Abstract
Measurement of IgG subclass concentrations is a standard laboratory test run as part of a panel to investigate the suspicion of antibody deficiency. The assessment is clinically important when total IgG is within the normal age-specific reference range. The measurement is useful for diagnosis of IgG subclass deficiency, to aid the diagnosis of specific antibody deficiency, as a supporting test for the diagnosis of common variable immunodeficiency, as well as for risk stratification of patients with low IgA. The measurement of IgG subclasses may also help determine a revaccination strategy for patients and support patient management. In certain circumstances, the measurement of IgG subclasses may be used to monitor a patient’s humoral immune system. In this review, we discuss the utility of measuring IgG subclass concentrations.
Keywords: IgG subclass, low IgA, specific antibody deficiency, common variable immunodeficiency
Differentiating individuals with recurrent infections (RIs) because of a compromised immune system from those whose immune systems operate at the edge of normal function may be challenging, particularly in the case of pediatric age groups. Normal pediatric age groups may have many respiratory infections in any one year,1-3 and additionally, those individuals whose transient immune deficiency will resolve over time must be distinguished from those whose deficiency is more permanent. To resolve the above issues, detailed immunological analyses may help to ensure early diagnosis and appropriate treatment. This is imperative because of the high risk of RI, which may cause irreversible organ damage.
The measurement of IgG subclass antibodies (IgGSc) is included in a number of protocols and guidelines for investigating individuals presenting with RIs, lung diseases, and strong suspicion of an antibody deficiency (Table 1). These analyses are also helpful in 1) monitoring of transient versus permanent antibody deficiencies, 2) assessing the progression of mild immunodeficiencies into more severe forms, and 3) supporting some treatment decisions (summarized in Table 2).
Table 1.
Protocols and Guidelines Documenting the Measurement of IgGSc in Immunological Investigation in the Last 10 Years
| Reference | Title | Recommendation for IgGSc Measurement |
|---|---|---|
| Slatter and Gennery 111 | Clinical immunology review series: an approach to the patient with recurrent infections in childhood. | IgGSc testing as firstline testing alongside IgG, IgA and IgM |
| De Vries et al16 | Patient-centred screening for primary immunodeficiency, a multi-stage diagnostic protocol designed for non-immunologists: 2011 update. | IgGSc testing after IgG, IgA and IgM testing |
| Chang et al12 | Chronic suppurative lung disease and bronchiectasis in children and adults in Australia and New Zealand. | IgGSc testing as first-line testing alongside IgG, IgA and IgM |
| Ameratunga et al83 | New diagnostic criteria for common variable immune deficiency (CVID), which may assist with decisions to treat with intravenous or subcutaneous immunoglobulin | Measurement of IgG3 as supportive laboratory evidence |
| Ameratunga et al84 | New diagnostic criteria for CVID | Measurement of IgG3 as supportive laboratory evidence |
| Ladomenou and Gaspar19 | How to use immunoglobulin concentrations in investigating immune deficiencies | IgGSc testing after IgG, IgA, and IgM testing |
Older protocols and guidelines include the following:
De Vries et al14,
17
Cunningham Rundles13
Folds and Schmitz18
Table 2.
Summary of the Clinical Utilities of IgGSc Measurement
| Utility of IgGSc Measurements |
|---|
| Diagnosis |
| 1. Isolated IgGScD |
| 2. Specific antibody deficiency |
| 3. Clinically relevant IgGScD |
| 4. Transient hypogammaglobulinemia of infancy |
| Supporting information/risk of infection |
| 1. Patients with low IgA |
| 2. Common variable immunodeficiency |
| Monitoring |
| 1. Transient hypogammaglobulinemia of infancy/permanent antibody deficiency |
| 2. Progression to a more complex antibody deficiency |
| Treatment |
| 1. Support treatment decisions (revaccination, antibiotics, antibody replacement therapy, etc) |
IgGSc deficiency (IgGScD) is clinically and genetically heterogeneous, and may coexist with abnormal expression of more than 1 subclass antibody. Individuals with IgGScD may present with a combination of frequent or severe bacterial infections of the upper and lower respiratory tract,4-6 allergic asthma and allergic rhinitis,7,8 and autoimmune conditions.7,9-11
In this review, we discuss the clinical situations in which measurement of IgGSc has been reported to be of benefit.
Diagnosis of IgGSc Deficiency
The measurement of IgGSc has been employed as a screening tool for individuals presenting with multiple chronic illnesses or RI (Table 1). Some authors have reported measuring IgGSc as a frontline test alongside the measurement of total IgG, IgA, and IgM,12-15 but more commonly, the measurement of IgGSc is a second-line test.16-19 De Vries16 has suggested that IgGSc are measured after the measurement of IgG, IgA, and IgM usually if IgG is higher than 4 g/L and alongside the response to vaccination. Ladomenou and Gaspar19 have suggested that IgGSc concentrations are measured after the assessment of the response to vaccines. Further, IgGSc are useful as part of an evaluation in studying patients with a strong history of infections, normal major isotypes, and normal antibody responses. As discussed below, IgGSc are also recommended as second-line tests in patients with low IgA and should be monitored in IgGScD patients for progression to common variable immunodeficiency (CVID).20-25 Whether first or second line, the measurement of IgGSc is an important test that provides a more complete picture of the humoral immune system.
Concentrations of IgGSc are commonly measured using radial immunodiffusion (RID), enzyme-linked immunosorbent assay (ELISA), and the more automated methods of nephelometry and turibidimetry. All methods provide quantitative measurement of the IgGSc concentrations, and all assays provide a good level of sensitivity for detection of low IgGSc concentrations. RID is commonly used if the testing numbers are low, and nephelometric and turbidimetric assays are operated on automated high throughput analysers on which other laboratory assays are run. ELISA may use monoclonal antibodies for detection rather than the polyclonal antibodies used in RID and nephelometric or turbidimetric assays. Time to first result is slower with RID due to incubation times required for antigen-antibody ring formation. For nephelometric and turbidimetric assays, differences exists between manufacturers’ assays.26-29
Reference ranges for pediatric age groups and adult populations have been developed30,31 (Table 3), as well as some study-specific ranges.7,32,33 The diagnosis of IgGScD requires one or more IgGSc concentrations to be less than the fifth percentile in the presence of normal concentrations of IgG, IgA, and IgM.34 In patients older than 15 years of age, it has been reported the median IgG3 concentration in patients with IgG3 subclass antibodies deficiency (IgG3ScD) is approximately 0.19 g/L (range 0.10-0.34) and less than 0.10 to 0.24 g/L in patients with chronic sinusitis, and that IgG2 concentrations were less than 0.25 g/L in patients with low IgA and IgG2 subclass antibodies deficiency (IgG2ScD).7,32,33 All abnormal IgGSc concentrations should be confirmed with at least one additional measurement one month after the first test.34,35 IgGScD may be due to abnormal concentrations of one IgGSc only or different combinations of any of the four IgGSc.
Table 3.
