Abstract
Background
Cerebral amyloid angiopathy (CAA) is associated with hemorrhagic and nonhemorrhagic markers small vessel disease (SVD). A composite score to quantify the total burden of SVD on MRI specifically for CAA patients was recently developed. Brain network alterations related to individual MRI markers of SVD in CAA were demonstrated.
Objectives
Considering diffusion based network measures sensitive to detect different relevant SVD-related brain injury, we investigated if increased overall SVD injury on MRI corresponds to worse global brain connectivity in CAA.
Methods
Seventy-three patients (79.5% male, mean age 70.58±8.22 years) with a diagnosis CAA were considered. SVD markers in total MRI SVD score included: lobar cerebral microbleeds, cortical superficial siderosis (cSS), white matter hyperintensities (WMH) and centrum semiovale-enlarged perivascular spaces. Diffusion imaging based network reconstruction was made. The associations between total MRI SVD score and global network efficiency (GNE) were analyzed.
Results
A modest significant inverse correlation between total MRI SVD score and GNE existed (p=0.013; R2=0.07). GNE was related with the presence of cSS and moderate-severe WMHs.
Conclusions
An increased burden of SVD neuroimaging markers corresponds to more reductions in global brain connectivity, implying a possible cumulative effect of overall SVD markers on disrupted physiology. GNE was related with some components of the score, specifically cSS and moderate-severe WMHs.
Keywords: cerebral amyloid angiopathy, global network efficiency, neuroimaging markers, brain connectivity, global injury burden
Background and Objective
Cerebral amyloid angiopathy (CAA) represents a common cause of spontaneous lobar intracerebral hemorrhage (ICH) and cognitive impairment in the elderly [1]. CAA is associated with a high prevalence of markers of small vessel disease (SVD), including hemorrhagic and nonhemorrhagic markers [1]. Hemorrhagic and non-hemorrhagic markers often occur together, but the idea of addressing all features combined as a unitary measure of SVD has only gained attention recently [2–3]. A composite score to quantify the total burden of SVD on MRI was developed by Charidimou et al. in 2016 specifically for CAA patients, based on the key neuroimaging markers of the disease [3].
Brain network alterations related to individual MRI markers of SVD in CAA were recently demonstrated [4]. We hypothesized that overall SVD burden in CAA measured with the composite score would disrupt brain network connectivity. In turn, this could predict clinical severity of the disease and its phenotypic expression. Considering diffusion based network measures sensitive to detect different relevant SVD-related brain injury, we aimed to investigate if increased overall SVD injury on MRI (total MRI SVD score) corresponds to worse global brain connectivity (evaluated by diffusion imaging based network analysis) in CAA.
Materials and Methods
Cross-sectional data was obtained from an ongoing single-center cohort study on CAA. One-hundred-thirty-two patients with a diagnosis of CAA according to the Boston criteria presented to Massachusetts General Hospital (MGH) between March 2006 and July 2015 were eligible for the current study [5]. The Institutional Review Board approved the study and informed consent was obtained from all participants. Inclusion criteria were age ≥ 55 years; fulfillment of the Boston criteria for the diagnosis of probable or definite CAA [5]; diffusion weighted sequences available on 1.5 Tesla research brain MRI scan [4]. Out of 74 subjects, 73 patients, 5.5% with a diagnosis of definite CAA and 94.5% with probable CAA, were included in the analysis and one patient was excluded because MRI sequences were technically inadequate for diffusion imaging based network reconstruction.
MRI scans were rated for SVD markers by trained observers, according to STandards for ReportIng Vascular changes on nEuroimaging (STRIVE) [6]. Raters were blinded to all clinical data. Detection of each SVD marker was performed by 5 trained raters. Twenty randomly selected MRI scans were independently assessed by the 5 raters to evaluate inter-rater agreement. SVD markers included: lobar cerebral microbleeds (CMBs), and cortical superficial siderosis (cSS) (rated on susceptibility weighted images), white matter hyperintensities (WMH) (rated on FLAIR images) and centrum semiovale (CSO)-enlarged perivascular spaces (EPVS) (rated on T1 weighted images). More details of markers included in total MRI SVD score in CAA are described in the paper of Charidimou et al [3]. In our cohort, CSO-EPVS were rated on axial T1-weighted MR images, according to a validated 4-point visual scale [7]. We dichotomized degrees of EPVS into high (score 4, i.e. >20) and low (score 1 to 3) (EPVS intra- and inter-rater Cohen's Kappa agreement: 0.90±0.09 and 0.89±0.10, respectively).
The total MRI SVD score ranged from a minimum of 0 to a maximum of 6 points, by counting the presence of each of these 4 MRI features [i.e. lobar CMBs, cSS, WMH and CSO- EPVS], as described [3] (Table 1).
Table 1.
