Abstract
Purpose
To confirm the psychometric properties of the Athens Insomnia Scale (AIS) among Japanese chronic pain patients.
Patients and methods
In total, 144 outpatients were asked to complete questionnaires comprising the AIS and other study measures. According to the original article, the AIS has 2 versions: the AIS-8 (full version) and the AIS-5 (brief version). To validate the AIS-8 and AIS-5 among chronic pain patients, we confirmed: 1) factor structure by confirmatory factor analysis; 2) internal consistency by Cronbach’s a; 3) test–retest reliability using with interclass correlation coefficients; 4) known-group validity; 5) concurrent validity; and 6) cut-off values by receiver operating characteristic analysis. In addition, semi-structured interviews were conducted to assess the participants’ sleep disturbance. If the participants had any sleep complaints, including difficulty in initiating sleep, difficulty in maintaining sleep, and early morning awakening, they were defined as insomnia symptoms.
Results
A 2-factor model of the AIS-8 and 1-factor model of the AIS-5 demonstrated good fit. The AIS had adequate internal consistency and test–retest reliability. Patients with insomnia had a higher AIS score than those without insomnia. The sleep disturbance measured by the AIS was positively associated with pain intensity, disability, depression, anxiety, and pain catastrophizing, and negatively associated with pain-related self-efficacy. The cut-off values for detecting insomnia were estimated at 8 points in the AIS-8 and 4 points in the AIS-5.
Conclusion
The AIS-8 and AIS-5 had adequate reliability and validity in chronic pain patients.
Keywords: sleep disturbance, AIS, Japanese, insomnia symptom
Introduction
Sleep disturbance is a prevalent clinical complaint among individuals with chronic pain.1 In previous studies, 53%–89% of chronic pain patients had sleep complaints.2–6 Sleep disturbance is associated with greater pain intensity,3–6 disability,3 depression,3,5,6 and anxiety6 and also with pain-related cognition, such as greater pain catastrophizing and lower pain-related self-efficacy.7,8 Therefore, the evaluation and treatment of sleep disturbance are essential components of pain management.9
A self-report questionnaire can be used to easily assess difficulties in the sleep of chronic pain patients. The Athens Insomnia Scale (AIS)10,11 may be one of the useful measures because it assesses all 3 major insomnia symptoms (difficulties in initiating sleep [DIS], difficulties in maintaining sleep [DMS], and early morning awakening [EMA])12 and important sleep domains (sleep quality and quantity as well as daytime functioning).13 Furthermore, the AIS has only 8 items and takes few minutes to complete it. The AIS has been translated into many languages10,14–17 and has been validated in patients with insomnia, psychiatric disorders, and cancer.10,14–16 However, it has not yet been validated in chronic pain patients.
This study aimed to validate the application of the AIS in chronic pain patients. According to the original author, the AIS has 2 versions: AIS-8 and AIS-5.10 The AIS-8 is the full version, which comprises 8 items related to both nocturnal sleep problems and daytime dysfunction. By contrast, the AIS-5 is the brief version of the AIS and comprises 5 items assessing only nocturnal sleep problems.10,14 We hypothesized that: 1) the score of AIS-8 and AIS-5 are positively associated with measures of pain intensity, disability, depression, anxiety, and pain catastrophizing; 2) they have negative correlations with pain-related self-efficacy measures; and 3) patients with insomnia symptoms have higher AIS-8 and AIS-5 scores than patients without insomnia symptoms.
Materials and methods
Participants
This study had a cross-sectional design. Outpatients on their first visit to a pain management center in a university hospital were included as participants. The data of this study were retrospectively extracted from the clinical records between April 2014 and December 2016.
The inclusion criteria were as follows: 1) a history of pain lasting for ≥3 months and 2) age ≥20 years. The exclusion criteria were as follows: 1) difficulty in reading and writing Japanese and 2) incomplete interview records of sleep disturbance. For example, if the frequency of DMS was not recorded, the author would be unable to assess the patient’s sleep condition.
Measures
AIS
The AIS-8 is an 8-item self-report questionnaire that measures the intensity of sleep difficulties10,11 according to the International Statistical Classification of Disease and Related Health Problems-10th Revision (ICD-10) diagnostic criteria for insomnia.18 Five items assess difficulty in sleep introduction, awakening during the night, early morning awakening, total sleep duration, and overall sleep quality. Three items pertain to the next-day consequences of insomnia (sense of well-being during the day, functioning [physical and mental] during the day, and sleepiness during the day). The AIS-5 is the brief version of the AIS-8 and comprises only 5 items assessing nocturnal sleep problems. Respondents are required to rate positively if they have experienced sleep difficulties at least thrice per week during the last month. Each item is rated on a 4-point numerical rating scale (NRS; where 0= no problem at all and 3= very serious problem). Total scores range from 0 to 24 in the AIS-8 and from 0 to 15 in the AIS-5. Higher scores in these AIS measures indicate that responders have severe insomnia symptoms.