Mean (50th Percentile) IgGSc Concentration and Reference Intervals (2.5-97.5 Percentiles) With Respect to Age
Data obtained from Schauer et al31
| Age Range | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5-1 year | 1-1.5 years | 1.5-2 years | 2-3 years | 3-4 years | 4-6 years | 6-9 years | 9-12 years | 12-18 years | Adult | ||
| IgG1 (g/L) |
Mean | 2.9 | 3.5 | 4.0 | 4.5 | 4.8 | 5.0 | 5.7 | 6.0 | 5.8 | 5.0 |
| Reference interval | 1.4-6.2 | 1.7-6.5 | 2.2-7.2 | 2.4-7.8 | 2.7-8.1 | 3.0-8.4 | 3.5-9.1 | 3.7-9.3 | 3.7-9.1 | 2.8-8.0 | |
| IgG2 (g/L) |
Mean | 0.58 | 0.62 | 0.80 | 0.95 | 1.15 | 1.30 | 1.70 | 2.10 | 2.60 | 3.0 |
| Reference interval | 0.41-1.30 | 0.4-1.40 | 0.5-1.80 | 0.55-2.00 | 0.65-2.20 | 0.7-2.55 | 0.85-3.30 | 1.0-4.00 | 1.1-4.85 | 1.15-5.70 | |
| IgG3 (g/L) |
Mean | 0.41 | 0.42 | 0.44 | 0.46 | 0.48 | 0.50 | 0.54 | 0.58 | 0.63 | 0.64 |
| Reference interval | 0.11-0.85 | 0.12-0.87 | 0.14-0.91 | 0.15-0.93 | 0.16-0.96 | 0.17-0.97 | 0.20-1.04 | 0.22-1.09 | 0.24-1.16 | 0.24-1.25 | |
| IgG4 (g/L) |
Mean | 0.002 | 0.030 | 0.068 | 0.138 | 0.201 | 0.257 | 0.368 | 0.469 | 0.491 | 0.349 |
| Reference interval | 0.000- 0.008 | 0.000- 0.255 | 0.000- 0.408 | 0.006- 0.689 | 0.012- 0.938 | 0.017- 1.157 | 0.030- 1.577 | 0.043- 1.900 | 0.052- 1.961 | 0.052-1.250 | |
Knowledge of IgGSc concentrations is clinically important when total IgG, IgA, and IgM concentrations are within their normal ranges,17,19,33 and in individuals with RI, a high percentage may have normal IgG concentrations (Table 4).
Table 4.
Examples of the Measurement of IgG and IgGSc for Screening Individuals Presenting With Different RIs
| Type of Infection | Normal IgG (%) | Low IgGSc Concentration | Comments Regarding IgGScD | Reference |
|---|---|---|---|---|
| Chronic and recurrent ear, nose, and throat | 101/103 (98) | 4/101 (4) | Response to Pneumococcal vaccination normal in 1/4 IgGScD (25%) 1/4 IgGScD developed bronchiectasis |
Aghamohammadi et al78 |
| Upper respiratory tract infection with otitis media/lower respiratory tract infection with pneumonia | 22/55 (47)a | 22/22 (100) | IgGScD as follows: 3 IgG1, 8 IgG2, 11 IgG3. 3/7 patients with low IgA had IgGScD. | Bossuyt et al 71 |
| Recurrent bronchitis | 23/25 (92) | 17/25 (68) | IgGScD as follows: 2 IgG2, 4 IgG3 and 7 IgG4. 4 combined IgGScD. |
De Baets et al55 |
| Chronic and recurrent rhinosinusitis | 70/74 (95) | 33/70 (47) | 5 low IgG1, 3 low IgG2, 19 low IgG3, 6 low IgG4—7 with >1 low IgGSc | Scadding et al33 |
| Therapy refractory recurrent rhinosinusitis | 240/245 (98) | 17/240 (7) | IgGScD as follows: 5 IgG1, 10 IgG2, 1 IgG3, and 1 IgG4. 3/17 had an inadequate pneumovax response | May et al112 |
aIn 2 patients with low IgG2, they had low total IgG.
Mechanism of IgGScD
The human immunoglobulin heavy chain constant region (IGHC) is localized on chromosome 14 and contains 9 functional genes (μ-δ-γ3-γ1-Ψε-α1-Ψγ-γ2-γ4-ε-α2).36 The frequency of IGHC gene deletions in the Caucasian population is approximately 1.5%. The linkage pattern dictates that many of the deletions occur in discrete blocks such as IgG1 and IgG3, IgG2 and IgG4, and IgA1, IgG2, and IgG4, which may be extended to include IgG1 and IgE. IGHC gene deletions have been reported in the normal healthy population, calling into question the clinical relevance of IgGScD.36-41
Plebani et al41 reported 2 siblings that had IgGScD with undetectable serum concentrations of IgA1, IgG2, IgG4, and IgE due to a homozygous deletion. Serum IgG, IgG1, and IgG3 concentrations were higher than expected in normal individuals, and they displayed a normal IgG response to tetanus toxoid and polysaccharide antigens with increased IgG1 and IgG3 isotypes. Similarly Hammarstrom et al42 reported IgG3 and IgG4 compensation in an individual with IgG1 subclass antibody deficiency (IgG1ScD), and a shift in the isotype of antipolysaccharide antibodies to IgG1 and IgG3 in IgG2ScD individuals. The high level of homology of the IGHC gene favors unequal crossing-over events, and the compensatory mechanisms reported for the gene deletions may explain the lack of significant infections in the healthy population and thus suggest these gene deletions are nonpathogenic.
Immunoglobulin deficiencies are mainly caused by immunoregulatory dysfunctions such as aberrations in 5’ regulatory sequences, in 3’ regulatory sequences, or in the production or response to selected cytokines (reviewed in Pan and Hammarstrom36) are probably the cause of the more pathogenic IgGScD.
There may be prognostic importance in distinguishing between IgGScD caused by gene deletion and that due to immunoregulatory dysfunction as those with the latter may have the higher risk of RI.
IgGScD
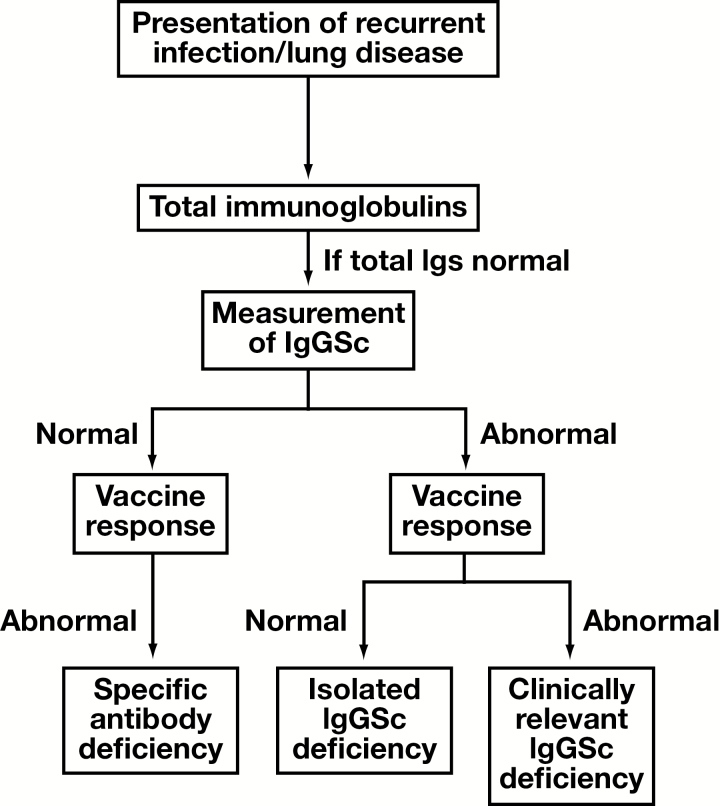
IgGScD is one of the most common primary antibody deficiencies (PADs) in both pediatric age groups and adults presenting with RI (Figure 1 and Table 4).43,44 The most common clinical associations of IgGScD are recurrent encapsulated bacterial and viral respiratory tract infections.7,45-47 The frequency of recurrent pneumonia is significantly higher in IgGScD than that observed in the normal population. Ozkan et al found that the rate of chronic pulmonary damage was 5 times higher in pediatric age groups with IgGScD than those with low IgA.46 It has been reported that bronchiectasis may already be present in as many as 10% of IgGScD pediatric patients at diagnosis.43 In a study of 350 adult IgGScD patients, 29% had asthma, 9% had asthma combined with chronic obstructive pulmonary disease (COPD), and 9% had COPD alone.48
Figure 1.
Algorithm for the measurement of IgGSc in individuals with suspicion of antibody deficiency.
