Figure 1.
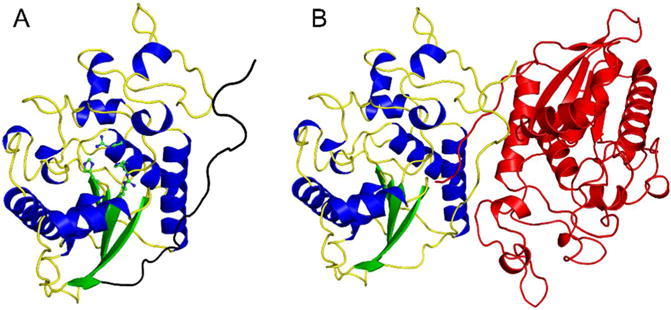
Overall structure of human Sts-1HP. The overall fold of the Sts-1HP protomer is shown (A) with the conserved active site histidine residues (H380 and H565) and arginine residues (R379 and R462) shown in ball and stick representation (carbon atoms colored green and nitrogen atoms colored blue). The dimerization domain is at the C-terminus of the protein and is colored black in this figure. The physiologically relevant dimeric form of Sts-1HP is given in (B) with one chain colored by secondary structure as in (A) and the other colored red.