Total MRI small vessel disease score: MRI markers, definitions and points.
| MRI marker | Definition | Score |
|---|---|---|
| Lobar CMBs | 2–4 CMBs | 1 point |
| ≥ 5 CMBs | 2 points | |
| cSS | Focal cSS (≤3 sulci) | 1 point |
| Disseminated cSS (≥4 sulci) | 2 points | |
| CSO-EPVS | Moderate-to severe, i.e. ≥20 CSO-EPVS | 1 point |
| WMH | Confluent deep WMH (Fazekas score 2 or 3) and/or irregular periventricular WMH extending into the deep white matter (Fazekas score 3) | 1 point |
| from Charidimou et al.3 | ||
CMBs=cerebral microbleeds, cSS=cortical superficial siderosis, CSO-EPVS=centrum semiovale - enlarged perivascular spaces, WMH=white matter hyperintensities
Total brain volume (TBV) was obtained from T1-weighted multi-echo MPRAGE (MEMPRAGE) scans using the FreeSurfer neuroimaging analysis software’s (version 5.3) segmentation algorithm (http://surfer.nmr.mgh.harvard.edu) as previously described [4].
Diffusion imaging based network reconstruction: High angular resolution diffusion imaging (HARDI) scans were collected and processed as previously described [4]. In brief, whole-brain fiber tractography was performed using deterministic streamline constrained spherical deconvolution. The cortical and subcortical gray matter were parcellated into 90 regions and the mean FA of the fiber tracts connecting each pair of brain regions was calculated. Because we are interested in the effect of SVD on structural connectivity independent of ICH, we limited our analysis to the network of the ICH-free hemisphere. For each hemispheric network we calculated the global efficiency of the network in accordance to our cross-sectional analysis [4].
The associations between total MRI SVD score (independent variable) and global network efficiency (GNE) (dependent variable) were analyzed using linear regression analysis adjusted for age and sex, and secondary for normalized TBV. Analyses were repeated using a stricter cut-off for the number of CMBs (i.e. 2–19 CMBs = 1 point; >=20 CMBs = 2 points). The association between presence of lacunes, as additional marker of SVD, and GNE was also analyzed.
Results
The study sample consisted of 73 patients (79.5% male, mean age 70.58±8.22 years). According to Boston criteria, 5.5% of patients were diagnosed with definite CAA and 94.5% patients with probable CAA. Among these 73 patients, 39.7% presented with ICH, 27.4% presented with a transient focal neurological episode, and 6.8% with mild cognitive deficits. The prevalence [n (%)] of neuroimaging markers in this cohort are: n=60 (82%) with ≥ 5 CMBs, n=18 (25%) with focal cSS and n=21 (29%) with disseminated cSS, n=45 (62%) with >20 CSO-EPVS, n=50 (69%) with moderate-severe WMH. The total MRI SVD score followed a normal distribution range (mean±SD: 3.9±1.40) range (1–6).
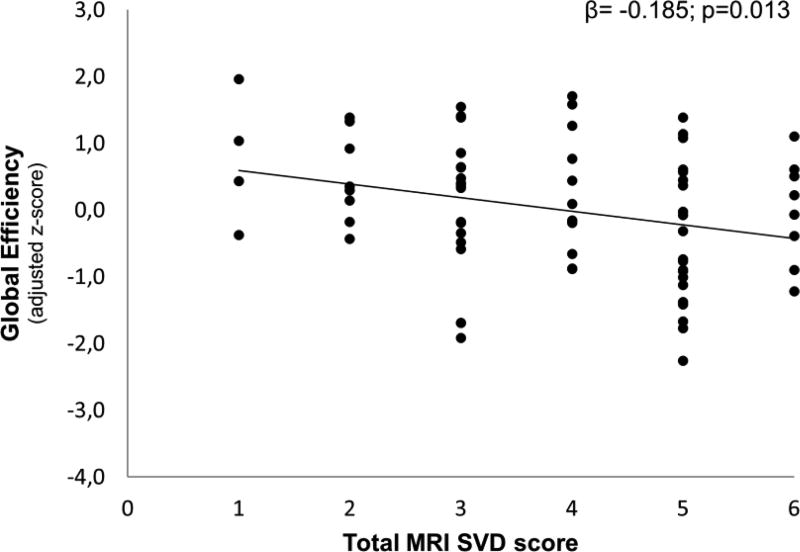
Figure 1 shows the association between total MRI SVD score and GNE. For each point increase in total MRI SVD score, GNE decreased with −0.185(−0.329;−0.041) SD (p=0.013; R2=0.07). Adjustment for normalized TBV did not change the results (p=0.030). Using a stricter cut-off score for the number of CMBs did also not notably change the results (β=−0.199, p=0.013). Of the individual SVD components of the total MRI SVD score, presence of cSS and moderate-severe WMHs were negatively associated with GNE (adjusted mean differences ± SEM: −0.013 ± 0.005 p=0.021 and −0.010 ± 0.005 p=0.046, respectively). The presence of lacunes, which was not included in the MRI SVD score, was also negatively associated with GNE (−0.604 ± 0.208, p=0.005) independent of the other SVD markers (i.e. the associations between GNE and cSS or WMH remained significant p<0.001).
Figure 1. Scatterplot of the association between total MRI SVD score and global network efficiency.
Relationship between the total MRI SVD score and global network efficiency in patients with CAA. Global efficiency is expressed as standardized z-scores adjusted for age and sex.