IgG2- and IgG3- are the most common IgGScDs,7,43,45-47 presumably in part because IgG1 deficiency can closely mirror IgG deficiency. IgG2ScD may be associated with some restricted responses to polysaccharide antigens, and in IgG3ScD, the response to Moraxella catarrhalis, Streptococcus pyogenes, and respiratory syncytial virus infection may be compromised.49,50 IgG4 is present at very low concentrations in healthy individuals; thus IgG4 subclass antibodies deficiency (IgG4ScD) may be difficult to diagnose. IgG2ScD is sometimes accompanied by low IgG4 concentrations,34 and a deficiency of IgG1 is more likely associated with low IgG3 concentrations. IgGScD has been observed as a comorbidity in other primary immunodeficiencies (PIDs), such as ataxia telangiectasia,51,52 and secondary immunodeficiencies, such as chronic lymphocytic leukemia.53
Some abnormalities of specific polysaccharide antibody production have been described in patients with IgG1ScD, IgG2ScD, and IgG3ScD.54-58 IgG2ScD patients have antibody responses to a restricted number of polysaccharides in the Pneumovax polysaccharide vaccine56,59,60 and the association between decreased IgG3 concentrations and lack of pneumovax response have been reported.61 De Gracia and colleagues reported that the prevaccination, postvaccination concentrations as well as fold-increase in antibody concentrations in response to the Haemophilus conjugate vaccine were significantly lower in individuals with IgGScD.62 Defective response to vaccination needs to be considered in cases of suspected IgGScD, as this may indicate a more severe antibody deficiency (clinically relevant IgGScD) rather than an isolated IgGScD.47 Likewise, normal IgGSc with defective response to vaccination may indicate a specific antibody deficiency (SAD, summarized in Figure 1). Along with the clinical presentation, these distinctions may support a reliable diagnosis and treatment regime.
Measurement of IgGSc in Other Antibody Deficiencies
IgGScD in Patients with Low IgA
The majority of patients with low IgA are asymptomatic, but approximately 30% present with infection, eg recurrent viral infections, recurrent otitis media, frequent sinopulmonary infections, or gastrointestinal infections.63 In addition, low IgA has been associated with abnormalities of antibody-mediated immunity, such as IgE deficiency,64 deficient serum IgGSc concentrations,65-68 and impaired antibody responses against both protein and polysaccharide antigens.65,69,70 In pediatric age groups presenting with RI, a percentage may have an IgGScD dependent on presentation or type of RI.21,55,71 The additional defective ability to produce normal IgGSc in the background of low IgA may lead to more frequent and more severe infections than those present in patients with normal IgA20-25,68 and is readily observed in patients who present with recurrent bronchitis.22,23,68,72-76 When patients with low IgA were grouped according to severity of infection (none, mild, and severe), although the IgA concentrations were not significantly different, mean IgG4 concentrations were lower in the group with mild infections and even lower in the group with severe infections compared to the group with no infections.65 A higher frequency of infection has also been reported in patients with low IgA and IgG2ScD compared to those with low IgA alone.32,77
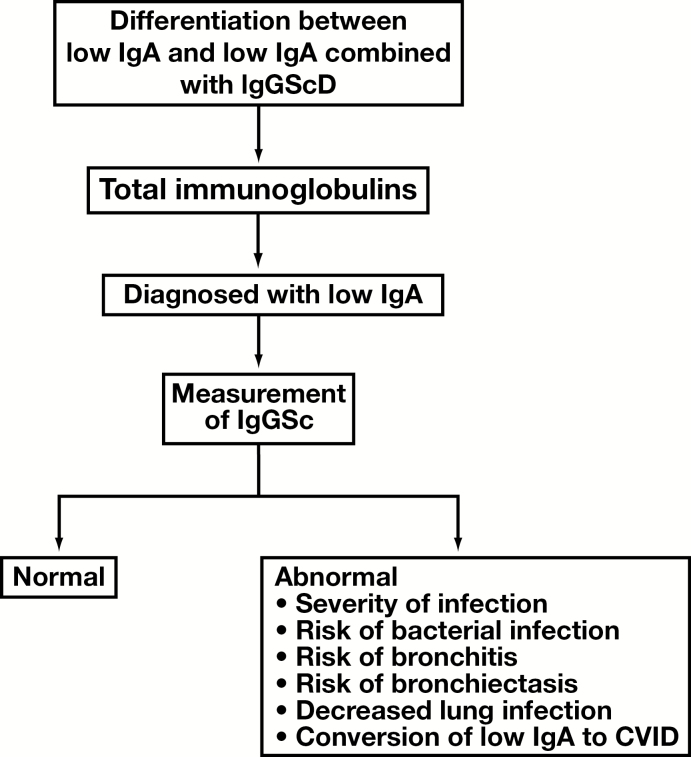
The high rates of infection in patients with low IgA and IgGScD result in decreased lung function,72 and in particular IgG4ScD correlates with the presentation of bronchiectasis.78 In some cases, low IgA may evolve into the more severe CVID, and a decrease in IgGSc concentrations may occur before a decrease in total IgG.20,78,79 An algorithm for the testing of IgGSc in individuals with low IgA and utility is shown in Figure 2.
Figure 2.
Algorithm for the measurement of IgGSc in individuals with low IgA.
Finally, and although it is outside the scope of this review, patients with low IgA may present with antibody-mediated autoimmune diseases that reflect a dysregulation of the humoral response.80 However, little evidence is found in the literature about the association of low IgA and IgGScD with autoimmunity.
IgGScD in CVID Patients
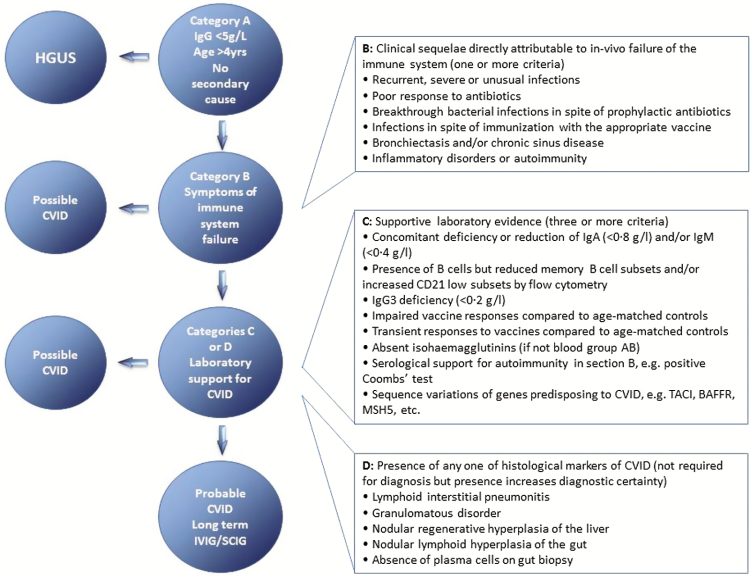
CVID is the most frequent clinically symptomatic primary antibody disorder in adults with a marked decrease of IgG and at least 1 of IgM or IgA. The prevalence is approximately 1:25,000 to 1:50,000, and in most patients the genetic cause remains undefined.81,82 The onset of CVID occurs after 4 years of age. Diagnosis requires prior exclusion of hypogammaglobulinemia and that patients have a poor response to vaccines.34,81 Ameratunga and colleagues have proposed updated criteria for the diagnosis of CVID, based on recent evaluation of the European Society of Immune Deficiencies (ESID) and the Pan American Group for Immune Deficiency (PAGID) definitions of CVID.83,84 The diagnostic algorithm requires sequential steps.
