Abstract
Intratracheal instillation serves as a model for inhalation exposure. However, for this, materials are dispersed in appropriate media that may influence toxicity. We tested whether different intratracheal instillation dispersion media influence the pulmonary toxicity of different nanomaterials. Rodents were intratracheally instilled with 162 µg/mouse/1620 µg/rat carbon black (CB), 67 µg/mouse titanium dioxide nanoparticles (TiO2) or 54 µg/mouse carbon nanotubes (CNT). The dispersion media were as follows: water (CB, TiO2); 2% serum in water (CB, CNT, TiO2); 0.05% serum albumin in water (CB, CNT, TiO2); 10% bronchoalveolar lavage fluid in 0.9% NaCl (CB), 10% bronchoalveolar lavage (BAL) fluid in water (CB) or 0.1% Tween-80 in water (CB). Inflammation was measured as pulmonary influx of neutrophils into bronchoalveolar fluid, and DNA damage as DNA strand breaks in BAL cells by comet assay. Inflammation was observed for all nanomaterials (except 38-nm TiO2) in all dispersion media. For CB, inflammation was dispersion medium dependent. Increased levels of DNA strand breaks for CB were observed only in water, 2% serum and 10% BAL fluid in 0.9% NaCl. No dispersion medium-dependent effects on genotoxicity were observed for TiO2, whereas CNT in 2% serum induced higher DNA strand break levels than in 0.05% serum albumin. In conclusion, the dispersion medium was a determinant of CB-induced inflammation and genotoxicity. Water seemed to be the best dispersion medium to mimic CB inhalation, exhibiting DNA strand breaks with only limited inflammation. The influence of dispersion media on nanomaterial toxicity should be considered in the planning of intratracheal investigations.
Introduction
Humans are exposed to a range of particles through inhalation. For the assessment of human toxicology, animal models are often employed to determine levels at which toxicity occurs. Inhalation in rodents is a model that resembles human inhalation. Intratracheal instillation and aspiration are alternative exposure models often employed, as these are cost-effective, use less test material and provide safety for the staff. Also, it has been suggested that intratracheal instillation studies are useful for ranking and grouping of nanomaterials (1–7). However, when using this administration method, a liquid dispersion medium has to be added as a carrier and dispersant. Dispersion media are often based on saline containing different proteins. These are added to decrease agglomeration size and improve stability of the aggregates for some nanomaterials (8,9). Conversely, for other materials, protein-free media may be best. CB Printex-90 has a primary agglomerate size in the micrometre range when dispersed in 10% acellular bronchiolar lavage fluid (BAL) in saline (4,10), whereas 50- to 100-nm agglomerates are observed in water (11,12).
Dispersion media may, however, influence the nature and severity of the toxicity. It has e.g. been proposed that dispersion media should be tested on how they affect the genotoxic properties of the dispersed particle. This is because the dispersion medium can participate in the formation of a protein corona on the material surface and thus influence the available surface area. The latter is a potential determinant of genotoxicity (13).
We aimed at assessing whether dispersion media in general influence toxicity. At the same time, we wanted to determine the best dispersion media to mimic inhalation toxicity for three specific nanomaterials. These were carbon black (CB), multiwalled carbon nanotubes (CNT) and titanium dioxide nanoparticles (TiO2). CB Printex-90 (14 nm of diameter) has been extensively characterised (6,10,14) and has at a dose of 8 mg/kg mouse body weight (bw) by intratracheal administration been used extensively as a positive control and reference particle in the toxicological testing of nanomaterials (4–6,12,13,15–18). Rodent inhalation of CB Printex-90 is associated with increased levels of DNA strand breaks in BAL cells (19) and in liver cells (11), although no effect was found on 8-Oxo-dG levels in DNA in rat lung following exposure to CB (20). CB has been evaluated as possibly carcinogenic to humans (IARC group 2B) based on animal studies (21). Rodent inhalation of CNTs increases neutrophil numbers in BAL fluid (22–25). Regarding genotoxicity, DNA strand breaks were increased in rats by inhalation of 0.94 mg/m3 multiwalled CNTs (26). Following inhalation in mice, DNA damage increased for straight but not tangled CNTs (27). Rodent inhalation of TiO2 increases BAL neutrophil numbers as shown e.g. in refs (28–31). Genotoxicity was found in one study (32), but not in another study (31). Carcinogenicity was found in one inhalation study (33), but not in another study (34). TiO2 has been evaluated as possibly carcinogenic to humans (IARC group 2B) based on animal studies (21).
We wanted to assess whether dispersion media influence pulmonary toxicity of nanomaterials. CB, CNT and TiO2 were tested in combination with different dispersion media by intratracheal instillation in mice and rats. BAL neutrophils and lung expression of inflammatory genes were measured to assess inflammation. The studies were mainly mice studies on CB, CNT and TiO2, supplemented with a confirmatory rat study on two of the dispersion media. Levels of DNA strand breaks were analysed by comet assay comparing tail lengths of BAL cells. The results were compared to data from the literature on studies using inhalation as well as intratracheal instillation.
Materials and methods
Materials
Printex-90 CB nanoparticles were a gift from Evonik Degussa (Essen, Germany). The diameter was 14 nm, and the specific surface area was 295–338 m2/g (19,35). It was composed of 99% C, 0.8% N and 0.01% H, and the total content of polycyclic aromatic hydrocarbons was 0.07 mg/g (35). CNT NM-400 are multiwalled CNTs (diameter 30 nm). Physical–chemical characteristics are described in refs (36–38). Titanium dioxide (TiO2) nanoparticles, one with endogenous negative surface charge (NRCWE-001) (NanoAmor, Houston, TX, USA) and one based on NRCWE-001 was modified to be positively charged (NRCWE-002) (38). A third TiO2 particle NRCWE-025 was similar to NRCWE-001 except for having a supplier reported size of 80 nm (39) and a measured size of 38 nm (40). Physical-chemical properties of the materials are detailed in Table 1.
Table 1.
Physical–chemical characterisation of the investigated nanomaterials dispersed in different media
| Nanomaterial | Dispersion medium | Size as Z-average (nm) | Dispersity | BET surface area (m2/g) |
|---|---|---|---|---|
| CB Printex-90 (declared size 14 nm; minor elements: N: 0.82%, H: 0.01%) | Water | 81.7 | 0.383 | 295, taken from (19) |
| – | 2% serum | 62.8 | 0.168 | – |
| – | 10% BAL in 0.9% NaCl | 1231 | 0.642 | – |
| – | 0.05% BSA in water and ethanol | 112 | 0.325 | – |
| – | 10% BAL in water | 4345 | 1.000 | – |
| – | 0.1% Tween-80 | 112 | 0.219 | – |
| CNT NM-400 [supplier information on size: 30 nm × 5 µm, no known coating, characterised in water with 2% foetal calf serum, TEM size: diameter: 5–35, length: 700–3000 nm; size in water 198 ± 85 nm; size in saline 124 ± 50 nm; measured by NanoSight tracking analysis (36,38,66)] | 2% serum | Not detectable by DLS | 298, taken from (38) | |
| – | 0.05% BSA in water and ethanol | Not detectable by DLS | 298, taken from (38) | |
| TiO2 NRCWE-001 [supplier information size (10 nm by XRD); known coating: none; phase: rutile (but ~6% anatase observed in one of two samples); primary characteristics by TEM; irregular euhedral particles detected (38)] | Water | 111 | 0.120 | 99, taken from (38) |
| – | 2% serum | 128 | 0.136 | – |
| – | 0.05% BSA in water and ethanol | 123 | 0.180 | – |
| NRCWE-002 [supplier information size (10 nm by XRD) and form: rutile; known coating: positively charged; phase: rutile; coating: positively charged; primary characteristics by TEM irregular euhedral particles detected by TEM (38)] | Water | 1232 | 0.251 | 84, taken from (38) |
| – | 2% serum | 146 | 0.194 | – |
| – | 0.05% BSA in water and ethanol | 149 | 0.217 | – |
| NRCWE-025 (described as rutile; XRD size supplier 80 nm; XRD size 38.1 nm; AFM particle size 35.3 nm) | Water | 187 | 0.153 | 28, value from (40) |
| – | 2% serum | 211 | 0.130 | – |
| – | 0.05% BSA in water and ethanol | 221 | 0.127 | – |
Dispersion procedures
Preparation of cell-free BAL fluid and serum
BAL was collected under anaesthesia (anaesthesia procedure described below). The lungs of unexposed C57BL/6 mice were flushed twice with 0.6 ml 0.9% NaCl solution, yielding a total of ~1 ml BAL fluid. Cells were next removed by centrifugation at 400 × g (10 min, 4°C). The final acellular fluid was stored at −20°C until use. C57BL/6JBomTac mouse serum was obtained from unexposed animals by removing coagulated cells from blood by centrifugation. Serum was stored at −20°C until use.
Sonication procedure
Unless otherwise stated below, sonication of all dispersions was performed on ice-bath for 16 min using a Branson Sonifier S-450D (Branson Ultrasonics Corp., Danbury, CT, USA) equipped with a 13-nm disruptor horn (model number: 101-147-037; Branson Ultrasonics Corp., Danbury, CT, USA). To assure particle homogeneity, the suspension was administered to the animals within 1 h after sonication. All dispersion medium control samples were done as described below for specific nanoparticles, except that no nanomaterials were included.
Preparation of CB, CNT and TiO2 in water
Particles were suspended in 0.2-mm filtered, γ-irradiated Nanopure Diamond UV water (Pyrogens: <0.001 EU/ml, total organic carbon: <3.0 ppb) (11). The amount of particles was 3.24 mg/ml corresponding to 162 µg/mouse at a volume of 50 µl.
Preparation of CB, CNT and TiO2 in 2% serum
Two percent serum diluted in Nanopure water was added to the weighed out nanomaterials. CB, CNT and TiO2 suspensions were 3.24 mg particles/ml, giving mass concentrations of 162 µg/mouse. For CNT the sonicated suspension was diluted into 54 µg CNT/mouse. For TiO2, the sonication suspension was 1.34 mg particles/ml to obtain a dose level of 67 µg/mouse.
Preparation of CB and TiO2 in BAL
The nanoparticles were suspended in 0.9% NaCl MilliQ water containing 10% v/v BAL. Particles were suspended in an amount of 3.24 mg/ml for CB to obtain a dose level of 162 µg/mouse and in an amount of 1.34 mg/ml for TiO2 (67 µg/mouse). The sonication procedure was done as described above, except the suspensions were sonicated by alternating 10-s pulses and 10 s pauses for 16 min. Preparation of CB in BAL in water was done in the same way except that no NaCl was added.
Preparation of CB in 0.1% Tween 80
The CB particles were dispersed by sonication in 0.1% Tween-80 [chemical name: polyoxyethylene (20) sorbitan mono-oleate] (Croda International, Goole, UK) at an amount of 3.24 mg/ml.
Preparation of CB in 0.05% bovine serum albumin (BSA)
CB was pre-wetted by ethanol 0.5% (v/v), followed by sonication in 0.05% w/v BSA water at a 2.56 mg/ml.
Material characterisation
The hydrodynamic size distributions of the different dispersions were determined by dynamic light scattering (DLS) using a Malvern Zetasizer Nano ZS (Malvern Instruments, UK) and the Dispersion Technology Software v5.0 (Malvern Instruments, UK). Size distributions were measured at 25°C directly on the instillation suspensions. Data were recorded as the mean value of six consecutively repeated analyses of each sample. For the calculation of hydrodynamic size and dispersity, standard optical and viscosity properties for water were used. Refractive and absorption indices of 2.020 and 2.000 were used for CB. These values were 2.903 and 0.10, respectively, for TiO2.
