data are used. In these models, we also adjusted for participant age, race, and age × race interaction.
Ethical Review
This study was approved by the Duke University Institutional Review Board (eIRB Pro00034414).
RESULTS
Query Results
We successfully geocoded 5828 of 6396 study subjects; race data were available for 4394 of these subjects. A total of 3527 subjects fell within the elliptical study area described in “Methods.” Of this group, 93.4% were either white (1928 [54.7%]) or black (1394 [39.2%]). The remaining subjects were Asian (191 [5.3%]), Native American (7 [0.2%]), or Hawaiian/Pacific Islander (7 [0.2%]). Of these subjects, 46 (1.3%) self-identified as Hispanic, and 44 of them designated their race as white. We dichotomized our overall cohort into 1887 non-Hispanic white subjects and 1640 minority subjects. We analyzed the small number of Hispanic subjects in the minority category.
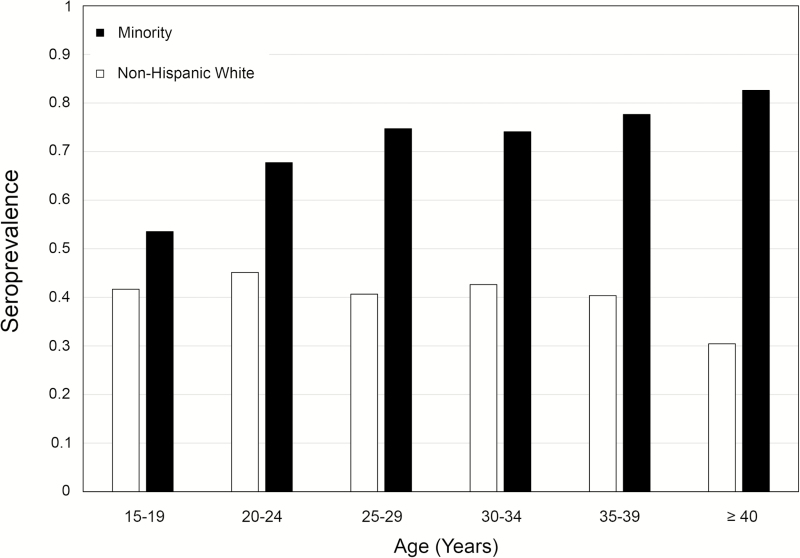
CMV infection was common in all groups, but its incidence was substantially higher among minority than among non-Hispanic white subjects (71.7% vs 41.9%, respectively; OR 3.76 [95% confidence interval (CI) 3.25–4.34]). Non-Hispanic white subjects had a 41.9% seroprevalence, and 70.9% of black subjects were seropositive; the rates were even higher among the remaining minority groups (Table 1). Among minority subjects, the CMV IgG seroprevalence increased from 50% among teenagers to more than 80% among women older than 40 years (Figure 2). In contrast, the seroprevalence did not increase among older non-Hispanic white women. Of the 3527 women, 23 had low-avidity IgG, which indicates recent primary infection (0.65% [95% CI, 0.41–0.98]). Five of these women were black, and 18 were non-Hispanic white.
Table 1.
Age Ranges and Testing Resultsa
| Race/Ethnicity | No. of Subjects | Age (y) | CMV Testing Results | ||||
|---|---|---|---|---|---|---|---|
| Median | Range | Primary | Seropositive (n) | Seronegative (n) | % Positive | ||
| White/Caucasian | 1887 | 32 | 15–59 | 18 | 770 | 1094 | 41.9 |
| Black/African American | 1394 | 27 | 15–47 | 5 | 984 | 405 | 70.9 |
| Hawaiian-Pacific | 7 | 34 | 21–40 | 0 | 7 | 0 | 100 |
| Asian | 191 | 33 | 21–44 | 0 | 159 | 32 | 83.2 |
| Native American | 7 | 31 | 27–36 | 0 | 5 | 2 | 71.4 |
| Hispanic or Latino | 46 | 30 | 19–38 | 0 | 30 | 16 | 65.2 |
Abbreviation: CMV, cytomegalovirus.
Race and ethnicity were self-identified by the patients and are listed here as they appeared when the patients registered. CMV testing results were defined as primary infection (positive IgM with low-avidity IgG or IgG seroconversion), seropositive (IgG positive/IgM negative or IgG positive/IgM positive with high-avidity IgG), or seronegative.
Figure 2.
Age distribution of cytomegalovirus (CMV) prevalence according to race. We categorized our subjects into age intervals of 5 years, beginning with 15- to 19-year-olds and ending with women aged 40 years or older. Prevalences varied between 30% and 45% among non-Hispanic white women, and no increase in prevalence with age was found. In contrast, more than 50% of teenaged minority women were seropositive, and this rate increased to more than 80% in the oldest age group.