The evaluations in category A are required to consider a diagnosis of CVID, and to differentiate between those with mild hypogammaglobulinemia of uncertain significance (HGUS) and those whose presentation is more suggestive of CVID (Figure 3). Category B details the clinical sequelae from the in vivo failure of the immune system. The determination of recurrent, severe, or unusual infections, particularly in the presence of prophylaxis and treatment, is important, as is excluding infection due to anatomical and functional defects. Category C is the provision of serological and genetic data to support the suggestion of CVID. Many CVID patients have a deficiency of IgG, IgA, and/or IgM, and many have reduced memory B cell subsets. The response to vaccination and concentration of isohemagglutinins are important to assess, as are the serological indications for autoimmunity. Determination of sequence variations in genes known for predisposing one to CVID is important. IgG3ScD may be considered a supportive marker for a defective immune system and should be given similar status to impaired memory B cells. The significance of reduction of any other IgGSc is currently unknown. The final category, D, is the identification of lesions that may be associated with CVID.
Figure 3.
Diagnostic and treatment algorithm for patients with hypogammaglobulinaemia and CVID. Data obtained from Ameratunga et al.83
Patients with probable CVID must fulfill all criteria in category A, at least 1 from category B, and 3 or more from category C. Category D does not need to be fulfilled, but strengthens the diagnosis of CVID. For patients who do not fulfill category A, one may consider HGUS. Many patients may not fulfill categories B and/or C, and may have possible rather than probable CVID. Some CVID patients present with severe hypogammaglobulinemia and significant autoimmunity, rather than an increased susceptibility to infections. These patients may have a diagnosis of CVID if they meet the criteria in category A as well as those in categories C or D.
The recent International Consensus Document (ICON) for common variable immunodeficiency disorders suggests that the diagnosis of CVID does not require the additional laboratory criteria reported in Ameratunga et al.83,84 Bonilla et al suggest that milder laboratory phenotypes such as IgGScD may evolve over time into CVID, and so monitoring of IgGScD patients may be applicable for early detection of any transformation.81
Measurement of IgGSc Concentrations to Aid the Diagnosis of Specific Antibody Deficiency
It is important to differentiate between isolated IgGScD, SAD, and clinically relevant IgGScD, since the decision process for treatment and management may differ. Individuals with SAD differ from those with isolated IgGScD and clinically relevant IgGScD, since they have normal IgGSc concentrations and always have an impaired response to pneumococcal polysaccharide vaccination.34,85 Patients with SAD are predisposed to sinopulmonary bacterial infections, sinusitis, otitis, bronchitis, and pneumonia with pathogens, including Streptococcus pneumoniae, Moraxella catarrhalis, Haemophilus influenzae, and Streptococcus aureus.
Patients with SAD are managed in a variety of ways, including vaccination with conjugated vaccines, prophylactic antibiotics, and occasionally with antibody replacement therapy. This may differ from the patient management employed for those with isolated IgGScD and certainly for those with clinically relevant IgGScD with respect to antibody replacement. This may be indicative of the differing severity of immune defect among these PIDs (Figure 3).
Initial treatment of patients with normal IgG but IgGScD or SAD may include antibiotics for the infections and vaccination with the pneumococcal conjugate vaccine.86 A clear purpose for antibody replacement therapy is reduced levels of serum IgG (<2 g/L) in patients with recurrent bacterial infections, but this may be more difficult to justify in the presence of normal total IgG. The justification may be strengthened, however, for patients with clinically relevant IgGScD, as both IgGSc concentrations and response to vaccination are compromised. Antibody replacement therapy may be indicated in SAD patients, or patients with isolated IgGScD or clinically relevant IgGScD if the patients present with severe and/or RI,87 even though prophylactic antibiotics have been administered.48 The decision-making process is governed by clinical presentation, may involve the patients response to treatment, and is a clinician-dependent justification. Measurement of IgGSc is required to support the clinical presentations and the decisions for appropriate treatment.87,88
Measurement of IgGSc as a Monitoring Tool
Differentiation Between Transient and Persistent Antibody Deficiencies
A challenging issue for the clinician is whether the clinical symptoms presented are the result of a slow maturing immune system or an underlying immune deficiency. Transient hypogammaglobulinemia of infancy (THI) is an antibody deficiency occurring in the first years of life that is characterized by a delay in immunoglobulin production that spontaneously recovers in early infancy. Only those patients whose clinical symptoms have resolved and IgG concentrations have normalized after the age of 4 years have definitive THI. The diagnosis is made a posteriori.34,89
Routine measurement and normalisation of IgGSc may differentiate those who have a transient immunodeficiency from those who may have a more persistent polyclonal immunodeficiency.43,90-94
During the follow-up of 24 pediatric age group patients with IgGScD over 40 months, 25% had no further RIs, which correlated with IgGSc normalisation in all individuals tested.43 Kutukculer and colleagues reported that the IgGSc concentrations in 67% of pediatric age group patients with low IgA and IgGScD and 30% of pediatric age group patients with isolated IgGScD will normalize to age-specific normal concentrations.95 It has been reported that the mean age for normalization of IgGSc concentrations in pediatric age group patients was between 4 and 6 years of age,91,96 and that low IgG2 concentrations may be more challenging to normalize, signifying a more permanent antibody deficiency.91,92
The measurement of IgGSc may serve as suitable diagnostic tool for THI to support the change in presentation that accompanies IgGSc normalization. Moschese and colleagues reported the normalization of IgG in 41/57 children with hypogammaglobulinemia thus suggesting the diagnosis of THI.89 Of these 41 children, 18 (44%) still had episodes of infection, 61% with upper respiratory tract infections and 5.5% with pneumonia. The indication of infections in individuals with THI and normal IgG may warrant the measurement of IgGSc.47,89,97 Stiehm et al has suggested that patients diagnosed with THI may develop IgGScD.47
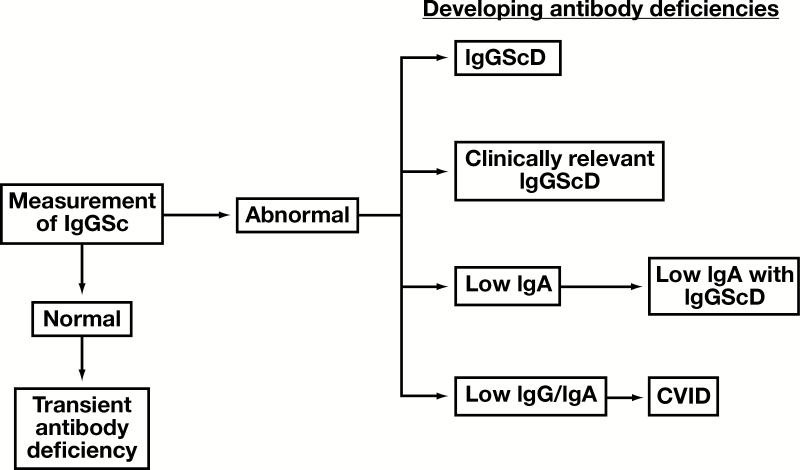
In addition to normalization of IgGSc suggesting a transient rather than permanent antibody deficiency, failure of the IgGSc concentrations to normalize may be a first step in identifying an underlying progressive immunodeficiency (Figure 4). In pediatric age group IgGScD patients, 11/24 (46%; >10 years of age) progressed to a more severe antibody deficiency; 1 developed low IgA, 3 developed IgG deficiency (IgGD), 3 developed IgGD with low IgA, and 4 developed CVID.43 Further reports have suggested that a IgGScD may develop after diagnosis of low IgA and correlate with severity of infection, and that the IgGScD may precede IgGD and gradual development of CVID.20,98-100 De Vries and colleagues have recommended that pediatric age groups with IgGScD should have their IgGSc concentrations measured every 1 to 2 years.14,101 ICON guidelines suggest that milder laboratory phenotypes such as IgGScD may evolve over time into more severe antibody deficiencies, and so monitoring of IgGScD patients may be applicable for early detection of any transformation.
Figure 4.