Animal housing
This investigation encompasses five animal studies, four using mice and one using rats. The parameters in these studies are detailed in Table 2. All animal procedures complied with the EC Directive 86/609/EEC and Danish law regulating experiments with animals (The Danish Ministry of Justice, Animal Experiments Inspectorate permission 2006/561–1123). Female C57BL/6J BomTac mice, 6–7 weeks of age, and with a body weight (bw) of 19 ± 1.5 g were obtained from Taconic Europe (Ejby, Denmark). Upon arrival, the animals were randomised either to cages containing control animals (n = 2–8) or to cages containing animals for nanomaterial administration (n = 2–8). The mice were allowed to acclimatise 1–2 weeks before the experiment. Food (Altromin no. 1324, Christian Petersen, Denmark) and tap water were provided ad libitum. Housing was polypropylene cages with Enviro-Dri bedding (Brogaarden, Gentofte, Denmark). Enrichment was MS blocks of wood (Brogaarden, Gentofte, Denmark) and hides (Mouse House, Scanbur, Karlslunde, Denmark). Housing was at a temperature of 20 ± 2°C and a humidity of 50 ± 20% with a 12 h light:12 h dark cycle (on from 6 a.m. to 6 p.m.). Female Sprague Dawley rats were purchased from Taconic Europe (Ejby, Denmark) and housed two in each cage. Housing conditions were as described for mice. The rats were 7 weeks old at arrival and 8 weeks old at the beginning of the experiment. The weight at the beginning of the experiment was in the range of 171–205 g.
Table 2.
Overview of animal experiments
| Investigation number | Particles | Animal species and number (N) of animals | Dispersion media tested | Exposure period and end points (comet assay system) |
|---|---|---|---|---|
| 1 | 162 µg/mouse (8 mg/kg bw) CB or 54 µg/mouse (2.7 mg/kg bw) CNT | Mouse | Nanopure water (CB) | 24 h: |
| n = 6 per group (in total n = 72) | 2% serum in Nanopure water (CB, CNT) | BAL neutrophils, Saa3 and Mcp-1 mRNA | ||
| 0.05% serum albumin in Nanopure water and powder pre-wetting with 0.5% (v/v) ethanol (CB, CNT) | DNA strand breaks (Andor Komet 6) | |||
| 10% BAL fluid in 0.9% NaCl (CB) | ||||
| 10% BAL fluid in Nanopure water (CB) | ||||
| 2 | 162 µg/mouse (8 mg/kg bw) CB | Mouse | 0.1% Tween-80 in Nanopure water | 24 h: |
| n = 7 per group (in total n = 14) | BAL neutrophilsDNA strand breaks (IMSTAR) | |||
| 3 | 162 µg/mouse (8 mg/kg bw) CB | Mouse | Nanopure water | 3, 5 and 28 days: |
| n = 8 per group (in total n = 144) | 2% serum in Nanopure water | BAL neutrophils | ||
| 0.05% serum albumin in Nanopure water and powder pre-wetting with 0.5% (v/v) ethanol | DNA strand breaks (IMSTAR) | |||
| 4 | 67 µg/mouse (3.3 mg/kg bw) TiO2 NRCWE-001, NRCWE-002 or NRCWE-025 | Mouse | For TiO2 particles: Nanopure water 2% serum in Nanopure water 0.05% serum albumin in Nanopure water and powder pre-wetting with 0.5% (v/v) ethanol | 24 h:BAL neutrophilsDNA strand breaks (IMSTAR) |
| 162 µg/mouse (8 mg/kg bw) CB | n = 8 per group (except NRCWE-001 in 2% serum n = 7) (in total n = 103) | |||
| For CB: 2% serum in Nanopure water | ||||
| 5 | 1620 µg/rat (5.4 mg/kg bw) CB | Rat | Nanopure water | 24 h: |
| n = 6 per group (in total n = 24) | 0.05% serum albumin in Nanopure water and powder pre-wetting with 0.5% (v/v) ethanol | BAL neutrophilsDNA strand breaks (Andor Komet 6) |
Animal procedures
At 8 weeks of age, the mice were administered particles or dispersion medium controls. This was done by single intratracheal instillation. The mice were anaesthetised by 4% isoflurane inhalation or alternatively by injection with a volume of 0.2 ml containing Hypnorm (fentanyl citrate 0.315 mg/ml and fluanisone 10 mg/ml from Janssen Pharma) and Dormicum (midazolam 5 mg/ml from Roche). The intratracheal instillation procedure is described in detail in ref. (5). A volume of 50-µl particle or vehicle control suspension was instilled followed by 150-µl air. This was done with a 250-µl SGE glass syringe (250F-LT-GT, MicroLab, Aarhus, Denmark). Afterwards, breathing was observed to assure that airways were not blocked. At euthanisation, the mice were anaesthetised using subcutaneous injection of Hypnorm/Dormicum/water at a ratio of 1:1:2. The start dose was 0.1 ml/25 g. The dose was increased if deemed necessary. The abdomen and thorax were opened, and it was observed whether macroscopic abnormalities such as discolourations, ascites or bleeding were present. To recover BAL, the lungs were flushed twice each using 1-ml saline/25 g bw using a 1- or 2-ml syringe. Each flush consisted of three down and up movements (each lasting 5–10 s). The final fluid volume that was recovered from the lungs was estimated and recorded. The BAL fluid was kept on ice until separation of fluid and cells by centrifugation at 400 × g at 4°C for 10 min (done within 2 h).
Regarding rats, these were anaesthetised by intubation with an 18-gauge 45-mm-long tube with 5% isoflurane at a flow rate of 200–250 ml/min. As we observed that rats may stop breathing if instilled in deep anaesthesia, we kept the rats intubated without isoflurane. Instillation was completed well before the animals gained consciousness. Breathing was observed; none of the rats stopped breathing. The rats were intratracheally instilled on a 40° slope with 500 µl of particle or vehicle control suspension, immediately followed by 500 µl of air. At the end of exposure, the animals were anaesthetised by subcutaneous injection of Hypnorm/Dormicum/water at a ratio 1:1:2 at a recommended volume of 0.3 ml/100 g. The animals were then euthanised by bleeding via a cut in the groin area. Next, BAL was prepared by flushing the lungs 10 times with 5-ml saline. Each volume flushed once and subsequently combined in one tube. BAL cells were recovered by centrifugation.
BAL cell procedure for comet assay and differential counting
Cells were resuspended in 100 µl HAMF12 medium with 10% foetal bovine serum (Prod numbers: 217654037 and 10106169, Invitrogen, Carlsbad, CA, USA). Forty microlitre of this suspension was mixed with 160 µl of HAMF12 medium with 10% foetal bovine serum containing 10% dimethyl sulfoxide (DMSO). This was stored at −80°C until comet assay analyses. For immune cell differential counting, 40 µl of the fresh suspension was collected on microscope slides by centrifugation at 60 × g for 4 min using a Cytofuge 2 (StatSpin, Bie and Berntsen, Rødovre, Denmark). Next, the cells were fixed on the slides in 96% ethanol and incubated with May-Grünwald-Giemsa stain. Two hundred cells were differentially counted. The number of neutrophils was recorded. The total number of cells in the suspension was measured with a NucleoCounter NC-100 (Chemometec, Allerød, Denmark) Live/Dead Assay.
Levels of DNA strand breaks
Levels of DNA strand breaks were assessed in BAL cells as tail length measured by comet assay using either the Andor Komet 6 assay as described (41) or the IMSTAR system as described (42) (see Table 2 for information on in which experiments these were used). In brief, for Andor Komet, the following procedure was done. BAL cells in freezing medium were thawed and immediately embedded in 0.6% agarose and loaded onto GelBond Film Slides (Lonza, Rockland, ME, USA). The slides were incubated overnight with 4°C cold lysing buffer (2.5 M NaCl, 10 mM Tris, 100 mM sodium-ethylenediaminetetraacetic acid (EDTA), 1% sodium sarcosinate, 10% DMSO, 1% Triton X-100, pH 10). The slides were then rinsed in cold electrophoresis buffer, alkaline treated with ice-cold electrophoresis buffer directly in the electrophoresis chamber (0.3 M NaOH, 1 mM sodium-EDTA, pH 13.2). Electrophoresis was conducted for 20 min on ice at 25 V (0.76 V/cm) and 192–196 mA (pH > 13). Then, the slides were rinsed in neutralisation buffer (0.4 M Tris, pH 7.5) and fixed for 1.5 h in 96% ethanol. Next, nuclei were stained with SYBR® Gold in TE buffer (10 mM Tris–HCl, 1 mM EDTA, pH 7.6). For IMSTAR, the same procedure was followed except, the agarose concentration was 0.7%, the slides were Trevigen Comet Slides (Trevigen, Gaithersburg, MD, USA), the electrophoresis was conducted at 38 V (1.2 V/cm) for 25–30 min and fixing was done for 5 min in 96% ethanol followed by 15 min at 45°C, nuclei were stained with SYBR® Green. Both tail length and percentage DNA in the tail were evaluated as measures of DNA strand breaks. Tail length was presented in graphs and used for the evaluation of results. Both end points were reported in tabulated form in Supplementary Material, available at Mutagenesis Online. Negative and positive controls were included in each run, namely A549 cells, exposed to 0 and 30 µM H2O2. To enable comparison between different data sets, comet data were normalised to the negative A549 controls included on each slide.
RNA isolation and quantitative PCR measurements
RNA was recovered from lung tissue by use of the NucleoSpin 96 RNA kit (Macherey-Nagel). The left lung was lysed in 2-ml RLT buffer, by homogenising for 2 × 60 s in a Tissuelyser (Qiagen, Denmark) containing a 5-mm stainless steel bead and run through a QIAshredder (Qiagen, USA). The remaining purification steps were done according to the manufacturer’s description. cDNA was next prepared using TaqMan reverse transcription reagents (Applied Biosystems, USA) according to the manufacturer’s description. Saa3 and Mcp-1 mRNA levels were determined using real-time quantitative PCR (qPCR) with 18S RNA as reference gene. Mcp-1 was determined as described (43). Saa3 was determined as described (15). qPCR was conducted with Universal Mastermix (Applied Biosystems, Nærum, Denmark). The qPCR was performed on an ABI PRISM® 7500 sequence detector (PE Biosystems, Foster City, CA, USA) as described previously (43). Data were normalised to 18S rRNA (prod. no. Mm03024053_m1 from Applied Biosystems) and multiplied with 107 to obtain readable numbers.
Statistics
Statistics were calculated using the software package Graph Pad Prism 7.02 (Graph Pad Software Inc., La Jolla, CA, USA). Data were tested for normality using the Shapiro–Wilk test. The t-test and the analysis of variance (ANOVA) test are robust against deviations in normality but somewhat sensitive to differences in standard deviation. The t-test and the ANOVA were not performed if the P-value of the Shapiro–Wilk test was very low (P < 0.001) or if the standard deviation was deemed very different using the F test (for two sample comparisons) or Brown–Forsythe test for three or more treatment groups (P < 0.001). In case of these described deviations in normality or in standard deviations, non-parametric Mann–Whitney test (two groups) or Kruskal–Wallis test (more than two groups) were applied. To assess differences in between groups in one-way ANOVA or Kruskal–Wallis test, multiple comparisons post tests were applied. These were Holm-Sidak’s multiple comparisons test (ANOVA) or Dunn’s multiple comparisons test (Kruskal–Wallis test).
Results
Effect of dispersion medium on agglomerate size
Six different dispersion media were tested. These included media that have previously been used for the dispersion of nanomaterials. These were water (11,12,15), 10% cell-free BAL in 0.9% NaCl (6), 2% serum (16,17,44), 0.1% Tween80 (45). In addition, we included two media that have not been used previously. These were 0.05% serum albumin in water and 10% cell-free BAL in water. The six different dispersion media yielded very different agglomeration sizes as measured by DLS (Table 1). The smallest CB particle agglomerates (measured as maximal number percentage) (18 nm) were observed in pure water, whereas agglomerates with mean sizes of 59 and 68 nm were observed in 2% mouse serum in water and 0.05% serum albumin in water, respectively. Ten percent BAL in 0.9% NaCl and 10% BAL in water both resulted in agglomerates in the micrometre range (1.2 and 4.3 µm, respectively). Z-average values of TiO2 particles are reported in Table 1.