The factor structure of the AIS-8 is different in populations. It has been reported as a 1-factor structure (item 1–8) or a 2-factor structure (item 1–5 and item 6–8) in previous studies.10,14,15,17 The factor structure of the AIS-5 is reported as a 1-factor structure only.14,15 Cronbach’s a coefficients for the Japanese version of the AIS-8 and AIS-5 were 0.88 and 0.85, respectively.14 The cut-off values for detecting insomnia among Japanese outpatients with chronic insomnia were estimated at 6 points in the AIS-8 and 4 points in the AIS-5.14
NRS
To assess the pain intensity, we determined the average score of 4 items on an 11-point NRS. The NRS ranges from 0 (no pain) to 10 (worst pain imaginable), and the 4 assessed items are as follows: 1) worst pain in the past 24 hours; 2) least pain in the past 24 hours; 3) average pain in the past 24 hours; and 4) current pain. The average score of these 4 items was used for data analysis.19,20
Pain Disability Assessment Scale (PDAS)
The PDAS is a self-report questionnaire assessing the degree of pain interference.21 It comprises 20 items, each of which is rated on a 4-point numerical rating scale. Higher scores (range from 0 to 60) indicate a greater degree of disability. Cronbach’s a coefficient of the total PDAS was 0.96.21
Hospital Anxiety and Depression Scale (HADS)
The HADS is a 14-item self-report questionnaire, and each item is rated on a 4-point numerical rating scale. It comprises 2 subscales measuring anxiety (HADS-A) and depression (HADS-D).22 Each subscale includes 7 items, and higher scores indicate a greater degree of anxiety or depression. Total scores range from 0 to 42 and each subscale ranges from 0 to 21. Cronbach’s a coefficients for the Japanese version of the HADS were 0.77 for HADS-A and 0.79 for HADS-D.23,24 The cut-off value was estimated at 11 points in total score and 8 points in HADS-A and HADS-D.25
Pain Catastrophizing Scale (PCS)
The PCS is a self-report questionnaire that assesses the degree of catastrophic thinking regarding pain. It comprises 13 items, each of which is rated on a 5-point numerical rating scale. The PCS comprises 3 subscales: rumination, magnification, and helplessness.26 Higher scores indicate greater levels of catastrophizing. The total scores range from 0 to 51. The Japanese version of the PCS has good internal consistency (Cronbach’s a coefficients were 0.80 for the rumination subscale, 0.65 for the magnification subscale, 0.81 for the helplessness subscale, and 0.89 for the total score).27
Pain Self-Efficacy Questionnaire (PSEQ)
The PSEQ is a 10-item self-report questionnaire that measures the confidence in performing activities despite pain.28 Each item is rated on a 7-point numerical rating scale. Higher scores indicate greater perceived self-efficacy among chronic pain patients. The total scores range from 0 to 60. The Cronbach’s a coefficient for the Japanese version of the PSEQ was 0.94.29
Patient’s Global Impression of Change (PGIC)
The PGIC is a self-report questionnaire that measures the change in patients’ overall conditions since treatment initiation.30 It is a 7-point categorical scale and is scored as follows: 1) very much improved; 2) much improved; 3) minimally improved; 4) no change; 5) minimally worse; 6) much worse; and 7) very much worse. Participants who visited the hospital again were asked to complete this questionnaire. In the present study, we used the PGIC for assessing the test–retest reliabilities of the AIS-8 and AIS-5.
Semi-structured interview for sleep disturbance
In a semi-structured interview conducted by psychologists, participants were questioned regarding their sleep condition. The items assessed through the interview were wake-up time, bedtime, DIS, DMS, EMA, and history of sleeping pill consumption. DIS was defined as requiring >30 min to fall asleep; the presence of DMS and EMA was defined as >3 episodes per week.31,32 The presence of insomnia was defined as at least 1 positive response to questions regarding DIS, DMS, or EMA.31–34
Procedure
Data were collected as part of routine care at a tertiary pain management center. First, the participants were informed that their questionnaire data and clinical records might be used for clinical and research purposes. If the participants agreed, they were asked to complete questionnaires using an e-tablet with a touch screen in the waiting room. After completing the questionnaires, the patients were consulted by a multidisciplinary team (physician, occupational therapist, physiotherapist, and psychotherapist). During the psychological assessment, the psychologist conducted semi-structured interviews regarding the sleep condition. The interviews were conducted by either of two psychotherapists. Patients who revisited the clinic within 100 days were asked to complete the questionnaires twice. This study was approved by the Institutional Review Board of the Osaka University Hospital (No. 13004-6), and written informed consent was obtained from all participants prior to study inclusion.
Statistical analysis
All statistical analyses were conducted using R software (version 3.4.1).35 Descriptive statistics were calculated to describe the demographic characteristics of the participants. The factor structures of the AIS-8 and AIS-5 were analyzed though confirmatory factor analysis using the package “lavaan” (version 0.5-23).36 To determine absolute fit indices, the chi-square goodness-of-fit index (χ2), the root mean square error of approximation (RMSEA), and the standardized root mean square residual (SRMR) were used.37 An acceptable fitting model was determined as that with a 0.05 threshold using non-significant χ2 tests,37 with a RMSEA of <0.08,38 and a SRMR of <0.10.38 To assess incremental fit indices, the comparative fit index (CFI) was used.37 An acceptable model was determined as that with a CFI of >0.95.37 In addition, the Akaike Information Criterion (AIC) was used as a parsimony fit index.37 The best model demonstrated the minimal value of AIC. The χ2 improvement was assessed between the 2 nested models of the AIS-8.