Summary for measuring IgGSc concentrations as a monitoring tool. Normalization of IgGSc may support the diagnosis of THI and exclude suspicion of a more permanent antibody deficiency. The failure of IgGSc concentrations to normalize may be indicative of a more permanent IgGScD or the evolution of a more severe antibody deficiency. The antibody defect may remain IgGScD or develop further into other deficiencies. Examples may include accompanying a progressive deficient response to vaccines developing into clinically relevant IgGScD, development of low IgA alone with IgGScD, or the further development of low IgG or IgA in the progression to CVID.
Influence on Treatment Regimes
Immunoglobulin Replacement Therapy
Current guidelines for the treatment of IgGScD suggest treating any allergic symptoms and support the administration of prophylactic antibiotics. In selected patients, cautious use of polyclonal human IgG may be required.87,88
Immunoglobulin prophylaxis reduces the frequency of RI in IgGScD patients5,48,102-105 and those with low IgA and low IgG2.72,106,107 Genel and colleagues used a prophylactic regime (oral immunomodulator bacterial extract OM-85 BV or benzathine penicillin) for patients with IgGScD alone and low IgA with IgGScD.102 At 12 months, the number of infections and antibiotic courses decreased significantly. In IgGScD patients receiving a prophylactic regime of antibiotic prophylaxis, oral bacterial lysate, and/or intravenous immunoglobulin, the frequency of RI decreased.95,97 Measurement of IgGSc may further identify individuals who may benefit from the appropriate treatment.
Revaccination
Reports suggest some correlation between IgG2ScD and the defective antibody response to polysaccharide antigens.24,56 Vaccination with the conjugated pneumococcal polysaccharide vaccine, however, has been shown to be effective in patients with IgG2ScD, since the response is directed towards the protein conjugated immunogen rather than the polysaccharide backbone.86,108 Identification of an IgG2ScD may illicit revaccination with the protein conjugated vaccine. The ICON study recommends impaired vaccine antibody responses for a diagnosis of CVID,81 while Ameratunga and colleagues suggest that the response to vaccination is not an important part of the diagnostic criteria.83,84 Goldacker and colleagues supported this further by reporting the response to vaccines in some CVID patients.109 Measurement of IgGSc may be of interest to determine who requires revaccination or further vaccination, and indeed whether to proceed with polysaccharide or conjugated polysaccharide vaccination.86,110
Conclusions
The measurement of IgGSc is a frequently requested laboratory test whose extensive clinical indication requires complete understanding.
Individuals are often referred to immunologists for the evaluation of reduced serum immunoglobulins. Normal values of IgG, IgA, and IgM are not always enough to exclude a more serious condition, and alongside the information obtained from measurement of specific antibody production and B lymphocyte populations, measurement of IgGSc can provide a more complete view of the function of the humoral immune system.19
Measurement of IgGSc may occur as a frontline test alongside the measurement of total immunoglobulins,12-15 but more commonly, the measurement of IgGSc is a second-line test16-19 given alongside or after the response to vaccines. Reference ranges for pediatric age groups and adult populations have been developed to aid identification of low concentrations, and a minimum of 2 measurements are required to confirm a diagnosis of IgGScD. Several methods are available for quantitation of the IgGSc concentrations, and several differences exist between manufacturers’ assays within a certain technique. This may influence quantitation, and therefore care must be taken to ensure serial monitoring for patients is performed within a single laboratory rather than across multiple laboratories to ensure a single technique is used.
Measurement of IgGSc can support the diagnosis of CVID and aid in the risk stratification of individuals with low IgA. The utility of the IgGSc is most prominent when combined with the response to vaccination. The discrimination between isolated IgGScD, SAD, and clinically relevant IgGScD is particularly important since this may influence treatments. There is clear utility for the measurement of IgGSc as a monitoring tool. The use alongside the measurement of total IgG may further aid differentiation between THI and a more permanent PAD. Prognostic information may be obtained by defining the cause of the deficiency.
IgGScD may influence frequency and severity of infection. The measurement of IgGSc should always be considered within a panel of other tests. In PADs the measurement of IgGSc and the response to vaccination provides a comprehensive window into the function of the immune system. LM
Disclosures
Antony R Parker, PhD, Markus Skold, PhD, and Stephen Harding, PhD, are employees of the Binding Site Group Limited, which manufactures IgG subclass assays.
Abbreviations
- COPD
chronic obstructive pulmonary disease
- CVID
common variable immunodeficiency
- ELISA
enzyme-linked immunosorbent assay
- ESID
European Society of Immune Deficiencies
- HGUS
hypogammaglobulinemia of uncertain significance
- ICON
International Consensus Document
- IgGD
IgG deficiency
- IgGSc
IgG subclass antibodies
- IgGScD
IgGSc deficiency
- IGHC
immunoglobulin heavy chain constant region
- PAD
primary antibody deficiency
- PAGID
Pan American Group for Immune Deficiency
- PID
primary immunodeficiency
- RI
recurrent infection
- RID
radial immunodiffusion
- SAD
specific antibody deficiency
- THI
transient hypogammaglobulinemia of infancy.
References
- 1. Fleming DW, Cochi SL, Hightower AW et al. Childhood upper respiratory tract infections: to what degree is incidence affected by day-care attendance? Pediatrics. 1987;79(1):55-60. [PubMed] [Google Scholar]
- 2. Glocker E, Ehl S, Grimbacher B. Common variable immunodeficiency in children. Curr Opin Pediatr. 2007;19(6):685-692. [DOI] [PubMed] [Google Scholar]
- 3. Monto AS, Napier JA, Metzner HL. The Tecumseh study of respiratory illness. I. Plan of study and observations on syndromes of acute respiratory disease. Am J Epidemiol. 1971;94(3):269-279. [DOI] [PubMed] [Google Scholar]
- 4. Buckley RH. Immunoglobulin G subclass deficiency: fact or fancy? Curr Allergy Asthma Rep. 2002;2(5):356-360. [DOI] [PubMed] [Google Scholar]
- 5. Herrod HG. Clinical significance of IgG subclasses. Curr Opin Pediatr. 1993;5(6):696-699. [DOI] [PubMed] [Google Scholar]
- 6. Schur PH, Borel H, Gelfand EW et al. Selective gamma-g globulin deficiencies in patients with recurrent pyogenic infections. N Engl J Med. 1970;283(12):631-634. [DOI] [PubMed] [Google Scholar]
- 7. Abrahamian F, Agrawal S, Gupta S. Immunological and clinical profile of adult patients with selective immunoglobulin subclass deficiency: response to intravenous immunoglobulin therapy. Clin Exp Immunol. 2010;159(3):344-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oxelius VA, Hanson LA, Björkander J et al. IgG3 deficiency: common in obstructive lung disease. Hereditary in families with immunodeficiency and autoimmune disease. Monogr Allergy. 1986;20:106-115. [PubMed] [Google Scholar]