Effect of dispersion medium on pulmonary inflammation
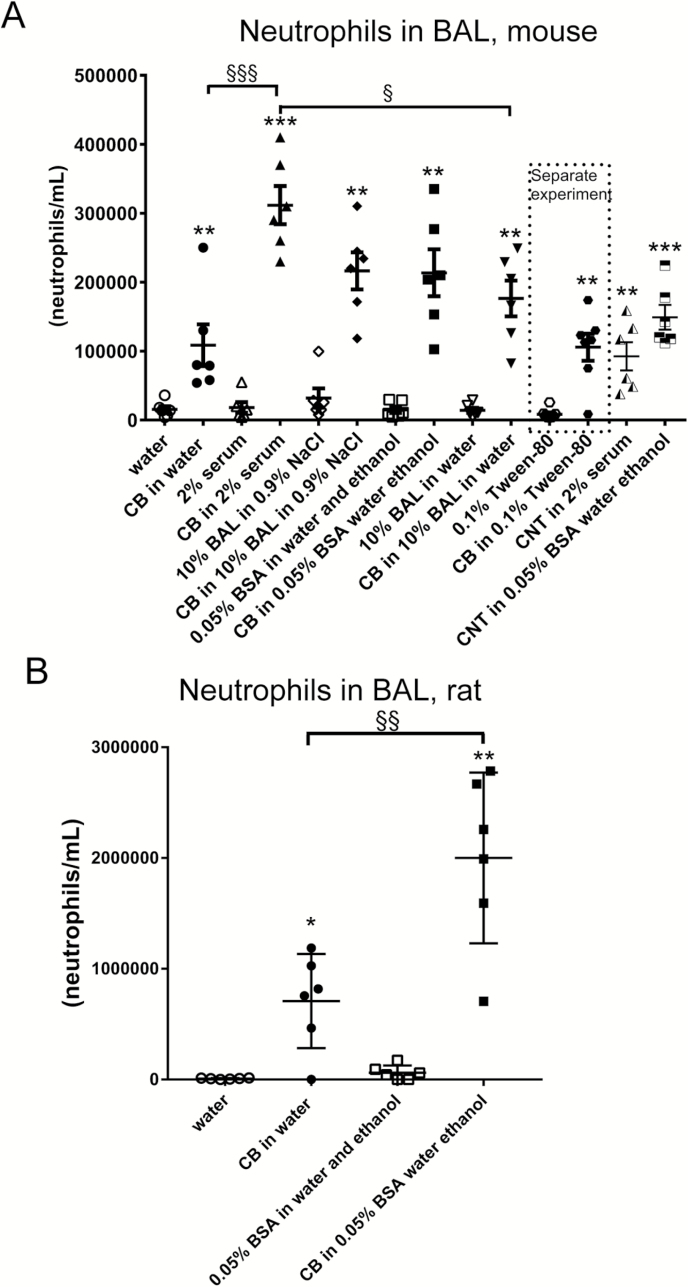
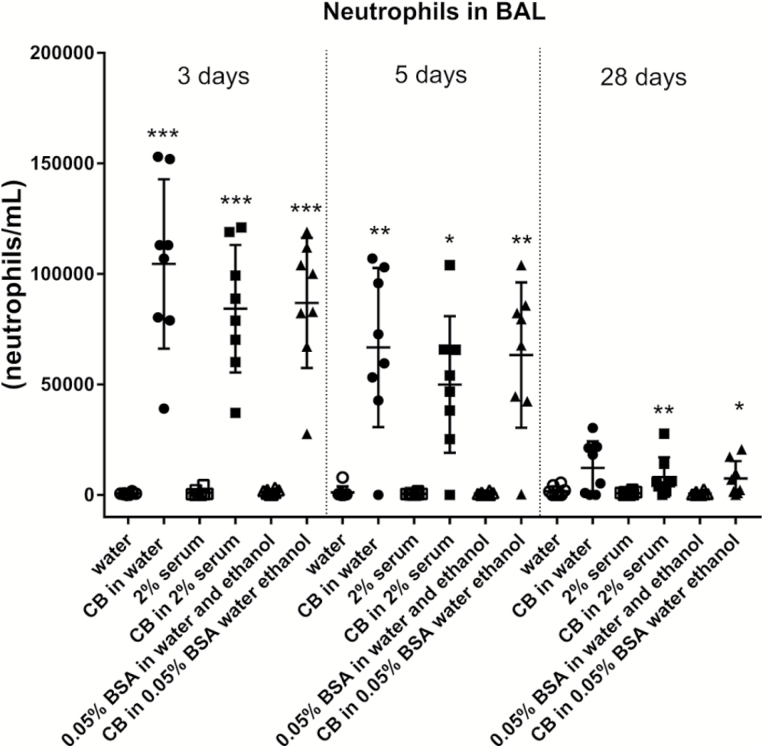
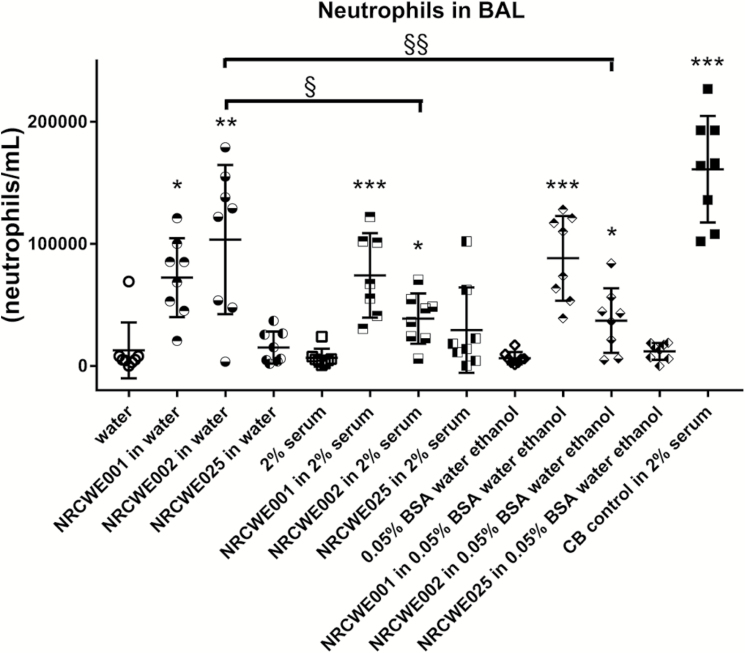
BAL neutrophil numbers were increased in mice exposed to CB regardless of dispersion medium (Figures 1–3). Higher neutrophil cell numbers were observed at 24 h with CB in 2% serum as compared to CB in water and as compared to CB in 10% BAL in water. At 3, 5 and 28 days, these inter-dispersion medium differences were not seen (Figure 2). The levels observed at Day 3 and onwards indicate a quick return from the additional and higher neutrophil influx observed with four of the dispersion media to the same level as observed with CB in water. In rats, CB in 0.05% BSA induced a higher response as compared to CB in water. For TiO2 in mice nanoparticles, increased BAL neutrophil numbers were observed for NRCWE-001 and NRCWE-002, but not for the larger TiO2 NP NRCWE-025 (Figure 3). NRCWE-002 in water gave a higher response as compared to this particle in 2% serum and as compared to this particle in 0.05% BSA (Figure 3). BAL neutrophil numbers were increased in mice exposed to CNT regardless of dispersion medium (Figure 1).
Fig. 1.
Effect of dispersion medium on neutrophils influx in BAL for CB and CNT 24 h after pulmonary administration to mice or rats. CB was administered intratracheally to mice at 162 µg/mouse (A) or at 1620 µg/rat (B). CNT was administered at 54 µg/mouse (A). Twenty-four hours later BAL fluid was prepared and the number of neutrophils established by differential counting. Data are mean and bars represent SD. ***, ** and * designate P-values of <0.001, <0.01 and <0.05, respectively, of t-test because data were approaching normality and were not having a highly different variation (details given in the Methods section). §§§, §§ and § designate P-values of <0.001, <0.01 and <0.05, respectively, of one-way ANOVA with Holm-Sidak’s multiple comparisons test because data were approaching normality and were not having a highly different variation (details given in the Methods section). The Tween-80 dispersion medium data are from an independent experiment and are thus not included in the multiple comparisons tests.
Fig. 2.
Effect of dispersion medium on neutrophil influx in BAL for CB 3, 5 and 28 days after pulmonary administration to mice. CB was administered intratracheally to mice at 162 µg/mouse. Three, five or twenty-eight days later BAL fluid was prepared and the number of neutrophils established by differential counting. Data are mean and bars represent SD. ***, ** and * designate P-values of <0.001, <0.01 and <0.05, respectively, of t-test in case of data approaching normality and not having a highly different variation (details given in the Methods section), otherwise of Mann–Whitney test.
Fig. 3.
Effect of dispersion medium on neutrophil influx in BAL for TiO2 and CB control 24 h after administration to mice. Three different TiO2 were administered intratracheally to mice at 67 µg/mouse. CB was administered at 162 µg/mouse. Twenty-four hours later BAL fluid was prepared and the number of neutrophils established by differential counting. Data are mean and bars represent SD. ***, ** and * designate P-values of <0.001, <0.01 and <0.05, respectively, of one-way ANOVA with Holm-Sidak’s multiple comparisons test of TiO2 particles against dispersion medium because data were approaching normality and were not having a highly different variation (details given in the Methods section).
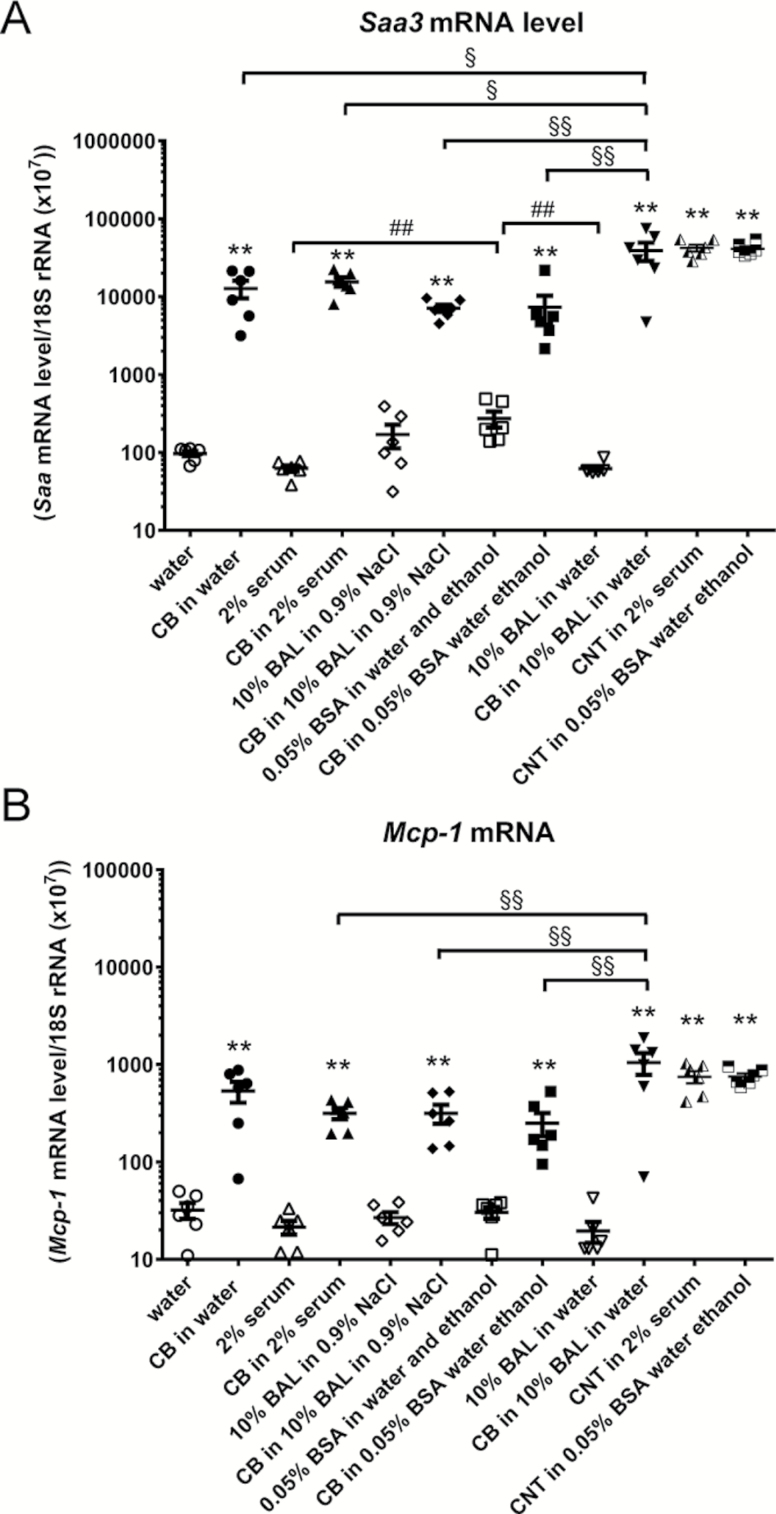
Pulmonary acute phase response and inflammation was further assessed for CB and CNTs by quantifying pulmonary Serum amyloid A-3 (Saa3) and Monocyte Chemoattractant Protein-1 (Mcp-1) mRNA levels. Pulmonary Saa3 mRNA levels were increased 24 h after instillation with CB in all five dispersion media. The Saa3 mRNA levels were higher with CB in 10% BAL in water as compared to all other dispersion media and the level was higher with 0.05% BSA as compared to 2% serum and to 10% BAL in water. The Saa3 mRNA level was also increased in both CNT groups (Figure 4). Mcp-1 mRNA levels were increased with CB in all dispersion media (Figure 4). The Mcp-1 mRNA levels were higher for CB in 10% BAL in water as compared to the other dispersion media, except CB in water. Mcp-1 mRNA levels were also increased in both CNT groups. A close correlation between Saa3 and Mcp-1 mRNA levels was observed.