Internal consistencies in the AIS-8 and AIS-5 were evaluated using Cronbach’s a. Test–retest reliability was determined in participants who responded with scores of 3 (minimally improved), 4 (no change), or 5 (minimally worse) on the PGIC at the second visit. To assess test–retest reliability, the interclass correlation coefficient (ICC) was calculated. By using the Pearson correlation coefficients, concurrent validity was evaluated between the AIS and other psychometric measures (the NRS, PDAS, HADS, PCS, and PSEQ) for all participants. Known-group validity was established using the independent t-test to compare the mean values of AIS scores between participants with insomnia and those without insomnia defined by semi-structured interview. In the present study, the significance level was set at p<0.05. Finally, the cut-off points for the AIS-8 and AIS-5 were established with receiver-operating characteristic (ROC) curves using the package “pROC” (version 1.10.0).39 The optimal cut-off value was determined based on the sensitivity and specificity for all participants. The diagnostic accuracy was evaluated by calculating the area under the curve (AUC). Generally, an AUC of 0.9–1.0 represents excellent, 0.8–0.9 represents good, 0.7–0.8 represents fair, and 0.6–0.7 represents poor discriminative ability.40
Results
Demographic characteristics of participants
In total, 144 participants completed the questionnaires and semi-structured interviews. Demographic valuables are shown in Table 1. Eighty-six participants were females (60.0%), and the mean age was 53.3 (SD =16.2) years. The average pain duration was 53.6 (SD =66.7) months. The most frequently reported pain sites were the lower limbs (68.1%), followed by upper shoulder and upper limbs (55.6%), and the lower back (51.4%). Furthermore, 45.0% of the participants reported experiencing pain at more than 3 sites. Participants were classified using the recently proposed International Association for the Study of Pain classification.41,42 A total of 61.8% of participants were categorized as Code 1 (chronic primary pain).
Table 1.
Demographic characteristics of the participants
| Demographic characteristics | Frequency (n) | % |
|---|---|---|
| Number of participants | 144 | |
| Age (years) | 53.3±16.2 | |
| Number of female | 86 | 60.0 |
| Pain durations (months) | 53.6±66.7 | |
| Pain sitea | ||
| Head | 37 | 25.7 |
| Cervical | 40 | 27.8 |
| Upper shoulder and upper limbs | 80 | 55.6 |
| Thoracic | 24 | 16.7 |
| Abdominal | 22 | 15.3 |
| Lower back | 74 | 51.4 |
| Lower limbs | 98 | 68.1 |
| Genital | 6 | 4.2 |
| >3 major sites | 65 | 45.0 |
| Classification of chronic pain according to new IASP classificationa | ||
| Code 1: chronic primary pain | 89 | 61.8 |
| Code 2: chronic cancer pain | 0 | 0.0 |
| Code 3: chronic postsurgical and posttraumatic pain | 30 | 20.8 |
| Code 4: chronic neuropathic pain | 15 | 10.4 |
| Code 5: chronic headache and orofacial pain | 4 | 2.8 |
| Code 6: chronic visceral pain | 0 | 0.0 |
| Code 7: chronic musculoskeletal pain | 6 | 4.2 |
| Received at least high school education | 128 | 88.9 |
| Marital status | ||
| Married | 76 | 52.8 |
| Single | 36 | 25.0 |
| Divorced | 18 | 12.5 |
| Remarried | 6 | 4.1 |
| Widowed | 8 | 5.6 |
| Work status | ||
| Working full time | 41 | 28.5 |
| Working part time | 7 | 4.9 |
| Not employed | 52 | 36.1 |
| Retired due to pain | 10 | 6.9 |
| Homemakers | 30 | 20.8 |
| Other | 4 | 2.8 |
| Taking sleep pills | 72 | 50.0 |
| Insomnia symptoms | 131 | 91.0 |
| Difficulty in initiating sleep | 69 | 47.9 |
| Difficulty in maintaining sleep | 109 | 75.7 |
| Early morning awakening | 26 | 18.1 |
Note:
Duplicate reports of pain site are included.
Abbreviation: IASP, International Association for the Study of Pain.
Most participants (88.9%) had received at least high school education. Marital status was as follows: married (52.8%), single (25.0%), divorced (12.5%), remarried (4.1%), and widowed (5.6%). Only 33.4% of the participants were working. A total of 50.0% of participants took medication for difficulty sleeping. The prevalence of DIS was 47.9%, of DMS was 75.7%, and of EMA was 18.1%. In total, 131 participants (91.0%) exhibited insomnia symptoms. The mean AIS-8 score was 10.8 (SD =5.7), mean AIS-5 score was 6.7 (SD =4.1), and mean daytime function (items 6–8) score was 4.1 (SD =2.3). The scores for other questionnaires are reported in Table 2.
Table 2.