- 9. Barton JC, Bertoli LF, Barton JC. Comparisons of CVID and IgGSD: referring physicians, autoimmune conditions, pneumovax reactivity, immunoglobulin levels, blood lymphocyte subsets, and HLA-A and -B typing in 432 adult index patients. J Immunol Res. 2014;2014:542706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sarmiento E, Mora R, Rodríguez-Mahou M et al. Autoimmune disease in primary antibody deficiencies. Allergol Immunopathol (Madr). 2005;33(2):69-73. [DOI] [PubMed] [Google Scholar]
- 11. Söderström T, Söderström R, Avanzini A et al. Immunoglobulin G subclass deficiencies. Int Arch Allergy Appl Immunol. 1987;82(3-4):476-480. [DOI] [PubMed] [Google Scholar]
- 12. Chang AB, Bell SC, Byrnes CA et al. Chronic suppurative lung disease and bronchiectasis in children and adults in Australia and New Zealand. Med J Aust. 2010;193(6):356-365. [DOI] [PubMed] [Google Scholar]
- 13. Cunningham-Rundles C. Immune deficiency: office evaluation and treatment. Allergy Asthma Proc. 2003;24(6):409-415. [PubMed] [Google Scholar]
- 14. de Vries E. Immunological investigations in children with recurrent respiratory infections. Paediatr Respir Rev. 2001;2(1):32-36. [DOI] [PubMed] [Google Scholar]
- 15. Slatter MA, Gennery AR. Clinical immunology review series: an approach to the patient with recurrent infections in childhood. Clin Exp Immunol. 2008;152(3):389-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Vries E. Patient-centred screening for primary immunodeficiency, a multi-stage diagnostic protocol designed for non-immunologists: 2011 update. Clin Exp Immunol. 2012;167(1):108-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Vries E. Patient-centred screening for primary immunodeficiency: a multi-stage diagnostic protocol designed for non-immunologists. Clin Exp Immunol. 2006;145(2):204-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Folds JD, Schmitz JL. 24. Clinical and laboratory assessment of immunity. J Allergy Clin Immunol. 2003;111(2 Suppl):S702-S711. [DOI] [PubMed] [Google Scholar]
- 19. Ladomenou F, Gaspar B. How to use immunoglobulin levels in investigating immune deficiencies. Arch Dis Child Educ Pract Ed. 2016;101(3):129-135. [DOI] [PubMed] [Google Scholar]
- 20. Aghamohammadi A, Mohammadi J, Parvaneh N et al. Progression of selective IgA deficiency to common variable immunodeficiency. Int Arch Allergy Immunol. 2008;147(2):87-92. [DOI] [PubMed] [Google Scholar]
- 21. Aytekin C, Tuygun N, Gokce S et al. Selective IgA deficiency: clinical and laboratory features of 118 children in Turkey. J Clin Immunol. 2012;32(5):961-966. [DOI] [PubMed] [Google Scholar]
- 22. Beard LJ, Ferrante A. IgG4 deficiency in IgA-deficient patients. Pediatr Infect Dis J. 1989;8(10):705-709. [DOI] [PubMed] [Google Scholar]
- 23. Beard LJ, Ferrante A, Oxelius VA et al. IgG subclass deficiency in children with IgA deficiency presenting with recurrent or severe respiratory infections. Pediatr Res. 1986;20(10):937-942. [DOI] [PubMed] [Google Scholar]
- 24. Edwards E, Razvi S, Cunningham-Rundles C. IgA deficiency: clinical correlates and responses to pneumococcal vaccine. Clin Immunol. 2004;111(1):93-97. [DOI] [PubMed] [Google Scholar]
- 25. Morell A, Muehlheim E, Schaad U et al. Susceptibility to infections in children with selective IgA- and IgA-IgG subclass deficiency. Eur J Pediatr. 1986;145(3):199-203. [DOI] [PubMed] [Google Scholar]
- 26. Bossuyt X, Mariën G, Meyts I et al. Determination of IgG subclasses: a need for standardization. J Allergy Clin Immunol. 2005;115(4):872-874. [DOI] [PubMed] [Google Scholar]
- 27. Ludwig-Kraus B, Kraus FB. Similar but not consistent: revisiting the pitfalls of measuring IgG subclasses with different assays. J Clin Lab Anal. 2017. doi: 10.1002/jcla.22146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parker AR, Hughes RG, Mead GP et al. Reply to “Pediatric reference intervals for immunoglobulin G and its subclasses with Siemens immunonephelometric assays.” Clin Biochem. 2011;44(8-9):745-746. [DOI] [PubMed] [Google Scholar]
- 29. Wilson C, Ebling R, Henig C et al. Significant, quantifiable differences exist between IgG subclass standards WHO67/97 and ERM-DA470k and can result in different interpretation of results. Clin Biochem. 2013;46(16-17):1751-1755. [DOI] [PubMed] [Google Scholar]
- 30. Lepage N, Huang SH, Nieuwenhuys E et al. Pediatric reference intervals for immunoglobulin G and its subclasses with Siemens immunonephelometric assays. Clin Biochem. 2010;43(7-8):694-696. [DOI] [PubMed] [Google Scholar]
- 31. Schauer U, Stemberg F, Rieger CH et al. IgG subclass concentrations in certified reference material 470 and reference values for children and adults determined with the binding site reagents. Clin Chem. 2003;49(11):1924-1929. [DOI] [PubMed] [Google Scholar]
- 32. French MA, Denis KA, Dawkins R et al. Severity of infections in IgA deficiency: correlation with decreased serum antibodies to pneumococcal polysaccharides and decreased serum IgG2 and/or IgG4. Clin Exp Immunol. 1995;100(1):47-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scadding GK, Lund VJ, Darby YC et al. IgG subclass levels in chronic rhinosinusitis. Rhinology. 1994;32(1):15-19. [PubMed] [Google Scholar]
- 34. Bonilla FA, Khan DA, Ballas ZK et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. 2015;136(5):1186-1205.e1. [DOI] [PubMed] [Google Scholar]
- 35. Wasserman RL, Sorensen RU. Evaluating children with respiratory tract infections: the role of immunization with bacterial polysaccharide vaccine. Pediatr Infect Dis J. 1999;18(2):157-163. [DOI] [PubMed] [Google Scholar]
- 36. Pan Q, Hammarström L. Molecular basis of IgG subclass deficiency. Immunol Rev. 2000;178:99-110. [DOI] [PubMed] [Google Scholar]
- 37. Lefranc G, Chaabani H, Van Loghem E et al. Simultaneous absence of the human IgG1, IgG2, IgG4 and IgA1 subclasses: immunological and immunogenetical considerations. Eur J Immunol. 1983;13(3):240-244. [DOI] [PubMed] [Google Scholar]
- 38. Lefranc MP, Lefranc G. Human immunoglobulin heavy-chain multigene deletions in healthy individuals. FEBS Lett. 1987;213(2):231-237. [DOI] [PubMed] [Google Scholar]
- 39. Lefranc MP, Lefranc G, Rabbitts TH. Inherited deletion of immunoglobulin heavy chain constant region genes in normal human individuals. Nature. 1982;300(5894):760-762. [DOI] [PubMed] [Google Scholar]
- 40. Migone N, Oliviero S, de Lange G et al. Multiple gene deletions within the human immunoglobulin heavy-chain cluster. Proc Natl Acad Sci U S A. 1984;81(18):5811-5815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Plebani A, Ugazio AG, Meini A et al. Extensive deletion of immunoglobulin heavy chain constant region genes in the absence of recurrent infections: when is IgG subclass deficiency clinically relevant? Clin Immunol Immunopathol. 1993;68(1):46-50. [DOI] [PubMed] [Google Scholar]
- 42. Hammarström L, Carbonara AO, DeMarchi M et al. Subclass restriction pattern of antigen-specific antibodies in donors with defective expression of IgG or IgA subclass heavy chain constant region genes. Clin Immunol Immunopathol. 1987;45(3):461-470. [DOI] [PubMed] [Google Scholar]
- 43. Schatorjé EJ, de Jong E, van Hout RW et al. The challenge of immunoglobulin-G subclass deficiency and specific polysaccharide antibody deficiency—a Dutch Pediatric Cohort Study. J Clin Immunol. 2016;36(2):141-148. [DOI] [PubMed] [Google Scholar]