Fig. 4.
Saa3 and Mcp-1 mRNA levels were increased in lung tissue 24 h following pulmonary exposure to CB and CNT in mice. CB was administered intratracheally to mice at 162 µg/mouse. CNT was administered at 54 µg/mouse. Twenty-four hours later the animals were euthanised and lung processed for Saa3 (A) and Mcp-1 (B) mRNA measurement by qPCR. Data are mean and bars represent SD. ***, ** and * designate P-values of <0.001, <0.01 and <0.05, respectively, of t-test in case of data approaching normality and not having a highly different variation (details given in the Methods section), otherwise by Mann–Whitney test. §§§, §§ and § designate P-values of <0.001, <0.01 and <0.05, respectively, of different CB groups against each other of one-way ANOVA with Holm-Sidak’s multiple comparisons test in case of data approaching normality and not having a highly different variation (details given in the Methods section), otherwise of Kruskal–Wallis test with Dunn’s multiple comparisons test. ###, ## and # designate P-values of <0.001, <0.01 and <0.05, respectively, of different dispersion medium groups against each other of one-way ANOVA with Holm-Sidak’s multiple comparisons test in case of data approaching normality and not having a highly different variation (details given in the Methods section), otherwise of Kruskal–Wallis test with Dunn’s multiple comparisons test. The Tween-80 dispersion medium data are from an independent experiment and are thus not included in the multiple comparisons tests.
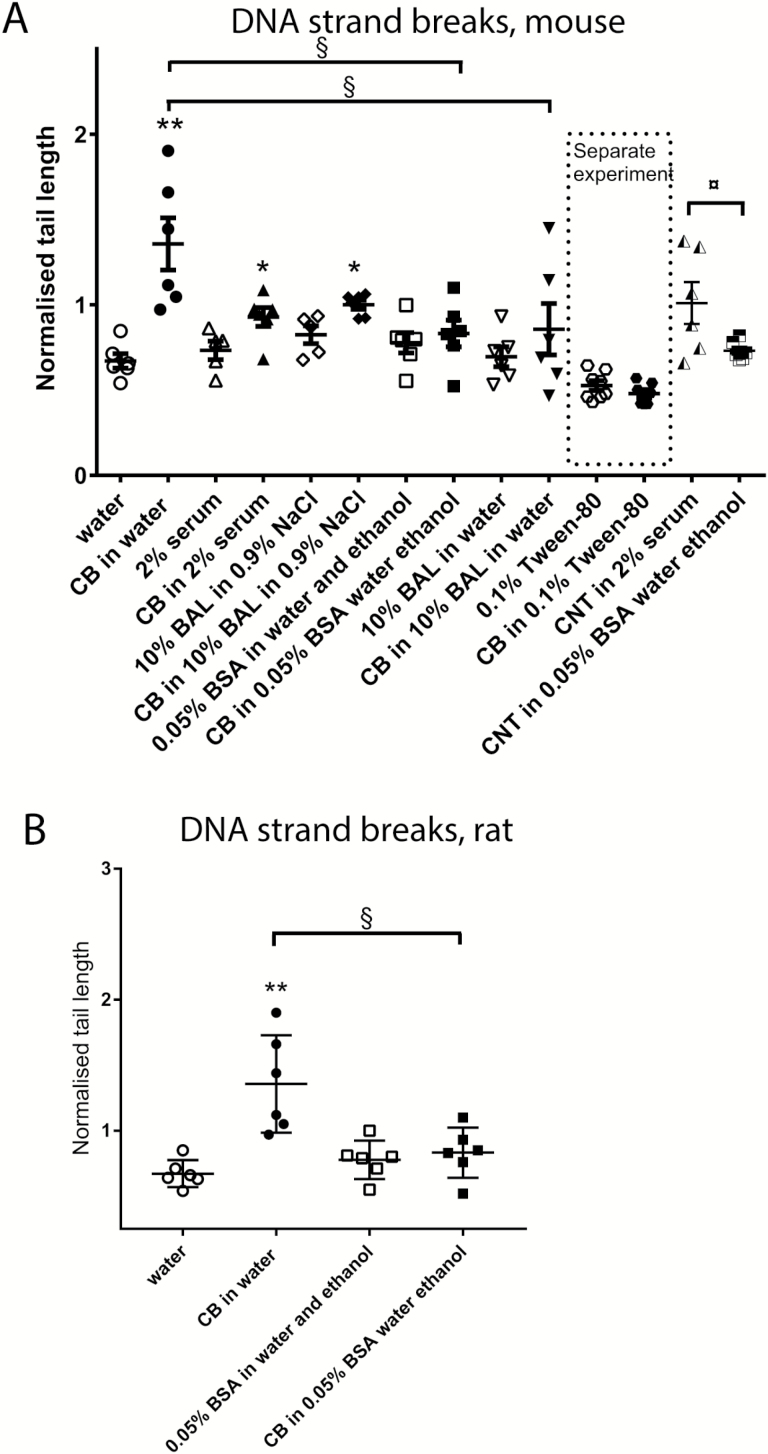
Effect of dispersion medium on DNA strand breaks in BAL cells
CB in water increased levels of DNA strand breaks both in mice and rats 24 h after exposure (Figure 5). CB in 2% serum increased levels of DNA strand breaks in mice in one experiment (Figure 5) but not in another (Figure 7). CB in 10% BAL in NaCl increased levels of DNA strand breaks in mice (Figures 5). There was no effect of CB in 0.05% BSA in water with ethanol pre-wetting in mice or rats (Figure 5) and no effect of CB in 10% BAL in water or of CB in 0.1% Tween-80 (Figure 5).
Fig. 5.
Levels of DNA strand breaks are increased in BAL cells by CB in some dispersion media 24 h after administration to mice or rats. CB was administered intratracheally to mice at 162 µg/mouse (A) or at 1620 µg/rat (B). CNT was administered at 54 µg/mouse (A). Twenty-four hours later BAL fluid was prepared, and tail length was measured by comet assay. Data are mean and bars represent SD. ***, ** and * designate P-values of <0.001, <0.01 and <0.05, respectively, of t-test in case of data approaching normality and not having a highly different variation (details given in the Methods section), otherwise of Mann–Whitney test. §§§, §§ and § designate P-values of <0.001, <0.01 and <0.05, respectively, of one-way ANOVA with Holm-Sidak’s multiple comparisons test in case of data approaching normality and not having a highly different variation (details given in the Methods section), otherwise of Kruskal–Wallis test with Dunn’s multiple comparisons test. The Tween-80 dispersion medium data are from an independent experiment and are thus not included in the multiple comparisons tests.
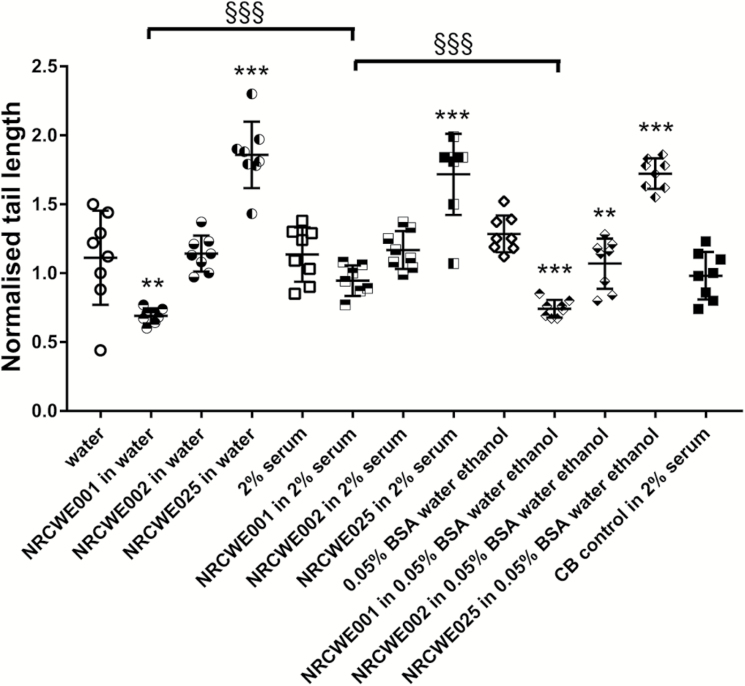
Fig. 7.
DNA strand break levels in BAL cells 24 h after pulmonary exposure to TiO2 and CB in mice. Three different TiO2 were administered intratracheally to mice at 67 µg/mouse. CB was administered at 162 µg/mouse. Twenty-four hours later BAL fluid was prepared, and tail length was measured by comet assay. Data are mean and bars represent SD. ***, ** and * designates P-values of <0.001, <0.01 and <0.05, respectively, of one-way ANOVA with Holm-Sidak’s multiple comparisons test of TiO2 particles against dispersion medium in case of data approaching normality and not having a highly different variation (details given in the Methods section), otherwise of Kruskal–Wallis test with Dunn’s multiple comparisons test. §§§, §§ and § designate P-values of <0.001, <0.01 and <0.05, respectively, of TiO2 data of each TiO2 particle type against each other of one-way ANOVA with Holm-Sidak’s multiple comparisons test in case of data approaching normality and not having a highly different variation (details given in the Methods section), otherwise of Kruskal–Wallis test with Dunn’s multiple comparisons test.