Spatial Models
CMV Seroprevalence
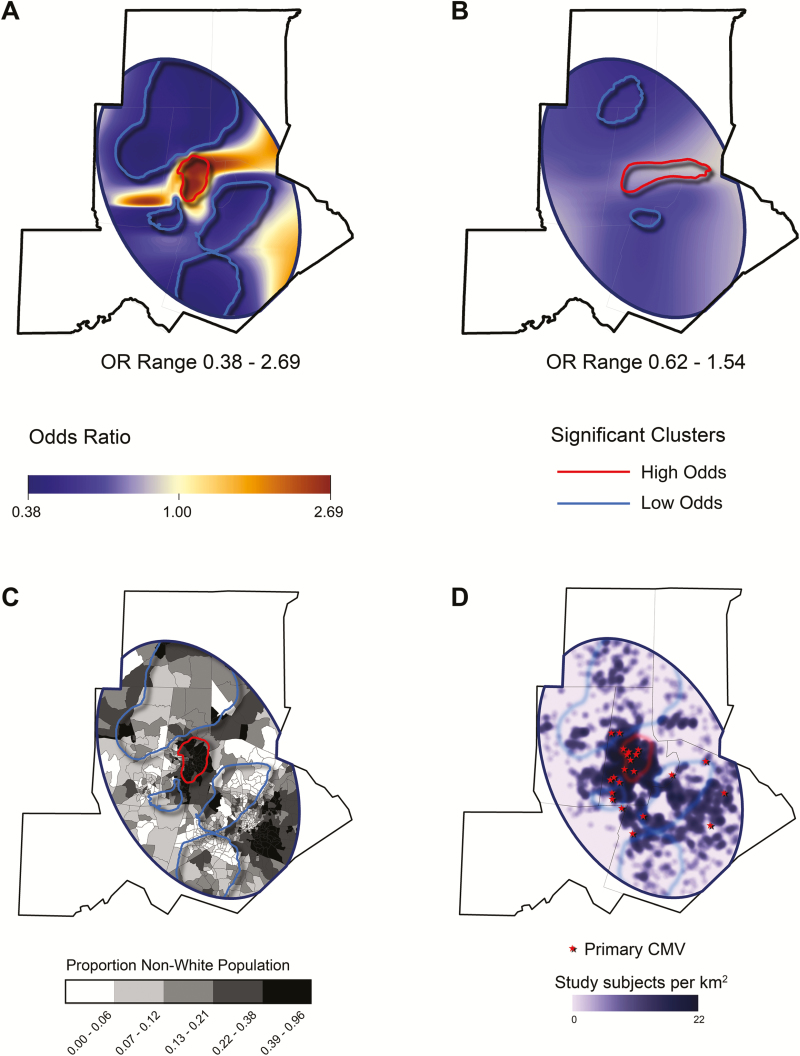
The optimal span sizes for our GAMs were 0.15 for the unadjusted GAM and 0.60 for the adjusted model. Our unadjusted GAM (Figure 3A) computed an OR range from 0.38 to 2.69, with a statistically significant cluster of high OR overlying the city of Durham. Much of the surrounding suburban and rural area fell within low-OR clusters. Adjusting our model for subject race, age, and race × age interaction blunted the OR range (0.62–1.54) (Figure 3B). However, even after this adjustment, significant clustering of high odds in urban Durham and low odds in the surrounding areas remained, which suggests that racial segregation did not adequately account for the geographic heterogeneity we observed. The high-OR cluster largely encircled neighborhoods with a high proportion of black people in the general population, whereas the opposite was true of the low-OR clusters (Figure 3C).
Figure 3.
Generalized additive model (GAM) showing geographic variation and clustering of cytomegalovirus (CMV) seropositivity. This analysis was similar to logistic regression, but in the GAM, the response variable was correlated with a 2-dimensional spatial smoothing function, which results in a continuous odds ratio (OR) surface. The response variable in this model was the binary result of CMV testing (CMV seropositive vs uninfected). Areas with significantly high or low odds of CMV are circled with a contour, which represents a 2-tailed P value of .05. (A) Unadjusted GAM. Our unadjusted model identified a cluster with significantly high odds of CMV seropositivity overlying the city of Durham; several large clusters with significantly low odds of CMV seropositivity were found also. (B) Adjusted GAM. Adjusting for subject race and age diminished the OR range, but clustering was still observed in a similar distribution, which suggests that the heterogeneous distribution of CMV cannot be explained solely by racial segregation. (C) Odds clusters generated by our unadjusted GAM superimposed on a census tract map. Census tracts are shaded according to the proportion of the population that is nonwhite. The high-odds cluster circumscribed the high minority urban tracts in Durham, whereas the low-odds clusters encircled areas with lower minority populations. (D) Geographic distribution of cases of primary CMV infection. The approximate location of the subjects with primary CMV infection, as defined by low anti-CMV IgG avidity, are displayed. Most of the 23 women with primary CMV infection lived in the densely sampled areas of Durham County. To preserve anonymity, only approximate subject locations are shown.