- 44. Stiehm ER. The four most common pediatric immunodeficiencies. J Immunotoxicol. 2008;5(2):227-234. [DOI] [PubMed] [Google Scholar]
- 45. Ocampo CJ, Peters AT. Antibody deficiency in chronic rhinosinusitis: epidemiology and burden of illness. Am J Rhinol Allergy. 2013;27(1):34-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ozkan H, Atlihan F, Genel F et al. IgA and/or IgG subclass deficiency in children with recurrent respiratory infections and its relationship with chronic pulmonary damage. J Investig Allergol Clin Immunol. 2005;15(1):69-74. [PubMed] [Google Scholar]
- 47. Stiehm RE. The four most common pediatric immunodeficiencies. Adv Exp Med Biol. 2007;601:15-26. [DOI] [PubMed] [Google Scholar]
- 48. Olinder-Nielsen AM, Granert C, Forsberg P et al. Immunoglobulin prophylaxis in 350 adults with IgG subclass deficiency and recurrent respiratory tract infections: a long-term follow-up. Scand J Infect Dis. 2007;39(1):44-50. [DOI] [PubMed] [Google Scholar]
- 49. Ferrante A, Beard LJ, Feldman RG. IgG subclass distribution of antibodies to bacterial and viral antigens. Pediatr Infect Dis J. 1990;9(8 Suppl):S16-S24. [PubMed] [Google Scholar]
- 50. Goldblatt D, Scadding GK, Lund VJ et al. Association of Gm allotypes with the antibody response to the outer membrane proteins of a common upper respiratory tract organism, Moraxella catarrhalis. J Immunol. 1994;153(11):5316-5320. [PubMed] [Google Scholar]
- 51. Aucouturier P, Bremard-Oury C, Griscelli C et al. Serum IgG subclass deficiency in ataxia-telangiectasia. Clin Exp Immunol. 1987;68(2):392-396. [PMC free article] [PubMed] [Google Scholar]
- 52. Ochs HD. The Wiskott-Aldrich syndrome. Clin Rev Allergy Immunol. 2001;20(1):61-86. [DOI] [PubMed] [Google Scholar]
- 53. Freeman JA, Crassini KR, Best OG et al. Immunoglobulin G subclass deficiency and infection risk in 150 patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2013;54(1):99-104. [DOI] [PubMed] [Google Scholar]
- 54. Ambrosino DM, Schiffman G, Gotschlich EC et al. Correlation between G2m(n) immunoglobulin allotype and human antibody response and susceptibility to polysaccharide encapsulated bacteria. J Clin Invest. 1985;75(6):1935-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. de Baets F, Pauwels R, Schramme I et al. IgG subclass specific antibody response in recurrent bronchitis. Arch Dis Child. 1991;66(12):1378-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Siber GR, Schur PH, Aisenberg AC et al. Correlation between serum IgG-2 concentrations and the antibody response to bacterial polysaccharide antigens. N Engl J Med. 1980;303(4):178-182. [DOI] [PubMed] [Google Scholar]
- 57. Sorensen RU, Hidalgo H, Moore C et al. Post-immunization pneumococcal antibody titers and IgG subclasses. Pediatr Pulmonol. 1996;22(3):167-173. [DOI] [PubMed] [Google Scholar]
- 58. Umetsu DT, Ambrosino DM, Quinti I et al. Recurrent sinopulmonary infection and impaired antibody response to bacterial capsular polysaccharide antigen in children with selective IgG-subclass deficiency. N Engl J Med. 1985;313(20):1247-1251. [DOI] [PubMed] [Google Scholar]
- 59. Barrett DJ, Ayoub EM. IgG2 subclass restriction of antibody to pneumococcal polysaccharides. Clin Exp Immunol. 1986;63(1):127-134. [PMC free article] [PubMed] [Google Scholar]
- 60. Freijd A, Hammarström L, Persson MA et al. Plasma anti-pneumococcal antibody activity of the IgG class and subclasses in otitis prone children. Clin Exp Immunol. 1984;56(2):233-238. [PMC free article] [PubMed] [Google Scholar]
- 61. Barton JC, Bertoli LF, Barton JC et al. Selective subnormal IgG3 in 121 adult index patients with frequent or severe bacterial respiratory tract infections. Cell Immunol. 2016;299:50-57. [DOI] [PubMed] [Google Scholar]
- 62. De Gracia J, Rodrigo MJ, Morell F et al. IgG subclass deficiencies associated with bronchiectasis. Am J Respir Crit Care Med. 1996;153(2):650-655. [DOI] [PubMed] [Google Scholar]
- 63. Ludvigsson JF, Neovius M, Hammarström L. Risk of infections among 2100 individuals with IgA deficiency: a Nationwide Cohort Study. J Clin Immunol. 2016;36(2):134-140. [DOI] [PubMed] [Google Scholar]
- 64. Polmar SH, Waldmann TA, Balestra ST et al. Immunoglobulin E in immunologic deficiency diseases. I. Relation of IgE and IgA to respiratory tract disease in isolated IgE deficiency, IgA deficiency, and ataxia telangiectasia. J Clin Invest. 1972;51(2):326-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. French MA, Harrison G. An investigation into the effect of the IgG antibody system on the susceptibility of IgA-deficient patients to respiratory tract infections. Clin Exp Immunol. 1986;66(3):640-647. [PMC free article] [PubMed] [Google Scholar]
- 66. Ones U, Güler N, Somer A et al. Low immunoglobulin G3 levels in wheezy children. Acta Paediatr. 1998;87(4):368-370. [PubMed] [Google Scholar]
- 67. Out TA, van Munster PJ, De Graeff PA et al. Immunological investigations in individuals with selective IgA deficiency. Clin Exp Immunol. 1986;64(3):510-517. [PMC free article] [PubMed] [Google Scholar]
- 68. Oxelius VA, Laurell AB, Lindquist B et al. IgG subclasses in selective IgA deficiency: importance of IgG2-IgA deficiency. N Engl J Med. 1981;304(24):1476-1477. [DOI] [PubMed] [Google Scholar]
- 69. De Graeff PA, The TH, van Munster PJ et al. The primary immune response in patients with selective IgA deficiency. Clin Exp Immunol. 1983;54(3):778-784. [PMC free article] [PubMed] [Google Scholar]
- 70. Lane PJ, MacLennan IC. Impaired IgG2 anti-pneumococcal antibody responses in patients with recurrent infection and normal IgG2 levels but no IgA. Clin Exp Immunol. 1986;65(2):427-433. [PMC free article] [PubMed] [Google Scholar]
- 71. Bossuyt X, Moens L, Van Hoeyveld E et al. Coexistence of (partial) immune defects and risk of recurrent respiratory infections. Clin Chem. 2007;53(1):124-130. [DOI] [PubMed] [Google Scholar]
- 72. Björkander J, Bake B, Oxelius VA et al. Impaired lung function in patients with IgA deficiency and low levels of IgG2 or IgG3. N Engl J Med. 1985;313(12):720-724. [DOI] [PubMed] [Google Scholar]
- 73. Cunningham-Rundles C, Oxelius VA, Good RA. IgG2 and IgG3 subclass deficiencies in selective IgA deficiency in the United States. Birth Defects Orig Artic Ser. 1983;19(3):173-175. [PubMed] [Google Scholar]
- 74. Klemola T, Seppälä I, Savilahti E. Serum IgG subclass levels in paediatric clinic patients with variable degrees of IgA deficiency. J Clin Lab Immunol. 1988;25(1):29-34. [PubMed] [Google Scholar]
- 75. Plebani A, Monafo V, Avanzini MA et al. Relationship between IgA and IgG subclass deficiencies: a reappraisal. Monogr Allergy. 1986;20:171-178. [PubMed] [Google Scholar]
- 76. Ugazio AG, Out TA, Plebani A et al. Recurrent infections in children with “selective” IgA deficiency: association with IgG2 and IgG4 deficiency. Birth Defects Orig Artic Ser. 1983;19(3):169-171. [PubMed] [Google Scholar]
- 77. Preud’homme JL, Hanson LA. IgG subclass deficiency. Immunodefic Rev. 1990;2(2):129-149. [PubMed] [Google Scholar]
- 78. Aghamohammadi A, Moin M, Karimi A et al. Immunologic evaluation of patients with recurrent ear, nose, and throat infections. Am J Otolaryngol. 2008;29(6):385-392. [DOI] [PubMed] [Google Scholar]