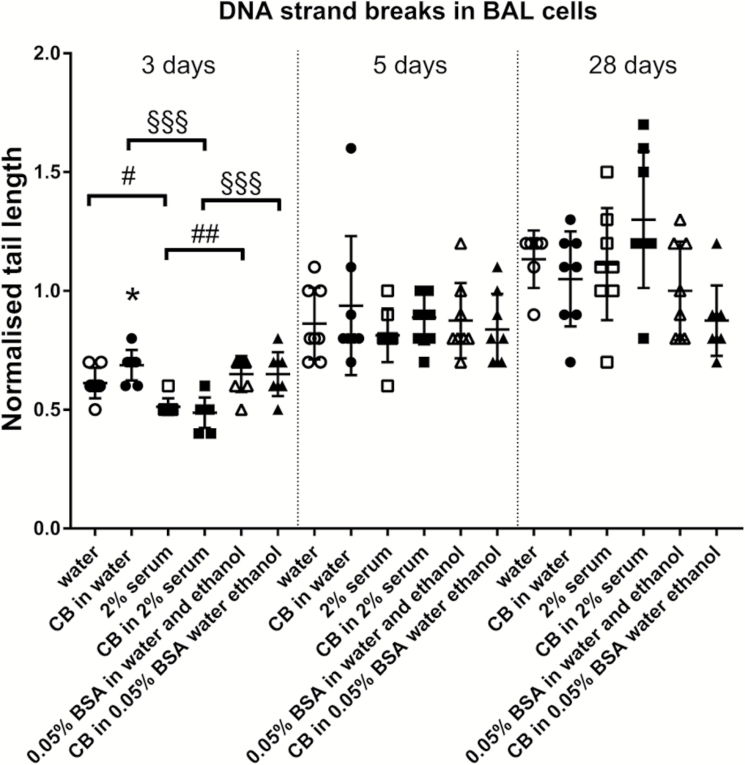
CB in water also increased levels of DNA strand breaks 3 days after exposure (Figure 6), whereas CB in other dispersion media had no effects. No effects were recorded at later time points. A dispersion medium effect was observed at Day 3 as both CB in different dispersion media and dispersion media alone displayed different levels of DNA strand breaks (Figure 6).
Fig. 6.
Only CB dispersed in water dispersion medium increases levels of DNA strand breaks in BAL 3 days after administration to mice. CB was administered intratracheally to mice at 162 µg/mouse. Three, five or twenty-eight days later BAL fluid was prepared, and tail length was measured by comet assay. Data are mean and bars represent SD. ***, ** and * designate P-values of <0.001, <0.01 and <0.05, respectively, of t-test in case of data approaching normality and not having a highly different variation (details given in the Methods section), otherwise of Mann–Whitney test. §§§, §§ and § designates P-values of <0.001, <0.01 and <0.05, respectively, of CB groups of one-way ANOVA with Holm-Sidak’s multiple comparisons test in case of data approaching normality and not having a highly different variation (details given in the Methods section), otherwise of Kruskal–Wallis test with Dunn’s multiple comparisons test. ###, ## and # designate P-values of <0.001, <0.01 and <0.05, respectively, of dispersion medium groups of one-way ANOVA with Holm-Sidak’s multiple comparisons test in case of data approaching normality and not having a highly different variation (details given in the Methods section), otherwise of Kruskal–Wallis test with Dunn’s multiple comparisons test.
No increased levels of DNA strand breaks were observed for CNTs at 24 h when compared to their respective dispersion medium controls. However, the level of DNA strand breaks was higher for CNT in 2% serum as compared to 0.05% BSA in water with ethanol pre-wetting (Figure 5). Regarding TiO2, NRCWE-001 administration was associated with a lower level of DNA strand breaks as compared controls. This was seen for water dispersion medium and for 0.05% BSA dispersion medium, but not for the 2% serum dispersion medium. Lower levels of DNA strand breaks were also observed for NRCWE-002, but only in the 0.05% BSA dispersion medium. NRCWE-025 increased levels of DNA strand breaks with all three dispersion media (Figure 7).
The above-described DNA strand break levels were assessed as tail length. Overall, a similar pattern was seen using % DNA in the tail (Supplementary Tables S1–S4, available at Mutagenesis Online).
Discussion
For the assessment of nanomaterial toxicity, it is often desirable to use alternative exposure methods to inhalation, but still observe effects that closely mimic those observed following inhalation exposure. Intratracheal instillation has been developed as a cost-effective method that delivers the desired amount of particles directly into the deeper part of the lungs (17,46). However, one drawback of this model is that the particles have to be stably dispersed in an appropriate medium before instillation to ensure uniform dosing of exposed animals. Correctly made, this ensures acceptable agglomerate size for alveolar deposition, but may affect the extent and nature of the toxicological response measured. The aim of this study was to test whether different intratracheal instillation dispersion media influence the pulmonary toxicity of CB, CNT and TiO2 nanomaterials. For this, we conducted intratracheal instillation studies using different dispersion media and compared the responses on inflammation and DNA strand breaks with those observed in previous experiments by inhalation.
CB
CB was included because we consistently have seen more inflammation following intratracheal instillation compared to inhalation. By inhalation, the nano-sized CB Printex 90 causes low levels of inflammation. In a previous study, mice were exposed by nose-only inhalation to aerosolised CB and diesel-exhaust particles (DEP) at 20 mg/m3 for 90 min for four consecutive days. DEP, but not CB particles, increased BAL neutrophil influx. Both particles induced increased levels of DNA strand breaks in BAL cells and thus CB-induced genotoxicity seemed to be independent of inflammation (19). In another study, CB Printex-90 was investigated in dams and offspring following the inhalation of 42 mg/m3 1 h daily during gestation days 8–18 or given by intratracheal instillation (in water dispersion medium) on gestation days 7, 10, 15 or 18. Increased BAL neutrophil numbers were observed after inhalation of an estimated deposited total dose of 287 µg/mouse and with a total instilled dose of 268 µg/mouse. However, instillation caused more neutrophil influx (control: 4 × 103 neutrophils vs. CB: 1.1 × 106) than inhalation (control: 1 × 103 neutrophils vs. CB: 1.2 × 104). CB, by inhalation, increased DNA strand breaks in liver. No increase was observed in this endpoint following intratracheal instillation (11). Furthermore, Jacobsen et al. compared CB inhalation to intratracheal instillation in wild-type mice and ApoE−/− mice. Three and twenty-four hours later, inflammation was assessed. Following 90-min inhalation at a concentration of 60 mg/m3, a small non-significant 5-fold increase in neutrophils was recorded. Following instillation, on the contrary, large increases in neutrophils were recorded with doses of 18 and 54 µg/mouse in ApoE−/− mice and also at 54 µg/mouse in C57 mice, 24 h after exposure. The instillation dispersion medium was 10% BAL fluid in 0.9% NaCl (3). Another study in rats only included CB inhalation exposure with mass concentrations of 1, 7 or 50 mg/m3 for 6, 5 or 13 weeks. BAL neutrophils were increased at the two highest doses. Moreover, Hprt-gene mutations were investigated at 13 weeks and found to be increased also at the two highest doses (47). Also, to support a potentially low inflammation of CB as compared to other nanomaterials is the observation that inhalation of CB Printex caused lower neutrophil influx than inhalation of the TiO2 NP UV-Titan L181 in the same experimental setup even though the estimated deposited dose was considerably higher for CB than for TiO2 (11,48).
The findings of genotoxicity are supported by other reports. Based on genotoxicity/mutagenicity/carcinogenicity observed in animal studies, CB has been classified as possibly carcinogenic to humans by IARC (21). Rats were exposed to CB at an average of 11.6 mg/m3 for 104 weeks, and increased formation of lung tumours was found (33). Mutagenicity caused by CB at a similar level to that observed by DEP has been shown in vitro (35,49). In addition, data on oxidative stress (50,51), reactive oxygen species production (6,52,53) and a detailed analysis of mutations caused by CB point to that a high reactive oxygen species production is the cause of the observed oxidatively damaged DNA and mutations (54).
Based on the data described above, intratracheal instillation of CB in a suitable dispersion medium should result in genotoxicity and limited inflammation. Although we know that as the dose rate is very high using intratracheal instillation compared to inhalation, it will not be possible to eliminate the inflammation completely.
Regarding inflammation in the current investigation, we found that CB in all dispersion media induced inflammation as seen by neutrophil influx. The inflammation induction was supported by mRNA level measurements of the cytokine MCP-1 and the acute phase protein Saa3. Two inflammatory markers Saa3 and MCP-1 were upregulated at the mRNA level by CB. However, assessing neutrophil influx 24 h after exposure, CB dispersed in 2% serum induced more inflammation as compared to CB in water and CB in 10% BAL in water. In rats, CB in 0.05% BSA caused higher inflammation than CB in water. This suggests that pure water and 10% BAL in water could be suitable dispersion media for dispersion of CB. Of note, there were no differences in-between the dispersion media at Day 3 and later suggesting that the effect of the medium was neutralised by Day 3 after exposure.
Regarding DNA strand breaks, CB in water increased DNA strand break levels in BAL cells in both mice and rats 24 h after exposure (Figure 5). Moreover, DNA strand breaks were also detected using this dispersion medium at 3 days after exposure (Figure 6). Increased levels of DNA strand breaks were also observed after 24 h with CB in 2% serum and with CB in 10% BAL in NaCl.
In the current investigation, taking all data from studies on CB together it seems that water is the dispersion medium for which the strongest and most consistent effect on genotoxicity is observed, and this is combined with a low level of inflammation.
Agglomeration size was not a predictor of toxicity. CB had the smallest agglomeration size when dispersed in water as compared to the other dispersion media. Nevertheless, small agglomerates were also observed in 2% serum (inducing significantly more inflammation) and 0.1% serum albumin (where CB did not induce DNA strand breaks), whereas CB forms large aggregates in 10% BAL in NaCl. This suggests that agglomeration size is not a determinant of inflammation or genotoxicity as such. Agglomeration size in air is an important determinant deposited dose (55).
Previous studies using water as a dispersion medium have demonstrated genotoxicity in both presence and absence of inflammation. Mice were exposed by intratracheal instillation to CB dispersed in water at 162 µg/mouse. Levels of DNA strand breaks in BAL cells were increased at 3 h and at 3 days, but not at 1 day. Neutrophils were increased at all time points 3 h, 1 day, 2 days, 3 days and later (56). Low doses of CB dispersed in water as dispersion medium have also shown increased DNA strand breaks in doses as low as 0.67 µg/mouse at 28 days and 2 µg/mouse at 3 and 28 days. No effect on DNA strand break levels was seen at 162 µg/mouse and all doses increased the neutrophil influx at 24 h (15).
Two dispersion media, 0.9% NaCl containing 10% BAL and 2% serum, displayed DNA strand breaks in the current investigation. However, genotoxicity was not seen in independent investigations with CB in 2% serum (16,17). This together with a high level of inflammation suggests that 2% serum is a less suitable dispersion medium for simulating inhalation of CB. For 0.9% NaCl containing 10% BAL, DNA damage has been demonstrated (5,10). Even though CB in this dispersion medium may be rather inflammogenic as neutrophil influx was increased at all doses (5,10), this dispersion medium may be an acceptable alternative to water for the simulation of CB inhalation.
TiO2
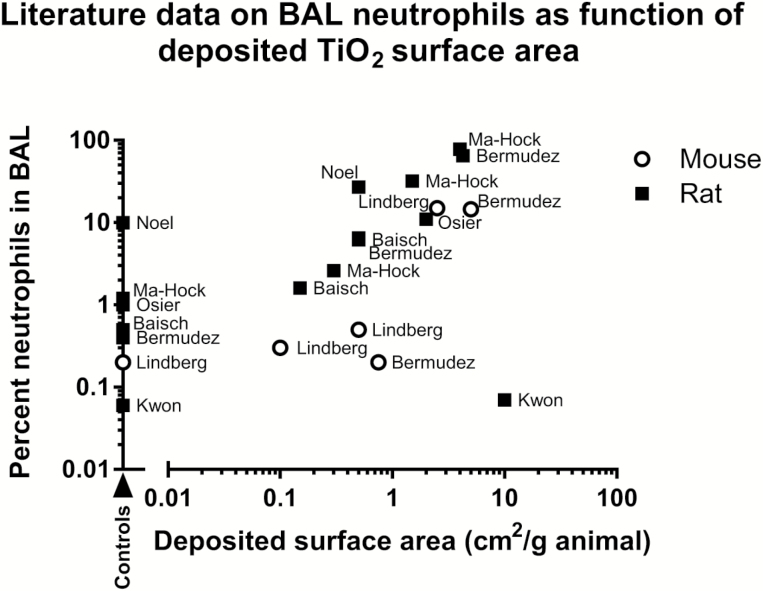
Inhaled TiO2 particles induce dose-dependent inflammation (7,29,31,57). Moreover, inflammation in terms of neutrophil influx depends on the deposited surface area (58,59). Literature data on neutrophil influx as function of surface area from a range of inhalation studies are depicted in Figure 8 and detailed in Table 3.