Mean, SD, and ranges of scores of psychological measures
| Measure | All patients (N=144)
|
Patients with insomnia (N=131)
|
Patients without insomnia (N=13)
|
|||
|---|---|---|---|---|---|---|
| Mean±SD | Range | Mean±SD | Range | Mean±SD | Range | |
| AIS-8 (0–24) | 10.8±5.7 | 0–24 | 11.4±5.6 | 0–24 | 5.2±3.4 | 0–11 |
| AIS-5-nocturnal sleep problem (0–15) | 6.7±4.1 | 0–15 | 7.1±4.1 | 0–15 | 2.7±2.5 | 0–7 |
| AIS-daytime dysfunction (0–9) | 4.1±2.3 | 0–9 | 4.3±2.2 | 0–9 | 2.5±1.9 | 0–6 |
| NRS (0–10) | 6.0±2.0 | 0.3–10.0 | 6.2±1.9 | 1.8–10.0 | 4.4±2.5 | 0.3–7.8 |
| PDAS (0–60) | 28.9±14.8 | 0–60 | 29.7±15.0 | 0–60 | 20.5±10.3 | 5–34 |
| HADS-total (0–42) | 19.8±9.2 | 1–42 | 20.4±9.3 | 1–42 | 13.1±5.3 | 5–23 |
| HADS-anxiety (0–21) | 9.3±4.8 | 0–21 | 9.6±4.8 | 0–21 | 6.2±3.0 | 1–10 |
| HADS-depression (0–21) | 10.4±5.2 | 1–21 | 10.8±5.3 | 1–21 | 6.8±2.9 | 3–13 |
| PCS-total (0–52) | 38.1±10.0 | 11–52 | 38.8±9.7 | 11–52 | 31.1±10.9 | 13–46 |
| PCS-rumination (0–20) | 17.0±3.5 | 0–20 | 17.1±3.6 | 0–20 | 16.2±3.2 | 11–20 |
| PCS-magnification (0–12) | 7.3±3.2 | 0–12 | 7.6±3.1 | 0–12 | 4.8±2.8 | 1–9 |
| PCS-helplessness (0–20) | 13.7±4.6 | 0–20 | 14.1±4.3 | 1–20 | 10.1±5.9 | 0–18 |
| PSEQ (0–60) | 20.1±14.1 | 0–60 | 19.9±14.4 | 0–60 | 23.0±11.3 | 0–42 |
Abbreviations: AIS, Athens Insomnia Scale; HADS, hospital anxiety and depression scale; NRS, numerical rating scale; PCS, pain catastrophizing scale; PDAS, pain disability assessment scale; PSEQ, pain self-efficacy questionnaire.
Confirmatory factor analysis
Fit indices revealed that the 1-factor model of the AIS-8 was a poor fit to the data: χ2 (20)=121.315, p<0.001, SRMR =0.082, RMSEA =0.188 (90% CI: 0.157–0.221), CFI =0.840, and AIC =2687.137. Conversely, the 2-factor model of the AIS-8 was an acceptable fit to the data: χ2 (19)=36.325, p<0.05, SRMR =0.045, RMSEA =0.080 (90% CI: 0.039–0.119), CFI =0.973, and AIC =2623.297. Furthermore, the model fit significantly improved for the 2-factor model (Δχ2 [1]=84.99; p<0.001). Therefore, we adopted the 2-factor structure for the AIS-8 (Figure 1). The factor loading for item 8 was low in the 2-factor model. However, the fit indices were poor if item 8 was removed: χ2 (13)=32.852, p<0.01, SRMR =0.051, RMSEA =0.103 (90% CI: 0.060–0.148), and CFI =0.949. Thus, the 2-factor model retaining item 8 was finally selected.
Figure 1.
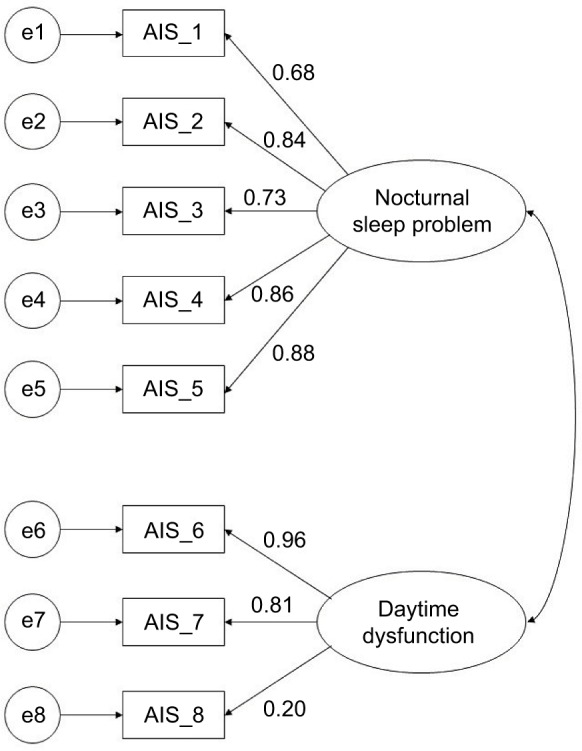
A 2-factor model of AIS-8.
Abbreviation: AIS, Athens Insomnia Scale.
For the AIS-5, fit indices revealed that the 1-factor model was marginally acceptable: χ2 (5)=12.495, p<0.05, SRMR =0.045, RMSEA =0.102 (90% CI: 0.031–0.175), CFI =0.983, and AIC =1602.073. To improve the model fit, modification indices suggested the addition of error covariances between items 1 and 5. These items provide a more subjective evaluation of nocturnal sleep problems than items 2–4. Therefore, items 1 and 5 were permitted to covary. With the addition of this error covariance, the model showed a good fit: χ2 (4)=4.007, p=0.41, SRMR =0.013, RMSEA =0.004 (90% CI: 0.000–0.126), CFI =1.000, and AIC =1595.526 (Figure 2).