- 79. Aghamohammadi A, Cheraghi T, Gharagozlou M et al. IgA deficiency: correlation between clinical and immunological phenotypes. J Clin Immunol. 2009;29(1):130-136. [DOI] [PubMed] [Google Scholar]
- 80. Singh K, Chang C, Gershwin ME. IgA deficiency and autoimmunity. Autoimmun Rev. 2014;13(2):163-177. [DOI] [PubMed] [Google Scholar]
- 81. Bonilla FA, Barlan I, Chapel H et al. International Consensus Document (ICON): common variable immunodeficiency disorders. J Allergy Clin Immunol Pract. 2016;4(1):38-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gathmann B, Mahlaoui N, Gérard L et al. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J Allergy Clin Immunol. 2014;134(1):116-126. [DOI] [PubMed] [Google Scholar]
- 83. Ameratunga R, Woon ST, Gillis D et al. New diagnostic criteria for common variable immune deficiency (CVID), which may assist with decisions to treat with intravenous or subcutaneous immunoglobulin. Clin Exp Immunol. 2013;174(2):203-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ameratunga R, Woon ST, Gillis D et al. New diagnostic criteria for CVID. Expert Rev Clin Immunol. 2014;10(2):183-186. [DOI] [PubMed] [Google Scholar]
- 85. Orange JS, Ballow M, Stiehm ER et al. Use and interpretation of diagnostic vaccination in primary immunodeficiency: a working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2012;130(3 Suppl):S1-24. [DOI] [PubMed] [Google Scholar]
- 86. Sorensen RU, Leiva LE, Giangrosso PA et al. Response to a heptavalent conjugate Streptococcus pneumoniae vaccine in children with recurrent infections who are unresponsive to the polysaccharide vaccine. Pediatr Infect Dis J. 1998;17(8):685-691. [DOI] [PubMed] [Google Scholar]
- 87. Maarschalk-Ellerbroek LJ, Hoepelman IM, Ellerbroek PM. Immunoglobulin treatment in primary antibody deficiency. Int J Antimicrob Agents. 2011;37(5):396-404. [DOI] [PubMed] [Google Scholar]
- 88. Bonagura VR. Using intravenous immunoglobulin (IVIG) to treat patients with primary immune deficiency disease. J Clin Immunol. 2013;33(Suppl 2):S90-S94. [DOI] [PubMed] [Google Scholar]
- 89. Moschese V, Graziani S, Avanzini MA et al. A prospective study on children with initial diagnosis of transient hypogammaglobulinemia of infancy: results from the Italian Primary Immunodeficiency Network. Int J Immunopathol Pharmacol. 2008;21(2):343-352. [DOI] [PubMed] [Google Scholar]
- 90. Gaschignard J, Levy C, Chrabieh M et al. Invasive pneumococcal disease in children can reveal a primary immunodeficiency. Clin Infect Dis. 2014;59(2):244-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Karaca NE, Aksu G, Gulez N et al. New laboratory findings in Turkish patients with transient hypogammaglobulinemia of infancy. Iran J Allergy Asthma Immunol. 2010;9(4):237-243. [PubMed] [Google Scholar]
- 92. Roberton DM, Colgan T, Ferrante A et al. IgG subclass concentrations in absolute, partial and transient IgA deficiency in childhood. Pediatr Infect Dis J. 1990;9(8 Suppl):S41-S45. [PubMed] [Google Scholar]
- 93. Shackelford PG, Granoff DM, Madassery JV et al. Clinical and immunologic characteristics of healthy children with subnormal serum concentrations of IgG2. Pediatr Res. 1990;27(1):16-21. [DOI] [PubMed] [Google Scholar]
- 94. Shackelford PG, Granoff DM, Polmar SH et al. Subnormal serum concentrations of IgG2 in children with frequent infections associated with varied patterns of immunologic dysfunction. J Pediatr. 1990;116(4):529-538. [DOI] [PubMed] [Google Scholar]
- 95. Kutukculer N, Karaca NE, Demircioglu O et al. Increases in serum immunoglobulins to age-related normal levels in children with IgA and/or IgG subclass deficiency. Pediatr Allergy Immunol. 2007;18(2):167-173. [DOI] [PubMed] [Google Scholar]
- 96. Roberton DM, Björkander J, Henrichsen J et al. Enhanced IgG1 and IgG3 responses to pneumococcal polysaccharides in isolated IgA deficiency. Clin Exp Immunol. 1989;75(2):201-205. [PMC free article] [PubMed] [Google Scholar]
- 97. Karaca NE, Karadeniz C, Aksu G et al. Clinical and laboratory evaluation of periodically monitored Turkish children with IgG subclass deficiencies. Asian Pac J Allergy Immunol. 2009;27(1):43-48. [PubMed] [Google Scholar]
- 98. Carvalho Neves Forte W, Ferreira De Carvalho Júnior F, Damaceno N et al. Evolution of IgA deficiency to IgG subclass deficiency and common variable immunodeficiency. Allergol Immunopathol (Madr). 2000;28(1):18-20. [PubMed] [Google Scholar]
- 99. Español T, Catala M, Hernandez M et al. Development of a common variable immunodeficiency in IgA-deficient patients. Clin Immunol Immunopathol. 1996;80(3 Pt 1):333-335. [DOI] [PubMed] [Google Scholar]
- 100. Gutierrez MG, Kirkpatrick CH. Progressive immunodeficiency in a patient with IgA deficiency. Ann Allergy Asthma Immunol. 1997;79(4):297-301. [DOI] [PubMed] [Google Scholar]
- 101. de Vries E, Driessen G. Educational paper: primary immunodeficiencies in children: a diagnostic challenge. Eur J Pediatr. 2011;170(2):169-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Genel F, Kutukculer N. Prospective, randomized comparison of OM-85 BV and a prophylactic antibiotic in children with recurrent infections and immunoglobulin A and/or G subclass deficiency. Curr Ther Res Clin Exp. 2003;64(8):600-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Jehangir A, Bennett K, Fareedy SB et al. Recurrent C. difficile in a patient with IgG deficiency. Case Rep Gastrointest Med. 2015;2015:356293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Meyts I, Bossuyt X, Proesmans M et al. Isolated IgG3 deficiency in children: to treat or not to treat? Case presentation and review of the literature. Pediatr Allergy Immunol. 2006;17(7):544-550. [DOI] [PubMed] [Google Scholar]
- 105. Snowden JA, Milford-Ward A, Reilly JT. Symptomatic IgG3 deficiency successfully treated with intravenous immunoglobulin therapy. Postgrad Med J. 1994;70(830):924-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Grey HM, Kunkel HG. H chain subgroups of myeloma proteins and normal 7S gamma-globulin. J Exp Med. 1964;120:253-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Oxelius VA. Chronic infections in a family with hereditary deficiency of IgG2 and IgG4. Clin Exp Immunol. 1974;17(1):19-27. [PMC free article] [PubMed] [Google Scholar]
- 108. Zielen S, Bröker M, Strnad N et al. Simple determination of polysaccharide specific antibodies by means of chemically modified ELISA plates. J Immunol Methods. 1996;193(1):1-7. [DOI] [PubMed] [Google Scholar]
- 109. Goldacker S, Draeger R, Warnatz K et al. Active vaccination in patients with common variable immunodeficiency (CVID). Clin Immunol. 2007;124(3):294-303. [DOI] [PubMed] [Google Scholar]
- 110. Paris K, Sorensen RU. Assessment and clinical interpretation of polysaccharide antibody responses. Ann Allergy Asthma Immunol. 2007;99(5):462-464. [DOI] [PubMed] [Google Scholar]
- 111. Slatter MA, Bhattacharya A, Flood TJ et al. Polysaccharide antibody responses are impaired post bone marrow transplantation for severe combined immunodeficiency, but not other primary immunodeficiencies. Bone Marrow Transplant. 2003;32(2):225-229. [DOI] [PubMed] [Google Scholar]
- 112. May A, Zielen S, von Ilberg C et al. Immunoglobulin deficiency and determination of pneumococcal antibody titers in patients with therapy-refractory recurrent rhinosinusitis. Eur Arch Otorhinolaryngol. 1999;256(9):445-449. [DOI] [PubMed] [Google Scholar]