The high-OR cluster contained 720 (43.9%) of the minority subjects and 169 (9.0%) of the non-Hispanic white subjects. The non-Hispanic white subjects residing in this high-OR cluster were significantly more likely to be CMV seropositive than those residing outside it (53% vs 42%, respectively; OR 1.55 [95% CI, 1.12–2.16]). Minority subjects who lived in the 4 low-risk clusters were significantly less likely to be CMV seropositive than minority subjects who lived elsewhere (63% vs 74%, respectively; OR 0.60 [95% CI, 0.42–0.86]).
Primary CMV Infection
We found 23 women with primary CMV infection, 18 non-Hispanic white and 5 black subjects. When compared with the underlying susceptible (CMV-negative) populations of 1110 non-Hispanic white and 405 black subjects, we did not find a significantly different incidence of primary CMV infection (0.016 vs 0.012, respectively; P = .2344). Four of the 5 black women lived within the high-odds cluster in Durham (Figure 3D). The remaining subjects were scattered throughout the study region, although many of them were either within or near the high-odds cluster. The local OR of CMV seropositivity was 1.2 (95% CI, 1.1–1.3), which suggests that cases of primary CMV infection were more likely to occur in neighborhoods with a higher-than-average CMV seroprevalence. The optimal span size for our unadjusted model of primary CMV infection versus CMV seronegativity was 0.65. This model did not reveal significant clustering of primary CMV infection with respect to CMV-seronegative women (P = 0.077, analysis of variance comparing the spatial and nonspatial models).
Community-Level Predictors
Community-level (either tract or block group) measures of median family income were associated inversely with CMV seroprevalence (Table 2). For example, those who lived in a census tract with the highest median income were approximately one-third as likely to be CMV seropositive (OR 0.58; P < .001). Adjusting for individual age and race diminished the magnitude of the associations, but median family income remained a statistically significant predictor, which suggests that there is an independent effect of community income (OR 0.65; P = 0.002). In contrast, average household size and population density were not associated with CMV prevalence. Although the percentage of the population that reported nonwhite race was associated with CMV before adjustment for individual-level factors, we observed no statistically significant associations after adjustment for participant race and age.
Table 2.
Association of Select Community-Level Variables With CMV Seropositivitya
| Community Variable | Community Level–Only Model | Community- and Individual-Level Model | ||
|---|---|---|---|---|
| OR | P | OR | P | |
| Median family income | ||||
| Quartile 1 | Reference | Reference | ||
| Quartile 2 | 0.78 | .03 | 0.81 | .05 |
| Quartile 3 | 0.67 | .001 | 0.74 | .01 |
| Quartile 4 | 0.58 | <.0001 | 0.65 | .002 |
| Average household size | ||||
| Quartile 1 | Reference | Reference | ||
| Quartile 2 | 0.88 | .06 | 0.88 | .18 |
| Quartile 3 | 0.93 | .49 | 0.99 | .93 |
| Quartile 4 | 0.97 | .73 | 1.03 | .78 |
| Population density | ||||
| Quartile 1 | Reference | Reference | ||
| Quartile 2 | 1.18 | .10 | 1.39 | .23 |
| Quartile 3 | 1.16 | .15 | 1.45 | .11 |
| Quartile 4 | 1.12 | .29 | 1.49 | .12 |
| Percent nonwhite | ||||
| Quartile 1 | Reference | Reference | ||
| Quartile 2 | 1.33 | .01 | 0.99 | .90 |
| Quartile 3 | 1.87 | <.0001 | 1.14 | .36 |
| Quartile 4 | 1.81 | <.0001 | 0.92 | .61 |
Abbreviation: CMV, cytomegalovirus.
In this analysis, we took into account both individual variables and variables aggregated at the census-tract level. Median family income was associated inversely with the prevalence of CMV IgG even after we adjusted for patient race and age.