Fig. 8.
Data from the literature illustrating BAL neutrophil percentage as function of deposited TiO2 surface area in the lungs. Data from studies described in Table 3 are presented in graphical form. Percentage neutrophils in BAL were assessed either immediately after exposure or up to 24 h after exposure as detailed in Table 3. The name of the first author of the used reference is added as a label of each data point. Some data from refs (32,57) are not depicted because the percentage of neutrophils was zero, and only values higher than zero can be plotted on the logarithmic y scale.
Table 3.
Overview of TiO2 inhalation data in the literature
| Animal species | TiO2 size (nm) | Form | Doses | BET surface area (m2/g) | Deposited dose and calculated deposited surface area (m2/animal) | Inflammation (neutrophils increased in BAL fluid at end of exposure) | Genotoxicity/ carcinogenicity | Reference |
|---|---|---|---|---|---|---|---|---|
| Mouse | 10, but reported to be aggregated | Anatase | 271 mg/m3 for 1 h | 173 | Estimated deposited dose (by authors): 91 µg | No (assessed 24 h after exposure) | Yes in lung tissue | (32) |
| Estimated deposited surface area: 160 cm2 | ||||||||
| Mouse | Anatase crystallite size: 41 nm and brookite size 7 nm, but particles consisted of agglomerates of 10–60 nm crystallites with an average primary particle size of 21 nm | 74% anatase, 26% brookite | 5 days, 4 h/day at 0.8, 7.2 or 28.5 mg/ m3 | 61 | Measured retained TiO2 per animal 2.7, 18 and 84 µg | Yes at highest dose (assessed immediately after exposure) | No effect on DNA damage | (31) |
| Deposited surface areas: 2, 10 and 50 cm2 | ||||||||
| Rat | 5 nm, but when tested showed large agglomerates >100 nm, and small aggregates <100 nm | <10% rutile, >90% anatase | 6 h at 2 or 7 mg/m3 | 210 | Calculated lung burden (by authors) 14 and 51 µg | Yes at high dose but only with large aggregates (assessed 16 h after exposure) | Not measured | (30) |
| Estimated deposited surface areas: 30 and 100 cm2 | ||||||||
| Rat | Anatase phase particles were 52 nm and rutile phase particles were 61 nm, but in article measured and reported to be 79 nm | 5.5% rutile, 94.5 (not written directly in article) of anatase | 2 weeks, 6 h/day for 5 days/week at 11.4 mg/m3 | Not reported but estimated to be 20 based on TiO2 surface areas reported in other studies (07,29–33,57,67–69) | Estimated delivered dose (by authors): 10.3 mg/rat | No effect (assessed 24 h after exposure) | Not measured | (28) |
| Estimated deposited surface area: 2000 cm2 | ||||||||
| Rat | 25 ± 8 | 14% rutile, 86% anatase | 2, 10 or 50 mg/ m3 6 h/ day for 5 days | 51 | Measured lung burden TiO2 118, 545 and 1635 µg/lung (by current authors considered to be per 2 lungs) | Effect at two highest doses (assessed at the day of the last exposure) | Not measured | (29) |
| Deposited surface areas: 60, 300 and 800 cm2 | ||||||||
| Rat | Mass median aerodynamic diameter of 1.1 µm. The used particle, Bayertitan T, has a size of 1800 nm (70) | 99.5% rutile | 5 mg/m3 for 18 h per day, 5 days/week for up to 24 months | 1.9 | Reported retained mass 2.7 mg/lung | Not measured | Tumours were investigated, but no differences found | (34) |
| Deposited surface area 50 cm2 | ||||||||
| Rat | 15–40 nm, mass median aerodynamic diameter was ~1.5 µm | P25, CAS no. 13463- 67-7, 80% anatase and 20% rutile | 18 h/day, 5 days per week for up to 24 months (7.2 mg/m3 for first 4 months and then 14.8 mg/ m3 for 4 months, and 9.4 mg/m3 for 16 months) | 48 | Retained mass in rat at 24 months: 39 mg | Not measured | Lung tumours were increased | (33) |
| Deposited surface area in rat: 19000 cm2 | ||||||||
| Rat | 25 | Aeroxide P25 80% anatase, 20% rutile | 13 or 33 mg/ m3 for 4 h | 57 | Lung burden of 44 or 170 µg | Effect at highest dose (assessed 20 h after exposure) | Not measured | (07) |
| Estimated surface area deposited: | ||||||||
| Low dose: 25 cm2 | ||||||||
| High dose: 100 cm2 | ||||||||
| Rat and mouse | 21 | P-25 particles (ratio between rutile and anatase not reported) | 0.5, 2 or 10 mg/ m3 6 h/day, 5 days/ week for 13 weeks | Not provided, estimated to be 50 based on refs (07,29–33,57,67–69) | Deposited doses: | Yes, effect but only at highest dose (assessed immediately after exposure) | Not measured | (57) |
| High-dose rat: 11 mg/g dried lung = 1.7 mg/ lung (a female rat dried lung weight of 150 mg was used) | ||||||||
| Mid-dose rat: 1.4 (rat) = 0.2 mg/lung | ||||||||
| High-dose mouse: 11 mg/g dried lung = 0.2 mg/lung (a female mouse dried lung weight of 17 mg was used) | ||||||||
| Mid-dose mouse: 1.7 (mouse) mg/g dried lung weight = 0.03 mg/lung | ||||||||
| Estimated surface area in the lungs: | ||||||||
| Mid-dose rat: 100 cm2 | ||||||||
| High-dose rat: 850 cm2 | ||||||||
| Mid-dose mouse: 15 cm2 | ||||||||
| High-dose mouse: 100 cm2 | ||||||||
| Rat | 21 | 125 mg/m3 for 2 h | Not provided, estimated to be 50 based on refs (07,29–33,57,67–69) | Determined lung burden: 765 µg | Yes, effect | Not measured | (71) | |
| Estimated surface area dosed: 400 cm2 |
Regarding genotoxicity, published data are not consistent. Mice were exposed to 10-nm TiO2 through inhalation at a dose of 271 mg/m3 for 1 h with a deposited surface area 160 cm2. Increased levels of DNA strand breaks were observed (32). Rats were exposed to P25 TiO2 for 18 h/day, 5 days/week for 24 months at an average exposure of 10 mg/m3. Lung tumours were increased at the deposited surface area of 1.9 m2 (33). However, there are a number of studies in which no genotoxicity was found. Mice were exposed to TiO2 (74% anatase, 26% brookite for 5 days, 4 h/day) at 0.8, 7.2 or 28.5 mg/m3. Deposited surface areas were 2, 10 and 50 cm2. No genotoxicity was observed in epithelial lung cells nor were there any increases in polychromatic erythrocyte micronuclei (31). In another study, rats were exposed to 99.5% rutile TiO2 at 5 mg/m3 for 18 h/day, 5 days/week for up to 24 months (deposited surface area: 50 cm2) and no difference in tumour development was observed (34).
In the current study, using intratracheal instillation TiO2 in water, TiO2 in 2% serum in water and TiO2 in 0.05% BSA with pre-wetting in ethanol induced similar inflammation, except the 38-nm TiO2, perhaps because of a low deposited surface area at the used dose (67 µg/mouse). We found the 38-nm unmodified rutile particle to increase levels of DNA strand breaks with all three dispersion media. Thus, increased levels of DNA strand breaks were observed for NRCWE-025 as has also been observed for CB (15).
In another study using two of the same TiO2 particles, NRCWE-001 and NRCWE-002, the dispersion medium used was water. The doses were 18, 54 and 162 µg/mouse. Neutrophils in BAL were increased at the two highest doses for both particles. DNA strand breaks were decreased with NRCWE-002 at the all three doses at 1 day, but increased at high dose at Day 3 (60). In addition, there are range of studies with TiO2 in different forms and sizes investigated. The ones utilising the same dispersion media as those in the current investigation are summarised in Table 4. In general, TiO2 induce pulmonary inflammation. DNA damage was found in one study (6).
Table 4.
Overview of TiO2 intratracheal instillation data from studies in the literature using some of the dispersion media also investigated in the current study
| Animal species | TiO2 size (nm) | Form | Coating | Dispersion medium | Doses | Inflammation (BAL neutrophil increase) at 24 h | DNA damage in BAL cells | Reference |
|---|---|---|---|---|---|---|---|---|
| Mouse | 10 | Rutile (same as NRCWE-001) | None | Water | 18, 54 and 162 µg/ mouse | Yes, at two highest doses | BAL cells: no effect (increasing at two lowest doses at 3 days only tail length) | (60) |
| Mouse | 10 | Rutile (same as NRCWE-002) | Positively charged (amino-TiO2) | Water | 18, 54 and 162 µg/ mouse | Yes, at two highest doses | BAL cells: decreased tail length 24 h but increased at highest dose at 3 days (tail length and tail percentage) | (60) |
| Mouse | 288 | Rutile | Al and polyalcohol | 10% BAL in NaCl | 54 µg/mouse | No | BAL cells: yes | (06) |
| Mouse | 20.6 | Rutile | Si, Al, Zr, polyalcohol | 10% BAL in NaCl | 54 µg/mouse | Yes | BAL cells: yes | (06) |
| Mouse | 19 | 7.8% rutile/92% anatase | None | 10% BAL in NaCl | 54 µg/mouse | Yes | BAL cells: no | (06) |
| Mouse | 10 | Rutile (same as NRCWE-002) | Positively charged (amino-TiO2) | Water | 8, 32, 64 or 128 µg/ mouse | Yes, at highest dose | Not measured | (09) |
| Mouse | 10 | Rutile (same as NRCWE-002) | Positively charged (amino-TiO2) | 2% serum | 8, 32, 64 or 128 µg/ mouse | Yes, at two highest doses | Not measured | (09) |
| Rat | 15 | ‘Clear crystalline form’ | Not reported | Water | 0.2 (0.66 mg/kg bw) or 1 mg (3.3 mg/kg bw) | Yes, at the highest dose | Not measured, but no lung tumours at 24 months | (72) |
| Rat | 15 | Anatase | None | Water | 1 mg/rat | No, but only tested at 3 days | Not measured | (73) |
| Rat | 14 | Rutile | None | Water | 1 mg/rat | No, but only tested at 3 days | Not measured | (73) |
| Rat | 14 | Amorphous (anatase crystallite size 6.1) | Not reported | Water | 1 mg/rat | Yes, but only tested at 3 days | Not measured | (73) |
| Rat | 28 | P25 (anatase crystallite size 23.5 and rutile crystallite size 35.9 nm) | Not reported | Water | 1 mg/rat | Yes but only tested at 3 days | Not measured | (73) |
Overall, we found no consistent dispersion medium-dependent effects on TiO2-induced inflammation or genotoxicity.