Figure 2.
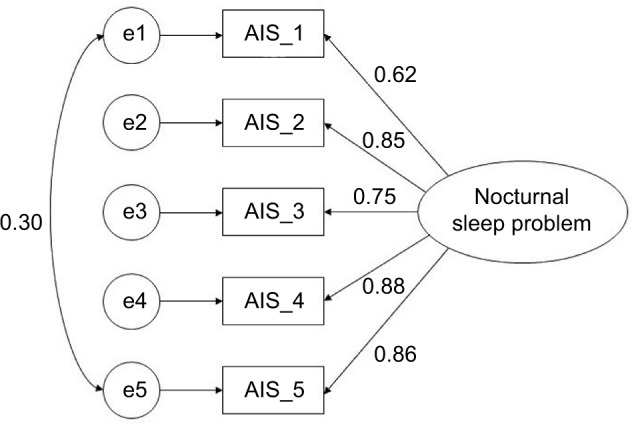
A one-factor model of AIS-5.
Abbreviation: AIS, Athens Insomnia Scale.
Internal consistency and test–retest reliability
Cronbach’s a coefficient for the AIS-8 total score was 0.87 and the AIS-5 was 0.89. In subscales, Cronbach’s a coefficient was 0.89 for nocturnal sleep problems and 0.66 for daytime dysfunction. Test–retest reliability was analyzed in 43 participants (19 males and 24 females). The mean period between the first and second surveys was 81.4 (SD =19.3; range =21–98) days. The overall ICC was 0.64 (95% CI: 0.43 to 0.79) for the AIS-8, 0.72 (95% CI: 0.45 to 0.80) for the AIS-5 and nocturnal sleep problems, and 0.54 (95% CI: 0.29 to 0.72) for daytime dysfunction.
Concurrent validity
Correlation analyses revealed that the AIS-8 had moderate correlations with the PDAS, HADS-A, HADS-D, and PSEQ, and the AIS-5 had moderate correlations with the HADS-A and HADS-D. Moreover, the AIS-8 was weakly associated with the NRS and PCS, and the AIS-5 had weak correlations with the NRS, PDAS, PCS, and PSEQ (Table 3).
Table 3.
Correlation coefficients between AIS and the other psychological measures
| Measure | AIS-8 (95% CI) | AIS-5 (95% CI) |
|---|---|---|
| NRS | 0.36 (0.21 to 0.50) | 0.35 (0.19 to 0.48) |
| PDAS | 0.46 (0.33 to 0.58) | 0.37 (0.22 to 0.51) |
| HADS-anxiety | 0.54 (0.41 to 0.65) | 0.42 (0.28 to 0.55) |
| HADS-depression | 0.64 (0.53 to 0.73) | 0.52 (0.39 to 0.63) |
| PCS-total | 0.36 (0.21 to 0.49) | 0.26 (0.10 to 0.41) |
| PCS-rumination | 0.23 (0.07 to 0.38) | 0.17 (0.01 to 0.33) |
| PCS-magnification | 0.37 (0.22 to 0.50) | 0.27 (0.11 to 0.42) |
| PCS-helplessness | 0.35 (0.19 to 0.48) | 0.24 (0.08 to 0.39) |
| PSEQ | −0.47 (−0.59 to −0.33) | −0.35 (−0.48 to −0.19) |
Abbreviations: AIS, Athens Insomnia Scale; HADS, hospital anxiety and depression scale; NRS, numerical rating scale; PCS, pain catastrophizing scale; PDAS, pain disability assessment scale; PSEQ, pain self-efficacy questionnaire.
Known-group validity
Based on the semi-structured interview data, the participants were divided into an insomnia group (n=131) and non-insomnia group (n=13). The mean AIS-8 score was 11.4 (SD =5.6) for those with insomnia and 5.2 (SD =3.4) for those without insomnia. In addition, the mean AIS-5 score was 7.1 (SD =4.0) in participants with insomnia and 2.7 (SD =2.5) in participants without insomnia. Independent t-tests revealed higher AIS scores in participants with insomnia than in those without insomnia in both the AIS-8 and AIS-5 (AIS-8: t=3.93, df=142, p<0.001; AIS-5: t=3.81, df=142, p<0.001).
Cut-off point
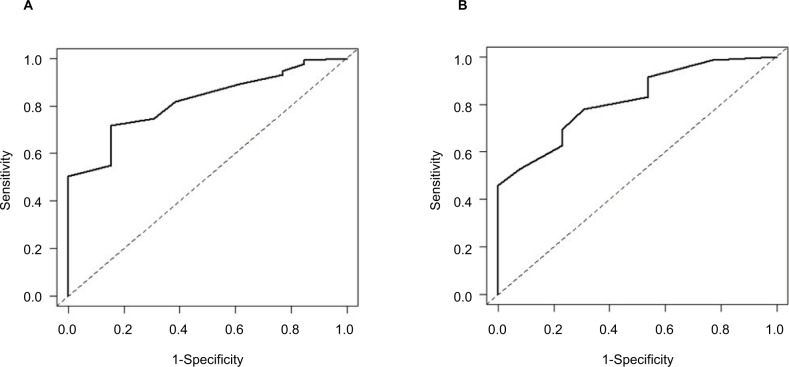
Based on the ROC analysis, the cut-off value of the AIS-8 for insomnia was estimated at 8 points, with 72% sensitivity and 85% specificity. Furthermore, the cut-off value of the AIS-5 was estimated at 4 points, with 78% sensitivity and 70% specificity (Table 4). The AUC was 0.82 (95% CI: 0.72 to 0.91) for the AIS-8 and 0.82 (95% CI: 0.71 to 0.92) for the AIS-5. The ROC curves are shown in Figure 3.