DISCUSSION
CMV seropositivity is significantly higher among women who live in the urban neighborhoods of Durham. Although CMV seropositivity is markedly more common among minority than among white populations, racial segregation does not adequately explain the heterogeneous geography found for CMV. Women from low-income neighborhoods were significantly more likely to be CMV seropositive, even after controlling for individual race.
Among minority women, the CMV seroprevalence increased with age, a phenomenon we did not observe among the non-Hispanic white women in our cohort. These findings raise concern that infants born to these mothers are at a disproportionately high risk of congenital CMV infection, because both newly and previously infected mothers can transmit CMV to their infants [8]. Previous research identified a higher risk of congenital CMV among minority infants [9–11]. Our geospatial analyses add to this body of knowledge by showing that this risk might be concentrated heavily in individual communities, particularly those that already experience disparities in healthcare access and public health.
Newly acquired cases of CMV occurred at similar proportions in white and in black women. We did not identify any cases of primary CMV infection among other racial or ethnic groups, likely because of their underrepresentation in our cohort. As shown in Figure 3D, most of the new cases were either within the high-CMV-prevalence cluster in Durham or nearby, and the local OR of CMV seropositivity was higher than that of the baseline at which these primary infection cases occurred. Our spatial analyses did not identify statistically significant clustering of subjects with primary CMV infection (when compared with seronegative subjects). However, the small number of patients with primary CMV infection in our cohort certainly limited our ability to detect such a pattern.
It is important to reiterate that our spatial data are limited to patient residences and do not necessarily represent where our subjects acquired CMV. Thus, women who live near the high-odds cluster, even if they do not reside within it, might be at increased risk of exposure to CMV, because their occupational and social exposures are closer to higher-prevalence areas. Non-Hispanic white women were more likely to be CMV seronegative and therefore susceptible to primary infection; proximity to the high-odds cluster might therefore have resulted in increased risk for these women. In contrast, the distribution of these relatively rare cases of primary CMV infection might also have been a function of our spatial sampling density; as shown in Figure 3D, our overall cohort of study subjects was concentrated most heavily in this area.
It is not sufficient to ascribe the clustering of CMV risk simply to racial segregation. After we adjusted for race, we still observed a pattern of high odds clustering in Durham and low odds clustering in the wealthier surrounding communities. Non-Hispanic white subjects within the high-odds cluster had a significantly higher risk of CMV seropositivity than in other neighborhoods elsewhere, and minority subjects who lived in low-odds clusters had a significantly lower risk than minorities elsewhere, which suggests that there are important unmeasured variables in these geographic settings for which race is merely a surrogate. Neighborhood income was associated inversely with CMV seroprevalence even after we adjusted for patient race. Last, there is no known intrinsic susceptibility (or resistance) to CMV that can be ascribed to race. It is most plausible that certain social factors, including household composition, crowding, contact with children, and socially segregated sexual networks, are associated with CMV risk and that these risks themselves segregate alongside both race and poverty [12, 13].
Limitations and potential biases exist in this study. The relationship between maternal and congenital CMV is complex, and additional research is needed to confirm whether they share the same geospatial and demographic distributions. We did not have data on our subjects’ mobility (ie, previous residences and geographic exposures); our geographic analyses were restricted to the subjects’ recorded residential addresses, which clearly do not account entirely for the totality of their exposures. Similarly, we did not have individual socioeconomic data; we used neighborhood data to make inferences about associations of wealth and living conditions with CMV risk. Last, reliably documenting primary CMV infection in pregnancy remains challenging [3]. IgG avidity is the most reliable means of identifying primary infection in this population, but its sensitivity for predicting fetal infection is influenced by gestational age, and its specificity has varied in different studies [3]. However, our serologic testing was performed prospectively at a central laboratory, which should have minimized the risk of measurement errors.
Our study is one of very few spatial studies of CMV and, to our knowledge, the only spatial study of CMV in pregnant women. Nonspatial studies performed in different regions have reported substantially higher CMV-seropositivity rates among various minority populations than among white populations [14–19]. It is likely, then, that our findings are generalizable to other regions with comparable racial and socioeconomic segregation.
We conclude from our study that it is important to account for the heterogeneous risk landscape of CMV in models of CMV prevention. Long-term sequelae of symptomatic congenital CMV can be ameliorated with early detection and antiviral therapy in the neonatal period [20]. Newborn CMV screening would identify many infants who would benefit from measures such as antiviral therapy and early interventions. Several congenital CMV screening and treatment strategies, including hearing-based infant CMV testing, universal CMV screening, and the development of a maternal CMV vaccine, have been studied or considered. A geographically or demographically targeted intervention would likely not be practicable, and it is most likely that any widely adopted strategy would be universal rather than targeted. If, then, we are to pursue a universal strategy for preventing congenital CMV, accounting for geographic heterogeneity will be instrumental in understanding how such a program will benefit the communities at the highest risk.