CNT
Inhaled CNT are highly inflammogenic. In a 13-week subchronic inhalation study in rats (6 h/day, 5 days/week), 0.4, 1.5 and 6 mg/m3 (10 nm in diameter 200–300 nm in length) multiwalled CNT increased BAL neutrophils, whereas 0.1 mg/m3 had no effect (23). DNA damage was seen in two inhalation studies (26,27). A 104-week carcinogenic study was conducted with multiwalled CNT in rats using 0.02, 0.2 and 2 mg/m3. Carcinogenicity was found at the two highest doses (61). Sargent et al. found multiwalled CNT to promote lung adenocarcinoma by inhalation in mice (62). In contrast, multiwalled CNTs in a 90-day inhalation study in rats did not induce genotoxicity in spite of the induction of inflammation (63). These data suggest that a dispersion medium for CNT intratracheal instillation should allow for both inflammation and DNA damage.
The dispersion of CNTs is highly challenging since unmodified CNTs are highly hydrophobic and cannot be stably dispersed in water or ionic buffers. Dispersion of CNTs in water will often result in uneven and unstable dispersion (64), something that could result in little control of the delivered dose. Therefore, CNTs were only studied in dispersion media, which contained proteins to assist dispersion. CNT was found to be equally inflammogenic in the two tested dispersion media, 2% serum and 0.05% serum albumin. The studied CNT, NM-400, did not induce DNA strand breaks as compared to dispersion medium controls. We have previously shown that multiwalled CNT-induced DNA strand breaks depends on the diameter of the multiwalled CNT such that thicker multiwalled CNTs induce more DNA damage than thin multiwalled CNTs (17). NM-400 is a relatively thin MWCNT and thus not expected to induce DNA damage. Thus, both studied dispersion media were acceptable for single instillation studies. However, it is noted that the use of protein as dispersion agent constitutes a problem in studies using repeated exposures as the repeated exposure to protein-containing dispersion medium can induce an allergic inflammatory reaction (65).
Conclusion
Water seems to be the best dispersion medium for observing a genotoxic effect of CB to mimic the genotoxic effects observed after inhalation exposure. Ten percent BAL in NaCl could be suggested as an alternative dispersion medium for CB. No dispersion-dependent effects were observed for TiO2. For CNTs both investigated dispersion media, CNT in 2% serum or CNT in 0.05% BSA with ethanol pre-wetting were similar. Our results suggest that it is important to consider the influence of dispersion medium when using intratracheal instillation as a model of inhalation exposure.
Supplementary data
Supplementary data are available at Mutagenesis Online.
Funding
This work was supported by the Danish Centre for Nanosafety II; the European Union project: NANOGENOTOX (grant number n°2009 21 01) and the European Union Seventh Framework Programme (FP7/2007–2013) GLADIATOR (grant number FP7-604000).
Acknowledgements
Technical assistance from Michael Guldbrandsen, Lisbeth Meyer Petersen, Lourdes Pedersen, Anne-Karin Asp, Eva Terrida, Natacha Synnøve Olsen, Elzbieta Christiansen was greatly appreciated.
Conflict of interest statement: None declared.
References
- 1. Warheit D. B., Reed K. L. and Webb T. R (2003) Pulmonary toxicity studies in rats with triethoxyoctylsilane (OTES)-coated, pigment-grade titanium dioxide particles: bridging studies to predict inhalation hazard. Exp. Lung Res., 29, 593–606. [DOI] [PubMed] [Google Scholar]
- 2. Søs Poulsen S., Jacobsen N. R., Labib S. et al. (2013) Transcriptomic analysis reveals novel mechanistic insight into murine biological responses to multi-walled carbon nanotubes in lungs and cultured lung epithelial cells. PLoS One, 8, e80452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jacobsen N. R., Møller P., Jensen K. A., Vogel U., Ladefoged O., Loft S. and Wallin H (2009) Lung inflammation and genotoxicity following pulmonary exposure to nanoparticles in ApoE-/- mice. Part. Fibre Toxicol., 6, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saber A. T., Koponen I. K., Jensen K. A., Jacobsen N. R., Mikkelsen L., Møller P., Loft S., Vogel U. and Wallin H (2012) Inflammatory and genotoxic effects of sanding dust generated from nanoparticle-containing paints and lacquers. Nanotoxicology, 6, 776–788. [DOI] [PubMed] [Google Scholar]
- 5. Saber A. T., Jacobsen N. R., Mortensen A. et al. (2012) Nanotitanium dioxide toxicity in mouse lung is reduced in sanding dust from paint. Part. Fibre Toxicol., 9, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saber A. T., Jensen K. A., Jacobsen N. R., Birkedal R., Mikkelsen L., Møller P., Loft S., Wallin H. and Vogel U (2012) Inflammatory and genotoxic effects of nanoparticles designed for inclusion in paints and lacquers. Nanotoxicology, 6, 453–471. [DOI] [PubMed] [Google Scholar]
- 7. Baisch B. L., Corson N. M., Wade-Mercer P., Gelein R., Kennell A. J., Oberdörster G. and Elder A (2014) Equivalent titanium dioxide nanoparticle deposition by intratracheal instillation and whole body inhalation: the effect of dose rate on acute respiratory tract inflammation. Part. Fibre Toxicol., 11, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Casanova A., Carriere M. and Herlin-Boime N (2011) Dispersion of aeroxil P25 TiO2 nanoparticle in media of biological interest for the culture of eukaryotic cells. J. Biomed. Nanotechnol., 7, 24–25. [DOI] [PubMed] [Google Scholar]
- 9. Vranic S., Gosens I., Jacobsen N. R. et al. (2017) Impact of serum as a dispersion agent for in vitro and in vivo toxicological assessments of TiO2 nanoparticles. Arch. Toxicol., 91, 353–363. [DOI] [PubMed] [Google Scholar]
- 10. Bourdon J. A., Saber A. T., Jacobsen N. R. et al. (2012) Carbon black nanoparticle instillation induces sustained inflammation and genotoxicity in mouse lung and liver. Part. Fibre Toxicol., 9, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jackson P., Hougaard K. S., Boisen A. M. et al. (2012) Pulmonary exposure to carbon black by inhalation or instillation in pregnant mice: effects on liver DNA strand breaks in dams and offspring. Nanotoxicology, 6, 486–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kyjovska Z. O., Jacobsen N. R., Saber A. T., Bengtson S., Jackson P., Wallin H. and Vogel U (2015) DNA strand breaks, acute phase response and inflammation following pulmonary exposure by instillation to the diesel exhaust particle NIST1650b in mice. Mutagenesis, 30, 499–507. [DOI] [PubMed] [Google Scholar]
- 13. Donaldson K., Poland C. A. and Schins R. P (2010) Possible genotoxic mechanisms of nanoparticles: criteria for improved test strategies. Nanotoxicology, 4, 414–420. [DOI] [PubMed] [Google Scholar]
- 14. Jacobsen N. R., Pojana G., White P. et al. (2008) Genotoxicity, cytotoxicity, and reactive oxygen species induced by single-walled carbon nanotubes and C(60) fullerenes in the FE1-Mutatrade markMouse lung epithelial cells. Environ. Mol. Mutagen., 49, 476–487. [DOI] [PubMed] [Google Scholar]
- 15. Kyjovska Z. O., Jacobsen N. R., Saber A. T., Bengtson S., Jackson P., Wallin H. and Vogel U (2015) DNA damage following pulmonary exposure by instillation to low doses of carbon black (Printex 90) nanoparticles in mice. Environ. Mol. Mutagen., 56, 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saber A. T., Mortensen A., Szarek J. et al. (2016) Epoxy composite dusts with and without carbon nanotubes cause similar pulmonary responses, but differences in liver histology in mice following pulmonary deposition. Part. Fibre Toxicol., 13, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Poulsen S. S., Jackson P., Kling K. et al. (2016) Multi-walled carbon nanotube physicochemical properties predict pulmonary inflammation and genotoxicity. Nanotoxicology, 10, 1263–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jacobsen N. R., Stoeger T., van den Brule S. et al. (2015) Acute and subacute pulmonary toxicity and mortality in mice after intratracheal instillation of ZnO nanoparticles in three laboratories. Food Chem. Toxicol., 85, 84–95. [DOI] [PubMed] [Google Scholar]
- 19. Saber A. T., Bornholdt J., Dybdahl M., Sharma A. K., Loft S., Vogel U. and Wallin H (2005) Tumor necrosis factor is not required for particle-induced genotoxicity and pulmonary inflammation. Arch. Toxicol., 79, 177–182. [DOI] [PubMed] [Google Scholar]
- 20. Danielsen P. H., Loft S., Jacobsen N. R., Jensen K. A., Autrup H., Ravanat J. L., Wallin H. and Møller P (2010) Oxidative stress, inflammation, and DNA damage in rats after intratracheal instillation or oral exposure to ambient air and wood smoke particulate matter. Toxicol. Sci., 118, 574–585. [DOI] [PubMed] [Google Scholar]
- 21. WHO. (2010) Carbon black, titanium dioxide, and talc. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 93 IARC, Lyon, France. [PMC free article] [PubMed] [Google Scholar]
- 22. Ellinger-Ziegelbauer H. and Pauluhn J (2009) Pulmonary toxicity of multi-walled carbon nanotubes (Baytubes) relative to alpha-quartz following a single 6h inhalation exposure of rats and a 3 month post-exposure period. Toxicology, 266, 16–29. [DOI] [PubMed] [Google Scholar]
- 23. Pauluhn J. (2010) Subchronic 13-week inhalation exposure of rats to multiwalled carbon nanotubes: toxic effects are determined by density of agglomerate structures, not fibrillar structures. Toxicol. Sci., 113, 226–242. [DOI] [PubMed] [Google Scholar]
- 24. Shvedova A. A., Kisin E., Murray A. R. et al. (2008) Inhalation vs. aspiration of single-walled carbon nanotubes in C57BL/6 mice: inflammation, fibrosis, oxidative stress, and mutagenesis. Am. J. Physiol. Lung Cell. Mol. Physiol., 295, L552–L565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kinaret P., Ilves M., Fortino V. et al. (2017) Inhalation and oropharyngeal aspiration exposure to rod-like carbon nanotubes induce similar airway inflammation and biological responses in mouse lungs. ACS Nano, 11, 291–303. [DOI] [PubMed] [Google Scholar]
- 26. Kim J. S., Sung J. H., Song K. S. et al. (2012) Persistent DNA damage measured by comet assay of Sprague Dawley rat lung cells after five days of inhalation exposure and 1 month post-exposure to dispersed multi-wall carbon nanotubes (MWCNTs) generated by new MWCNT aerosol generation system. Toxicol. Sci., 128, 439–448. [DOI] [PubMed] [Google Scholar]
- 27. Catalán J., Siivola K. M., Nymark P. et al. (2016) In vitro and in vivo genotoxic effects of straight versus tangled multi-walled carbon nanotubes. Nanotoxicology, 10, 794–806. [DOI] [PubMed] [Google Scholar]
- 28. Kwon S., Yang Y. S., Yang H. S., Lee J., Kang M. S., Lee B. S., Lee K. and Song C. W (2012) Nasal and pulmonary toxicity of titanium dioxide nanoparticles in rats. Toxicol. Res., 28, 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ma-Hock L., Burkhardt S., Strauss V., Gamer A. O., Wiench K., van Ravenzwaay B. and Landsiedel R (2009) Development of a short-term inhalation test in the rat using nano-titanium dioxide as a model substance. Inhal. Toxicol., 21, 102–118. [DOI] [PubMed] [Google Scholar]
- 30. Noël A., Maghni K., Cloutier Y., Dion C., Wilkinson K. J., Hallé S., Tardif R. and Truchon G (2012) Effects of inhaled nano-TiO2 aerosols showing two distinct agglomeration states on rat lungs. Toxicol. Lett., 214, 109–119. [DOI] [PubMed] [Google Scholar]