Discussion
In the current study we examined whether an increased burden of SVD in patients with CAA was associated with greater impairments in structural network efficiency. We showed a modest significant inverse correlation between total MRI SVD score and GNE in CAA patients. Among elements within the MRI SVD score, GNE was related with the presence of cSS and moderate-severe WMHs.
We applied the composite SVD score of overall SVD load by summing some MRI features of SVD created by extensive work of two groups. Our results showed more severe CAA injury on MRI corresponds to more reductions in global brain connectivity, implying a possible cumulative effect of overall SVD markers on disrupted physiology [4]. However, not quite unexpectedly, not every SVD component of the total MRI SVD score seemed to significantly contribute to the reduction in network efficiency. Possible explanations for this finding are: 1. the cut-off score for each SVD marker within the score was not optimal for this sample (e.g. almost all patients have CMBs); 2. a possible ceiling effect due to advanced CAA severity in all patients; 3. some CAA SVD markers (e.g. cortical microinfarcts, cSS) are markers of cortical brain injury, whereas the network measures may be more sensitive to injury in the subcortical white matter; 4. individual SVD markers do not add up in a synergistic fashion but represent shared diffuse underlying pathology; 5. the sample size is small and type II error, particularly for less prevalent markers or for those markers with weaker associations with GNE (CMBs and CSO-EPVS) is possible.
The possible cumulative effect of overall SVD markers on disrupted physiology in CAA patients could somehow predict the clinical severity of the disease and its phenotypic expression.
An important limitation of this preliminary study are the small sample size and the lack of control group. The cross-sectional nature of the study precludes us from investigating longitudinal associations between SVD burden and GNE. Moreover, we are unable to determine whether the observed associations are causal in nature.
Further research with larger CAA cohorts and control groups focusing on the association between burden of SVD neuroimaging markers and impairments in structural network efficiency are needed.
Highlights.
-
✓
Overall SVD injury on MRI corresponds to worse global brain connectivity in CAA
-
✓
Inverse correlation between total MRI SVD score and network efficiency exists
-
✓
Cumulative effect of overall SVD markers on disrupted physiology is possible
-
✓
Global network efficiency is related with some components of the MRI SVD score
Acknowledgments
This work was supported by the NIH (grants R01AG047975, R01AG026484, P50AG005134, K23AG02872605).
Boulouis G. was supported by a J. William Fulbright Research Scholarschip and a Monahan Foundation Biomedical Research Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors and their individual contributions to the manuscript
Raffaella Valenti: project concept and design, imaging analysis, data interpretation, data and statistical analysis, write up
Yael D. Reijmer: project concept and design, imaging analysis, data interpretation, data and statistical analysis, write up, critical revisions
Andreas Charidimou: project concept and design, data interpretation, data and statistical analysis, write up, critical revisions
Gregoire Boulouis: imaging analysis, data collection, data analysis, critical revisions
Sergi Ramirez Martinez: data analysis, critical revisions
Li Xiong: project concept and design, data interpretation, statistical analysis, critical revisions
Panagiotis Fotiadis: imaging analysis
Michael Jessel: data collection and management
Alison M. Ayres: data collection and management
Grace Riley: data collection and management
Leonardo Pantoni: critical revisions
Mahmut Edip Gurol: data collection
Steven M. Greenberg: funding, critical revisions
Anand Viswanathan: project concept and design, funding, write up, critical revisions
Conflict of Interest
The authors report no disclosures relevant to this work.
References
- 1.Greenberg SM, Al-Shahi Salman R, Biessels GJ, van Buchem M, Cordonnier C, Lee JM, et al. Outcome markers for clinical trials in cerebral amyloid angiopathy. Lancet Neurol. 2014;13:419–428. doi: 10.1016/S1474-4422(14)70003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Staals J, Booth T, Morris Z, Bastin ME, Gow AJ, Corley J, et al. Total MRI load of cerebral small vessel disease and cognitive ability in older people. Neurobiol Aging. 2015;36:2806–2811. doi: 10.1016/j.neurobiolaging.2015.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charidimou A, Martinez-Ramirez S, Reijmer YD, Oliveira-Filho J, Lauer A, Roongpiboonsopit D, et al. Total Magnetic Resonance Imaging Burden of Small Vessel Disease in Cerebral Amyloid Angiopathy: An Imaging-Pathologic Study of Concept Validation. JAMA Neurol. 2016;73:994–1001. doi: 10.1001/jamaneurol.2016.0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reijmer YD, Fotiadis P, Martinez-Ramirez S, Salat DH, Schultz A, Shoamanesh A, et al. Structural network alterations and neurological dysfunction in cerebral amyloid angiopathy. Brain: a journal of neurology. 2015;138:179–188. doi: 10.1093/brain/awu316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56:537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- 6.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. The Lancet Neurology. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez-Ramirez S, Pontes-Neto OM, Dumas AP, Auriel E, Halpin A, Quimby M, et al. Topography of dilated perivascular spaces in subjects from a memory clinic cohort. Neurology. 2013;80:1551–1556. doi: 10.1212/WNL.0b013e31828f1876. [DOI] [PMC free article] [PubMed] [Google Scholar]