Notes
Disclaimer. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
Financial support. Dr Lantos was supported by the National Center for Advancing Translational Sciences of the NIH under award KL2 TR001115, Dr Permar was supported by the NIH Director’s New Innovator Award DP2 grant HD075699, and Dr Swamy was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal Fetal Medicine Units Network grant HD068258.
Potential conflicts of interest. Dr Permar has a nonfinancial consulting agreement with Pfizer Vaccines. All other authors: No reported conflicts.
All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Cannon MJ, Davis KF. Washing our hands of the congenital cytomegalovirus disease epidemic. BMC Public Health 2005; 5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lantos PM, Permar SR, Hoffman K, Swamy GK. The excess burden of cytomegalovirus in African American communities: a geospatial analysis. Open Forum Infect Dis 2015; 2:ofv180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lazzarotto T, Guerra B, Lanari M, et al. New advances in the diagnosis of congenital cytomegalovirus infection. J Clin Virol 2008; 41:192–7. [DOI] [PubMed] [Google Scholar]
- 4. Prince HE, Leber AL. Validation of an in-house assay for cytomegalovirus immunoglobulin G (CMV IgG) avidity and relationship of avidity to CMV IgM levels. Clin Diagn Lab Immunol 2002; 9:824–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Munro SC, Hall B, Whybin LR, et al. Diagnosis of and screening for cytomegalovirus infection in pregnant women. J Clin Microbiol 2005; 43:4713–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vieira V, Bartell S, Bliss R. Mapping Smoothed Effect Estimates From Individual-Level Data. Available at: https://cran.r-project.org/web/packages/MapGAM/MapGAM.pdf Accessed September 1, 2016
- 7. United States Census: Maps & Data. Available at: https://www.census.gov/geo/maps-data/ Accessed January 27, 2017
- 8. Pass RF, Anderson B. Mother-to-child transmission of cytomegalovirus and prevention of congenital infection. J Pediatric Infect Dis Soc 2014; 3 Suppl 1:S2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bristow BN, O’Keefe KA, Shafir SC, Sorvillo FJ. Congenital cytomegalovirus mortality in the United States, 1990–2006. PLoS Negl Trop Dis 2011; 5:e1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fowler KB, Pass RF. Sexually transmitted diseases in mothers of neonates with congenital cytomegalovirus infection. J Infect Dis 1991; 164:259–64. [DOI] [PubMed] [Google Scholar]
- 11. Fowler KB, Pass RF. Risk factors for congenital cytomegalovirus infection in the offspring of young women: exposure to young children and recent onset of sexual activity. Pediatrics 2006; 118:e286–92. [DOI] [PubMed] [Google Scholar]
- 12. Dowd JB, Palermo TM, Aiello AE. Family poverty is associated with cytomegalovirus antibody titers in U.S. children. Health Psychol 2012; 31:5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Griffiths P, Baboonian C, Ashby D. The demographic characteristics of pregnant women infected with cytomegalovirus. Int J Epidemiol 1985; 14:447–52. [DOI] [PubMed] [Google Scholar]
- 14. Colugnati FA, Staras SA, Dollard SC, Cannon MJ. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infect Dis 2007; 7:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stadler LP, Bernstein DI, Callahan ST, et al. Seroprevalence and risk factors for cytomegalovirus infections in adolescent females. J Pediatric Infect Dis Soc 2013; 2:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stadler LP, Bernstein DI, Callahan ST, et al. Seroprevalence of cytomegalovirus (CMV) and risk factors for infection in adolescent males. Clin Infect Dis 2010; 51:e76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sohn YM, Oh MK, Balcarek KB, et al. Cytomegalovirus infection in sexually active adolescents. J Infect Dis 1991; 163:460–3. [DOI] [PubMed] [Google Scholar]
- 18. Gaffey MJ, Tucker RM, Fisch MJ, Normansell DE. The seroprevalence of cytomegalovirus among Virginia state prisoners. Public Health 1989; 103:303–6. [DOI] [PubMed] [Google Scholar]
- 19. Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the National Health and Nutrition Examination Surveys, 1988–2004. Clin Infect Dis 2010; 50:1439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kimberlin DW, Jester PM, Sánchez PJ, et al. ; National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Valganciclovir for symptomatic congenital cytomegalovirus disease. N Engl J Med 2015; 372:933–43. [DOI] [PMC free article] [PubMed] [Google Scholar]