Table 4.
Sensitivity and specificity values of the AIS
| Measure | Cut-off score | Sensitivity (%) | Specificity (%) | AUC (95% CI) |
|---|---|---|---|---|
| AIS-8 | 7 | 75 | 69 | 0.82 (0.72 to 0.91) |
| 8a | 72a | 85a | ||
| 9 | 66 | 85 | ||
| AIS-5 | 3 | 83 | 46 | 0.82 (0.71 to 0.92) |
| 4a | 78a | 70a | ||
| 5 | 69 | 76 |
Note:
Optimal cutoff score values are shown in bold.
Abbreviations: AIS, Athens Insomnia Scale; AUC, area under the curve.
Figure 3.
ROC curve of the AIS-8 and AIS-5.
Notes: (A) ROC curve of the AIS-8. (B) ROC curve of the AIS-5.
Abbreviations: AIS, Athens Insomnia Scale; ROC, receiver-operating characteristic.
Discussion
The present study aimed to examine the psychometric properties of the AIS-8 and AIS-5 in Japanese chronic pain patients. The 2-factor model of the AIS-8 and 1-factor model of the AIS-5 showed a good fit. The AIS-8 and AIS-5 had adequate reliability. All the hypotheses were supported. Thus, the AIS was confirmed to exhibit concurrent validity and known-group validity. According to the ROC analysis, the cut-off value of the AIS-8 was estimated at 8 points and AIS-5 was estimated at 4 points. Our data illustrated that the AIS is a measure with good reliability and validity in chronic pain patients.
A 2-factor model of the AIS-8 showed better fit indices than a 1-factor in this study. The factor structure of the AIS-8 in the present study is similar to that in a prior study conducted at a sleep clinic recruiting individuals with insomnia in Japan.14 According to the developers of the original version of the AIS, items 1–5 are based on the criterion A for the diagnosis of insomnia according to ICD-10 and items 6–8 are based on the criterion C of the ICD-10.10,18 Based on this notion, it is reasonable that the 2-factor model of the AIS-8 was an acceptable fit to the data.
In this study, the factor loading for sleepiness during the day (item 8) was lower than that in previous studies (0.45–0.63).10,14,15 Although the reason is uncertain, some explanations are available. First, chronic pain patients might concentrate on their pain rather than their sleepiness in the daytime and do not mind daytime sleepiness. Second, because 66.6% of participants were currently not at work, they might be less bothered by their daytime sleepiness. Further studies are needed to examine the factor loading for item 8 in chronic pain patients.
We confirmed that the AIS-8 and AIS-5 have high internal consistency. The Cronbach’s a value was similar to that of the Japanese version for an insomnia sample (AIS-8: a=0.88 and AIS-5: a=0.85).14 However, the overall ICCs of the AIS-8 and AIS-5 were low (AIS-8: ICC =0.64 and AIS-5: ICC =0.72). The ICC was 0.89 for AIS-8 and 0.88 for AIS-5 in the original version.10 This difference might have occurred due to the longer interval in the present study.
The score of AIS was positively associated with pain intensity, disability, depression, anxiety, and pain catastrophizing, and negatively associated with self-efficacy in the current study. In previous studies, sleep disturbance was associated with greater pain intensity,3–6 disability,3 depression,3,5,6 anxiety,6 and pain catastrophizing.7 In addition, poor sleep quality was associated with worse levels of self-efficacy in patients with fibromyalgia.8 Therefore, the correlation between the AIS and other psychometric questionnaires is applicable. Concurrent validity was confirmed for the AIS.
The cut-off value of the AIS-8 was higher than that of the original and Japanese versions (6 points for both) for an insomnia sample.10,14 By contrast, the cut-off value of the AIS-5 was the same as that of the Japanese version for patients with insomnia.14 The cut-off value of only the AIS-8 was higher than that of the original version because the current participants with chronic pain might have rated their daytime dysfunction (items 6–8) more positively than participants with primary insomnia. Actually, the mean daytime dysfunction score in this study was higher than that of the primary insomnia group (3.65; SD =1.95).14 According to Dueñas’s review, chronic pain severely affects the patients’ daily activities and quality of life.43 Many chronic pain patients are less able to exercise, walk, work outside home, attend social activities, and do household chores.2 Hence, because of pain, chronic pain patients with insomnia would feel more daytime dysfunction than patients with primary insomnia.
Limitations
This study has some limitations. First, the number of patients without insomnia is low (N=13), and the selection bias might have occurred in this study. The low number of non-insomnia patients may be due to the participants in this study being recruited in a tertiary care setting, in which many patients may experience severe pain and insomnia. In previous studies, 92.9% chronic pain patients reported difficulty with sleep in tertiary setting,4 while 74.9% patients with chronic pain reported sleep disturbance in primary care settings.44 The patients in current study would experience more insomnia than those in primary or secondary care settings.