- 31. Lindberg H. K., Falck G. C., Catalán J. et al. (2012) Genotoxicity of inhaled nanosized TiO2 in mice. Mutat. Res., 745, 58–64. [DOI] [PubMed] [Google Scholar]
- 32. Larsen S. T., Jackson P., Poulsen S. S., Levin M., Jensen K. A., Wallin H., Nielsen G. D. and Koponen I. K (2016) Airway irritation, inflammation, and toxicity in mice following inhalation of metal oxide nanoparticles. Nanotoxicology, 10, 1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Heinrich U., Fuhst R., Rittinghausen S., Creutzenberg O., Bellmann B., Koch W. and Levsen K (1995) Chronic inhalation exposure of Wistar rats and two different strains of mice to diesel engine exhaust, carbon black, and titanium dioxide. Inhal. Toxicol., 7, 533–556. [Google Scholar]
- 34. Muhle H., Kittel B., Ernst H., Mohr U. and Mermelstein R (1995) Neoplastic lung lesions in rat after chronic exposure to crystalline silica. Scand. J. Work. Environ. Health, 21 (Suppl. 2), 27–29. [PubMed] [Google Scholar]
- 35. Jacobsen N. R., Saber A. T., White P. et al. (2007) Increased mutant frequency by carbon black, but not quartz, in the lacZ and cII transgenes of muta mouse lung epithelial cells. Environ. Mol. Mutagen., 48, 451–461. [DOI] [PubMed] [Google Scholar]
- 36. Jackson P., Kling K., Jensen K. A., Clausen P. A., Madsen A. M., Wallin H. and Vogel U (2015) Characterization of genotoxic response to 15 multiwalled carbon nanotubes with variable physicochemical properties including surface functionalizations in the FE1-Muta™ mouse lung epithelial cell line. Environ. Mol. Mutagen., 56, 183–203. [DOI] [PubMed] [Google Scholar]
- 37. Poulsen S. S., Saber A. T., Williams A. et al. (2015) MWCNTs of different physicochemical properties cause similar inflammatory responses, but differences in transcriptional and histological markers of fibrosis in mouse lungs. Toxicol. Appl. Pharmacol., 284, 16–32. [DOI] [PubMed] [Google Scholar]
- 38. Kermanizadeh A., Pojana G., Gaiser B. K. et al. (2013) In vitro assessment of engineered nanomaterials using a hepatocyte cell line: cytotoxicity, pro-inflammatory cytokines and functional markers. Nanotoxicology, 7, 301–313. [DOI] [PubMed] [Google Scholar]
- 39. Gomez V., Levin M., Saber A. T. et al. (2014) Comparison of dust release from epoxy and paint nanocomposites and conventional products during sanding and sawing. Ann. Occup. Hyg., 58, 983–994. [DOI] [PubMed] [Google Scholar]
- 40. Halappanavar S., Saber A. T., Decan N. et al. (2015) Transcriptional profiling identifies physicochemical properties of nanomaterials that are determinants of the in vivo pulmonary response. Environ. Mol. Mutagen., 56, 245–264. [DOI] [PubMed] [Google Scholar]
- 41. Dybdahl M., Risom L., Bornholdt J., Autrup H., Loft S. and Wallin H (2004) Inflammatory and genotoxic effects of diesel particles in vitro and in vivo. Mutat. Res., 562, 119–131. [DOI] [PubMed] [Google Scholar]
- 42. Jackson P., Pedersen L. M., Kyjovska Z. O., Jacobsen N. R., Saber A. T., Hougaard K. S., Vogel U. and Wallin H (2013) Validation of freezing tissues and cells for analysis of DNA strand break levels by comet assay. Mutagenesis, 28, 699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saber A. T., Jacobsen N. R., Bornholdt J., Kjaer S. L., Dybdahl M., Risom L., Loft S., Vogel U. and Wallin H (2006) Cytokine expression in mice exposed to diesel exhaust particles by inhalation. Role of tumor necrosis factor. Part. Fibre Toxicol., 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Poulsen S. S., Saber A. T., Mortensen A. et al. (2015) Changes in cholesterol homeostasis and acute phase response link pulmonary exposure to multi-walled carbon nanotubes to risk of cardiovascular disease. Toxicol. Appl. Pharmacol., 283, 210–222. [DOI] [PubMed] [Google Scholar]
- 45. Bengtson S., Knudsen K. B., Kyjovska Z. O. et al. (2017) Differences in inflammation and acute phase response but similar genotoxicity in mice following pulmonary exposure to graphene oxide and reduced graphene oxide. PLoS One, 12, e0178355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mikkelsen L., Sheykhzade M., Jensen K. A., Saber A. T., Jacobsen N. R., Vogel U., Wallin H., Loft S. and Møller P (2011) Modest effect on plaque progression and vasodilatory function in atherosclerosis-prone mice exposed to nanosized TiO2. Part. Fibre Toxicol., 8, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Driscoll K. E., Carter J. M., Howard B. W., Hassenbein D. G., Pepelko W., Baggs R. B. and Oberdörster G (1996) Pulmonary inflammatory, chemokine, and mutagenic responses in rats after subchronic inhalation of carbon black. Toxicol. Appl. Pharmacol., 136, 372–380. [DOI] [PubMed] [Google Scholar]
- 48. Hougaard K. S., Jackson P., Jensen K. A. et al. (2010) Effects of prenatal exposure to surface-coated nanosized titanium dioxide (UV-Titan). A study in mice. Part. Fibre Toxicol., 7, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jacobsen N. R., Møller P., Cohn C. A., Loft S., Vogel U. and Wallin H (2008) Diesel exhaust particles are mutagenic in FE1-MutaMouse lung epithelial cells. Mutat. Res., 641, 54–57. [DOI] [PubMed] [Google Scholar]
- 50. Stone V., Shaw J., Brown D. M., Macnee W., Faux S. P. and Donaldson K (1998) The role of oxidative stress in the prolonged inhibitory effect of ultrafine carbon black on epithelial cell function. Toxicol. In Vitro, 12, 649–659. [DOI] [PubMed] [Google Scholar]
- 51. Møller P., Jensen D. M., Christophersen D. V. et al. (2015) Measurement of oxidative damage to DNA in nanomaterial exposed cells and animals. Environ. Mol. Mutagen., 56, 97–110. [DOI] [PubMed] [Google Scholar]
- 52. Sharma A. K., Jensen K. A., Rank J. et al. (2007) Genotoxicity, inflammation and physico-chemical properties of fine particle samples from an incineration energy plant and urban air. Mutat. Res., 633, 95–111. [DOI] [PubMed] [Google Scholar]
- 53. Høgsberg T., Jacobsen N. R., Clausen P. A. and Serup J (2013) Black tattoo inks induce reactive oxygen species production correlating with aggregation of pigment nanoparticles and product brand but not with the polycyclic aromatic hydrocarbon content. Exp. Dermatol., 22, 464–469. [DOI] [PubMed] [Google Scholar]
- 54. Jacobsen N. R., White P. A., Gingerich J., Møller P., Saber A. T., Douglas G. R., Vogel U. and Wallin H (2011) Mutation spectrum in FE1-MUTA™ Mouse lung epithelial cells exposed to nanoparticulate carbon black. Environ. Mol. Mutagen., 52, 331–337. [DOI] [PubMed] [Google Scholar]
- 55. Oberdorster G. (1996) Significance of particle parameters in the evaluation of exposure-dose-response relationships of inhaled particles. Inhal. Toxicol., 8 (Suppl), 73–89. [PubMed] [Google Scholar]
- 56. Husain M., Kyjovska Z. O., Bourdon-Lacombe J. et al. (2015) Carbon black nanoparticles induce biphasic gene expression changes associated with inflammatory responses in the lungs of C57BL/6 mice following a single intratracheal instillation. Toxicol. Appl. Pharmacol., 289, 573–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bermudez E., Mangum J. B., Wong B. A., Asgharian B., Hext P. M., Warheit D. B. and Everitt J. I (2004) Pulmonary responses of mice, rats, and hamsters to subchronic inhalation of ultrafine titanium dioxide particles. Toxicol. Sci., 77, 347–357. [DOI] [PubMed] [Google Scholar]
- 58. Oberdörster G., Oberdörster E. and Oberdörster J (2005) Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect., 113, 823–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stoeger T., Schmid O., Takenaka S. and Schulz H (2007) Inflammatory response to TiO2 and carbonaceous particles scales best with BET surface area. Environ. Health Perspect., 115, A290–A291; author reply A291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wallin H., Kyjovska Z. O., Poulsen S. S., Jacobsen N. R., Saber A. T., Bengtson S., Jackson P. and Vogel U (2017) Surface modification does not influence the genotoxic and inflammatory effects of TiO2 nanoparticles after pulmonary exposure by instillation in mice. Mutagenesis, 32, 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kasai T., Umeda Y., Ohnishi M., Mine T., Kondo H., Takeuchi T., Matsumoto M. and Fukushima S (2016) Lung carcinogenicity of inhaled multi-walled carbon nanotube in rats. Part. Fibre Toxicol., 13, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sargent L. M., Porter D. W., Staska L. M. et al. (2014) Promotion of lung adenocarcinoma following inhalation exposure to multi-walled carbon nanotubes. Part. Fibre Toxicol., 11, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pothmann D., Simar S., Schuler D. et al. (2015) Lung inflammation and lack of genotoxicity in the comet and micronucleus assays of industrial multiwalled carbon nanotubes Graphistrength(©) C100 after a 90-day nose-only inhalation exposure of rats. Part. Fibre Toxicol., 12, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Grunlan J. C., Liu L. and Kim Y. S (2006) Tunable single-walled carbon nanotube microstructure in the liquid and solid states using poly(acrylic acid). Nano Lett., 6, 911–915. [DOI] [PubMed] [Google Scholar]
- 65. Christophersen D. V., Jacobsen N. R., Andersen M. H. et al. (2016) Cardiovascular health effects of oral and pulmonary exposure to multi-walled carbon nanotubes in ApoE-deficient mice. Toxicology, 371, 29–40. [DOI] [PubMed] [Google Scholar]
- 66. Cao Y., Jacobsen N. R., Danielsen P. H. et al. (2014) Vascular effects of multiwalled carbon nanotubes in dyslipidemic ApoE-/- mice and cultured endothelial cells. Toxicol. Sci., 138, 104–116. [DOI] [PubMed] [Google Scholar]
- 67. Rossi E. M., Pylkkänen L., Koivisto A. J., Nykäsenoja H., Wolff H., Savolainen K. and Alenius H (2010) Inhalation exposure to nanosized and fine TiO2 particles inhibits features of allergic asthma in a murine model. Part. Fibre Toxicol., 7, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rossi E. M., Pylkkänen L., Koivisto A. J. et al. (2010) Airway exposure to silica-coated TiO2 nanoparticles induces pulmonary neutrophilia in mice. Toxicol. Sci., 113, 422–433. [DOI] [PubMed] [Google Scholar]
- 69. Gallagher J., Heinrich U., George M., Hendee L., Phillips D. H. and Lewtas J (1994) Formation of DNA adducts in rat lung following chronic inhalation of diesel emissions, carbon black and titanium dioxide particles. Carcinogenesis, 15, 1291–1299. [DOI] [PubMed] [Google Scholar]
- 70. Creutzenberg O., Hansen T., Ernst H., Muhle H., Oberdörster G. and Hamilton R (2008) Toxicity of a quartz with occluded surfaces in a 90-day intratracheal instillation study in rats. Inhal. Toxicol., 20, 995–1008. [DOI] [PubMed] [Google Scholar]
- 71. Osier M., Baggs R. B. and Oberdörster G (1997) Intratracheal instillation versus intratracheal inhalation: influence of cytokines on inflammatory response. Environ. Health Perspect., 105 (Suppl 5), 1265–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yoshiura Y., Izumi H., Oyabu T. et al. (2015) Pulmonary toxicity of well-dispersed titanium dioxide nanoparticles following intratracheal instillation. J. Nanopart. Res., 17, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Okada T., Ogami A., Lee B. W., Kadoya C., Oyabu T. and Myojo T (2016) Pulmonary responses in rat lungs after intratracheal instillation of 4 crystal forms of titanium dioxide nanoparticles. J. Occup. Health, 58, 602–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.