Second, this study did not apply any other insomnia scales (such as the Pittsburgh Sleep Quality Index45 and Insomnia Severity Index46) or objective measures of sleep (e.g., actigraphy and polysomnography). In previous studies, other insomnia scales and Actiwatch parameters have been measured to confirm the validity of the AIS.14,16,17 Furthermore, the semi-structured interview for insomnia was brief. The diagnostic criteria of Diagnostic and Statistical Manual of Mental Disorders-IV-Text Revision were used to divide participants into insomnia and non-insomnia groups in previous studies.16,17
In spite of these limitations, we confirmed good psychometric properties of the AIS in chronic pain patients. The AIS is widely used for assessing the risk of insomnia,12 because it is short and assesses important sleep domain.12,13 The AIS is expected to assess insomnia symptom of chronic pain patients not only in research settings, but also in clinical settings.
Conclusion
This study demonstrated that the AIS-8 and AIS-5 have excellent reliability and validity in chronic pain patients. The cut-off value for screening insomnia was estimated at 8 points in the AIS-8 and 4 points in the AIS-5. The AIS has high utility because it assesses all the 3 major insomnia symptoms and takes only a few minutes to complete. The AIS is useful for assessing insomnia symptoms of chronic pain patients in research and clinical practice.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.Menefee LA, Cohen MJM, Anderson WR, Doghramji K, Frank ED, Lee H. Sleep disturbance and nonmalignant chronic pain: a comprehensive review of the literature. Pain Med. 2000;1(2):156–172. doi: 10.1046/j.1526-4637.2000.00022.x. [DOI] [PubMed] [Google Scholar]
- 2.Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 3.McCracken LM, Iverson GL. Disrupted sleep patterns and daily functioning in patients with chronic pain. Pain Res Manag. 2002;7(2):75–79. doi: 10.1155/2002/579425. [DOI] [PubMed] [Google Scholar]
- 4.McCracken LM, Williams JL, Tang NKY. Psychological flexibility may reduce insomnia in persons with chronic pain: a preliminary retrospective study. Pain Med. 2011;12(6):904–912. doi: 10.1111/j.1526-4637.2011.01115.x. [DOI] [PubMed] [Google Scholar]
- 5.Smith MT, Perlis ML, Smith MS, Giles DE, Carmody TP. Sleep quality and presleep arousal in chronic pain. J Behav Med. 2000;23(1):1–13. doi: 10.1023/a:1005444719169. [DOI] [PubMed] [Google Scholar]
- 6.Tang NKY, Wright KJ, Salkovskis PM. Prevalence and correlates of clinical insomnia co-occurring with chronic pain. J Sleep Res. 2007;16(1):85–95. doi: 10.1111/j.1365-2869.2007.00571.x. [DOI] [PubMed] [Google Scholar]
- 7.Roberts MB, Drummond PD. Sleep problems are associated with chronic pain over and above mutual associations with depression and catastrophizing. Clin J Pain. 2016;32(9):792–799. doi: 10.1097/AJP.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 8.Miró E, Martínez MP, Sánchez AI, Prados G, Medina A. When is pain related to emotional distress and daily functioning in fibromyalgia syndrome? The mediating roles of self-efficacy and sleep quality. Br J Health Psychol. 2011;16(4):799–814. doi: 10.1111/j.2044-8287.2011.02016.x. [DOI] [PubMed] [Google Scholar]
- 9.Nicholas M, Keefe FJ, Lautenbacher S. Pain, anxiety, depression, and psychological evaluation and treatment. In: Claudia LS, Mark SW, Steven PC, Michaela K, editors. Pain 2016 Refresher Courses, 16th World Congress on Pain. Washington, DC: IASP Press; 2016. pp. 379–398. [Google Scholar]
- 10.Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res. 2000;48(6):555–560. doi: 10.1016/s0022-3999(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 11.Soldatos CR, Dikeos DG, Paparrigopoulos TJ. The diagnostic validity of the Athens Insomnia Scale. J Psychosom Res. 2003;55(3):263–267. doi: 10.1016/s0022-3999(02)00604-9. [DOI] [PubMed] [Google Scholar]
- 12.Chiu HY, Chang LY, Hsieh YJ, Tsai PS. A meta-analysis of diagnostic accuracy of three screening tools for insomnia. J Psychosom Res. 2016;87:85–92. doi: 10.1016/j.jpsychores.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Wells GA, Li T, Kirwan JR, et al. Assessing quality of sleep in patients with rheumatoid arthritis. J Rheumatol. 2009;36(9):2077–2086. doi: 10.3899/jrheum.090362. [DOI] [PubMed] [Google Scholar]
- 14.Okajima I, Nakajima S, Kobayashi M, Inoue Y. Development and validation of the Japanese version of the Athens Insomnia Scale. Psychiatry Clin Neurosci. 2013;67(6):420–425. doi: 10.1111/pcn.12073. [DOI] [PubMed] [Google Scholar]
- 15.Gómez-Benito J, Ruiz C, Guilera G. A Spanish version of the Athens Insomnia Scale. Qual Life Res. 2011;20(6):931–937. doi: 10.1007/s11136-010-9827-x. [DOI] [PubMed] [Google Scholar]
- 16.Sun JL, Chiou JF, Lin CC. Validation of the Taiwanese version of the Athens Insomnia Scale and assessment of insomnia in Taiwanese cancer patients. J Pain Symptom Manage. 2011;41(5):904–914. doi: 10.1016/j.jpainsymman.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 17.Jeong HS, Jeon Y, Ma J, et al. Validation of the Athens Insomnia Scale for screening insomnia in South Korean firefighters and rescue workers. Qual Life Res. 2015;24(10):2391–2395. doi: 10.1007/s11136-015-0986-7. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization . The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization; 1992. [Google Scholar]
- 19.Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 20.Jensen MP, Turner JA, Romano JM, Fisher LD. Comparative reliability and validity of chronic pain intensity measures. Pain. 1999;83(2):157–162. doi: 10.1016/s0304-3959(99)00101-3. [DOI] [PubMed] [Google Scholar]
- 21.Yamashiro K, Arimura T, Iwaki R, Jensen MP, Kubo C, Hosoi M. A multidimensional measure of pain interference: reliability and validity of the Pain Disability Assessment Scale. Clin J Pain. 2011;27(4):338–343. doi: 10.1097/AJP.0b013e318204858a. [DOI] [PubMed] [Google Scholar]
- 22.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 23.Kitamura T. Hospital anxiety and depression scale (in Japanese) Seisinka Sindangaku. 1993;4(3):371–372. [Google Scholar]
- 24.Kugaya A, Akechi T, Okuyama T, Okamura H, Uchitomi Y. Screening for psychological distress in Japanese cancer patients. Jap J Clin Oncol. 1998;28(5):333–338. doi: 10.1093/jjco/28.5.333. [DOI] [PubMed] [Google Scholar]
- 25.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524–532. [Google Scholar]
- 27.Matsuoka H, Sakano Y. Assessment of cognitive aspect of pain: development, reliability, and validation of Japanese version of pain catastrophizing scale (in Japanese) J Psychosom Med. 2007;47(2):95–102. [Google Scholar]
- 28.Nicholas MK. The pain self-efficacy questionnaire: taking pain into account. Eur J Pain. 2007;11(2):153–163. doi: 10.1016/j.ejpain.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Adachi T, Nakae A, Maruo T, et al. Validation of the Japanese version of the pain self-efficacy questionnaire in Japanese patients with chronic pain. Pain Med. 2014;15(8):1405–1417. doi: 10.1111/pme.12446. [DOI] [PubMed] [Google Scholar]
- 30.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 31.Nakata A, Ikeda T, Takahashi M, et al. Sleep-related risk of occupational injuries in Japanese small and medium-scale enterprises. Ind Health. 2005;43(1):89–97. doi: 10.2486/indhealth.43.89. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi M, Iwakiri K, Sotoyama M, et al. Work schedule differences in sleep ploblems of nursing home caregivers. Appl Ergon. 2008;39(5):597–604. doi: 10.1016/j.apergo.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Kaneita Y, Oshida T, Osaki Y, et al. Insomnia among Japanese adolescents: a nationwide representative survey. Sleep. 2006;29(12):1543–1550. doi: 10.1093/sleep/29.12.1543. [DOI] [PubMed] [Google Scholar]
- 34.Nakata A, Haratani T, Takahashi M, et al. Job stress, social support, and prevalence of insomnia in a population of Japanese daytime workers. Soc Sci Med. 2004;59(8):1719–1730. doi: 10.1016/j.socscimed.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 35.R Core Team . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Accessed October 04, 2017]. Available from: https://www.R-project.org/ [Google Scholar]
- 36.Rosseel Y. lavaan: an R package for structural equation modeling. [Accessed October 04, 2017];J Stat Softw. 2012 48(2):1–36. Available from: http://www.jstatsoft.org/v48/i02/ [Google Scholar]
- 37.Hooper D, Coughlan J, Mulle MR. Structural equation modelling: guidelines for determining model fit. EJBRM. 2008;6(1):53–60. [Google Scholar]
- 38.Schermelleh-Engel K, Moosbrugger H, Muller H. Evaluating the fit of structural equation models: tests of significance and descriptive goodness-of fit measures. MPR Online. 2003;8(2):23–74. [Google Scholar]
- 39.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. [Accessed October 04, 2017];BMC Bioinformatics. 2011 12:73. doi: 10.1186/1471-2105-12-77. Available from: http://www.biomedcentral.com/1471-2105/12/77/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240(4857):1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 41.Treede RD, Reif W, Barke A, et al. A classification of chronic pain for ICD-11. Pain. 2015;156(6):1003–1007. doi: 10.1097/j.pain.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takura T, Shibata M, Inoue S, et al. Socioeconomic value of intervention for chronic pain. J Anesth. 2016;30(4):553–561. doi: 10.1007/s00540-016-2162-9. [DOI] [PubMed] [Google Scholar]
- 43.Dueñas M, Ojeda B, Salazar A, Mico JA, Failde I. A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res. 2016;28(9):457–467. doi: 10.2147/JPR.S105892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salazar A, Dueñas M, Mico JA, et al. Undiagnosed mood disorders and sleep disturbances in primary care patients with chronic musculoskeletal pain. Pain Med. 2013;14(9):1416–1425. doi: 10.1111/pme.12165. [DOI] [PubMed] [Google Scholar]
- 45.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 46.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]